#long qt syndrome type 1
Explore tagged Tumblr posts
Link
Panera SETTLES wrongful death suit Update on The Ivy League student with heart condition died after consuming the highly caffeinated drink. #PaneraSettlement #SarahKatz #WrongfulDeathLawsuit #ChargedLemonade #CaffeineHealthRisk #Panera #paneralemonade Subscribe👇: https://vist.ly/3miamtv 👀👇: https://vist.ly/3miamtu
#panera bread#sarah katz#21-year-old#ivy league student#death#charged lemonade#highly caffeinated#heart condition#long qt syndrome type 1#cardiac arrest#wrongful death lawsuit#marketed#plant-based and clean#as much caffeine#dark roast coffee#elizabeth crawford#plaintiffs#settlement#menu transformation#nutrition information update.
0 notes
Text
Forecasting the Size of Long QT Syndrome Market
Market Overview –
The long QT syndrome market is anticipated to grow at a 5.2% CAGR from 2022 to 2030, or USD 3,933.02 million.
The Long QT Syndrome (LQTS) market encompasses medical interventions and devices designed to diagnose, treat, and manage this cardiac disorder characterized by abnormal heart rhythms. LQTS increases the risk of sudden cardiac arrest or arrhythmias, posing serious health threats. The market includes genetic testing, medications, implantable cardioverter-defibrillators (ICDs), and lifestyle modifications aimed at reducing the risk of life-threatening events.
The Long QT Syndrome market is characterized by advancements in understanding and managing this cardiac disorder. With a focus on long QT disorder, healthcare providers emphasize early diagnosis and personalized treatment approaches to mitigate the risk of life-threatening arrhythmias. As awareness grows, there's a rising demand for innovative therapies and genetic testing, driving market growth and research endeavors.
A key driver of the Long QT Syndrome market is the growing awareness of inherited cardiac disorders and advances in genetic testing technology. Early detection through genetic screening allows for timely interventions, such as medication adjustments or implantation of ICDs, to prevent cardiac events and improve patient outcomes.
Advancements in medical devices, such as miniaturized ICDs and wearable cardiac monitors, have revolutionized the management of Long QT Syndrome. These devices provide continuous monitoring and early detection of arrhythmias, offering peace of mind to patients and caregivers while improving overall safety.
The COVID-19 pandemic has impacted the Long QT Syndrome market, with disruptions in routine medical care, delayed diagnoses, and challenges in accessing specialized cardiac services. However, telemedicine and remote monitoring technologies have emerged as valuable tools in managing Long QT Syndrome, enabling patients to receive timely care and support from healthcare providers.
Segmentation –
The global long QT syndrome market is segmented on the basis of type, diagnosis, treatment, and end users. On the basis of the type, the market is segmented into long QT syndrome type 1, long QT syndrome type 2, long QT syndrome type 3, and others. On the basis of the diagnosis, the market is categorized into tests, electrocardiogram (ECG), genetic testing, and others.
On the basis of the treatment, the market is segmented into medication, surgical procedures, and others. On the basis of the end user, the market is segmented into hospitals & clinics, diagnostic labs, research organizations, and others.
Regional Analysis –
Regional analysis of the Long QT Syndrome (LQTS) market reveals variations in diagnosis rates, treatment options, and healthcare infrastructure across different regions. In developed regions like North America and Europe, where there is greater awareness of genetic disorders and access to specialized cardiac care, the market for LQTS diagnostics and management is well-established, with genetic testing, beta-blockers, and implantable cardioverter-defibrillators (ICDs) being standard of care.
Conversely, in developing regions with limited access to advanced cardiac diagnostics and therapies, such as parts of Africa and Asia-Pacific, the market for LQTS is still emerging, with challenges related to underdiagnosis and limited treatment options. Moreover, cultural attitudes towards genetic testing and preventive healthcare influence patient behaviors and healthcare-seeking patterns across different regions. As awareness of LQTS and its genetic basis increases globally, there is a growing opportunity for market expansion through education, advocacy, and investment in cardiac care infrastructure to improve outcomes for LQTS patients worldwide.
Key Players –
Long QT syndrome key players include Invitae Corporation (U.S.), GeneDx (U.S.), Asper Biogene (Estonia), Boston Scientific Corporation (U.S.), Laboratory Corporation of America Holdings (U.S.), Pfizer Inc. (U.S.), Zydus Pharmaceuticals, Inc. (U.S.), Aralez Pharmaceuticals Inc. (Canada), AstraZeneca (U.K.), Torrent Pharmaceuticals Limited (India), Lupin Pharmaceuticals, Inc. (U.S.), Cipla Inc. (India), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), and others.
Related Reports –
Pain Patch
Electronic Trial Master File Systems
Neurology Devices
Medical Payment Fraud Detection
For more information visit at MarketResearchFuture
#Long QT Syndrome#Long QT Syndrome Size#Long QT Syndrome Share#Long QT Syndrome Growth#Long QT Syndrome Trends
0 notes
Text
(Dr Ramji Mehrotra) What are Inherited Cardiac Conditions?
Inherited cardiac conditions are a group of cardiovascular disorders that are caused by genetic mutations or abnormalities passed down from parents to their children. These conditions can affect the structure or function of the heart and may occur at various stages of life, from infancy to adulthood. Understanding the nature of inherited cardiac conditions is crucial for early detection, proper management, and preventive measures.
One prominent example of an inherited cardiac condition is hypertrophic cardiomyopathy (HCM). HCM is characterized by the thickening of the heart muscle, particularly in the left ventricle. This abnormal thickening can disrupt the heart's pumping ability and lead to symptoms such as chest pain, shortness of breath, and palpitations. In some cases, HCM can cause sudden cardiac arrest, especially during intense physical activity.
According to Dr. Ramji Mehrotra, leading cardiac surgeon in NCR region, genetic mutations in genes responsible for the structure of cardiac muscle proteins are often associated with HCM. Diagnosis typically involves a combination of clinical evaluation, imaging tests like echocardiography, and genetic testing to identify specific mutations. Management may include medications to alleviate symptoms, lifestyle modifications, and, in severe cases, surgical interventions.
Another inherited cardiac condition is Long QT syndrome (LQTS), which affects the electrical system of the heart. Individuals with LQTS have an abnormality in the ion channels responsible for regulating the heart's electrical activity. This can lead to irregular and potentially life-threatening arrhythmias, including a specific type known as Torsades de Pointes.
Symptoms of LQTS may include fainting, seizures, and sudden cardiac arrest. Genetic testing can identify mutations associated with LQTS, and electrocardiograms (ECGs) are used to evaluate the heart's electrical activity. Treatment typically involves medications to prevent arrhythmias, lifestyle modifications, and the avoidance of certain medications that can prolong the QT interval.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiac condition that affects the structure and function of the heart's right ventricle. In ARVC, the normal muscle tissue in the right ventricle is progressively replaced by fatty or fibrous tissue, leading to abnormal heart rhythms and, in some cases, heart failure. Genetic mutations involving proteins responsible for maintaining the structural integrity of heart cells are often associated with ARVC. Diagnosis may involve a combination of clinical evaluation, imaging tests like cardiac MRI, and genetic testing. Treatment typically focuses on managing arrhythmias, preventing sudden cardiac death, and addressing heart failure symptoms through medications, implantable devices like defibrillators, and lifestyle modifications.
Marfan syndrome is an inherited connective tissue disorder that can also affect the cardiovascular system. It is caused by mutations in the FBN1 gene, which leads to abnormalities in the production of the protein fibrillin-1. Fibrillin-1 is a crucial component of connective tissue that provides strength and elasticity to various structures, including the heart's valves and aorta.
In individuals with Marfan syndrome, the aorta can be weakened and dilated, increasing the risk of aortic dissection or rupture. Diagnosis involves clinical evaluation, imaging tests like echocardiography, and genetic testing to confirm the presence of FBN1 mutations. Treatment may include medications to manage symptoms, regular monitoring of the aorta's size, and surgical interventions to repair or replace the affected valves or aorta if necessary.
Dr. Ramji Mehrotra says that understanding the genetic basis of inherited cardiac conditions, along with appropriate diagnostic strategies and management approaches, is crucial for early detection, prevention of complications, and optimal care.
0 notes
Text
A medical youtuber named Medlife Crisis, who has a video about Long Qt Syndrome, (type 2), a heart disease that can literally make you die of fright, ends the video with a LOUD, and sudden, phone ring. Earlier on in the video, he straight up used someone getting a call from their mom and dying from it as an example. Additionally, he says that about 1 in 7000 people have Long Qt Syndrome.
That video currently has 68k views. While there does appear to be treatment for it, the fact that he would even do that in the first place could still mean that someone with it would’ve died FROM A VIDEO ABOUT IT.
Hell, someone with it could’ve looked up videos about their own condition, found this, and died because of it.
This is made even worse by the fact that, while I don’t know much in depth about the disease, is something that some people might dismiss as extreme anxiety. Even if I am wrong on that, it’s still something that legitimately might’ve caused a few deaths.
And in the comments, his only response, as far as I can tell, to people talking about how that could’ve actually killed someone, is this:

I don’t want to say that jump scares in general should be banned or anything, but when it’s on a video about how something like that could kill someone is when I think completely crosses the line. Note: While this is important, YOU ARE NOT A BAD PERSON IF YOU DON’T REBLOG IT. I want to make that very clear.
#long qt syndrome#lqts#LGT#medlife crisis#Medical#serious#important#warning#death tw#death trigger warning#tw: death#Scared to death: The rare heart disease that means a fright can be fatal#(<-Video title)
60 notes
·
View notes
Text
So they changed their tune after a wrongful death suit. There's actually a second one too, which is the one I heard about first. They also dropped the 30 oz to 237mg of Caffiene according to some stories but I'm not sure if that was after the first (October) suit or the second (December) suit.
Panera Bread is evidently taking a page out of the Cocaine Era of Coca-Cola
Caffeine In Beverages (See Google)
20 fl oz. Coke | 53 mg
8 fl. oz. Coffee | 95 mg
16 fl oz. Monster | 160 mg
20 fl. oz. Panera* | 260 mg
30 fl. oz. Panera* | 389 mg
*Panera Charged Lemonade Mango Yuzu Citrus
Maximum Recommended Daily Intake for a Healthy Adult 400 mg (Caffeine has a half life in the body of about 4-6 hours if what I saw online was correct.)
Companies with the American Beverage Association are not legally required to add this label (the FDA asked nicely so they could avoid expensive courts) and it looks as follows.

I should note the FDA does require labels to say if caffeine is present.
This is how the Charged Lemonades are served at Panera

Notice the labels only list the amount, absolutely zero further information. They are also only labeled as "Charged Lemonade" and not as Energy Drinks, which they actually contain significantly more caffeine than.
Be Informed and Be Aware
This info blew up over on TikTok after a woman made a video after having 4-5 of them without noticing the caffeine content. She had been doing this for several days it seems, going there and working on her laptop while drinking her Lemonade. When I say to you in the video she looked like her body was about to short circuit? Yeah. Video is linked below.
Now, back to my initial statement, why do I mention back when Coke had Coke? Well because caffeine is addictive, I'm no puritan about caffeine myself, but I at least know what I'm getting myself into with my tea, coke, and occasionally a Monster (about 1-3 a month). Furthermore it's a stimulant and can make you feel more awake and productive. Finally the larger sizes just barely skirt that max intake limit. I believe this is intentional, to bring customers back again and again.
https://www.tiktok.com/t/ZTRsaCEAk/
20 notes
·
View notes
Text
What You Need To Know About Nuedexta
Nuedexta for treatment of Pseudobulbar affect (PBA) and other uses.
The drug known as Nuedexta is made up of a combination of dextromethorphan hydrobromide/ quinidine sulfate which is prescribed for treating PBA (pseudobulbar affect). This medication was developed and manufactured by a company by the name of Avanir Pharmaceuticals. This company received authorization to market Nuedexta from the EC (European Commission) in June 2013, for treating the condition PBA. Avanir Pharmaceuticals obtained approval from the FDA (US Food and Drug Administration) for Nuedexta for treating PBA in the month of October 2010.
Symptoms Of Pseudobulbar Effect
PBA which is short for Pseudobulbar Affect is classified as a type of neurological disorder that emanates from a neurological condition like Parkinson's disease, amyotrophic lateral sclerosis, multiple sclerosis, stroke or a traumatic injury to the brain. This condition is the cause of uncontrolled and sudden episodes of laughing or crying involuntarily. This condition has been estimated to affect over 1 million people across the U.S.
The Mechanism Of Action Associated With Nuedexta
Nuedexta is made up of a mixture of two medications, which includes quinidine sulfate and dextromethorphan hydrobromide. Exact mechanisms of action associated with the drug is still not completely known, yet it is said that it works in the way of regulating the excitatory neurotransmissions that occur through the Sigma-1 Receptor agonist activity along with the NMDA receptor antagonist activity. This medication also works on slowing the breakdown of a chemical known as dextromethorphan found inside the brain. Nuedexta drug is in a class that includes opium alkaloids along with its derivatives.
Side Effects Of Nuedexta
To date, a number of side effects regarded as serious have been reported with this medication. Some of the common types of side effects linked to Nuedexta include:
- Vomiting
- Diarrhea
- Fainting/dizziness
- Stomach pain
- Difficulties with breathing
-Irregular or fast heartbeat
- Cough
- Fever
- Weakness
- Swelling of the lower legs, ankles, feet or hands
- Muscle spasms
- Painful, difficult or frequent urination
This list is not the complete version of Nuedexta side effects. It is advisable to ask for advice medically or call a doctor if you are experiencing side effects that are not going away.
Nuedexta Interactions
It is important that you let your physician know about any other non-prescription and prescription medications that you are taking, this also includes herbal supplements and vitamins. MAO inhibitors shouldn't be taken with or within 2 weeks after or before taking Nuedexta.
Important Information
This medication should not be taken in patients who have experienced heart failure, a heart condition known as AV block, or you have a history associated with Long AT syndrome or heart rhythm disorders. You should also not take Nuedexta when you have used MAO inhibitors within the last 14 days. This can result in a dangerous interaction. The MAO inhibitors can include linezolid, isocarboxazid, phenelzine, methylene blue injection, rasagiline, phenelzine, tranylcypromine, selegiline, tranylcypromine among others. You should also refrain from using Nuedexta if you currently take quinine, mefloquine, or quinidine or you have in the past experienced allergic reactions or severe medical issues by taking one or more of these drugs.
Certain drugs are able to interact with Nuedexta, which is why they should never be used together. You need to disclose to your doctor about any medication that you use, along with those that you stop or start using over the course of your treatment with Nuedexta. This medication can be dangerous if you decide to use it while taking Quin-G, Lariam or Qualaquin, or when you have experienced allergic reactions.
Before You Take This Medicine
- Nuedexta should not be a medication that you should be using when you have already had an allergic reaction to quinidine or dextromethorphan. You should also not be using Nuedexta if you have experienced heart failure.
- A history linked to a life-threatening heart-rhythm disorder.
- A history linked to Long QT syndrome.
- You have been diagnosed with a heart condition known as AV Block unless you now have a pacemaker.
- If you are already taking quinine, quinidine or mefloquine.
Nuedexta usually starts with a 1 capsule dose every day at the same time for a period of 7 days. In the second week, you will start taking 1 capsule twice a day (every 12 hours).
1 note
·
View note
Photo

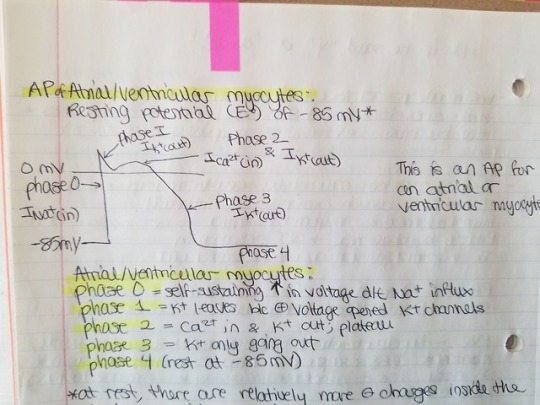

It’s hard to remember all the antiarrhythmic drugs because of this classification system. But there’s no other way. 😩 Drugs that prolong phase 0 in ventricular and atrial myocytes also prolong (widen) the QRS complex. Drugs that prolong phases 1 to 3 prolong the QT interval, which can lead to Torsades de Pointes. Drugs that slow conduction through the AV node (CCBs and beta blockers) can cause heart block (first degree or Mobitz type 1/Wenckebach). AV nodal block = increased PR interval (it’s taking longer for the impulse to go through the AV node).
Blocking Na+ channels prolongs phase 0, which widens the QRS complex. Blocking K+ channels prolongs phases 1-3, which prolongs the QT interval. Slowing AV node conduction = prolonged PR interval.
Class Ic only affects the Na+ channels. Class Ia blocks Na+ and K+ channels. Class Ib shrinks action potential duration and effective refractory period, but doesn’t affect QRS complex.
Class Ib binds Na+ channels better when the HR is fast because these drugs bind better to Na+ channels in depolarized tissue. Faster HR means depolarization is happening faster, so more drug can bind and thus you get increased toxicity. So class Ib drugs have increased toxicity if the HR is fast. All the class I antiarrhythmics exhibit use dependence, meaning the drugs bind better to Na+ channels that are in use (open or inactivated). In ischemic tissue, the myocytes are more depolarized because they can't maintain the -85 mV membrane potential. Thus, class Ib drugs work best for ischemic tachycardia.
Amiodarone has the lowest risk of causing torsades de pointes of all the class III drugs. It also has class I, II, and IV effects, so it’s very effective. It has the most effects of all the antiarrhythmics. It is lipid soluble, so it takes a long time to be eliminated from the body. It has a lot of adverse effects--the one I remember most and the reason pts can die from amiodarone is pulmonary fibrosis. It has iodine in it, so it can cause hypothyroidism or hyperthyroidism. It has worse side effects the longer you use it, so it’s not given to young pts. It also causes photosensitivity, increased LFTs, blue man syndrome (blue face), corneal deposits (look like cat whiskers; benign effect). So you need to do baseline LFTs, PFTs, thyroid tests, CXR before starting pts on amiodarone.
Sotalol and dofetilide especially cause QT interval prolongation. Sotalol has beta block effects too. Sotalol and dofetilide work best when K+ channels are at rest, which occurs during bradycardia. This is reverse use dependence. So sotalol and dofetilide work best in pts with bradycardia; that also means increased risk of toxicity in such pts. Ibutilide may be given IV for chemical cardioversion but it has lots of side effects.
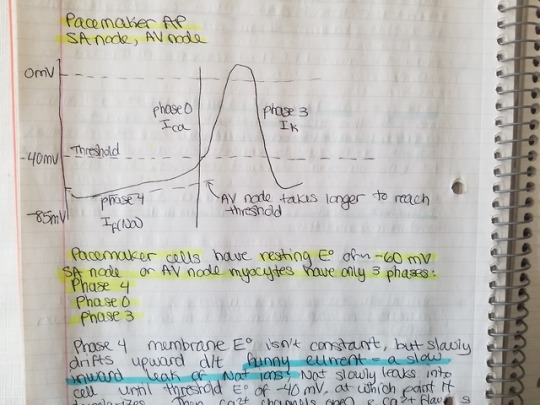
Class II (beta blockers) work on myocytes in the SA and AV nodes. So they can slow the HR. Recall that the action potential in pacemaker cells looks different from the one for ventricular or atrial myocytes. The action potential for pacemaker cells has phase 4, phase 0, and phase 3. Beta blockers prolong phase 4 (resting potential, funny current). Beta blockers make the cell take longer to depolarize. So they decrease HR and conduction through AV node. Decreased conduction through AV node = PR prolongation, which can cause AV nodal block. Unlike other antiarrhythmics, beta blockers improve mortality.
Class IV = Calcium Channel Blockers (CCBs). Block Ca2+ channels in phase 0-> slow HR & AV node conduction. Also prolong PR interval. Same effect as beta blockers.
Adenosine binds to purinergic receptors in the AV node and vascular smooth muscle. In AV node, it activates K+ channels, hyperpolarizing myocytes. It also blocks Ca2+ influx. Slows AV node conduction. Treats SVT (like AVNRT). Theophylline and caffeine block adenosine receptors. In the vascular smooth muscle, adenosine causes vasodilation. So it's normal for adenosine to cause flushing, dyspnea, and CP, which resolves rapidly.
Magnesium can help manages torsades because it blocks Ca2+ influx into cells. Ca2+ influx into myocytes causes early afterdepolarizations, which cause torsades. So blocking Ca2+ stops the early afterdepolarizations.
Atropine is a muscarinic receptor antagonist. I always remember that it's used to block the parasympathetic nervous system; so people who have been poisoned by nerve gas have overactivation of the parasympathetic nervous system (lacrimation, diarrhea) and atropine blocks that. Atropine blocks the parasympathetic nervous system--> increased conduction through AV node, so it treats bradycardia due to AV block by increasing HR and impulse conduction through AV node. Since it blocks PSNS, AEs include dry mouth, constipation, urinary retention, and confusion in elderly pts.
Digoxin blocks the Na+/K+ ATPase. This stops the pumping of K+ into the cell and Na+ out of the cell. The increased Na+ in the cell will make it so that the Na+/Ca2+ exchanger will stop pumping Na+ into the cell in exchange for Ca2+ out of the cell. Thus there will be more Ca2+ in the cell--> increased contractility. Digoxin increases parasympathetic tone--> suppressed AV node conduction. So digoxin increases contractility and slows conduction through AV node. Digoxin competes with K+ for binding to the Na+/K+ ATPase, so when you have hypokalemia, there's less K+ for digoxin to compete with and thus increased effects of digoxin--> increased toxicity. So loop diuretics like furosemide, which cause K+ loss would increase digoxin toxicity. Digoxin AEs: N/V, abdominal pain, lethargy, fatigue, scotomas, loss of color vision, blindness, cardiac arrhythmias. Digoxin causes more Na+ to be inside myocytes, so their membrane potential is more positive and they can spontaneously depolarize (automaticity)--> extra beats. Digoxin can cause hyperkalemia since it can poison the Na+/K+ ATPase--> too much extracellular K+. Digibind is the antidote; it's an antibody (digoxin antigen binding fragment, Fab). Digibind treats the hyperkalemia.
#antiarrhythmics#antiarrhythmic drugs#pharm#pharmacology#cardio#cardiology#Torsades de Pointes#TdP#procainamide#quinidine#lidocaine#mexiletine#flecainide#propafenone#amiodarone#sotalol#dofetilide#ibutilide#verapamil#diltiazem#CCBs#CCB#calcium channel blocker#adenosine#atropine#magnesium#digoxin#digibind
8 notes
·
View notes
Text
Confession #4,511
Hi, i have Long QT syndrome type 1 and i was diagnosed at age 13 despite it being genetic. is it bad that i think i’m lucky that i’m asymptomatic? But then Like i love my friends and going out with them and doing stupid things or being stupid with them but then i remember the faulty ticker and say sorry guys i’m gonna sit out for a bit and get eye rolls in response or i get asked if i want kids and then get judgemental looks for saying no because the heart conditions genetic but i don’t show it
#chronic-confessions#chronic illnesss#chronic community#spoonie#spoon theory#long qt syndrome#unsupportive friends
10 notes
·
View notes
Text
Buy reputable cheap levitra

What is Generic Levitra?
Original brand name Levitra contains the dynamic fixing vardenafil. It treats erectile dysfunction by expanding blood flow to the p***s during s**ual excitement, in this manner instigating an erection. It is favored by many over its nearest competitor, Viagra, on account of the fact that it produces fewer side effects and lasts a few hours longer.
Levitra is a renowned brand name ED treatment designed to induce hard, long-lasting erections in impotent men. Its main benefits beyond its effectiveness are that it has relatively fewer side effects when compared to its nearest competitor, Viagra. It also lasts slightly longer at an average of 8-10 hours.
Levitra, like other brand name ED treatments, remains too expensive for many men to buy in-store. Fortunately, generic Levitra is available from select online drug stores. It contains vardenafil, a similar dynamic fixing present in brand name Levitra is accessible from select online pharmacies. It contains vardenafil, a similar dynamic fixing present in brand name Levitra, and is therefore identical in every way to the original. It simply costs a lot less as it does not bear an expensive brand name.
ED Affects Relationships
When either you or your partner experiences impotence, it can put immense strain on your relationship. Physical closeness is imperative to your bliss inside a relationship and a scarcity in that department can be dooming. By using conventional Levitra, you can guarantee that you don't pass up the associations that physical contact and s**ual interaction forge.
Once a man has experienced impotence, he may find himself pulling away from any sort of physical intimacy or contact. This is because the fear of disappointing both himself and his partner may prevent him from risking embarrassment. Vardenafil tablets can provide you with the confidence that you need to be intimate with your partner and keep your relationship healthy.
It is additionally normal for ladies whose accomplices are encountering ED to start to remove themselves. This is on the grounds that, while they intentionally know that their accomplice's weakness isn't their deficiency, they subconsciously worry that they are doing something wrong in the bedroom or that they are unattractive. Generic Levitra can prevent these concerns by ensuring a man’s s**ual performance.
How Does Generic Levitra Work?
The dynamic fixing in Levitra is called vardenafil and is a phosphodiesterase type 5 (PDE-5) inhibitor. PDE-5 is a compound discharged in the male body after peak has been come to. It effectively counteracts the effects of another enzyme called cyclic guanosine monophosphate.
During s**ual arousal, levels of cGMP within a man’s body rise. This triggers an increased rate of blood flow to the p***s and causes an erection. While cGMP remains active in your body, you will be able to effectively maintain an erection and satisfy your s**ual partner.
A man experiences ED when his body rashly discharges PDE-5 into his system and the rate of blood flow to his p***s drops to normal levels. At the point when a PDE-5 inhibitor, for example, vardenafil is available in your system, the discharge and activity of PDE-5 in your body will be seriously confined and you will probably accomplish and keep up an erection for as long as you remain aroused.
Note that nonexclusive Levitra is not a Spanish fly and subsequently does not incite an erection independently. Its only function is to make it possible for you to attain an erection should you become aroused and hence arousal is necessary for an erection to be produced.
Use of Levitra
Read the Patient Information Leaflet given by your pharmacist before you begin taking vardenafil and each time you get a refill. On the off chance that you have any inquiries. Accept this prescription by mouth as directed by your doctor, usually as needed. Take vardenafil, with or without food, about 1 hour before s**ual activity. Do not take more than once daily. Doses should be taken at least 24 hours apart.
The measurements depends on your ailment, response to treatment, and different drugs you might take.
Avoid eating grapefruit or drinking grapefruit juice while using this medication unless your doctor or pharmacist says you may do so safely. Grapefruit can increase the chance of side effects with this medicine.
How should I take genetic Levitra?
Take Levitra exactly as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
The tablets can be taken with or without nourishment.
Levitra is taken just when needed, around an hour prior s**ual activity. The medication can help achieve an erection when s**ual incitement happens. An erection won't happen just by taking a pill. On the off chance that your erection is difficult or keeps going longer than 4 hours. A drawn out erection can harm the p***s. Staxyn should not be used in place of Levitra. Avoid medication errors by using only the form and strength prescribes.
Store at room temperature away from dampness and heat.
On the off chance that you get restorative consideration for heart issues, tell your guardians when you last took vardenafil.
What You Can Do
If you are in a relationship in which ED is a factor, there are a number of things that you can do to ensure that the bond among you and your accomplice stays solid. Right off the bat, you might need to utilize a medicine such as nonexclusive Levitra to guarantee that both you and your partner can accomplish s**ual satisfaction.
It is also important that you keep communication channels honest and open to prevent a void from growing between you. Consider speaking to a relationship therapist about your issues and consider changing your definition of intimacy to meet your capabilities.
Benefits of buying Levitra online
Many e-pharmacies sell this medication without prescription at affordable prices that are sometimes further reduced if you buy it in larger quantities. Unlimited access to 24/7 live customer services in the form of live chat or monitored contact forms is also provided. Your order should arrive discreetly wrapped in 2-5 working days after payment if you are in the UK, and in 5-7 working days if you are in the EU. Buy effective treatment for erectile dysfunction online today, for the s*x life you deserve.
Usage and Dosage Instructions for Levitra Tablets
When you take your single 20mg dosage, it is best taken with a full glass of water about 30 to 60 minutes before you decide to engage in any s**ual activity. The effects will then last for up to 8 hours. It is important as to not take more than a single 20mg dosage within a 24 hour period, just to avoid any temporary and easily treatable side effects.
Before taking this medicine
You ought not to use Levitra in the event that you are susceptible to vardenafil, or on the off chance that you take different medications to treat pneumonic blood vessel hypertension, such as riociguat.
Levitra isn't affirmed for men more youthful than 18 years of age. Do not take vardenafil if you are also using a nitrate drug for chest pain or heart problems. This includes nitroglycerin, isosorbide dinitrate, and isosorbide mononitrate. Nitrates are also found in some recreational drugs such as amyl nitrate or nitrite. Taking Levitra with a nitrate medicine can cause a sudden and serious decrease in blood pressure.
Tell your doctor if you have ever had:
Heart disease, heart rhythm problems
A heart attack, stroke, or congestive heart failure
Long QT syndrome
High or low blood pressure
Levitra side effects
Get emergency medical help if you have signs of an allergic reaction to Levitra: hives, difficult breathing, and swelling of your face, lips, tongue, or throat.
Stop taking Levitra and get emergency medical help if you have:
Heart attack symptoms - chest pain or pressure, pain spreading to your jaw or shoulder, nausea, sweating
Vision changes or sudden vision loss
Erection is painful or lasts longer than 4 hours irregular heartbeat
Swelling in your hands, ankles, or feet
Shortness of breath
A light-headed feeling, like you might pass out
P***s erection that is painful or lasts 4 hours or longer
Seizure
Common Levitra side effects may include:
flushing
stuffy nose, sinus pain
headache, dizziness
stomach upset
back pain
Buy long lasting Levitra online
Do not miss out on another chance to enjoy some vigorous love making. Purchase Levitra online from our reputable erectile wellness pharmacy and we will ensure that your order is delivered post haste.
1 note
·
View note
Text
Global Cardiovascular Partnering 2014-2021: Deal trends, players and financials
Bharat Book Bureau Provides the Trending Market Research Report on “Global Cardiovascular Partnering 2014-2021: Deal trends, players and financials” under Life Sciences Category. The report offers a collection of superior market research, market analysis, competitive intelligence and Market reports.
Global Cardiovascular Partnering 2014 to 2021 provides the full collection of Cardiovascular disease deals signed between the world’s pharmaceutical and biotechnology companies since 2014.
Trends in Cardiovascular partnering deals Financial deal terms for headline, upfront and royalty by stage of development Cardiovascular partnering agreement structure Cardiovascular partnering contract documents Top Cardiovascular deals by value Most active Cardiovascular dealmakers
Most of the deals included within the report occur when a licensee obtains a right or an option right to license a licensor’s product or technology. More often these days these deals tend to be multi-component including both a collaborative R&D and a commercialization of outcomes element.
The report takes readers through the comprehensive Cardiovascular disease deal trends, key players and top deal values allowing the understanding of how, why and under what terms companies are currently entering Cardiovascular deals.
The report presents financial deal terms values for Cardiovascular deals, where available listing by overall headline values, upfront payments, milestones and royalties enabling readers to analyse and benchmark the value of current deals.
The initial chapters of this report provide an orientation of Cardiovascular dealmaking trends.
Chapter 1 provides an introduction to the report.
Chapter 2 provides an overview of the trends in Cardiovascular dealmaking since 2014 covering trends by year, deal type, stage of development, technology type and therapeutic indication.
Chapter 3 includes an analysis of financial deal terms covering headline value, upfront payment, milestone payments and royalty rates.
Chapter 4 provides a review of the leading Cardiovascular deals since 2014. Deals are listed by headline value. The chapter includes the top 25 most active Cardiovascular dealmakers, together with a full listing of deals to which they are a party. Where the deal has an agreement contract published at the SEC a link provides online access to the contract.
Chapter 5 provides comprehensive access to Cardiovascular deals since 2014 where a deal contract is available, providing the user with direct access to contracts as filed with the SEC regulatory authorities. Each deal title links via Weblink to an online version of the deal record contract document, providing easy access to each contract document on demand.
Chapter 6 provides a comprehensive directory of all Cardiovascular partnering deals by specific Cardiovascular target announced since 2014. The chapter is organized by specific Cardiovascular therapeutic target. Each deal title links via Weblink to an online version of the deal record and where available, the contract document, providing easy access to each contract document on demand.
In addition, a comprehensive appendix is provided with each report of all Cardiovascular partnering deals signed and announced since 2014. The appendices are organized by company A-Z, stage of development at signing, deal type (collaborative R&D, co-promotion, licensing etc) and technology type. Each deal title links via Weblink to an online version of the deal record and where available, the contract document, providing easy access to each contract document on demand.
The report also includes numerous tables and figures that illustrate the trends and activities in Cardiovascular partnering and dealmaking since 2014.
In conclusion, this report provides everything a prospective dealmaker needs to know about partnering in the research, development and commercialization of Cardiovascular technologies and products.
Report scope
Global Cardiovascular Partnering 2014-2021: Deal trends, players and financials is intended to provide the reader with an in-depth understanding and access to cardiovascular trends and structure of deals entered into by leading companies worldwide.
Global Cardiovascular Partnering 2014-2021: Deal trends, players and financials includes:
Trends in cardiovascular dealmaking in the biopharma industry since 2014 Access to headline, upfront, milestone and royalty data Access to over 850 cardiovascular deal records The leading cardiovascular deals by value since 2014
The report includes deals for the following indications: Abdominal aortic aneurysm, Angina, Arrhythmia, Atrial fibrillation, Long QT syndrome, Supraventricular Tachycardia, Ventricular fibrillation, Ventricular tachycardia, Atherosclerosis, Breathlessness, Cardiogenic shock, Cardiomyopathy (heart muscle disease), Chest pain, Congenital heart disease, Congestive heart failure, Coronary artery disease, Ductus arteriosus, Fatigue, Hypercholesterolemia, Hypertension, Intermittent claudication, Ischemic heart disease, Limb ischemia, Marfan's Syndrome, Myocardial Infarction, Oedema (excess fluid), Palpitations, Peripheral arterial disease, Thrombus (blood clot), Valvular heart disease, Aortic stenosis, Restenosis, Varicose veins, plus other cardiovascular indications.
In Global Cardiovascular Partnering 2014-2021: Deal trends, players and financials, the available deals are listed by:
Company A-Z Headline value Stage of development at signing Deal component type Specific therapy target
Each deal title links via Weblink to an online version of the deal record and where available, the contract document, providing easy access to each contract document on demand.
The Global Cardiovascular Partnering 2014-2021: Deal trends, players and financials report provides comprehensive access to available deals and contract documents for over 850 cardiovascular deals.
Browse our full report with Table of Content : https://www.bharatbook.com/report/897789/global-cardiovascular-partnering-deal-trends-players-and-financials About Bharat Book Bureau: Bharat Book is Your One-Stop-Shop with an exhaustive coverage of 4,80,000 reports and insights that includes latest Market Study, Market Trends & Analysis, Forecasts Customized Intelligence, Newsletters and Online Databases. Overall a comprehensive coverage of major industries with a further segmentation of 100+ subsectors.
Contact us at: Bharat Book Bureau Tel: +91 22 27810772 / 27810773 Email: [email protected] Website: www.bharatbook.com
0 notes
Text
Long QT Syndrome Market Overview, Economic Impact, Dynamics and SWOT Analysis To 2027
Market Highlights
The market of Long QT syndrome (LQTS) is driven by factors like the prevalence of cardiovascular diseases & diabetes, and growing geriatric population. Moreover, rising per capita healthcare expenditure and increasing R&D expenses by the key players is fuelling the market growth over the forecast period. However, lack of awareness and stringent FDA approvals followed by the high cost of the surgical therapeutics pertaining to this disorder are restraining the market growth.
The global long QT syndrome market is expected to grow at a CAGR of 8.50% during the forecast period.
Segmentation
The global long QT syndrome market is segmented on the basis of type, diagnosis, treatment, and end users. On the basis of the type, the market is segmented into long QT syndrome type 1, long QT syndrome type 2, long QT syndrome type 3, and others. On the basis of the diagnosis, the market is categorized into tests, electrocardiogram (ECG), genetic testing, and others.
On the basis of the treatment, the market is segmented into medication, surgical procedures, and others. On the basis of the end user, the market is segmented into hospitals & clinics, diagnostic labs, research organizations, and others.
Regional Analysis
The Americas dominate the global long QT syndrome market. A well-developed healthcare sector, high per capita healthcare expenditures, increasing number of patients suffering from cardiovascular diseases and diabetes are the major drivers for long QT syndrome market in the Americas. Moreover, the presence of developed economies like the U.S. and Canada boosts the market growth within this region.
Europe holds the second largest market share. Availability of funds for research, huge patient population, and government support for research & development will drive the market. Regionally, Europe is divided into Western Europe and Eastern Europe. The Western Europe leads the market within the Europe. However, Eastern Europe is estimated to be the fastest growing region.
Browse Full Report @ https://www.marketresearchfuture.com/reports/long-qt-syndrome-market-5303 .
Asia Pacific is the fastest growing market. Increasing prevalence of diabetes and growing geriatric population are the major driving factors for the market growth within the region. Moreover, the presence of developing healthcare sector and developing economies within the region are boosting the market growth.
The Middle East & Africa holds the least share of the market. Presence of poor economies and stringent government policies especially in the Africa region are the restraining factors for market growth within the region. The Middle East holds a major share of the market within the region. This can be attributed due to huge healthcare expenditures by the developed economies of the region.
Key Players
The key players for the global long QT syndrome market are Invitae Corporation (U.S.), GeneDx. (U.S.), Asper Biogene (Estonia), Boston Scientific Corporation (U.S.), Laboratory Corporation of America Holdings (U.S.), Pfizer Inc. (U.S.), Zydus Pharmaceuticals, Inc. (U.S.), Aralez Pharmaceuticals Inc. (Canada), AstraZeneca (U.K), Torrent Pharmaceuticals Limited (India), Lupin Pharmaceuticals, Inc. (U.S.), Cipla Inc. (India), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), and others.
Browse Related Reports at:
Telehealth Market Trends, Size, Growth, Share, Application and Analysis by 2024 | MRFR
Migraine Drugs Market by Type, Drug Class, Growth Analysis Till 2027 | MRFR
Patient Registry Software Market Size, Share, Type, Global Analysis, 2027 | MRFR
0 notes
Note
You definitely don't have to answer if you don't want to but what heartproblem do you have? : /
not a problem love! been talking about it for twenty years so might as well.
it’s an arrhythmia disorder called lqts (long QT syndrome) and it basically means that there’s a faulty electric wiring in my genes that makes my heartbeat irregular. your heartbeat has a QT interval, and mine is prolonged. you have different types of it (one type only occurs when you’re in physical extortion/straining yourself, the second with extreme emotions, third in rest).
we discovered I had this when I was seven through a story I won’t bore you with and I’ve been taking beta blockers ever since (which people with anxiety also get prescribed sometimes, double cheers fnfnf). I have type 1, which means that with working out I have to be careful, with sharp differences between hot and cold, and especially with working out in heat. (This sounds like I’m an omega, Jesus) swimming can be dangerous in case it goes wrong so I’m never allowed to go alone.
It’s fine, I just have to be careful with holidays and sports in the summer and it means I’ll never be a professional athlete but I never really had that dream anyway 😂
#it means I get dizzy very easily#always have cold feet because of my meds#and if I get dizzy in heat or in the water I need to get to safety ASAP#and that people in high school who loved sports and holidays to warm places found me boring or frustrating fnfnf#for me it’s ok though I’m fine with it but it gets annoying sometimes and it IS scary sometimes#messages
3 notes
·
View notes
Text
ECG MONITORING SYSTEMS MARKET ANALYSIS(2020-2027)
An electrocardiogram (ECG or EKG) records the electrical signal from the heart to check for different heart conditions. It is the most important test for interpretation of the cardiac rhythm, detection of myocardial ischemia and infarction, conduction system abnormalities, preexcitation, long QT syndromes, atrial abnormalities, ventricular hypertrophy, pericarditis, and other conditions.
The global ECG monitoring systems market is estimated to account for US$ 1,102.5 Mn in terms of value in 2020 and is expected to reach US$ 1,847.2 Mn by the end of 2027.
Global ECG Monitoring Systems Market: Drivers
Increasing prevalence of cardiovascular diseases is expected to propel growth of the global cardiovascular monitoring and diagnostic devices market over the forecast period. For instance, according to the American Heart Association's Heart and Stroke Statistics 2019 Update, around 48% of all adults in the U.S. suffered from some type of cardiovascular disease in 2016.
Moreover, increasing geriatric population is also expected to aid in growth of the market. For instance, according to the U.S. Census Bureau, the U.S. geriatric population is expected to reach 77 million by 2034.
ECG Monitoring Equipment held dominant position in the global ECG monitoring systems market in 2019, accounting for 56.5% share in terms of value, followed by Holter Monitoring Systems and ECG Stress Testing Systems, respectively.
Figure 1. Global ECG Monitoring Systems Market Share (%), by Value, by Equipment Type, 2019

Global ECG Monitoring Systems Market: Restraints
Inaccuracy of wearable ECG devices is expected to hinder growth of the market. For instance, according to a research published in April 2019 by the Harvard Medical School, 30% of wearable devices yield inaccurate results.
Moreover, handheld ECG devices offer limited information compared to conventional lead ECGs, which is also expected to limit growth of the market.

Global ECG Monitoring Systems Market: Opportunities
R&D in ambulatory ECGs is expected to offer lucrative growth opportunities for players in the global ECG monitoring systems market. For instance, in February 2020, researchers at University of Electronic Science and Technology of China developed an adaptive cancellation algorithm based on multi-inertial sensors to suppress motion artifacts in ambulatory ECGs.
Moreover, increasing investment in development of new devices is also expected to aid in growth of the market. For instance, in December 2019, Biotricity Inc., a medical diagnostic and consumer healthcare technology company, raised US$ 6 million for FDA Filing for its patch solution within the first 6 months of 2020, which will be a novel product in the field of Holter monitoring.
Request Sample Free Copy of Report here: https://www.coherentmarketinsights.com/insight/request-sample/4027
Download PDF Brochure: https://www.coherentmarketinsights.com/insight/request-pdf/4027
The global ECG monitoring systems market was valued at US$ 1,024.4 Mn in 2019 and is forecast to reach a value of US$ 1,847.2 Mn by 2027 at a CAGR of 7.7% between 2020 and 2027.
Figure 2. Global ECG Monitoring Systems Market Value (US$ Mn), and Y-o-Y Growth (%), 2019-2027

Market Trends/Key Takeaways
The market is witnessing development of web-based remote cardiac monitoring solutions. For instance, in November 2019, Bittium Corporation, a Finland-based cardiology and neurology device company, presented Bittium HolterPlus, a web-based remote cardiac monitoring solution, consisting of a Bittium Faros ECG device and Bittium HolterPlus mobile application, at Medica 2019, a leading international trade fair for the medical sector that was held in Germany.
R&D in ECG-enabled smartwatch is aiding in growth of the market. For instance, in February 2020, Johnson & Johnson launched a new, virtual clinical study to determine the efficacy of Apple’s iPhone and ECG-enabled smartwatch in reducing the risk of stroke and detecting cases of atrial fibrillation.
Global ECG Monitoring Systems Market: Competitive Landscape
Major players operating in the global ECG monitoring systems market include, GE Healthcare, Philips Healthcare, Nihon Kohden, Schiller AG, Opto Circuits (India) Limited, Opto Circuits (India) Limited, Welch Allyn, Fukuda Denshi Co., Ltd., OSI Systems, Lidco Group plc, Biotricity Inc., Bittium Corporation, St. Jude Medical Inc., VivaQuant, and Johnson & Johnson.
Global ECG Monitoring Systems Market: Key Developments
Major players in the market are focused on approval and launch of new devices to expand their product portfolio. For instance, in July 2020, AliveCor released KardiaCare, a digital subscription service that offers a suite of features that helps users interpret their heart data, monitor risk factors, identify symptom triggers and measure the impact of lifestyle changes.
Similarly, in January 2020, VivaLNK, a China-based provider of connected healthcare solutions for in-patient and remote patient monitoring, received the U.S. FDA clearance for its Continuous ECG platform comprising of reusable wearable ECG sensors and associated software development kit
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/ecg-monitoring-systems-market-4027
#ECG Monitoring Systems Market#CoherentMarketInsights#MarketResearch#Industryanalysis#BusinessConsulting
0 notes
Text
Things To Know About Sudden Cardiac Dysfunction
Sudden Cardiac Dysfunction/Arrest is one of the largest causes of natural death in the world today that occurs most commonly in adults (individuals in the age group of 35-45 years), and occurs twice as much in males than females. This kind of heart related disorder leads to the sudden cessation of the heart functions, loss of breathing, and unconsciousness. A victim would require prompt attention or death might occur.
Sudden Cardiac Dysfunction Symptoms:
There are certain symptoms of sudden cardiac dysfunction, including
1. Shortness of breath
2. Dizziness
3. Sudden collapse
4. Palpitations
5. No pulse
6. Loss of consciousness
7. Weakness
8. Rapid heartbeat
Remember in most of the cases, sudden cardiac arrests occur without any prior signs and symptoms.
Causes:
The most common cause of a sudden cardiac arrest is an abnormal heart rhythm, also known as arrhythmia. The heart function ceases to function during a sudden cardiac arrest. It is our heart’s electrical system that controls the rate and rhythm of the heartbeat. If something goes amiss, the heart will start beating fast and in irregular fashion. Often these arrhythmias are harmless, but some types can lead to sudden cardiac arrest, particularly if it happens in the ventricles (lower chambers of the heart). These racing, erratic electrical impulses cause ventricular fibrillation (it is the most common life-threatening arrhythmia), and the heart is unable to pump blood anymore. Death will occur within minutes if left untreated.

Heart conditions that can lead to a sudden cardiac arrest include coronary artery disease, enlarged heart, congenital disease, heart attack (can cause scarring of the heart tissue), electrical system issues (the problem is in the heart’s electrical system itself, and are called primary heart rhythm abnormalities, including conditions such as Brugada syndrome and long QT syndrome) (certain electrical abnormalities, such as Long QT syndrome may cause sudden cardiac arrest in children and young people),and other causes include heart medications, and recreational drug use.
Risk Factors:
The two leading risk factors include previous heart attack and coronary artery disease (CAD). Risk factors for CAD include smoking, family history of cardiovascular disease, high cholesterol or an enlarged heart.
Other factors contributing to a person’s risk of sudden cardiac arrest and consequent death include smoking, obesity, family history of CAD, high cholesterol, high blood pressure, diabetes, chronic kidney disease, and sedentary lifestyle.
Emergency Treatments for Sudden Cardiac Dysfunction:
CPR- CPR is the acronym for cardiopulmonary resuscitation, which is a lifesaving procedure that involves performing repeated chest compressions and breathing into the mouth of the patient which helps supply oxygen to their lungs and brain.
Defibrillation- Defibrillation is restoring the normal rhythm of the heart by giving electric shock on the chest of the patient (with an AED device that is available in most public places these days).
Sudden Cardiac Arrest survival rates are as high as 90% provided the above measures are taken in the first few minutes.
To sign up for a CPR class, contact CPR Cincinnati in Ohio at 513-828-3488.
0 notes
Text
What are the Risperdal Side Effects?
What Is Risperdal Why Use It And What Are Its Side Effects? It is one of the drugs used in case of risperdal psychological disorder. Risperdal; It was put up for sale in 1994 and is often preferred for the solution of many mental problems. It is used in obsessive or schizophrenic patients, in individuals who are extremely irritable due to autism, and also plays a role in the treatment of many diseases. Risperdal is effective in the following situations; Evolution, blurring, feeling, seeing or hearing something (hallucination), paranoia (extreme skepticism), aggression, social and emotional shyness; It is effective in solving problems that change behavior, emotions or thoughts. Besides, Risperdal; It provides relief of feelings of guilt, mental breakdown, anxiety and nervousness experienced by individuals with this psychology. It plays a role in the correction of mood disorders. It is used in the treatment of suddenly occurring and chronic conditions. It is effective in improving symptoms of flood attacks during mood swings (bipolar disorder). It is effective in eliminating psychological problems that progress with aggressive attitudes and other destructive attitudes. It is used to control unrest caused by autistic disorder in some children and adolescents. For example in these people; Damage to the body, anger crises, aggressive symptoms, sudden changes in mood are observed and relief can be achieved thanks to Risperdal. How to Use Risperdal? Risperdal; for oral use. Risperdal can be taken before meals or after meals. Tablets should be swallowed with as much liquid (1 glass of water) as needed. How long you should continue using Risperdal, the appropriate dose rate and other usage instructions will be informed by your physician. Always act in accordance with your physician's instructions. Do not increase your dose or discontinue your medication without your doctor's approval. The daily dose to be taken in risperdal treatment varies from 0.50 mg to 6 mg depending on the discomfort experienced. Follow your doctor's recommendations. What are the Side Effects of Risperdal? Like all medicines, there may be side effects in people who are sensitive to the ingredients in Risperdal. If one of the following stops, stop using Risperdal and tell your doctor immediately or contact the emergency department of the nearest hospital: Rash on the skin, itching Shortness of breath Swelling in the face, tongue and trachea (sudden hypersensitivity reaction) These are all very serious side effects. If you have one of these, you have a serious allergy to Risperdal. You may need an emergency medical intervention or hospitalization. These very serious side effects are all very rare. If you notice any of the following, tell your doctor immediately or contact the emergency department of the nearest hospital: Flicker Involuntary muscle contractions, inactivity, impaired voluntary movements, fainting Irregularities in blood pressure; For example, your blood pressure drop Irregular heartbeat, palpitations, chest pain QT prolonged syndrome (a condition that can lead to serious arrhythmias and sudden death in the heart) Transient ischemic attack (a reversible type of stroke caused by transient occlusion of the vessels leading to the brain) and similar brain vessels related diseases, suppression of consciousness Sleep apnea syndrome (temporary respiratory arrest during sleep) Weakness in the arms and legs and difficulty speaking (paralysis), referral Lung inflammation (pneumonia), severe abdominal and back pain (pancreatitis) Gastroenteritis (gastrointestinal infection with diarrhea and vomiting) High blood sugar or exacerbation of existing diabetes (diabetes) Jaundice (yellowing of the eyes and skin) Change in some laboratory tests (elevated milk hormone (prolactin) in the blood, elevated liver enzymes, sugar in the urine) Blood clotting in the veins, especially in the legs (including symptoms such as swelling, pain and redness of the legs), moving from the blood vessels to the lungs, causing chest pain and difficulty breathing. These are all serious side effects. Emergency medical attention may be required. Serious side effects are very rare. If you notice any of the following, tell your doctor: Upper and lower respiratory tract infection, flu, inflammation of the air cavities in the facial bones (sinusitis), viral infection, tonsillitis, bronchial inflammation, established infection, cellulite, subcutaneous abscess Diabetes mellitus Fluid buildup in the body (edema) Anemia, neutropenia (decrease in the number of fragmented cells in the blood) Increased appetite or lack of appetite Insomnia, sleepiness, mental distress (depression), anxiety, irritability, restlessness, decreased sexual desire, lack of orgasm, blind feelings Headache, vertigo (dizziness due to impaired balance), dizziness, sedation (calmness), weakness, distraction, unresponsiveness to stimuli, abnormal coordination, less alert sensation, involuntary movements in the face and lips (tardive dyskinesia) Inflammation of the eyes (conjunctivitis), blurred vision, eye bleeding, eye swelling, eyelid swelling, dry eye, increased tear, disturbance from light or light, increased intraocular pressure (glaucoma) Ear pain, tinnitus, otitis media) Flushing (sudden reddening of the face) Nasal congestion, nosebleeds, cough, wheezing Nausea, constipation, digestive disorders, vomiting, diarrhea, increased salivary secretion, dry mouth, abdominal pain, stomach upset, difficulty swallowing, toothache Dry skin, dandruff, rash on the skin (erythema), acne, eczema Back, joint, neck, muscle pain, pain in extremities, joint swelling, muscle weakness, skeletal muscle destruction (rhabdomyolysis) Incontinence, painful urination, inflammation of the urinary tract, frequent urination Swelling in the breasts (gynecomastia), erection related disorders, disorders in sperm discharge Continuous milk and milk-like discharge from the nipple, irregularities in the monthly period Fatigue, weakness, irregularities in body temperature, feeling abnormal, stagnation Not being able to urinate or emptying the bladder fully Low blood sugar (hypoglycaemia) These are mild side effects of RISPERDAL. Post-Marketing Data The following are very rare side effects that may affect less than 1 in 10,000 people: Agranulocytosis (decrease in the number of white blood cells), thrombocytopenia (decrease in platelet-blood flake-number) Disorders in hormone secretion that allows water to be reabsorbed through the kidneys (improper antidiuretic hormone secretion, including symptoms such as nausea, vomiting, anorexia, headache, weakness, hypersensitivity to stimuli) Excessive water consumption Flood watch (mania) Bowel obstruction Alopecia (hair loss) Prolonged painful erection in men (priapism) Lower body temperature These are mild side effects of RISPERDAL. If you encounter any side effects not mentioned in this leaflet, inform your doctor or pharmacist. Read the full article
0 notes
Text
Management of Ventricular Tachycardia
Sustained vs. Nonsustained ventricular tachycardia: ventricular tachycardia with duration less than 30 seconds is classified as nonsustained. Sustained ventricular tachycardia has a duration of more than 30 seconds
Monomorphic ventricular tachycardia: all QRS complexes display the same morphology (minor differences are allowed).This indicates that the impulses originate in the name ectopic focus. In structural heart disease (CAD, HF, cardiomyopathy, valvular disease, etc.) monomorphic ventricular tachycardia is typically caused by re-entry.

Polymorphic ventricular tachycardia: QRS complexes with varying morphology or varying electrical axis. The rhythm may be irregular. Polymorphic ventricular tachycardia is typically very fast (100-320 beats per minute) and unstable. There are several types of polymorphic ventricular tachycardia. The most common cause is myocardial ischemia. The second most common cause is prolonged QTc interval (Long QT syndrome).

Treatment in the emergency setting
Unconscious patients: start CPR immediately.
Hemodynamically unstable patients (hypotension, angina, heart failure, shock, presyncope/syncope): the patient should be treated immediately with electrical cardioversion (during anesthesia).
Beta-blockers are administered intravenously, unless the patient has bradycardia induced ventricular tachycardia. In this scenario, amiodarone is preferred after cardioversion)
Hypokalemia and hypomagnesaemia should be corrected rapidly
Causes underlying the ventricular tachycardia must be targeted; heart failure, ischemia, hypotension, hypokalemia, etc. can be treated rapidly.
Hemodynamically stable patients may be treated pharmacologically with amiodarone, lidocaine, sotalol, or procainamide. Only one of these drugs are administered and a loading dose is followed by an infusion.
Amiodarone is the primary choice with a loading dose of 150 mg IV bolus over 10 minutes. An infusion at 1 mg/min over 6 hours, followed by 0.5mg/min over 18 hours. The loading dose may be repeated every 10-15 minutes if needed. The maximum dose of amiodarone is 2.2 g/day. If amiodarone fails, electrical cardioversion should be considered before pharmacological alternatives are considered.
Intermittent ventricular tachycardia with frequent capture beats should be treated pharmacologically.
Polymorphic ventricular tachycardia should be considered unstable and treated immediately with electrical cardioversion. Beta-blockers may be administered if the ECG does not show long QT interval. If the ECG does show long QT interval, the condition is classified as torsade de pointes, for which treatment varies:
Torsade de pointes in a hemodynamically stable patient
Magnesium sulfate 1g is given over 60 seconds, repeated every 5-10 minutes if indicated. If continuous infusion is necessary, the does is 5-10 mg/min.
All medications/drugs that may cause or aggravate the arrhythmia must be stopped.
Potassium infusion in patients with hypokalemia.
Correction of bradycardia; atropine IV 1-2 mL at 0.5 mg/mL. Isoprenaline (isoproterenol) may also be used, 0.01 mcg/kg/min, which is titrated up until bradycardia resolves. Note that isoprenaline must be administered carefully because it activates beta adrenergic receptors, which may aggravate the arrhythmia.
Temporary transcutaneous or transvenous pacemaker.
Torsade de pointes with hemodynamic compromise
Start with 150 J (biphasic shock) and increase by 50 J for each shock
Ventricular fibrillation and cardiac arrest is treated with conventional resuscitation
Long-term treatment of ventricular tachycardia: patients with preserved LV function and asymptomatic nonsustained ventricular tachycardias may be adequately treated with beta-blockers. Sotalol (may cause QT prolongation) and amiodarone may be considered. Verapamil is contraindicated. If the individual has suffered a MI, and ICD should be considered. Amiodarone appears to be the most effective drug in preventing new episodes of ventricular tachycardia.
x x
#cardiac#nclex cardiac#cardiology#ecg#ecg interpretation#ecg checklist#ecg made easy#advanced practice nursing#advanced pathophysiology#advanced patho#patho#pathophysiology#anatomy#anatomy help#telemetry#telemetry nursing#medical school#ekg#internal medicine#ventricular tachycardia#dysrhythmias#acls#heart disease#heart#nursing school#biomedical sciences#premed#biomed#science#heart rhythm
1 note
·
View note