#hydroxychloroquine manufacturers in india
Explore tagged Tumblr posts
Text
India's Pharmaceutical Market: Key Trends & Drivers Explained

The Indian pharmaceutical industry is set for remarkable growth, expected to reach $65 billion by 2024 and $130 billion by 2030, up from its current $50 billion valuation, according to Invest India. As a leading exporter, India serves over 200 countries, supplying more than 50% of Africa’s generic drugs, about 40% of the United States’ generic drug demand, and 25% of the UK’s medicines. India also accounts for around 60% of global vaccine demand and provides 70% of the World Health Organization’s essential immunization vaccines, including DPT, BCG, and Measles. This expansion highlights India’s crucial role in the global healthcare sector, highlighting its robust export capabilities and significant contributions to vaccine supply.
India’s Rising Importance in The Global Pharmaceutical Landscape
India’s pharmaceutical industry sees exports accounting for over $25 billion, supplying 20% of global generic medicines demand. This growth positions India with about 13% of the global pharmaceutical market share. Also, according to the Indian Brand Equity Foundation (IBEF), the nation is the third-largest producer of active pharmaceutical ingredients (APIs), holding 8% of the global market share and manufacturing over 500 APIs.
According to the Department of Pharmaceuticals, Indian pharmaceutical firms are key players in the United States and European Union (EU) prescription drug sectors, with the highest number of FDA-approved manufacturing plants outside the US. As the world’s largest supplier of generic medicines, the nation meets 20% of global demand by volume. Globally valued at $42 billion, India’s pharmaceutical industry saw nearly 5% year-on-year growth in FY23, reaching $49.78 billion. This growth, driven by an 8% increase in exports and a 6% rise in domestic market growth from FY18 to FY23, underscores India’s role as a major pharmaceutical hub.
The sector further ranks among India’s top ten industries attracting foreign investment, with exports reaching highly regulated markets like the US, Western Europe, Japan, and Australia. During the global health crisis, India demonstrated its capability by supplying around 45 tons and 400 million hydroxychloroquine tablets to 114 countries.
Driving Forces in India’s Pharmaceutical Market
India’s pharmaceutical industry is driven by population growth, urbanization, and an increasing prevalence of chronic diseases. Rising healthcare expenditures, supported by both public and private sectors, further boost the industry growth. In this regard, government initiatives like ‘Ayushman Bharat Yojana’ significantly enhance medication accessibility.
Additionally, schemes like the Production Linked Incentive (PLI) scheme promote domestic manufacturing to reduce import dependency, while the Development of Pharmaceutical Industry (DPI) scheme enhances efficiency and competitiveness through sub-schemes for Bulk Drugs and Medical Devices. These efforts aim to elevate India’s global pharma presence and provide affordable, quality healthcare solutions. Increased investments in research and development (R&D) for new drugs further reinforce India’s significant role in global pharmaceutical innovation.
Major companies, such as Sun Pharma and Mankind, are expanding their market reach by deploying 12,000 medical representatives in urban and rural areas to engage with healthcare professionals.
Regulatory Environment & Advancing Tech in The Indian Pharmaceutical Industry
The pharmaceutical industry in India operates under stringent regulatory oversight to ensure drug safety, efficacy, and quality. The Central Drugs Standard Control Organization (CDSCO) , under the Ministry of Health & Family Welfare, controls the manufacture, import, distribution, and drug sales through the Drugs & Cosmetics Act 1940. The Drugs and Magic Remedies (Objectionable Advertisement) Act 1954 regulates drug advertising, prohibiting claims of miraculous properties.
Further, Good Clinical Practice (GCP) guidelines, developed in collaboration with the Drugs Controller General of India (DCGI) and the Indian Council for Medical Research (ICMR) , set standards for human subject research aligning with international norms like the Declaration of Helsinki and World Health Organization (WHO) guidelines. Its regulatory framework also aligns with international guidelines, including the International Conference on Harmonization (ICH) standards and regulations from bodies like the US FDA and the European Medicines Agency (EMA) , ensuring compliance with global standards.
In recent years, the Indian pharmaceutical sector has been at the forefront of technological innovation, harnessing artificial intelligence (AI), big data analytics, telemedicine, and the Internet of Medical Things (IoMT) to revolutionize healthcare delivery. PharmEasy, launched in 2015, stands as a prime example that has democratized healthcare access by seamlessly connecting patients with nearby pharmacies and diagnostic centers. Similarly, Cipla is digitizing its pharmaceutical sales approach by equipping medical representatives with iPads for e-detailing. This digital transformation enhances sales effectiveness via streamlined communication and interactive engagement with healthcare providers. This wave of innovation is supported by initiatives like the Scheme for Promotion of Research and Innovation in the Pharma MedTech Sector (PRIP) , launched in 2023.
Global Interest Supports the Indian Pharma Sector
The Indian pharmaceutical sector attracts significant foreign direct investment (FDI) due to liberalized policies, allowing up to 100% FDI for Greenfield projects and up to 74% for Brownfield ventures. Since April 2000, the sector has drawn around $22.52 billion in FDI equity inflows, supported by over 10,000 Pradhan Mantri Bhartiya Janaushadhi Kendras nationwide. Major global players such as AstraZeneca, Dr. Reddy’s, and Pfizer have heavily invested in India’s pharmaceutical industry, leveraging its manufacturing and regulatory strengths.
Hence, this sector is set for significant growth in the next decade, driven by its role in global trade and compliance with GMP standards from WHO and USFDA. As a leading producer of generics, India expects around 912% increase in medicine spending over the next five years, placing it among the top global markets. Growth will focus on chronic therapies like cardiovascular and anti-cancer treatments. Besides, pharma companies will adopt FMCG-like strategies, manage diverse channels, and leverage the influence of pharmacists and patient empowerment. Government initiatives and expanding access to low-cost generics will further support this growth.
0 notes
Text
Antiprotozoal drugs Market Projected to Show Strong Growth

Advance Market Analytics added research publication document on Worldwide Antiprotozoal drugs Market breaking major business segments and highlighting wider level geographies to get deep dive analysis on market data. The study is a perfect balance bridging both qualitative and quantitative information of Worldwide Antiprotozoal drugs market. The study provides valuable market size data for historical (Volume** & Value) from 2018 to 2022 which is estimated and forecasted till 2028*. Some are the key & emerging players that are part of coverage and have being profiled are Enzon Pharmaceuticals (United States), Sanofi (France), Pfizer, Inc. (United States), Sun Pharmaceutical Industries Ltd. (India), GlaxoSmithKline plc (United Kingdom), Novartis AG (Switzerland), Ipca Laboratories Ltd. (India), Merck KGaA (Germany), F. Hoffmann La-Roche Ltd. (Switzerland). Get free access to Sample Report in PDF Version along with Graphs and Figures @ https://www.advancemarketanalytics.com/sample-report/165708-global-antiprotozoal-drugs-market Keep yourself up-to-date with latest market trends and changing dynamics due to COVID Impact and Economic Slowdown globally. Maintain a competitive edge by sizing up with available business opportunity in Antiprotozoal drugs Market various segments and emerging territory.
Market Drivers
Increasing prevalence of protozoan infections
Favourable government support to treat malaria
Availability of generic version at a cheaper price
Opportunities:
Ongoing research and development for Drug Development to Protozoan Diseases
Have Any Questions Regarding Global Antiprotozoal drugs Market Report, Ask Our Experts@ https://www.advancemarketanalytics.com/enquiry-before-buy/165708-global-antiprotozoal-drugs-market Analysis by Type (Chloroquine, Pyrimethamine, Mefloquine, Hydroxychloroquine, Metronidazole, Atovaquone, Others), Form (Liquid, Tablet, Injectable form), Distribution (Online Pharmacy, Offline Pharmacy)
Competitive landscape highlighting important parameters that players are gaining along with the Market Development/evolution
• % Market Share, Segment Revenue, Swot Analysis for each profiled company [Enzon Pharmaceuticals (United States), Sanofi (France), Pfizer, Inc. (United States), Sun Pharmaceutical Industries Ltd. (India), GlaxoSmithKline plc (United Kingdom), Novartis AG (Switzerland), Ipca Laboratories Ltd. (India), Merck KGaA (Germany), F. Hoffmann La-Roche Ltd. (Switzerland)]
• Business overview and Product/Service classification
• Product/Service Matrix [Players by Product/Service comparative analysis]
• Recent Developments (Technology advancement, Product Launch or Expansion plan, Manufacturing and R&D etc)
• Consumption, Capacity & Production by Players The regional analysis of Global Antiprotozoal drugs Market is considered for the key regions such as Asia Pacific, North America, Europe, Latin America and Rest of the World. North America is the leading region across the world. Whereas, owing to rising no. of research activities in countries such as China, India, and Japan, Asia Pacific region is also expected to exhibit higher growth rate the forecast period 2023-2028. Table of Content Chapter One: Industry Overview Chapter Two: Major Segmentation (Classification, Application and etc.) Analysis Chapter Three: Production Market Analysis Chapter Four: Sales Market Analysis Chapter Five: Consumption Market Analysis Chapter Six: Production, Sales and Consumption Market Comparison Analysis Chapter Seven: Major Manufacturers Production and Sales Market Comparison Analysis Chapter Eight: Competition Analysis by Players Chapter Nine: Marketing Channel Analysis Chapter Ten: New Project Investment Feasibility Analysis Chapter Eleven: Manufacturing Cost Analysis Chapter Twelve: Industrial Chain, Sourcing Strategy and Downstream Buyers Read Executive Summary and Detailed Index of full Research Study @ https://www.advancemarketanalytics.com/reports/165708-global-antiprotozoal-drugs-market Highlights of the Report • The future prospects of the global Antiprotozoal drugs market during the forecast period 2023-2028 are given in the report. • The major developmental strategies integrated by the leading players to sustain a competitive market position in the market are included in the report. • The emerging technologies that are driving the growth of the market are highlighted in the report. • The market value of the segments that are leading the market and the sub-segments are mentioned in the report. • The report studies the leading manufacturers and other players entering the global Antiprotozoal drugs market. Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Middle East, Africa, Europe or LATAM, Southeast Asia. Contact US : Craig Francis (PR & Marketing Manager) AMA Research & Media LLP Unit No. 429, Parsonage Road Edison, NJ New Jersey USA – 08837 Phone: +1 201 565 3262, +44 161 818 8166 [email protected]
#Global Antiprotozoal drugs Market#Antiprotozoal drugs Market Demand#Antiprotozoal drugs Market Trends#Antiprotozoal drugs Market Analysis#Antiprotozoal drugs Market Growth#Antiprotozoal drugs Market Share#Antiprotozoal drugs Market Forecast#Antiprotozoal drugs Market Challenges
0 notes
Text
Hydroxychloroquine Market Segmentation, Research Methodology And Revenue Growth Forecast Till 2030
Global Hydroxychloroquine Market Size research report 2023 offers in-depth assessment of revenue growth, market definition, segmentation, industry potential, influential trends for understanding the future outlook and current prospects for the market.
Get a Sample Copy of the Report at – https://www.fortunebusinessinsights.com/hydroxychloroquine-market-102706
Hydroxychloroquine is used as a first line treatment of diseases such as rheumatoid arthritis, malaria, and other autoimmune diseases such as lupus. The growth of hydroxychloroquine market is predominantly influenced by the increasing cases of COVID-19 cases world-wide. Major countries like U.S and countries in Europe have approved the use of this drug for the treatment of this pandemic disease until a designated vaccine is discovered. India is one of the largest manufacturers and exporters of hydroxychloroquine, and the companies have ramped up the production capacities of the drug to meet its growing demand. The increase in the cases of malaria in developing countries of Africa and Asia Pacific is estimated to drive this market in the coming years.
0 notes
Text
Quality Assurance in Antiviral Drug Supply: The Indian Perspective
The global demand for antiviral drugs has never been more evident than in the midst of a pandemic. As the world grappled with the challenges posed by viral outbreaks, the role of pharmaceutical companies in ensuring a consistent and reliable supply of antiviral medications became paramount. In this article, we explore the crucial aspect of quality assurance in antiviral drug supply from the Indian perspective. India's pharmaceutical industry has made significant strides in providing high-quality antiviral drugs to the world, and its commitment to ensuring the safety and efficacy of these medications is a cornerstone of its success.

The Significance of Quality Assurance in Antiviral Drug Supply
Quality assurance in antiviral drug supply encompasses a range of measures and practices that ensure the products meet the highest standards of quality, safety, and efficacy. This is critical not only for the well-being of patients but also for the reputation and competitiveness of pharmaceutical companies and the healthcare industry as a whole.
1. Patient Safety and Health
Quality assurance is fundamentally about protecting patient safety and health. Antiviral drugs, which are often used to treat serious and life-threatening conditions, must meet stringent quality standards to avoid harm or adverse effects.
2. Regulatory Compliance
Pharmaceutical products, including antiviral drugs, are subject to strict regulations and standards enforced by health authorities worldwide. Compliance with these regulations is essential to avoid legal repercussions and to ensure the trust of healthcare professionals and patients.
3. Global Reputation
The reputation of a country's pharmaceutical industry is closely linked to the quality of its products. Consistently high-quality antiviral drugs help build trust and credibility on the global stage.
India's Role in Global Antiviral Drug Supply
India is a key player in the global pharmaceutical industry, renowned for its expertise in generic drug manufacturing. Its ability to produce affordable, high-quality medicines has made it a primary source of antiviral drugs, serving both domestic and international markets. This prominence in the global pharmaceutical landscape underscores the importance of quality assurance in India's antiviral drug supply.
1. Export of Antiviral Drugs
India is a significant exporter of pharmaceutical products, including antiviral drugs, to countries across the world. During the COVID-19 pandemic, the Indian pharmaceutical industry played a crucial role in supplying medications like remdesivir and hydroxychloroquine to nations in need.
2. Cost-Effective Production
India's cost-effective drug production capabilities have made antiviral drugs more accessible to developing countries, effectively contributing to the global effort to combat viral diseases.
3. Expertise and Innovation
The Indian pharmaceutical industry is characterized by a strong emphasis on research and development, fostering innovation in the production of antiviral drugs. This has led to the development of novel drug formulations and treatment options.
Ensuring Quality in Antiviral Drug Supply
To ensure quality in the supply of antiviral drugs, Indian pharmaceutical companies adhere to a rigorous set of practices and standards. These practices encompass the entire drug manufacturing and distribution process.
1. Good Manufacturing Practices (GMP)
GMP guidelines, as stipulated by regulatory authorities, serve as the foundation for maintaining quality in pharmaceutical production. This includes stringent controls over raw materials, production processes, quality control, and packaging.
2. Quality Control and Testing
Pharmaceutical companies employ state-of-the-art quality control laboratories to rigorously test the raw materials, intermediates, and finished products. Quality control teams analyze the chemical composition, stability, and efficacy of antiviral drugs.
3. Documentation and Traceability
Comprehensive documentation of the manufacturing process, from the procurement of raw materials to the distribution of finished products, ensures traceability and accountability. This documentation is vital for regulatory compliance and quality assurance.
4. Training and Skill Development
The pharmaceutical workforce in India receives continuous training and skill development to remain up-to-date with the latest advancements in pharmaceutical manufacturing and quality assurance.
Challenges in Quality Assurance
While India has made remarkable strides in ensuring quality in its antiviral drug supply, there are challenges that must be addressed:
1. Regulatory Compliance
Meeting the diverse and evolving regulatory requirements of various countries can be challenging. Ensuring uniform compliance with global standards is an ongoing process.
2. Supply Chain Vulnerabilities
Disruptions in the supply chain, as witnessed during the COVID-19 pandemic, can impact the availability of critical raw materials and affect production schedules.
3. Counterfeit Medications
The pharmaceutical industry, including antiviral drugs, is vulnerable to counterfeit products. Stricter measures and technologies are needed to combat counterfeiting.
The Way Forward
As India continues to play a crucial role in the global supply of antiviral drugs, there are several initiatives and strategies that can further strengthen the commitment to quality assurance:
1. Collaboration with Regulatory Authorities
Collaboration between Indian pharmaceutical companies and regulatory authorities can streamline the compliance process and ensure a more robust framework for quality assurance.
2. Research and Development Investment
Increased investment in research and development can yield innovative solutions, such as new formulations and drug delivery methods, further enhancing the quality of antiviral drugs.
3. Supply Chain Resilience
Pharmaceutical companies can work to develop more resilient supply chains to better manage disruptions and ensure the consistent supply of critical raw materials.
4. International Cooperation
Engaging in international cooperation to establish common standards and practices can help streamline the process of exporting high-quality antiviral drugs to countries worldwide.
Conclusion
Quality assurance in antiviral drug supply is of paramount importance, not only for the patients who rely on these medications but also for the reputation and standing of the Indian pharmaceutical industry on the global stage. India's role in producing affordable and effective antiviral drugs is an example of the positive impact of the country's pharmaceutical expertise. By continually improving quality assurance practices and addressing challenges, India can maintain its pivotal position in global healthcare while ensuring the highest standards of patient safety and product quality. Credits : https://pharmaknowledgehubb.blogspot.com/2023/10/blog-post.html
#PharmaceuticalIndustry#AntiviralDrugs#QualityAssurance#PatientSafety#RegulatoryCompliance#GlobalPharmaceuticals#IndianPharmaceuticals#HealthcareQuality
0 notes
Text

An appreciation of the intellectual renaissance which occurred in India during the second half of the nineteenth century, flowing into the twentieth, is vital in order to understand the genesis of modern science in our country. It was during the Renaissance that modern science took root and flourished to a remarkable extent in a brilliant group of scientists. Acharya Prafulla Chandra Ray was one of them. The epitome of scientific attitude and human values was born on 2nd August 1861. The world was looking for ‘ Hydroxychloroquine’ for the treatment of Covid 19 and India was the leading manufacturer of hydroxychloroquine. The man behind hydroxychloroquine is Acharya Prafulla Chandra Ray, the ‘Father of Indian chemistry’. And ‘ Bengal Chemical’ is the leading manufacturer company of hydroxychloroquine, which is the first pharmaceutical company in India established by Acharya Prafulla Chandra Ray. He published around 150 research papers during his lifetime. His publication on “ Mercurous Nitrile” and his derivatives brought him recognition from all over the world. This Indian chemist, scientist, entrepreneur, and Swadeshi leader presented India in the world and made India proud.
For more information, you can visit our Instagram & Facebook, Or Contact us: +91 7908369443
#AcharyaPrafullaChandraRay#IndianScientist#Chemist#Educationist#Entrepreneur#Chemistry#Science#IndianHistory#ChemistryInIndia#ScienceInIndia#PrafullaChandraRay#IndianChemist#ChemicalIndustry#Innovation#ChemistryNobelPrize#thinkaginlab
0 notes
Text
Plandemic
In February 2022, a year after lockdowns were imposed on the public, a large group of Canadians decided they had had enough. They organized a peaceful protest, blocking roads in an effort to demand an end to lockdowns, the removal of vaccine mandates, and the restoration of personal freedoms. This convoy, made up mostly of truck drivers but also concerned citizens, called on the government to listen to their pleas. How did the government respond? By freezing the protesters' bank accounts. Millions of dollars belonging to hardworking truck drivers were frozen simply because they were peacefully protesting—a move that Prime Minister Trudeau evidently did not support.
This protest took place in the broader context of worldwide vaccine mandates, where governments across the globe insisted on mandatory COVID-19 vaccinations. After introducing the experimental COVID-19 vaccine, authorities rolled out aggressive campaigns, pushing the narrative that mass vaccination was the only way to bring the pandemic under control. However, many people had reservations due to the lack of long-term safety studies, rushed development timelines, and concerns about potential side effects. Governments and media outlets, instead of addressing these concerns openly, doubled down on mandates and restrictions, further polarizing society.
In countries like the United States, strict mandates were implemented, declaring that anyone who refused the vaccine would not be allowed to work, attend school, or even enter certain public spaces. This authoritarian approach led to widespread unrest, as many felt their personal freedom and bodily autonomy were being trampled upon. The justification for these drastic measures rested on the vaccine being safe and effective. However, as more data emerged, cracks in this narrative began to show.
Despite mass vaccination efforts, the number of COVID-19 cases continued to rise, and what followed was even more concerning: a growing number of vaccine-related injuries. Reports of adverse effects, ranging from mild reactions to more severe complications such as myocarditis, blood clots, and neurological disorders, began to surface. Many of these cases were dismissed as rare or coincidental by the authorities, but the sheer number of people coming forward told a different story. Social media platforms and alternative media outlets were flooded with testimonies from people who claimed they or their loved ones had been injured by the vaccine. Yet, mainstream media and government agencies were slow to acknowledge these incidents.
In many cases, vaccine mandates were enforced even as reports of injury increased. Governments continued to promote the vaccine as the only solution, and those who questioned or refused it faced severe consequences, including job loss, social ostracization, and restricted access to everyday services. This led to widespread distrust in public health institutions. Instead of taking responsibility, governments and pharmaceutical companies remained largely unaccountable, shielding themselves with legal protections that prevented vaccine manufacturers from being sued for any injuries caused by the vaccine.
It became increasingly clear that the blanket approach of mass vaccination was a failure. People were still getting sick, despite being vaccinated, and in some cases, those who had received the vaccine were more vulnerable to certain variants of the virus. Meanwhile, effective alternative treatments like Ivermectin, hydroxychloroquine, and vitamin D, which had shown promise in treating COVID-19, were dismissed or even censored. In fact, during the height of the pandemic, platforms like YouTube and Facebook actively removed content that discussed these alternatives, further limiting the public’s access to potentially life-saving information.
One notable example is India, where Ivermectin was used as a first-line treatment in many states to great success. Infection rates dropped significantly in areas where the drug was used, in stark contrast to the Western approach of relying solely on vaccines. This divergence in strategies highlighted the flaws in the global push for vaccination as the only solution.
The backlash against vaccine mandates and the growing awareness of vaccine injuries sparked protests around the world. From Canada to Australia, Europe to the United States, people began to push back against government overreach and demand accountability for the policies that had caused so much harm. The message was clear: governments had failed to protect their citizens, and instead of admitting their mistakes, they were doubling down on ineffective and harmful measures.
Ultimately, the rush to mandate the COVID-19 vaccine without proper safety studies or respect for individual choice resulted in a public health and political disaster. The long-term consequences of these actions are still being felt, as governments try to rebuild trust with citizens who feel betrayed. By prioritizing control over honest discourse, they exacerbated the crisis rather than solving it. As more information comes to light, the world continues to grapple with the fallout from one of the most controversial public health responses in modern history.
0 notes
Photo

Hydroxychloroquine no wonder drug for treating COVID-19, can be fatal: Experts Image Source : AP Hydroxychloroquine no wonder drug for treating COVID-19, can be fatal: Experts As countries around the world explore the potential of hydroxychloroquine to treat COVID-19 patients, several experts have sounded a note of warning to say it is not a wonder drug and may even be fatal in some cases.
#hydroxychloroquine#hydroxychloroquine coronavirus#hydroxychloroquine for covid 19#hydroxychloroquine in hindi#hydroxychloroquine in india#hydroxychloroquine manufacturers in india#hydroxychloroquine structure#hydroxychloroquine sulfate#hydroxychloroquine tablet uses#hydroxychloroquine tablets#hydroxychloroquine uses
0 notes
Text
World malaria day: मलेरिया की दवाओं का बड़ा उत्पादक देश कैसे बना भारत?
World malaria day: मलेरिया की दवाओं का बड़ा उत्पादक देश कैसे बना भारत?

[ad_1]
मलेरिया दिवस 2020: भारत मलेरिया की दवाओं का बड़ा उत्पादक देश है – फोटो : Pexels
ख़बर सुनें
ख़बर सुनें
आज विश्व मलेरिया दिवस है। इस साल इसकी थीम Zero malaria starts with me यानी कि ‘जीरो मलेरिया की शुरुआत मुझसे’ है। मच्छर से फैलने वाली यह बीमारी भारत में लोगों की मौत का कारण बनती है। विश्व स्वास्थ्य संगठन की रिपोर्ट के मुताबिक, भारत में हर साल मलेरिया के कारण दो लाख से ज्यादा…
View On WordPress
#Chloroquine#Health & Fitness Hindi News#Health & Fitness News in Hindi#Hydroxy Chloroquine#Hydroxychloroquine#hydroxychloroquine company#hydroxychloroquine covid#hydroxychloroquine for coronavirus#hydroxychloroquine formula#hydroxychloroquine india#hydroxychloroquine manufacturer in india#hydroxychloroquine manufacturers in india#hydroxychloroquine price in india#hydroxychloroquine tablets uses#icmr hydroxychloroquine#Lifestyle news in hindi#malaria caused by#malaria day#malaria symptoms#medicine#Paracetamol#poster on world malaria day#trump hydroxychloroquine#World malaria day#world malaria day 2019 theme#world malaria day 2020#world malaria day 2020 theme#world malaria day images#world malaria day poster#world malaria day quotes
0 notes
Photo

Hydroxychloroquine dangerous for heart ailments: FDA wans against side effects of malaria drug Image Source : PTI FDA warns against side effects of hydroxychloroquine The US Food and Drug Administration has issued a safety communication regarding the known side effects of malaria drug hydroxychloroquine and chloroquine touted by President Donald Trump for treating coronavirus.
#ailments#dangerous#drug#effects#FDA#Heart#hydroxychloroquine#hydroxychloroquine coronavirus#hydroxychloroquine for covid 19#hydroxychloroquine in hindi#hydroxychloroquine india#hydroxychloroquine manufacturer#hydroxychloroquine side effects#hydroxychloroquine structure#hydroxychloroquine sulphate#hydroxychloroquine tablet#hydroxychloroquine tablet uses#hydroxychloroquine uses#Malaria#Side#wans
0 notes
Text
The politics of a pandemic, how not to manage coronavirus
No man is an island, Entire of itself, Every man is a piece of the continent, A part of the main. If a clod be washed away by the sea, Europe is the less. As well as if a promontory were. As well as if a manor of thy friend's Or of thine own were: Any man's death diminishes me,
Because I am involved in mankind, And therefore never send to know for whom the bell tolls.
It tolls for thee.
John Donne
1624
The poet John Donne warned of the dangers of isolation and imagining oneself as self-sufficient, without need of community. It was true 500 years ago; it still holds true today. No man is an island…every man is a part of the main. As wave upon wave of SARS-CoV-2 reached every continent, even Antarctica, most of us have tried to isolate ourselves on this crowded planet - with mixed results.
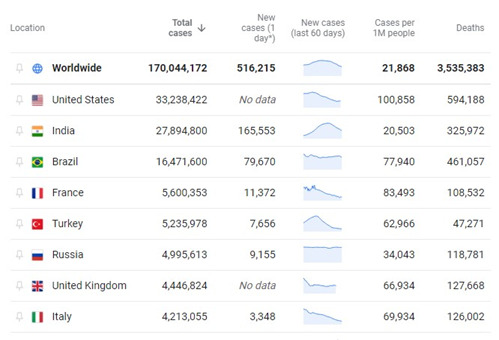
As of May 30, 2021, by every metric, the United States was leading the world in the number of cases and deaths from COVD-19. Brazil and India are catching up quickly. In the US, the underlying tension between public health and personal liberty has had disastrous consequences. As successful as the vaccine roll-out has been, and even with the numbers of new cases, hospitalizations, and deaths dropping, this is no time to be complacent.
India, with a population of over 1 billion, and Brazil, a pariah among countries in Latin America for its poor response to the pandemic, cause or should cause great concern to everyone everywhere. Not having the resources of rich countries, they will require help to manage the tragic situation their leaders have put their populations in and it is in our interest to do so because...the bell tolls for thee.
India
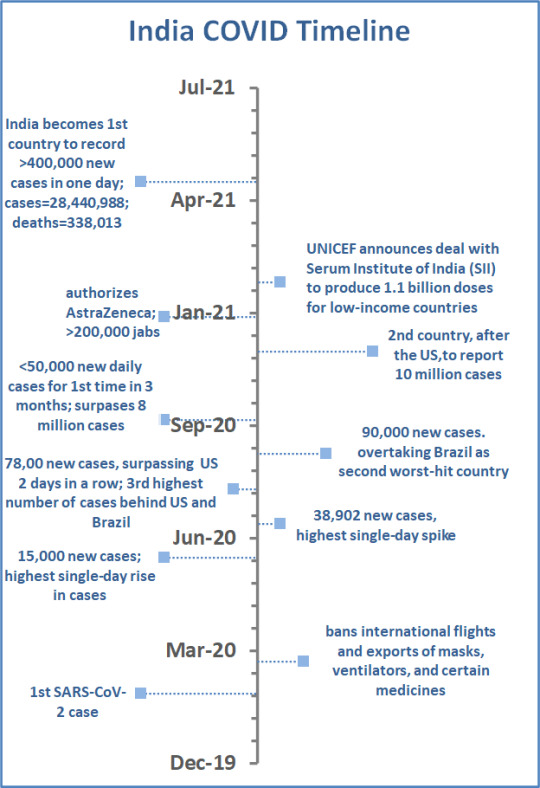
When the World Health Organization (WHO) declared COVID-19 a global pandemic in March 2020, there had been 330,000 cases and 30,000 deaths from SARS-CoV-2 reported worldwide. In the early days of the pandemic, India was considered a model of how to manage the worst public health crisis in recent memory. India responded with a strict lockdown. International flights and exports of masks, ventilators, and certain medicines were banned. As a result, India did not see the same initial explosion in new cases and deaths compared to other countries.
Three months later, India’s Prime Minister Narendra Modi began easing lockdown restrictions - like the American football player who does the end-zone dance on the two-yard line—not a good idea. When the lockdown lifted, many Indians stopped taking precautions. Mr. Modi allowed large gatherings, including campaigns in state elections that he attended, without wearing a mask, at rallies of thousands of mask-less supporters, to help his governing Bharatiya Janata party. Large religious festivals resumed drawing millions of people as well. By July 2020, India had seen 600,000 cases and 17,834 deaths due to COVID. An editorial from The Lancet, said that Mr. Modi “seemed more intent on removing criticism” on social media than “trying to control the pandemic.” Sound familiar?
As recently as March 2021, India’s health minister assured the public that they had reached the pandemic’s “endgame”.
The New York Times reported in May 2021 that India was responsible for more than half of the world’s daily COVID cases, setting a record-breaking pace of 400,000 new cases in one day. Researchers believe the B.1.1.7 variant and the delta variant, which are also major variants in Britain and the US, are to blame for the surge. Clinics across India report desperate shortages of hospital beds, protective equipment, and oxygen.[1]
Just to add to the global disaster, India is one of the world’s leading vaccine manufacturers. It is struggling to inoculate its own citizens; less than 10% of Indians have gotten even one dose.[2] In September 2020, Serum Institute of India (SII) received $150 million from the Bill and Melinda Gates foundation to accelerate production of Oxford University’s AstraZenica (AZ) vaccine and the American vaccine Novavax as soon as the WHO granted regulatory approval. Under the original terms of the agreement, 50% of vaccines would be earmarked for India and the remainder would go to other low- and middle-income countries.[3]Currently, exports of vaccines from India have been shut down.
Brazil
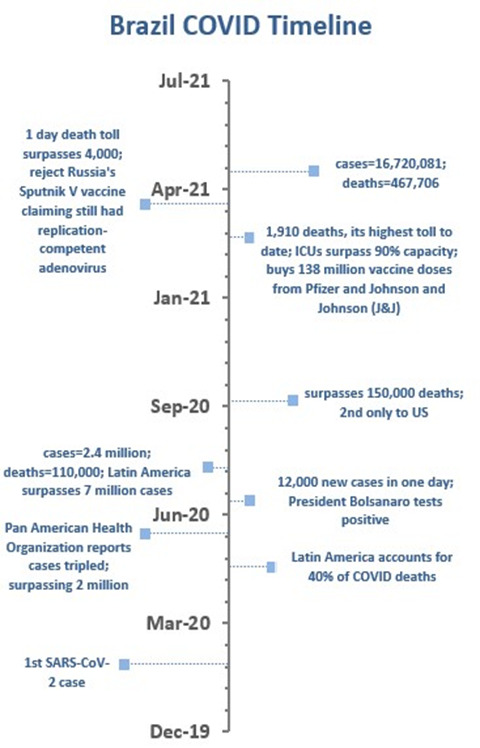
In an editorial from The Lancet, dated May 9, 2020, the president of Brazil, Jair Bolsanoro, was criticized for allowing the SARS-CoV-2 virus to spread widely while presenting himself as a “messiah” touting unproven medicines like hydroxychloroquine, with support from his rightwing allies.
At the time, Brazil had the most cases (105,000) and deaths (72,88) in Latin America. Estimates suggest the death rate was doubling every five days. When asked by a reporter about the rapidly increasing numbers of COVID-19 cases, Mr. Bolsanaro responded: “So what? What do you want me to do?”[4]
In March 2021, Brazil’s pandemic spiraled out of control. Its Latin American neighbors grounded flights, closed land borders, and regional sports events were canceled in attempts to stop the P.1 variant (and approximately 90 other variants) from spreading to their populations.
The British Medical Journal reported that 400,000 Brazilians have died from COVID-19—13% of deaths worldwide.[5] Some models predict the death toll in Brazil will reach half a million this month. That trajectory could be an indicator for what is to come for its neighbors. As Paraguay’s director of health surveillance, Guillermo Sequera, has said: “When Brazil sneezes, Paraguay gets a cold.”[6]
COVAX
With a fast-moving pandemic, no one is safe, unless everyone is safe.
author unknown. Retrieved from: https://www.who.int/initiatives/act-accelerator/covax
COVAX is an initiative dedicated to equitable access to a vaccine, particularly to healthcare workers and those most at risk. To date (5/31/2021), COVAX has shipped more than 77 million COVID-19 vaccines to 127 participants. It is co-led by[7]:
CEPI-Coalition for Epidemic Preparedness Innovations. The governing board has 12 voting members; four investors and eight independent members with competencies in industry, global health, science, resource mobilization, and finance—and five observers (17 total). Financial support comes from public sources including US Agency for International Development (USAID).
Gavi, the Vaccine Alliance-a public/private partnership which has helped to vaccinate 760 million children in the world’s poorest countries.[8] It ensures that infrastructure is in place and technical support is available to make sure that COVID-19 vaccines can be safely delivered to support the participation of 92 lower-middle and lower-income economies. It is part of the health systems work of Access to COVID-19 Tools (ACT) Accelerator effort, focusing on areas where it has expertise and experience, such as keeping vaccines at the correct temperature.
World Health Organization (WHO)
United Nations International Children’s Emergency Fund (UNICEF)
COVAX hopes to get 280 million doses of vaccines to Latin America but has been hit with delays to eight manufacturers (including SII) it has deals with and does not expect to deliver them until the end of 2021.[8]This has led South American nations to look to China’s Coronavac and Russia’s Sputnik V vaccine supplies. One study found that Coronovac was only 50% effective after a single dose. The Biden administration has pledged to purchase 500 million doses of Pfizer BioNTech vaccine to give to COVAX; the first 200 million doses will be distributed this year, with the subsequent 300 million in the first half of next year.[10]
My Take
In what can only be called being one step ahead of the game, armed robbers in Hong Kong stole $16,000 worth of toilet paper as coronavirus sparked panic-buying of essential goods a month before WHO declared a global pandemic in March 2020.[11] (Good times)
In July 2020, President Trump formally notified Congress and the United Nations that the US was withdrawing from WHO because of course he did.
Several articles, including one from the Journal of the American Medical Association (JAMA)[12] have compared weekly deaths in the US that would be expected from historical trends with COVID and non-COVID deaths from March 2020 until January 2021. There was an increase of 22.9% of all-cause mortality. This far exceeds expectations. Excess deaths attributed to non-COVID causes could be the result of deaths that were, in fact, COVID but misclassified. They might also be due to delayed care, an overwhelmed healthcare system, or behavioral health crises. On the other side of the ledger, no doubt at least some of the deaths that would have been anticipated from non-COVID causes might have died from the coronavirus instead. Which is to say, these are at best estimates of the mortality rates. During surges in various parts of the US, deaths from several non-COVID diseases like heart disease and Alzheimer’s increased. Either way, the excess deaths could have been helped with a better response to the pandemic early on.[13]
For those “give me liberty, or give me death” fans, do I really need to point out that Patrick Henry was referring to his own death, not the deaths of millions all over the world? My parents’ generation made many sacrifices during WWII, including blood and treasure, and considered it worth the price to defeat Hitler. Wearing a mask to defeat a virus? Really? Who have we become?
It comes as a surprise to no one that the countries with the largest death tolls to date, the US, India, and Brazil, are also countries in which partisan politics was the priority over public health measures. It isn’t a good idea. Why don’t we just stop?
[1] What to know about India’s coronavirus crisis. What is behind the explosion of new coronavirus cases that is overwhelming the South Asian country? NY Times, May 25, 2021. Retrieved from: https://www.nytimes.com/article/india-coronavirus-cases-deaths.html
[2] ibid
[3]Raghavan, P. 2020. $150 million dollar shot for serum production of COVID vaccine, India Express.
[4]Lancet editorial. September 19, 2020. COVID-19 in Brazil: “So what?”, Lancet, 395: 1461. doi: 10.1016/s0140-6736(20)31095-3
[5]Taylor, L. 5/20/2021. COVID-19: How the Brazilian variant took hold of South America, BMJ 2021, 373: n1277. doi: 10.1136/bmj.n1277
[6]ibid
[7]World Health Organization: COVAX Working for global equitable access to COVID-19 vaccines. Retrieved from: https://www.who.int/initiatives/act-accelerator/covax
[8]Raghavan. Op cit.
[9]Taylor. Op cit.
[10] Page, T, Rauhala, E. Jun 9, 2021. Biden administration to buy 500 million Pfizer coronavirus vaccine doses to donate to the world, Washington Post, retrieved from: https://www.washingtonpost.com/politics/biden-vaccine-donate/2021/06/09/c2744674-c934-11eb-93fa-9053a95eb9f2_story.html
[11]www.thinkglobalhealth.org/article1
[12]Woolf, SH, Chapman, DH, Sabo, RT, Zimmerman, EB. May 4,2021.Excess deaths from COVID-19 and other causes in the US, March 1 2020, to January 2, 2021,JAMA, 325(17): 1786-1789. doi: 10.1001/jama.2021.5199
[13]ibid
2 notes
·
View notes
Text
Coronavirus: Brazil President Bolsonaro invokes Indian epic Ramayana in Covid-19 letter to Modi, requesting hydroxychloroquine export
India is the largest manufacturer of HCQ, an anti-malarial drug that is also considered on

In a letter to the Indian Prime Minister Narendra Modi, seeking supply of the anti-malarial drug hydroxychloroquine, Brazilian President Jair Bolsonaro cited a story from the ancient Indian epic Ramayana. The letter mentions how the Hindu deity Hanuman brought holy medicine from the Himalayas to save the life of Hindu deity Rama's brother, according to online news reports shared on April 8. On April 9, he also thanked PM Modi after the Indian premier obliged to Brazil's request.
The Brazilian President wrote: "Just as ... Hanuman brought the holy medicine from the Himalayas to save the life of ... Rama's brother Lakshmana, and Jesus (PBUH) healed those who were sick and restored the sight to Bartimeu, India and Brazil will overcome this global crisis by joining forces and sharing blessings for the sake of all peoples."
India is the largest manufacturer of HCQ, an anti-malarial drug that is also considered one of the possible cures for Covid-19. The coronavirus pandemic has infected more than 1.4 million people and resulted in the death of over 83,000 across the world.
India had earlier banned the export of HCQ, and only allowed a partial lift on the ban after US President Donald Trump requested an urgent supply. Trump had said that he didn’t like Modi’s decision to ban the export of the drug. India is now supplying the drug to the United and several other countries hit by the pandemic.
Brazil's envoy to India Ambassador André Aranha Correa do Lago said, "President Bolsonaro is a religious man and he saw how religious was Prime Minister Modi also. So he thought it was interesting to show in his letter how two religious men with very strong traditions could find examples in their religions that were most appropriate for the case."
Continue reading.
#brazil#india#coronavirus#covid 19#politics#religion#brazilian politics#indian politics#jair bolsonaro#narendra modi#international politics#foreign policy#mod nise da silveira
4 notes
·
View notes
Text
Indian Economy after Covid: a positive outlook
In comparison with different nations, India appears to have a higher possibility of pulling off with lesser inadvertent blow-back, atleast for the time being.
A few variables may play in or out, over the span of this critical situation and these might end up being good for India to turn into a significant exchange and trade player in the global market.
Since the US, Japan and several other countries have decided to shift their manufacturing units from China, India seems to be the next best alternative.
In 2019, India had reduced it's corporate tax by 25% and for new manufacturing firms it was down to 15%.
Currently more than 300 international companies are interested in setting up their manufacturing units in India.
Also, since India is the only country supplying Hydroxychloroquine, the antimalarial drug to selected foreign countries, the pharmaceutical industry in India can also expect rise in investments in the future.

April 28th,2020
#after lockdown#economy recovery#economics#economy#positive outlook#recovery#startup india#india#indian#make in india
3 notes
·
View notes
Text
India Bans Export Of Potential Drug for COVID-19
President Trump has repeatedly called for America not to be dependent on foreign supplies and medicines. A huge case in point surfaced this week as India locked down its 1.3 billion citizens over the coronavirus. But it was their other action that sent ripples through the medical community: they banned the exportof hydroxychloroquine and all its formulations. India is the largest manufacturer of…
View On WordPress
#anti-malarial drugs#ban exports of drugs#Cadila Healthcare LTD#Coronavirus#COVID-19#generic drugs#hydroxychloroquine#India#malaria#quarantine#Uncle Sam&039;s Misguided Children
4 notes
·
View notes
Text
FabiFlu
Over nine million people have tested positive for COVID-19 so far, out of which, 4,25,000 are Indians. With the advent of such a disastrous pandemic, the need for it’s treatment becomes all the more important.
Glenmark Pharmaceuticals is a research-led pharmaceutical company in India which has received approval for officially manufacturing and marketing Favipiravir, an antiviral drug to be used for treatment of mild to moderate COVID-19 patients. After this approval, Favipiravir has become the first approved oral medication for the treatment of COVID-19 in India. This drug shall be available by the name Fabiflu and is priced at Rs, 103 per tablet. Glenmark has also become the first Indian pharma company to have received the approval for conducting phase 3 clinical trials on COVID-19 patients with mild to moderate symptoms.
Considering a minimum of two strips per patient, Glenmark said it will be able to provide Fabiflu to about 82,500 patients in the first month itself. They will then scale up and meet the healthcare needs of the country.
It is an oral product which means it does not require hospitalization. This will reduce the burden on the hospitals and it’s staff to some extent. The medicine is said to result in the rapid reduction in viral load within four day and provide faster symptomatic and radiological improvement.
Following are certain drugs which are being used to treat COVID-19 in different countries-
1. Favipiravir: the Japanese Flu drug
Favipiravir is an antiviral drug which was developed by a Japanese pharma company, Fujifilm Toyama Chemical, to treat influenza and thus, it is also known as the Japanese Flu drug. Favipiravir was approved as an experimental treatment for COVID-19 infections in China in February 2020. According to scientists, the drug shows antiviral activity against all the subtypes of influenza virus strains including influenza A, B, and even avian influenza.
2. Remdesivir: the Ebola drug
Remdesivir was first developed by Gilead Sciences, a US-based pharma company, during the outbreak of ebola. Since the drug did not work well on ebola patients, it was tested against coronavirus strains, SARS and MERS, in the year 2017. The research showed that Remdesivir had the ability to stop the enzyme RNA polymerase which was necessary for virus multiplication. This ensured that the virus could not spread in the body. Since then, it has been tested in various countries for its effectiveness against COVID-19 infection.
3. Hydroxychloroquine: the Anti-malarial drug
Hydroxychloroquine (HCQ) was first developed in India for the treatment of malaria, however, the drug is also a disease-modifying anti-rheumatic drug (DMARD) which helps in reducing swelling and pain in arthritis patients. Some laboratory and in-vivo studies have suggested that HCQ has the ability to prevent COVID-19 infection. Since then, many nations including India, have been using hydroxychloroquine as a prophylactic drug.
4. Tocilizumab: the Rheumatoid arthritis drug
Tocilizumab is an anti-rheumatoid arthritis drug which is being tested against SARS-CoV-2 virus by the National Cancer Institute in Naples and the trial is called TOCIVID-19. Tocilizumab is actually a humanized IgG1 monoclonal antibody which has the ability to mimic the natural antibodies produced by the immune system of the body in response to the bad microorganisms that enter the body. The trial is ongoing and the drug is being used to treat pneumonia caused by the COVID-19 infection.
5. Ivermectin: the Anti-parasitic drug
Ivermectin is a drug which is used for the treatment of head lice and intestinal worms. On testing the drug against the novel coronavirus, scientists found that ivermectin could restrict the growth of SARS-CoV-2 virus present in a cell within 48 hours and kills it eventually. Ivermectin has also been effective against other viruses such as dengue, influenza and even HIV. The medication is still under trial.
REFERENCES:
https://www.indiatvnews.com/health/glenmark-fabiflu-covid-medicine-to-treat-mild-to-moderate-coronavirus-cases-price-availability-in-stores-627881
https://www.firstpost.com/health/covid-19-treatment-fabiflu-by-glenmark-hcq-remdesivir-and-other-drugs-that-are-being-used-to-treat-coronavirus-patients-8515091.html
1 note
·
View note
Link
What kind of #Friend #threatens #another "Friend" ? Answer: #America and #DonaldTrump. If #USA/ #america didn't build factories to produce Hydroxychloroquine, Because their greedy, #bloodsucking profiteering #bankers outsourced everything because it'll be cheap to make and profitable to sell... that's their fault. and now they are paying the price. What #India have to do anything with it? They would have easily taken license and start producing in their homeland easy!! Hel most americans are even concerned about side effects of #Hydroxychloroquine and denying it... plus America have enough stockpile for their entire population, Then Why They Need More? India is just securing it's stock, which is ofcourse not enough for protection of it's population. The Indian #Government is so called "elected" to protect #Indian lives and Not American Lives. We are going through a crisis too, not like we're in heaven while whole world is burning! Most of the Indians are very angry after current Government of India of #Modi "partially" lifted the ban after trump's threat of "maybe retaliation" And trump, what is "maybe retaliation" ? Drop nukes on us or starve us to death? that's your grand plan? #Doctors in India are terrified after this decision, Factories are closed due to lockdown.. How will India even meet the requirements of it's own population? Many slammed Trump and america saying, " there's no place of threat and hostility in friendship"... Many even advocated to blacklist #UnitedStatesOfAmerica of Hydroxychloroquine Exportation from #India and are ready to get nuked or face their "retaliation" after such shameful threats by trump and shameful actions taken by GOI, when there's not enough supplies for people of the country. Hydroxychloroquine maker and one of the oldest manufacturers of pharmaceutical supplies, "Bengal Chemicals" doesn't have enough supplies to make it and their existing stockpiles are almost finished. So, thanks to you trump for showing how a true friendship looks like , and thank you modi for spinelessly bowing down to america. i hope that's what so called #nationalists do, shown their true colors. Trump, next time instead of threatening #India because of your own mistakes and taking away funds from World Health Organization, try stop wasting money on those worthless #DefenseContractors , on worthless #MilitaryIndustrialComplex , and those worthless #Falseflag wars on overseas, you're supposedly "fighting". Then you'll have enough money to fix your financial situation of your country and eventually will be able to build factories of emergency supplies that in future you won't have to threaten another to get your "emergency/healthcare supplies". 🤬😉🤔 https://www.indiatimes.com/news/india/after-donald-trump-threatens-retaliation-india-lifts-ban-on-export-of-anti-malarial-drug-hydroxychloroquine-510240.html
2 notes
·
View notes
Text
Antibiotics Manufacturing Companies in India
Oxi Pharma is the best manufacturer of Antibiotics Manufacturing Companies in India. Oxi Pharma has been a well-known company for its production of high-quality Antioxidant Manufacturers in India, which are prepared with all natural ingredients. These medicines have been used to treat infections caused by bacteria. Well, the Azithromycin tablets we produce can be used solo or if it is combined with other medicine is included amongst the medicine to treat coronavirus. In a study it is proved that Azithromycin with Hydroxychloroquine is used to treat patients with coronavirus or on the patients who have symptoms of coronavirus.
For More infornation:-https://www.oxipharma.in/antibiotic-medicine-manufacturer-india
Contact Us:+91-9548355063
mail:[email protected]
Location:-Near DBS College,Selaqui Dehradun
0 notes