#molecular diagnostics laboratory
Explore tagged Tumblr posts
Text
#Greenarray is a Genomic research & molecular diagnostics laboratory located in Pune#molecular diagnostic laboratory in pune#genomics company in india#laboratory#greenarray#pune#microarray system#molecular biology training course in pune#incubation centre in pune#molecular diagnostic laboratory#covid-19 rtpcr testing#Greenarray#Genomic research#molecular diagnostics laboratory in Pune#Genetic test#molecular diagnostics laboratory
0 notes
Text
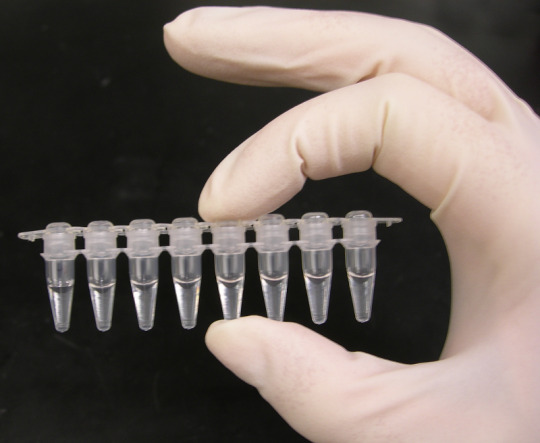
Polymerase chain reaction
“Photo of a strip of PCR tubes, each tube contains a 1000uL (1mL) reaction.” - via Wikimedia Commons
#science#biology#genetics#pcr#pcr tubes#polymerase chain reaction#molecular biology#molecular methods#molecular diagnostics#wikipedia#wikipedia pictures#wikimedia commons#stem#stemblr#stemcore#science aesthetic#sciencecore#lab aesthetic#labcore#laboratory aesthetic#biochemistry#biochemical#biochem#bio
44 notes
·
View notes
Text
The United States IVD market is experiencing robust growth, driven by the increasing prevalence of chronic and infectious diseases, including diabetes, cardiovascular disorders, cancer, and infectious diseases such as COVID-19. This, in turn, has heightened the need for accurate and timely diagnostic tools like IVD, thus creating lucrative growth opportunities for the market.
#United States In Vitro Diagnostics Market Report by Test Type (Clinical Chemistry#Molecular Diagnostics#Immunodiagnostics#Hematology#and Others)#Product (Reagent and Kits#Instruments)#Usability (Disposable IVD Devices#Reusable IVD Devices)#Application (Infectious Disease#Diabetes#Cancer/Oncology#Cardiology#Autoimmune Disease#Nephrology#End User (Hospitals Laboratories#Clinical Laboratories#Point-of-care Testing Centers#Academic Institutes#Patients#and Region 2025-2033
1 note
·
View note
Text
The Impact of Molecular Testing on Modern Cancer Therapy
Molecular biology laboratories have revolutionized cancer diagnosis and treatment. This video explores the basics of cancer genetics and the power of molecular diagnostics. Discover how molecular biomarkers like RNA analysis, immunohistochemistry, and next-generation sequencing (NGS) are transforming cancer care. Learn about groundbreaking advancements in molecularly targeted therapies and the use of CRISPR-Cas9 gene editing technology. Join us as we delve into the future of cancer treatment, where molecular biology laboratories are making precision and personalized medicine a reality.
#molecular biology laboratory#medical diagnostics lab#molecular virology test lab Dubai#diagnostic medical laboratory
1 note
·
View note
Text
#longest phase of cell cycle#molecular diagnostics laboratories#molecular biology#molecular diagnostic
0 notes
Text
Also preserved on our archive
First detection of XEC in Virginia
By Jean Jadhon
ROANOKE, Va. (WDBJ) - A new COVID-19 variant has been detected in half of U.S. states, including Virginia.
It’s the X.E.C. variant.
Some of the earliest cases were reported from samples tested at the Fralin Biomedical Research Institute at Virginia Tech Carilion in Roanoke.
Carla Finkielstein is the director of the Molecular Diagnostics Laboratory and professor at Virginia Tech’s Fralin Biomedical Research Institute. She oversees the lab where they’re tracking the virus.
“We have detected a new variant that is called XEC,” said Finkieltein. The cases we identified all came from Southwest Virginia.”
But Finkielstein says the virus may not have originated here.
“I want to make sure we don’t link the cases to Southwest Virginia because this could be simply people that traveled to Europe and arrived [back] and they live in Southwest Virginia.
Scientists at the Fralin Biomedical Research Institute at Virginia Tech Carilion in Roanoke have been testing samples since the pandemic began; the tests are completed in the same day in an intricate process.
Here they conduct genomic surveillance, tracking the virus at the molecular level. The idea is to catch any changes in the virus early.
“The reason is you want to catch something that is super new immediately that pops up,” said Finkielstein. “So you can report it to the health department.”
The information is also entered into a federal database.
So far Finkielstein says the new variant doesn’t seem to present in patients much differently than past variants of COVID-19.
“It shows kind of the typical symptoms of COVID we’ve seen before. Maybe this one seems to have a little bit more of a sore throat which is a little different,” said Finkielstein.
The lab tests samples from all over Virginia. Finkielstein predicts a surge in COVID cases this winter and is advising everyone to get their COVID vaccine and to wear a mask if you feel sick out of courtesy to your co-workers and friends and to help prevent the spread of the virus.
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#still coviding#sars cov 2#wear a respirator
39 notes
·
View notes
Text
Best Diagnostic Center In Singasandra Bangalore
Life Care Diagnostics

Why choose the best diagnostic center in Singasandra Bangalore?
If you are searching for best diagnostic center in Singasandra Bangalore, you have gotten onto the right track. Any diagnostic center plays a big role in the timely diagnosis and management of diseases, and it must be the right one for you. Accurate reports, state-of-the-art technology, and experienced professionals make a huge difference in delivering healthy solutions. We shall discuss the main services, some technology, and why it is vital to have the best quality diagnostic center for you.
Diverse Types of Tests and Services
These kinds of highly developed diagnostic capabilities must cover any and all tests and services for ailments that could affect any modern individual.
Pathology tests (blood tests, urine tests, biopsy).Radiology services (digital X-ray, ultrasound, CT scan, and MRI).Cardiac investigations (ECG-echocardiogram-treadmill stress test).
Preventive health check-ups (general check-up or annual health check-up, followed by diabetic screening and cholesterol screening).Special tests (genetic screening, allergy testing, and hormone testing).
Cutting-Edge Technology and Equipment
Increasingly sophisticated technology has left little room for doubt or error in the medical testing efficiencies at the latest diagnostic center in Singasandra Bangalore. The following avant-garde technologies are used:
Laboratory Automation: Appropriately regarded as facilitating the highest degree of precision and reliability of test results.
Imaging at High Resolution: The MRI, 3D ultrasound, and digital X-ray recently introduced have unparalleled image quality.
Molecular Diagnostics: Early disease identification through DNA or RNA techniques.
Qualified and Experienced Medical Professionals
The backbone of diagnostic center is its skilled set of healthcare professionals. The best in the diagnostic business of Singasandra Bangalore comprises:
Certified pathologists and radiologists who duly discuss and analyze test results. Competent lab technicians who follows standard medical protocol. Experienced technical staff who attend to patients during the entire testing schedule. Expert consultants for report interpretation and further treatment recommendations.
Conclusion
Whatever your Best Diagnostic Center needs, the most reliable and technologically advanced center is here to deliver accurate and timely results: Lifecare Diagnostic, Singasandra Bangalore. With expert professionals, state-of-the-art equipment, and a commitment to excellence, we ensure the best diagnostic experience for you and your loved ones.
2 notes
·
View notes
Text
WHO press release suggests I was
Meryl Nass Oct 30, 2024 Here is what I wrote last night:
“The WHO never once said what its teams are going to actually do. Or if any doctors or nurses were part of teams. They are not sending in imprecise PCR kits to gin up the cases and deaths, are they?”
And this morning, it looks like that might be exactly the plan:
Wednesday, 30 October 2024
News Release
WHO lists additional mpox diagnostic tests for emergency use
As part of ongoing efforts to enhance quality-assured testing options, the World Health Organization (WHO) has listed two additional mpox in vitro diagnostics under its Emergency Use Listing (EUL) procedure. WHO’s EUL is based on the review of quality, safety and performance data in compliance with international standards while addressing the specific needs of low- and middle-income countries (LMICs).
Polymerase Chain Reaction (PCR) testing, which detects viral DNA, is considered the gold standard for diagnosing mpox infection.
WHO listed the Xpert Mpox, a real-time PCR test manufactured by Cepheid under its EUL procedure, on 25 October. This test is designed for use on compatible GeneXpert systems. The Xpert Mpox test is easy to operate and delivers results in under 40 minutes. Once the cartridge is placed in the system, the process is fully automated, with real-time PCR detecting viral DNA of monkeypox virus clade II. The GeneXpert system is a near-point-of-care testing option, which can support decentralized testing.
Another PCR-based option, the cobas MPXV assay, developed by Roche Molecular Systems, Inc., was listed on 14 October 2024. It is intended for use on the cobas 6800/8800 Systems. This tool is a real-time PCR test capable of detecting both mpox clades and delivering results in under 2 hours. It can process multiple samples simultaneously and is suitable for clinical laboratories that handle large volumes of tests.
“Ensuring global access to mpox diagnostic tests that meet WHO standards for quality, safety and performance is essential for efficient and effective testing in settings affected by mpox outbreaks,” said Dr Rogerio Gaspar, WHO Director for Regulation and Prequalification. “Rapid access to those listed products is critical not only for prompt diagnosis and timely treatment but also for effectively containing the spread of the virus."
WHO previously listed Alinity m MPXV assay, manufactured by Abbott Molecular Inc. under EUL on 3 October.
2 notes
·
View notes
Text

@angelofthemornings Making this a proper reply-post because as a reply it might end up being too big.
The field is very vast, but the most common would be the person that runs the tests a physician asks you to and signs the paper to validate the results - we can be found doing your bloodwork, urine, biochem analysis (which all falls under clinical analysis/clinical pathology), and many other fields like radiology (x-rays, tomography, etc), acupuncture, embryology... here in Brazil we can have up to 29 licenses, each one corresponding to a different field. Some of these you can come out of university ready to work due to experience in your internship, but some others will require a specialization course or a master's - like acupuncture and radiology.
My license is in clinical pathology, but I've also spent a year in a specialization/expertise program (after getting my bachelor's degree) in laboratorial surveillance for diseases of public health interest, with a focus on diagnostic tools of immunology (serology tests) and molecular biology (mostly real time Polymerase Chair Reaction - PCR).
So I'm both licensed to work in any hospital or particular lab to run tests (with the objective of a diagnosis), and to work with the government in public health surveillance (where the objective here isn't a diagnosis, it is to confirm cases of a disease in a certain population and keep an eye out on how it behaves throughout the year, epidemiology).
I was about to decide between a Master's or a "direct" Doctorate (a Doctorate that "skips" a Master's and lasts longer than the usual Doctorate program) but then the pandemic came and many internship deals crashed, unless you were in virology, as that became the main and only focus of research at the time. I tried for a spot in the State's Strategic Lab, but it was interview-only and for only 1 candidate - I was placed 4th.
With that, my grandma's health also began to deteriorate, so I've been staying home since then to help care for her.
But 4 years later, I've started to job hunt again, and I'm now afraid I might be overqualified (and thus, "more expensive") to employers, as so far I havent gotten any return from my applications. I quite miss and wish to return to academia, sometimes, and with the State's lab, but I have a love-hate relationship with academia and it takes them forever to open up sign-ins for employees. Working in public health was my best time though, really, so if the opportunity arises, I'll be trying my chance there again - I did leave in good terms, and a lot o the chief researchers there wanted to work with me.
#Lay replies#angelofthemornings#I found and fixed an error in their database while doing my analysis to graduate the expertise program#said error might have screwed up another chief researcher's work so it was quite the big deal#GOD I want a position there to open up SO BAD because Im 99% sure they'd take me in like PLEASE WE WORKED TOGETHER SO WELL#IM BEGGIN THE STARS
9 notes
·
View notes
Text
Home PCR Tests: A Closer Look at the PCR Test At Home Dubai Option
The COVID-19 pandemic sparked major growth in the development and usage of diagnostic and antibody tests that patients can self-administer from home. Home PCR tests in particular enable private, convenient detection of active coronavirus infections. For those wondering whether accurate PCR Test At Home Dubai kits are available, exploring the leading options provides helpful guidance.

How Do Home PCR Tests for COVID-19 Work?
The PCR (polymerase chain reaction) technique is the gold standard for directly detecting the presence of the COVID-19 virus from respiratory samples. Home PCR test kits allow patients to collect their own nasal or saliva samples and perform the PCR assay without visiting a clinic.
PCR tests work by identifying the specific genetic material of the COVID-19 virus. Users collect a sample, mix it with chemical reagents, and insert the solution into the test kit for analysis. Results are displayed indicating whether viral genetic material was detected based on any color change reaction on the test strips.
Kits include step-by-step instructions to ensure patients perform the easy, quick tests properly using non-invasive nasal swabs or saliva collection. Many provide results within 10-30 minutes.
Here is a video from MedCram Youtube Channel about At Home Rapid COVID 19 Tests and False Positives (Coronavirus Antigen Tests). Watch the video
youtube
Benefits of At-Home PCR Testing
Here are some of the major advantages of having access to accurate home PCR tests for COVID-19:
Convenience: Test from the privacy of your residence without traveling to clinics.
Speed: Get results rapidly within minutes rather than waiting days for lab tests.
Self-Administered: Users can collect their own sample comfortably rather than relying on technicians.
Affordability: Individual kits are very competitively priced.
Detection Reliability: PCR technology directly identifies viral presence with high accuracy.
Ease of Use: Tests have simple, straightforward instructions for patients of all ages.
Infection Verification: Confirms active infections unlike antibody tests.
Having the option to privately, quickly, and accurately test for possible COVID-19 infections at home provides significant peace of mind during the pandemic.
How Reliable Are Home PCR Tests?
Many people reasonably wonder whether DIY home PCR test kits can match the reliability of lab-based PCR tests. The good news is that leading home PCR kits on the market have very high accuracy.
Most kits have published sensitivity and specificity above 90% when compared to lab PCR tests. High quality home tests analyze samples using comparable PCR methodology and match labs in detecting positives and negatives.
Furthermore, unlike Rapid PCR Test At Home kits some vendors offer, full home PCR tests analyze the sample through many amplification cycles to maximize accuracy. With good sampling collection, top home PCR kits offer laboratory-grade results conveniently at home.
Leading Home PCR Test Kit Options
For those exploring PCR Test At Home Dubai choices, here are some of the top-rated home PCR kits to consider:
Cue Health PCR Test: Cue offers an FDA-authorized home PCR test delivering highly accurate results in 20 minutes with nasal swab samples.
Lucira Check It PCR Test: This is a single-use PCR kit with 98% validated accuracy that provides molecular-level detection from nasal samples in 30 minutes or less.
Ellume COVID-19 Home Test: This over-the-counter home kit uses a mid-turbinate nasal sample and provides an amplified PCR digital reading of positive or negative in 15 minutes on a connected analyzer.
Pixel by LabCorp PCR Test: Pixel is a monitored at-home nasal PCR test analyzed through LabCorp with over 98% accuracy returning results within 1-2 days.
Doximity's Covid-19 PCR Test: Doximity partners with qualified labs for monitored video-observed PCR testing with 97%+ accuracy and results in 24 hours.
All these options allow for convenient, accurate at-home COVID-19 testing using PCR with trusted partners. Kits can be purchased online and shipped directly to your home in Dubai.
When Are Home PCR Tests Recommended?
The CDC recommends utilizing home PCR tests in situations such as:
If you have any symptoms of COVID-19. Home testing allows quick confirmation.
After exposure events to quickly check for possible infection.
Before visiting individuals at higher risk for severe illness.
Before travel or group events for added assurance.
For frequent screening in schools or workplaces.
Even fully vaccinated individuals should test if they experience COVID-like symptoms or have a known exposure. Home PCR tests make quick detection fast and easy.
Home PCR Tests Offer Accuracy and Convenience
High quality Home PCR Tests have become an important tool in the fight against COVID by making reliable diagnostic testing accessible outside of clinics. There are excellent PCR Test At Home Dubai options available matching the standards of lab PCR sensitivity and specificity. Home PCR kits allow people to conveniently and confidently check themselves for possible COVID-19 infections from the privacy of home. As the technology continues advancing, home collection PCR will likely take on an increasingly vital role supporting public health and safety.
2 notes
·
View notes
Text
#Genomic research & molecular diagnostics laboratory in Pune#molecular diagnostic laboratory#genomics company in india#laboratory#molecular biology training course in pune#greenarray#molecular diagnostic laboratory in pune#incubation centre in pune#pune#microarray system#covid-19 rtpcr testing#Genomic research#molecular diagnostics laboratory in Pune#Greenarray#Genetic test
0 notes
Text

Proteomics
“TECAN Genesis 2000 robot preparing Ciphergen SELDI-TOF protein chips for proteomic pattern analysis. Highly accurate cancer prognosis can be accomplished by identifying protein patterns of specific cancers using this device.” - via Wikimedia Commons
#tecan genesis 2000#seldi-tof#proteomics#science#biotechnology#biology#biochemistry#biochemical assay#cancer biology#cancer diagnostics#diagnostics#prognostics#medicine#oncology#medblr#wikipedia#wikipedia pictures#wikimedia commons#stemblr#sciencecore#laboratory aesthetic#science aesthetic#lab aesthetic#laboratory automation#automation#machinery#laboratory equipment#laboratory#laboratory science#molecular biology
21 notes
·
View notes
Text
United States point of care diagnostics market size is projected to exhibit a growth rate (CAGR) of 6.90% during 2024-2032. Numerous advancements in portable and handheld diagnostic devices have enhanced the convenience and user-friendliness of testing, which is primarily driving the market growth.
#United States Point of Care Diagnostics Market Report by Product Type (Blood-Glucose Monitoring Kit#Cardio-Metabolic Monitoring Kit#Pregnancy and Fertility Testing Kit#Infectious Disease Testing Kit#Cholesterol Test Strip#Hematology Testing Kit#and Others)#Platform (Lateral Flow Assays#Dipsticks#Microfluidics#Molecular Diagnostics#Immunoassays)#Prescription Mode (Prescription-Based Testing#OTC Testing)#End User (Professional Diagnostic Centers#Home Care#Research Laboratories#and Region 2024-2032
0 notes
Text
Laboratory Proficiency Testing Market Size, Growth Outlook 2035
The Laboratory Proficiency Testing Market Size was estimated at 2.21 (USD Billion) in 2024. The Laboratory Proficiency Testing Market Industry is expected to grow from 2.33 (USD Billion) in 2025 to 3.76 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 5.48% during the forecast period (2025 - 2034).
Market Overview
The Laboratory Proficiency Testing Market is experiencing significant growth, driven by the increasing focus on quality assurance in clinical and diagnostic laboratories, regulatory compliance requirements, and advancements in analytical testing. Proficiency testing (PT) is crucial in ensuring the accuracy, reliability, and standardization of laboratory testing procedures across various industries, including healthcare, environmental testing, pharmaceuticals, and food safety.
With the rise in accreditation requirements for diagnostic laboratories, the adoption of external quality assessment (EQA) programs has surged. Additionally, the growing demand for molecular diagnostics and microbiology testing has further expanded the scope of proficiency testing in modern laboratories.
Market Size and Share
The Laboratory Proficiency Testing Market Size was estimated at 2.21 (USD Billion) in 2024. The Laboratory Proficiency Testing Market Industry is expected to grow from 2.33 (USD Billion) in 2025 to 3.76 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 5.48% during the forecast period (2025 - 2034).North America holds the largest market share due to strict regulatory guidelines, high adoption of clinical laboratory accreditation programs, and an increasing number of reference laboratories. The Asia-Pacific region is anticipated to experience the fastest growth due to rising investments in laboratory infrastructure and growing awareness about quality assurance in diagnostic labs.

Market Drivers
Stringent Regulatory & Compliance Requirements: Government and private healthcare bodies mandate the use of proficiency testing programs to ensure laboratory accuracy.
Growing Demand for Accurate Diagnostic Testing: With the increasing prevalence of infectious diseases, chronic conditions, and genetic disorders, the need for error-free laboratory testing has risen.
Technological Advancements in Laboratory Automation: The integration of AI-powered laboratory testing solutions and automated analytical instruments has improved efficiency in testing.
Rising Adoption of External Quality Assurance (EQA) Programs: Laboratories worldwide are increasingly participating in EQA schemes to benchmark their performance and maintain certification.
Expansion of Molecular & Microbiology Testing: The growing use of PCR-based diagnostics, NGS, and microbiology assays has expanded proficiency testing applications.
Challenges and Restraints
High Costs Associated with Proficiency Testing Programs: Many laboratories, especially in developing regions, struggle with the affordability of external quality assessment services.
Limited Awareness in Emerging Economies: Lack of proper knowledge about the benefits of quality control testing in laboratories affects market penetration.
Challenges in Standardization of Testing Procedures: Variability in laboratory testing protocols across different regions can hinder the effectiveness of proficiency testing programs.
Market Trends
Increased Use of Digital & AI-Driven Proficiency Testing Solutions: AI and machine learning algorithms for laboratory quality control are improving proficiency testing accuracy.
Rising Adoption of Proficiency Testing in Food & Water Safety: Beyond healthcare, environmental testing laboratories are increasingly utilizing PT programs.
Expansion of Molecular & Genetic Proficiency Testing Programs: NGS-based laboratory testing assessments are gaining traction in precision medicine research.
Integration of Cloud-Based Laboratory Quality Control Solutions: Many labs are adopting cloud-based PT data management systems for real-time performance tracking.
Regional Analysis
North America: Dominates the market due to strong regulatory policies, high participation in CAP proficiency testing programs, and the presence of leading diagnostic companies.
Europe: Growing demand for ISO 15189-accredited laboratories and increased government funding for EQA programs support market growth.
Asia-Pacific: Rising awareness about laboratory accreditation and expansion of diagnostic facilities drive regional growth.
Rest of the World: The adoption of proficiency testing services in pharmaceutical and clinical laboratories is gradually increasing.
Segmental Analysis
By Industry:
Clinical Diagnostics
Pharmaceuticals & Biotechnology
Environmental & Water Testing
Food & Beverage Testing
By Technology:
Microbiology
Molecular Diagnostics
Immunology
Clinical Chemistry
Haematology
By End-User:
Hospitals & Clinical Laboratories
Research & Academic Institutes
Pharmaceutical Companies
Government & Regulatory Agencies
Key Market Players
Thermo Fisher Scientific
College of American Pathologists
International Accreditation Services
Quality Control Solutions
BD
Roche Diagnostics
Recent Developments
Expansion of Proficiency Testing Programs: CAP launched new molecular pathology PT programs to support genetic and infectious disease testing.
Integration of AI in Laboratory Quality Control: Bio-Rad introduced an AI-powered laboratory proficiency testing software to streamline data analysis.
Collaborations for Global Standardization: Thermo Fisher partnered with regulatory bodies to develop universal PT guidelines for clinical laboratories.
For more information, please visit us at marketresearchfuture.
#Laboratory Proficiency Testing Market Size#Laboratory Proficiency Testing Market Share#Laboratory Proficiency Testing Market Growth#Laboratory Proficiency Testing Market Analysis#Laboratory Proficiency Testing Market Trends#Laboratory Proficiency Testing Market Forecast#Laboratory Proficiency Testing Market Segments
0 notes
Text
Advanced Molecular Diagnostics: COVID-19 Rapid Tests and CXCR4 Mutation Detection Kits

Accurate diagnostic testing plays a crucial role in controlling infectious diseases and advancing cancer research. Rapid COVID-19 antigen tests help detect infections early, while CXCR4 mutation analysis is essential for understanding disease progression and developing targeted therapies. 3B BlackBio Biotech provides high-quality solutions like the COVID Rapid Antigen Test and CXCR4 Mutation Detection Kit, ensuring quick and reliable results for healthcare professionals and researchers.
COVID Rapid Antigen Test: Fast and Effective Detection
The COVID-19 pandemic has highlighted the need for rapid and efficient testing. The COVID-19 Ag Test by 3B BlackBio Biotech is designed for quick and accurate detection of SARS-CoV-2 antigens, making it an essential tool in preventing the spread of the virus. Unlike traditional PCR tests that require laboratory processing, this antigen test delivers results within minutes, making it ideal for point-of-care testing in hospitals, clinics, airports, and workplaces.
Early detection is critical in managing outbreaks and preventing further transmission. The ability to identify positive cases quickly allows healthcare providers to isolate infected individuals and take necessary precautions. The COVID-19 Ag Test is easy to use, requiring a simple nasal or throat swab, and provides results in just 15-20 minutes. Its high sensitivity and specificity ensure accurate detection, making it a valuable tool for large-scale screening efforts.
Frequent testing in high-risk environments such as hospitals, public transport hubs, and densely populated areas can significantly reduce the spread of the virus. As travel restrictions ease and workplaces reopen, reliable antigen testing remains a crucial part of health and safety protocols.
CXCR4 Mutation Detection: Advancing Cancer Research
The CXCR4 gene is a key player in cancer development and progression. It encodes a chemokine receptor that regulates immune responses and cell migration. Mutations in CXCR4 are often associated with hematological malignancies such as chronic lymphocytic leukemia (CLL) and other cancers. These mutations can lead to uncontrolled cell growth, increased drug resistance, and poor patient prognosis. Identifying CXCR4 mutations helps doctors determine the best treatment strategies and improve patient outcomes.
Understanding CXCR4 mutations allows researchers and oncologists to assess disease severity and predict treatment response. Patients with CXCR4 mutations may benefit from targeted therapies designed to inhibit the effects of the mutated receptor, improving overall survival rates. Since these mutations influence how cancer cells interact with their environment, testing for CXCR4 abnormalities provides valuable insights into disease mechanisms and therapy resistance.
CXCR4 Mutation Detection Kit: Precision in Genetic Testing
The CXCR4 Mutations Detection Kit by 3B BlackBio Biotech is an advanced real-time PCR test that accurately identifies CXCR4 mutations. Genetic testing plays a significant role in precision medicine, and this kit ensures high sensitivity and specificity in mutation detection. The test is compatible with standard PCR instruments and delivers fast results, enabling timely and informed treatment decisions.
Cancer research continues to evolve, with genetic testing becoming an essential tool for developing personalized therapies. The CXCR4 Mutation Detection Kit supports oncologists and researchers in identifying CXCR4-related abnormalities, allowing them to tailor treatments based on an individual’s genetic profile.
Conclusion
Accurate and timely diagnostics are essential in managing infectious diseases and advancing cancer treatment. The COVID Rapid Antigen Test by 3B BlackBio Biotech provides a fast and effective solution for COVID-19 detection, helping prevent the spread of the virus. The CXCR4 PCR Kit is a powerful tool in oncology, aiding in precision medicine and targeted cancer therapies. As healthcare continues to evolve, reliable testing solutions remain the backbone of disease prevention and treatment advancements. 3B BlackBio Biotech is committed to providing high-quality diagnostic kits that empower healthcare professionals and researchers in their fight against diseases.
#3b blackbio biotech#3b blackbio#CXCR4 Mutation Detection Kit#COVID Rapid Antigen Test#CXCR4 PCR Kit
0 notes
Text
The Role of ANAB Accredited Labs in Ajman’s Healthcare and Pharmaceutical Sectors | +971 554747210
Ajman’s healthcare and pharmaceutical industries are critical to public health and economic growth. With an increasing focus on quality, safety, and compliance, these sectors rely on ANAB accredited labs to ensure that medical devices, pharmaceuticals, and healthcare products meet international standards.
An ANAB accredited lab in Ajman operates under the stringent guidelines of ISO/IEC 17025, ensuring that all tests and calibrations adhere to globally recognized quality assurance protocols. These labs play a vital role in protecting public health, ensuring regulatory compliance, and supporting innovation in the healthcare sector.
What is an ANAB Accredited Lab?
The ANSI National Accreditation Board (ANAB) accredits laboratories that meet strict quality, technical competency, and operational integrity requirements. ANAB accreditation assures that the testing, calibration, and certification processes performed in these labs meet global regulatory standards, including those set by:
UAE Ministry of Health and Prevention (MOHAP)
Emirates Authority for Standardization and Metrology (ESMA)
World Health Organization (WHO)
U.S. Food and Drug Administration (FDA)
European Medicines Agency (EMA)
How ANAB Accredited Labs Support Ajman’s Healthcare and Pharmaceutical Sectors
1. Pharmaceutical Testing and Quality Assurance
Ensuring that pharmaceutical products meet the highest purity, potency, and safety standards is a top priority for manufacturers. ANAB accredited labs conduct rigorous testing to:
Verify active pharmaceutical ingredient (API) purity
Detect contaminants and heavy metals
Assess stability and shelf life
Evaluate dissolution and bioavailability
Conduct microbiological analysis to prevent contamination
By partnering with an ANAB accredited lab, pharmaceutical companies in Ajman can ensure that their products meet global safety and efficacy standards, reducing the risk of recalls and regulatory non-compliance.
2. Medical Device Testing and Validation
Medical devices, including surgical instruments, diagnostic equipment, and implants, must adhere to strict safety and performance guidelines. ANAB accredited labs provide:
Material composition analysis to verify biocompatibility
Sterilization validation to ensure infection control
Electrical and mechanical safety assessments
Performance testing for device reliability
Failure analysis to detect potential defects
These services help medical device manufacturers in Ajman comply with ISO 13485 standards, ensuring patient safety and product effectiveness.
3. Biochemical and Microbiological Testing
The presence of bacteria, viruses, and other microorganisms in healthcare settings can pose serious risks. ANAB accredited labs in Ajman perform:
Pathogen screening in pharmaceutical and healthcare environments
Endotoxin and pyrogen testing to ensure sterility
Water quality analysis for medical facilities
Environmental monitoring in pharmaceutical manufacturing plants
These tests help prevent infections, improve patient outcomes, and ensure compliance with health and safety regulations.
4. Blood and Tissue Sample Analysis
Accurate testing of blood, plasma, and tissue samples is critical for disease diagnosis and treatment. ANAB accredited labs offer:
Clinical chemistry and hematology testing
Molecular diagnostics for genetic screening
Toxicology testing for drug monitoring
Histopathology analysis for disease detection
These advanced diagnostic services contribute to early disease detection, personalized medicine, and improved patient care in Ajman.
5. Compliance with International Pharmaceutical Regulations
Pharmaceutical companies operating in Ajman must comply with local and international regulatory frameworks to distribute products globally. ANAB accredited labs help ensure compliance with:
Good Manufacturing Practices (GMP)
Good Laboratory Practices (GLP)
Good Clinical Practices (GCP)
U.S. FDA, EMA, and WHO guidelines
Through precise testing and validation, ANAB accredited labs assist companies in meeting stringent international requirements, facilitating global market access for Ajman-based pharmaceutical firms.
6. Water and Air Quality Testing in Healthcare Facilities
Hospitals, clinics, and pharmaceutical production sites require strict water and air quality control to prevent contamination and infection. ANAB accredited labs provide:
Legionella and bacterial testing in hospital water systems
Airborne particle monitoring in cleanrooms and operating rooms
Sterile environment validation for pharmaceutical production
HVAC system inspections to maintain air purity
These environmental monitoring services help maintain safe and sterile conditions, ensuring the health and well-being of patients and healthcare workers.
Key Benefits of Working with an ANAB Accredited Lab in Ajman
1. Ensured Quality and Accuracy
ANAB accredited labs guarantee high-precision testing and validation, reducing errors and ensuring reliable results.
2. Regulatory Compliance and Certification
Pharmaceutical and healthcare companies benefit from seamless regulatory compliance, avoiding costly penalties and legal challenges.
3. Improved Patient Safety
By ensuring drug purity, device safety, and contamination control, ANAB accredited labs play a crucial role in protecting public health.
4. Competitive Advantage in Global Markets
Companies working with ANAB accredited labs can obtain international certifications, making it easier to export products and expand into global markets.
5. Reduced Risk of Recalls and Product Failures
Through rigorous testing and validation, ANAB accredited labs help prevent costly recalls, legal issues, and reputational damage.
Conclusion
The role of ANAB accredited labs in Ajman’s healthcare and pharmaceutical sectors is indispensable. These labs ensure that pharmaceuticals, medical devices, and healthcare facilities meet the highest standards of quality, safety, and regulatory compliance.
By providing advanced testing, certification, and compliance services, ANAB accredited labs help protect public health, support innovation, and enhance Ajman’s reputation as a hub for high-quality healthcare and pharmaceutical manufacturing.
0 notes