#medical device compliance
Explore tagged Tumblr posts
Text

AI Consulting Service for Medical Devices
Artificial intelligence software algorithms to learn from the real-world use of the medical device to improve its performance. We offer specialized healthcare AI consulting services that help companies achieve their goals. To more details, reach our website.
#device consulting#fda compliance strategies#quality system development#risk remediation#compliance audits#medical device compliance
0 notes
Text

0 notes
Text
Why CDSCO Registration is Crucial for Your Medical Products
CDSCO registration is mandatory for all medical devices and pharmaceutical products being sold or imported into India. This process ensures your product meets the necessary health and safety standards, gaining approval for market access. Navigating the regulatory environment can be complex, but with the right guidance, it becomes a manageable task. Let us help you through every step of the CDSCO registration process, from documentation to certification. Get in touch to begin your registration today!

#CDSCO#CDSCO Registration#Regulatory Compliance#Medical Product Approval#Medical Devices#CDSCO Compliance
0 notes
Text
//healthcareeverything.com/navigating-regulatory-compliance-in-medical-device-manufacturing/
#BestHealthcareDigitalMagazine#TopHealthandWellnessMagazines#tophealthmagazines#Medical Device#Navigating Regulatory Compliance#Device Manufacturing
0 notes
Text
Medical Device Labeling: The Important Role Of Medical Device Label In Patient Care

Medical devices sold in the United States must comply with labeling requirements set by the Food and Drug Administration (FDA). All labels must contain specific information about the device such as its intended use, any potential risks or side effects, and instructions for use. Device labels provide critical safety information that helps ensure devices are used properly. The FDA regulates labeling to protect patient health.
Labels Must Clearly Identify The Device
First and foremost, a Medical Device Labeling must clearly identify the specific device. This includes stating the product name and any applicable product codes or reference numbers. Having a clear device name and identification numbers helps providers and facilities properly select, use, and track the intended device. Any trade or brand names must also be included but must not overshadow the core product identity information.
Instructions For Use Must Be Clear And Complete
Detailed instructions for use are extremely important for medical devices to be operated safely and as intended. Labels must provide step-by-step directions on how the device is to be used, prepared, fitted, applied, implanted, or operated. Pictures, diagrams or other illustrative guides can help visualize proper technique when words alone may not fully explain the process. Instructions must account for all reasonably foreseeable uses. Omitting any necessary steps could result in misuse leading to patient harm.
Packaging Labels Provide Sterility And Shelf Life Assurances
For devices distributed in single-use sterile packaging, labels must affirm the method used to sterilize the contents as well as the expiration date or shelf life. This information guarantees the continued sterility and integrity of the device up until the noted expiration date. Devices like surgical tools and implants must remain free of contaminants when used. Packaging labels demonstrate the steps taken to achieve and maintain sterile conditions.
Potential Adverse Reactions And Hazards Must Be Disclosed
Device labels must include a complete list of known or reasonably foreseeable adverse health effects or hazards from use. This involves describing any potential allergic reactions, biological risks, toxicology concerns and interactions with other devices, drugs or substances. Precautions, contraindications and any use limitations due to patient risks or conditions should also be detailed. Making providers aware of safety issues enables them to properly assess risks and benefits for individual patients.
Symbols Standardize Hazard And Safety Communications
Pictograms and symbols play an important role in medical device labeling by allowing concepts to be recognized universally without language barriers. Common symbols indicate requirements such as “Do Not Reuse”, “Sterilized Using Irradiation”, “Keep Away From Heat or Flames”, and many others defined by International Standards Organization (ISO) regulations. Having standardized symbols helps labeling information be consistently interpreted.
Product Labels Remain With The Device
Device manufacturers must ensure all labeling remains affixed or adjacent to the medical device itself throughout distribution and use. Shipping labels serve to identify devices during transportation but do not replace the requirement for complete labeling on the finished able product. Device labels need to be available anytime and anywhere the device is utilized to provide critical use and safety guidance to providers.
As described, medical device labelingserves the fundamental purpose of guiding appropriate and safe use while communicating potential risks. Complying with comprehensive FDA labeling policies supports quality patient care.
Get more insights on this topic: https://www.pressreleasebulletin.com/medical-device-labeling-device-labeling-regulations-ensuring-patient-safety-and-compliance/
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Medical Devices#Regulatory Compliance#Product Labeling#FDA Regulations#UDI (Unique Device Identification)#MDR (Medical Device Regulation)#Labeling Standards#Risk Management#Health Canada Regulations#Clinical Labeling
0 notes
Text
Vicki Partridge: Your Expert in Medical Device Regulations
I'm Vicki Partridge, bringing over 30 years of expertise in Biological Research and Australian medical device regulations. With 15 years at the Therapeutic Goods Administration (TGA), I've delved deep into legislative requirements, consulted with global industry leaders and the WHO, and ensured compliance through rigorous audits. In 2012, I ventured into consulting, offering specialized guidance and regulatory training worldwide.
As a dedicated medical equipment consultant, I specialize in navigating Australia's intricate regulatory landscape. From TGA submissions to compliance documentation and beyond, I assist medical and dental device manufacturers in achieving market approval efficiently. Join me on this journey of ensuring safety and compliance in the medical device industry. Let's connect and explore how I can support your regulatory needs!
#compliance consultant#medical compliance#regulatoryaffairs#regulatorycompliance#medicaldevice#medical devices#medical#medical device regulation#medical equipment suppliers
1 note
·
View note
Text
For manufacturers of in vitro diagnostic (IVD) devices seeking to market their products in the European Union, ensuring MDR compliance is a critical requirement. The EU Medical Device Regulation (MDR) has brought about significant changes in the regulatory landscape, and manufacturers must navigate these new rules to achieve and maintain compliance. Before divulging details, it is essential to understand IVD compliance.
0 notes
Text
youtube
Microchip: Ultra Low-Power ULP Connected Wearable Activity Monitor Demo
https://www.futureelectronics.com/m/microchip . High-end wearable activity trackers can take step count, temperature, light and other movement and environment measurements. Microchip’s Ultra Low-Power or ULP Connected, Wearable Activity Monitor Demonstration Board can be used as the starting point for the design of medical home monitoring, patient tracking and drug delivery compliance devices. https://youtu.be/hIcIEwWiHoU
#Microchip#Ultra Low-Power#ULP#Connected Wearable#Activity Monitor#wearable activity trackers#step count#medical home monitoring#patient tracking#drug delivery compliance devices#Youtube
0 notes
Text
youtube
Microchip: Ultra Low-Power ULP Connected Wearable Activity Monitor Demo
https://www.futureelectronics.com/m/microchip . High-end wearable activity trackers can take step count, temperature, light and other movement and environment measurements. Microchip’s Ultra Low-Power or ULP Connected, Wearable Activity Monitor Demonstration Board can be used as the starting point for the design of medical home monitoring, patient tracking and drug delivery compliance devices. https://youtu.be/hIcIEwWiHoU
#Microchip#Ultra Low-Power#ULP#Connected Wearable#Activity Monitor#wearable activity trackers#step count#medical home monitoring#patient tracking#drug delivery compliance devices#Youtube
1 note
·
View note
Text
0 notes
Text
Navigating the World of Medical Devices: A Guide to Classifying Your Product

Picture a world filled with incredible innovations, each one a solution to a unique problem. Now imagine yourself in the center of this world, holding your sparkling new product, pondering that all-important question: “Is this amazing creation of mine a medical device?”
It’s not as simple as it seems, but fear not. This guide, infused with the wisdom of an experienced medical device consultant, will be your playful companion in unraveling the many layers of this puzzle. So let’s embark on this delightful exploration, shall we?
Understanding the Basics of a Medical Device
Welcome to the world of medical devices. Here, products come in all shapes and sizes, each one uniquely designed to address a specific need. But what exactly qualifies as a medical device?
Well, it’s not as simple as throwing a stethoscope on a table and calling it a day. Medical devices range from the simple, such as thermometers, to the complex, like life-saving ventilators. But they can also include more ambiguous items, such as software designed to assist with medical decision-making.
A medical device consultant can be your guide in this maze. This expert can help you understand essential characteristics, like the purpose of diagnosing, preventing, monitoring, or alleviating diseases.
But that’s not all. These consultants also delve into the nuances of invasiveness, chemical actions within the body, and the fine line between general wellness products and medical devices. It’s a vibrant world, filled with intrigue, and your medical device consultant is the perfect partner to explore it with you.
Consult the Medical Device Consultant: Why Professionals Matter
In the world of medical devices, a medical device consultant is the wise sage you’ve been seeking. These professionals aren’t just experts in their field; they’re strategic allies, equipped with the know-how to transform your creative visions into tangible realities.
Whether it’s understanding the intricate landscape of regulations, dissecting the varying classifications, or crafting a clear pathway for your product, they are there every step of the way.
Think of them as your guide into the regulatory mountain, providing insights, ensuring you avoid pitfalls, and helping you make informed decisions. They know the shortcuts, the best paths, and the potential dangers that lurk along the way. With their assistance, what might initially appear as an insurmountable peak becomes an enjoyable ascent, filled with learning and triumph.
The ABCs of Regulations and Standards
The world is a diverse tapestry of rules, especially when it comes to medical devices. Different regions have their own guidelines, and navigating this multifaceted landscape can feel like deciphering an ancient scroll. In the U.S., you’ll encounter the FDA; in Europe, the CE marking; in Australia, the TGA. Each has its unique requirements, classifications, and procedures.
A medical device consultant is your knowledgeable translator in this world, taking those complex rules and turning them into a language you can understand. They’ll help you dance through the bureaucratic waltzes, ensuring that your product complies with the right rules in the right places.
Whether it’s understanding the differences between Class I, II, and III devices or navigating post-market surveillance, they are there to make sure your product not only compiles but excels in the regulatory environment.
Don’t Break the Bank: Financial Considerations
Finances in the world of medical devices can quickly turn into a roller coaster if you’re not careful. There are application fees, registration costs, testing expenditures, and more to consider. And these are just the obvious ones! Hidden costs can lurk around every corner, ready to throw your budget off course.
Your medical device consultant is like your financial co-pilot in this thrilling ride, ensuring you know exactly what to expect at every twist and turn. They’ll guide you through budgeting, identifying areas where you might save or need to invest more, and help you create a financial roadmap tailored to your unique journey.
From the initial planning stages to post-launch, they are there to ensure your financial decisions align with your goals, steering you clear of unnecessary detours and roadblocks.
Testing, One, Two, Three: Proper Verification and Validation
If you think about the world of medical devices as a grand theater, testing is the dress rehearsal before the big premiere. It’s the time when your product must prove itself, demonstrating that it can perform as intended, safely, and effectively.
This phase is filled with various tests, from biocompatibility for items that interact with the body to electrical safety for powered devices. It’s not just about passing the tests but understanding why each one is necessary and what it reveals about your product.
Well, enter the medical device consultant, your director in this critical phase. They’ll orchestrate the tests, ensuring each one is appropriate, relevant, and conducted with precision. Whether guiding you through pre-clinical trials or assisting with clinical evaluations, their expertise ensures that when the curtain rises on your product, it shines in the spotlight, ready to make its mark on the world.
Eyes on the Prize: Keeping Compliance on Track
The launch of a medical device isn’t the final bow; it’s the beginning of a continuing narrative. Regulatory compliance is a never-ending commitment, requiring ongoing vigilance, quality checks, and adaptations to ever-changing regulations. It’s akin to a garden that must be continually tended to ensure it thrives.
In this ongoing saga, a medical device consultant is your dedicated gardener, equipped with the knowledge and tools to nurture your product’s ongoing success. They’ll help you monitor quality, remain aligned with current regulations, and ensure that your product continues to meet the standards that allowed it to reach the market in the first place.
It’s not just about maintaining the status quo but continuously evolving and improving, and your medical device consultant is there to make sure you never lose sight of the prize.
Conclusion
From the exciting spark of an idea to the triumphant launch and beyond, the path to understanding if your product qualifies as a medical device is filled with discovery, challenges, and growth. But you’re not alone on this path; the guidance of a seasoned medical device consultant ensures that your journey is not just successful but thoroughly enjoyable.
So keep exploring, keep innovating, and remember that in the world of medical devices. Every question is a door to new possibilities, and every challenge is an opportunity to shine.
Read More :
1 note
·
View note
Text
0 notes
Text
Medical Device Design Engineer: Shaping Healthcare Innovations
Explore the pivotal role of a Medical Device Design Engineer in shaping healthcare innovations. Learn about conceptualization, prototyping, regulatory compliance, collaboration, and more. Discover how these engineers are redefining patient care with creativity and technical brilliance.
#Medical Device Design Engineer#Medical Device Protocols#Healthcare Product Development#Regulatory Compliance in Healthcare#Healthcare Device Manufacturing#Healthcare Engineering#Medical Device Innovation#Medical Product Design#Healthcare Technology#Medical Equipment Design
0 notes
Text
Medical Grade Thermocouple Wire Market Overview and Regional Outlook Study 2017 – 2032

The market for medical grade thermocouple wire is witnessing steady growth due to the increasing demand for accurate and reliable temperature measurement in the medical industry. Thermocouples are widely used in various medical applications, including monitoring body temperature, laboratory testing, and medical equipment calibration.
Market Overview of Medical Grade Thermocouple Wire:
Technological Advancements: Advancements in thermocouple wire technology, such as the development of high-precision sensors and miniaturization, are driving the market growth. These advancements enable accurate temperature measurements in medical devices.
Healthcare Industry Expansion: The expanding healthcare industry, including hospitals, clinics, and research laboratories, is creating a substantial demand for medical grade thermocouple wire. These wires are widely used in medical devices, such as patient monitoring systems, incubators, and surgical equipment.
Stringent Regulations: The medical industry is subject to strict regulations regarding patient safety and product quality. Medical grade thermocouple wires comply with these regulations, ensuring reliability and accuracy in temperature measurement, which is a key factor driving their adoption.
Increasing Awareness: Growing awareness among healthcare professionals and researchers about the benefits of using thermocouple wires in medical applications is boosting the market. These wires offer advantages such as high accuracy, fast response time, and compatibility with various medical devices.
Key Factors Driving the Medical Grade Thermocouple Wire Market:
Rising Focus on Patient Safety: The healthcare industry's increasing emphasis on patient safety and comfort is driving the demand for medical grade thermocouple wires. These wires help in precise temperature monitoring, ensuring optimal patient care.
Growing Demand for Minimally Invasive Procedures: The rising preference for minimally invasive procedures requires accurate temperature sensing and monitoring during surgeries. Medical grade thermocouple wires enable real-time temperature measurements, enhancing the safety and success rates of such procedures.
Advancements in Medical Device Technologies: The continuous advancements in medical device technologies, such as wearable devices and remote patient monitoring systems, are fueling the demand for medical grade thermocouple wires. These wires play a crucial role in temperature sensing within these devices.
Increasing Research and Development Activities: The ongoing research and development activities in the medical field, including the development of new drugs, medical treatments, and diagnostic techniques, rely on accurate temperature measurements. Medical grade thermocouple wires are essential components in such research activities.
Growing Geriatric Population: The global increase in the geriatric population is driving the demand for medical devices and healthcare services. Medical grade thermocouple wires find extensive applications in temperature monitoring devices used for elderly care, such as patient monitors and wearable health trackers.
COVID-19 Impact: The COVID-19 pandemic has increased the demand for medical devices and equipment, including temperature monitoring systems. Thermocouple wires are used in various COVID-19-related applications, such as vaccine storage and transport, ICU patient monitoring, and fever detection systems.
Expansion of Home Healthcare: The rising trend of home healthcare services and the need for portable medical devices have led to an increased demand for medical grade thermocouple wires. These wires enable temperature monitoring in portable devices used for home healthcare purposes.
Increasing Adoption of Wireless Thermocouple Systems: The market is witnessing a shift towards wireless thermocouple systems, which offer convenience, ease of use, and reduced clutter. Medical grade thermocouple wires are an integral part of these wireless systems, driving their demand.
Collaborations and Partnerships: Key players in the medical device industry are entering into collaborations and partnerships to develop innovative solutions. Such collaborations involving medical grade thermocouple wire manufacturers contribute to market growth.
Growing Focus on Precision Medicine: The increasing focus on precision medicine, which involves personalized treatment based on individual characteristics, requires accurate temperature monitoring in various medical procedures. Medical grade thermocouple wires play a vital role in delivering precise temperature measurements for effective treatment outcomes.
We recommend referring our Stringent datalytics firm, industry publications, and websites that specialize in providing market reports. These sources often offer comprehensive analysis, market trends, growth forecasts, competitive landscape, and other valuable insights into this market.
By visiting our website or contacting us directly, you can explore the availability of specific reports related to this market. These reports often require a purchase or subscription, but we provide comprehensive and in-depth information that can be valuable for businesses, investors, and individuals interested in this market.
“Remember to look for recent reports to ensure you have the most current and relevant information.”
Click Here, To Get Free Sample Report: https://stringentdatalytics.com/sample-request/medical-grade-thermocouple-wire-market/3577/
Market Segmentations:
Global Medical Grade Thermocouple Wire Market: By Company • TE Wire&Cable • Johnson Matthey • Heraeus • Sandvik (Kanthal) • BASF • OMEGA Engineering • Belden • Pelican Wire • National Instruments • Indutrade (Pentronic) • Pyromation • Dwyer Instruments • Tempco • Durex Industries • Marlin Manufacturing Corporation • Multi/Cable Corporation • Ellab • Temprel • Thermo-Electra • Hayashidenko Global Medical Grade Thermocouple Wire Market: By Type • Type T • Type K • Other Global Medical Grade Thermocouple Wire Market: By Application • Pharmaceutical Enterprise • Hospital • Other Global Medical Grade Thermocouple Wire Market: Regional Analysis All the regional segmentation has been studied based on recent and future trends, and the market is forecasted throughout the prediction period. The countries covered in the regional analysis of the Global Medical Grade Thermocouple Wire market report are U.S., Canada, and Mexico in North America, Germany, France, U.K., Russia, Italy, Spain, Turkey, Netherlands, Switzerland, Belgium, and Rest of Europe in Europe, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, China, Japan, India, South Korea, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), and Argentina, Brazil, and Rest of South America as part of South America.
Visit Report Page for More Details: https://stringentdatalytics.com/reports/medical-grade-thermocouple-wire-market/3577/
Reasons to Purchase Medical Grade Thermocouple Wire Market Report:
• To obtain insights into industry trends and dynamics, including market size, growth rates, and important factors and difficulties. This study offers insightful information on these topics.
• To identify important participants and rivals: This research studies can assist companies in identifying key participants and rivals in their sector, along with their market share, business plans, and strengths and weaknesses.
• To comprehend consumer behaviour: these research studies can offer insightful information about customer behaviour, including preferences, spending patterns, and demographics.
• To assess market opportunities: These research studies can aid companies in assessing market chances, such as prospective new goods or services, fresh markets, and new trends.
• To make well-informed business decisions: These research reports give companies data-driven insights that they may use to plan their strategy, develop new products, and devise marketing and advertising plans.
In general, market research studies offer companies and organisations useful data that can aid in making decisions and maintaining competitiveness in their industry. They can offer a strong basis for decision-making, strategy formulation, and company planning.
Medical Grade Thermocouple Wire Market Research Report Contains Answers to your following Questions
Which Manufacturing Technology is Used for Medical Grade Thermocouple Wire? What Developments Are Going On in That Technology? Which Trends Are Causing These Developments?
Who Are the Global Key Players in This Medical Grade Thermocouple Wire Market? What's Their Company Profile, Their Product Information, and Contact Information?
What Was Global Market Status of Medical Grade Thermocouple Wire Market? What Was Capacity, Production Value, Cost and PROFIT of Medical Grade Thermocouple Wire Market?
What Is Current Market Status of Medical Grade Thermocouple Wire Industry? What's Market Competition in This Industry, Both Company, and Country Wise? What's Market Analysis of Medical Grade Thermocouple Wire Market by Taking Applications and Types in Consideration?
What Are Projections of Global Medical Grade Thermocouple Wire Industry Considering Capacity, Production and Production Value? What Will Be the Estimation of Cost and Profit? What Will Be Market Share, Supply and Consumption? What about Import and Export?
What Is Medical Grade Thermocouple Wire Market Chain Analysis by Upstream Raw Materials and Downstream Industry?
What Is Economic Impact On Medical Grade Thermocouple Wire Industry?
What are Global Macroeconomic Environment Analysis Results? What Are Global Macroeconomic Environment Development Trends?
What Are Market Dynamics of Medical Grade Thermocouple Wire Market? What Are Challenges and Opportunities?
What Should Be Entry Strategies, Countermeasures to Economic Impact, Marketing Channels for Medical Grade Thermocouple Wire Industry?
Click Here, To Buy Premium Report: https://stringentdatalytics.com/purchase/medical-grade-thermocouple-wire-market/3577/?license=single
About US:
Stringent Datalytics offers both custom and syndicated market research reports. Custom market research reports are tailored to a specific client's needs and requirements. These reports provide unique insights into a particular industry or market segment and can help businesses make informed decisions about their strategies and operations.
Syndicated market research reports, on the other hand, are pre-existing reports that are available for purchase by multiple clients. These reports are often produced on a regular basis, such as annually or quarterly, and cover a broad range of industries and market segments. Syndicated reports provide clients with insights into industry trends, market sizes, and competitive landscapes. By offering both custom and syndicated reports, Stringent Datalytics can provide clients with a range of market research solutions that can be customized to their specific needs
Contact US:
Stringent Datalytics
Contact No - +1 346 666 6655
Email Id - [email protected]
Web - https://stringentdatalytics.com/
#Medical Grade Thermocouple Wire#Thermocouple Technology#Temperature Measurement#Medical Devices#Healthcare Industry#Biomedical Applications#Thermocouple Sensors#Medical Equipment#Temperature Monitoring#Diagnostic Devices#Surgical Instruments#Patient Care#Clinical Research#Temperature Sensing#Biotechnology#Laboratory Instruments#Quality Control#Regulatory Compliance#Medical Instrumentation#Patient Monitoring#Invasive Procedures#Medical Testing#Precision Measurement#Life Sciences#Remote Monitoring#Sterilization Processes#Heat Management#Data Logging#Therapeutic Devices#Research and Development.
0 notes
Text
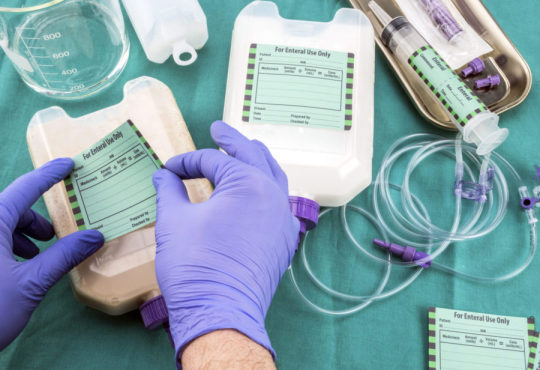
#Medical Devices#Regulatory Compliance#Product Labeling#FDA Regulations#UDI (Unique Device Identification)#MDR (Medical Device Regulation)#Labeling Standards#Risk Management#Health Canada Regulations#Clinical Labeling#Device Instructions for Use#Packaging and Labeling#Medical Device Safety
0 notes