#eclinical solutions
Explore tagged Tumblr posts
Text
0 notes
Text
The global market for eClinical solutions is estimated at USD 9.85 Billion in 2023 and is projected to reach USD 37.16 Billion by 2033, growing at a CAGR of 14.2% from 2024 to 2033. This comprehensive report provides an in-depth analysis of market trends, drivers, and forecasts, helping you make informed business decisions.
0 notes
Text
Transforming Healthcare with eClinicalWorks EHR Integration Solutions
In the ever-evolving field of healthcare, seamless integration of electronic health records (EHR) is essential for enhancing patient care and operational efficiency. eClinicalWorks, a prominent provider of cloud-based healthcare solutions, offers advanced EHR integration solutions designed to unify disparate systems and streamline healthcare delivery. These solutions are pivotal in improving care coordination, data accuracy, and overall healthcare management.
eClinicalWorks integration solutions provide a comprehensive framework for connecting various healthcare technologies, including EHR systems, practice management software, and other clinical tools. This integration addresses a common challenge in healthcare: the fragmentation of patient information across multiple systems. By consolidating data into a unified platform, eClinicalWorks enables healthcare providers to access a single, comprehensive view of patient information, thereby eliminating data silos and improving decision-making.
One of the key benefits of eClinicalWorks EHR integration is the enhancement of care coordination. The platform allows for real-time access to critical patient data, such as medical histories, treatment plans, and diagnostic results. This centralized access facilitates better communication and collaboration among healthcare providers, which is essential for managing complex cases and ensuring continuity of care. For example, when a patient transitions between different care settings or specialists, eClinicalWorks EHR ensures that all relevant information is available to the new care team, supporting a more coordinated approach to treatment.
Interoperability is another significant advantage of eClinicalWorks EHR integration solutions. The platform is designed to integrate seamlessly with a wide range of external systems, including laboratory information systems, radiology information systems, and pharmacy management systems. This broad interoperability enables efficient data exchange and reduces the risk of errors associated with manual data entry. For instance, integration with laboratory systems allows for the automatic updating of patient records with test results, ensuring that healthcare providers have the most current information for clinical decision-making.
Additionally, eClinicalWorks EHR integration extends to administrative functions, such as scheduling and billing. By connecting EHR systems with practice management software, the platform streamlines scheduling, billing, and insurance claims processing. This integration reduces administrative burdens and enhances operational efficiency, allowing healthcare providers to focus more on patient care.
In summary, eClinicalWorks EHR integration solutions play a crucial role in transforming healthcare delivery. By providing a unified platform that enhances care coordination, supports interoperability, and optimizes administrative processes, eClinicalWorks helps healthcare organizations improve patient outcomes and operational efficiency. As the healthcare industry continues to advance, eClinicalWorks remains a key player in driving technological integration and innovation.
#eclinicalworks integration#eclinical works ehr#emr eclinicalworks#eclinicalworks ehr#eclinicalworks ehr system#ehr eclinicalworks#eclinicalworks billing#eclinicalworks emr#eclinicalworks ehr integration#eclinicalworks emr integration#eclinicalworks integration solutions#eclinicalworks integration software#custom eclinicalworks integration solutions#ECLINICALWORKS HEALTHCARE SOFTWARE INTEGRATION SERVICES#ECLINICAL WORKS INTEGRATION SOLUTIONS
0 notes
Text
eClinical Solutions Market Size To Reach $22.7 Billion By 2030
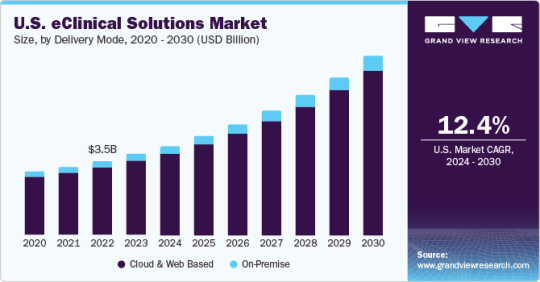
The global eClinical solutions market size is expected to reach USD 22.7 billion by 2030, growing at a CAGR of 14.1% from 2024 to 2030, according to a new report by Grand View Research, Inc. Increasing R&D activities by biopharma and pharma companies, application of software solutions in clinical trials, and expanding customer base are anticipated to fuel market growth. During COVID-19, clinical laboratories experienced high demand for COVID-19 tests. Clinical data management systems assisted these laboratories to seamlessly manage the huge influx of specimens daily. Technological advancements such as electronic data capture and Wi-Fi connectivity are projected to drive the market in the forthcoming years.
As the demand for tracking and analyzing clinical data increases, the need for effective clinical solutions rises. Unmet needs to manage efficient clinical development processes are poised to boost market growth over the forecast period. Moreover, digital transformation in the field of clinical trials and preference for data-centric approaches are providing a tremendous push to the market. Demand for integrated clinical IT solutions is increasing due to the massive volume of data generated during clinical development processes. eClinical solutions offer a single source of information that helps optimize the cost by eliminating redundant data entry and by reducing on-site verification and source data verification. Rising awareness regarding these advantages is projected to propel the market.
Increasing adoption of eClinical workflows in trials offers enormous potential in clinical development processes. These solutions can facilitate decision-making in each stage of development. It helps reduce cost and time between the development phase by utilizing seamless designs and identifying failing compounds. It offers rapid access to data and patient safety information, which helps make quick decisions. Market players engage in new product development and strategic alliances including partnership, agreement, promotional activities, etc. to keep market rivalry high. In August 2023, Sitero acquired Clarios eClinical technology suite to enhance clinical trial delivery. This acquisition demonstrates Sitero's dedication to remain at the forefront of innovation and offering its clients the best possible support and services.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/eclinical-solutions-market
eClinical Solutions Market Report Highlights
Based on product, the CTMS segment led the market in 2023 and accounted for a revenue share of around 20.2% owing to benefits such as centralized end-to-end management of clinical trial activities, elimination of reliance on manual processes, real-time status tracking, and maintenance of multiple databases, which cumulatively improve the overall efficiency of clinical trials
Web and cloud-based systems are anticipated to exhibit an exponential CAGR during the forecast period owing to integrated features such as flexibility, high accessibility, negligible handling costs, and easy data backup. Real-time data is available through these systems, which enables users to take quick decisions and provide high-quality information for risk-based monitoring
Based on development phase, the phase III segment held the largest market share of 53.3% in 2023. Phase I segment is expected to grow at the fastest CAGR over the forecast period
Based on end-use, the CRO segment held the largest revenue share in 2023. The segment is projected to rise at a remarkable CAGR during the forecast period owing to the growing inclination of pharmaceutical companies to reduce overall expenditure.
eClinical Solutions Market Segmentation
Grand View Research has segmented the global eClinical solutions market based on product, delivery mode, development phase, end-use, and region:
eClinical Solutions Product Outlook (Revenue, USD Million, 2018 - 2030)
Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
Clinical Trial Management Systems (CTMS)
Clinical Analytics Platforms
Randomization and Trial Supply Management (RTSM)
Clinical Data Integration Platforms
Electronic Clinical Outcome Assessment (eCOA)
Safety Solutions
Electronic Trial Master File (eTMF)
Electronic Consent (eConsent)
eClinical Solutions Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
Web and Cloud based
On-premise
eClinical Solutions Development Phase Outlook (Revenue, USD Million, 2018 - 2030)
Phase I
Phase II
Phase III
Phase IV
eClinical Solutions End-use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals/Healthcare providers
CROs
Academic Institutes
Pharma & Biotech Organizations
Medical Device Manufacturers
eClinical Solutions Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
S.
Canada
Mexico
Europe
K.
Germany
France
Italy
Spain
Netherlands
Sweden
Denmark
Rest of Europe (EU) {RoE}
Asia Pacific
Japan
China
India
South Korea
Australia
New Zealand
Taiwan
Hong Kong
Singapore
Thailand
Vietnam
Rest of Asia Pacific (RoAPAC)
Central & South America
Brazil
Argentina
Chile
Rest of Latin America (RoLA)
Middle East & Africa
South Africa
Saudi Arabia
UAE
Egypt
Qatar
Rest of Middle East & Africa (RoMEA)
List of Key Players in the eClinical Solutions Market
Fountayn, formerly known as Datatrak International, Inc.
Oracle
Calyx, formerly part of Parexel International Corporation
Medidata (Dassault Systemes)
CRF Health (Signant Health)
Clario (ERT and Bioclinica)
eClinicalWorks
Merative (IBM Watson Health)
Anju Software
eClinical Solutions
MaxisIT
IQVIA
Castor
Veeva Systems
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/eclinical-solutions-market
#eClinical Solutions Market#eClinical Solutions Market Trends#eClinical Solutions Market Size#eClinical Solutions Market Share
0 notes
Text
eClinical Solutions Market Report, Company Analysis, Business Challenges and Opportunitiese
IMARC Group has recently released a new research study titled “eClinical Solutions Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios. How big is the eClinical solutions market? The…

View On WordPress
0 notes
Text
eClinical Solutions and Software Market is expected to reach a valuation of US$ 29.1 Billion by 2033 | FMI
The global eClinical Solutions and Software Market is anticipated to reach a valuation of US$ 29.1 billion in 2033, rising up from an estimated US$ 9.3 billion in 2023. Valued at US$ 8.4 billion in 2022, the market witnessed a CAGR of 10.6% from 2017 to 2022. It is gauged that the eClinical solutions and software market will expand at a stellar CAGR of 12.1% from 2023 to 2033. This growth can be attributed to the surge in funding for clinical research and life sciences will enable the widespread usage of eClinical solutions and software which, in turn, will promote the overall market growth during the forecast period.
Gain complete access to the report @ https://www.futuremarketinsights.com/reports/eclinical-solutions-and-software-market
The expansion of research and development activities by biopharmaceutical and pharmaceutical businesses will prompt the use of eClinical solutions and software in various clinical trials which will foster growth for the market. This is primarily due to the fact that in order to address certain contemporary eClinical trial management needs, many businesses will require a comprehensive eClinical platform that will adapt to required needs. Hence, the prospects for the eClinical solutions and software market look bright in the upcoming years.
With the surge in the amount of data produced via the clinical development process, the need for efficient tracking and evaluation of clinical data will also rise. As a result of this, the demand for eClinical solutions and software amplifies in clinical trials which, in turn, supplements the overall market growth. The eClinical solutions and software increase efficiency, lessen the expenditure, and errors like duplicate entry are avoided due to the use of eClinical technologies. This factor bodes well for the expansion of the eClinical solutions and software market size. Other factors that will aid the market growth are the progress witnessed in the life sciences field and the outsourcing of clinical trials to contract research organizations (CROs). All of these factors propel the Clinical Data Management Tools market forward during the projection period.
Key Takeaways:
High costs and constant maintenance expenditure will prevent the growth of the eClinical solutions and software market during the assessment period.
Based on the service, the clinical data management systems (CDMS) category will dominate the global market with an estimated share of 22.6% for 2023 and 2033.
By delivery mode, the web-hosted (on-demand) will lead the market with a share of 67.3%.
The eClinical solutions and software market in the US will hold about 39.3% of the market share in 2023 due to increasing product launches by key players.
China’s eClinical solutions and software market will expand at a strong CAGR of 8.7% owing to greater medical needs.
Competitive Landscape
Oracle
Datatrak International, Inc.
Dassault Systemes
CRF Health
eClinicalWorks
Parexel International Corporation
Bioclinica
eClinical Solutions
IBM Watson Health
Anju Life Sciences Software
ERT Clinical
Key Market Segments Covered in eClinical Solutions and Software Industry Research
Solution:
Randomization & Trial Management (RTSM)
Clinical Data Management System (CDSM)
Clinical Trial Management System (CLMS)
Electronic Clinical Outcome Assessment (eCOA)
Electronic Trial Master File (eTMF)
Electronic Data Capture
Others
Delivery Mode:
Licensed Enterprise (On-premise) Solution
Cloud-based (SAAS) Solution
Web-hosted (on-demand) Solution
Clinical Trial:
Phase I
Phase II
Phase III
Phase IV
End User:
Contract Research Organization
Medical Device Companies
Pharma/Biotech Companies
Hospitals & Clinics
Others
0 notes
Text
0 notes
Text
Eclinical Solutions Rising Demand Attributed To Increasing Tracking And Analyzing Clinical Data
The global eClinical solutions market size is expected to reach USD 21.8 billion by 2030, according to a new report by Grand View Research, Inc. The market is expected to expand at a CAGR of 13.9% from 2023 to 2030. Increasing R&D activities by biopharma and pharma companies, application of software solutions in clinical trials, and expanding customer base are anticipated to fuel market growth. During the COVID-19 pandemic, clinical laboratories experienced high demand for COVID-19 tests. eClinical solutions, such as the clinical data management systems assisted these laboratories to seamlessly manage the huge influx of specimens daily. Technological advancements such as electronic data capture and Wi-Fi connectivity are projected to drive the market in the forthcoming years.
Gain deeper insights on the market and receive your free copy with TOC now @: eClinical Solutions Market Report
As the demand for tracking and analyzing clinical data increases, the need for effective clinical solutions rises. Unmet needs to manage efficient clinical development processes are poised to boost market growth over the forecast period. Moreover, digital transformation in the field of clinical trials and preference for data-centric approaches are providing a tremendous push to the market. Demand for integrated clinical IT solutions is increasing due to the massive volume of data generated during clinical development processes. eClinical solutions offer a single source of information that helps optimize the cost by eliminating redundant data entry and by reducing on-site verification and source data verification. Rising awareness regarding these advantages is projected to propel the market. Increasing adoption of eClinical workflows in trials offers enormous potential in clinical development processes. These solutions can facilitate decision-making in each stage of development. It also helps reduce cost and time between the development phase by utilizing seamless designs and by identifying failing compounds. In addition, it offers rapid access to data and patient safety information, which helps make quick decisions. Market players engage in new product development and strategic alliances including partnership agreements, promotional activities, and acquisitions to keep market rivalry high. For instance, in October 2020, Oracle entered into collaboration with FHI Clinical Inc. for improving clinical trial efficiency and to get therapies to market faster.
#eClinical Solutions Market Size & Share#Global eClinical Solutions Market#eClinical Solutions Market Latest Trends#eClinical Solutions Market Growth Forecast#COVID-19 Impacts On eClinical Solutions Market#eClinical Solutions Market Revenue Value
0 notes
Text
Medical research has a major problem: an alarmingly high number of trials are based on fake, fraudulent or misinterpreted data.
Research misconduct sleuths call them “zombie” studies. They look like real research papers but they’re rotten to the core. And when these studies go on to influence clinical guidelines, that is, how patients are treated in hospitals and doctors’ rooms, they can be dangerous.
Professor Ben Mol, head of the Evidence-based Women’s Health Care Research Group at Monash University, is a professional zombie hunter. For years, he has warned that between 20 and 30 per cent of medical trials that inform clinical guidelines aren’t trustworthy.
��I’m surprised by the limited response from people in my field on this issue,” he says. “It’s a topic people don’t want to talk about.”
The peer review process is designed to ensure the validity and quality of findings, but it’s built on the assumption that data is legitimate.
Science relies on an honour system whereby researchers trust that colleagues have actually carried out the trials they describe in papers, and that the resulting data was collected with rigorous attention to detail.
But too often, once findings are queried, researchers can’t defend their conclusions. Figures such as former BMJ editor Richard Smith and Anaesthesia editor John Carlise argue it’s time to assume all papers are flawed or fraudulent until proven otherwise. The trust has run out.
“I think we have been naive for many years on this,” Mol says. “We are the Olympic Games without any doping checks.”
How bad science gets into the clinic
Untrustworthy papers may be the result of scientists misinterpreting their data or deliberately faking or plagiarising their numbers. Many of these “zombie” papers emerge from Egypt, Iran, India and China and usually crop up in lower-quality journals.
The problem gets bad when these poor-quality papers are laundered by systematic reviews or meta-analyses in prestigious journals. These studies aggregate hundreds of papers to produce gold-standard scientific evidence for whether a particular treatment works.
Often papers with dodgy data are excluded from systematic reviews. But many slip through and go on to inform clinical guidelines.

My colleague Liam Mannix has written about an example of this with the hormone progesterone. Official guidelines held that the hormone could reduce the risk of pre-term birth in women with a shortened cervix.
But those guidelines were based on a meta-analysis largely informed by a paper from Egypt that was eventually retracted due to concerns about the underlying data. When this paper was struck from the meta-analysis, the results reversed to suggest progesterone had no preventative effect.
There’s a litany of other examples where discounting dodgy data can fundamentally alter the evidence that shapes clinical guidelines. That’s why, in The Lancet’s clinical journal eClinical Medicine, Mol and his colleagues have reported a new way to weed out bad science before it makes it to the clinic.
Holding back the horde
The new tool is called the Research Integrity in Guidelines and evIDence synthesis (RIGID) framework. It mightn’t sound sexy, but it’s like a barbed-wire fence that can hold back the zombie horde.
The world-first framework lays out a series of steps researchers can take when conducting a meta analysis or writing medical guidelines to exclude dodgy data and untrustworthy findings. It involves two researchers screening articles for red flags.
“You can look at biologically implausible findings like very high success rates of treatments, very big differences between treatments, unfeasible birth weights. You can look at statistical errors,” says Mol.
“You can look at strange features in the data, only using rounded numbers, only using even numbers. There are studies where out of dozens of pairs of numbers, everything is even. That doesn’t happen by chance.”
A panel decides if a paper has a medium to high risk of being untrustworthy. If that’s the case, the RIGID reviewers put their concerns to the paper’s authors. They’re often met with stony silence. If authors cannot address the concerns or provide their raw data, the paper is scrapped from informing guidelines.
The RIGID framework has already been put to use, and the results are shocking.
In 2023, researchers applied RIGID to the International Evidence-based Guidelines for Polycystic Ovary Syndrome (PCOS), a long misunderstood and misdiagnosed syndrome that affects more than 1 in 10 women. As a much maligned condition, it was critical the guidelines were based on the best possible evidence.
In that case, RIGID discounted 45 per cent of papers used to inform the health guidelines.
That’s a shockingly high number. Those potentially untrustworthy papers might have completely skewed the guidelines.
Imagine, Mol says, if it emerged that almost half of the maintenance reports of a major airline were faked? No one would be sitting around waiting for a plane to crash. There would be swift action and the leadership of the airline sacked.
#australia#women's health#medical misogyny#radblr#this feels particularly important with the huge gender data gap in medicine and the cass review's findings of bad research in the UK
78 notes
·
View notes
Link
0 notes
Text
Household Cleaners Market Growth: Key Trends Driving the Future of the Industry
The global electronic clinical outcome assessment solutions market size is anticipated to reach USD 4.12 billion by 2030, registering CAGR of 15.2% during the forecast period, according to a new report by Grand View Research, Inc. The key factors driving the market growth include increasing interoperability across eClinical solutions, surge in adoption owing to the COVID-19, use of telehealth, need to comply with changing regulations, and increasing complexity of data generated in clinical research.
The COVID-19 led to operational hurdles in clinical research activities including clinical trials. This included postponement of trials, recruitment challenges, and management problems. The pandemic, however, accelerated the adoption of enabling technologies for managing clinical trial operations and data. This boosted demand for the eClinical solutions including electronic clinical outcome assessment (eCOA) solutions. As patients were unable to visit trial sites, eCOA solutions emerged as a reliable solution to collect patient data. It also helped sites maintain compliance during the pandemic. For instance, IQVIA reported that its eCOA platform was deployed multiple times during the pandemic in clinical trials.
The complexity in healthcare information management is anticipated to fuel demand for the eCOA solutions in coming years. These solutions deliver accurate and timely health information and reduce burden of the patients enrolled in clinical trials. eCOA measures overall mental state, patient symptoms, and the progression of a disease. Electronic diaries and electronic patient reported outcomes (ePRO) are a part of eCOA platforms. Electronic diaries help document patient response. These are used as support systems for ePRO. eCOA solution from Cloudbyz for instance, includes ePRO and eDiary functionalities and supports electronic clinical outcome data, captured with compliance adherence.
Gather more insights about the market drivers, restrains and growth of the Electronic Clinical Outcome Assessment Solutions Market
Electronic Clinical Outcome Assessment Solutions Market Report Highlights
• The electronic clinical outcome assessment (eCOA) solutions market was valued at USD 1.36 billion in 2022 and is expected to expand at a CAGR of 15.2% during the forecast period
• Web &Cloud-based solutions are anticipated to grow at an exponential rate owing to the integrated features that include flexibility, high accessibility, negligible handling costs, and easy data backup. The added advantage of remote access to information also contributes to segment growth
• Contract research organizations dominated the eCOA solutions market as major pharmaceutical companies are focused on reducing expenditure on clinical trials
• North America held the largest market share owing to the local presence of well-established market players coupled with large number of ongoing research in this region
• Asia Pacific market is expected to show fastest growth during the forecast period owing to the increasing clinical research activities by the end users such as CROs and biopharmaceutical companies
• The companies are making significant investments to implement eClinical solutions in order to manage medical information, owing to the benefits it offers
Electronic Clinical Outcome Assessment Solutions Market Segmentation
Grand View Research has segmented the global electronic clinical outcome assessment solutions market based on the delivery mode, end user, and region:
eCOA Solutions Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
• On-premise
• Web & Cloud-based
eCOA Solutions End-user Outlook (Revenue, USD Million, 2018 - 2030)
• Hospitals/Healthcare Providers
• CROs
• Pharmaceutical & Biotechnology Firms
• Medical Device Companies
• Others
eCOA Solutions Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o Germany
o UK
o France
o Italy
o Spain
• Asia Pacific
o China
o India
o Japan
o Australia
o South Korea
• Latin America
o Brazil
o Mexico
• MEA
o South Africa
o Saudi Arabia
Order a free sample PDF of the Electronic Clinical Outcome Assessment Solutions Market Intelligence Study, published by Grand View Research.
#Household Cleaners Market#Household Cleaners Market Size#Household Cleaners Market Share#Household Cleaners Market Analysis#Household Cleaners Market Growth
0 notes
Text
0 notes
Link
0 notes
Text
0 notes