#covid 19 care home test kit collection
Explore tagged Tumblr posts
Text
Covid-19 Testing Los Angeles: Fast, Reliable, and Accessible Options

Covid-19 Testing Los Angeles: Quick access to reliable testing centers near you.
Covid-19 Testing Los Angeles: Ensure your safety with accurate and timely results.
Covid-19 Testing Los Angeles: Find the best locations for convenient testing.
Quick Access to Covid-19 Testing in Los Angeles
Covid-19 Testing in Los Angeles is essential for maintaining public health and safety during the ongoing pandemic. With the ever-evolving nature of the virus, regular testing is crucial to prevent outbreaks and ensure that infected individuals receive timely care. Whether you need a test for travel, work, or simply for peace of mind, Los Angeles offers numerous options to get tested quickly and efficiently.
Understanding Your Testing Options
Los Angeles is home to a wide array of testing sites, each catering to different needs and preferences. From drive-thru centers to walk-in clinics and at-home testing kits, there’s a solution for everyone. Here’s a breakdown of the most common types of Covid-19 tests available:
PCR Tests: Known for their high accuracy, PCR (Polymerase Chain Reaction) tests are the gold standard for Covid-19 testing. These tests detect the virus's genetic material and are typically processed in a laboratory, with results available within 24-72 hours.
Rapid Antigen Tests: These tests provide results within 15-30 minutes and are useful for quickly determining if you’re infectious. However, they are less accurate than PCR tests, especially in asymptomatic individuals.
At-Home Testing Kits: For those who prefer the convenience of testing at home, Los Angeles offers several FDA-approved at-home testing kits. These kits allow you to collect a sample and mail it to a lab for processing, with results typically available in a few days.
Process in Los Angeles
Los Angeles has made it easy for residents and visitors to access Covid-19 testing. Here’s what you need to know:
Location Selection: Choose a testing site that suits your needs, whether it’s a drive-thru for minimal contact, a walk-in clinic for convenience, or an at-home kit for privacy.
Booking an Appointment: While many testing sites in Los Angeles accept walk-ins, booking an appointment is recommended to avoid long wait times. Many sites offer online scheduling, making it easy to secure a spot at your preferred location.
Test Day: On the day of your test, bring a valid ID and any necessary documentation, such as your insurance card. Follow the instructions provided by the testing center staff to ensure the process goes smoothly.
Receiving Results: Depending on the type of test you choose, results can be available within minutes to a few days. PCR tests typically take longer, but they offer the most reliable results. Rapid tests are quicker but may require confirmation with a PCR test if the result is positive.
Frequently Asked Questions
1. How much does Covid-19 testing cost in Los Angeles?
Most Covid-19 testing in Los Angeles offer free testing, especially for those who meet certain criteria, such as having symptoms, being exposed to the virus, or needing a test for travel. However, some private clinics may charge a fee, so it's advisable to check with the specific testing site beforehand.
2. Do I need to make an appointment for Covid-19 testing in Los Angeles?
While many testing sites allow walk-ins, it’s generally a good idea to make an appointment. This can help reduce your wait time and ensure that you’re tested promptly. Most testing centers in Los Angeles offer easy online appointment booking.
3. What should I do if I test positive for Covid-19 in Los Angeles?
If you test positive for Covid-19, it’s important to follow public health guidelines to prevent the spread of the virus. This includes self-isolating for at least 10 days, notifying close contacts, and monitoring your symptoms. Seek medical attention if your symptoms worsen or if you are in a high-risk group.
The Importance of Testing
Covid-19 Testing in Los Angeles is not just about meeting travel requirements or workplace mandates; it’s about protecting your health and the health of those around you. Regular testing, especially if you’ve been exposed to the virus or are experiencing symptoms, plays a critical role in controlling the spread of Covid-19.
Los Angeles has taken significant steps to make testing accessible to everyone, with numerous testing centers spread across the city. Whether you’re a resident or a visitor, you’ll find that getting tested is straightforward and hassle-free. By staying vigilant and getting tested when necessary, you contribute to the collective effort to combat the pandemic.
“Testing is one of our most powerful tools in the fight against Covid-19. By getting tested regularly, we can identify cases early, isolate them, and prevent further transmission.”
In conclusion, Covid-19 testing in Los Angeles is a vital service that’s readily available to all. Whether you’re looking for peace of mind, need a test for travel, or have been exposed to the virus, Los Angeles offers a variety of testing options to suit your needs. Stay informed, stay safe, and make testing a regular part of your health routine.
0 notes
Text
Scanbase Presents Smartphone-Based, Computer Vision COVID-19 Testing
In the ongoing battle against the COVID-19 pandemic, at-home testing kits have emerged as indispensable tools, offering a multitude of advantages. The foremost among these is the unparalleled convenience they provide. By enabling individuals to collect samples and perform tests in the safety of their homes, these kits eliminate the need for travel to testing sites, reducing potential exposure.
Moreover, the accessibility of at-home testing kits is a crucial factor in addressing healthcare disparities. By reaching remote and underserved populations, these kits bridge gaps in access to testing, ensuring that everyone, regardless of their location, has the opportunity to undergo testing.
Timeliness is of the essence in managing the spread of the virus, and at-home COVID-19 testing kits, coupled with smartphone technology, play a pivotal role in this regard. Rapid results allow for swift isolation and contact tracing, contributing significantly to controlling the transmission of the virus.

Different Benefits & Applications of Innovative COVID-19 Testing Methods
A less evident but equally critical benefit is the reduction in the burden on healthcare systems. By shifting a portion of testing from healthcare facilities to homes, these kits free up valuable resources for critical care, helping healthcare providers manage their workload more efficiently.
Moreover, privacy is a fundamental aspect addressed by at-home testing kits. Individuals can undergo testing without disclosing their health status to others, reducing the potential for stigma and discrimination associated with COVID-19.
The integration of smartphone-based computer vision technology has further elevated the effectiveness and user-friendliness of at-home COVID-19 testing kits. Designed to be user-friendly, these kits typically include nasal swabs or saliva collection devices for painless sample collection.
Smartphone apps associated with the testing kits guide users through the sample collection process. Users capture images of their collected samples or test result areas using the smartphone's camera, following the app's instructions.
The real innovation lies in the application of sophisticated computer vision algorithms within the smartphone app. These algorithms are trained to accurately identify viral particles or markers indicative of the virus's presence, ensuring a high level of accuracy in the results.

Results In Minutes
Within minutes, users receive their test results on their smartphone screens. The app may also provide additional information, such as recommendations for isolation or further medical consultation. In essence, the marriage of at-home COVID-19 testing kits and smartphone-based computer vision technology represents a significant leap forward in our collective efforts to combat the pandemic.
0 notes
Text
Navigating COVID-19 Testing in Brownsville, TX- Your Comprehensive Guide
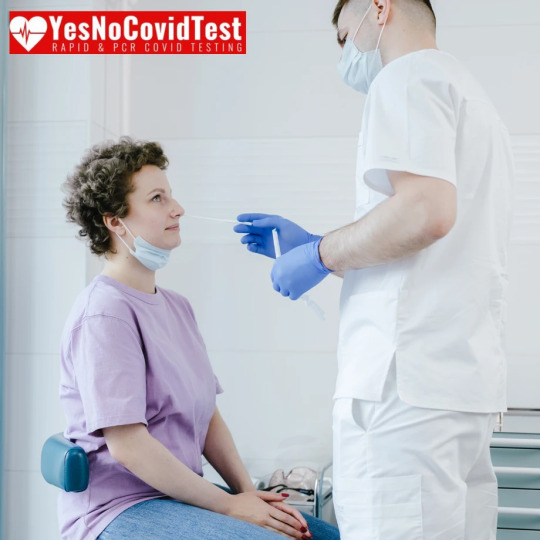
As we continue to grapple with the ongoing challenges posed by the COVID-19 pandemic, one crucial aspect of our collective response remains regular testing. Whether you're experiencing symptoms, planning a trip, or simply want to ensure your safety and that of those around you, knowing where to get tested in Brownsville, Texas, is essential. In this blog post, we'll provide you with a comprehensive guide to COVID test Brownsville TX.
Local Health Departments and Clinics
Local health departments and clinics in Brownsville play a pivotal role in COVID-19 testing. These facilities offer free testing services to the community. They are often centrally located and can provide reliable testing results. To access testing through these channels, check with the Brownsville Health Department or visit their website for the latest information on testing sites, hours of operation, and any eligibility criteria.
Pharmacies and Retail Clinics
Many national pharmacy chains, as well as local pharmacies, have stepped up to offer COVID-19 testing services. Walgreens, CVS, and Walmart, among others, have established testing sites across Brownsville. These locations often require online scheduling, and they may offer both PCR and rapid antigen tests. Be sure to verify the type of test available and any associated costs before your visit.
Urgent Care Centers
Urgent care centers in Brownsville have been actively participating in COVID-19 testing efforts. These facilities provide convenient options for testing, often with shorter wait times than larger testing centers. While costs can vary, many accept insurance, making testing accessible to a broader range of residents.
Community Testing Events
Keep an eye out for community testing events and mobile testing units that periodically visit Brownsville neighborhoods. These events, often organized by local health authorities and non-profit organizations, provide free or low-cost testing opportunities. Follow local news and public health announcements for information about upcoming events.
Home Testing Kits
Some pharmacies and online retailers offer at-home COVID-19 testing kits. These kits allow you to collect a sample at home and send it to a laboratory for analysis. While convenient, it's essential to carefully follow the instructions provided and consider shipping times when using this option.
Conclusion
Access to reliable COVID-19 testing is crucial in our efforts to control the spread of the virus. Brownsville, TX, offers a variety of testing options to meet the diverse needs of its residents. Whether you opt for a local health department, pharmacy, urgent care center, or a community testing event, remember to follow safety guidelines and check for the most up-to-date information on testing availability and requirements.
In these challenging times, knowing where and how to get tested for COVID-19 empowers individuals and communities to take proactive measures to protect themselves and others. Stay informed, stay safe, and together, we can overcome this public health crisis.
0 notes
Text
General HPV Testing Guidelines:
Although recommendations may vary based on location and individual health conditions, there are overarching guidelines to bear in mind:
1. Screening for Cervical Cancer: It is crucial for women to undergo regular cervical cancer screenings. Typically, this initiates at age 21 and recurs every three years. Beyond the age of 30, co-testing, which involves both a Pap test and an HPV DNA test, can be considered. If both results are normal, the testing interval can extend to every five years.
2. Men and At-Risk Populations: Routine HPV screening for men is not standard practice. However, individuals with specific risk factors, such as a compromised immune system or a history of genital warts, may necessitate more frequent testing.
3. Vaccination: HPV vaccination, typically administered during childhood and early adolescence, significantly reduces the risk of infection and associated cancers.
Evolving Trends in HPV Testing
In recent years, notable trends and advancements have emerged in the realm of HPV testing:
1. Self-Sampling Kits: Certain healthcare providers now offer self-collection kits, allowing individuals to gather their own samples for HPV testing at home. This option proves particularly convenient, especially during the COVID-19 pandemic when in-person healthcare visits were limited.
2. Primary HPV Testing: In select regions, primary HPV testing, which assesses the presence of high-risk HPV types without a Pap test, has gained preference for cervical cancer screening due to its proven effectiveness in detecting cervical abnormalities.
3. Point-of-Care Testing: Point-of-care HPV tests are increasingly accessible, enabling swift on-site testing in clinical settings. These tests offer quick results and immediate follow-up care if required.
You can consult some of the best hospitals in Mumbai, like H N Reliance Hospital to undergo regular health screenings for HPV. The hospital has some of the best specialist doctors, like fertility specialist Dr. Firuza Parikh, who can provide you with an accurate diagnosis and accurate treatment plan for your condition.
#HPV testing#HPV vaccination#cervical cancer#health checkup#health screening#best hospital in Mumbai
0 notes
Text
8 Remote Patient Monitoring Trends and Medical Devices in 2023
Remote Patient Monitoring (RPM) has emerged as a transformative technology in healthcare, enabling healthcare providers to monitor patients' health conditions and vital signs outside of traditional clinical settings. This technology has gained even more prominence in 2023, as advancements in medical devices and telehealth solutions continue to reshape the healthcare landscape. Reliable medical equipment sales in Denver helps medical experts with advanced RPM devices making the RPM landscape successful.
This article explores eight prominent trends in remote patient monitoring and the innovative medical devices that are driving these trends in 2023.
8 Remote Patient Monitoring Trends To Look For In 2023
Check out these RPM trends of 2023:
Integration of AI and Machine Learning
Artificial Intelligence (AI) and Machine Learning (ML) have become integral to remote patient monitoring. In 2023, these technologies are being used to analyze vast amounts of patient data, enabling early detection of anomalies and predicting potential health issues. AI-powered algorithms are capable of providing real-time insights into patient conditions, which assists healthcare providers in making informed decisions promptly. For instance, wearable devices equipped with AI can track trends in vital signs and alert medical professionals if any deviations from the norm occur.
Wearable Health Tech
Wearable devices are evolving beyond basic activity trackers to sophisticated health monitoring tools. In 2023, wearable devices have become more accurate and versatile, capable of monitoring a range of health parameters, including heart rate, blood pressure, oxygen saturation, glucose levels, and even ECG readings. These devices can transmit data to healthcare providers remotely, enabling them to monitor patients' health conditions in real-time without requiring them to visit medical facilities frequently. One can get such devices from medical equipment sales in Denver.
Telehealth Expansion
Telehealth has seen a massive expansion in 2023, driven by the ongoing need for remote healthcare services. RPM plays a crucial role in telehealth by allowing doctors to remotely monitor patients' conditions through virtual consultations. Patients can use various devices to measure their vital signs and share the data with healthcare providers during telehealth appointments. This trend has particularly been accelerated due to the COVID-19 pandemic, where virtual consultations have become the norm.
IoMT (Internet of Medical Things) Connectivity
The Internet of Medical Things (IoMT) has led to improved connectivity between medical devices, patients, and healthcare providers. In 2023, medical devices are increasingly interconnected, allowing seamless data exchange. This interconnectedness not only enhances remote patient monitoring capabilities but also aids in comprehensive patient care. For instance, patient data collected from wearable devices can be integrated with electronic health records, offering a holistic view of a patient's health history.
Remote Chronic Disease Management
RPM has shown great potential in managing chronic diseases such as diabetes, hypertension, and heart conditions. In 2023, medical devices are being designed with specific features to cater to patients with chronic illnesses. These are available on medical equipment sales in Denver. Continuous monitoring of vital signs and symptoms helps in early detection of complications, enabling timely interventions. Patients with chronic conditions can now maintain a higher quality of life while reducing hospital visits and associated costs.
Home-Based Medical Devices
Advancements in technology have enabled the development of sophisticated medical devices that can be used conveniently at home. From portable ultrasound devices to blood testing kits, patients can now perform various medical tests at home and share the results with healthcare professionals. This trend not only promotes patient convenience but also reduces the burden on healthcare facilities, making healthcare more accessible to remote and underserved populations.
Data Security and Privacy
As remote patient monitoring involves the transmission and storage of sensitive health data, data security and privacy have become paramount. In 2023, there is a heightened focus on implementing robust security measures to protect patient information. Encrypted communication channels, secure cloud storage, and compliance with data protection regulations are essential aspects of ensuring patient trust and maintaining the integrity of RPM systems. Reliable medical equipment sales in Denver ensure data safety in the advanced RPM devices.
Personalized Healthcare Insights
The wealth of data generated by remote patient monitoring devices allows for personalized healthcare insights. In 2023, medical devices are equipped with algorithms that analyze individual patient data to provide tailored health recommendations. Patients receive actionable insights to improve their health and well-being based on their unique health profiles. This personalized approach not only empowers patients to take control of their health but also enhances the overall effectiveness of remote patient monitoring.
Essential RPM Devices
Wearable Health Trackers: They provide real-time data to both patients and healthcare providers, helping in early detection of any abnormalities.
Connected Blood Pressure Monitors: These devices allow patients to measure their blood pressure at home and transmit the readings to healthcare providers.
Smart Glucose Monitors: Patients with diabetes can use these devices to measure their blood glucose levels and share the data with healthcare professionals.
Pulse Oximeters: Pulse oximeters measure the oxygen saturation in a patient's blood. They are commonly used to monitor patients with respiratory conditions like chronic obstructive pulmonary disease (COPD) and COVID-19.
Mobile ECG Monitors: These portable devices allow patients to record their electrocardiogram (ECG) readings at home. Get them on medical equipment sales in Denver.
Remote Temperature Monitors: Especially relevant in the context of infectious diseases, these devices enable patients to monitor their body temperature and share fever trends.
Telehealth Platforms: While not a single device, telehealth platforms are essential for RPM. These platforms facilitate virtual consultations between patients and healthcare providers, allowing for the review of remote monitoring data and discussion of treatment plans.
Weight Scales with Biometric Analysis: Advanced weight scales can not only measure weight but also analyze body composition, including metrics like body fat percentage and muscle mass.
Portable Spirometers: Spirometers measure lung function and are crucial for patients with respiratory conditions like asthma and COPD.
Home Sleep Apnea Monitors: Patients suspected of having sleep apnea can use these devices to record their sleep patterns and breathing during the night.
Continuous Glucose Monitoring (CGM) Systems: These systems provide real-time glucose data through a sensor implanted under the skin.
Remote Medication Dispensers: These devices help patients adhere to their medication schedules by dispensing the right doses at the right times.
Smart Inhalers: Designed for patients with asthma and other respiratory conditions, smart inhalers track medication usage and provide feedback on inhaler technique, ensuring optimal treatment outcomes.
Remote Cardiac Monitors: These specialized devices monitor the heart's electrical activity over an extended period, aiding in the diagnosis of cardiac arrhythmias and other heart conditions.
Telehealth Kits for Elderly Patients: These kits include a combination of devices like blood pressure monitors, thermometers, and tablets preloaded with telehealth apps.
Conclusion
The year 2023 has witnessed a remarkable transformation in remote patient monitoring, with innovative medical devices and technologies driving significant trends. From AI-powered analytics to wearable health tech and telehealth expansion, RPM is revolutionizing the way healthcare is delivered. These trends not only improve patient outcomes and quality of life but also offer more efficient and cost-effective healthcare solutions. One can get advanced RPM devices from reliable medical equipment sales in Denver. As technology continues to evolve, the future of remote patient monitoring holds even more exciting possibilities for enhancing patient care and reshaping the healthcare landscape.
0 notes
Text
Benefits of buying home medical tests

If you are someone who fears visiting a doctor, then the at home testing option is great for you as there are numerous benefits of using testing kits at home. Testing kits at home are nothing new as they have been used for many years. Not everyone has access to health insurance or lives close to a doctor's office. Many times people cannot go to an urgent care clinic that is more affordable as compared to a hospital. If you live in the Suburban area then visiting a doctor in the city can be quite difficult. This is why at home medical testing kits can be ordered online and used to perform any medical tests that are required. These tests are quite convenient as you don't have to take off from work and wait for a long time to see the doctor. When you use a home testing kit you don't have to leave your house as you can order your test online and take the test at home.
You can get instant results and can then visit the doctor if required. Testing at home is quite reasonable as compared to a visit to the doctor. While at-home medical kits are convenient, discreet and accessible. If you have a doubt about a disease, it is better to perform an at-home test and then if needed visit a doctor or seek advice online. At-home testing kits are becoming a big part of the healthcare system and have captured a lot of interest after the covid-19 pandemic. At-home tests are now available in a wide variety and are offered online by numerous companies. However, it is important to buy from a trustable supplier. It provides customers and patients with more options than ever before. These kits can help you avoid contact with others when you have communicable diseases as you will not have to leave home to get yourself tested. At-home test kits are used for numerous reasons like diagnosis of a health condition and infectious diseases like Covid-19. There are also home test-kits for diseases like diabetes that can help monitor blood sugar easily.
There are home test kits to perform food intolerance tests in case you feel you are allergic to certain types of foods. These kits are widely available nowadays online and these over-the-counter tests are delivered right to your doorstep. The kit includes all the instructions for collecting and testing the sample. All you have to do is follow the instructions carefully using the materials in the kit. If you are unsure about how to take the test, then talk to the customer care about your queries. Haled is an organisation that offers doorstep delivery of home food sensitivity test kits, along with numerous other self-test kits, so order your kits and perform the tests from the proximity of your home. Order your self-test kits online easily to diagnose yourself from diseases that you might feel you have and then visit the doctor at your convenience.
#Home Health Testing Kits#home health testing kits#testing kits at home#home test kit#Food Intolerance Test#Food Sensitivity Test
0 notes
Text
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET Growth, Industry Size-Share, Global Trends, Key Players Strategies and Upcoming Demand
Data Bridge Market Research analyses that the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET will project a compound annual growth rate (CAGR) of 5.7% during the forecast period of 2022-2028.
A world class AFRICA POINT-OF-CARE DIAGNOSTICS MARKET research report is formulated with the finest and advanced tools of collecting, recording, estimating and analysing market data. With the systematic and comprehensive market research study, this market research report offers the facts associated with any subject in the field of marketing for Healthcare industry. It gives superior ideas and solutions in terms of product trends, marketing strategy, future products, new geographical markets, future events, sales strategies, customer actions or behaviours. This AFRICA POINT-OF-CARE DIAGNOSTICS MARKET report has been prepared by considering several fragments of the present and upcoming market scenario.
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET Scope and Market Size
The Point-of-care diagnostics market is segmented on the basis of type, platform, end user and distribution channel.
On the basis of type, the Point-of-care diagnostics market is segmented into devices, software and services, consumables and kits, and accessories. In 2021, the devices segment in the point-of-care diagnostics market is expected to grow due to changing lifestyles and increasing chronic diseases, and a lack of awareness of over-the-counter point-of-care diagnostics devices.
On the basis of platform, the point-of-care diagnostics market is segmented into lateral flow assays (immunochromatography tests), dipsticks, microfluidics, molecular diagnostics, immunoassays. In 2021, the lateral flow assays/immunochromatography tests segment in the point-of-care diagnostics market is expected to grow due to advanced technologies and inventions.
On the basis of end user, the point-of-care diagnostics market is segmented into professional diagnostic centers, home care, research laboratories and other end users. In 2021, the professional diagnostic centers segment in the point-of-care diagnostics market is expected to grow due to growing patient emphasis on diagnosis operations. However, the high investment cost may hamper the Point-of-care diagnostics market growth.
Get the Free sample copy of the report here:
Some of the key questions answered in this report:
How has the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET performed so far and how will it perform in the coming years?
What has been the impact of COVID-19 on the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET?
What are the key regional markets?
What are the key driving factors and challenges in the industry?
What is the structure of the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET and who are the key players?
Market Analysis and Insights: AFRICA POINT-OF-CARE DIAGNOSTICS MARKET
The Point of care diagnostics market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing at a CAGR of 5.7% in the forecast period of 2021 to 2028 and is expected to reach USD 816.12 million by 2028. Increasing prevalence of chronic diseases and rising preference for homecare testing are anticipated to drive the growth of the market.
The Point-of-care diagnostics is characterized as a testing which can be done in close proximity to the patient where a medical decision can be made immediately including the results and monitoring. Point-of-care diagnostics is also called with-patient testing and allows physicians to accurately measure real-time, lab-quality diagnostic results within less than minutes as compared to hours. It ensures patients to receive the most effective and efficient care when and wherever needed. Additionally, Point-of-care diagnostics enables staff to make rapid decisions of treatments when diagnosing patients’ condition and the Point-of-care diagnostics simplifying the testing process.
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET - Regional Level Analysis
The countries covered in the point-of-care diagnostics market report are Kenya, Algeria, Nigeria, Morocco, Rwanda, Zambia, Uganda, Central African Republic, Chad, Ethiopia, Mauritania, Cameroon, Angola, Libya, DRC, Ghana, Malawi.
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET - Share Analysis:
Point-of-care diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to Point-of-care diagnostics market.
Key player - AFRICA POINT-OF-CARE DIAGNOSTICS MARKET
Some of the major players operating in the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET are BioTech Africa, Medtronic, Bio-Rad Laboratories, Inc., PTS Diagnostics, Trinity Biotech, Atlas Medical Uk, Abbott, Sysmex Corporation, bioMérieux SA, Quidel Corporation, BD, Sekisui Diagnostics, F. Hoffmann-La Roche Ltd, Beckman Coulter, Inc., ACON Laboratories, Inc., Nova Biomedical, Siemens Healthcare S.A.E. (a subsidiary of Siemens Healthineers AG), Instrumentation Laboratory, Abaxis, and Radiometer Medical ApS (a subsidiary of Danaher) among others. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Get Full Access of Report@
https://www.databridgemarketresearch.com/reports/africa-point-of-care-diagnostics-market MAJOR TOC OF THE REPORT
Chapter One: Introduction
Chapter Two: Scope and Market Size
Chapter Three: Analysis and Insights
Chapter Four: Country Level Analysis
Chapter Five: Share Analysis
Chapter Six: Key player
Get TOC Details:
https://www.databridgemarketresearch.com/toc/?dbmr=africa-point-of-care-diagnostics-market Top Trending Reports:
Global Autoclave Market
Global Proctoscope Market
Global Molecular Methods Market
Global Wound Debridement Market
Global Human Papilloma Virus Testing Market
Global Non-Small Cell Lung Cancer Market
Global CPAP Market
Global RNA Markers Market
Global Healthcare Cloud Computing Market
Global Medical Image Management Market
Global Pharmacogenetic Testing Market
Global Hemodialysis Services Market
Global Hemostatic Wound Dressing Market
Global Interventional Radiology Products Market
Global Lab Accessories Market
Global Label-free Array Systems Market
Global Laboratory Gas Generators Market
Global Life Science Analytics Market
Global Mediastinoscopes Market
Global Medical Alert System Market
Global EDS, WDS, EBSD, Micro-XRF Instruments Market
Global Direct-to-Consumer (DTC) Genetic Testing Market
Global Q- Polymerase Chain Reaction and D- Polymerase Chain Reaction Devices Market
Asia-Pacific (APAC) Q-PCR and D-PCR Devices Market
Europe Q-PCR and D-PCR Devices Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-386.8-2818
Email: [email protected]
0 notes
Text
FDA Authorizes First OTC At-Home Test to Detect Both Influenza and COVID-19 Viruses

FDA Authorizes First Over-the-Counter At-Home Test to Detect Both Influenza and COVID-19 Viruses. Silver Spring, Md., Feb. 24, 2023 - Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the first over-the-counter (OTC) at-home diagnostic test that can differentiate and detect influenza A and B, commonly known as the flu, and SARS-CoV-2, the virus that causes COVID-19. The Lucira COVID-19 & Flu Test is a single-use at-home test kit that provides results from self-collected nasal swab samples in roughly 30 minutes. "Today's authorization of the first OTC test that can detect Influenza A and B, along with SARS-CoV-2, is a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home," said Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health. "The FDA strongly supports innovation in test development, and we are eager to continue advancing greater access to at-home infectious disease testing to best support public health needs. We remain committed to working with test developers to support the shared goal of getting more accurate and reliable tests to Americans who need them." The Lucira COVID-19 & Flu Test is a single-use test for individuals with signs and symptoms consistent with a respiratory tract infection, including COVID-19. The test can be purchased without a prescription and performed completely at home using nasal swab samples self-collected by individuals ages 14 years and older or collected by an adult for individuals 2 years of age or older. The test works by swirling the sample swab in a vial that is placed in the test unit. In 30 minutes or less, the test unit will display the results that show whether a person is positive or negative for each of the following: Influenza A, Influenza B, and COVID-19. Individuals should report all results obtained to their healthcare provider for public health reporting and to receive appropriate medical care. In individuals with symptoms, the Lucira COVID-19 & Flu Test correctly identified 99.3% of negative and 90.1% of positive Influenza A samples, 100% of negative and 88.3% of positive COVID-19 samples, and 99.9% of negative Influenza B samples. Since there are currently not enough cases of Influenza B circulating to include in a clinical study, validation confirmed that the test can identify the virus in contrived specimens, and the EUA requires Lucira to continue to collect samples to study the test's ability to detect Influenza B in real-world settings. As with all rapid diagnostic tests, there is a risk of false positive and false negative results. Individuals who test positive for either flu or COVID-19 should take appropriate precautions to avoid spreading the virus and should seek follow-up care with their physician or healthcare provider as additional testing may be necessary. Negative results for SARS-CoV-2 and influenza B should be confirmed, if necessary for patient management, with an authorized or cleared molecular test performed in a CLIA-certified laboratory that meets the requirements to perform high or moderate complexity tests. Individuals who test negative and continue to experience symptoms of fever, cough, and/or shortness of breath may still have a respiratory infection and should seek follow-up care with their healthcare provider. The collective impact of COVID-19, flu, and RSV underscores the importance of diagnostic tests for respiratory viruses, and the FDA recognizes the benefits that home testing can provide. The agency will continue to use its authorities to increase the number of appropriately accurate and easy-to-use at-home tests available to the public, especially tests that detect these highly contagious respiratory viruses. Read the full article
0 notes
Text
How To Get a Same Day Rapid PCR Test in Miami
If you are in Miami and you need same day rapid PCR testing, there are a variety of options available to you. It is important that you choose a facility that is certified and reliable, as the accuracy of the results will depend upon it. While same day test results are convenient and fast, they may come with higher rates than other tests. The best way to find same day PCR test Miami is by researching your options online and selecting a facility that offers same day service at an affordable price.
Where to get tested
If you are looking for a reliable pcr covid test Miami same day results, there are many different testing centers to choose from. The health departments for most major cities will have reliable pcr covid tests available at designated times, or you can visit local urgent care clinics or private medical offices to get tested as well. It is important to consider the speed of results when selecting where to get tested and make sure that the center uses reliable pcr testing that delivers accurate results in a timely fashion.
The challenges of getting tested
Getting tested for COVID-19 can be time consuming and challenging. Between waiting to receive same day PCR test results, shortages in testing supplies, and not having a primary care provider to rely on, it can be difficult to identify whether or not an individual has been infected with the virus. Although same day PCR COVID test are becoming increasingly available across the country, limited access to these same day tests can put people in a precarious situation where their health is at risk -- without being aware of their actual condition. To ensure individuals have access to reliable testing methods, healthcare providers must prioritize investing in same day PCR testing capabilities.
Covid-19 testing at home – practical and convenient
The availability of Covid-19 testing at home is a powerful solution to the need for widespread and widespread access to medical testing procedures. Home-based diagnostic tests allow individuals to obtain same day PCR test results in their own homes, ensuring practical and convenient access to quick, safe, and reliable results. The convenience of not having to leave one’s home for testing allows for an easier return to daily life once it has been confirmed that an individual does not have Covid-19. Home testing also allows individuals who do not have access to reliable transportation or are home-bound due to health conditions the same opportunity as any other patient. With possibilities from self-collection kits, lab visits, and point-of-care testing facilities, it's clear that home covid-19 testing provides a great option for those looking for fast and reliable diagnosis.
Same Day Rapid PCR Tests In Miami – At Home
Same day rapid PCR testing is now available in Miami, offering residents the opportunity to get test results quickly from the comfort of their own homes. This service eliminates the need for individuals to travel to a laboratory, wait in line, and risk potentially being exposed to other infected individuals. With this convenient new option for how to get a rapid PCR test, getting tested for COVID-19 has never been easier. Informed by evidence-based practices and confirmatory lab results, customers can rest assured that they are receiving accurate knowledge about their health status. Miami is proud to provide this invaluable service to its citizens and remains committed to protecting its community during these uncertain times.
A more accurate alternative
Finding a reliable, accurate alternative is an important part of many businesses today. With advances in technology, people are constantly looking for options that provide quicker and more exact results. In many cases, a more accurate alternative can make all the difference. Organizations need to identify the best solution for their particular situation to ensure maximum efficiency and accuracy. Companies could benefit from searching for tools that can offer superior results – from better data handling capacity to more precise analysis of information. Taking time to find a more accurate alternative can have positive organizational effects ranging from improved customer service to increased competitiveness in the industry.
Concimed - COVID-19 & Monkeypox Testing Centers https://www.concimed.com/
Read more useful information in our blog https://www.concimed.com/blog
0 notes
Text
Stay Safe in 2021 by Getting Covid-19 Test at Home

Venturing outside has become dangerous in the wake of the global Covid-19 pandemic. New waves of mutated Covid-19 strains are bringing in new fears in the general population. So, it has become very important to get vaccinated and also perform timely covid-19 test at home whenever you experience symptoms.
The Value of Dependable Covid 19 Care Home Test Kit Collection
A dependable covid 19 care home test kit collection pack is all you need to allay the fears of a possible Covid-19 infection.
Nowadays, RT-PCR kits are considered to be the gold standards for testing. But it is also very important that you source these kits from the right place. As after testing, you are likely to mingle with your family and friends, it is very important that the test results are spot on.
At Citation Bioscience, we have dependable covid-19 test kits - in stock, which means you stay completely assured about the results after using the kits.
Instead of painful nasal swabs, our kits work with a spit sample that is deposited in a hazard-proof box and sent to a CLIA certified lab. The results are provided within 24-48 hours. Before getting the results, it is important that you self-isolate to prevent the spread of virus.
#covid-19 test home collection kit rapid pack#covid-19 test kits - in stock#covid 19 care home test kit collection#covid-19 test kits for sale#covid-19 test at home
0 notes
Text
The Choice Between Covid-19 Antibody Test Kit and RT-PCR Kits in 2021

In 2021, Covid-19 is running rampant and hampering the quality of life in so many ways. Experts believe that the best way to stop the negative impact of Covid-19 is testing and testing accurately. However, with so many options available in the market, it can be hard to choose a particular testing methodology. Primarily, people are confused between covid 19 antibody test kit and RT-PCR test kits. Let’s try to find the answers.
RT-PCR vs. Antibody vs. Antigen Testing
Primarily, there are three type of tests available: antibody, antigen and RNA testing also known as RT-PCR. Antibody testing tries to find the presence of antibodies in the body that are created as an immune system response. These tests are cheaper but provide rapid results.
Antigen testing takes longer than antibody testing but it tries to find the actual protein of the virus in the body. Lastly, RT-PCR testing looks for the RNA, which is the genetic material of the Covid-19 virus.
The Verdict
Initially, antigen and antibody testing kits were heavily used. These kits provided rapid results but the number of false negatives and false positives were really high. In contrast, the RT-PCR covid 19 care home test kit collection have proven to be the most effective. The results can be provided within 48 hours, which is a slightly longer duration but the accuracy of test is unmatched.
#covid 19 antibody test kit#covid-19 test home collection kit 6 kits#covid-19 home test kit courier collection kit service#covid-19 test home collection kit rapid pack#covid-19 test kits - in stock#covid 19 care home test kit collection
0 notes
Text
What's the Best Type of COVID-19 Test for Travel

One way to end the COVID-19 pandemic is through mass vaccination, but that’s far away.
Meanwhile, if you must travel, remember to take care to safeguard yourself and others. You're less likely to obtain and spread COVID-19 if you're completely vaccinated, but foreign travel can still increase your risk of contracting new COVID-19 variations. If possible, the CDC recommends you do not travel unless you are fully vaccinated.
Before you Embark on your Journey:
Consider the following questions as you make travel arrangements.
-Have you received a COVID-19 vaccine? Vaccinate yourself whenever possible. -If the vaccine consists of two doses, you need to wait for two weeks before going about traveling. Any vaccination takes time for your body to start accordingly. -You'll be less likely to spread COVID-19 once you've been properly vaccinated, and you'll be able to travel freely within the United States.
Examine the Local Criteria, Limits, and Circumstances: Some state, local, and territorial governments have rules that require people to wear masks or get tested, as well as to stay at home for up to 14 days if they have recently traveled. To avoid unpleasant surprises and delays, check for limits at your destination and any potential stops along the way. Keep in mind that legislation might change fast based on local situations. It's also vital to remember that the COVID-19 situation differs per country, including the extent of distribution and presence of variants. As your vacation approaches, check back for updates.
Testing and Travel: People who have been vaccinatedThe CDC notes that if you have been completely vaccinated, you do not need to be tested before or after your vacation within the United States, nor do you need to be quarantined once you return. If you plan to travel outside of the United States, the CDC recommends that you get tested only if it is required at your destination. CDC recommends that you get tested only if your final destination’s requirements need you to. this is when you are traveling outside of the US. You must have a negative test within the past three days of your arrival or documentation of COVID-19 recovery within the last three months before entering the United States.Plan on having your blood drawn three to five days after your trip. You don’t necessarily need to quarantine when you reach home. Still, for safety purposes, you can look out for any symptoms & stay home if you feel so.
Types of Examinations: The purpose of COVID-19 tests is to check for present or previous infections. A viral test concludes whether or not you are currently infected. Antigen testing and nucleic acid amplification tests (NAATs) are two forms of viral tests that can be employed.If you have had a previous infection, an antibody test (also known as a serology test) may be able to tell you. Antibody tests must not be used to diagnose a present infection.
How to Check if You're Infected With COVID-19 Right Now: For the most up-to-date local information on testing, contact your healthcare practitioner or go to the websites of your state, tribal, local, and territory health departments. The kind of viral COVID-19 testing available may vary depending on where you are. If you have signs and symptoms of COVID-19 and can't get tested by a healthcare professional or public health authority, you and your healthcare provider might explore an at-home collection kit or an at-home test.
Understanding Your Results: -If you test positive, you should know what precautions to take to avoid infecting others. -If you test negative, you were most likely not contaminated when your sample was taken. The test result simply indicates that you were not infected with COVID-19 at the time of the test. Continue to take precautions to keep yourself safe.
Remember, Safety First: When illness strikes, even the best-laid plans may have to be abandoned. If you or any of your traveling companions: -Even if you don't have symptoms, you may be sick or suspect you have COVID-19. -A COVID-19 virus test is awaiting findings. -Even if you don't have symptoms, you've been diagnosed with COVID-19. -Have you been near someone with COVID-19, whether suspected or confirmed, in the last 14 days, even if they didn't show any symptoms?
#covid19rapidtestnearme#freecovid19testingnearme#covid19antibodytest#antibodytestforcovid19#rapidpcrcovidtestnearme#freecovid-19testingnearme#freecovid19testing#covid-19antibodytestnearme#freetestingforcovid19#covid 19 antibody test#covid 19 free testing#group covid testing#covid-19 pcr test
1 note
·
View note
Text
COVID-19: FDA issues authorization for first molecular non-prescription, at-home test

- By U.S. Food and Drug Administration (FDA) -
On March 5, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter (OTC) Use. The product is a molecular nucleic acid amplification test (NAAT) that is intended to detect genetic material from SARS-CoV-2 virus present in the nostrils. The test is the first molecular test authorized for at-home use without a prescription.
“The authorization of this new diagnostic test underscores FDA’s goal to continue supporting innovation in testing and providing flexibility to test developers with the aim of increasing the availability of accurate and reliable tests for all Americans,” said Acting FDA Commissioner Janet Woodcock, M.D. “The FDA will continue to expand Americans’ access to testing to help us in the fight against this pandemic, which has claimed over half a million lives in the United States.”
Cue COVID-19 Test for Home and Over The Counter (OTC) Use test is authorized for non-prescription home use for the qualitative detection of nucleic acid from SARS-CoV-2 in anterior nasal (nasal) swab specimens collected with the Cue Sample Wand. This test is intended for use in adults (self-swabbing) or children two years of age or older (swabbed by an adult) with or without symptoms or other epidemiological reasons to suspect COVID-19.
The authorized test includes: the single-use Cue COVID-19 Test Cartridge, the single-use Cue Sample Wand nasal swab, the Cue Cartridge Reader (used by the Cue Health Monitoring System, provided separately), and the Cue Health Mobile Application (App) that is downloaded onto compatible mobile smart devices, like a smart cell phone. The reusable, battery-operated Cue Cartridge Reader runs the Cue Test Cartridge and communicates results directly to the Cue Health App in about 20 minutes. The mobile application requires individuals to create an account, and in the future will be updated to include capability to report test results as appropriate to public health authorities to monitor disease prevalence.
Cue COVID-19 Test for Home and Over The Counter (OTC) Use correctly identified 96% of positive samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms. Cue Health expects to produce more than 100,000 tests per day by summer 2021.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” said Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health. “Cue COVID-19 Test for Home and Over-the-Counter (OTC) Use provides access to accurate and reliable testing at-home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public.”
The FDA has authorized more than 330 tests and collection kits for a variety of uses, users and locations to provide a wide array of test options. FDA has also prioritized review and authorization of EUA requests taking into account a variety of factors, as discussed in the Emergency Use Authorization of Medical Products and Related Authorities Guidance, such as the public health need for the product and the availability of the product, with the goal of expanding overall US testing capacity and patient access to tests. The FDA has, for example, prioritized review of EUA requests for tests where authorization would increase testing accessibility (e.g., point-of-care (POC) tests, home collection tests, and at-home tests) or would significantly increase testing capacity (e.g., tests that reduce reliance on test supplies and high-throughput, widely distributed tests). Tests with EUA authorization can be found on our website at In Vitro Diagnostic EUAs.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation and for regulating tobacco products.
--
Source: U.S. Food and Drug Administration (FDA)
Read Also
COVID-19: Novel biosensing platform detects antibodies within seconds
1 note
·
View note
Text
Quarantine Q&A
quarantine q & a
tagged by @edenfalling
Are you staying home from work/school? Working from home. So much working. I wrote about it on DW. I’m a regulatory consultant to the food and pharmaceutical industry with a specialty in supply chain logistics and cannabis. I’ve been working 14-16 hours a day trying to help get medical supplies from Point A to Point B, helping companies convert their operations to making hand sanitizer, dealing with the congressional COVID-19 relief legislation, poring through contracts to try to cancel them under Force Majeure clauses and many many many many conference calls. I fortunately have a colleague who is watching and summarizing the shit show that is the daily White House COVID-19 briefing. I collect all the day’s news and send it out to clients at about 11 PM. Then go to bed and dream about N95 masks from China stuck in a loading dock in Anchorage.
If you’re staying home, who’s with you? My spousal unit who is working even longer than I am, is here. He works in consumer financial protection. My spawn, is also working a lot -- finishing college junior year and an internship remotely. Plus the dogs, Thorin Corgishield and Komo.
Are you a homebody? Sort of? I have to get out at least twice a day to walk the dogs. No one else will do it though my spawn helps.
What movies have you watched recently? What shows are you watching? Very little. I’m watching Top Chef and Spring Baking Championship on the treadmill and whatever movies are on. I sort of started Westworld and Picard. I’m re-watching The Expanse with my spawn and re-watched the last season of Good Place. I’m working A LOT so there’s not much time.
Did an event that you were looking forward to get cancelled? Funny you should ask. I had travel planned to big client meetings where I was going to be making big presentations. I was really looking forward to them. Now, they are cancelled all the way into July.
What music are you listening to? I don’t listen to music, ever.
What are you reading?
HA HA. Instructions on how to import products FDA has NOT approved into the U.S. Stalking Federal Express entry information at ports around the country to track clients’ shipments. Department of Justice antitrust memos and FDA-fTC warning letters against people selling fraudulent COVID-19 products. Reading CDC and OSHA documents on facility cleaning. Reading Emergency Use Authorizations for diagnostic test kits. So many hotel contracts. Emergency stay at home and public health orders in California, Florida, and Illinois. The Defense Production Act. Guidances on how to make hand sanitizer when you aren’t a drug manufacturer. Reading hundreds of pages of the CARES Act -- not all of it just pieces. Following certain Twitter feeds for critical information -- FDA, CMS, HHS, Dr. Gottlieb, loads of others.
When I can’t stand it anymore, I go back and read some of my own fic -- or nice comments people have left. Absolute lifesavers.
What are you doing for self care? Playing Pokemon Go and Jurassic World. Working out pretty regularly. Drinking more alcohol. Walking the dogs. Bitching with my female clients. I mean -- I’m older than compost and my spawn can make food and take care of his own learning. I don’t have to work all these hours and then teach my kids too.
Hey, I’m bitching but I know I’ve got it really, really good. We have a really competent governor and I have confidence in him, I’m still employed, my spouse is still employed, we have health insurance and I’m not responsible for little kids or elderly parents. I’m trying to be present for those who are struggling.
Tag someone else!
Um. @wingedflight @larmalot @syrena-of-the-lake
14 notes
·
View notes
Text
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET Growth, Industry Size-Share, Global Trends, Key Players Strategies and Upcoming Demand
Data Bridge Market Research analyses that the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET will project a compound annual growth rate (CAGR) of 5.7% during the forecast period of 2022-2028.
A world class AFRICA POINT-OF-CARE DIAGNOSTICS MARKET research report is formulated with the finest and advanced tools of collecting, recording, estimating and analysing market data. With the systematic and comprehensive market research study, this market research report offers the facts associated with any subject in the field of marketing for Healthcare industry. It gives superior ideas and solutions in terms of product trends, marketing strategy, future products, new geographical markets, future events, sales strategies, customer actions or behaviours. This AFRICA POINT-OF-CARE DIAGNOSTICS MARKET report has been prepared by considering several fragments of the present and upcoming market scenario.
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET Scope and Market Size
The Point-of-care diagnostics market is segmented on the basis of type, platform, end user and distribution channel.
On the basis of type, the Point-of-care diagnostics market is segmented into devices, software and services, consumables and kits, and accessories. In 2021, the devices segment in the point-of-care diagnostics market is expected to grow due to changing lifestyles and increasing chronic diseases, and a lack of awareness of over-the-counter point-of-care diagnostics devices.
On the basis of platform, the point-of-care diagnostics market is segmented into lateral flow assays (immunochromatography tests), dipsticks, microfluidics, molecular diagnostics, immunoassays. In 2021, the lateral flow assays/immunochromatography tests segment in the point-of-care diagnostics market is expected to grow due to advanced technologies and inventions.
On the basis of end user, the point-of-care diagnostics market is segmented into professional diagnostic centers, home care, research laboratories and other end users. In 2021, the professional diagnostic centers segment in the point-of-care diagnostics market is expected to grow due to growing patient emphasis on diagnosis operations. However, the high investment cost may hamper the Point-of-care diagnostics market growth.

Get the Free sample copy of the report here:
Some of the key questions answered in this report:
How has the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET performed so far and how will it perform in the coming years?
What has been the impact of COVID-19 on the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET?
What are the key regional markets?
What are the key driving factors and challenges in the industry?
What is the structure of the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET and who are the key players?
Market Analysis and Insights: AFRICA POINT-OF-CARE DIAGNOSTICS MARKET
The Point of care diagnostics market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing at a CAGR of 5.7% in the forecast period of 2021 to 2028 and is expected to reach USD 816.12 million by 2028. Increasing prevalence of chronic diseases and rising preference for homecare testing are anticipated to drive the growth of the market.
The Point-of-care diagnostics is characterized as a testing which can be done in close proximity to the patient where a medical decision can be made immediately including the results and monitoring. Point-of-care diagnostics is also called with-patient testing and allows physicians to accurately measure real-time, lab-quality diagnostic results within less than minutes as compared to hours. It ensures patients to receive the most effective and efficient care when and wherever needed. Additionally, Point-of-care diagnostics enables staff to make rapid decisions of treatments when diagnosing patients’ condition and the Point-of-care diagnostics simplifying the testing process.
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET - Regional Level Analysis
The countries covered in the point-of-care diagnostics market report are Kenya, Algeria, Nigeria, Morocco, Rwanda, Zambia, Uganda, Central African Republic, Chad, Ethiopia, Mauritania, Cameroon, Angola, Libya, DRC, Ghana, Malawi.
AFRICA POINT-OF-CARE DIAGNOSTICS MARKET - Share Analysis:
Point-of-care diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to Point-of-care diagnostics market.
Key player - AFRICA POINT-OF-CARE DIAGNOSTICS MARKET
Some of the major players operating in the AFRICA POINT-OF-CARE DIAGNOSTICS MARKET are BioTech Africa, Medtronic, Bio-Rad Laboratories, Inc., PTS Diagnostics, Trinity Biotech, Atlas Medical Uk, Abbott, Sysmex Corporation, bioMérieux SA, Quidel Corporation, BD, Sekisui Diagnostics, F. Hoffmann-La Roche Ltd, Beckman Coulter, Inc., ACON Laboratories, Inc., Nova Biomedical, Siemens Healthcare S.A.E. (a subsidiary of Siemens Healthineers AG), Instrumentation Laboratory, Abaxis, and Radiometer Medical ApS (a subsidiary of Danaher) among others. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Get Full Access of Report@
https://www.databridgemarketresearch.com/reports/africa-point-of-care-diagnostics-market MAJOR TOC OF THE REPORT
Chapter One: Introduction
Chapter Two: Scope and Market Size
Chapter Three: Analysis and Insights
Chapter Four: Country Level Analysis
Chapter Five: Share Analysis
Chapter Six: Key player
Get TOC Details:
https://www.databridgemarketresearch.com/toc/?dbmr=africa-point-of-care-diagnostics-market Top Trending Reports:
Global Autoclave Market
Global Proctoscope Market
Global Molecular Methods Market
Global Wound Debridement Market
Global Human Papilloma Virus Testing Market
Global Non-Small Cell Lung Cancer Market
Global CPAP Market
Global RNA Markers Market
Global Healthcare Cloud Computing Market
Global Medical Image Management Market
Global Pharmacogenetic Testing Market
Global Hemodialysis Services Market
Global Hemostatic Wound Dressing Market
Global Interventional Radiology Products Market
Global Lab Accessories Market
Global Label-free Array Systems Market
Global Laboratory Gas Generators Market
Global Life Science Analytics Market
Global Mediastinoscopes Market
Global Medical Alert System Market
Global EDS, WDS, EBSD, Micro-XRF Instruments Market
Global Direct-to-Consumer (DTC) Genetic Testing Market
Global Q- Polymerase Chain Reaction and D- Polymerase Chain Reaction Devices Market
Asia-Pacific (APAC) Q-PCR and D-PCR Devices Market
Europe Q-PCR and D-PCR Devices Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-386.8-2818
Email: [email protected]
#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET#Global AFRICA POINT-OF-CARE DIAGNOSTICS MARKET#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET size#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET share#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET analysis#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET growth#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET demand#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET research#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET research report#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET report#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET overview#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET opportunity#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET segmentation#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET value#AFRICA POINT-OF-CARE DIAGNOSTICS MARKET insight
0 notes
Text
Macro-post #4: Our Impact.
youtube
On Thursday, November 19, 2020, my practicum group and I officially ended our practicum by presenting our work to our class. With the help of our preceptor and owner of On Target Preparedness, I feel we have fulfilled our duty of meeting our assigned competencies and deliverables given by our professor (Dr. Tillman). Below are the assigned competencies, deliverables, and ways we tackled each task.
Deliverables:
1. Design educational material and activities related to COVID-19 for distribution to Latinx/Hispanic communities in Harnett and surrounding counties
2. Collect assessment data related to the Latinx/Hispanic COVID-19 outreach efforts from various stakeholders (health departments, advocacy orgs, nonprofits, healthcare systems, state/federal
agencies, etc.)
3. Create an advocacy campaign promoting improved emergency response outreach to Hispanic/Latinx communities.
Competencies:
R1. Differentiate the impacts of social determinants of health that contribute to health disparities in rural communities as compared with urban communities
F2. Select quantitative and qualitative data collection methods appropriate for a given public health context
F7. Assess population needs, assets, and capacities that affect communities' health
F14. Advocate for political, social, or economic policies and programs that will improve health in diverse populations
F19. Communicate audience-appropriate public health content, both in writing and through oral presentation
For deliverable one, we created a Spanish and English COVID-19 informational brochure that highlighted the virus's symptoms, the importance of following safety regulations, local resources, state resources, the importance of wearing a mask, and testing sites. We decided to print more Spanish brochures than English because our target was the Hispanic/Latinx communities at our tabling events. We recruited undergraduate students from Campbell University's Hispanic Association to help us communicate efficiently who are not proficient in English. For this part of our project, Michelle and I tackled the competency F19 because we communicated with our target audience in both writing and Spanish.
My practicum group member Sophia came up with the idea of creating COVID Care Kits, which was an excellent idea because we learned that many members of the Hispanic and Latinx population did not have the proper equipment to fight the pandemic. Many did not have a reusable mask, disinfectant wipes, hand sanitizer, hand soap, and more. Although we ran into some doubt from our preceptor regarding creating the kits, we believed in our teammate and helped make it happen. We met early in the morning to go to local businesses in Montgomery County to inform the owners of our goals and asked for any donations towards the events. Initially, we did not think many people would have donated, but that was not our reality. The community was very supportive of our efforts; therefore, they donated gracefully. With our care kits, we created a bilingual COVID Coloring Book for the children to understand why it is necessary to wash their hands, explain our dangerous germs, and more. We made over 200 COVID Care Kits to give to the Hispanic/Latinx population in Montgomery County. Additionally, the Public Health Department gave us 500 reusable wipes to give out at our last tabling event. I believe this was definitely a part of our efforts to create a campaign to improve emergency response outreach to the Hispanic and Latinx populations in Montgomery County (competency F7).
Mason was the group member in charge of the tabling events. I'm glad we selected Mason because he got the job done. He contacted the two businesses where we hosted the tabling events with the help of Michelle because La Cosecha owner is a Spanish speaker. Our tabling event overall brought every deliverable together to meet our goals. At our first tabling event we noticed that many people were not wearing their mask, which was not surprising since we hypothesized that they did not have the resources.Our tabling event was hosted at a Hispanic market. We really touched many people from or targeted population. Some were sharing their stories of how they contracted the disease, how they had to pay for COVID testing, and stories of their families back home in their home country. It was definely and eye opener and really made my experience a member of OTP worth every minute. Our second tabling event was interesting. Because it was hosted at the local flea market we encounter alot of track and ended earlier than the last event. We engaged with a variety of demographic with the community. After our first event, we were given instructions to ask the people about the amount of help or information they received from their local health department. Over 80% of the people said they “did not hear anything” from the Montgomery Health Department. This was surprising because the department stated that they gave over 10,000 mask to the citizen. Being the public health students that we are we hypothesized that the department was not successfully reaching the Hispanic/Latinx populations! Tragic!
Overall, I’m grateful to have this experience to work hands-on in a community and make a small but mighty impact on the lives of others. I grew closer to my group members and learned the importance of working with others who are different. I was grateful to be the leader of ensuring our brochures got to the Montgomery County School District. Our brochure was given to over 3,000 young scholar and out of that 1,000 were Hispanic. I feel that my group has come a long way. We experienced some setbacks, but we remained motivated and got the job done. I hope OTP continues our efforts and impact many others through this pandemic.
1 note
·
View note