#charles p boyle
Explore tagged Tumblr posts
Text





Dress from Horizons West (1952) worn by Julie Adams, costume design by Rosemary Odell
#horizons west#1952#western#rosemary odell#julie adams#50s movies#1950s film#budd boetticher#charles p. boyle#charles p boyle#early 50s#robert ryan#raymond burr#dress#silver dress#white dress#period piece#period drama
16 notes
·
View notes
Text


Band of Brothers Birthdays
January
1 John S. Zielinski Jr. (b. 1925)
21 Richard D. “Dick” Winters (b. 1918)
26 Herbert M. Sobel (b. 1912)
30 Clifford Carwood "Lip" Lipton (b. 1920)
31 Warren H. “Skip” Muck (b. 1922) & Robert B. Brewer (b. 1924)
February
8 Clarence R. Hester (b. 1916)
18 Thomas A. Peacock (b. 1920)
23 Lester A. “Les” Hashey (b. 1925)
March
1 Charles E. “Chuck” Grant (b. 1922)
2 Colonel Robert L. “Bob” Strayer (b. 1910)
4 Wayne “Skinny” Sisk (b. 1922)
10 Frank J. Perconte (b. 1917)
13 Darrell C. “Shifty” Powers (b. 1923)
14 Joseph J. “Joe” Toye (b. 1919)
24 John D. “Cowboy” Halls (b. 1922)
26 George Lavenson (b. 1917) & George H. Smith Jr. (1922)
27 Gerald J. Loraine (b. 1913)
April
3 Colonel Robert F. “Bob” Sink (b. 1905) & Patrick S. “Patty” O’Keefe (b. 1926)
5 John T. “Johnny” Julian (b. 1924)
10 Renée B. E. Lemaire (b. 1914)
11 James W. Miller (b. 1924)
15 Walter S. “Smokey” Gordon Jr. (b. 1920)
20 Ronald C. “Sparky” Speirs (b. 1920)
23 Alton M. More (b. 1920)
27 Earl E. “One Lung” McClung (b. 1923) & Henry S. “Hank” Jones Jr. (b. 1924)
28 William J. “Wild Bill” Guarnere (b. 1923)
May
12 John W. “Johnny” Martin (b. 1922)
16 Edward J. “Babe” Heffron (b. 1923)
17 Joseph D. “Joe” Liebgott (b. 1915)
19 Norman S. Dike Jr. (b. 1918) & Cleveland O. Petty (b. 1924)
25 Albert L. "Al" Mampre (b. 1922)
June
2 David K. "Web" Webster (b. 1922)
6 Augusta M. Chiwy ("Anna") (b. 1921)
13 Edward D. Shames (b. 1922)
17 George Luz (b. 1921)
18 Roy W. Cobb (b. 1914)
23 Frederick T. “Moose” Heyliger (b. 1916)
25 Albert Blithe (b. 1923)
28 Donald B. "Hoob" Hoobler (b. 1922)
July
2 Gen. Anthony C. "Nuts" McAuliffe (b. 1898)
7 Francis J. “Frank” Mellet (b. 1920)
8 Thomas Meehan III (b. 1921)
9 John A. Janovec (b. 1925)
10 Robert E. “Popeye” Wynn (b. 1921)
16 William S. Evans (b. 1910)
20 James H. “Moe” Alley Jr. (b. 1922)
23 Burton P. “Pat” Christenson (b. 1922)
29 Eugene E. Jackson (b. 1922)
31 Donald G. "Don" Malarkey (b. 1921)
August
3 Edward J. “Ed” Tipper (b. 1921)
10 Allen E. Vest (b. 1924)
15 Kenneth J. Webb (b. 1920)
18 Jack E. Foley (b. 1922)
26 Floyd M. “Tab” Talbert (b. 1923) & General Maxwell D. Taylor (b. 1901)
29 Joseph A. Lesniewski (b. 1920)
31 Alex M. Penkala Jr. (b. 1924)
September
3 William H. Dukeman Jr. (b. 1921)
11 Harold D. Webb (b. 1925)
12 Major Oliver M. Horton (b. 1912)
27 Harry F. Welsh (b. 1918)
30 Lewis “Nix” Nixon III (b. 1918)
October
5 Joseph “Joe” Ramirez (b. 1921) & Ralph F. “Doc” Spina (b. 1919) & Terrence C. "Salty" Harris (b. 1920)
6 Leo D. Boyle (b. 1913)
10 William F. “Bill” Kiehn (b. 1921)
15 Antonio C. “Tony” Garcia (b. 1924)
17 Eugene G. "Doc" Roe (b. 1922)
21 Lt. Cl. David T. Dobie (b. 1912)
28 Herbert J. Suerth Jr. (b. 1924)
31 Robert "Bob" van Klinken (b. 1919)
November
11 Myron N. “Mike” Ranney (b. 1922)
20 Denver “Bull” Randleman (b. 1920)
December
12 John “Jack” McGrath (b. 1919)
31 Lynn D. “Buck” Compton (b. 1921)
Unknown Date
Joseph P. Domingus
Richard J. Hughes (b. 1925)
Maj. Louis Kent
Father John Mahoney
George C. Rice
SOURCES
Military History Fandom Wiki
Band of Brothers Fandom Wiki
Traces of War
Find a Grave
#this is going off who was on on the show#i double checked the dates and such but if you notice any mistakes please let me know :)#band of brothers#easy company#hbo war#not gonna tag everyone lol#mine: misc#yep it's actually Halls and not Hall#i've seen Terrence Harris's name spelled with as Terence but wenand t with two Rs s#since that's how it's spelled on photos of memorials and on his gravestone#I’ll do the pacific next! should be significantly shorter since there’s far fewer characters 😅
206 notes
·
View notes
Text
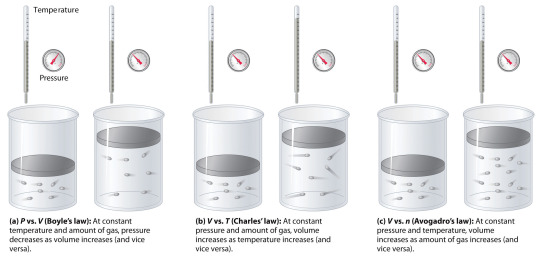
Gas Laws
A gas law is, simply put, a physical law that describes the behavior of gases. There are multiple gas laws, including the following four laws that are more well known than others:
Boyle's Law relates the pressure and volume of a gas. Published by Robert Boyle in 1662 it states that "the volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature". One mathematical representation of this law is P1 x V1 = P2 x V2.
Charles' Law was published in 1787 by Jacques Charles. It relates temperature and volume and states that "volume of a given fixed mass of a dry gas is directly proportional to its absolute temperature at a constant pressure". One mathematical representation of this law is V1/T1 = V2/T2
Gay-Lussac’s Law was published by Joseph Louis Gay-Lussac in 1808 and relates pressure and temperature, stating: "The pressure exerted by a given mass and constant volume of an ideal gas on the sides of its container is directly proportional to its absolute temperature." One mathematical representation of this law is P1/T1 = P2/T2.
Finally, Avogadro's Law relates the amount and volume of a gas. It was published in 1811 by Amedeo Avogadro and states that the "volume occupied by an ideal gas at a constant temperature is directly proportional to the number of molecules of the gas present in the container." One mathematical representation of this law is V1/n1 = V2/n2.
Sources/Further reading: (Image source - LibreTexts) (NIH) (Jove) (Wikipedia)
*P - pressure; V - volume; T - temperature; n - amount of a substance
57 notes
·
View notes
Text
so either im lowkey going insane or the character charles boyle from b99 is a fucking. chemistry joke.
because in gas laws, there's a law called Boyles's Law that states P1V1=P2V2 (p is pressure v is volume it just shows the relationship in gas changes ykw im gonna stop talking now :))
and there's another law called Charles's Law that states that (V1/T1)=(V2/T2) (v is volume t is temperature it shows the inverse relationship between the two yeah im gonna shut up now srry)
so basically just sciency chemistry stuff but still.
Boyles's Law. Charles's Law.
#yeah i think this is just me going crazy#whatever#b99#brooklyn 99#charles boyle#chemistry#i guess?#idk man#mae-rants
5 notes
·
View notes
Text
The gas laws were developed in eighteenth century when scientist realized that there was some relationship that was there between the volume, pressure and the temperature of given sample of gas. This relationship was postulated to be true to all gases irrespective of its physical features. Normally have a similar behavior to the colossal extent of conditions because all gases tend to have their molecules that are widely spaced. The equation that is obeyed by gases is called the equation of state that is derived from the kinetic theory of gases. There are various gas laws, but all are considered to be some particular forms of the ideal gas equation which contains some constant (s). There are different gas laws that exist and are summarized by the equation of state (Meyer, 2011). These gas laws include Boyle’s law, Charles law, Gay – Lussac’s law and other gas law. Part 1: Boyle’s Law Purpose Experiment to investigate the correlation between pressure and volume of a gas. Background The Boyle’s law was derived, finalized and published in 1662. The law states that when gas is at constant temperature, the product obtained from the volume, and the pressure of a certain mass of gas that is confined in a closed system is always a constant. The pressure gauge can be used to verify this statement together with a variable container capacity. The law can also be derived from the kinetic theory of ideal gases. For instance, if a gas container has a fixed number of molecules in it and its volume is reduced more molecules will collide per unit time per given area. This aspect results in a higher pressure in the container (Wang, 2013). The mathematical expression of the law is as shown below: …. (1) PV = k1 or P1V1 = P2V2 …… (2) Where, P – The pressure of the gas. V – The volume of the gas. K1 – The constant of the equation. Procedure 1. Reset the pump and give it a firm push. Wait until the value stabilizes. Observe whether the values of the pump after stabilizing the same? 2. Select the light species of the box on the right-hand corner. You can notice that the pump turns red. At that time, the pump is given a press. Again, wait for the values to stabilize and observe the results. 3. In the box entitled box control move the arrow and remove it and find what happens. The height of the gas container should be 5.3 nm, and the depth is 230 nm. Data Table 1: values of gas pressure and volume at constant temperature. 100 heavy particles constant temperature 300 k° length volume pressure nm nm^3 atm Pascal 9.00 10971.0 0.40 40530.00 8.50 10361.5 0.42 42556.50 8.00 9752.0 0.45 45596.25 7.50 9142.5 0.49 49649.25 7.00 8533.0 0.51 51675.75 6.50 7923.5 0.55 55728.75 6.00 7314.0 0.60 60795.00 5.50 6704.5 0.65 65861.25 Analysis Graph 1: pressure against volume at constant temperature Discussion When the pump is pressed it some while to come to the stable state. When the pump gives a substantial push the volume of the gas reduced. For this experiment volume in an independent variable, since it does depend on the other parameter to change. The pressure is the dependent variable as it depends on the volume change for it to vary. The graph was plotted for the values of the pressure, and the volume showed that the pressure of the gas varies inversely proportionally to the volume of the gas. Conclusion The relationship between the pressure and the volume of gas under constant temperature is summarized by the Boyles Law. This law states the under constant conditions of temperature, gas will have its pressure vary inversely proportional to the volume. Part 2a: Experiment on Charles’ Law Purpose Determination of the relationship between temperature and volume at constant pressure. Background Charles’ Law is also referred to as the law of volumes. It was founded in 1787 by a scientist by the name Jacques Charles. It states that the amount of given mass of gas at constant conditions of pressure is directly proportional to the absolute temperature when in a closed system. The mathematical expression of the law is as shown below (Kotz, Treichel and Townsend, 2010). V T ……… (2) V/T = k2 … (3) It can be summarized as V1/T1 = V2/T2 …… (4) In the above T is the Kelvin temperature of the gas, while V is the resulting volume of the gas at given temperature. Procedure N.B.: To hold pressure constant, the container will be filled some percentage with some gas in it before the start of the experiment. This step is done to ensure that the atmospheric pressure inside the container is not equal to zero. 1. Heat the container while measuring the corresponding values of the volume while keeping the pressure constant (don’t add any gas molecules to the container). 2. Record the readings for various values of the volumes corresponding to the temperature. Data Table 2: Values of volume and temperature of a gas at constant pressure. 100 heavy particles constant pressure (0.22 atm) temperature length Volume Kelvin° nm nm^3 450 9.2 11214.8 400 7.9 9630.1 350 6.3 7679.7 300 5.9 7192.1 250 4.8 5851.2 200 4.5 5485.5 150 3.5 4266.5 100 2.2 2681.8 Analysis The data was plotted in a graph of volume against temperature. The following figure was obtained. Graph 2: Graph of volume against temperature. Discussion In this experiment, the temperature component is an independent variable, and the volume is the dependent variable where it depends on the temperature. In this experiment, there are 2 factors that are held constant, which are pressure and the number of the molecules of the gas. The graph obtained of the volume against temperature is a straight line that does not pass through the origin. Conclusion The relationship of the volume and the temperature of a gas at constant number of molecules and constant pressure are illustrated by the Charles’ law. The law holds for all gases that obey the equation of state. Part 2b: Gay- Lussac’s Law Experiment Purpose Read the full article
0 notes
Text
Alphabetical Character Encyclopedia
Here is the Alphabetized character encyclopedia. There will be spoilers throughout, so read at your own discretion.
Chronological encyclopedia
Master Post
A
Abezithibod
Abraham Van Helsing
Achasiah
Adam Frankenstein
Adelaide Sallow
Albert Dirks
Albrecht Schindewolf/Allan Erkstrom
Alfonso Pari
Amadeus Briggs
Amanda Briggs
Amon, Duke of Hell
Andelin Sylvia Lillemor Lindbek
Andrew Gruell (The Ragdoll of Old)
Antares Briggs
Antonio Pari
Aoife Crawford (ULTRAMagic Devil)
Spiritus Magni Aphrodite
Asclepius, Son of Hermes
Spiritus Magni Athena
Auda Willfort
Aureolus Schindewolf (ULTRAMagic Disciple)
B
Bai’sin
Barna Schindewolf
Barry Esko Boyle (ULTRAMagic Hunter)
Baphomet
The Beast of Old
Beauregard
Berislav Briggs
Bethany Briggs
Bileth, King of Inferno
Blood-Wraith Raynot
Blythe “Witchblade” Finely
Boris Lazarov
Brendan Devilfay
Brenna Thompson
Bronislav
Brooklyn Langley
Brunehilde Skull Thrasher
Spiritus Magni Brutus
King Brutus IV
Buster Ash
C
Caius
Carol the Traveler
Charles Blackwell Ford
Chernobog
Claudius Alfieri
Cliff Steele
The Colossus of Old
The Conspirator of Old
Cordelia Willfort
Corentin Schindewolf
The Crimson Abyss
Cronus (ULTRAMagic Reaper)
D
Daniel
Darkness
David Livesey (ULTRAMagic Magistrate)
Deimos (Eustorgio, The Mage of Old)
Desislav Robles
Dionysus, Lord of Madness
Dolus & Iocus
Donia Albronda
Doppelganger
Dragoslava Raynot
General Drazhan Thornefield
Spiritus Magni Drusa
Dunja Schindewolf
E
Ekaterina Moore
Elaine Gabriella O'Nessie
Eleanor Albronda
Duke Eligio Moretti
Ellen the Wayward
Empress Eliza-Rex/Eloise
King Englehart Schindewolf
Erika Storm
Sir Erling Vang
Dr. Ethan Luminate
Evan Dunn
The Evangelist of Old
F
Fausta Dracul
Faustus Ashman
The Fear of Old
Fenrir
Folkvar Haugen
The Forest of Old
G
Gabriella Pari
Gostislav Robles
The Great Unspeaker
The Grass God
Gratiana Arlotti
The Grave God
Grendel Bombastus Scarfe
Gustav Dahlberg
H
Hades, Lord of the Underworld
Hannibal Skull Thrasher
Hanzou Nagasawa
Heinrik Rofocale
Herman Lydon
Hermes Trismegistus
The Hunger of Old
The Hydra of Old
I
Ignatius Darren Ford (ULTRAMagic Infinity)
Inkblot Fischer
K
Karnage the Traitor
Katsuko Yoshinaga
Konrad Strobel (ANTIMagic Hex)
Kresimira Raynot
Kyu #9
L
Leif/Tyrant (The Dragon of Old)
Leonardo Hammond O'Nessie
Logan Bonneville
Loki
Lucifuge Rofocale
Ludwig Leichenberg (Rebirth - Red Suit)
M
Mable Acheson
The Madman of Old
Chief Magnus Scully
Mal (The Malformed of Old)
Marion "Tanya" Devilfay
Mary Pickford
Maya Athenon
Mayhem Highland
Spiritus Magni Maxima
Maximus Raynot (The War Machine of Old)
Mazatl Nakahara/Adrien Irons
Mercurius II
Milan Proch
Milosh Proch
Mira Ashman
Mizuki Kitagawa
The Monolith of Old
Morana Dracul
Morrigan Devilfay
Mortis Theodore Kidd
N
Nathaniel "Haunt" Fernsby
The Night God
O
Spiritus Magni Octavia
Octavius
Odin
Sir Odo Ash, Knight of the Unlight
Olivia Briggs
Orion
Ornias
P
Persephone, Lady of Darkness
Proteus (The Ocean of Old)
Q
Quasar Sagan (ULTRAMagic Quasar)
R
Duke Radovan Raynot
Raguel
Randalph Theoprastus Scarfe
Razor Scully
Rebis
Regnault
Dr. Reynard Woodall
Richard Callahan/Rostislav Dracul (ULTRAMagic Richter)
Captain Roger/Spiritus Magni Manius
Duchess Rose Raynot
Ryota Tsukumo (ANTIMagic Inferno)
S
Saul Bonneville
Seishin Mikoto
The Screaming God
Shigeko Tsukumo (ANTIMagic Mania)
Chief Sigmund Willfort
Six-Eared Macaque
Skari Willfort
The Sludge God
King Sten Haugen
Stolas, Prince of Inferno
Stolon, Duke of Inferno
Sun Wukong
T
Taro Miyazaki
Teunis Van Hautum (ULTRAMagic Ex)
The Taffy of Old
Theobold
Thor
Thora Willfort
Threrth
ULTRAMagic Thunder
Tiberius Philipus Skull (ULTRAMagic Scholar)
Timothy Finnegan
Torunn Craddock
Trevor MacQuoid
Trumna Wintergate
Tusk Willfort
U
ULTRAMagic Ultimatum
Umbra
V
Valentina Pari
Vexation (Ermenrich Denzell)
Victor Frankenstein
Vlad III Dracula
Vlad IV Dracul
Vladislav Velimir Dracul
Vlastimir Bartholomew Dracul
Voltage Tessla
W
Walter Nithercott (ULTRAMagic Walker)
The Watchman of Old
Weaver Craddock
Will-15
Wilhelm
William Ford II (ULTRAMagic Shadow)
Sir Wolfgang Bramson
X
The Xanthous King
Xavier Dufort
Y
Dire Fairy Yale
Queen Yngvild Haugen
Z
Zal-Rint
Zoltan Tenebrae Raphael Dracul
2 notes
·
View notes
Text
Gas Constant
One of the fundamental states of matter is the gaseous state, commonly known as gas. Gases possess distinct characteristics that set them apart from other states of matter. Despite being highly compressible, gases evenly distribute pressure on all sides. They lack a definite shape and volume, instead adapting to the shape of the container they occupy. Moreover, gases readily mix due to minimal interactions between their intermolecular forces.
The behavior of gases is governed by several sets of laws derived from experimental studies conducted under various conditions, including temperature, pressure, and volume. This discussion centers around a specific constant known as the gas constant (R) and its significance.
The gas constant (R) is a physical constant expressed in units of energy per temperature increase per mole. It is also referred to as the molar gas constant or universal gas constant. The value of R is equivalent to that of the Boltzmann constant, although the latter is expressed in terms of the pressure-volume product. This constant plays a crucial role in understanding the behavior of gases and their properties under different circumstances.
Ideal Gas
An ideal gas is a theoretical concept used in physics and chemistry to simplify the behavior of gases under certain conditions. It is a hypothetical model of a gas that follows specific ideal gas laws, making its behavior easy to analyze and calculate.
The behavior of real gases deviates from the ideal gas model at high pressures and low temperatures, where intermolecular forces become significant, and the volume of gas particles becomes non-negligible. However, real gases behave similarly to ideal gases under many conditions, especially at low pressures and high temperatures.
An ideal gas can be defined as the theoretical representation of a gas comprising point particles that do not reveal any alterations during intermolecular movements. The ideal gas follows all three fundamental laws as given by Charles, Avogadro, Boyle, and Gay Lussac.
V ∝ 1/P with T and n as constant (From Boyle's law)
V ∝ T with P and n as constant (From Charles law)
Finally, V ∝ n with T and n as constant (From Avogadro's law)
Combining all three of these gives V ∝ n X T/P
Mathematically, this can be expressed as,
PV = nRT
Here,
P is the pressure
V is the volume
T is the temperature
R is the Ideal Gas Constant, and
n is the amount of substance
Gas Constant Laws
The gas constant r is a vital factor for numerous principles and laws of physics. It is used in various laws as a combination of a constant and equations as a fundamental factor.
Boyle's Law: Boyle's Law is a fundamental gas law that describes the relationship between the pressure and volume of a gas at a constant temperature. It states that when the volume of a gas decreases, its pressure increases, and vice versa, as long as the temperature remains constant. In mathematical terms, Boyle's Law can be expressed as:
P ∝ 1/V
where P is the pressure of the gas, and V is its volume. The law implies that the product of the pressure and volume of a given amount of gas is constant, provided the temperature remains unchanged.
Charles's Law: Charles's Law, also known as the law of volumes, relates the volume of a gas to its temperature at constant pressure. It states that the volume of a gas will increase or decrease in direct proportion to its absolute temperature as long as the pressure remains constant. In mathematical terms, Charles's Law can be represented as:
V ∝ T
where V is the volume of the gas, and T is its absolute temperature (measured in Kelvin). As the temperature of a gas rises, its volume will expand, and conversely, as the temperature decreases, the volume will contract.
Avogadro's Law: Avogadro's Law states that under the same temperature and pressure conditions, equal volumes of different gases contain an equal number of molecules. This law is based on the idea that the volume of a gas is directly proportional to the number of molecules it contains. In mathematical terms, Avogadro's Law can be expressed as:
V ∝ n
where V is the volume of the gas, and n is the number of gas molecules. It implies that at constant temperature and pressure, the number of molecules to the volume ratio is constant for all gases.
Gay-Lussac's Law: Gay-Lussac's Law, also known as the pressure-temperature law, describes the relationship between the pressure and temperature of a gas at constant volume. It states that the pressure of a fixed amount of gas is directly proportional to its absolute temperature, provided the volume remains constant. Mathematically, Gay-Lussac's Law can be represented as:
P ∝ T
where P is the pressure of the gas, and T is its absolute temperature. This law implies that if the temperature of a gas increases, its pressure will also increase, and if the temperature decreases, the pressure will decrease, assuming the volume remains constant.
0 notes
Text
I js ran into this one but anyways :p
Marauders ☆ Sirius Black
MCU ☆ Loki
RRverse ☆ Alex Fierro
Nowhere, On Air ☆ Jess
Six Of Crows ☆ Wylan Van Eck
Xmen ☆ Charles Xavier
The Maze Runner ☆ Newt
B99 ☆ Charles Boyle
Arcane ☆ Mel Medarta
Guardians Of The Galaxy ☆ Rocket
(Yes I know GOTG are in the MCU I just ran out of fandoms)
@mireyaaaaaaaaaa @the-official-failure @peapea-0405 @boldofyoutoassumeicanspell
(That's all the ppl I know help-) (obv anyone else who would like to join)
10 fandoms, 10 characters!
list ten favorite characters from 10 different fandoms and tag 10 people!
(ty for the tag @bloopf1sh and @apurplesloth ! I'm making a new chain bc my Tumblr crashes when I try to reblog the original one lol--)
Stranger Things ★ Mike Wheeler
One Piece ★ Sabo
Hunter x Hunter ★ Kuruta Kurapika
Bungo Stray Dogs ★ Dazai Osamu
My Hero Academia ★ Himiko Toga
Avatar - The Last Airbender ★ Toph Beifong
Seraph of the End ★ Mikaela Hyakuya
Genshin Impact ★ Venti
Pokemon ★ James
Omniscient Reader's Viewpoint ★ Kim Dokja
Npt<3 @sykatz @pythoness94 @purple-racoon-80 @miwiromantics @scroofy-was-here @sillylittlerock @the--last-great-american-dynasty @its-celery @i-eat-homeless-people and anyone else! :)
307 notes
·
View notes
Text
Far Cry 5 as B99 Cause i was inspired and there were clips lol | Thank you @yeetslovescheese and @toadsmoss for inspo. As you can see I never let anything go :p @racheljo47 @mrspaigeomega @i-am-the-balancing-point | *credits to original owners
#ik this isnt what the post was about but it kinda diverged#also theres probably a bunch of mistakes i made so beware :p#far cry 5 memes#fc5#far cry 5#brooklyn nine nine#b99#b99 x fc5#deputy rook#deputy hudson#deputy pratt#jacob seed#faith seed#john seed#joseph seed#sheriff whitehorse#the seeds#the deputy#jake peralta#charles boyle#raymond holt#rosa diaz#amy santiago#terry jeffords#gina linetti#my weird videos :p
617 notes
·
View notes
Text
Elizabeth reacts to Jane and Bingley getting engaged:


gifs source (blog deactivated)
Pride and Prejudice Chapter 55: Mr. Bingley proposes to Jane, is accepted, and everyone is happy
View the full series of P&P chapter memes here
#p&p chapter memes#p&p ch55#pride and prejudice memes#pride and prejudice#jane austen memes#jane austen#english lit memes#jane bennet#mr bingley#elizabeth bennet#brooklyn nine nine#charles boyle#gifs#my stuff
92 notes
·
View notes
Text










Saloon interiors from Horizons West (1952)
Art Direction by Robert Clatworthy, Bernard Herzbrun Set Decoration by Russell A. Gausman, Joseph Kish Costume Design by Rosemary Odell
#saloon#set design#interiors#interior decor#horizons west#1952#western#50s movies#1950s film#wild west#budd boetticher#old west#charles p. boyle#charles p boyle#early 50s#robert ryan#dennis weaver#raymond burr#sam harris#(not that one)#dan poore#bar#decor#movie sets
11 notes
·
View notes
Note
On your B99-WandaVision stuff, I actually like to imagine Billy and Teddy being mini versions of Holt and Kevin. Just a cute wholesome preteen romance.
But then six years later Tommy and Jimmy are on a road trip for some reason, and Tommy keeps getting texts from David, and he admits ‘I’m bisexual.’
Honestly this headcanon could just be because I can completely imagine Tommy’s actor making the same face Rosa did during the ‘Bye Rosa’ scene.
Tommy: *tells the family he’s bisexual*
Tommy: Uncle P found out on the road trip, and I was positive he was not going to be able to keep it a secret.
*flashback*
Peter: Bye Tommy! I mean, not bi, but bye! I mean, see ya! I mean, have fun only having sex with girls, just banging chicks left and right.
Tommy:
*end flashback*
Peter: I just stopped saying ‘bye’ altogether.
#anon#b99!hexagang#wandavision#incorrect xmen quotes#brooklyn 99#uncle p#peter maximoff#tommy maximoff#tommy shepherd#rosa diaz#charles boyle
106 notes
·
View notes
Text
So I made a thing
Brooklyn 99 x Pride & Prejudice (2005)


#b99#pride and prejudice#p & p#pride and prejudice 2005#brooklyn 99#brooklyn nine nine#elizabeth bennet#mister darcy#jake peralta#amy Santiago#gina linetti#ray holt#hitchcock and scully#mister bingley#jane bennet#lydia bennet#mister bennet#mrs bennet#rosa diaz#charles boyle#ilovecorgibutts
39 notes
·
View notes
Text

Ideal Gas Law
In 1834, Benoît Paul Émile Clapeyron stated a combined version of previous gas laws (Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law) known as the ideal gas law or the general gas expression. Though it can be used in many situations to describe the behavior of real gases, it is a theoretical equation relating pressure, volume, temperature, and amount of a substance.
The most common form of the equation is PV = nRT, with P = pressure, V = volume, n = the amount or number of moles of a substance, R = the ideal gas constant, and T = temperature. However, it can also be stated using Boltzmann's constant and/or Avogadro's constant, as well as a variety of other forms.
Sources/Further Reading: (Image source - Wikipedia) (Khan Academy) (LibreTexts) (HyperPhysics)
23 notes
·
View notes
Text
Have some B-bone Boyle
#you know that's my smallest bone!#gina and charles#gina linetti#brooklyn nine nine#b99#brooklyn 99#chelsea peretti#charles boyle#boyle#joe lo truglio#s p i n e#(that last tag was purely for my own amusement)
15 notes
·
View notes
Photo


the mark of the renegade (us, fregonese 51)
#the mark of the renegade#hugo fregonese#ricardo montalban#andrea king#gilbert roland#charles p. boyle
13 notes
·
View notes