#medicine for multiple myeloma
Explore tagged Tumblr posts
Text
My therapist asking me if I had a youtube channel for general cancer info last week honestly got me thinking. Professionally I AM a cancer educator and my audience is other healthcare professionals, but god so daunting to think of presenting to audiences larger than 50 or so at a time :S it has always been a passion to digest high level research and then translate it into understandable language - - I do it a lot for family+friends dealing with ominous or confusing medical news. Idk! He put it really well when he said even my "basic" knowledge can help people better understand some of the most life-altering medical realities affecting them.
#Creepy chatter#Idk lol...i talk thru a lot of complex cancer processes walking thru my apartment to make sure it's accessibly worded#But 80% of that ends up in my noggin and I focus on the more topic specific stuff#Iirc I have multiple myeloma/leukemia/lymphoma on my education docket next but I could spend hours talking about bone marrow alone#If you don't know bone marrow you don't really know myeloma or leukemia after all. They both originate in there!#Gave a breakdown of the exocrine/endocrine pancreas function last week and duuuuuuuuuuuuudes!#To see that act as a successful foundation to the understanding of pancreatic neuroendocrine tumors was so fulfilling!#These topics CAN be accessible and it's my favorite part of my role. Idk if I would end up w a yt channel but#I already talk to myself about neoplasms 8 - 10hrs a day already 🤔#Cancer cw#Medical cw#Sorry if I've forgotten those recently! I am medicine brained more than usual this time of year
14 notes
·
View notes
Text
How Lenalid 25 Capsule Treats
Lenalid 25 Capsule is a medication containing lenalidomide, an immunomodulatory drug used to treat multiple myeloma and myelodysplastic syndromes (MDS). It works by stimulating T cells to attack cancer cells and modifying the immune system. Lenalid 25 Capsule is also used to treat lepra reaction, a complication of leprosy. However, this medication can cause kidney problems as a potential side effect, so patients must be closely monitored.
A phthalimide and piperidone derivative that acts as an immunomodulatory drug. It is primarily used to treat blood-related disorders such as multiple myeloma and myelodysplastic syndromes (MDS) by stimulating the immune system to attack cancer cells.
Patients can purchase Lenalid 25 Capsule from various online pharmacies, including Gomeds online. However, it is important to consult with a healthcare provider before taking this medication to ensure it is safe and appropriate for the individual patient’s condition.
#cancer medicine#gomedsonline#gomeds online#gomeds#myelodysplastic syndromes#Lenalid 25 Capsule#bortezomib#immunosuppressants#lenalidomide#plasma cells#multiple myeloma#MDS#Side Effects of Lenalid 25 Capsule
1 note
·
View note
Note
Okay! Now it's your turn! Tell me about your DNP project (or any of your dream projects) that you could share at a conference
Loadedddd question, haha!
I technically don't have to land on a project until this coming Spring term (April 2025), but I have been thinking about it. Since my DNP is associated with also becoming an NP, our instructors would like us to focus on how to implement things on the provider side of things versus nursing (though I think that is a bit unfair, considering the majority of my knowledge is nursing, and I'm also here to improve patient lives and outcomes, not just the provider process... but ya know).
So far my ideas include the process of implementing and managing blood glucose in stem cell transplant patients who have acute/chronic graft versus host disease, are in the inpatient setting, and are on high-dose steroids. Steroid-induced DM, or just exacerbate someone's DM! Right now, it is pretty well known that we are okay with blood glucose levels below 180 (typically between 140-180 is tolerated), but steroids make life a lot more tricky, especially when these patients can be on these steroids for *months*. I'm hoping I get to do a rotation on the transplant unit to find out their process (since I don't currently work there). This one is directly tied to how providers order and institute blood glucose management (insulin, sliding scales, etc), and I believe we can always do better when it comes to managing blood glucose.
My second idea (that likely has NO literature on it whatsoever, so it is unlikely to be a process I get to change any time soon, plus it is directly tied to nursing medication administration and not the provider side of medicine) is how we administer Daratumumab (a monoclonal antibody for multiple myeloma). It is frequently given as a subcutaneous injection these days (IV formulation still available), and it is 15 mLs over 5 minutes into the stomach once a week for about 8 weeks, then every two weeks for another 8 weeks, and then once a month until progression of disease. In this case, as someone who has given this drug hundreds of times at two different institutions, I wanted to see if I could implement a new quality improvement on how the drug is administered (a straight needle versus a butterfly needle).
Anecdotally, I have heard from nurses and patients that the butterfly needle is typically preferred. Some patients reported decreased discomfort with the butterfly needle, both during administration and hours after, and others didn't even notice. If you push the med too fast (15 mLs is a lot at once!), there can be discomfort. As for the nurses, we have better control over the syringe and have better ergonomics with a butterfly needle. When using a straight needle, you are holding the needle and syringe up against the skin and are leaning over the patient, which can be uncomfortable for the back and shoulders. You have the ability to sit back more and have more steady control over pushing the syringe when utilizing the butterfly needle. (We typically taped the butterfly needle down, which I think also decreases unnecessary needle movement). At least from what I have seen, there is no standard across the board for administration (just subcutaneous into the abdominal area).
I would love to compile some data on patient and nursing feedback on both administration techniques to get the ball rolling (but it sounds more research-based than quality improvement, and I guess I kind of disagree, but my instructors would argue with me... especially after this summer term. However, it is nursing-focused, not provider-focused, and there is unlikely to be any literature (multiple myeloma is a very small subset of blood cancer, and I pigeon-holed myself on data during my first research course in this program. I ran with it, but it was slim evidence to work with, and it was just around multiple myeloma and stem cell transplantation. I doubt anyone has even considered nursing preference in administration technique for one specific immunotherapy drug *laughs*). I'll get to this one day! Unfortunately, while I talked about it a lot with my fellow nurses in both Denver and here in Portland, I never had a way to implement a change or gather data (though before I left Denver, we changed from the straight technique to the butterfly needle. No idea what data was behind it, but the pharmacy drew up the drug and provided it with the butterfly needle. Daratumumab isn't hazardous, so my Portland job just gave us the bottle, lol)
I am also interested in palliative care (though that might be a project too big to chew for the size we have to limit ourselves to. My mentor Patti literally did her project on the barriers to mass transfusion protocol documentation. All she did was obtain qualitative data from the trauma ICU nurses voluntarily). Our mock project for our quality improvement course this summer was on palliative care in heart failure, and I know my classmate wants to take that and run with it. I also am far more interested in palliative care in oncology. But I couldn't say what I would want to do for a project other than something in palliative care and oncology.
Our instructors (and the class ahead of us, haha) would also love it if we took a previous project and continued it, but none of the current third years have a project I want to continue (no oncology!). I don't want to do a project I am not invested in or interested in.
I may come up with other ideas once we get further into our management courses and start clinicals in February (kajsdlkfjklajsdfl), but I am pretty passionate about DM treatment/management, stem cell transplants, oncology, and palliative care.
(I almost signed up for the elective about submitting abstracts and posters, but I chickened out and went with Institutional Racism. As I said, I have lowered my expectations, lmao. It has been a weird year. I have met some very impressive nursing greats - I effing met the creator of the Tanner's Model of Clinical Judgement. Mind. Blown!)
#pluto#dnp school#nursing#dnp project#dnp project ideas#multiple myeloma#oncology#palliative care#diabetes mellitus#about me#DNP student#insanity
3 notes
·
View notes
Photo
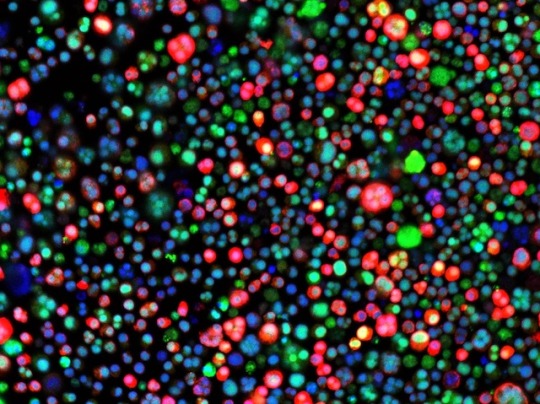
In a Bind
Multiple myeloma is a blood cancer affecting plasma cells, white blood cells that produce antibodies, causing them to multiply abnormally in the bone marrow. Right now, only half of patients can expect to survive five years after diagnosis, so better treatments are sorely needed. Promising targets include fatty acid-binding proteins (FABPs), a multi-functional family of proteins that can pick up lipids, involved in a variety of processes. A recent study found that cultured myeloma cells (pictured, their nuclei in blue) expressed high levels of one member in particular, FABP5 (in red); blocking it reduced cell proliferation, while patients with higher FABP5 levels tended to experience worse outcomes. Inhibiting FABPs more broadly appeared even more effective, improving survival in some mice with myeloma. To build on these results, further research needs to investigate why not all mice responded so well, and whether inhibiting FABPs would be safe and effective in humans.
Written by Emmanuelle Briolat
Image from work by Mariah Farrell and colleagues
Center for Molecular Medicine, Maine Health Institute for Research, Scarborough, ME, USA
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in eLife, March 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#myeloma#haemato-oncology#haematology#bone marrow#oncology#white blood cells#fatty acid binding proteins#cancer#blood cancer#immunofluorescence
10 notes
·
View notes
Text
Got an email from Feds 4 Medical Freedom that may interest everyone. I copied and pasted what I could...
DOCTOR’S NOTE
An old adage goes, "The enemy of my enemy is my friend." And, so, it is with ivermectin, the antiparasitic drug that emerged from the COVID pandemic as the 'best in show' blue ribbon winner for the prevention and treatment of SARS CoV-2-associated spike protein disease.
First proven to be a safe and effective treatment for River Blindness and Elephantitis, ivermectin rapidly became the most important drug for the control of parasitic infections in humans, winning for its Japanese discoverer, Satoshi Omura of Kitasato University, the Nobel Prize in Physiology or Medicine in 2015. Then, the late (great) Dr. Vladimir Zelenko brought ivermectin into the limelight during the early days of the COVID pandemic, saving thousands of lives in the process. But it turns out that ivermectin has more tricks up its therapeutic sleeve than we ever imagined.
There is now a wealth of laboratory research showing that ivermectin has great potential in the treatment of a wide range of cancers: melanoma, sarcoma, multiple myeloma, leukemia, glioblastoma, and cancers of the breast, ovary, prostate, lung, pancreas, esophagus, and colon. The various pathways by which ivermectin kills cancer cells (while leaving normal cells alone) are as impressive as the number of different cancers it seems capable of nailing.
But while the laboratory data about the efficacy of ivermectin in killing cancer cells is converging and compelling, it suffers from a major problem: it's a drug that has been off patent for decades. As a result, there's no patent-pay day fortune to be made by the pharmaceutical industry were it to spend the (average) $2.3 billion dollars required to repurpose ivermectin and have it approved by the FDA for use in cancer patients.
Fortunately, there are a few (unfriendly) countries that are not interested in spending a fortune inadequately treating patients with cancer, which is about where the world stands today. Notably, China and Iran have moved ahead to evaluate ivermectin in human clinical trials. The trick in the United States and in other western pharma-controlled countries will be to find a way to conduct and fund clinical trials apart from traditional funding sources, which can be counted on to look the other way for as long as possible.
Cancer is our enemy; has been for decades. Ivermectin appears to be its enemy, too. Could it be that ivermectin is our friend? We ought to find out, we need to find out, we need to know as soon as possible.
Reference: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5835698/
Questions? Email Doc Ruddy at [email protected]
3 notes
·
View notes
Text
Cancer Immunotherapy Market Size, Share And Trends Report, 2030

Cancer Immunotherapy Market Growth & Trends
The global cancer immunotherapy market size is expected to reach USD 224.30 billion by 2030, registering a CAGR of 8.3% from 2024 to 2030, according to a new report by Grand View Research, Inc. The rising adoption of the immunotherapy over other therapy options for cancer owing to its targeted action is anticipated to increase the adoption during the forecast period. Moreover, increasing regulatory approvals from authoritarian establishments for novel immunotherapy used for oncology is also expected to further fuel the market growth. For instance, in October 2021, the U.S. FDA approved Tecentriq (atezolizumab), of Genentech, Inc for the treatment of NSCLC.
Moreover, the robust product pipeline of the immunotherapy medicines for oncology is one of the major driving factors for strong growth of the market. For instance, (phase-III), developed by AstraZeneca for the treatment of first-line stage small cell lung malignancy. Immuno-oncology agents have shown promising results with improved survival rates and less toxicity. Such clinical trial results are expected to increase the introduction of novel therapeutic options in the coming years.
Increasing adoption of the combination therapies to treat cancer is further expected to increase demand for the immunotherapy. Combination therapies target multiple pathways within the tumor microenvironment that can potentially increase effectiveness of the immunotherapeutic treatment. Companies are mainly emphasizing development of the targeted treatments as novel regimens for the oncology disorder treatment.
The introduction of immunotherapy has aided the treatment options for the malignancies of breast, brain, bladder, lymphomas, and others. Although the usage of this therapy is minimal as compared to chemotherapy, radiotherapy, and surgery. Immunotherapy is anticipated to emerge as the leading treatment strategy for the malignancies during the next few years. The alarming rise in oncology incidence rates has provoked global collaboration on oncology drugs and other therapies. For instance, in March 2020, Astellas Pharma and CytomX collaborated to expand pipeline of the next-generation Immuno-oncology treatments. This collaboration has helped companies to strengthen their immune-oncology portfolio.
Furthermore, development and approval of the novel immunotherapy treatments for relapsed and refractory malignancies are accelerating the cancer immunotherapy market expansion. For instance, in February 2022, the Janssen Pharmaceutical Companies of Johnson & Johnson announced the approval of CARVYKTI from the U.S. FDA for treatment of the refractory multiple myeloma after four or more lines of treatment.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/cancer-immunotherapy-market
Cancer Immunotherapy Market Report Highlights
Monoclonal antibodies segment held the largest market share in 2023 owing to rising investments in the R&D of monoclonal antibodies
By product, the oncolytic viral therapies & cancer vaccines sub-segment is anticipated to grow at the fastest rate owing to their greater clinical benefit to patients with advanced-stage malignancies
Based on application, lung cancer dominated the market owing to the rise in prevalence of the disease and increasing adoption of immunotherapy for the treatment
The prostate cancer sub-segment is likely to register the fastest CAGR due to the increasing awareness about prostate cancer and rising product launch
The hospitals & clinics segment led the market in 2023 owing to the increasing demand for immunotherapeutic medicines in hospitals and increasing hospitalization of cancer patients
Asia Pacific is expected to exhibit the fastest growth during the forecast period due to the growing establishment of healthcare, and high unmet medical needs
Key market players are continuously involved in the development of novel treatments and geographical expansion, in order to expand their footprint in the global market.
Cancer Immunotherapy Market Segmentation
Grand View Research has segmented the global cancer immunotherapy market based on product, application, distribution, end use, and region:
Cancer Immunotherapy Product Outlook (Revenue, USD Million, 2018 - 2030)
Monoclonal Antibodies
Immunomodulators
Oncolytic Viral Therapies & Cancer Vaccines
Cancer Immunotherapy Application Outlook (Revenue, USD Million, 2018 - 2030)
Lung Cancer
Breast Cancer
Colorectal Cancer
Melanoma
Prostate Cancer
Head & Neck Cancer
Ovarian Cancer
Pancreatic Cancer
Others
Cancer Immunotherapy Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
Cancer Immunotherapy End Use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals & Clinics
Cancer Research Centers
Others
Cancer Immunotherapy Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
List Of Key Players Cancer Immunotherapy Market
Pfizer Inc.
AstraZeneca
Merck & Co., Inc
Hoffmann-La Roche Ltd
Bristol-Myers Squibb Company
Novartis AG
Lilly
Johnson & Johnson Services, Inc
Immunocore, Ltd
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/cancer-immunotherapy-market
#Cancer Immunotherapy Market#Cancer Immunotherapy Market Size#Cancer Immunotherapy Market Share#Cancer Immunotherapy Market Trends
0 notes
Text
High-Risk Smoldering Multiple Myeloma: The Critical Link Between MGUS and Multiple Myeloma

What is High-Risk Smoldering Multiple Myeloma?
High-risk smoldering multiple myeloma (HR-SMM) is an advanced precursor stage between monoclonal gammopathy of undetermined significance (MGUS) and active multiple myeloma. Patients diagnosed with HR-SMM have a significantly higher likelihood of developing symptomatic multiple myeloma within two years. Diagnosis is based on specific biomarkers, including abnormal plasma cell counts, elevated monoclonal protein levels, and genetic mutations. Early detection of HR-SMM is crucial, as it provides an opportunity to explore new treatments for HR-SMM that may slow disease progression.
Symptoms of Smoldering Multiple Myeloma
Unlike active multiple myeloma, smoldering multiple myeloma often presents no noticeable symptoms. However, some patients may experience mild signs such as fatigue, anemia, or occasional bone pain. Regular monitoring is essential to track disease development and determine the right time for intervention. While multiple myeloma remains incurable, early treatment strategies can delay its progression, and the risk of recurrence highlights the need for continuous observation of disease markers.
Advancements in Treating High-Risk Smoldering Multiple Myeloma
Traditionally, HR-SMM has been managed through active monitoring, but new treatments for HR-SMM are now being explored to slow or even prevent disease progression. Immunotherapy, particularly Revlimid-based regimens, has demonstrated promising results in clinical studies. Additionally, monoclonal antibodies and proteasome inhibitors like Carfilzomib are emerging as effective treatment options.
For eligible patients, multiple myeloma stem cell transplant remains a potential treatment strategy, offering extended remission and delaying disease advancement. Researchers are also developing innovative chemotherapy and targeted therapies to suppress disease progression. The multiple myeloma drugs market continues to expand, with cutting-edge treatments such as CAR-T cell therapy and novel therapeutic agents currently under investigation in clinical trials.
Conclusion
High-risk smoldering multiple myeloma represents a pivotal stage in disease progression, making early diagnosis and proactive intervention essential. The evolving multiple myeloma drugs market is driving the development of advanced therapies, providing patients with more options for disease management. With continuous monitoring, innovative treatments, and emerging medical breakthroughs, individuals with HR-SMM have a better chance of delaying the onset of active multiple myeloma and improving their long-term prognosis.
Latest Blogs by DelveInsight:
Pfizer’s ABRYSVO Outpaces GSK’s AREXVY with Expanded FDA Approval – But Can It Sustain the Momentum?
5 Promising Exosome-based Therapies Paving the Way for Personalized Medicine
FDA Grants Orphan Status to MDL-101 for LAMA2-CMD; Pfizer’s ABRYSVO Approved for High-Risk Adults (18-59); KIND’s AND017 Gains Orphan Designation for Sickle Cell Disease; HiberCell’s HC-7366 Fast-Tracked for AML; ORLYNVAH Approved for Uncomplicated UTIs
7 Key Technologies Pioneering Cybersecurity in the Healthcare Sector
About DelveInsight
DelveInsight is a leading provider of market research and consulting services specializing in the life sciences and healthcare industries. Our insights help pharmaceutical, biotechnology, and medical device companies navigate competitive landscapes and achieve long-term success.
Contact: Kanishk
Email: [email protected]
0 notes
Text
Melphalan is widely used for the treatment of blood cancer (multiple myeloma), ovarian cancer, and eye cancer. Indian Generic Medicines (IGM) legally help facilitate the export of Melphalan from India. We ensure safe and legal delivery to international locations. With WHO-GDP certification, IGM guarantees high-quality medications and hassle-free export services. Our expertise in global logistics ensures timely and readily access to new and life-saving therapeutic drugs. Kindly contact IGM today via Call/WhatsApp: +91 8130290915 to arrange Melphalan export services.
0 notes
Link
0 notes
Text
Dr. Pooja Gupta: Leading Medical Oncologist in Gurgaon
In recent years, the need for skilled medical oncologists has increased, as cancer remains one of the most serious health challenges worldwide. In Gurgaon, Dr. Pooja Gupta stands out as a leading medical oncologist, known for her expertise in treating various types of cancer and providing compassionate care to her patients.
Who is Dr. Pooja Gupta?
Dr. Pooja Gupta is a highly qualified and experienced medical oncologist based in Gurgaon, India. With years of training and clinical experience, she has gained a reputation for delivering the best oncological care. She specializes in diagnosing, treating, and managing cancer, focusing on both medical treatments and the psychological well-being of her patients.
Her approach is patient-centric, ensuring that each patient receives personalized treatment options tailored to their specific needs. Dr. Gupta’s compassionate approach has made her a trusted name in the field of oncology.
Areas of Expertise
Dr. Pooja Gupta specializes in various types of cancer treatments, ensuring that patients receive the most appropriate care. Some of her areas of expertise include:
1. Breast Cancer
Breast cancer is one of the most common types of cancer in women. Dr. Gupta is an expert in the diagnosis and treatment of breast cancer, utilizing the latest advancements in medical oncology. Her treatments include chemotherapy, targeted therapies, and hormone therapies, all aimed at improving the quality of life and survival rate for patients.
2. Lung Cancer
Lung cancer can often go undetected in its early stages, making early detection and expert care critical. Dr. Gupta offers a range of treatments for lung cancer, including chemotherapy, radiation therapy, and immunotherapy, depending on the type and stage of cancer.
3. Colorectal Cancer
Colorectal cancer is another area of expertise for Dr. Gupta. She is committed to diagnosing colorectal cancer in its early stages and offering a variety of treatments, including surgery, chemotherapy, and targeted therapies.
4. Blood Cancers (Leukemia, Lymphoma, Myeloma)
Dr. Gupta has extensive experience in treating blood cancers, including leukemia, lymphoma, and multiple myeloma. She uses a combination of chemotherapy, immunotherapy, and stem cell transplants to provide effective care.
5. Gynecological Cancers
Dr. Pooja Gupta is skilled in treating cancers related to female reproductive organs, such as ovarian, cervical, and uterine cancer. She works closely with gynecologists and other specialists to provide a multidisciplinary approach for the best possible outcomes.
Why Choose Dr. Pooja Gupta?
1. Expertise in Advanced Oncology Treatments
Dr. Gupta is well-versed in the latest treatments available for cancer, including immunotherapy, targeted therapy, and precision medicine. She uses these cutting-edge methods to enhance the effectiveness of cancer treatments and reduce side effects for patients.
2. Patient-Centered Care
Dr. Gupta understands that a cancer diagnosis can be overwhelming for both patients and their families. She takes the time to listen to each patient's concerns and provides emotional support along with medical treatment. Her compassionate approach ensures that patients feel comfortable throughout their treatment journey.
3. Personalized Treatment Plans
Cancer treatment is not one-size-fits-all. Dr. Gupta believes in creating personalized treatment plans for each patient based on their specific type of cancer, stage of disease, and overall health. This ensures that patients receive the most effective and appropriate treatment for their condition.
4. Comprehensive Follow-up Care
Cancer treatment doesn’t end after chemotherapy or surgery. Dr. Gupta emphasizes the importance of follow-up care to monitor the patient's progress, detect recurrence early, and manage any long-term side effects of treatment.
Dr. Pooja Gupta’s Approach to Oncology
Dr. Pooja Gupta takes a holistic approach to cancer care. She focuses not only on the medical aspect of cancer treatment but also on the emotional and mental health of her patients. Her emphasis on a multidisciplinary approach, including collaboration with surgeons, radiologists, and psychologists, ensures the best possible outcomes for her patients.
Her clinic in Gurgaon is equipped with the latest medical technology to provide patients with world-class care. Moreover, Dr. Gupta believes in the power of patient education and provides her patients with all the information they need to understand their diagnosis and treatment options.
Conclusion
Dr. Pooja Gupta is undoubtedly one of the leading medical oncologists in Gurgaon. Her dedication to providing high-quality care, combined with her extensive experience in treating various cancers, makes her a trusted name in oncology. If you or a loved one are seeking expert cancer care in Gurgaon, Dr. Pooja Gupta is the professional to turn to. Her patient-first approach, advanced treatment options, and compassionate care will guide you through every step of your cancer journey.
For more information or to book an appointment, you can visit Dr. Pooja Gupta’s clinic or contact her team directly. Start your journey toward recovery with the guidance and expertise of one of Gurgaon’s top oncologists.
1 note
·
View note
Text
Choosing the Right Oncologist: Tips for Patients and Families

An in-depth and early cancer diagnosis may change a life. Thus, selecting the right oncologist is one of the most important decisions to make among the many. With medical expertise, guidance, and support, the oncologist plays a vital role in the cancer treatment journey. Meet the top oncologist in Siliguri who works in a comprehensive cancer care team, offering all-inclusive health support.
Understand the Types of Oncologists (Cancer Specialists)
The specialty area of medicine known as oncology is focused on the diagnosis, management, and study of cancer.
The following types and sub-types of oncologists are useful to know before you start your search:
Medical Oncologists. These cancer doctors specialize in treating cancer using medications such as chemotherapy, immunotherapy, and targeted therapy.
Radiation Oncologists. They focus on treating cancer with radiation therapy, which destroys malignant cells.
Surgical Oncologists. Cancer surgeons perform surgeries to remove tumors or biopsy suspicious growths.
Pediatric Oncologists. Cancer experts specialize in treating cancer in children.
Neuro Oncologist. Treatment of malignancies of the brain, spine, and peripheral nerves is the focus of neuro-oncologists.
Hematologist-Oncologist. They specialize in the diagnosis and treatment of blood malignancies, such as multiple myeloma, leukemia, and lymphoma.
Ask or Request Referrals
Begin by seeking advice from your specialist or general care physician because they have bigger resources in this regard. Based on their personal experiences, friends, relatives, and cancer support organizations can also offer insightful information.
Check Experience and Credentials
Always consider a board-certified oncologist in Siliguri with extensive experience in treating any type of cancer. You may verify credentials with organizations like national or international. Wise to know about the oncologist's length of practice and whether they have worked for reputable cancer clinics, their experience, and certification.
Assess Accessibility and Availability
Since cancer treatment sometimes requires several sessions, it's important to choose an oncologist in Siliguri who is easily reachable. Think about where their treatment facility and office are located, the simplicity of appointment scheduling, etc. Also, the availability of nurses, physicians, and other doctors is vital at the cancer hospital in Siliguri.
Research the Cancer Hospital
An oncologist's clinic or hospital location in Siliguri may have an effect on the standard of care a patient receives. Consider receiving treatment in a cancer facility in Siliguri approved by the National Cancer Institute (NCI), as these facilities usually provide the most recent treatments and clinical trials. Examine the Siliguri cancer hospital's rankings, reviews, and patient satisfaction to ensure it meets all required health needs.
Examine Testimonials and Reviews
Online patient reviews and testimonials might offer valuable information about the treatment and service. Examine the feedback for any recurrent themes, such as manners, communications, supportive care, doctors’ expertise, and overall service in cancer care. Keep in mind that each person's experience is unique, so include reviews in decision-making.
Know Available Treatments and Clinical Trials
A reputable oncologist will discuss with you all of your treatment options, including the benefits and drawbacks of each. If suitable, they may also suggest participating in clinical trials that might provide access to better treatment outcomes. Before deciding on a course of therapy, don't hesitate to get a second opinion. A trustworthy oncologist will your desire for comfort and could even suggest other specialists for other viewpoints. Consult the best oncologists in Siliguri today by following this guideline.
0 notes
Text
Blood Cancer Treatment Market Size & Forecast 2025-2035
Blood Cancer Treatment Market Overview The Blood Cancer Treatment Market was valued at USD 38.2 billion in 2024 and is projected to grow to USD 85.5 billion by 2035 , at a CAGR of 7.6% from 2025 to 2035. This market encompasses therapies addressing blood-related cancers such as leukemia, lymphoma, and multiple myeloma. Common treatment options include chemotherapy, radiation therapy, immunotherapy (e.g., CAR-T cell therapy), targeted therapy, and stem cell transplantation. Innovations in these areas are helping to improve patient outcomes while personalizing treatment approaches. Access the full report here: https://www.metatechinsights.com/industry-insights/blood-cancer-treatment-market-1154 Key Market Dynamics Rising Incidences of Blood Cancers - Blood cancer rates are increasing globally due to environmental and genetic factors. - Regions such as North America and Europe report higher incidences of leukemia, lymphoma, and myeloma. For instance, the NIH estimates that over 474,519 new cases of leukemia were reported globally in 2020. - Contributing factors include chemical exposure (e.g., benzene) and radiation exposure, as noted by WHO and the U.S. Environmental Protection Agency. Innovative Therapies Transforming Care - CAR-T cell therapy is a breakthrough, with remission rates exceeding 80% in patients with relapsed acute lymphoblastic leukemia. - New-generation sequencing enables precise, genetics-based treatment approaches, improving survival rates. For example, targeted therapies like imatinib (Gleevec) have achieved a 10-year survival rate of over 90% for chronic myeloid leukemia. - Recent funding initiatives, such as the Leukemia & Lymphoma Society's $5 million investment, aim to accelerate research on immunotherapies. Cost Barriers and Accessibility Challenges - Advanced therapies like CAR-T treatment cost over $373,000 per patient , excluding additional hospital expenses. - The financial burden is particularly challenging for patients in low-income regions, leading to disparities in access to lifesaving treatments. Regional Insights North America - Leads the market with advanced healthcare infrastructure and widespread adoption of personalized medicine. In 2022, approximately 1.6 million people in the U.S. were undergoing treatment for blood cancers. Asia-Pacific - Expected to grow rapidly due to rising awareness and improved healthcare infrastructure in countries like China and India. Innovations in immunotherapy and targeted treatments are gaining traction in the region. Competitive Landscape Key players include Roche , Novartis , Amgen , and Gilead Sciences . Roche leads with a strong oncology portfolio, while Novartis is expanding its CAR-T therapies. Increased investment in clinical trials and collaborations is driving innovation across the sector. For detailed insights, visit: Click Here
0 notes
Text
Blood Cancer Treatment Market Size & Forecast 2025-2035
Blood Cancer Treatment Market Overview
The Blood Cancer Treatment Market was valued at USD 38.2 billion in 2024 and is projected to grow to USD 85.5 billion by 2035, at a CAGR of 7.6% from 2025 to 2035. This market encompasses therapies addressing blood-related cancers such as leukemia, lymphoma, and multiple myeloma. Common treatment options include chemotherapy, radiation therapy, immunotherapy (e.g., CAR-T cell therapy), targeted therapy, and stem cell transplantation. Innovations in these areas are helping to improve patient outcomes while personalizing treatment approaches.
Access the full report here: https://www.metatechinsights.com/industry-insights/blood-cancer-treatment-market-1154
Key Market Dynamics
Rising Incidences of Blood Cancers
Blood cancer rates are increasing globally due to environmental and genetic factors.
Regions such as North America and Europe report higher incidences of leukemia, lymphoma, and myeloma. For instance, the NIH estimates that over 474,519 new cases of leukemia were reported globally in 2020.
Contributing factors include chemical exposure (e.g., benzene) and radiation exposure, as noted by WHO and the U.S. Environmental Protection Agency.
Innovative Therapies Transforming Care
CAR-T cell therapy is a breakthrough, with remission rates exceeding 80% in patients with relapsed acute lymphoblastic leukemia.
New-generation sequencing enables precise, genetics-based treatment approaches, improving survival rates. For example, targeted therapies like imatinib (Gleevec) have achieved a 10-year survival rate of over 90% for chronic myeloid leukemia.
Recent funding initiatives, such as the Leukemia & Lymphoma Society's $5 million investment, aim to accelerate research on immunotherapies.
Cost Barriers and Accessibility Challenges
Advanced therapies like CAR-T treatment cost over $373,000 per patient, excluding additional hospital expenses.
The financial burden is particularly challenging for patients in low-income regions, leading to disparities in access to lifesaving treatments.
Regional Insights
North America
Leads the market with advanced healthcare infrastructure and widespread adoption of personalized medicine. In 2022, approximately 1.6 million people in the U.S. were undergoing treatment for blood cancers.
Asia-Pacific
Expected to grow rapidly due to rising awareness and improved healthcare infrastructure in countries like China and India. Innovations in immunotherapy and targeted treatments are gaining traction in the region.
Competitive Landscape
Key players include Roche, Novartis, Amgen, and Gilead Sciences. Roche leads with a strong oncology portfolio, while Novartis is expanding its CAR-T therapies. Increased investment in clinical trials and collaborations is driving innovation across the sector.
For detailed insights, visit: https://www.metatechinsights.com/industry-insights/blood-cancer-treatment-market-1154.
0 notes
Text
Hematologic Malignancies Market Size, Share, Trends, Growth and Competitive Analysis
"Global Hematologic Malignancies Market – Industry Trends and Forecast to 2029
Global Hematologic Malignancies Market, By Type (Leukaemia, Lymphoma, Myeloma), Therapy Type (Chemotherapy, Immunotherapy, Targeted Therapy), Diagnosis (Blood Tests, Biopsy, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Capsules, Injections, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- **Type**: The hematologic malignancies market can be segmented based on the type of malignancy, including leukemia, lymphoma, and multiple myeloma. Leukemia is a cancer of the blood cells, while lymphoma affects the lymphatic system. Multiple myeloma, on the other hand, is a cancer that forms in a type of white blood cell called a plasma cell.
- **Treatment**: Segmentation based on treatment modalities includes chemotherapy, immunotherapy, targeted therapy, stem cell transplant, and others. Chemotherapy is a common treatment for hematologic malignancies that involves the use of drugs to kill cancer cells. Immunotherapy utilizes the body's immune system to fight cancer cells, while targeted therapy focuses on specific molecules involved in cancer growth.
- **End-User**: The market can also be segmented by end-user, such as hospitals, specialty clinics, research institutes, and others. Hospitals are the primary point of care for hematologic malignancies patients, where they receive diagnosis, treatment, and follow-up care. Specialty clinics may offer specialized treatments or clinical trials for these conditions.
**Market Players**
- **Roche**: A leading player in the hematologic malignancies market, Roche offers a range of innovative therapies and diagnostic tools for leukemia, lymphoma, and multiple myeloma. The company's commitment to research and development has resulted in groundbreaking treatments that improve patient outcomes.
- **Johnson & Johnson**: With a focus on cutting-edge oncology therapies, Johnson & Johnson has made significant advancements in the treatment of hematologic malignancies. The company's portfolio includes novel drugs that target specific cancer pathways, providing new options for patients.
- **Novartis**: Known for its expertise in precision medicine, Novartis has developed several targeted therapies for hematologic malignancies. By identifying genetic mutations driving cancer growth, Novartis delivers personalized treatments that are more effective and less toxic for patients.
- **AbbVie**:AbbVie is a key player in the hematologic malignancies market, known for its strong focus on developing innovative therapies for various types of blood cancers. The company's robust pipeline includes potential treatments for leukemia, lymphoma, and multiple myeloma, leveraging cutting-edge technologies and research to address unmet medical needs in this space. AbbVie's commitment to oncology research and development has led to the introduction of novel treatment options that aim to improve patient outcomes and quality of life.
In the competitive landscape of the hematologic malignancies market, AbbVie distinguishes itself through a combination of strategic partnerships, investments in research, and a patient-centric approach to drug development. The company's collaborative efforts with academic institutions, research organizations, and other industry partners have resulted in the acceleration of novel therapeutic solutions for blood cancers. By prioritizing patient needs and engaging in meaningful dialogue with healthcare providers, AbbVie continues to shape the future of hematologic oncology with a focus on personalized medicine and targeted therapies.
AbbVie's portfolio of hematologic malignancy treatments encompasses a diverse range of modalities, including small molecule inhibitors, monoclonal antibodies, and immunotherapies. These innovative therapies target specific pathways and molecular mechanisms involved in the development and progression of blood cancers, offering new hope for patients who may not have responded to conventional treatments. By leveraging its expertise in precision medicine and biomarker-driven approaches, AbbVie continues to advance the field of hematologic oncology with a strong emphasis on tailored treatment regimens that consider individual patient characteristics and disease profiles.
In addition to its focus on drug development, AbbVie also plays a crucial role in raising awareness about hematologic malignancies and promoting early detection and diagnosis. Through educational initiatives, patient advocacy programs, and community engagement efforts, the company strives to empower patients, caregivers, and healthcare professionals with the knowledge and resources needed to effectively manage blood cancers. By fostering a culture of collaboration and knowledge-sharing, AbbVie contributes to the overall**Global Hematologic Malignancies Market Analysis**
- **Type**: The global hematologic malignancies market, segmented by type, includes leukemia, lymphoma, and multiple myeloma. With advancements in precision medicine and targeted therapies, the market is witnessing a shift towards personalized treatment regimens tailored to the specific type of malignancy, driving growth in the segment.
- **Therapy Type**: The market segmented by therapy type comprises chemotherapy, immunotherapy, and targeted therapy, among others. The rising prevalence of hematologic malignancies and the increasing adoption of novel treatment approaches are driving the demand for innovative therapies, leading to significant market growth in this segment.
- **Diagnosis**: Diagnostic modalities such as blood tests, biopsies, imaging tests, and others play a crucial role in the early detection and management of hematologic malignancies. The emphasis on early diagnosis and personalized medicine is driving the market for diagnostic tools, contributing to the overall growth of the hematologic malignancies market.
- **Route of Administration**: Different routes of administration, including oral, parenteral, and others, offer varied options for delivering hematologic malignancy treatments. The convenience and efficacy of different administration routes influence patient compliance and treatment outcomes, shaping the market dynamics in this segment.
- **Dosage Form**: The market segmented by dosage form includes tablets, capsules, injections, and others. The availability of diverse dosage forms caters to patient preferences and treatment needs, promoting adherence and enhancing the overall therapeutic outcomes in
Key points covered in the report: -
The pivotal aspect considered in the global Hematologic Malignancies Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global Hematologic Malignancies Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global Hematologic Malignancies Market.
The Global Hematologic Malignancies Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Thermal Imaging Cameras Market Baby Food Market Thin Film Encapsulation Market Paper Coating Materials Market Protein Engineering Market Psoriasis Treatment Market Whole Exome Sequencing Market Std Diagnostics Market Medication Delivery Systems Market Lane Keep Assist System Market Liquid Synthetic Rubber Market Mainframe Market Myxoid Round Cell Liposarcoma Drug Market Hematology Analyzer Market Low Differential Pressure Sensor Market Biofuel Enzyme Market Aroma Ingredients Market Coconut Water Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes