Don't wanna be here? Send us removal request.
Text
MIT researchers develop advanced machine learning models to detect pancreatic cancer

MIT researchers develop advanced machine learning models to detect pancreatic cancer. MIT CSAIL researchers develop advanced machine-learning models that outperform current methods in detecting pancreatic ductal adenocarcinoma. Prismatic perspectives pancreatic cancer The path forward The first documented case of pancreatic cancer dates from the 18th century. Since then, researchers have embarked on a long and difficult journey to better understand this elusive and deadly disease. To date, early intervention is the most effective cancer treatment. Unfortunately, due to its location deep within the abdomen, the pancreas is particularly difficult to detect early on. Scientists from the MIT Computer Science and Artificial Intelligence Laboratory (CSAIL), as well as Limor Appelbaum, a staff scientist in the Department of Radiation Oncology at Beth Israel Deaconess Medical Center (BIDMC), wanted to better identify potential high-risk patients. They set out to create two machine-learning models for the early detection of pancreatic ductal adenocarcinoma (PDAC), the most common type of cancer. To gain access to a large and diverse database, the team collaborated with a federated network company and used electronic health record data from multiple institutions across the United States. This vast data set contributed to the models' reliability and generalizability, making them applicable to a wide range of populations, geographical locations, and demographic groups. The two models—the “PRISM” neural network and the logistic regression model (a statistical technique for probability)—outperformed current methods. The team’s comparison showed that while standard screening criteria identify about 10 percent of PDAC cases using a five-times higher relative risk threshold, Prism can detect 35 percent of PDAC cases at this same threshold. Using AI to detect cancer risk is not a new phenomenon; algorithms analyze mammograms, CT scans for lung cancer, and assist in the analysis of Pap smear tests and HPV testing, to name a few applications. “The PRISM models stand out for their development and validation on an extensive database of over 5 million patients, surpassing the scale of most prior research in the field,” says Kai Jia, an MIT PhD student in electrical engineering and computer science (EECS), MIT CSAIL affiliate, and first author on an open-access paper in eBioMedicine outlining the new work. “The model uses routine clinical and lab data to make its predictions, and the diversity of the U.S. population is a significant advancement over other PDAC models, which are usually confined to specific geographic regions, like a few health-care centers in the U.S. Additionally, using a unique regularization technique in the training process enhanced the models' generalizability and interpretability.” “This report outlines a powerful approach to use big data and artificial intelligence algorithms to refine our approach to identifying risk profiles for cancer,” says David Avigan, a Harvard Medical School professor and the cancer center director and chief of hematology and hematologic malignancies at BIDMC, who was not involved in the study. “This approach may lead to novel strategies to identify patients with high risk for malignancy that may benefit from focused screening with the potential for early intervention.”
Prismatic perspectives pancreatic cancer
The journey toward the development of PRISM began over six years ago, fueled by firsthand experiences with the limitations of current diagnostic practices. “Approximately 80-85 percent of pancreatic cancer patients are diagnosed at advanced stages, where cure is no longer an option,” says senior author Appelbaum, who is also a Harvard Medical School instructor as well as radiation oncologist. “This clinical frustration sparked the idea to delve into the wealth of data available in electronic health records (EHRs).” The CSAIL group’s close collaboration with Appelbaum made it possible to understand the combined medical and machine learning aspects of the problem better, eventually leading to a much more accurate and transparent model. “The hypothesis was that these records contained hidden clues — subtle signs and symptoms that could act as early warning signals of pancreatic cancer,” she adds. “This guided our use of federated EHR networks in developing these models, for a scalable approach for deploying risk prediction tools in health care.” Both PrismNN and PrismLR models analyze EHR data, including patient demographics, diagnoses, medications, and lab results, to assess PDAC risk. PrismNN uses artificial neural networks to detect intricate patterns in data features like age, medical history, and lab results, yielding a risk score for PDAC likelihood. PrismLR uses logistic regression for a simpler analysis, generating a probability score of PDAC based on these features. Together, the models offer a thorough evaluation of different approaches in predicting PDAC risk from the same EHR data. One paramount point for gaining the trust of physicians, the team notes, is better understanding how the models work, known in the field as interpretability. The scientists pointed out that while logistic regression models are inherently easier to interpret, recent advancements have made deep neural networks somewhat more transparent. This helped the team to refine the thousands of potentially predictive features derived from EHR of a single patient to approximately 85 critical indicators. These indicators, which include patient age, diabetes diagnosis, and an increased frequency of visits to physicians, are automatically discovered by the model but match physicians' understanding of risk factors associated with pancreatic cancer.
The path forward
Despite the promise of the PRISM models, as with all research, some parts are still a work in progress. U.S. data alone are the current diet for the models, necessitating testing and adaptation for global use. The path forward, the team notes, includes expanding the model's applicability to international datasets and integrating additional biomarkers for more refined risk assessment. “A subsequent aim for us is to facilitate the models' implementation in routine health care settings. The vision is to have these models function seamlessly in the background of health care systems, automatically analyzing patient data and alerting physicians to high-risk cases without adding to their workload,” says Jia. “A machine-learning model integrated with the EHR system could empower physicians with early alerts for high-risk patients, potentially enabling interventions well before symptoms manifest. We are eager to deploy our techniques in the real world to help all individuals enjoy longer, healthier lives.” Jia wrote the paper alongside Applebaum and MIT EECS Professor and CSAIL Principal Investigator Martin Rinard, who are both senior authors of the paper. Researchers on the paper were supported during their time at MIT CSAIL, in part, by the Defense Advanced Research Projects Agency, Boeing, the National Science Foundation, and Aarno Labs. TriNetX provided resources for the project, and the Prevent Cancer Foundation also supported the team. Source: MIT Read the full article
0 notes
Text
Inhalable sensors could enable early lung cancer detection
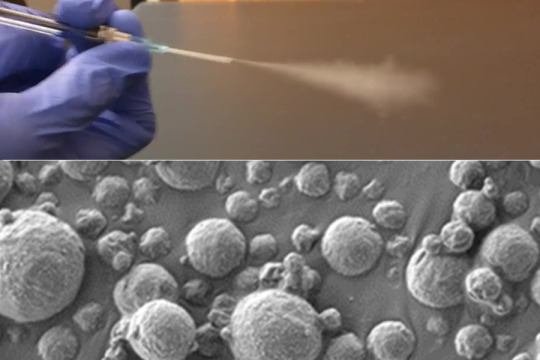
Inhalable sensors could enable early lung cancer detection. The diagnostic, which requires only a simple urine test to read the results, could make lung cancer screening more accessible worldwide. Inhalable particles Accurate diagnosis MIT, January 5, 2024 - Using a new MIT technology, diagnosing lung cancer could be as simple as inhaling nanoparticle sensors and then taking a urine test to see if a tumor is present. The new diagnostic uses nanosensors that can be delivered via inhaler or nebulizer. When the sensors come into contact with cancer-linked proteins in the lungs, they generate a signal that accumulates in the urine and can be detected with a simple paper test strip. This method has the potential to replace or supplement the current gold standard for lung cancer diagnosis, low-dose computed tomography (CT). According to the researchers, it could have a particularly large impact in low- and middle-income countries where CT scanners are not widely available. “Around the world, cancer is going to become more and more prevalent in low- and middle-income countries. The epidemiology of lung cancer globally is that it’s driven by pollution and smoking, so we know that those are settings where accessibility to this kind of technology could have a big impact,” says Sangeeta Bhatia, the John and Dorothy Wilson Professor of Health Sciences and Technology and of Electrical Engineering and Computer Science at MIT, and a member of MIT’s Koch Institute for Integrative Cancer Research and the Institute for Medical Engineering and Science. Bhatia is the senior author of the paper, which appears today in Science Advances. Qian Zhong, an MIT research scientist, and Edward Tan, a former MIT postdoc, are the lead authors of the study.
Inhalable particles
To help diagnose lung cancer as early as possible, the U.S. Preventive Services Task Force recommends that heavy smokers over the age of 50 undergo annual CT scans. However, not everyone in this target group receives these scans, and the high false-positive rate of the scans can lead to unnecessary, invasive tests. Bhatia has spent the last decade developing nanosensors for use in diagnosing cancer and other diseases, and in this study, she and her colleagues explored the possibility of using them as a more accessible alternative to CT screening for lung cancer. These sensors consist of polymer nanoparticles coated with a reporter, such as a DNA barcode, that is cleaved from the particle when the sensor encounters enzymes called proteases, which are often overactive in tumors. Those reporters eventually accumulate in the urine and are excreted from the body. Previous versions of the sensors, which targeted other cancer sites such as the liver and ovaries, were designed to be given intravenously. For lung cancer diagnosis, the researchers wanted to create a version that could be inhaled, which could make it easier to deploy in lower resource settings. “When we developed this technology, our goal was to provide a method that can detect cancer with high specificity and sensitivity, and also lower the threshold for accessibility, so that hopefully we can improve the resource disparity and inequity in early detection of lung cancer,” Zhong says To achieve that, the researchers created two formulations of their particles: a solution that can be aerosolized and delivered with a nebulizer, and a dry powder that can be delivered using an inhaler. Once the particles reach the lungs, they are absorbed into the tissue, where they encounter any proteases that may be present. Human cells can express hundreds of different proteases, and some of them are overactive in tumors, where they help cancer cells to escape their original locations by cutting through proteins of the extracellular matrix. These cancerous proteases cleave DNA barcodes from the sensors, allowing the barcodes to circulate in the bloodstream until they are excreted in the urine. In the earlier versions of this technology, the researchers used mass spectrometry to analyze the urine sample and detect DNA barcodes. However, mass spectrometry requires equipment that might not be available in low-resource areas, so for this version, the researchers created a lateral flow assay, which allows the barcodes to be detected using a paper test strip. The researchers designed the strip to detect up to four different DNA barcodes, each of which indicates the presence of a different protease. No pre-treatment or processing of the urine sample is required, and the results can be read about 20 minutes after the sample is obtained. “We were really pushing this assay to be point-of-care available in a low-resource setting, so the idea was to not do any sample processing, not do any amplification, just to be able to put the sample right on the paper and read it out in 20 minutes,” Bhatia says.
Accurate diagnosis
The researchers put their diagnostic system to the test in mice that have been genetically modified to develop lung tumors similar to those seen in humans. The sensors were given to the mice 7.5 weeks after the tumors formed, which corresponds to stage 1 or 2 cancer in humans. The researchers measured the levels of 20 different sensors designed to detect different proteases in their first set of experiments in mice. The researchers identified a combination of only four sensors that was predicted to provide accurate diagnostic results using a machine learning algorithm. They then tested that combination in a mouse model and discovered that it could detect early-stage lung tumors accurately. For use in humans, it’s possible that more sensors might be needed to make an accurate diagnosis, but that could be achieved by using multiple paper strips, each of which detects four different DNA barcodes, the researchers say. The researchers now plan to analyze human biopsy samples to see if the sensor panels they are using would also work to detect human cancers. In the longer term, they hope to perform clinical trials in human patients. A company called Sunbird Bio has already run phase 1 trials on a similar sensor developed by Bhatia’s lab, for use in diagnosing liver cancer and a form of hepatitis known as nonalcoholic steatohepatitis (NASH). In parts of the world where there is limited access to CT scanning, this technology could offer a dramatic improvement in lung cancer screening, especially since the results can be obtained during a single visit. “The idea would be you come in and then you get an answer about whether you need a follow-up test or not, and we could get patients who have early lesions into the system so that they could get curative surgery or lifesaving medicines,” Bhatia says The research was funded by the Johnson & Johnson Lung Cancer Initiative, the Howard Hughes Medical Institute, the Koch Institute Support (core) Grant from the National Cancer Institute, and the National Institute of Environmental Health Sciences. Source: MIT Read the full article
0 notes
Text
MIT Engineers develop a vibrating, ingestible capsule that might help treat obesity
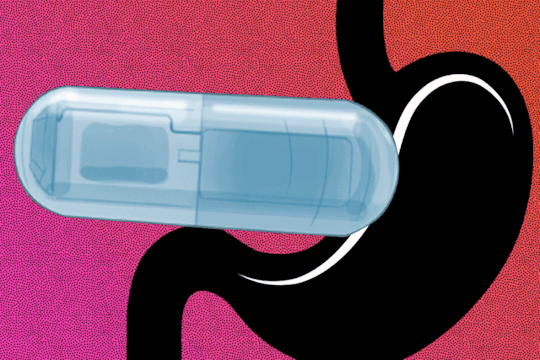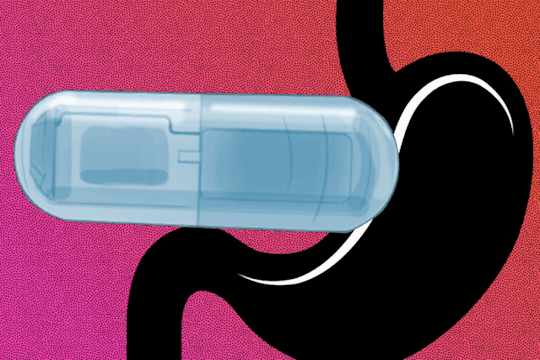
MIT Engineers develop a vibrating, ingestible capsule that might help treat obesity Swallowing the device before a meal could create a sense of fullness, tricking the brain into thinking it’s time to stop eating. When you eat a large meal, your stomach sends signals to your brain that cause you to feel full, allowing you to realize it's time to stop eating. These messages can also be sent by a stomach full of liquid, which is why dieters are often advised to drink a glass of water before eating. MIT engineers have developed a new method for capitalizing on this phenomenon, employing an ingestible capsule that vibrates within the stomach. These vibrations activate the same stretch receptors that detect when the stomach is distended, giving the illusion of fullness. The researchers discovered that giving this pill to animals 20 minutes before eating not only stimulated the release of hormones that signal satiety, but also reduced the animals' food intake by about 40%. Scientists still have a lot to learn about the mechanisms that influence human body weight, but if further research shows that this technology can be used safely in humans, such a pill could offer a minimally invasive way to treat obesity, according to the researchers. “For somebody who wants to lose weight or control their appetite, it could be taken before each meal,” says Shriya Srinivasan PhD ’20, a former MIT graduate student and postdoc who is now an assistant professor of bioengineering at Harvard University. “This could be really interesting in that it would provide an option that could minimize the side effects that we see with the other pharmacological treatments out there.” Srinivasan is the lead author of the new study, which appears today in Science Advances. Giovanni Traverso, an associate professor of mechanical engineering at MIT and a gastroenterologist at Brigham and Women’s Hospital, is the senior author of the paper.
A sense of fullness
When the stomach stretches, specialized cells known as mechanoreceptors detect it and send signals to the brain via the vagus nerve. As a result, the brain increases the production of insulin as well as other hormones like C-peptide, Pyy, and GLP-1. All of these hormones work together to aid digestion, feeling full, and stopping eating. At the same time, ghrelin, a hunger-promoting hormone, decreases. Srinivasan became interested in controlling this process as a graduate student at MIT by artificially stretching the mechanoreceptors that line the stomach with vibration. Previous research had shown that applying vibration to a muscle can create the illusion that the muscle has stretched further than it actually has. “I wondered if we could activate stretch receptors in the stomach by vibrating them and having them perceive that the entire stomach has been expanded, to create an illusory sense of distension that could modulate hormones and eating patterns,” Srinivasan says. As a postdoc at MIT's Koch Institute for Integrative Cancer Research, Srinivasan collaborated closely with Traverso's lab, which has pioneered many novel approaches to drug and electronic device delivery. Srinivasan, Traverso, and their colleagues created a capsule the size of a multivitamin that contains a vibrating element for this study. When the pill, which is powered by a small silver oxide battery, enters the stomach, acidic gastric fluids dissolve the capsule's gelatinous membrane, completing the electronic circuit that activates the vibrating motor. In an animal study, the researchers discovered that when the pill begins to vibrate, it activates mechanoreceptors, which send signals to the brain via vagus nerve stimulation. The researchers monitored hormone levels while the device was vibrating and discovered that they mirrored hormone release patterns seen after a meal, even when the animals had fasted. The researchers then examined how this stimulation affected the animals' appetites. They discovered that when the pill was activated for about 20 minutes before the animals were given food, they consumed 40% less on average than when it was not activated. The animals also gained weight at a slower rate when they were given the vibrating pill. “The behavioral change is profound, and that’s using the endogenous system rather than any exogenous therapeutic. We have the potential to overcome some of the challenges and costs associated with delivery of biologic drugs by modulating the enteric nervous system,” Traverso says. The current version of the pill is designed to vibrate for about 30 minutes after arriving in the stomach, but the researchers plan to explore the possibility of adapting it to remain in the stomach for longer periods of time, where it could be turned on and off wirelessly as needed. In the animal studies, the pills passed through the digestive tract within four or five days. The study also found that the animals did not show any signs of obstruction, perforation, or other negative impacts while the pill was in their digestive tract.
An alternative approach
According to the researchers, this type of pill could provide an alternative to current approaches to treating obesity. Nonmedical interventions such as diet and exercise are not always effective, and many existing medical interventions are quite invasive. Gastric bypass surgery and gastric balloons, which are no longer widely used in the United States due to safety concerns, are examples. Drugs such as GLP-1 agonists can also help with weight loss, but the majority of them must be injected and are therefore out of reach for many people. According to Srinivasan, the MIT capsules could be produced at a low enough cost that they would be accessible to people who do not have access to more expensive treatment options. “For a lot of populations, some of the more effective therapies for obesity are very costly. At scale, our device could be manufactured at a pretty cost-effective price point,” she says. “I’d love to see how this would transform care and therapy for people in global health settings who may not have access to some of the more sophisticated or expensive options that are available today.” The researchers now plan to explore ways to scale up the manufacturing of the capsules, which could enable clinical trials in humans. Such studies would be important to learn more about the devices’ safety, as well as determine the best time to swallow the capsule before to a meal and how often it would need to be administered. Other authors of the paper include Amro Alshareef, Alexandria Hwang, Ceara Byrne, Johannes Kuosmann, Keiko Ishida, Joshua Jenkins, Sabrina Liu, Wiam Abdalla Mohammed Madani, Alison Hayward, and Niora Fabian. The research was funded by the National Institutes of Health, Novo Nordisk, the Department of Mechanical Engineering at MIT, a Schmidt Science Fellowship, and the National Science Foundation. Read the full article
0 notes
Text
Samsung Announces New Medications Tracking Feature for Samsung Health App

Samsung Announces New Medications Tracking Feature for Samsung Health App. Users can now utilize the Samsung Health app to conveniently track medication regimens and receive useful tips about intake Samsung Electronics today announced a new Medications tracking feature that will be added to the Samsung Health app to help users manage their health more comprehensively. The new feature will help users easily keep track of both their prescription and over-the-counter medications and provide important, relevant information and tips about these medications. In particular, it can assist those who take medications regularly and those taking supplements for general well-being. “Samsung Health aims to help people better understand and manage their health through a holistic platform by connecting devices, services and people,” said Hon Pak, Vice President and Head of Digital Health Team, MX Business at Samsung Electronics. “With the addition of the new Medications tracking feature, we believe users will be able to more conveniently manage their medications, improve adherence, and ultimately maintain better health overall.” Upon entering the name of a select medication into Samsung Health, the Medications feature will provide users with detailed information that includes general descriptions as well as possible side effects. Adverse reactions that could occur from drug-to-drug interactions, or if taken alongside certain food and substances such as caffeine and alcohol, are also provided. One example of this is that if a user is taking the prescription drug Simvastatin, Samsung Health will warn the user that the drug has been linked to serious side effects when combined with grapefruit juice. Users can even log the shape and color of their medications, allowing them to easily differentiate between the pills they are taking. Dosage, time of consumption and other details can also be added to avoid any potential confusion. Users can set up alerts that remind them both when to take their medications and when they should consider refilling them. These alerts are fine-tuned to the individual user so the Medications feature is able to prioritize medications depending on their importance, with Samsung Health sending reminders ranging from “gentle” to “strong” depending on how important or urgent a given prescription is. For crucial medications, users can set a “strong” reminder that will display a full-screen alert on their smartphone accompanied by a long tone. For supplements like vitamins, a simple pop-up reminder will appear that will not disturb the user. Galaxy Watch users will also receive reminders right on their wrist so they can stay on top of their medication schedules, even when away from their phones. The Samsung Health app provides a range of advanced health offerings spanning sleep management, mindfulness programs and irregular heart rhythm detection capabilities. With the addition of the Medications tracking feature, Samsung Health delivers a truly holistic wellness experience that helps users maintain healthier lifestyles while keeping on top of their medication regimens. The Medications tracking feature will first be available on Samsung Health app in the U.S. via the app updates rolling out later this week. Read the full article
0 notes
Text
Bolivia’s hypergravity blood cell test for astronaut health

Bolivia’s hypergravity blood cell test for astronaut health. An all-female team from Bolivia is the latest international group to use ESA's hypergravity-generating Large Diameter Centrifuge, with access sponsored by the United Nations and ESA. The researchers are looking into whether the high gravity levels experienced during rocket launches contribute to the anemia that many astronauts suffer from in space. Based at ESA’s ESTEC technical canter in the Netherlands, the LDC is an 8-meter-diameter, four-arm centrifuge that gives researchers access to a range of hypergravity up to 20 times Earth gravity for weeks or months at a time. Access to the LDC was arranged through HyperGES, part of the Access to Space for All initiative sponsored by ESA and the United Nations Office of Outer Space Affairs, UNOOSA. At its fastest, the centrifuge rotates at up to 67 revs per minute, with its six gondolas placed at different points along its arms weighing in at 130 kg, and each capable of accommodating 80 kg of payload.

ESA's Large Diameter Centrifuge The five-strong all-female team from Universidad Católica Boliviana ‘San Pablo’ in La Paz, Bolivia, have spent two weeks studying the effects of hypergravity on red blood cells. Astronauts are known to experience numerous adverse health effects while in space. Among them – observed since the earliest days of orbital flight – is anemia, which can cause dizziness and weakness. Recent research shows this anemia is linked to ‘hemolysis'—in’ – in plain words that red blood cells are destroyed at a much higher rate than the usual recycling of old and damaged cells. This finding became the starting point for the team’s research.

Preparing_samples_for_testing Team leader Dr. Georgina Chávez explains: “As part of a biology class I assigned a very simple task which many people might do in school: testing how osmosis affects red blood cells. By putting low-salinity ‘hypotonic’ solution in the fluid surrounding the red blood cells, then water moves into the cells and they eventually burst. We saw the paper in Nature on the hemolytic anemia suffered by astronauts, and the parallels with our testing raised our interest. It is possible that hypergravity exposure causes cellular membranes to become fragile, leading to hemolysis.

Red_blood_cells_undergoing_hemolysis_after_hypergravity_exposure “What is well known is that the distribution of body fluid changes in weightlessness, so this might promote the same kind of destruction of red blood cells. But most of the existing studies have been done in microgravity. We thought, well actually astronauts go through two big bodily changes during spaceflight: to make it into microgravity they first experience a brief but intense period of hypergravity. So we decided to investigate the effects that this initial phase might have in terms of leading to hemolysis in space later.”

Samples_placed_in_LDC For hypergravity testing the team prepared various levels of hypotonic solutions to perform ‘osmotic fragility testing’ on samples that were exposed to 7.5 and 15 Earth gravities as well as normal gravity as a control. The hypergravity spins continue for differing test periods of 10 minutes, 30 minutes and 60 minutes, with samples stored at temperatures of 25°C and body temperature. “It typically takes a few minutes of high-gravity rocket flight to make it into orbit,” notes team member Daira Quenta. “We want to see how the amount of time spent in hypergravity might make a difference.”

Hypergravity_centrifuge_in_motion The team had been inspired by previous Bolivian participation in other UNOOSA programs, notably the DropTES (Drop Tower Experiment Series) which enables international researchers to utilize the ZARM drop tower in Bremen, Germany, with a 146-m shaft for brief microgravity testing. “We made the connection – if they can do it, we can do it. The opportunity got us motivated.” Team member Marcia Carrasco comments “We’re training to become biochemistry and bioprocess engineers, which is really quite vocational,” explains team member Belen Flores. “This career encompasses a wide range of options, we might for instance end up working for the pharmaceuticals sector or food companies. We don’t typically have the chance undertake scientific investigations like this, so this has been exciting.” “We are an all-female team, which wasn’t intentional, but they are all brilliant students, and it’s fair to say females are a bit more organised!” Dr. Chavez adds “We didn’t think too much about gender at the time, but we have been part of some important initiatives subsequently, including Matilda, an organisation encouraging female Latin America students to go into STEM subjects. And we’ve been featured on Pica, which is a popular TV show for Bolivian teenagers, raising a lot of interest.” Team member Natalia Agramont says Source: ESA Read the full article
0 notes
Text
MIT engineers design a robotic replica of the heart’s right chamber

MIT engineers design a robotic replica of the heart’s right chamber. The realistic model could aid the development of better heart implants and shed light on understudied heart disorders. Robotic Replica - A ballet of beats A heart’s shelf-life December 08, 2023 - MIT engineers have created a robotic replica of the right ventricle of the heart that mimics the beating and blood-pumping action of a living heart. The robo-ventricle is made up of real heart tissue and synthetic, balloon-like artificial muscles that allow scientists to control the ventricle's contractions while also observing how its natural valves and other intricate structures work. The artificial ventricle can be programmed to simulate both healthy and diseased states. The researchers manipulated the model to simulate right ventricular dysfunction conditions such as pulmonary hypertension and myocardial infarction. The model was also used to test cardiac devices. For example, the researchers implanted a mechanical valve to repair a naturally malfunctioning valve, then observed how the ventricle's pumping changed as a result. They claim that the new robotic right ventricle, or RRV, can be used as a realistic platform for studying right ventricle disorders and testing devices and therapies to treat them. “The right ventricle is particularly susceptible to dysfunction in intensive care unit settings, especially in patients on mechanical ventilation,” says Manisha Singh, a postdoc at MIT’s Institute for Medical Engineering and Science (IMES). “The RRV simulator can be used in the future to study the effects of mechanical ventilation on the right ventricle and to develop strategies to prevent right heart failure in these vulnerable patients.” Singh and her colleagues report details of the new design in an open-access paper appearing today in Nature Cardiovascular Research. Her co-authors include Associate Professor Ellen Roche, who is a core member of IMES and the associate head for research in the Department of Mechanical Engineering at MIT; along with Jean Bonnemain, Caglar Ozturk, Clara Park, Diego Quevedo-Moreno, Meagan Rowlett, and Yiling Fan of MIT; Brian Ayers of Massachusetts General Hospital; Christopher Nguyen of Cleveland Clinic; and Mossab Saeed of Boston Children’s Hospital.
Robotic Replica - A ballet of beats
The right ventricle is one of the heart’s four chambers, along with the left ventricle and the left and right atria. Of the four chambers, the left ventricle is the heavy lifter, as its thick, cone-shaped musculature is built for pumping blood through the entire body. The right ventricle, Roche says, is a “ballerina” in comparison, as it handles a lighter though no-less-crucial load. “The right ventricle pumps deoxygenated blood to the lungs, so it doesn’t have to pump as hard,” Roche notes. “It’s a thinner muscle, with more complex architecture and motion.” This anatomical complexity has made it difficult for clinicians to accurately observe and assess right ventricle function in patients with heart disease. “Conventional tools often fail to capture the intricate mechanics and dynamics of the right ventricle, leading to potential misdiagnoses and inadequate treatment strategies,” Singh says To improve understanding of the lesser-known chamber and speed the development of cardiac devices to treat its dysfunction, the team designed a realistic, functional model of the right ventricle that both captures its anatomical intricacies and reproduces its pumping function. The model includes real heart tissue, which the team chose to incorporate because it retains natural structures that are too complex to reproduce synthetically. “There are thin, tiny chordae and valve leaflets with different material properties that are all moving in concert with the ventricle’s muscle. Trying to cast or print these very delicate structures is quite challenging,” Roche explains
A heart’s shelf-life
In the new study, the team reports explanting a pig’s right ventricle, which they treated to carefully preserve its internal structures. They then fit a silicone wrapping around it, which acted as a soft, synthetic myocardium, or muscular lining. Within this lining, the team embedded several long, balloon-like tubes, which encircled the real heart tissue, in positions that the team determined through computational modeling to be optimal for reproducing the ventricle’s contractions. The researchers connected each tube to a control system, which they then set to inflate and deflate each tube at rates that mimicked the heart’s real rhythm and motion. To test its pumping ability, the team infused the model with a liquid similar in viscosity to blood. This particular liquid was also transparent, allowing the engineers to observe with an internal camera how internal valves and structures responded as the ventricle pumped liquid through. They found that the artificial ventricle’s pumping power and the function of its internal structures were similar to what they previously observed in live, healthy animals, demonstrating that the model can realistically simulate the right ventricle’s action and anatomy. The researchers could also tune the frequency and power of the pumping tubes to mimic various cardiac conditions, such as irregular heartbeats, muscle weakening, and hypertension. “We’re reanimating the heart, in some sense, and in a way that we can study and potentially treat its dysfunction,” Roche says To show that the artificial ventricle can be used to test cardiac devices, the team surgically implanted ring-like medical devices of various sizes to repair the chamber’s tricuspid valve — a leafy, one-way valve that lets blood into the right ventricle. When this valve is leaky, or physically compromised, it can cause right heart failure or atrial fibrillation, and leads to symptoms such as reduced exercise capacity, swelling of the legs and abdomen, and liver enlargement. The researchers surgically manipulated the robo-ventricle’s valve to simulate this condition, then either replaced it by implanting a mechanical valve or repaired it using ring-like devices of different sizes. They observed which device improved the ventricle’s fluid flow as it continued to pump. “With its ability to accurately replicate tricuspid valve dysfunction, the RRV serves as an ideal training ground for surgeons and interventional cardiologists,” Singh says. “They can practice new surgical techniques for repairing or replacing the tricuspid valve on our model before performing them on actual patients.” Currently, the RRV can simulate realistic function over a few months. The team is working to extend that performance and enable the model to run continuously for longer stretches. They are also working with designers of implantable devices to test their prototypes on the artificial ventricle and possibly speed their path to patients. And looking far in the future, Roche plans to pair the RRV with a similar artificial, functional model of the left ventricle, which the group is currently fine-tuning. “We envision pairing this with the left ventricle to make a fully tunable, artificial heart, that could potentially function in people,” Roche says. “We’re quite a while off, but that’s the overarching vision.” This research was supported, in part, by the National Science Foundation. Source: MIT Read the full article
1 note
·
View note
Text
NVIDIA BioNeMo Enables Generative AI for Drug Discovery on AWS
NVIDIA BioNeMo Enables Generative AI for Drug Discovery on AWS. Pharma and techbio companies can access the NVIDIA Clara healthcare suite, including BioNeMo, now via Amazon SageMaker and AWS ParallelCluster, and the NVIDIA DGX Cloud on AWS. New to AWS: NVIDIA BioNeMo Advances Generative AI for Drug Discovery Also Available on AWS: NVIDIA Clara for Medical Imaging and Genomics November 28, 2023 - Leading pharmaceutical and biotech companies' researchers and developers can now easily deploy NVIDIA Clara software and services for accelerated healthcare via Amazon Web Services. The initiative, announced today at AWS re:Invent, allows healthcare and life sciences developers who use AWS cloud resources to integrate NVIDIA-accelerated offerings such as NVIDIA BioNeMo—a generative AI platform for drug discovery—which is coming to NVIDIA DGX Cloud on AWS and is currently available via the AWS ParallelCluster cluster management tool for high-performance computing and the Amazon SageMaker machine learning service. AWS is used by thousands of healthcare and life sciences companies worldwide. They can now use BioNeMo to build or customize digital biology foundation models with proprietary data, scaling up model training and deployment on AWS using NVIDIA GPU-accelerated cloud servers. Alchemab Therapeutics, Basecamp Research, Character Biosciences, Evozyne, Etcembly, and LabGenius are among the AWS users who have already started using BioNeMo for generative AI-accelerated drug discovery and development. This collaboration provides them with additional options for rapidly scaling up cloud computing resources for developing generative AI models trained on biomolecular data. This announcement extends NVIDIA’s existing healthcare-focused offerings available on AWS — NVIDIA MONAI for medical imaging workflows and NVIDIA Parabricks for accelerated genomics.
New to AWS: NVIDIA BioNeMo Advances Generative AI for Drug Discovery
BioNeMo is a domain-specific framework for digital biology generative AI, including pretrained large language models (LLMs), data loaders, and optimized training recipes that can help advance computer-aided drug discovery by speeding target identification, protein structure prediction, and drug candidate screening. Drug discovery teams can use their proprietary data to build or optimize models with BioNeMo and run them on cloud-based high-performance computing clusters. One of these models, ESM-2, a powerful LLM that supports protein structure prediction, achieves almost linear scaling on 256 NVIDIA H100 Tensor Core GPUs. Researchers can scale to 512 H100 GPUs to complete training in a few days instead of a month, the training time published in the original paper. Developers can train ESM-2 at scale using checkpoints of 650 million or 3 billion parameters. Additional AI models supported in the BioNeMo training framework include small-molecule generative model MegaMolBART and protein sequence generation model ProtT5. BioNeMo’s pretrained models and optimized training recipes — which are available using self-managed services like AWS ParallelCluster and Amazon ECS as well as integrated, managed services through NVIDIA DGX Cloud and Amazon SageMaker — can help R&D teams build foundation models that can explore more drug candidates, optimize wet lab experimentation and find promising clinical candidates faster
Also Available on AWS: NVIDIA Clara for Medical Imaging and Genomics
Project MONAI, cofounded and enterprise-supported by NVIDIA to support medical imaging workflows, has been downloaded more than 1.8 million times and is available for deployment on AWS. Developers can harness their proprietary healthcare datasets already stored on AWS cloud resources to rapidly annotate and build AI models for medical imaging. These models, trained on NVIDIA GPU-powered Amazon EC2 instances, can be used for interactive annotation and fine-tuning for segmentation, classification, registration, and detection tasks in medical imaging. Developers can also harness the MRI image synthesis models available in MONAI to augment training datasets. To accelerate genomics pipelines, Parabricks enables variant calling on a whole human genome in around 15 minutes, compared to a day on a CPU-only system. On AWS, developers can quickly scale up to process large amounts of genomic data across multiple GPU nodes. More than a dozen Parabricks workflows are available on AWS HealthOmics as Ready2Run workflows, which enable customers to easily run pre-built pipelines. Read the full article
0 notes
Text
A new ultrasound wearable device can measure how full your bladder is

A new ultrasound wearable device can measure how full your bladder is The wearable device, designed to monitor bladder and kidney health, could be adapted for earlier diagnosis of cancers deep within the body. Ultrasound wearable device - Wearable monitoring Bladder volume MIT researchers have created a patch-like wearable ultrasound monitor that can image organs within the body without the use of an ultrasound operator or the application of gel. The researchers demonstrated that their patch can accurately image the bladder and determine how full it is in a new study. According to the researchers, this could make it easier for patients with bladder or kidney disorders to determine whether these organs are functioning properly. This method could also be used to monitor other organs within the body by relocating the ultrasound array and adjusting the frequency of the signal. Such devices may be able to detect cancers that form deep within the body, such as ovarian cancer, earlier. “This technology is versatile and can be used not only on the bladder but any deep tissue of the body. It’s a novel platform that can do identification and characterization of many of the diseases that we carry in our body,” says Canan Dagdeviren, an associate professor in MIT’s Media Lab and the senior author of the study. Lin Zhang, an MIT research scientist; Colin Marcus, an MIT graduate student in electrical engineering and computer science; and Dabin Lin, a professor at Xi’an Technological University, are the lead authors of a paper describing the work, which appears today in Nature Electronics.
Ultrasound wearable device - Wearable monitoring
Dagdeviren's lab, which specializes in the development of flexible, wearable electronic devices, recently created an ultrasound monitor that can be incorporated into a bra and used to screen for breast cancer. The team used a similar approach in the new study to develop a wearable patch that can adhere to the skin and take ultrasound images of organs located within the body. The researchers decided to focus on the bladder for their first demonstration, partly inspired by Dagdeviren's younger brother, who was diagnosed with kidney cancer a few years ago. He had difficulty completely emptying his bladder after having one of his kidneys surgically removed. Dagdeviren wondered if an ultrasound monitor that shows how full the bladder is could help patients like her brother or others with bladder or kidney problems. “Millions of people are suffering from bladder dysfunction and related diseases, and not surprisingly, bladder volume monitoring is an effective way to assess your kidney health and wellness,” she says Currently, the only way to measure bladder volume is to visit a medical facility and use a traditional, bulky ultrasound probe. Dagdeviren and her colleagues wanted to create a wearable option for patients to use at home. To accomplish this, the researchers created a flexible patch of silicone rubber embedded with five ultrasound arrays made from a new piezoelectric material developed specifically for this device. The arrays are arranged in the shape of a cross, allowing the patch to image the entire bladder, which measures approximately 12 by 8 centimeters when full. The patch's polymer is naturally sticky and adheres gently to the skin, making it simple to attach and detach. When applied to the skin, underwear or leggings can help keep it in place.
Bladder volume
The researchers demonstrated that the new patch could capture images comparable to those taken with a traditional ultrasound probe in a study conducted with collaborators from the Center for Ultrasound Research and Translation and the Department of Radiology at Massachusetts General Hospital and that these images could be used to track changes in bladder volume. The researchers recruited 20 patients with varying BMIs for the study. The subjects were photographed with a full bladder, then a partially empty bladder, and finally a completely empty bladder. The new patch produced images of comparable quality to traditional ultrasound, and the ultrasound arrays worked on all subjects regardless of body mass index. Because the field of view is large enough to encompass the entire bladder, no ultrasound gel or pressure is required when using this patch, as with a regular ultrasound probe. The researchers connected their ultrasound arrays to the same type of ultrasound machine used in medical imaging centers to view the images. The MIT team is now developing a portable device the size of a smartphone that could be used to view the images. “In this work, we have further developed a path toward clinical translation of conformable ultrasonic biosensors that yield valuable information about vital physiologic parameters. Our group hopes to build on this and develop a suite of devices that will ultimately bridge the information gap between clinicians and patients,” says Anthony E. Samir, director of the MGH Center for Ultrasound Research and Translation and Associate Chair of Imaging Sciences at MGH Radiology, who is also an author of the study The MIT team also hopes to create ultrasound devices that can image other organs in the body, such as the pancreas, liver, or ovaries. The frequency of the ultrasound signal must be adjusted based on the location and depth of each organ, which necessitates the development of new piezoelectric materials. For some of these deep-seated organs, the device may be more effective as an implant rather than a patch. “For whatever organ that we need to visualize, we go back to the first step, select the right materials, come up with the right device design and then fabricate everything accordingly,” before testing the device and performing clinical trials, Dagdeviren says “This work could develop into a central area of focus in ultrasound research, motivate a new approach to future medical device designs, and lay the groundwork for many more fruitful collaborations between materials scientists, electrical engineers, and biomedical researchers,” says Anantha Chandrakasan, dean of MIT’s School of Engineering, the Vannevar Bush Professor of Electrical Engineering and Computer Science, and an author of the paper. The research was funded by a National Science Foundation CAREER award, a 3M Non-Tenured Faculty Award, the Sagol Weizmann-MIT Bridge Program, Texas Instruments Inc., the MIT Media Lab Consortium, a National Science Foundation Graduate Research Fellowship, and an ARRS Scholar Award. Source: MIT Read the full article
0 notes
Text
Mobile healthcare dominates as most commonly used component in decentralized clinical trials, reveals GlobalData

Mobile healthcare dominates as most commonly used component in decentralized clinical trials, according to GlobalData. The COVID-19 pandemic catalyzed the adoption of decentralized clinical trials (DCTs) even though they have been in use for decades. DCTs offer the advantage of enabling patients to take part in clinical studies from the convenience of their homes, eliminating the need for them to travel to clinical sites. This not only reduces the burden on patients but also leads to higher participation rates. Against this backdrop, out of the virtual components used in clinical trials, mobile healthcare is the most commonly used component, with 47% of DCTs using this element, reveals GlobalData, a leading data and analytics company. GlobalData’s latest report “Thematic Intelligence: Digital Transformation and Emerging Technologies in the Healthcare Industry,” reveals that web-based technology is the second most commonly used virtual component, with 23% of DCTs using it for activities such as electronic data collection (eCOA, eConsent, eDiary, ePRO, and questionnaire). Mobile healthcare consists of activities such as remote patient monitoring, remote drug delivery, telemedicine, and home nursing.

“As technologies continue to improve, it has become easier to collect, transfer, and store data electronically. After the COVID-19 pandemic, there has been a drastic increase in patients becoming more comfortable with using gadgets. As people were forced to adapt to social distancing measures and lockdowns, many became more comfortable with using technologies for various purposes, including healthcare.” Shiva Narayana, Associate Project Manager, Pharma at GlobalData, comments: Progress in network technologies, connected gadgets, medical wearables, sensors, data analytics algorithms, and software is reshaping the healthcare and clinical trial environments. These breakthroughs are facilitating the more effective gathering of data and the provision of advanced healthcare through a variety of means. With more patients using technologies such as wearable devices, smartphone apps, or other remote monitoring tools to collect and transmit data such as vital signs, medication adherence, and symptoms, clinicians have an opportunity to access real-world data and gain timely insights. “By introducing virtual components, the study sponsors or clinical research organizations (CROs) can drive more cost-effective and efficient clinical trials. With fewer geographical constraints and increased patient engagement, decentralized trials can potentially be completed more quickly. However, there are also challenges and considerations in implementing decentralization in clinical trials, such as ensuring data privacy and security, addressing the digital divide, and maintaining the integrity of the study.” Shiva Narayana concludes Read the full article
0 notes
Text
'Lab on a chip' genetic test device can identify viruses within three minutes

'Lab on a chip' genetic test device can identify viruses within three minutes with top-level accuracy. Lab on a chip - How LoCKAmp works Scope to track outbreaks via wastewater A virus diagnosis device that gives lab-quality results within just three minutes has been invented by engineers at the University of Bath, who describe it as the "world's fastest COVID test." The prototype LoCKAmp device uses innovative "lab on a chip" technology and has been proven to provide rapid and low-cost detection of COVID-19 from nasal swabs. The research team, based at the University of Bath, say the technology could easily be adapted to detect other pathogens, such as bacteria—or even conditions like cancer. The device works by rapidly releasing and amplifying genetic material from a nasal swab sample by carrying out a chemical reaction to produce a result, which can be viewed on a smartphone app. Unlike lateral flow assay tests, commonplace during the pandemic, the LoCKAmp employs the same 'gold standard' genetic-based testing techniques previously reserved for lab-based PCR (polymerase chain reaction) tests, thus enabling rapid testing at laboratory-scale standards for the first time. As well as its accuracy, the speed of the LoCKAmp sets it apart. With results shown within three minutes, the research team say that to their knowledge, this makes LoCKAmp the fastest COVID-19 test reported to date. Made with off-the-shelf components and factory-manufactured printed circuit boards, the prototype device could be made on a mass scale quickly and at low cost, presenting care providers and public health bodies around the world with an effective new tool in virus detection. The research team says a commercial partner with the relevant design and manufacturing expertise could quickly redesigned the LoCKAmp into a small, portable device—with great potential for use in remote health care settings. The research team is already engaging with academic and commercial partners and would welcome further approaches as it seeks to bring LoCKAmp into production. The device and how it works is detailed in the research paper LoCKAmp: lab-on-PCB technology for Read the full article
0 notes
Text
UK hospital AI-enhanced cancer treatment is better for patients
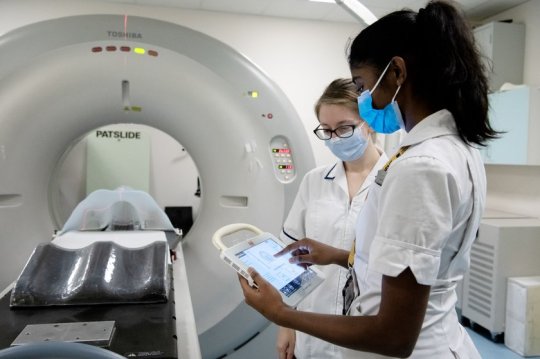
UK hospital AI-enhanced cancer treatment is better for patients. AI-enhanced cancer treatment - Two-year pilot highlights benefits Cambridge Cancer Research Hospital Nov 1, 2023 - Addenbrooke's is the first NHS hospital to use an AI-enhanced superhot needle treatment to pinpoint and destroy life-threatening tumors in one go, including hard-to-reach cancers. The treatment, known as thermal ablation, is highly-targeted and can treat multiple small tumors with less risk to surrounding healthy tissue. The target area is segmented, that is mapped in advance, using CT images. These images show the clinician where to guide the needles during the procedure. But gases and blood in the treated area can make this mapping process less accurate meaning the more hard-to-find tumors potentially remain only partially treated. This can lead to local tumors recurrence, often not detected until post-treatment scans. When this happens, a patient may need to go through the process again. Using AI to train computers to do the mapping makes the treatment quicker, less invasive for the patient, and more accurate. It also means the treatment is carried out in a CT-suite rather than in an operating theatre. Therefore, further scans can be taken during the actual treatment, providing near real-time monitoring which reduces the potential need for further treatment. Combining the precision of AI and thermal ablation means hard-to-reach or very small tumors can be more easily and effectively treated without the need for repeat treatments. This means we can treat more patients and save and improve more lives. We are really grateful to ACT for making us the first NHS hospital to be able to offer this AI-enhanced treatment. Dr Nadeem Shaida, Addenbrooke's Consultant Interventional Radiologist https://youtu.be/cxcQLyV_cjU?si=9YlLC7ZeHNmIezow
AI-enhanced cancer treatment - Two-year pilot highlights benefits
The hospital's charity, Addenbrooke's Charitable Trust (ACT), bought the £250,000 thermal ablation machine following a two-year pilot involving 50 patients with liver cancer. Less than half of those treated during the pilot needed further treatment. The hospital is now looking at how this treatment can be expanded to kidney and other cancer patients. Patients diagnosed with cancer deserve access to powerful, life-saving treatments. Generous donations from our supporters help us to make such treatments possible, making Addenbrooke's even better. Shelly Thake, CEO, Addenbrooke's Charitable Trust
Cambridge Cancer Research Hospital
The development comes as Addenbrooke's forges ahead with plans to build the Cambridge Cancer Research Hospital, a world-class facility that will bring NHS staff and scientists together to deliver personalized precision treatments to patients. Construction work on the new hospital’s site is due to begin in 2024. The hospital is set to be the first delivered in the East of England as part of the Government’s New Hospital Program. Read the full article
0 notes
Text
Shanghai Unveils Advanced Synchrotron-Based Proton Therapy Facility for Enhanced Cancer Treatment

Shanghai Unveils an Advanced Synchrotron-Based Proton Therapy Facility for Enhanced Cancer Treatment CHINA, October 29, 2023 - Proton therapy is an advanced form of radiation therapy that uses protons instead of X-rays to treat cancer. Due to its precise nature, it significantly reduces damage to surrounding normal tissue. The Bragg peak of proton beams ensures that there's almost no dose after the peak, protecting essential organs from unnecessary radiation. With over 103 proton therapy centers operational worldwide and more than 280,000 patients treated, this technology is revolutionizing the way cancer is treated. This study is published in Nuclear Science and Techniques Volume 34, researchers from Shanghai Institute of Applied Physics, Chinese Academy of Sciences and Shanghai Advanced Research Institute, Chinese Academy of Sciences, has been successfully developed and certified by the National Medical Products Administration (NMPA). This cutting-edge proton therapy facility, based on synchrotron technology, is a significant leap forward in the realm of China made high-tech medical apparatus for cancer treatment. The SAPT facility is located at the Shanghai Ruijin Hospital Proton Therapy Center. The construction of this sophisticated facility encompassed two phases. In the initial phase, four beam lines were developed in three treatment rooms. The second phase saw the introduction of a 360° rotating gantry room. The facility's accelerator system includes a 7-MeV proton LINAC, a synchrotron, two rotating gantries, and corresponding transport beamlines. Each of the facility’s four treatment rooms is equipped with a beam delivery system, a positioning system, image-guided radiotherapy (IGRT), a treatment control system (TCS), a treatment plan system (TPS), an oncology information system (OIS), and a quality assurance (QA) system. With the pinpoint accuracy of the whole system, treatment is precise, ensuring optimal results with minimal side effects. Following rigorous third-party inspections and tests for medical devices, the SAPT facility underwent a clinical trial with 47 patients. These patients, aged between 32 and 80, had tumors located in various regions, including the chest, abdomen, spine, and head and neck. Results were promising: the local control rate of the tumor was 100%, and no severe adverse reactions were noted. The SAPT facility demonstrated remarkable reliability, with an availability rate of over 98.5% during the clinical trial period. With the SAPT facility now officially registered and certified, it will open its doors for patient treatment in July 2023. This facility is expected to enhance the cancer treatment power in the region, offering a more precise method with less side effects to tackle various forms of tumors. As the need for such advanced treatment grows globally, the successful establishment and operation of the SAPT facility in Shanghai serve as a beacon of hope and an innovation model in the medical world. Funding information: The work is supported by the major projects for strategic emerging industries of the Shanghai local government and National Key R&D Program of China. More Information: DOI: 10.1007/s41365-023-01293-1 Read the full article
0 notes
Text
Advanced laser spectroscopy detects falsified vaccines

Advanced laser spectroscopy detects falsified vaccines. Prevent, detect, respond Tackling an important problem New uses for advanced Raman Spectroscopy technology Promising future applications An international consortium of multidisciplinary researchers and specialists has developed a new method to counter the problem of COVID-19 vaccine falsification. There have been numerous instances of vaccine supply chains being infiltrated by falsified products, both for vaccines before the pandemic and for COVID-19 around the globe. In response to this, the Science and Technology Facilities Council (STFC) Central Laser Facility (CLF) and partners have demonstrated the use of a specialized laser spectroscopy technique to rapidly verify falsified vaccines.
Prevent, detect, respond
Apart from endangering the public, by not effectively protecting people from COVID-19, falsified vaccines also risk undermining trust in vaccines. As such, it is critically important that we maintain integrity of supply chains by detecting falsified products effectively. To address this global health issue, the World Health Organization (WHO) member states adopted a prevent, detect, and respond strategy. Although critical, the detection of falsified vaccines currently relies on analysis that can only be performed in specialized laboratories.
Tackling an important problem
In response to the need to develop new methods to counter falsified vaccines, a consortium of world-leading experts convened in 2020, consisting of representatives from: - University of Oxford's Nuffield Department of Medicine, Department of Biochemistry, Department of Chemistry and Kavli Institute for Nanoscience Discovery - STFC, part of UK Research and Innovation (UKRI) - WHO, Geneva - Agilent Technologies - Serum Institute of India - University of Huddersfield - University of East London In line with WHO strategy, this multidisciplinary and multi-institutional research consortium has developed a new method of effectively and efficiently detecting falsified vaccines. Their study demonstrates the viability of the handheld spatially offset Raman spectroscopy (SORS) technique to rapidly authenticate COVID-19 vaccines through unopened vaccine vials. The full study can be read in the journal Vaccine.
New uses for advanced Raman Spectroscopy technology
SORS performs chemical analysis by shining a laser light into an intact vial of the vaccine and inspecting the light emanating from the vial to indicate the presence of different ingredients. SORS was originally invented and developed into a spin-out company by CLF, which was acquired in 2017 by Agilent Technologies, where the technique continues to be developed for various applications. SORS devices are currently used to screen for hazardous substances at airports and used widely by fire officers, the military, border protection, and law enforcement. Only minor modifications in sample compartment and software adaption are required for its deployment in the field to tackle falsified vaccines. The fact that SORS can effectively screen for falsified vaccines without opening the vial is a major advantage in terms of speed of detection and the ability to use vaccines that pass the SORS testing. "SORS is poised to become an important tool to protect against falsified vaccines. Since first being developed at the STFC Central Laser Facility, this innovative technology continues to find new areas of application which now include empowering governments and health care organizations to safeguard public health. It is a prime example of the enormous benefits of responding to global health challenges with strategic technological innovation." Professor John Collier, director of STFC CLF, said "The use of the handheld Resolve's SORS technology for swift and non-intrusive analysis within sealed containers is ground-breaking, attributed to both SORS technology, and the inherent sensitivity of the optical design. This approach not only advances vaccine authentication but also sets the stage for future high sensitivity analysis within sealed containers across diverse fields." Dr. Rob Stokes, field detection marketing director at Agilent Technologies, said
Promising future applications
Although this study focuses only on COVID-19 vaccines, the method it describes may also be used for authenticating other vaccines, liquid and solid medicines. Further research is needed with more vaccines and to evaluate its effectiveness at various points in supply chains. With the increasing importance of vaccines for many diseases with pandemic potential and their inequitable distribution, innovative tools to empower inspectors in detecting criminal falsification such as these are a vital asset. More information: Sara Mosca et al, Innovative method for rapid detection of falsified COVID-19 vaccines through unopened vials using handheld Spatially Offset Raman Spectroscopy (SORS), Vaccine (2023). DOI: 10.1016/j.vaccine.2023.10.012 Journal information: Vaccine Source: Medical Research Council Read the full article
0 notes
Text
Live streaming platform 'Twitch' poses risks for minors who may be manipulated

Live streaming platform 'Twitch' poses risks for minors who may be manipulated or preyed upon, says new research. A popular live streaming platform, Twitch, poses risks to minors who can interact with adult strangers and donate money to streamers without the supervision of a parent or guardian, according to research presented during the 2023 AAP National Conference & Exhibition at the Walter E. Washington Convention Center. The abstract, "Predator Paradise: Analyzing the Ease of Accessibility to Minors on Twitch," found that young users feel a false sense of safety on the platform, as a significant proportion were willing to reveal personal information despite having no knowledge of who might be listening. The nature of the live streaming platform makes it particularly risky, as there is no way to take back information that minor streamers reveal while on Twitch. "Twitch is an exciting platform; however, it may present hidden dangers for minor users," said Ruth Milanaik, MD, FAAP, principal investigator of the study. "Parents need to supervise all interactions on this platform to best protect their child." To conduct the research, trained coders entered Twitch.tv, searched popular video games, and scrolled to view current live streams that appeared to be conducted by minors who had their cameras on and showed their faces. No accounts were created for this study. The participants analyzed data collected on 100 minor Twitch streamers with 1,755,452 followers. Youth streamers provided their names (47%) and stated their location 50% of the time. About 38% provided detailed schedules of when they would be live, and 64% linked and encouraged viewers to follow their other public social media. Viewers were able to donate money to 37% of streamers. "The donation system is quite scary to me," said Fiona Dubrosa, visiting scholar at Cohen's Children Medical Center, Northwell Health in Rego Park, N.Y. "The idea that anyone can donate money to streamers of any age seems very manipulative, and I do not think that it is widely known of the disturbing ways this could be utilized. Twitch must create a safer platform." The researchers conclude that popular websites like Twitch can serve as a breeding ground for voyeuristic consumption of underage streamers and encourage pediatricians and caregivers to be aware of the potential dangers to children. This research was conducted by the Teen Trends Consortium at Cohen's Children Medical Center, Northwell Health. Teen Trends Consortium is a group of researchers aged 18–24 that focuses solely on the most salient issues facing pediatric populations, including substance use and technology. Source: American Academy of Pediatrics Read the full article
0 notes
Text
Drones delivering opioid overdose reversal kits could reach people more quickly than ambulances

Researchers from King's College London used real-world data of fatal opioid overdoses where a bystander was present to show that commercial off-the-shelf drones could have reached 78% of cases within seven minutes—the benchmark time for the arrival of emergency services for Category 1 calls in England—a huge increase on the 14% reached by ambulances. The researchers also found that by increasing the speeds of the drones and designing specialist cargo cradles, an estimated 98% of overdoses could be reached within seven minutes. The study, "An evaluation of naloxone transit for opioid overdose using drones: A case study using real-world coroner data" is published today (Oct. 13) in Addiction. Naloxone is a life-saving drug that reverses or blocks the effects of opioids and rapidly restores normal breathing. "Take-home" naloxone kits are increasingly available from community pharmacies and drug treatment services, but a supply is not always readily accessible. Paramedics routinely carry naloxone and aim to attend emergencies in seven minutes; however, this can be impacted by factors such as ambulance waiting times or the location of a patient, such as a music festival. The modeling suggests the naloxone kit would be delivered to the site of the overdose, and the bystander would administer the medicine by nasal spray. Paramedics would attend the scene as usual and deliver the patient to urgent care. "When a person overdoses and stops breathing, every second counts. Naloxone is very effective when given at the first signs of overdose and is easy to use. This study shows that drones can get naloxone to the site of an opioid overdose more quickly than paramedics in an ambulance. This could make a huge difference to people's survival," says lead author Dr. Caroline Copeland, from the School of Cancer & Pharmaceutical Sciences. "Bystanders leaving the scene of overdoses occurs due to fear of prosecution as illegal drugs are often present at the scene. If naloxone can reach those who need it before paramedics and law enforcement, bystanders may be encouraged to help before leaving." She added "Drones have the potential to revolutionize medicine delivery. A robust drone network can deliver naloxone kits efficiently. The drones under evaluation have collision detection technology so they don't fly into buildings or through air space, and are deployed using a standalone drone station." Dr. Paul Royall, from the School of Cancer & Pharmaceutical Sciences, first author and co-founder of Drone Mat Lab said More information: Paul G. Royall et al., An evaluation of naloxone transit for opioid overdose using drones: A case study using real-world coroner data, Addiction (2023). DOI: 10.1111/add.16361 Journal information: Addiction Source: King's College London Read the full article
0 notes
Text
Patient comfort in AI-driven healthcare linked to familiarity, finds GlobalData

Patient comfort in AI-driven healthcare is linked to familiarity, finds GlobalData. Oct 09, 2023 - As the healthcare landscape undergoes transformation due to factors like aging populations, rising chronic diseases, and shifts in lifestyle choices, technology, particularly artificial intelligence (AI), is playing an increasingly vital role. A recent survey highlights how patients’ familiarity with AI significantly influences their comfort level when considering healthcare facilities utilizing this technology, according to GlobalData, a leading data and analytics company. GlobalData’s recent survey, “Thematic Intelligence: AI in Clinical Practice—Patient Perspective 2023,” reveals that out of surveyed patients who were familiar with AI, 60% were either very or quite comfortable with using healthcare facilities that use AI, while of the ones who were not familiar with AI, only 7% were. “AI is already successfully used to detect image-based diseases such as cancer, and the technology is continuously evolving to enable much wider use cases within healthcare. Successful use cases can encourage further adoption and investment in this technology. “Together with the development of a robust regulatory framework, it is imperative to prioritize patient education regarding the technology. This education should aim to enhance comprehension of AI’s utilization, its potential advantages, and associated adoption risks, ultimately fostering increased trust in AI. Enhanced knowledge empowers individuals to make informed decisions and mitigate biases linked to this technology.” Urte Jakimaviciute, Senior Director of Market Research at GlobalData, comments

The survey data also reveals that younger surveyed patients (18–55 years old) were more likely to be familiar with AI than older (56+ years old) patients, with more than 50% of them rating their knowledge as moderately or very familiar. Younger generations tend to be introduced to technology earlier in their lives and, as a result, have more knowledge and confidence in technologies like AI. “While AI adoption is inevitable, it must be inclusive. Even though younger generations may drive the use and adoption of AI, building a fair and ethical AI system will need intergenerational collaboration. AI use will need to address generation-wide issues, and therefore it is essential to ensure that all generations can benefit from the adoption of AI.” Jakimaviciute concludes Read the full article
0 notes
Text
Medtech company MedAlliance acquired by Cordis for USD 1.135 billion

Medtech company MedAlliance acquired by Cordis for USD 1.135 billion. MedAlliance DEB technology Cordis GENEVA, Oct. 2, 2023 - Swiss-based medical technology company MedAlliance has been acquired by Cordis for a 2022 investment of $35M and a 2023 upfront closing payment of $200M, together with regulatory achievement milestones of up to $125M and commercial milestones of up to $775M through 2029, for a total consideration of up to $1.135 Billion. Cordis is a worldwide leader in the development and manufacture of interventional cardiovascular and endovascular technologies. MedAlliance's innovative and revolutionary sustained sirolimus drug-eluting balloon (DEB) program, SELUTION SLR™ (Sustained Limus Release), has provided a flagship product family which complements Cordis' existing product portfolio, together with their sales, marketing and distribution expertise. Cordis customers will benefit from the extensive clinical study program and publication plan that have been executed by MedAlliance to further Cordis' heritage of bringing innovative products to patients. "The Cordis acquisition will accelerate access to this breakthrough technology for patients around the globe suffering from coronary and peripheral disease," said Jeffrey B. Jump, Founder, Chairman and CEO of MedAlliance. "I want to thank our entire MedAlliance team – including physicians, distributors and clinical patients who have succeeded in disrupting the coronary and peripheral markets to provide a safe and effective new technology. The arsenal of SELUTION SLR™ DEB clinical data is designed to change medical practice and improve patient outcomes." "Nearly twenty years ago, Cordis introduced CYPHER®, the first drug-eluting stent, transforming cardiovascular treatment for patients around the world," said Shar Matin, Cordis CEO. "As a newly independent company, we are beyond proud to further our legacy of innovation and market disruption with MedAlliance and the first MicroReservoir sirolimus drug-eluting balloon, SELUTION SLR." "We are excited to report on the positive SELUTION SLR data to date, with first-hand experience of the impressive clinical outcomes from patients treated with SELUTION SLR in Japan, India, Europe, and South America. We are now leading the effort to emulate these results in the United States. This technology has the distinct opportunity to change the treatment paradigm for patients suffering from cardiovascular and peripheral vascular disease," commented George Adams, Principal Investigator of the US SELUTION4SFA IDE study; Director, Cardiovascular and Peripheral Vascular Research; Rex Hospital Inc., Raleigh, North Carolina, United States. SELUTION SLR was awarded CE Mark Approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary artery disease in May 2020 MedAlliance was the first drug-eluting balloon company to receive FDA Breakthrough Designation status. In addition to the BTK and superficial femoral artery (SFA) indications for which the company received FDA IDE approval in May and August 2022, MedAlliance received coronary in-stent restenosis (ISR) IDE approval in October 2022 and de novo coronary artery lesions approval in January 2023. Subsequent to achieving IDE Status, three FDA studies involving SELUTION SLR are currently enrolling with a fourth, involving patients with coronary de novo artery disease, planned to start in the next few weeks. The latter will complement the substantial experience gained with the ground-breaking SELUTION DeNovo trial in Europe, which has now enrolled over 1,660 patients, half way towards a planned 3,326 patients. SELUTION DeNovo compares the treatment strategy using SELUTION SLR versus any limus drug-eluting stent . This study is designed to change medical practice, as the majority of de novo coronary lesions are currently treated with a permanent metallic stent.
MedAlliance DEB technology
MedAlliance's unique DEB technology involves MicroReservoirs which contain a mixture of biodegradable polymer intermixed with the anti-restenotic drug sirolimus applied as a coating on the surface of an angioplasty balloon. These MicroReservoirs provide controlled and sustained release of the drug for up to 90 days. MedAlliance's proprietary CAT™ (Cell Adherent Technology) enables the MicroReservoirs to be coated onto balloons and efficiently transferred to adhere to the vessel lumen when delivered via expansion of the balloon. SELUTION SLR is commercially available in Europe, Asia, the Middle East, and the Americas (outside USA) and most other countries where the CE Mark is recognized. Over 40,000 units have been used for patient treatments in routine clinical practice or as part of coronary clinical trials.
Cordis
Cordis is a worldwide leader in the development and manufacturing of interventional cardiovascular technologies with a more than 60-year history of pioneering breakthrough therapies to treat millions of patients. With a reputation for clinical acumen, training, and service, Cordis has a legacy of innovation in high-quality and minimally invasive cardiovascular products, building a strong global footprint with operations in more than 70 countries around the world. Read the full article
0 notes