#clinical trial development
Explore tagged Tumblr posts
Text



Little lazy thing I made
#angel clinical trial#lee clinical trial#clinical trial game#lee smith#angel martinez#I hc that once Lee and Angel get together lee can become very handsy. (with angels consent ofc. )#like once he starts he has a really hard time stopping#this develops over time
37 notes
·
View notes
Text

#tooth regrowth#japan#medical research#dentistry#innovation#healthcare#drug development#clinical trials#science
139 notes
·
View notes
Text
Healthcare systems are flawed and frustrating and bureaucratic and I absolutely understand why people blame that for why people have an urge to turn to "alternative medicine" grifters instead.
But please, please stop.
A fundamental difference between ethical medical practitioners and grifters is that an ethical medical practitioner recognizes the limits of their own knowledge and resources. They will tell you "I don't know what's wrong," and "There's no treatment for this". They know that modern medicine can't fix everything. Sometimes there just isn't a treatment. Sometimes partial effectiveness is as good as it gets. Sometimes managing symptoms is all science knows how to do so far. Sometimes the honest answer is that the damage is permanent and you're going to have to learn to live with disability. Yes, there are bad doctors out there who are unhelpful and/or make things worse, but there are also simply limits to what modern medicine can do.
And understandably, that's frustrating! It sucks to get told that whatever problems you're having are insoluble or unfixable. People want answers and solutions and at least a chance that whatever they're struggling with will go away.
Which is where grifters come in. A grifter will always tell you that your problems can all be solved, your suffering will all go away, you just have to give them money and do what they tell you. They are happy to say anything that will keep you coming back to them, to offer false hope. And no one else is willing to make these promises of health and happiness and wholeness, because honest people know that it's a lie and not everything can be fixed. So the desperate keep going back to grifters, drifting from one con to the next or accumulating them like collectibles, sure that somewhere out there is someone who can perform the miracle they so desperately want.
I don't know what the solution to this is, other than maybe general mental health support to help people come to terms with having disabilities and illnesses and generally less-than-perfect health. But asking doctors to be dishonest, to promise miracles they can't perform, is not it.
#rants#healthcare#I have various chronic illness issues#and some of them have become a lot more manageable in the last twenty years with new medical developments#but that means I also recognize that when I was suffering from them as a child#those treatments weren't available!#and some of those diagnosis were almost unheard of#I started having intermittent knee problems as an underweight eight year old#HEDS was basically unknown at the time and I certainly wasn't a severe enough case to be identified#the only allergy treatment that has really worked for me was developed within the last twenty years and still isn't out of clinical trials#that's not anyone's fault and my childhood doctors weren't hiding things or lying#it was just the reality of the limits of modern medicine
9 notes
·
View notes
Text
Been reading a lot about diseases and public health and I fear it's making me weird
#claire chit chats#im reading dr fauci's memoir rn and its v good#a lot of it is the aids crisis and not just the political side but the clinical trials and vaccine development#i am excited to see if he talks about ebola tho since he was in charge during the 2014 outbreak
7 notes
·
View notes
Text

As we grow, we collaborate with trusted partners and clients who resonate with our vision and play a key role in propelling us forward. A commitment to quality and operational excellence lies at the heart of our processes. Our offerings span Pharmaceuticals, Agro solutions, Specialty Chemicals, along with comprehensive CRO and CDMO services.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#pharma industry#pharma#pharmaceutical#big pharma#contract development#custom synthesis#custom development
2 notes
·
View notes
Text
Just realised I'm actually studying cancer cure
#point is this stuff is real but there's not yet enough data to go to phase 3 clinical trials#and I kinda stopped being surprised that they work because i keep seeing the same results like come on guys it works go on put it on people#(they can't but probably will soon)#but I've spent two? three? years reading and cataloguing and writing what other people have done#in a week i will start doing my own thing#and I'm here#developing a cure for cancer#and feeling overwhelmed because in a year i will have to find a real job#like#that sounds unbelievable#back to ferroptosis this paper won't write itself
7 notes
·
View notes
Text
Is the Drug Discovery Services Market Ready for a Revolution?

Introduction: A Transformative Era for Drug Discovery Services
The global drug discovery services market is experiencing unprecedented growth, fueled by the rising demand for innovative therapeutics, breakthrough technologies, and evolving research dynamics. The drug discovery sector is projected to escalate from $17.47 billion in 2025 to approximately $29.45 billion by 2032. This robust expansion is propelled by several key factors: the escalating need for personalized medicine, the increasing prevalence of chronic diseases, and the growing investment in biotechnology and pharmaceutical R&D.
Pharmaceutical companies and biotech firms are increasingly outsourcing drug discovery processes to specialized contract research organizations (CROs), aiming to enhance efficiency, mitigate costs, and accelerate time-to-market. The increasing importance of precision medicine is reshaping the landscape, with an emphasis on developing treatments tailored to individual genetic profiles.
Request Sample Report PDF (including TOC, Graphs & Tables): https://www.statsandresearch.com/request-sample/40632-global-drug-discovery-services-market
Market Dynamics: Drivers, Challenges, and Opportunities
Key Drug Discovery Services Market Drivers
Technological Advancements: The integration of artificial intelligence (AI), machine learning (ML), high-throughput screening (HTS), and computational modeling is revolutionizing drug discovery processes. These innovations significantly enhance target identification, drug screening, and lead optimization, expediting the discovery of novel therapeutics.
Increased R&D Investments: Pharmaceutical companies and biotech firms are ramping up investments in R&D, particularly in the development of biologics, small molecules, and RNA-based therapeutics. The focus on targeted therapies and gene editing technologies like CRISPR is further propelling this market.
Chronic Disease Prevalence: The rise in chronic diseases such as oncology, cardiovascular diseases, and neurological disorders is driving the demand for innovative drug discovery solutions. With the global aging population and a surge in lifestyle-related diseases, the need for novel and effective treatments has never been greater.
Government Initiatives and Regulatory Support: Regulatory bodies around the world are providing expedited approval pathways for breakthrough therapies, further fueling the demand for innovative drug discovery services. Initiatives like Fast Track and Breakthrough Therapy Designation are accelerating drug development timelines.
Get up to 30% Discount: https://www.statsandresearch.com/check-discount/40632-global-drug-discovery-services-market
Challenges Facing the Drug Discovery Services Market
Despite its rapid growth, the drug discovery services sector is not without its challenges. The high costs associated with drug development, regulatory hurdles, and long clinical trial timelines are significant obstacles. Moreover, the high failure rates of drug candidates in the discovery phase and clinical trials continue to pose risks for investors and stakeholders.
The complexity of intellectual property rights, evolving regulations, and the challenge of maintaining data privacy in global markets also create barriers to entry for new players in the market.
Segmentation Analysis: Breaking Down the Market by Service, Drug Type, and Therapeutic Area
By Service Type
Biology Services: Accounting for 35% of the market share, biology services dominate the landscape, driven by increasing demand for target identification, biomarker research, and assay development. The projected CAGR for biology services is 11.8%, reflecting the increasing reliance on advanced biological research in drug discovery.
Medicinal Chemistry Services: Holding a 30% market share, medicinal chemistry services are expected to grow at a CAGR of 12.5%. This growth is attributed to the rising emphasis on small-molecule drug discovery and AI-driven screening methods that enhance the efficacy of lead compounds.
Toxicology Services: As drug safety is paramount, the toxicology services segment is gaining traction, particularly in the preclinical development phase, ensuring the safety of drug candidates before they proceed to clinical trials.
Preclinical Development: Preclinical services are essential for evaluating a drug’s pharmacokinetics, toxicity, and efficacy in animal models. This segment continues to expand, driven by the increasing complexity of drugs under development.
By Drug Type
Small Molecules: The small molecules segment, accounting for 55% of the market share, is the dominant player due to the long-standing role small molecules play in treating chronic diseases. Small molecules have established manufacturing processes, high market penetration, and cost-effective production. This segment is projected to grow at a CAGR of 10.5%.
Biologics: Biologics, including monoclonal antibodies, gene therapies, and cell therapies, are on the rise, with a projected CAGR of 13.1%. The biologics segment is gaining ground, driven by the increasing focus on immunotherapies, personalized medicine, and next-generation vaccines.
By Therapeutic Area
Oncology: Oncology remains the largest therapeutic area, contributing over 42% of the market share. The rise in cancer cases, coupled with the demand for targeted treatments, is spurring growth in this segment. Immunotherapies and precision oncology are transforming the landscape.
Neurology: With a market share of 15%, the neurology sector is poised for robust growth, with significant breakthroughs in Alzheimer's, Parkinson's, and multiple sclerosis treatments.
Infectious Diseases: The need for novel treatments in the face of rising antimicrobial resistance (AMR) and emerging pathogens is driving the growth of the infectious diseases sector. This area represents approximately 12% of the market, expanding at a CAGR of 10.8%.
By Process Stage
Target Discovery & Validation: Accounting for 30% of the market, this stage benefits from the latest advances in genomics and proteomics, enabling more precise identification of drug targets. The projected CAGR of 11.7% reflects the growing importance of early-stage discovery in streamlining drug development.
Lead Optimization: This segment, responsible for refining drug candidates, is expected to grow at a CAGR of 11.3%, as pharmaceutical companies focus on improving efficacy, safety, and bioavailability before clinical trials.
Preclinical Development: The preclinical development stage represents a significant portion of the market. The growing reliance on 3D cell culture models, organ-on-chip technologies, and animal models is driving innovation in this area.
Regional Insights: Global Dynamics and Emerging Markets
North America
North America is the leading market for drug discovery services, driven by significant R&D investments, the presence of major contract research organizations (CROs), and an advanced healthcare infrastructure. The U.S., in particular, is home to many of the world’s largest pharmaceutical companies and has been a key player in driving the global drug discovery market.
Europe
Europe follows closely as the second-largest market, with strong contributions from both government initiatives and collaborations between academic institutions and pharmaceutical companies. The European market benefits from a robust regulatory environment that encourages innovative research.
Asia Pacific
Asia Pacific is the fastest-growing region, with countries like China, India, Japan, and South Korea emerging as key players in the global drug discovery space. The region benefits from cost-effective research, an expanding middle class, and a growing demand for innovative therapeutics.
Competitive Landscape: Key Players and Strategic Developments
The drug discovery services market is highly competitive, with major players continuously expanding their service offerings to meet the rising demand for specialized drug development solutions.
WuXi AppTec: This leading CRO has significantly enhanced its service portfolio through strategic acquisitions and partnerships, expanding its capabilities in biologics and gene therapies.
Charles River Laboratories: A key player in preclinical research, Charles River provides comprehensive drug discovery services, including toxicology and medicinal chemistry services.
Evotec AG: With its strong presence in high-throughput screening, Evotec is well-positioned to capitalize on the growth of AI-driven drug discovery.
Recent Developments
WuXi Biologics sold its vaccine manufacturing facility in Ireland to Merck & Co. for $500 million in January 2025, shifting its focus towards more strategic areas of drug discovery.
Halozyme Therapeutics proposed a $2 billion acquisition of Evotec in late 2024, aiming to combine drug-delivery technologies with proprietary drug discovery platforms.
Purchase Exclusive Report: https://www.statsandresearch.com/enquire-before/40632-global-drug-discovery-services-market
Future Outlook: Shaping the Future of Drug Discovery
The global drug discovery services market is poised for continued growth, driven by the increasing emphasis on precision medicine, AI and machine learning technologies, and innovations in biologics and small molecules. As new research tools, platforms, and collaborations emerge, the market will continue to evolve, offering exciting opportunities for innovation and scientific breakthroughs.
To remain competitive, industry stakeholders must adapt to evolving regulatory environments, embrace next-generation technologies, and focus on developing personalized and targeted therapies that cater to the unique needs of patients worldwide.
Our Services:
On-Demand Reports: https://www.statsandresearch.com/on-demand-reports
Subscription Plans: https://www.statsandresearch.com/subscription-plans
Consulting Services: https://www.statsandresearch.com/consulting-services
ESG Solutions: https://www.statsandresearch.com/esg-solutions
Contact Us:
Stats and Research
Email: [email protected]
Phone: +91 8530698844
Website: https://www.statsandresearch.com
#Drug Discovery Services#Drug Discovery Market#Pharmaceutical Services#Clinical Trials#Drug Development#Drug Discovery Outsourcing#Global Drug Discovery Market#AI in Drug Discovery#Precision Medicine#Drug Discovery CRO#Biopharma Market Trends#Drug Discovery Market Size#Drug Discovery Industry Report
1 note
·
View note
Text

Intrinseque Health - Clinical Supply Chain We Build and Execute Complex Clinical Supply Plans
Intrinseque Health is an EN ISO 13485 Certified Global clinical trial support services provider committed to the utmost in service delivery to drug development organizations (Pharmaceutical, Biotechnology, Medical Device & Contract Research Organizations (CROs)). Our team of industry professionals has over 300 years of combined experience supporting global clinical trials across a wide array of therapeutic areas. This vast experience enables us to empathize with our Customers while providing best-in-class solutions to overcome the hurdles and pain-points of conducting a clinical trial.
Regions & countries throughout the world will often present a unique set of regulatory and logistical challenges. It is our responsibility to understand and overcome these while ensuring that your products, supplies, equipment and services are available where needed to ensure study timelines are met. Intrinseque Health utilizes an operational methodology that is based on proven, cost-effective clinical supply chain strategy for each clinical trial. Our practice is to engage with our customers, early and often to ensure implementation of a robust clinical supply plan, resulting in the most successful study start-up and initiation.
#clinical supply#clinical trials#health#healthcare#supplychainmanagement#clinical supply chain#clinical supply chain management#drug development
2 notes
·
View notes
Text
How does one develop vaccines for emerging infectious diseases?
Emerging infectious diseases pose significant threats to global public health. Rapid and effective development of vaccines is crucial in mitigating the impact of these diseases. This article explores the process of developing vaccines for emerging infectious diseases, highlighting the key steps involved and the challenges faced. Understanding the development process is essential to appreciate the…
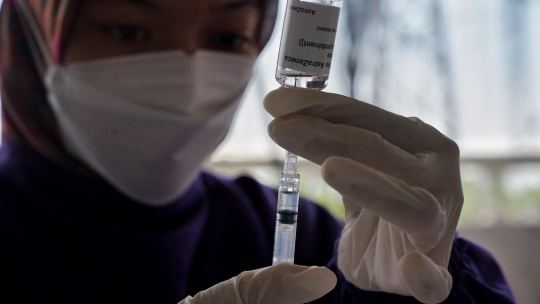
View On WordPress
#clinical trials#community immunity#disease outbreak control#Emerging infectious diseases#Equitable access#global health security#multidisciplinary approach#pathogen identification#preclinical studies#preparedness and response#public health collaboration#rapid response#regulatory approval#research and development#safety and efficacy#target antigen selection#vaccine design#vaccine development#Vaccine distribution#vaccine production
1 note
·
View note
Text

Zenovel provides expert Clinical Operations (CO) and Non-Clinical Operations (NCO) support, ensuring efficient, compliant, and successful drug trials and clinical studies from planning to completion.
#drug trials#clinical studies#drug development#good manufacturing practices guidelines#risk based monitoring clinical trials#clinical research center#quality assurance in clinical trials
0 notes
Text
Global Neurology Clinical Trials Market: Emerging Trends, Innovations, and Growth Projections for 2025 and Beyond
Across the world, neurological diseases are placing an ever-growing burden on healthcare systems and patients alike. Responding to this urgent challenge, the Global Neurology Clinical Trials Market is experiencing remarkable growth. From advanced drug development to digital trial technologies, this sector is transforming how treatments for brain disorders are discovered, tested, and brought to patients.
Market Landscape
In 2024, the Global Neurology Clinical Trials Market is valued at USD 5,549.3 million. Over the next several years, it is expected to expand at a compound annual growth rate (CAGR) of 5.8%, reaching USD 9,231.2 million by 2033. This growth trajectory underscores the urgent need for breakthroughs in treating conditions such as Alzheimer’s, Parkinson’s, Huntington’s, epilepsy, and multiple sclerosis.
The surge in clinical research activity is fueled by scientific advances, increased funding, and an evolving regulatory landscape that encourages innovation. With new tools and technologies emerging rapidly, the future of neurology trials looks promising—and potentially life-changing for millions worldwide.
Receive a Free PDF Sample Copy for In-Depth Analysis@ https://dimensionmarketresearch.com/report/neurology-clinical-trials-market/request-sample/
Major Growth Drivers
Increasing Prevalence of Neurological Disorders
Neurological diseases rank among the leading causes of disability and death globally. For example, Alzheimer’s disease currently affects over 55 million people, and that number is poised to rise dramatically in coming decades. The pressing need for more effective treatments is pushing clinical research to the forefront, driving investment and new trial initiatives.
Advancements in Therapeutic Strategies
Innovations in gene therapy, biologics, and precision medicine are redefining neurology trials. Modern research focuses on tailoring treatments to specific patient profiles, utilizing biomarkers and genetic data to identify who is most likely to benefit. This targeted approach not only increases the chances of trial success but also speeds up development timelines.
Rise of Digital and Decentralized Trials
Technology is revolutionizing clinical research. Remote monitoring tools, mobile apps, and wearable devices now enable decentralized trial models, making participation more accessible to patients—especially those with mobility or cognitive challenges. These digital solutions enhance patient engagement, improve data quality, and reduce costs.
Growing Financial Support
Governments, non-profit organizations, and private investors are significantly increasing funding for neurological research. Recognizing the enormous societal costs of these diseases, stakeholders are eager to support new discoveries. Pharmaceutical and biotech firms are also pouring resources into neurology, aiming to develop new drugs and secure a share of this expanding market.
Regional Dynamics
North America
North America is set to remain the dominant force in the Global Neurology Clinical Trials Market, projected to capture 48.10% of the market in 2024. The region benefits from strong research institutions, extensive funding channels, and an established regulatory framework. In the U.S., organizations such as the National Institutes of Health (NIH) continue to drive significant investments into neurological studies.
Europe
Europe stands as a major player in the neurology trials arena. Countries like Germany, the United Kingdom, and France are key contributors, with robust research networks and a supportive regulatory environment. Collaborative efforts between academia and industry are propelling the region’s neurology research forward.
Asia-Pacific
Asia-Pacific is increasingly becoming a significant region for neurology clinical research. Growing healthcare investments, larger patient pools, and strengthening clinical research infrastructure are positioning countries like China, India, and Japan as emerging leaders in the neurology trials market.
Explore Detailed Research Methodology@ https://dimensionmarketresearch.com/report/neurology-clinical-trials-market/request-methodology/
Challenges Ahead
Despite strong momentum, the Global Neurology Clinical Trials Market faces considerable hurdles:
Patient Recruitment: Neurological trials often involve elderly patients or individuals with cognitive impairments, making recruitment and retention complex.
High Costs: Neurology trials can be expensive due to specialized imaging, long follow-up periods, and intricate data collection needs.
Regulatory Hurdles: Navigating diverse regulatory environments across countries can slow down trial processes.
Ethical Considerations: Trials involving vulnerable populations require careful handling of consent and patient protection issues.
Innovations such as adaptive trial designs and digital tools are helping address these challenges, making research more efficient and patient-friendly.
Looking Forward
The future of the Global Neurology Clinical Trials Market is bright. Technological advancements, deeper understanding of disease mechanisms, and the rise of precision medicine will continue to transform the field. As research becomes more sophisticated and efficient, stakeholders stand to unlock new therapeutic possibilities that could revolutionize how neurological conditions are treated.
For organizations investing in this space, the coming decade offers significant opportunities to contribute to transformative breakthroughs and capture value in a market poised for sustained growth.
Frequently Asked Questions
1. How large is the Global Neurology Clinical Trials Market in 2024? It’s valued at USD 5,549.3 million.
2. What growth rate is forecasted for this market? The market is projected to grow at a CAGR of 5.8% through 2032.
3. Which region leads the market? North America is expected to hold 48.10% of the market in 2024.
4. What’s fueling the market’s expansion? Key drivers include rising disease prevalence, new therapeutic technologies, digital health innovations, and increased investment.
5. What are the key challenges in neurology trials? Recruitment difficulties, high operational costs, complex regulatory requirements, and ethical considerations pose challenges to market growth.
Conclusion
The Global Neurology Clinical Trials Market is at a pivotal moment. Driven by scientific innovation, expanding investments, and an urgent need for new treatments, the sector is rapidly evolving. As technologies and research methodologies advance, neurology trials are becoming more efficient and impactful, bringing hope for breakthroughs that could transform millions of lives worldwide.
#Neurology#Clinical Trials#Pharma Research#Healthcare Innovation#Neuroscience#Biotech#Life Sciences#Drug Development#Market Research#Healthcare Trends#Digital Health#CRO#Clinical Research#Neurodegeneration#Precision Medicine#Global Health#Medical Research#Clinical Development#Alzheimers#Healthcare Business
0 notes
Text
Hi everyone, I wanted to share some promising developments about a nasal COVID-19 vaccine:

"THIS IS HUGE! Researchers have developed a nasal COVID-19 vaccine that BLOCKS transmission of the virus. This suggests vaccines delivered directly to the nose or mouth could play a CRITICAL role in containing the spread of respiratory infections. Phase I clinical trials HAVE BEEN APPROVED!"
Link to said study:
Link to thread on Bluesky: /profile/sailorrooscout.bsky.social/post/3kyoj6hgihr2v
20K notes
·
View notes
Text
What Factors Are Driving the Growth of the Cardiac Safety Services Market in 2024?
The Cardiac Safety Services Market Size was valued at USD 740 Million in 2023 and is projected to reach an impressive USD 1,888.34 Million by 2032, expanding at a robust CAGR of 11.48% during the forecast period of 2024–2032. This significant growth trajectory reflects the rising demand for cardiac safety monitoring in drug development and the increasing prevalence of cardiovascular diseases globally. Cardiac Safety Services Market Size
https://www.snsinsider.com/assets/images/report/1731997958-709192537.png
The surge in outsourcing activities by pharmaceutical and biotechnology companies, coupled with stringent regulatory requirements from bodies like the FDA and EMA, are major growth catalysts. Additionally, technological advancements in cardiac imaging and diagnostics, such as ECG and telemetry monitoring, are boosting the adoption of cardiac safety services across preclinical and clinical trials.
Increased Regulatory Scrutiny Fueling Market Expansion
As regulatory agencies demand more rigorous cardiac evaluations during drug trials, companies are seeking specialized services to ensure compliance. The Cardiac Safety Services Market has emerged as a critical support industry, offering ECG/Holter monitoring, Thorough QT (TQT) studies, and integrated cardiac safety data analysis. These services help identify cardiovascular risks early in the drug development process, ultimately improving patient safety and reducing late-stage trial failures.
North America Leading, Asia-Pacific Emerging
Geographically, North America remains the dominant market, driven by the presence of major CROs, cutting-edge research institutions, and favorable regulatory frameworks. Asia-Pacific, on the other hand, is projected to witness the highest growth due to increased R&D spending, expanding clinical trial infrastructure, and rising demand for cost-effective cardiac safety solutions.
Key Players and Competitive Landscape
Key market participants include Bioclinica, Celerion, Medpace, Banook Group, Laboratory Corporation of America Holdings, Certara, and Richmond Pharmacology, among others. These companies are continually enhancing their cardiac safety portfolios through strategic partnerships, technology integrations, and service expansions.
Strategic Developments Boosting Market Dynamics
Medpace recently expanded its cardiac safety capabilities by integrating artificial intelligence into its ECG interpretation services.
Certara has been focusing on improving modeling and simulation solutions for predicting cardiac toxicity.
Bioclinica launched a cloud-based cardiac safety platform to streamline data management and regulatory compliance.
These innovations underline the industry's shift toward precision medicine and real-time cardiac monitoring.
Growing Demand for Cardiac Safety in Oncology and CNS Trials
With the rising complexity of oncology and CNS drug trials, cardiac safety has become an essential component of study protocols. As more drugs with potential cardiotoxic effects enter clinical pipelines, pharmaceutical sponsors are investing in high-quality cardiac safety solutions to mitigate risks and ensure market approval.
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Email: [email protected]
0 notes
Text

We continue to move forward through meaningful partnerships with respected clients and collaborators who align with our mission and support our advancement. Our commitment to delivering quality and operational excellence remains at the core of everything we do. Our offerings span Pharmaceuticals, Agro-based solutions, Specialty Chemicals, and comprehensive CRO and CDMO services. OctaneX Labs also partners with clients for contract research synthesis across all aspects of Organic and Medicinal Chemistry.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#science#cdmo services#chemical synthesis#chemistry#healthcare#cro services#contract research#custom development projects#custom synthesis#custom content#custom development#cdmo lab#cdmo telangana company
0 notes
Text

MMS, an award-winning CRO, acquires Exploristics and KerusCloud to expand biostatistics & data science capabilities. latest pharma news today
#latest pharma news today#news related to pharma industry#new developments in pharmaceutical industry#MMS acquisition#biostatistics CRO#MMS Holdings#CRO acquisition#Exploristics#KerusCloud#biostatistics services#data science in pharma#pharma CRO news#clinical research organization#pharmaceutical data analytics#drug development statistics#clinical trial simulation#pharma data science#latest pharma news#pharmaceutical industry trends#CRO mergers and acquisitions
1 note
·
View note
Text
Inside Drug Discovery Chemistry: From Molecules to Modern Medicine
Introduction: Understanding Drug Discovery Chemistry Drug discovery chemistry is the science that helps turn small chemical substances into medicines that people use to treat diseases. It plays a big role in modern healthcare by helping create drugs that are safe, effective, and affordable. The process may sound complex, but don’t worry—we’ll explain everything in a simple way. From the first…
#Drug Development Process#Drug Discovery Chemistry#How drugs are discovered#Medicinal chemistry and drug design#Phases of clinical trials#Role of Chemistry in Medicine
0 notes