#pathogen testing
Text
these crazy bastards are using magnets to isolate bacteria from whole blood samples
#and it only takes an hour!#which is definitely way better than the usual 48ish hours it takes to get a pure culture#the paper was published yesterday. these scientists are using magnetic nanoparticles coated in a protein that binds to a bunch of pathogens#then used a magnet to separate the bacteria from the blood#then used a multiplex assay to ID the pathogen and microtiter plates for susceptibility testing#their average turnaround time was ~13 hours!!!!!#it usually takes about 60 hours from inoculation to ID/AST so that TAT difference is kind of crazy#i'm not sure how cost effective this is but i'm excited to see where it goes#okay that's all sorry
2 notes
·
View notes
Text
no sink in the lab i work in which already annoyed me from a biosafety perspective but i've just done a first aid course where they showed some really nasty burn pictures and now all i can think about is we have three sources of extreme heat and no running water :/
#have to go through two sets of secure doors to get to the nearest one and i don't like that!#we also have one first aid kit for the whole building. which is kept on a different floor to where the labs are.#i want one per lab ideally but there should at least be one for this floor#in an emergency i don't want to have to run up two flights of stairs into an office area while covered in pathogenic bacteria#anyway. got 99 on the cpr test and 100 on the overall first aid test so i think that means i can do necromancy now
2 notes
·
View notes
Text


you're not gonna believe this, but i have a solution to this issue
#vegan#veganism#animal testing#animal trafficking#monkey#monkies#pathogen#bioterrorism#*pretends to be shocked*#cdc#the guardian
7 notes
·
View notes
Text
Food Pathogen Testing Market Size, Share and Forecast [Latest]
0 notes
Text
The Legionella Testing Market is set to grow significantly with key advancements in water testing

The Legionella testing market involves identification and quantification of Legionella bacteria that causes Legionellosis or Pontiac fever. Legionella testing helps diagnose infections and also monitor the effectiveness of water treatments. The growing complexity and scale of water systems and increasing regulations for testing of potable and recycled water have driven the demand for Legionella testing products and services.
The global legionella testing market size was valued at US$ 312.6 Mn in 2023 and is expected to reach US$ 553.3 Mn by 2030, grow at a compound annual growth rate (CAGR) of 8.5% from 2023 to 2030.
Key Takeaways
Key players operating in the Legionella Testing market are Eurofins Scientific, ALS Limited, Bio-Rad Laboratories, IDEXX Laboratories, Aqua Legion UK, Palintest, LuminUltra, Special Pathogens Laboratory, Romer Labs, Real Time Lab Services, Abbott, Beckman Coulter, Inc., BD, Pro Lab Diagnostics Inc. These players are focusing on new technologies and product launches to consolidate their position in the market.
The growing complexity of building water systems, increasing awareness about Legionella, and strict regulations have been fueling the Legionella Testing Market Demand. Various industries and sectors are implementing preventive Legionella testing plans to safeguard public health.
The Legionella testing market is expanding globally with increasing awareness in developing nations. Key players are focusing on partnerships, acquisitions, and geographical expansions to enter new markets and leverage lucrative opportunities.
Market Key Trends
Adoption of automated and integrated platforms: There is a growing adoption of automated and integrated platforms for Legionella Testing Companies that offer multiplexed detection with high throughput. Automation enables standardization and efficiency in testing.
Increasing preference for PCR-based methods: Polymerase chain reaction (PCR)-based methods are increasingly becoming the standard for Legionella testing due to advantages like sensitivity, accuracy, standardization, and ability to detect low colony-forming units. Real-time PCR is widely adopted for same-day results.
Porter’s Analysis
Threat of new entrants: Cost of equipment and accreditation requirements limit new entrants.
Bargaining power of buyers: Variety of test methods give buyers options to choose from and negotiate on pricing.
Bargaining power of suppliers: Standardization of testing protocols provides less differentiation in supplies, increasing buyer power.
Threat of new substitutes: No effective substitutes available for accurate and rapid detection of Legionella bacteria currently exist.
Competitive rivalry: High level of competition exists among existing players to gain market share through expanding service capabilities and geographic reach.
Geographical Regions
North America accounts for the largest share of the Legionella testing market currently, supported by stringent regulations and awareness levels regarding Legionella detection and prevention.
The Asia Pacific region is poised to experience the fastest growth over the forecast period due to increasing incidence of Legionnaires' disease, rising healthcare expenditures, and growing adoption of advanced water testing methods across countries like China and India.
Get more insights on Legionella Testing Market
Unlock More Insights—Explore the Report in the Language You Prefer
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights.
(LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
#Coherent Market Insights#Legionella Testing Market#Legionella Testing#Legionella Bacteria#Waterborne Pathogens#Legionella Prevention#Water Quality#Legionella Testing Market Demand#Legionella Testing Market Trends
0 notes
Text
The food pathogen safety testing equipment and supplies market is experiencing significant global growth due to growing concerns about food safety and its impact on consumer health. Stricter regulations and high-profile foodborne illness outbreaks have increased public awareness, driving the demand for advanced testing solutions.
#Food Pathogen Safety Testing Equipment and Supplies Market#Food Pathogen Safety Testing Equipment and Supplies#Food Pathogen Safety Testing Equipment and Supplies Market Size#Food Pathogen Safety Testing Equipment and Supplies Market Share#Food Pathogen Safety Testing Equipment and Supplies Market Growth#Food Pathogen Safety Testing Equipment and Supplies Market Trends#Food Pathogen Safety Testing Equipment and Supplies Market Forecast#Food Pathogen Safety Testing Equipment and Supplies Market Analysis#Food Pathogen Safety Testing Equipment and Supplies Market Report#Food Pathogen Safety Testing Equipment and Supplies Market Scope#Food Pathogen Safety Testing Equipment and Supplies Market Overview#Food Pathogen Safety Testing Equipment and Supplies Market Outlook#Food Pathogen Safety Testing Equipment and Supplies Market Drivers#Food Pathogen Safety Testing Equipment and Supplies Industry#Food Pathogen Safety Testing Equipment and Supplies Companies
0 notes
Text
Food Testing in Ahmedabad | Equinox Labs
Get complete food testing services in Ahmedabad to help ensure your food product's nutritional labeling and shelf life. Read more.....

#EquinoxLabs#CommitmentToSafety#BIScertification#FoodManufacturing#Fssaiupdates#Fssailicense#FoodHandlers#FoodSafety#SafeFood#FoodTesting#worldfoodsafety#foodindustry#foodsecurity#foodquality#fssairegistration#horeca#foodbusinesses#Food testing labs in Hyderabad#food testing in Hyderabad#food quality testing in Hyderabad#food microbiology testing in Hyderabad#nabl accredited labs in Hyderabad#food pathogen testing in Hyderabad#food nutrition testing in Hyderabad#shelf life testing labs in Hyderabad#sensory evaluation labs in Hyderabad#FSSAI-approved labs in Hyderabad#Best food testing in Hyderabad#Nutritional labeling in Hyderabad#shelf life testing in Hyderabad
0 notes
Text
The food pathogen safety testing equipment and supplies market is estimated to be valued at USD 7.4 billion in 2023 and is projected to reach USD 10.4 billion by 2028, at a CAGR of 7.1% from 2023 to 2028.
#Food Pathogen Safety Testing Equipment and Supplies Market#Food Pathogen Safety Testing Equipment and Supplies#Food Pathogen Safety Testing Equipment and Supplies Market Size#Food Pathogen Safety Testing Equipment and Supplies Market Share#Food Pathogen Safety Testing Equipment and Supplies Market Growth#Food Pathogen Safety Testing Equipment and Supplies Market Trends#Food Pathogen Safety Testing Equipment and Supplies Market Forecast#Food Pathogen Safety Testing Equipment and Supplies Market Analysis#Food Pathogen Safety Testing Equipment and Supplies Market Report#Food Pathogen Safety Testing Equipment and Supplies Market Scope#Food Pathogen Safety Testing Equipment and Supplies Market Overview#Food Pathogen Safety Testing Equipment and Supplies Market Outlook#Food Pathogen Safety Testing Equipment and Supplies Market Drivers#Food Pathogen Safety Testing Equipment and Supplies Industry#Food Pathogen Safety Testing Equipment and Supplies Companies
0 notes
Text
Animal Feed Testing Market - Forecast (2023 - 2028)
Animal Feed Testing involves the inspection and testing of animal feed. Animal feed testing is performed to understand the quality, nutritional value, contamination levels, formulation, and many other parameters. The market for animal feed testing has been broadly classified based on the type of feed and type of testing.
#animal feed testing market#animal feed testing market size#animal feed testing#pathogen testing#feed ingredient analysis#pesticides and fertilizers analysis#nutritional labeling#proximate analysis
0 notes
Text

All Food Testing Services in Bengaluru, Karnataka
All food testing services in Bengaluru. FSSAI and NABL-approved lab and well equipped for food microbial testing, nutritional labeling, and shelf life testing
#EquinoxLabs#CommitmentToSafety#BIScertification#FoodManufacturing#Fssaiupdates#Fssailicense#FoodHandlers#FoodSafety#SafeFood#FoodTesting#worldfoodsafety#foodindustry#foodsecurity#foodquality#fssairegistration#horeca#foodbusinesses#food nutrition testing in Bengaluru#food nutrition testing in Bangalore#Food Pathogen Testing In Bengaluru#food quality testing in Bengaluru#Food testing in Bangalore#Food testing in Bengaluru#Food testing laboratory in Bengaluru#Food testing labs in Bangalore#Food testing labs in Bengaluru#Food testing Service in Bangalore#Food testing Service in Bengaluru#FSSAI-approved labs in Bengaluru#FSSAI-approved labs in Bangalore
0 notes
Text
do you know how free i feel rn. like ok i had to take a bit of a pay cut accepting this job but i get to work in a lab where i handle plants & chemicals, doing chemistry shit (which i focused on in my associate's), i will finally get pto, i can have a normal human schedule and not have to work graves anymore, and most importantly of all, i can stop literally handling piss shit & blood all day
#don't get me wrong my current job has been good and is honestly interesting as hell#seeing the workings of a hospital lab has been fascinating#like damn every time ive gotten tested for strep this is what's going on????#but i have gotten sick. so. many. times.#and like my immune system is just not cut out for being around pathogens all day#like we literally test for tuberculosis and tularemia and yersinia etc#and i've been there for 6 months and gotten covid twice in that time span#nevermind the fact that it's graves and nevermind the fact that its just . not the field im passionate abt#i am so fucking excited#not just bc its funny weed lab job like. oh my god im so excited to expand my cv to include chem lab experience#also i never want to cut & grind up someone's toe ever again. thanks#rambles
1 note
·
View note
Text
Chef WK, lead charcuterie specialist in Alberta Canada
Table of contents
1. Control Program Requirements for Fermented Meat Products
2. Facility and Equipment Requirements
3. Starter Culture
4. Chemical Acidification
5. Water Activity Critical Limits
6. Time and Temperature for Fermented Products
7. Fermentation Done at a Constant Temperature
8. Examples of Degree-hours at constant room temperatures
9. Fermentation Done at Different Temperatures
10. Fermentation done at Different temperatures
11. What happens if fermentation fails to hit critical limit?
12. E. coli and Salmonella Control in Fermented Sausages
13. Options for E. coli validation
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
Control Program Requirements for Fermented Meat Products
The producer must have a program in place to assess the incoming product. This program should outline specifications for the incoming ingredients. This may include criteria including receiving temperature, farm/ supplier, lot code or packed on date, species/cut etc.
2. Facility and Equipment Requirements
Equipment used in the fermentation process must be included in the operator's prerequisite control programs. These must include the following elements:
Temperature in the fermentation, drying and smoking chambers must be uniform and controlled to prevent any fluctuation that could impact on the safety of the final product.
Fermentation, drying and smoking chambers must be equipped with a shatter resistant indicating thermometer, (or equivalent), with graduations of 1°C or less. If mercury thermometers are used, their mercury columns must be free from separations. All thermometers must be located such that they can be easily read.
Fermentation and smoking chambers must be equipped with a recording thermometer for determining degree-hours calculations in a reliable manner. Recording thermometers are also preferable in drying and aging rooms but, in these rooms, it may be sufficient to read and record the temperatures 2 times a day.
Drying and aging rooms must be equipped with humidity recorders in order to prevent uncontrolled fluctuations of the relative humidity. The only alternative to an automatic humidity recorder in these rooms would be for the company to manually monitor and record ambient humidity twice a day (morning and afternoon) every day with a properly calibrated portable humidity recorder.
For routine monitoring, accurate measurement electronic pH meters (± 0.05 units) should be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument as well as recommended sample preparation and testing be followed.
When the aw of a product is a critical limit set out in the HACCP plan for a meat product, accurate measurement devices must be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument be followed.
3. Starter Culture
The operator must use a CFIA approved starter culture. This includes Freeze-dried commercially available culture as well as back-slopping (use of previously successful fermented meat used to inoculate a new batch). When performing back-slopping, the operator must have a control program in place to prevent the transmission of pathogens from when using the inoculum from a previous batch to initiate the fermentation process of a new batch. These must include:
The storage temperature must be maintained at 4°C or less and a pH of 5.3 or less.
Samples for microbiological analysis must be taken to ensure that the process is in line with the specifications.
The frequency of sampling is to be adjusted according to compliance to specifications.
Any batch of inoculum which has a pH greater than 5.3 must be analysed to detect at least Staphylococcus aureus. Only upon satisfactory results will this inoculum be permitted for use in back slopping.
This can be an expensive and a time exhaustive process and is generally avoided due to food safety concerns. AHS does not allow back-slopping.
[Chef WK was in communication with the U of A to get his method, a starter mix, studied.]
4. Chemical Acidification
If product is chemically acidified by addition of citric acid, glucono-delta-lactone or another chemical agent approved for this purpose, controls must be in place and records kept to ensure that a pH of 5.3 or lower is achieved by the end of the fermentation process. These acids are encapsulated in different coatings that melt at specific temperatures, which then release the powdered acids into the meat batter and directly chemically acidulate the protein.
Summer sausage is a very common chemically acidified product. The flavor profile tends to be monotone and lacking depth.
5. Water Activity Critical Limits
The aw may be reduced by adding solutes (salt, sugar) or removing moisture.
Approximate minimum levels of aw (if considered alone) for the growth of:
molds: 0.61 to 0.96
yeasts: 0.62 to 0.90
bacteria: 0.86 to 0.97
Clostridium botulinum: 0.95 to 0.97
Clostridium perfringens: 0.95
Enterobacteriaceae: 0.94 to 0.97
Pseudomonas fluorescens: 0.97
Salmonella: 0.92 - 0.95
Staphylococcus aureus: 0.86
parasites: Trichinella spiralis��will survive at an aw of 0.93 but is destroyed at an aw of 0.85 or less.
The above levels are based on the absence of other inhibitory effects such as nitrite, competitive growth, sub-optimum temperatures, etc., which may be present in meat products. In normal conditions, Staphylococcus aureus enterotoxins are not produced below aw 0.86, although in vacuum packed products this is unlikely below aw 0.89.
6. Time and Temperature for Fermented Products
Certain strains of the bacteria Staphylococcus aureus are capable of producing a highly heat stable toxin that causes illness in humans. Above a critical temperature of 15.6°C, Staphylococcus aureus multiplication and toxin production can take place. Once a pH of 5.3 is reached, Staphylococcus aureus multiplication and toxin production are stopped.
Degree-hours are the product of time as measured in hours at a particular temperature multiplied by the "degrees" measured in excess of 15.6°C (the critical temperature for growth of Staphylococcus aureus). Degree-hours are calculated for each temperature used in the process. The limitation of the number of degree-hours depends upon the highest temperature in the fermentation process prior to the time that a pH of 5.3 or less is attained.
The operator is encouraged to measure temperatures at the surface of the product. Where this is not possible, the operator should utilize fermentation room temperatures. The degree hour calculations are based on fermentation room temperatures. Temperature and humidity should be uniform throughout the fermentation room.
A process can be judged as acceptable provided the product consistently reaches a pH of 5.3 using:
fewer than 665 degree-hours when the highest fermentation temperature is less than 33°C;
fewer than 555 degree-hours when the highest fermentation temperature is between 33° and 37°C; and
fewer than 500 degree-hours when the highest fermentation temperature is greater than 37°C.
This means that as the temperature increases, the amount of time that you have available to reach 5.3 or under is shorter. The warmer the temperature, the sharper the log growth phase of bacteria, which equates to more overshoot in lactic acid production, faster.
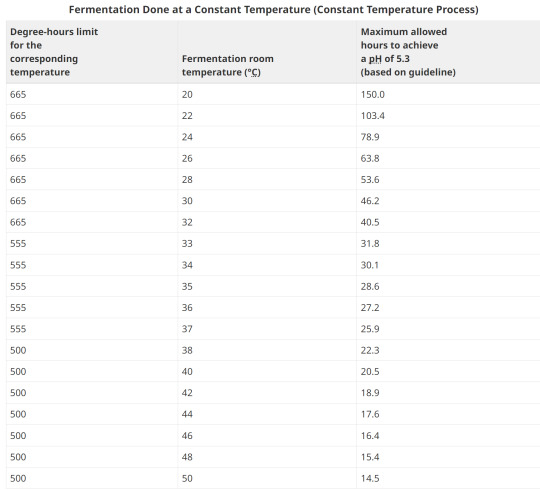
8. Examples of Degree-hours at constant room temperatures
Example 1:
Fermentation room temperature is a constant 26°C. It takes 55 hours for the pH to reach 5.3.
Degrees above 15.6°C: 26°C - 15.6°C = 10.4°C
Hours to reach pH of 5.3: 55
Degree-hours calculation: (10.4°C) x (55) = 572 degree-hours
The corresponding degree-hours limit (less than 33°C) is 665 degree-hours.
Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
Example 2:
Fermentation room temperature is a constant 35°C. It takes 40 hours for the pH to reach 5.3.
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C
Hours to reach pH of 5.3: 40
Degree-hours calculation: (19.4°C) x (40) = 776 degree-hours
The corresponding degree-hours limit (between 33 and 37°C) is 555 degree-hours.
Conclusion: Example 2 does not meet the guideline because its degree-hours exceed the limit
9. Fermentation Done at Different Temperatures
When the fermentation takes place at various temperatures, each temperature step in the process is analyzed for the number of degree-hours it contributes. The degree-hours limit for the entire fermentation process is based on the highest temperature reached during fermentation.
Example 1:
It takes 35 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for second 10 hours and 35°C for the final 15 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C
Hours to reach pH of 5.3: 15
Degree-hours calculation: (19.4°C) x (15) = 291 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 291 = 519
The highest temperature reached = 35°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
10. Fermentation done at Different temperatures
Example 2:
It takes 38 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for the second 10 hours and 37°C for the final 18 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 37°C - 15.6°C = 21.4°C
Hours to reach pH of 5.3: 18
Degree-hours calculation: (21.4°C) x (18) = 385.2 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 385.2 = 613.2
The highest temperature reached = 37°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)
Conclusion: Example 2 does not meet the guidelines because its degree-hours exceed the limit.
11. What happens if fermentation fails to hit critical limit?
What happens if the batch takes longer than degree-hours allows? For restaurant level production, it's always safer to discard the product. The toxin that Staph. Aureus produces is heat stable and cannot be cooked to deactivate. In large facilities that produce substantial batches, the operator must notify the CFIA of each case where degree-hours limits have been exceeded. Such lots must be held and samples of product submitted for microbiological laboratory examination after the drying period has been completed. Analyses should be done for Staphylococcus aureus and its enterotoxin, and for principal pathogens, such as E. coli O157:H7, Salmonella, and Clostridium botulinum and Listeria monocytogenes.
If the bacteriological evaluation proves that there are fewer than 104 Staphylococcus aureus per gram and that no enterotoxin or other pathogens are detected, then the product may be sold provided that it is labelled as requiring refrigeration.
In the case of a Staphylococcus aureus level higher than 104 per gram with no enterotoxin present the product may be used in the production of a cooked product but only if the heating process achieves full lethality applicable to the meat product.
In the case where Staphylococcus aureus enterotoxin is detected in the product the product must be destroyed.
12. E. coli and Salmonella Control in Fermented Sausages
Business' that manufacture fermented sausages are required to control for verotoxinogenic E. coli including E. coli O157:H7 and Salmonella when they make this type of product. This includes:
establishments which use beef as an ingredient in a dry or semi-dry fermented meat sausage;
establishments which store or handle uncooked beef on site;
Establishments which do not use beef and do not obtain meat ingredients from establishments which handle beef are not currently required to use one of the five options for the control of E. coli O157:H7 in dry/semi-dry fermented sausages.
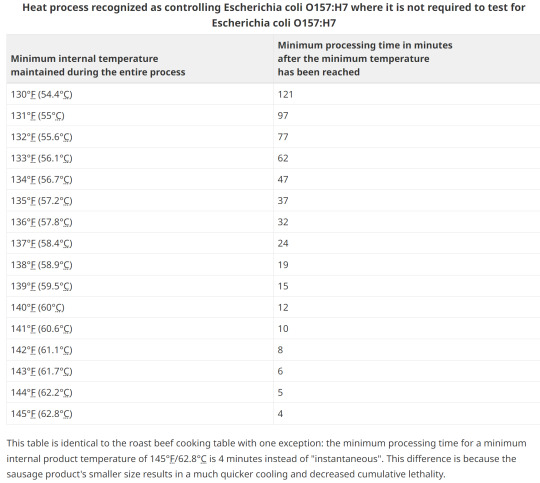
Any processed RTE product containing beef or processed in a facility that also processed beef, must be subjected to a heat treatment step to control E. coli O157:H7. Heating to an internal temperature of 71°C for 15 seconds or other treatment to achieve a 5D reduction is necessary. This is a CFIA requirement and is not negotiable.
Uncooked air dried products produced as RTE, must meet shelf stable requirements as detailed for Fermented-Dry products.
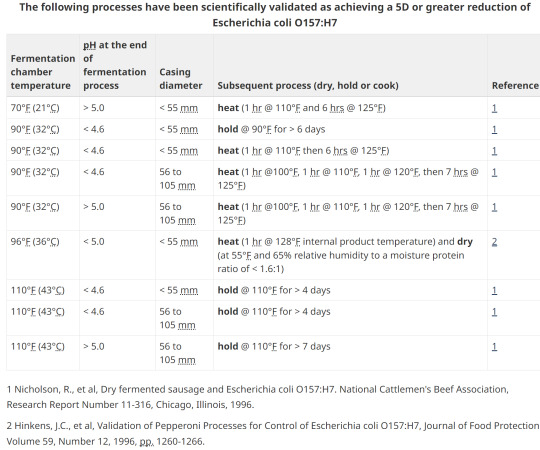
13. Options for E. coli validation
Without lab testing, the two main methods of validation are with heat treating by either low temp and a long duration, or various hotter processing temperatures for a shorter timeframe.
A challenge study to validate a process can take 1 year and over $100,000!
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
The aw and pH values are critical in the control of pathogens as well as to ensure shelf-stability in all semi-dry and dry fermented meat products. Each batch must be tested for aw and/or pH in order to verify that the critical limits are met.
Although aw measurement is mandatory only for shelf stable products, it is strongly recommended that the producer determine the aw values achieved for each product type they manufacture and for each product. Once this has been established, frequent regular checks should be made to ensure consistency. In the U.S., they rely on moisture to protein ratio and have set targets. This lab-tested value is a direct correlation of the % water to % meat protein and not aw. This gives more consistency to common names. For example, to legally call a product "jerky" it must have a MPR of 0.75:1 or lower.
Remember your ABCs:
Always be compliant.
-AND-
Documentation or it didn't happen.
(tags)
Charcuterie,Fermented Meat,Food Safety,Starter Culture,Chemical Acidification,Water Activity,Fermentation Process,Degree-Hours Method,Foodborne Pathogens,Meat Processing Guidelines,Chef WK Alberta Canada,Food Industry Standards,pH Critical Limits,Thermal Processing,Food Preservation,Food Microbiology,Sausage Fermentation,Charcuterie Expertise,Fermented Meats ,Food Safety Standards,Food Processing Guidelines,Starter Cultures,Chemical Acidification,Water Activity (a_w),Critical Limits,Degree-Hours Method,Foodborne Pathogens,Meat Processing Equipment,Processing Facility Requirements,Hazard Analysis and Critical Control Points (HACCP),Food Preservation Techniques,Temperature Control,Pathogen Reduction,Food Industry Compliance,Documentation Practices,Heat Treatment,pH Control,Food Stability,Consistency in Production,Microbial Testing,Real-time Monitoring,Process Validation,Regulatory Requirements,Verotoxigenic E. coli,Lethality Standards,Product Labelling,Spoilage Prevention,Enterotoxin Detection,Shelf-Stable Products,Moisture to Protein Ratio (MPR)
#Charcuterie#Fermented Meat#Food Safety#Starter Culture#Chemical Acidification#Water Activity#Fermentation Process#Degree-Hours#Meat Processing Guidelines#Thermal Processing#Food Preservation#Food Microbiology#Sausage Fermentation#Starter Cultures#Critical Limits#Meat Processing#Food Preservation Techniques#Temperature Control#Pathogen Reduction#Food Industry#Heat Treatment#pH Control#Food Stability#Microbial Testing#Real-time Monitoring#Process Validation#Spoilage Prevention#Enterotoxin Detection#Shelf-Stable Products#Moisture to Protein Ratio (MPR)
1 note
·
View note
Text
Respiratory Pathogen Testing Kits Market Share, Overview, Competitive Analysis and Forecast 2031
#Respiratory Pathogen Testing Kits Market#Respiratory Pathogen Testing Kits Market Scope#Respiratory Pathogen Testing Kits Market Size#Respiratory Pathogen Testing Kits Market Share
0 notes
Text
Food Pathogen Testing Market by Type (E.coli, Salmonella, Campylobacter, Listeria), Technology (Traditional, Rapid), Food Type (Meat & poultry, Dairy, Processed food, Fruits & Vegetables, Cereals & Grains), & by Region - Global Forecasts to 2028
0 notes
Text

All Food Testing Services in Bengaluru, Karnataka
All food testing services in Bengaluru. FSSAI and NABL-approved lab and well equipped for food microbial testing, nutritional labeling, and shelf life testing
#EquinoxLabs#CommitmentToSafety#BIScertification#FoodManufacturing#Fssaiupdates#Fssailicense#FoodHandlers#FoodSafety#SafeFood#FoodTesting#worldfoodsafety#foodindustry#foodsecurity#foodquality#fssairegistration#horeca#foodbusinesses#food nutrition testing in Bengaluru#food nutrition testing in Bangalore#Food Pathogen Testing In Bengaluru#food quality testing in Bengaluru#Food testing in Bangalore#Food testing in Bengaluru#Food testing laboratory in Bengaluru#Food testing labs in Bangalore#Food testing labs in Bengaluru#Food testing Service in Bangalore#Food testing Service in Bengaluru#FSSAI-approved labs in Bengaluru#FSSAI-approved labs in Bangalore
0 notes