#cmo manufacturing
Explore tagged Tumblr posts
Text
Transforming Pharmaceutical Access and Insights | Chemxpert Database
Discover excellence with Chemxpert Database, your trusted source for medicine wholesale price lists and connections to top API pharmaceutical companies. Explore advancements in controlled release technology, cutting-edge drug research, and seamless contract manufacturing pharmaceutical solutions. Gain insights into leading generic pharmaceutical companies and locate medical companies near me with ease. Empower your business with precise data and reliable resources to thrive in the dynamic pharmaceutical industry. Chemxpert Database: where innovation meets opportunity!
#top third party pharma manufacturers in India#drugs price list#CMO manufacturing#pharma services#pharmaceutical companies in Poland#pharmacy business#top 10 api pharma companies in world
1 note
·
View note
Text
https://www.futureelectronics.com/p/semiconductors--memory--RAM--dram--component-dram/as4c16m16sa-6bin-alliance-memory-8054874
Types of DRAM, dynamic random access memory, dram chip manufacturers,
AS4C4M16S 256-Mbit (16 M x 16) 3.6 V High-Speed CMOS Synchronous DRAM - TFBGA-54
#Alliance Memory#AS4C16M16SA-6BIN#RAM#DRAM#Component DRAM#DRAM suppliers#dram memory cell#Types of DRAM#dynamic random access memory#dram chip manufacturers#chip#High-Speed CMOS Synchronous DRAM#dram chip
1 note
·
View note
Text
It is actually negative surprising to me that a group that encourages or forces intimacy and admissions and is looking for more men might accidentally support a lot of trans men in their very real feelings and transition
It’s also negative surprising to me that in such a group people can be abused while some have wholly positive experiences lol like for the same reason I’m not ruling out that one woman was abused just bc her mom and sister say it was always dandy
#cmo's log#this is a spoiler for#escaping twin flames#like I also totally believe it could be manufactured memories that is a thing#can you imagine being in an environment that not only accepts your transition but encourages you every step of the way that you’re meeting#your destiny and shut
1 note
·
View note
Text
ontract Manufacturing in the Generic Pharma Sector: Trends and Analysis
The global generic pharmaceuticals contract manufacturing market size is expected to reach USD 106.9 billion by 2030, registering a CAGR of 5.8% over the forecast period, according to a new report by Grand View Research, Inc. Cost-saving and time-saving benefits associated with the implementation of outsourcing is responsible for driving the industry. A significant number of people globally suffer from chronic diseases. For instance, the CDC states that 6 in 10 adults in the U.S. suffer from at least one chronic disease and 4 in 10 adults suffer from two or more chronic diseases. Chronic diseases are required to be treated for a long time. The high cost of medicines is increasing the demand for cost-effective generic drugs for the treatment of chronic diseases.
Generic Pharmaceuticals Contract Manufacturing Market Report Highlights
The branded generics segment held the largest share in 2021due to the preference for branded generics among physicians. Some branded generic manufacturers offer benefits and gifts to physicians for boosting their product sales. This further contributes to the demand for branded generic manufacturing in the market
The API product segment held the largest share in 2021. The growing demand for generic drugs is supporting the demand for generic API contract manufacturing
The parenteral route of administration segment is expected to grow at the fastest CAGR over the forecast period due to the bioavailability of parenteral drugs over other formulations
The oncology segment is expected to register the fastest CAGRfrom 2022 to 2030 owing to the high cost of cancer drugs contributing to the demand for cost-effective generic medicines
Asia Pacific is expected to record the highest CAGR over the forecast period mainly due to the low cost of generic drug manufacturing
Gain deeper insights on the market and receive your free copy with TOC now @: Generic Pharmaceuticals Contract Manufacturing Market Report
This is expected to support the industry's growth post-pandemic. There is an improvement in the regulatory approval of generic drugs. For instance, in 2021, the FDA approved 93 generic drugs, and by October 2022, the regulatory authority approved over 95 generic drugs. Such improvements are expected to have a positive impact on the manufacturing of generic drugs and; thus, support the industry growth. The Japanese government is constantly trying to improve the generic pharmaceuticals market in the country. The government is also taking measures to improve the supply of generics in the country and is also encouraging medical institutes to promote the use of generic drugs.
This is expected to improve CMO activities for generics in the coming years. Global spending on medicines is also on the rise. According to the data provided in a report published by IQVIA in April 2021, global spending on medicine is expected to increase in the next 4-5 years. The report states that global spending on medicine accounted for USD 1, 265 billion in 2020 and is going to reach USD 1,580-1,610 billion by 2025. This is also expected to improve the demand for generic drugs owing to their cost efficiency, thereby supporting the industry in growth.
#Pharma Contract Manufacturing#Generic Pharmaceuticals#Pharma Industry#Drug Manufacturing#Pharmaceutical Supply Chain#Outsourcing#Supply Chain Management#Pharmaceutical Partnerships#Pharmaceutical sourcing#Healthcare Manufacturing#Drug Development#API Production#CRO#CMO#Pharmaceutical Trends
0 notes
Text



Conn - Multi-Vider
"All the way back in 1967, C.G. Conn wanted in on the decidedly nascent effects scene, and they wanted to do so with a bang. The company partnered with Jordan Electronics of Alhambra, CA to release an octave effect for wind instruments. The resulting circuit is a truly interesting piece of gear history. It needs to be said that Conn went into manufacturing, thereby ending its partnership with Jordan (at least according to all the paperwork) and the result was two different MultiViders. The differences on the surface are minute: the first model is grey and looks like a piece of dictation equipment, offering “bright” and “dark” input modes, a top-mounted Sensitivity control, and a plethora of battery gadgets. By contrast, the much cooler-looking model “914” did away with the frequency selector, opting for a switch called Unison and a power supply input.
Both models contain “Soprano,” “Bass” and “Sub Bass” switches, and corresponding volume for each. The 914’s Unison mode is essentially a dry signal control. The “grey box” model is a little more convoluted about it but the job is effectively identical. However, the way these two models go about these identical tasks in different—yet similar—ways.
This original “grey box” model contains a duo of ersatz flip-flop circuits, which the unit relies on for its octave down effects. The circuit utilizes some rather intense gain staging to convert the signal to a crude square wave and then use the flip-flops to divide the frequency in half and then in half again. In the later 914 model, much of this circuit is switched to a CD4013 chip, an all-in-one CMOS device. It’s interesting that the first draft of the MultiVider contains what amounts to a discrete imagining of the CD4013, and what it all adds up to is the first-ever octave effect for an electronic instrument. There’s also a wah inductor on the 914, which is connected to the sub-octave circuit somehow; I dare not remove the board due to extreme rocker switch fragility. I love stuff like this.
For as cool as this whole thing sounds, there are some drawbacks, as one might expect with the first pedal of any type. As previously stated, the MultiVider is a horns-only instrument, as is to be used with Conn’s proprietary woodwind pickup. While the “grey box” model serves up a battery option, the 914 is adapter-only, and it’s a doozy—only a 12-volt eighth-inch style phone plug will do. Thankfully there are workarounds for both; if you can solder, the power situation is a cinch and the microphone issue can be circumnavigated by hitting the MultiVider with a hotter input signal. Even then, a large belt clip on the back of the unit dictates its preferred method of implementation. With all that said, synth players are at an automatic advantage with modernizing the MultiVider.
Of course, the MultiVider was an advanced device for its time, and so it was used by artists that had explored brass instruments to their fullest. In particular, the MultiVider was used by Zappa’s band, the Mothers of Invention. It was also used by Miles Davis on 1970’s The Complete Jack Johnson Sessions. Of course there are others, but with a resume like that, stick to your strengths."
cred: catalinbread.com/blogs/kulas-cabinet/conn-multivider
30 notes
·
View notes
Text
Industry 4.0 and the Demand for Smart Manufacturing in Pharma: A New Era for CMOs
The pharmaceutical industry is experiencing a paradigm shift with the advent of Industry 4.0 and smart manufacturing technologies. For contract manufacturing organizations (CMOs), these improvements are not just optional but necessary to remain competitive in a rapidly evolving marketplace. By leveraging cutting-edge technology, CMOs can increase productivity, reduce costs, and deliver better results for their customers. Let’s explore how Industry 4.0 is transforming medicine and the critical role CMOs play in this new era
What is Industry 4.0 in Pharma?
Industry 4.0 refers to the integration of advanced technologies such as the Internet of Things (IoT), artificial intelligence (AI), robotics, data analytics, etc. In medicine, these technologies enable real-time monitoring, predictive maintenance and automated quality control for smarter and more efficient production
For CMOs, adopting Industry 4.0 means embracing these innovations to streamline operations and meet the stringent demands of pharmaceutical procurement. With an increased focus on quality and speed, smart manufacturing gives CMOs a competitive advantage by ensuring accuracy and compliance.
Key benefits of Industry 4.0 for CMOs
1. Improved productivity
Industry 4.0 technology empowers CMOs to optimize production processes. Using IoT-enabled devices and sensors, production lines can be monitored in real-time, in order to instantly identify bottlenecks and malfunctions. This ensures minimal downtime and increases productivity.
2. Quality control
AI-powered quality assurance systems help CMOs identify deficiencies early in the process.
Real-time data collection and analysis ensures that each batch meets the highest standards, reducing the risk of recalls and compliance issues.
3. Debt reduction
Smart design reduces waste, energy consumption and labor costs. Predictive maintenance enabled by the IoT reduces device failures and extends device life, saving CMOs operating costs.
4. Quick time to market
With automated processes and flexible workflows, CMOs can reduce development cycles. This is especially important in the pharmaceutical industry, where time to market can determine the success of a product.
Projects 4.0 Technologies for CMO transformation
1. Internet of Things (IoT) .
IoT devices provide seamless communication between devices, systems and people. For CMOs, IoT ensures real-time visibility into production, inventory levels, and supply chain management.
2. Artificial Intelligence (AI) .
AI-driven analytics provide CMOs with actionable insights, enabling them to streamline their processes and improve decision-making. Machine learning algorithms can predict demand, optimize resource allocation, and improve process efficiency.
3. Robotics and Automation
Automation technologies are transforming medicine. Robots perform tasks with repeatedly increasing accuracy, freeing up humans for more subtle activities to improve stability.
4. Digital twins
Digital twins are virtual replicas of physical objects or systems. CMOs can use these to simulate and optimize processes, reduce trial-and-error methodologies, and accelerate innovation.
Challenges for CMOs to adopt Industry 4.0
While the benefits are undeniable, CMOs face several challenges in implementing Industry 4.0 technologies:
Significant initial investment: Implementing smart manufacturing processes requires significant upfront costs, which can be a barrier for smaller CMOs.
Skills Gap: The transition to Industry 4.0 requires a workforce with advanced technology skills, which may require significant training and recruitment efforts.
Data Security Issues: As manufacturing becomes increasingly digital, ensuring data security and protecting intellectual property becomes increasingly important.
The future of CMOs is in the industry 4.0 era
Adopting Industry 4.0 technologies is not a luxury but a necessity for CMOs to remain competitive. As pharmaceutical companies demand faster, superior solutions and lower costs, CMOs need to invest in smarter manufacturing processes to meet these expectations
The future of pharmaceuticals is collaboration and innovation. By partnering with technology providers and taking advantage of advanced systems, CMOs can position themselves as leaders in the industry. As Industry 4.0 continues to evolve, CMOs that embrace this shift will not only survive but thrive in this time of change
conclusion
Industry 4.0 and smart manufacturing are reshaping the medical landscape, creating unparalleled opportunities for CMOs to advance their capabilities. From real-time analytics to AI-driven analytics, these technologies empower CMOs to deliver exceptional value to their clients. By overcoming challenges and investing in innovation, CMOs can play a key role in shaping the future of medicine.
2 notes
·
View notes
Text
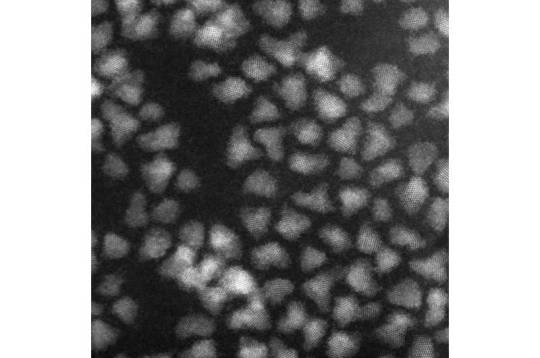
Environmentally-friendly InSb/InP colloidal quantum dots for fast and sensitive short-wave infrared photodetectors
Applications such as LIDAR, 3D imaging for mobile devices, automotive and augmented/virtual reality or night vision for surveillance, rely on the development of short-wave infrared (SWIR) photodetectors. These devices are capable of seeing in the region of the spectrum that is invisible to our eye since they operate in the spectral window of 1-2 µm. The SWIR light sensor industry has been dominated for years by epitaxial technology, mainly based on devices made of indium gallium arsenide (InGaAs). However, several factors such as high production costs, low-scale manufacturability and incompatibility with CMOS have confined the epitaxial technology to niche and military markets. In contrast, the potential of SWIR photodetectors made of colloidal quantum dots (CQDs), nanoscale semiconductor materials, has attracted significant interest in recent years due to their appealing features, such as low cost and compatibility with CMOS architecture, among others.
Read more.
8 notes
·
View notes
Text
Cutting to the "shopping list" section:
We focused on using “off the shelf” components and open-source software to develop the wireless endoscope. This significantly lowered the cost with the goal to make the device accessible for resource-constrained environments. While the technology described is applicable to a variety of rigid lens applications, we attempted to replicate the 4 mm cystoscope lens and separately a clip-on universal endoscope camera.
2.1 Hardware systems
A miniature single-board computer (SBC) module the Raspberry Pi Zero W (Raspberry Pi Foundation, Caldecote, UK) was used because of its size, its low cost (US$10) and its ability to handle high-definition (720p) video.
A 3.7-mm tube camera (model: 1001LG, Shenzhen Eastern International Corporation Limited, Shenzhen, China) was used. It delivers 1280 × 720 high-definition video using a 1/7″ colour CMOS sensor. Lens construction allows a wide (115 degree) field of view and an extended depth of field allowing object in the range of 5 to 50 mm to be in focus. Connectivity is via a USB 2.0 interface with the SBC. The camera is certified IP67 waterproof and the manufacturer is ISO 13485:2016 certified for the design and manufacturer of medical endoscope cameras.
Illumination is via 6 high luminous 0603 white colour LEDs incorporated into the tube camera. The system was powered by a 1200mAh lithium polymer battery and incorporated into a fireproof acrylonitrile butadiene styrene enclosure.
For the clip-on wireless camera module, an 18–35 mm optical zoom coupler (Ouman Medical, Jiangsu Ouman Electronic Equipment Co., ltd, Jiangsu, China) and an 8-megapixel camera module (model: IMX219, Arducam) were used.
2.2 Software systems
Open-source Linux software was used on the SBC as follows:
1. The SBC runs on the Raspbian Pi operating system Lite, a minimal image of Debian Buster [9]. 2. The SBC Wi-Fi module is placed in monitor mode (“hotspot”) using RaspAP [10]. This makes wireless video transmission possible. 3. The video signal is streamed via the UV4L module of “Video 4 Linux 2” [11].
4. Lastly, the wireless video signal is viewed on a standard computer via any internet browser. Figure 2 illustrates the entire software setup.
Fig. 2:
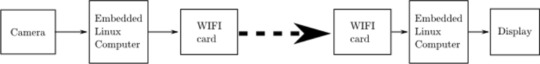
7 notes
·
View notes
Text
Fresh? Healthy? Safe? – Just a joke!

The vibrant glow of Frooti, the fizzy pop of Bindu Fizz, the golden promise of frozen fries and smiley faces. These products, marketed heavily towards children and teenagers, hold a certain allure. But beneath the flashy packaging and catchy jingles lies a harsh reality: these foods are detrimental to our health.
Frooti
There have been instances from the past few months that the juice inside of tetra packs turns into jelly, the consumers have recorded and uploaded the videos on social media platforms and till date frooti continues to sing “Mango Frooti, Fresh and Juicy”.
Bindu Fizz – a factory of sugar
The product claims that if quenches thirst and relieves acidity inside after the consumer eats junk.
Recently, there was a survey done which showed that one bottle of Bindu fizz consists sugar that an average consumer should not consume in a day. But shamingly they are saying
“Cool toh bindu hai.”
Smilies and Fries
Frozen fries and smiley faces are deep-fried in unhealthy fats, increasing cholesterol levels, and raising the risk of stroke and heart disease. Each serving of frozen fries can contain up to 17 grams of fat, nearly half the daily recommended intake for adults.
That means in one serving they are consuming double the number of calories they should.
Yet with no utter disguise they sing “fresh Banega baat banegi.”
Frozen Meat
Frozen meat products are often loaded with sodium, contributing to high blood pressure and kidney problems. Processed meats like chicken nuggets and hot dogs can contain up to 500 mg of sodium per serving, exceeding the recommended daily intake for adults.
Yet again your product sells under the tagline of “Juicy, Delicious… Must be Licious!”
Task at Hand
Your company is the manufacturer of all these products, you are the CMO of this company. Now your task is to re-brand all these products so that you can provide more value for the customers to save the deteriorating image for the same.
Deliverables
1) PPT (Detailed description of future products and services, Rebranding Budget, CSR activities)
2) New (Logo, Tagline & Poster) for each product.
3) Instagram Handle
Submission Time : 1:15 PM
2 notes
·
View notes
Text
Upgrading Your Computer's Memory: A 4-Year Journey in Laptop, Desktop, and Motherboard Memory
In today's fast-paced digital world, computers play an essential role in our daily lives. Whether you're a professional, a student, or a casual user, the performance of your computer greatly depends on its memory. Over the past four years, I've gained valuable experience in upgrading and replacing memory in various computer systems, including laptops, desktop PCs, and motherboards. In this article, I'll share insights and tips to help you enhance your computing experience.

Laptop Notebook Memory:
Laptops are known for their portability, but they often come with limited memory. Upgrading laptop memory can significantly boost performance. When choosing a laptop memory module, consider compatibility with your laptop's model and the operating system. DDR4 and DDR3L are common memory types, but ensure that you choose the right one.
Desktop PC Computer Memory:
Desktop PCs offer more flexibility for memory upgrades. Adding or replacing memory modules in a desktop is relatively straightforward. When upgrading desktop memory, focus on the type of RAM (DDR3, DDR4, etc.) and the memory speed. Faster memory can enhance multitasking and gaming performance.
Motherboard Memory:
Motherboard memory, also known as BIOS or CMOS memory, is vital for storing system settings and configurations. In some cases, you may need to replace a motherboard's memory component if it's causing issues. Ensure you're cautious when handling the motherboard and consult the manufacturer's guidelines to avoid any mishaps.
Dell Laptop Memory:
Dell laptops are popular for their quality and reliability. Upgrading memory in a Dell laptop can be a game-changer, making your laptop faster and more responsive. Always check for compatibility with your specific Dell laptop model to ensure the memory module fits perfectly.
Replacing and Upgrading Memory:
Upgrading memory is a cost-effective way to extend the life of your computer. Before starting, back up your data to prevent any potential data loss. Consult your computer's manual or the manufacturer's website for instructions on how to replace or upgrade memory. Make sure you have the necessary tools and components.
In conclusion, upgrading and replacing memory in your computer can transform your computing experience. My four years of experience in this field have taught me the importance of compatibility, research, and careful handling of computer components. With the right memory upgrade, your laptop, desktop, or motherboard can become faster and more efficient, ensuring that your computer keeps up with the demands of today's digital world.
2 notes
·
View notes
Text

Innovative Solutions in Controlled Release Drug Research and Manufacturing
Chemxpert Database empowers generic pharmaceutical companies with cutting-edge data on controlled release drug research and contract manufacturing. As a trusted partner in medicine supply, Chemxpert simplifies the complexities of pharmaceutical development by offering unparalleled insights into the latest advancements. Whether you're seeking opportunities in drug formulation or sourcing reliable manufacturing, Chemxpert connects you to the industry's best resources. Elevate your pharmaceutical business with Chemxpert's expertise in streamlining processes for enhanced efficiency and market reach.
#pharma companies in Singapore#top third party pharma manufacturers in India#drugs price list#cmo manufacturing#pharma services#pharmaceutical companies in Poland
1 note
·
View note
Text
Price: [price_with_discount] (as of [price_update_date] - Details) [ad_1] FUJIFILM X-T5 Mirrorless Camera (Black) A portable and powerful multimedia mirrorless camera, the FUJIFILM X-T5 features the newly developed 40MP APS-C X-Trans CMOS 5 HR BSI sensor for simply stunning results. Comparable in size to the original X-T1 and lighter than its predecessor, this camera provides a classic, dial-based layout and cutting-edge technology that includes a seven-stop in-body image stabilization system, Pixel Shift Multi-Shot mode for 160MP files, and action-freezing shutter speeds up to 1/180,000 sec from the electronic shutter. In addition to its versatile suite of stills capabilities, the X-T5 is also a highly capable moviemaking machine, recording up to 6.2K in 4:2:2 10-bit color internally or 12-bit ProRes RAW and Blackmagic RAW via HDMI. FUJIFILM XF 16-80mm f/4 R OIS WR Lens Well-suited for a wide variety of shooting situations, the FUJIFILM XF 16-80mm f/4 R OIS WR is a versatile 24-120mm-equivalent zoom, spanning wide-angle to medium-telephoto, and featuring a constant f/4 maximum aperture. Complementing this flexible design is an advanced optical layout, which includes a trio of aspherical elements and one ED aspherical element that help to minimize a variety of aberrations in order to produce high sharpness and clarity. A Super EBC coating also improves contrast and color neutrality by reducing flare and ghosting when working in strong lighting conditions. Also benefitting use in a variety of situations is a quick and quiet autofocus system along with a six stop-effective image stabilization system that minimizes the appearance of camera shake. Additionally, the lens is fully weather-sealed for working in inclement conditions. Batteries : 1 Lithium Ion batteries required. Is Discontinued By Manufacturer : No Product Dimensions : 8.8 x 7.8 x 7.8 cm; 950 g Date First Available : 9 January 2023 Manufacturer : FUJIFILM ASIN : B0BRY4T25X Item model number : 203400 Country of Origin : Japan Manufacturer : FUJIFILM, FUJIFILM INDIA PRIVATE LIMITED, Andheri East, Mumbai, Maharashtra-400069, For Support Mail- [email protected] contact- 0124-4325551 Packer : FUJIFILM INDIA PRIVATE LIMITED, Andheri East, Mumbai, Maharashtra-400069, For Support Mail- [email protected] contact- 0124-4325551 Importer : FUJIFILM INDIA PRIVATE LIMITED, Andheri East, Mumbai, Maharashtra-400069, For Support Mail- [email protected] contact- 0124-4325551
Item Weight : 950 g Item Dimensions LxWxH : 8.8 x 7.8 x 7.8 Centimeters Net Quantity : 1 Count Included Components : Camera Kit Generic Name : Mirrorless Camera with Lens The high-resolution 40.2MP X-Trans CMOS 5 HR sensor has an enhanced image – processing algorithm that boosts resolution without compromising the signal-to-noise ratio, delivering astonishing image quality. 20 Film Simulation modes inside of X-T5 digitally replicate the look of the classic photographic film stocks developed by Fujifilm for over 85 years. Reproduce the classic colors and tones that Fujifilm are known for. X-T5 is packed with many features such as pixel shift which give 160mp image after compiling Shift Multi-Shot|fastest shutter - 1/180000|2x digital teleconverter for photo|wireless tethering|camera to cloud with frame.io|touchtracking and dial operation for photo and video|quick lever to switch from photo to video or otherwise. 6.2K movies can be recorded internally at 30p in 4:2:2 10-bit color, delivering high-definition footage with rich color detail. In addition, you can record 4k 60fps and FHD 240fps. X-T5 is packed with many features such as AI deep learning|high resolution viewfinder|touchtracking|3-way tilting lcd|switch for change focus from single to continuous|intelligent hybrid ai af|micro hdmi type d| dual card slot (dual sdxc uhs-2)|740 frames battery life (cipa standard)|can run on power back (type -c) which makes it perfect hybrid camera for photo and video. X-T5 is a high resolution professional camera suits for every occasion and need be it wedding|fashion|portrait|landscape|wildlife|commercial|filmmaking|documentary|street|travel|lifestyle [ad_2]
0 notes
Text
Sensors Market Opportunities: Growth, Share, Value, Size, Industry Analsis and Forecast by 2031
"Sensors Market Size And Forecast by 2031
The report further examines the innovative strategies adopted by Sensors Market these top players, such as embracing cutting-edge technologies, prioritizing customer-centric approaches, and optimizing operational efficiency. By analyzing case studies and real-world applications, the study demonstrates how these companies have adapted to changing market demands and regulatory landscapes. Their ability to innovate and respond to emerging challenges underscores their importance in shaping the trajectory of the Sensors Market.
Data Bridge Market Research analyses that the Global Sensors Market which was USD 218.25 Million in 2023 is expected to reach USD 447.82 Billion by 2031 and is expected to undergo a CAGR of 9.40% during the forecast period of 2023 to 2031
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-sensors-market
Which are the top companies operating in the Sensors Market?
The Top 10 Companies in Sensors Market are prominent leaders known for their strong influence and significant market share. These include well-established companies which have built a reputation for their high-quality products and services. These companies are recognized for their innovation, customer satisfaction, and ability to adapt to market trends, playing a key role in shaping the growth and direction of the Sensors Market.
**Segments**
- By Type: The global sensors market can be segmented by type into temperature sensors, pressure sensors, motion sensors, image sensors, proximity sensors, level sensors, chemical sensors, humidity sensors, and others. Temperature sensors are vital for monitoring and controlling temperature in various industries. Pressure sensors find applications in automotive, healthcare, and industrial sectors. Motion sensors are widely used in consumer electronics and security systems. Image sensors are essential for capturing images in devices such as smartphones and cameras. Proximity sensors are crucial for object detection in automotive and industrial automation. Chemical sensors are utilized for detecting specific gases or chemical compounds in the environment. Humidity sensors play a key role in climate control and weather monitoring.
- By Technology: In terms of technology, the sensors market can be categorized into MEMS (Micro-Electro-Mechanical Systems) technology, infrared technology, CMOS (Complementary Metal-Oxide-Semiconductor) technology, and others. MEMS technology enables miniaturization of sensors, making them suitable for compact devices like smartphones and wearables. Infrared technology is commonly used for temperature sensing and night vision applications. CMOS technology is prevalent in image sensors for capturing high-quality digital images.
- By Application: The sensors market can also be segmented based on application areas such as automotive, healthcare, consumer electronics, industrial automation, aerospace and defense, environmental monitoring, and others. In the automotive sector, sensors are used for engine management, driver assistance systems, and vehicle safety features. Healthcare applications include medical monitoring devices, diagnostics, and wearable health trackers. Consumer electronics rely on sensors for gesture recognition, virtual reality experiences, and fitness tracking. Industrial automation utilizes sensors for monitoring and controlling manufacturing processes. Aerospace and defense sectors use sensors for navigation, surveillance, and weapon systems. Environmental monitoring involves sensors for detecting pollutants, weather conditions, and natural disasters.
**Market Players**
- Some of the key players in the global sensors market include: - Honeywell International Inc. - Infineon Technologies AG - Texas Instruments Incorporated - NXP Semiconductors - STMicroelectronics - TE Connectivity - Analog Devices, Inc. - OMRON Corporation - Broadcom Inc. - Siemens AG
These companies are actively involved in product innovations, strategic partnerships, and mergers and acquisitions to strengthen their market position and cater to the evolving needs of industries utilizing sensor technologies.
https://www.databridgemarketresearch.com/reports/global-sensors-marketThe global sensors market is experiencing a significant growth trajectory propelled by the advancements in technology across various industries. One of the emerging trends in the market is the increasing demand for IoT (Internet of Things) and AI (Artificial Intelligence) applications, which rely heavily on sensor data for decision-making and automation. This trend is fostering the development of smart sensors that can collect and transmit data seamlessly in interconnected systems. The integration of sensors with data analytics and cloud computing is driving efficiency and cost-effectiveness in diverse sectors like manufacturing, healthcare, transportation, and agriculture.
Moreover, the deployment of sensors for predictive maintenance is gaining traction as industries seek to enhance operational efficiency and reduce downtime. Predictive maintenance utilizes sensor data to anticipate equipment failures before they occur, thereby enabling proactive maintenance measures and avoiding costly unplanned shutdowns. This approach is particularly crucial in sectors such as manufacturing, where production disruptions can have significant financial implications.
Furthermore, the sensors market is witnessing a surge in demand for environmental monitoring solutions due to growing concerns about pollution, climate change, and natural disasters. Sensors play a vital role in detecting and measuring various environmental parameters, including air quality, water quality, soil conditions, and seismic activities. Governments and organizations worldwide are increasingly investing in sensor technologies to monitor environmental health and mitigate risks associated with pollution and climate variability.
Additionally, the rising adoption of autonomous vehicles and robotics is driving the demand for advanced sensor technologies such as LiDAR (Light Detection and Ranging) and radar for precise navigation and obstacle detection. These sensors enable autonomous systems to perceive their surroundings accurately and make real-time decisions to ensure safe and efficient operations.
In terms of market dynamics, intense competition among key players is leading to continuous innovation and product development in the sensors market. Companies are focusing on enhancing sensor accuracy, reliability, and energy efficiency to meet the evolving requirements of end-users. Strategic collaborations and partnerships between sensor manufacturers and technology providers are also shaping the market landscape by fostering technological synergies and expanding market reach.
Overall, the global sensors market is poised for robust growth driven by the pervasive adoption of sensor technologies across industries and the increasing emphasis on data-driven decision-making and automation. With ongoing technological advancements and the proliferation of IoT applications, the sensors market is expected to witness sustained expansion in the coming years, opening up new opportunities for market players to capitalize on the evolving needs of a digitized world.**Segments**
Global Sensors Market, By Component (Microcontrollers, Digital-to-Analog Converter (DAC), Amplifiers, Analog-to-Digital Converter (ADC), Transceivers), Type (Radar Sensor, Optical Sensor, Biosensor, Touch Sensor, Image Sensor, Pressure Sensor, Temperature Sensor, Proximity and Displacement Sensor, Level Sensor, Motion and Position Sensor, Humidity Sensor, Accelerometer and Speed Sensor, and Others), Technology (CMOS, MEMS, NEMS, and Others), End User (Electronics, IT and Telecom, Industrial, Automotive, Aerospace and Defense, Healthcare, and Others) – Industry Trends and Forecast to 2031.
The global sensors market is witnessing significant growth and evolution across various segments. Microcontrollers, DACs, amplifiers, ADCs, and transceivers form essential components of sensor systems, enabling signal processing and communication functionalities. The diverse types of sensors available, including radar, optical, biosensors, touch sensors, image sensors, pressure sensors, temperature sensors, proximity sensors, motion sensors, and more, cater to a wide range of applications in different industries. Technologies such as CMOS, MEMS, NEMS, and others are driving innovation and miniaturization in sensor development. End-user industries like electronics, IT and telecom, industrial automation, automotive, aerospace and defense, healthcare, and other sectors are increasingly relying on advanced sensor technologies to enhance operational efficiency and enable smart solutions.
**Market Players**
- Honeywell International Inc. (U.S.) - DENSO CORPORATION (Japan) - OmniVision (U.S.) - Alpha MOS (France) - AMETEK.Inc. (U.S.) - AlphaSense Inc. (U.S.) - BorgWarner Inc. (U.S.) - Figaro Engineering Inc. (Japan) - Emerson Electric Co. (U.S.) - GENERAL ELECTRIC (U.S.) - Industrial Scientific (U.S.) - SAMSUNG (South Korea) - Teledyne Monitor Labs (TML) - STMicroelectronics (Switzerland) - NXP Semiconductors (Netherlands) - Infineon Technologies AG (Germany) - Qualcomm Technologies, Inc. (U.S.) - Microchip Technology Inc. (U.S.) - Texas Instruments Incorporated (U.S.) - Bosch Sensortec GmbH (Germany) - Johnson Controls (Ireland) - Sony Semiconductor Solutions Corporation (Japan)
Key market players in the sensors industry are driving innovation, expansion, and market competitiveness through product development, strategic partnerships, and mergers and acquisitions. These companies are at the forefront of technological advancements, catering to the increasing demand for advanced sensor solutions across diverse industries. With a focus on enhancing sensor performance, accuracy, and efficiency, market players are continuously improving sensor capabilities to meet the evolving needs of end-users. Collaborations between sensor manufacturers and technology providers are fostering synergies and enabling the integration of sensors into various applications, paving the way for new opportunities and growth in the global sensors market.
The sensors market is poised for substantial growth as industries embrace digital transformation, IoT integration, and data-driven decision-making processes. The demand for sensors in autonomous systems, environmental monitoring, predictive maintenance, and smart applications is driving market expansion. With a relentless focus on innovation and technology advancement, market players are well-positioned to capitalize on the evolving landscape of sensor technologies. As sensor deployment continues to proliferate across industries, the global sensors market is set to experience sustained growth and development towards a more interconnected and digitized future.
Explore Further Details about This Research Sensors Market Report https://www.databridgemarketresearch.com/reports/global-sensors-market
Key Insights from the Global Sensors Market :
Comprehensive Market Overview: The Sensors Market is witnessing rapid growth, fueled by innovation and an increasing shift towards digital solutions.
Industry Trends and Projections: The market is forecasted to grow at a CAGR of X%, with trends such as automation and sustainability gaining momentum.
Emerging Opportunities: Growing demand for personalized and green technologies offers emerging business opportunities for new entrants.
Focus on R&D: Companies are heavily investing in research and development to create next-generation solutions and maintain competitive edges.
Leading Player Profiles: Dominant players the market with their advanced offerings and strategic expansions.
Market Composition: The market is a mix of established industry giants and innovative startups, fostering competition and rapid innovation.
Revenue Growth: Consistent revenue growth is driven by rising consumer demand, technological advancements, and new product introductions.
Commercial Opportunities: Expanding into untapped regions and investing in emerging technologies presents substantial commercial opportunities for businesses.
Find Country based languages on reports:
https://www.databridgemarketresearch.com/jp/reports/global-sensors-markethttps://www.databridgemarketresearch.com/zh/reports/global-sensors-markethttps://www.databridgemarketresearch.com/ar/reports/global-sensors-markethttps://www.databridgemarketresearch.com/pt/reports/global-sensors-markethttps://www.databridgemarketresearch.com/de/reports/global-sensors-markethttps://www.databridgemarketresearch.com/fr/reports/global-sensors-markethttps://www.databridgemarketresearch.com/es/reports/global-sensors-markethttps://www.databridgemarketresearch.com/ko/reports/global-sensors-markethttps://www.databridgemarketresearch.com/ru/reports/global-sensors-market
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975
Email:- [email protected]"
0 notes
Text
Amphotericin B Prices, News, Trend, Graph, Chart, Monitor and Forecast
Amphotericin B is a crucial antifungal medication widely used in the treatment of systemic fungal infections. As demand for antifungal treatments continues to grow, the market dynamics surrounding Amphotericin B prices are shaped by multiple factors, including raw material costs, supply chain challenges, regulatory frameworks, and global healthcare needs. The pricing of Amphotericin B is influenced by the cost of production, which includes active pharmaceutical ingredients (APIs) and excipients, as well as the complexities of the fermentation process involved in its synthesis. The drug is primarily derived from Streptomyces nodosus, and any disruption in the availability of this raw material can impact production costs, leading to fluctuations in market prices. Additionally, manufacturers must adhere to stringent quality control regulations and Good Manufacturing Practices (GMP), which further contribute to pricing variations across regions.
The global market for Amphotericin B is driven by increasing incidences of fungal infections, particularly among immunocompromised individuals, including cancer patients, organ transplant recipients, and those with HIV/AIDS. The growing prevalence of mucormycosis, also known as black fungus, has led to a surge in demand, particularly in developing countries with inadequate healthcare infrastructure. This rising demand, coupled with supply shortages during critical health crises, has caused significant price volatility. Manufacturers have responded by ramping up production and exploring alternative formulations, such as liposomal Amphotericin B, which offers better efficacy and reduced toxicity but comes at a higher cost. The introduction of generics has also played a key role in market pricing, as increased competition often results in more affordable options for consumers.
Get Real time Prices for Amphotericin B: https://www.chemanalyst.com/Pricing-data/amphotericin-b-1447
Regional disparities in Amphotericin B prices are evident due to differences in healthcare policies, government subsidies, and market regulations. In countries where healthcare systems provide financial support for essential medicines, patients can access the drug at relatively lower prices. On the other hand, in regions with high import duties and limited domestic production, prices tend to be significantly higher. The presence of major pharmaceutical companies and contract manufacturing organizations (CMOs) in certain regions also affects pricing strategies. North America and Europe are key markets with established regulatory frameworks that ensure drug safety and efficacy, but pricing pressures due to reimbursement policies and generic competition can lead to cost reductions. In contrast, emerging markets in Asia-Pacific and Latin America face challenges related to affordability, making price negotiations with manufacturers crucial for government agencies and healthcare providers.
Technological advancements and innovations in drug formulation have contributed to pricing variations in the Amphotericin B market. The development of liposomal Amphotericin B, which reduces nephrotoxicity and enhances patient outcomes, has led to a premium pricing structure compared to conventional formulations. Pharmaceutical companies investing in research and development (R&D) to improve drug delivery mechanisms and extend shelf life are also influencing market trends. The competition between branded and generic versions has created price disparities, with generic manufacturers focusing on cost-effective production to gain a competitive edge. While generic options provide affordability, regulatory challenges related to bioequivalence and market approvals can sometimes delay their availability in certain regions.
Government policies and pricing regulations play a pivotal role in shaping the Amphotericin B market. Price control measures imposed by regulatory bodies in different countries influence the accessibility and affordability of the drug. In markets where pharmaceutical pricing is strictly regulated, companies may face challenges in maintaining profitability, leading to potential supply constraints. On the other hand, in deregulated markets, pricing is often dictated by demand-supply dynamics and competitive forces. Health organizations and non-governmental organizations (NGOs) have been actively involved in ensuring that essential medicines like Amphotericin B remain accessible, particularly in low-income regions where affordability remains a key concern.
The competitive landscape of the Amphotericin B market is marked by the presence of established pharmaceutical giants and emerging players seeking to expand their market share. Companies are engaging in strategic collaborations, mergers, and acquisitions to strengthen their distribution networks and enhance production capabilities. The role of contract manufacturing organizations has grown significantly, enabling pharmaceutical firms to optimize costs and improve supply chain efficiency. The increasing focus on sustainable manufacturing practices and regulatory compliance has also influenced pricing structures, with companies investing in eco-friendly production processes to align with global standards.
Market forecasts suggest that Amphotericin B prices will continue to be influenced by evolving healthcare trends, regulatory developments, and technological innovations. The rising burden of fungal infections and the need for effective antifungal treatments are expected to drive sustained demand for the drug. While price fluctuations may persist due to raw material costs and supply chain dynamics, ongoing efforts to enhance production capacities and streamline distribution channels are likely to contribute to price stabilization. Pharmaceutical companies are also exploring novel formulations and combination therapies to improve treatment efficacy while addressing cost concerns.
The future of the Amphotericin B market will be shaped by collaborative efforts between governments, pharmaceutical companies, and healthcare organizations to ensure the drug remains accessible and affordable. Investments in research, infrastructure development, and strategic partnerships will play a crucial role in determining pricing trends. The increasing adoption of digital technologies in supply chain management is expected to enhance market transparency and improve price predictability. As healthcare systems worldwide continue to prioritize the management of infectious diseases, Amphotericin B will remain a vital component of antifungal therapy, with pricing dynamics reflecting the balance between innovation, affordability, and regulatory compliance.
Get Real time Prices for Amphotericin B: https://www.chemanalyst.com/Pricing-data/amphotericin-b-1447
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Amphotericin B#Amphotericin B Price#Amphotericin B Prices#India#united kingdom#united states#Germany#business#research#chemicals#Technology#Market Research#Canada#Japan#China
0 notes
Text
Hybrid Bonding Technology: Transforming the Semiconductor Industry
The semiconductor industry is evolving rapidly, driven by the need for increased performance, miniaturization, and energy efficiency. Among the many advancements, hybrid bonding technology has emerged as a game-changer in chip packaging and interconnect solutions. This cutting-edge technique is revolutionizing device integration, enhancing chip density, and improving electrical and thermal performance.
This blog provides an in-depth analysis of the hybrid bonding technology market, highlighting key trends, growth drivers, market segmentation, competitive landscape, and future prospects.
Understanding Hybrid Bonding Technology
Hybrid bonding is an advanced wafer-level packaging technique that enables direct interconnection between semiconductor devices at the molecular level. Unlike traditional bonding methods, hybrid bonding eliminates the need for solder or adhesives, reducing interconnect resistance and improving electrical performance. This technology is widely used in 3D ICs, MEMS, CMOS image sensors, and high-performance computing applications.

Market Overview
The global hybrid bonding technology market is experiencing significant growth, driven by increasing demand for high-performance computing, AI-driven applications, 5G infrastructure, and advanced semiconductor packaging. According to industry reports, the market was valued at approximately $250 million in 2022 and is expected to grow at a CAGR of 21.5% from 2023 to 2030.
Key Market Drivers
Rising Demand for Advanced Packaging: Hybrid bonding enables higher chip integration, boosting performance for AI, 5G, and IoT applications.
Growth in High-Performance Computing (HPC): The increasing need for efficient data processing and storage solutions is driving adoption.
Miniaturization Trends: Semiconductor manufacturers are focusing on reducing device size while enhancing functionality.
Improvements in Power Efficiency: Hybrid bonding reduces interconnect resistance, leading to lower power consumption and improved thermal management.
Expansion of CMOS Image Sensors: The adoption of hybrid bonding in image sensors enhances resolution and performance, benefiting industries like automotive and consumer electronics.
Market Segmentation
By Application:
3D ICs & Memory Stacking – Used in high-density memory and logic devices.
CMOS Image Sensors – Enhancing image resolution and efficiency.
MEMS & Sensors – Improving the performance of microelectromechanical systems.
High-Performance Computing – Boosting AI-driven applications and data centers.
By End-User Industry:
Consumer Electronics – Smartphones, wearables, and advanced imaging devices.
Automotive – Enabling next-gen ADAS and autonomous vehicle technologies.
Telecommunications – Supporting 5G and next-gen networking infrastructure.
Healthcare & Medical Devices – Enhancing biomedical sensors and imaging solutions.
By Region:
North America: Leading market due to strong semiconductor R&D and manufacturing hubs.
Europe: Growing investments in semiconductor packaging and automotive electronics.
Asia-Pacific: Rapid expansion of semiconductor fabrication in China, Taiwan, and South Korea.
Rest of the World: Increasing adoption of advanced semiconductor technologies.
Competitive Landscape
Several major players are investing in hybrid bonding technology, including:
TSMC – Leading in advanced packaging solutions.
Intel Corporation – Driving innovation in 3D stacking and chiplet technologies.
Samsung Electronics – Expanding hybrid bonding applications in memory and processors.
Sony Corporation – Advancing hybrid bonding in CMOS image sensors.
Amkor Technology – Enhancing semiconductor packaging and interconnect solutions.
Challenges and Future Prospects
Despite its rapid adoption, hybrid bonding faces challenges such as high initial costs, complex manufacturing processes, and the need for precision alignment. However, ongoing research and advancements in automated bonding technologies, AI-driven defect detection, and enhanced process scalability are expected to overcome these hurdles.
Conclusion
Hybrid bonding technology is set to redefine semiconductor packaging, offering higher performance, better efficiency, and superior interconnect solutions. As demand for AI, 5G, and IoT-driven applications grows, hybrid bonding will play a crucial role in enabling next-generation semiconductor innovations.
The future of semiconductor technology lies in advanced packaging solutions like hybrid bonding. Companies investing in this technology today are poised to lead the next wave of computing advancements.
Stay ahead of the curve—explore the potential of hybrid bonding technology and unlock new opportunities in the semiconductor industry!
0 notes
Text
The Rise of CMOs: How Outsourcing is Transforming Pharma Manufacturing
https://jpcdn.it/img/e030d2be4cc14e79bf66420ff3b18cbe.jpg
In recent years, the pharmaceutical industry has undergone a significant transformation, with Contract Manufacturing Organizations (CMOs) playing a pivotal role in this evolution. As companies face increasing pressure to reduce costs, accelerate time-to-market, and navigate complex regulatory landscapes, CMOs have emerged as strategic partners, reshaping the way drugs are developed and manufactured. This blog explores the rise of CMOs and how outsourcing is driving innovation and efficiency in the pharmaceutical sector.
The Role of CMOs in Pharma Manufacturing
CMOs are third-party organizations that provide manufacturing services to pharmaceutical and biotechnology companies. These services range from drug development and formulation to large-scale commercial production. By outsourcing manufacturing to CMOs, pharmaceutical companies can focus on their core competencies, such as drug discovery and marketing, while leveraging the specialized expertise and advanced infrastructure of their CMO partners.
Key Drivers of CMO Growth
Cost Efficiency:
Establishing and maintaining in-house manufacturing facilities is capital-intensive. CMOs allow companies to avoid these upfront costs by providing access to state-of-the-art facilities and equipment.
Outsourcing also helps companies optimize operational expenses, as CMOs often operate at scale, reducing per-unit production costs.
Accelerated Time-to-Market:
Speed is critical in the competitive pharmaceutical landscape. CMOs have the expertise and resources to streamline production processes, enabling faster development and commercialization of drugs.
Their experience in navigating regulatory approvals ensures compliance without unnecessary delays.
Flexibility and Scalability:
CMOs offer flexible solutions that adapt to changing market demands. Companies can scale production up or down without the need for significant internal adjustments.
This flexibility is especially valuable for smaller biotech firms that may not have the resources to build their own facilities.
Access to Expertise:
CMOs specialize in specific areas, such as biologics, small molecules, or advanced drug delivery systems. Partnering with them provides access to cutting-edge technology and technical know-how.
Many CMOs invest heavily in R&D, which translates into innovative solutions for their clients.
Global Reach:
With a network of facilities across multiple regions, CMOs enable pharmaceutical companies to access global markets efficiently. This is particularly important for meeting regulatory requirements in different countries.
How CMOs Are Transforming the Industry
Advancing Biologics Manufacturing:
The rise of biologics and biosimilars has created a demand for specialized manufacturing capabilities. CMOs with expertise in cell and gene therapies, monoclonal antibodies, and vaccines are leading this transformation.
Driving Digitalization:
Many CMOs are adopting digital technologies, such as automation, artificial intelligence, and IoT, to enhance production efficiency and quality control. These advancements ensure consistency and reduce errors in manufacturing.
Fostering Innovation:
CMOs often act as innovation hubs, collaborating with pharmaceutical companies to develop novel formulations and delivery systems. This collaboration accelerates the introduction of new therapies to the market.
Promoting Sustainability:
Environmental concerns are driving CMOs to adopt sustainable manufacturing practices. From reducing waste to using renewable energy, CMOs are contributing to a greener pharmaceutical industry.
Challenges and Considerations in Outsourcing
While CMOs offer numerous benefits, outsourcing also comes with its challenges:
Quality Control: Ensuring consistent product quality across different CMO facilities can be complex.
Regulatory Compliance: Pharmaceutical companies must ensure their CMOs adhere to stringent regulatory standards.
Intellectual Property (IP) Protection: Safeguarding proprietary information and formulations is critical when working with external partners.
Dependence on Partners: Relying heavily on a single CMO can pose risks if there are disruptions in their operations.
To mitigate these challenges, companies must establish robust contractual agreements, conduct thorough due diligence, and maintain strong communication with their CMO partners.
The Future of CMOs in Pharma
As the pharmaceutical industry continues to evolve, the role of CMOs is expected to grow even further. Key trends shaping the future include:
Increased Focus on Personalized Medicine: CMOs will play a crucial role in manufacturing small-batch, customized therapies for individual patients.
Expansion of Emerging Markets: The demand for affordable medicines in developing countries will drive CMO growth in these regions.
Integration of AI and Automation: Advanced technologies will enable CMOs to deliver even greater efficiency, quality, and speed.
Conclusion
The rise of CMOs has transformed pharmaceutical manufacturing, enabling companies to innovate, scale, and compete in an increasingly complex market. By leveraging the expertise and capabilities of CMOs, pharmaceutical firms can focus on their strengths while delivering high-quality medicines to patients around the world. As the industry continues to embrace outsourcing, CMOs will remain at the forefront of this transformation, driving progress and redefining the future of pharma manufacturing.
0 notes