#arthropoda collection no. 4
Explore tagged Tumblr posts
Text

COLA!!!. Arthropoda Collection. No. 4.
#insect photography#insect#photography#photos#photo#insects#moth#moths#arthropoda#arthropoda collection#arthropoda collection no. 4#perspective#perspective photography#surrealist photography#arthropods
10 notes
·
View notes
Text
Round 2 - Arthropoda - Ostracoda




(Sources - 1, 2, 3, 4)
Ostracoda are a class of crustaceans commonly known as “Seed Shrimp,” grouped into 7 orders. Ostracods are unique among crustaceans for their carapace which consists of two valves, superficially resembling the shell of a tiny clam.
Ostracods live in both freshwater and marine environments, and some even live in humid soils. Most live either as plankton or in sediment. The ostracod body is not clearly divided into segments, consisting of a head and thorax region. Their head has two pairs of well-developed antennae that are used to swim through water, a pair of mandibles, and a pair of maxillae. The thorax has three main appendages. The first pair’s function varies between species. It can be used for feeding, for walking, or for mating. The second pair is mainly used for locomotion, and the third is used for walking or cleaning, and it is reduced or absent in some species. Ostracods sense the world around them via sensitive hairs on their bodies and appendages, though the order Myodocopina also has compound eyes, and the Podocopids have simple eyes as larvae. They have a wide variety of diets and can be carnivores, herbivores, or scavengers, though most are filter feeders.
Male ostracods have two penises, corresponding to two genital openings (gonopores) on the female. Mating often occurs in a swarm, with large groups of females joining large groups of males. Some species are wholly or partially parthenogenetic, consisting almost entirely of females. Some species care for their young, releasing them after their first moult, some release their eggs directly into the water, while others glue their eggs to vegetation or a firm surface. Glued eggs often do not hatch until conditions are favorable. Larvae add on more legs with each moult until achieving their adult form.

Propaganda under the cut:
Giant Ostracods, the genus Gigantocypris (image 3), live in deep, cold oceans. They can get up to 3.2 cm (1.3 in) across, which is enormous for an ostracod. They use their large, mirror-like eyes to locate small prey.
Ostracod sperm is huge, sometimes up to six times the length of the male ostracod itself! They are usually coiled up within the testis prior to mating.
Some ostracods, such as Vargula hilgendorfii, are bioluminescent. These ostracods are called "blue sand" or "blue tears" and glow blue in the dark. The Japanese army actually collected these ostracods during WWII to use as lights for reading maps at night! Ostracods usually use this bioluminescence to distract predators, shooting out glowing mucus while they escape:

Some ostracod species use pulses of light to attract mates. In some, the male will release small balls of bioluminescent mucus, allowing the female to follow the light trail to find him. In one species hundreds of thousands of males synchronize their light display, and when one male creates a pattern of light, the new pattern will spread out as the neighboring males repeat it.
65 notes
·
View notes
Text
Unravelling large-scale patterns and drivers of biodiversity in dry rivers | Nature Communications
See on Scoop.it - EntomoNews
More than half of the world’s rivers dry up periodically, but our understanding of the biological communities in dry riverbeds remains limited. Specifically, the roles of dispersal, environmental filtering and biotic interactions in driving biodiversity in dry rivers are poorly understood. Here, we conduct a large-scale coordinated survey of patterns and drivers of biodiversity in dry riverbeds. We focus on eight major taxa, including microorganisms, invertebrates and plants: Algae, Archaea, Bacteria, Fungi, Protozoa, Arthropods, Nematodes and Streptophyta. We use environmental DNA metabarcoding to assess biodiversity in dry sediments collected over a 1-year period from 84 non-perennial rivers across 19 countries on four continents. Both direct factors, such as nutrient and carbon availability, and indirect factors such as climate influence the local biodiversity of most taxa. Limited resource availability and prolonged dry phases favor oligotrophic microbial taxa. Co-variation among taxa, particularly Bacteria, Fungi, Algae and Protozoa, explain more spatial variation in community composition than dispersal or environmental gradients. This finding suggests that biotic interactions or unmeasured ecological and evolutionary factors may strongly influence communities during dry phases, altering biodiversity responses to global changes. Over half the world’s rivers dry periodically, yet little is known about the biological communities in dry riverbeds. This study examines biodiversity across 84 non-perennial rivers in 19 countries using DNA metabarcoding. It finds that nutrient availability, climate and biotic interactions influence the biodiversity of these dry environments.
Image : Twelve metazoan phyla were detected, with the highest relative abundances corresponding to Arthropoda (8 ± 12%) followed by Nematoda (3 ± 4%). Sequences assigned to Arthropoda included Collembola (3 ± 7%), Ostracoda (2 ± 6%), and Arachnida (2 ± 3%, corresponding mainly to mites). Around 4% of the reads corresponded to Streptophyta.
------
NDÉ
Traduction
Plus de la moitié des rivières du monde s'assèchent périodiquement, mais notre compréhension des communautés biologiques dans les lits des rivières asséchées reste limitée. En particulier, les rôles de la dispersion, du filtrage environnemental et des interactions biotiques dans le maintien de la biodiversité dans les rivières asséchées sont mal compris.
Nous menons ici une étude coordonnée à grande échelle sur les modèles et les moteurs de la biodiversité dans les lits de rivières asséchées. Nous nous concentrons sur huit taxons principaux, dont les micro-organismes, les invertébrés et les plantes : Algues, Archaea, Bactéries, Champignons, Protozoaires, Arthropodes, Nématodes et Streptophytes.
Nous utilisons le métabarcodage de l'ADN environnemental pour évaluer la biodiversité dans les sédiments secs collectés sur une période d'un an dans 84 rivières non pérennes dans 19 pays sur quatre continents. Des facteurs directs, tels que la disponibilité des nutriments et du carbone, et des facteurs indirects, tels que le climat, influencent la biodiversité locale de la plupart des taxons.
La disponibilité limitée des ressources et les phases de sécheresse prolongées favorisent les taxons microbiens oligotrophes. La co-variation entre les taxons, en particulier les bactéries, les champignons, les algues et les protozoaires, explique davantage la variation spatiale de la composition de la communauté que la dispersion ou les gradients environnementaux.
Ce résultat suggère que les interactions biotiques ou des facteurs écologiques et évolutifs non mesurés peuvent fortement influencer les communautés pendant les phases d'assèchement, modifiant les réponses de la biodiversité aux changements globaux.
------
Plus de la moitié des rivières du monde s'assèchent périodiquement, mais on sait peu de choses sur les communautés biologiques présentes dans les lits des rivières asséchées.
Cette étude examine la biodiversité de 84 rivières non pérennes dans 19 pays en utilisant le métabarcodage de l'ADN. Elle révèle que la disponibilité des nutriments, le climat et les interactions biotiques influencent la biodiversité de ces environnements secs.
Traduit avec DeepL.com (version gratuite)
0 notes
Text
Round 2 - Arthropoda - Diplura




(Sources - 1, 2, 3, 4)
The Diplura (commonly called “Two-pronged Bristletails”) were classically placed together with collembolans and proturans in the class “Entognatha.” However, most recent research points to Entognatha being paraphyletic (ie, grouped together based on morphological similarities and not so much how closely related they actually are.) It would appear that collembolans, proturans, diplurans, and insects all evolved the hexapodous body structure independently.
With that out of the way, there are around 800 species of diplurans, grouped into 10 families. They can be 2–50 millimetres (0.08 – 2 in) long. They lack eyes, wings, and pigment. Unlike proturans, they have long antennae with bead-like segments. Their most distinguishing feature is the pair of cerci projecting backwards from their last abdominal segment. These cerci may be long and thin or short and pincer-like. Some diplurans have the ability to shed their cerci to escape from predators. They have biting mouthparts concealed within their head, consisting of mandibles with several apical teeth. Diplurans with short cerci are carnivorous predators, using their pincer-like cerci to capture springtails, isopods, small myriapods, insect larvae, and other diplurans. Those with long cerci are omnivores, eating soil fungi, mites, springtails, other small soil invertebrates, and/or detritus.
Like collembolans and centipedes, male diplurans lay spermatophores for females to find. These sperm packets are held off the ground by short stalks. After collecting spermatophores, the female will lay eggs on stalks within a crevice or cavity in the ground. Most diplurans will abandon their eggs after laying them, but some will remain to protect their eggs and newborns. Upon hatching, nymphs looks like smaller, less hairy adults.
Diplurans do not have a very good fossil record. One apparent dipluran, Testajapyx, dates to the Carboniferous. Testajapyx had compound eyes and mouthparts resembling those of true insects.

(source)
Propaganda under the cut:
As soil-dwelling organisms, diplurans play a role in indicating soil quality and as a measure of the impact of human activities such as farming.
Diplurans can regenerate lost legs, antennae, and cerci over the course of several moults.
25 notes
·
View notes
Text
Expéditions Corse : carnet de bord des scientifiques
See on Scoop.it - EntomoScience
En mai 2019, les scientifiques de La Planète Revisitée entament en Corse un programme d’expéditions de 3 ans conduit par le Muséum national d’Histoire naturelle, en partenariat avec la Collectivité de Corse et l’Agence française pour la Biodiversité. Ce programme a pour objectif de réaliser un échantillonnage des espèces d’algues et d’invertébrés marins et terrestres de l’île.
→ Carnet de bord des scientifiques | Expéditions Corse https://laplaneterevisitee-corse.mnhn.fr/fr/carnet-bord/carnet-bord-scientifiques
-------
NDÉ
La publication
La Planète Revisitée en Corse 2019-2021 : un grand inventaire de la biodiversité négligée dans une île méditerranéenne.Bulletin de la Société entomologique de France, 128 (4), 2023 : 353-382. ISSN 0037-928X https://doi.org/10.32475/bsef_2285 eISSN 2540-2641
Résumé. – De 2019 à 2021, des missions scientifiques de terrain ont été organisées en Corse par le Muséum national d’Histoire naturelle, l’Office français de la biodiversité et la Collectivité de Corse dans le cadre du programme d’exploration naturaliste “La Planète Revisitée”. Cet article présente le contexte, l’état des connaissances biogéographiques et taxinomiques avant le début du programme, les objectifs, les méthodes ainsi que les premiers résultats obtenus. L’objectif était d’établir un inventaire moderne des espèces présentes dans une série de sites représentatifs de différents écosystèmes et d’enrichir les collections d’histoire naturelle de nouveaux spécimens et nouvelles espèces, et en y associant des codes-barres ADN standards, utiles à leur identification. Pendant les trois ans, 19 sites ont été inventoriés de façon semi-standardisée dont trois ont bénéficié d’un dispositif de piégeage d’ampleur. L’accent a été mis sur l’échantillonnage des espèces forestières, ainsi que sur les pièges à interception et les assiettes colorées pour les diptères et les hyménoptères. Au total, 34 experts ont participé aux inventaires de terrain et plus de 80 ont contribué à l’étude du matériel. Les données d’observation sont mises à disposition dans l’Inventaire national du patrimoine naturel (http://www.inpn.fr), et pour les individus associés à des codes-barres ADN, les données des spécimens et les séquences ADN seront accessibles dans le Barcode of Life datasystems (BOLD : http://www.boldsystems.org). Début 2023, les jeux de données produits comprennent 31 100 données d’occurrence pour 3900 taxons d’arthropodes terrestres, ce qui représente une augmentation de 53 % des données publiques disponibles pour l’île. Les codes-barres ADN de plus de 6800 spécimens d’arthropodes ont été produits, ce qui représente 14 fois plus de séquences existantes pour les insectes de Corse que ce qui était disponible avant le début du programme. Jusqu’à présent, 148 signalements nouveaux et 12 espèces nouvelles pour la Science ont été publiés.
Keywords. – Arthropoda, Arachnida, Insecta, Crustacea Isopoda, expedition, sampling technique, survey protocol, survey strategy, map, sample processing, barcoding, integrative taxonomy.
0 notes
Text
Opposum Shrimp
I. Animalia
II. Bilateria
III. Protostomia
IV. Ecdysozoa
V. Arthropoda
VI. Crustacea
VII. Malacostraca
VIII. Eumalacostraca
IX. Peracarida
X. Mysida
XI. Mysis
XII. relicta
(Taken from: Integrated Taxonomic Information System, 2005)
The Opossum Shrimp (Mysida)
Their common name opossum shrimp comes from the presence of brood pouch in females, where they carry their larvae which are not free-swimming. This crustacean is like the kangaroo of the sea!
That’s a Mysid!
The cosmopolitans
They can be found in many types of aquatic environment, in freshwater and marine, deep sea, shallow coastal waters, lakes, rivers and underground waters. Mysids are utilized commercially in Japan, Southeast Asia, China and Korea, and can be found in different marine habitats such as Pelagic, Coastal and estuarine. Worldwide, there are 160 genera and over 1000 described species. (Porter 2016; Jones 2003)

Map of stations in Southeast Asia where Mysid specimens were recorded (Sawamoto 2013)
Life Cycle
Mysid Shrimp have mechanisms that help them in mating. In some species, receptive females release pheromones in the water to attract males. Mating takes place during copulation at night, where males deposit their sperm inside the brood pouch of a female. After mating, eggs are also deposited in the brood pouch and are fertilized. Depending on the species and water temperature, females brood the eggs that take weeks to several months to hatch. Young mysids are not released until they turn into well-developed juveniles.. Mysis relicta, one of the most studied mysid shrimp found in freshwater lakes, have a 1 to 2 year life cycle depending on the productivity of the lake. Eggs of Mysis hatch into fully developed young after 3-4 months. Typical lengths are observed in each stage or instar: I – 4.5 mm, II – 9 mm, III – 12 mm, IV – 16 mm. At stage IV, individuals reach sexual maturity. Some females have a fifth instar or stage, but males do not have. Fifth instar females have 22 mm total length. (Rudstam 2009)
Anatomy
Mysids are generally small and transparent crustaceans, ranging from about 6 to 350 mm in length, where the length of the majority of species is less than 25 mm. They have a pair of stalked eyes and two pairs of antennae on their head fused with up to four of the thoracic segments, and covered with a well-developed carapace. One of their distinct characteristics is the number of their thoracic appendages, where they have six to eight. Their pereopods are biramous or separated into two branches. Also, they have a distinctive pair of statocyst located in their tail fan or uropods. Statocyst is a sac-like organ that helps them maintain their balance and provide a sense on how they can orient themselves in their environment. Their common name Opossum Shrimp comes from their distinct characteristic or feature, having a marsupium or brood pouch. This is where the Mysids keep their eggs until they hatch and are released after reaching an early juvenile stage of development. The marsupium is a chamber formed by lamellae or oostegites on all or some of the second to eighth pairs of thoracic legs. (Lenihan 2017)
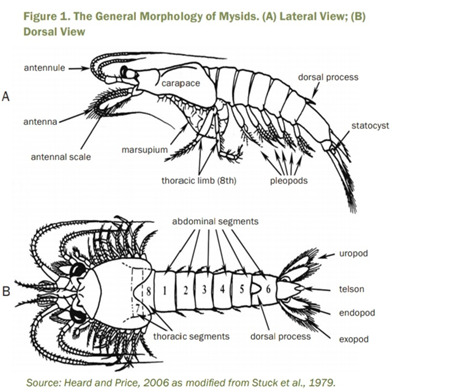
Photo from “Mysid (Americamysis bahia) Survival, Growth, and Fecundity Toxicity Tests” (EPA 2009)
Ecology
Mysids are present in almost all types of aquatic environment and are filter feeders and generally classified as omnivores, feeding on algae, detritus and zooplankton. They are very important in maintaining the balance of the aquatic ecosystem. Many fish and several species rely primarily on Mysids as their food. Mysids are rich in essential fatty acids that help in the survival of fishes. In cold regions, Mysids are a source of Polyunsaturated fatty acids that are important for fishes to have over-winter survival. Their abundance can form large shoals making them an important diet of several fish. Species like the Mysis relicta were introduced in the Kootenay lake in Canada, and it increased the growth rate and population of kokanee salmon. (Rudstam, 2009)
Sensitivity with a purpose
Mysids are very sensitive to a wide variety of contaminants. They are good indicators of harmful substances in the water. They are used as test organisms in monitoring the water quality. They are great help in wastewater management and ensuring a good quality of water in the estuarine and coastal areas since this is used in several water quality tests like the Mysid (Americamysis bahia) Survival, Growth, and Fecundity Toxicity Tests. This test is used by the U.S. Environmental Protection Agency to evaluate effluents in terms of its toxicity to biota and their impact on the natural environment. (Anderson et al. 2016; EPA 2009)
Relationship with humans
Mysids are very important especially in the aquaculture industry. They are used as food for different species of fish and a good source of nutrients for fishes being cultured like trout in the lakes of North America and Europe. Some species of Mysid are utilized as ingredients for fermented fish products. Shousuru is a Japanese fish sauce produced from fish meat added to 10% mysid, where the shrimp and mysid are used to color the sauce. (Ohshima et al. 2014)
Fun Facts
Little Cannibals
Mysids have cannibalistic behavior, where some eat weaker individuals. In fact, some parents may eat some of their babies!
Bloody Hell!
Hemimysis anomala or bloody-red shrimp is a species of Mysid Shrimp that can form reddish swarms in the shadows of piers and boats. They can spread and multiply rapidly and are considered “high-risks” for invasion of inland lakes in the US.
Invasion! Tragic Honeymoon
After mating, male Mysid (Mysis relicta) dies and the female lives for several months. A little sacrifice for the next generation.
References:
NOAA. n.d. Great Lakes New Invader: Bloody Red Shrimp (Hemimysis anomala). GLANSIS. Rretrieved from: https://www.glerl.noaa.gov/res/Programs/glansis/hemi_brochure.html#:~:text=Mysids%20are%20often%20called%20opossum,larger%20shrimps%20and%20other%20decapods.
Sawamoto, Shozo. 2013. Current Status of Mysid Taxonomy in Southeast Asia. Researchgate.net. Available from: https://www.researchgate.net/publication/307791935_CURRENT_STATUS_OF_MYSID_TAXONOMYIN_SOUTHEAST_ASIA
Rudstam, L.G. 2009. Other Zooplankton. Encyclopedia of Inland Waters. Science Direct. Available from: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/mysis
Anderson B., Philips B. 2016. Saltwater Toxicity Test. Science Direct. Available from: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/mysida
Jones, D.A. 2003. Characteristics of Crustacea. Science Direct. Available from: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/mysida
Porter, M.L. 2016. Collecting and Processing Mysids, Stygiomysids, and Lophogastrids. Journal of Crustacean Biology. Available at: https://academic.oup.com/jcb/article/36/4/592/2735746
Ohshima T, Giri A. 2014. FERMENTED FOODS-Traditional Fish Fermentation Technology and Recent Developments. Science Direct. https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/neomysis
Lenihan, Jamie. 2017. Opossum Shrimp. Vic High Marine. Available at: https://www.vichighmarine.ca/opossumshrimp/
EPA. 2009. Mysid (Americamysis bahia) Survival, Growth, and Fecundity Toxicity Tests. U.S. Environmental Protection Agency. Office of Wastewater Management. 1200 Pennsylvania Ave., NW Washington DC
Zooplankton of the Great Lakes. Central Michigan University. Available at: http://people.cst.cmich.edu/mcnau1as/zooplankton%20web/mysis/mysis.html
Order Mysidacea. Key to Australian Freshwater and Terrestrial Invertebrates. https://keys.lucidcentral.org/keys/v3/TFI/start%20key/key/crustacea%20key/Media/HTML/Mysidacea.html
10 notes
·
View notes
Photo

HUGE THANX 2 my friend @evilolaf 4 giving me this set of Giant Stag Beetles for my insect collection!!! 🙏😉 I had 2 soften them 2 stretch out & pin...the look great!!! 💪😎 #insectcollecting #insectcollection #insectcollector #Arthropoda #beetle #beetles #giantstagbeetle #lucanuselaphus #lucanidae #coleoptera #thegodbeast #godbeast #entomology #entopins #entomologypins #entomologycollection (at Olathe, Kansas) https://www.instagram.com/p/CDPbXEeAJyT/?igshid=gl3aqsrni0y5
#insectcollecting#insectcollection#insectcollector#arthropoda#beetle#beetles#giantstagbeetle#lucanuselaphus#lucanidae#coleoptera#thegodbeast#godbeast#entomology#entopins#entomologypins#entomologycollection
1 note
·
View note
Text
Animal/Bio-Diversity Facts!
I combined these two topics because there’s a lot of overlap, and I decided that taking notes on both really helped me understand what the other is trying to say. This will be a long post, strap yourself in.
Organisms are organized and classified via a system known as Taxonomy. This system was developed by a scientist named Carl Linnaeus. To identify individual organisms, binomial nomenclature is used. What this means is each organism is called by their genus and species name. For example, Homo sapien, Pyrrhura molinae, (Green cheek conure), and Betta splendens.
There were originally 6 taxa or levels of organization developed by Linnaeus; kingdom, phylum, class, order, family, genus, and species. The 20th century saw many changes to Linnaeus’ original system of organization. The 3 original kingdoms were expanded to 5; Monera, Protista, Fungi, Plantae, and Animalia, a 6th, Archaebacteria was added to represent extremophiles that were so intense they had to be separated from bacteria to give their coolness more merit.
Today's scientists added a 7th level, domain. We use a 3-domain system based on DNA analysis. These domains are eukarya, bacteria, and archaea. Monera stopped being used as the prokaryotes were split between bacteria and archaea. Archaea are in fact, not bacteria, and so were given their own domain.
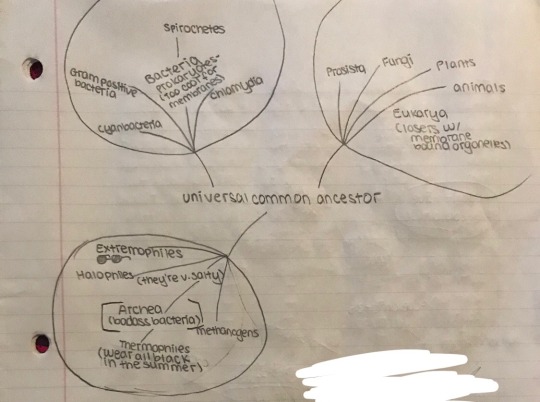
Here are some characteristics shared among members of the same domain:
Bacteria
All members of this domain are unicellular prokaryotes. This means that they lack internal membranes, like a nucleus, mitochondria, or chloroplasts)
Some are anaerobic (metabolize without oxygen) some are aerobic (metabolize with oxygen)
In the environment, some are decomposers, meaning they decompose and recycle dead organic material.
Some are pathogens, such as some strains of E.coli.
Speaking of E.coli, they also play a vital role in genetic engineering. E.coli is used to manufacture human insulin
Some reproduce using conjugation. This is a primitive process, where individuals exchange genetic material
They have a thick and rigid cell wall
Some, like blue-green algae, are autotrophic (make their own food) others are heterotropic (depend on complex organic substances for food)
Have no introns (noncoding segments of DNA)
Archaea
Also unicellular prokaryotes
Include extremophiles, which are organisms that live in extreme environments. Some examples are Methanogens (obtain energy by producing methane from hydrogen) Halophiles (thrive in extremely salty environments, such as the Dead Sea) and Thermophiles (thrive in extremely high temperatures, like Yellowstone's hot springs)
Have introns present in some of their genes
Eukarya
Have a nucleus and internal, membrane-bound organelles
Include: Protista, Fungi, Plantae, and Animalia
Moving into kingdoms, there are 4. These are the 4 mentioned above, fungi, Protista, Plantae, and Animalia. Here are some traits for each:
Protista
Most are unicellular, however, some are primitive multicellular organisms.
Include both heterotrophs (like amoeba, and paramecium) and autotrophs (like euglenas)
Move using different structures, such as pseudopods in amoeba, cilia in paramecium, and flagella in euglenas.
Include organisms not cool enough to sit with the fungi or Plantae kingdoms, like seaweed and slime mould.
Some, like algae and paramecium, carry out conjugation
Some can cause serious diseases like amoebic dysentery and malaria
Fungi
All are heterotrophic
Include unicellular and multicellular organisms
Able to digest extracellularly by secreting hydrolytic enzymes, and absorbing the nutrients via diffusion.
Are essential to the environment, as they are decomposers. They are saprobes, which mean they eat decaying organic matter.
They have cell walls, however, unlike plants whose cell walls are made of cellulose, their cell walls are made of chitin.
Lichens are fungi and algae living in a mutualistic, symbiotic relationship. Lichens are strong enough to withstand harsh, unforgiving environments, thus are often the pioneer organisms (the first to colonize a new environment).
They reproduce asexually by budding, like yeast, spore formation, like bread mould, or fragmentation (aka 1 parent breaks itself into several, living pieces), however, some can reproduce sexually.
Plantae
All are multicellular, nonmotile, and autotrophic.
Their cell walls, as mentioned above, are made of cellulose.
Plants can create their own food by photosynthesis, which uses chlorophyll a and b.
Their carbohydrates are stored as starch
They reproduce sexually by alternating between the gametophyte and sporophyte generations.
Some (tracheophytes) have vascular tissue while others (bryophytes) do not.
Animalia
All are heterotrophic, multicellular, and motile
Most reproduce sexually with a dominant diploid (2n) stage
In most, a sperm with a flagellum fertilizes a large, nonmotile egg.
Animals are classified, traditionally based on anatomical features (homologous structures) and embryonic development.
There are 35 phyla. Since I want to eat something today, I’ll go over the 9 the Barron’s SAT book describes, which are Porifera, cnidarians, Platyhelminthes, nematodes, annelids, molluscs, arthropods, echinoderms, and chordates.
Each animal phylum represents the evolution of a new, successful body plan. Some of these trends include specialisation of tissues, germ layers, body symmetry, the development of a head end, and body cavity formation.
Specialized cells, tissues, and organs
The cell is the basic unit of all life, for example, fat cells. Tissue is the next block up and is a collection of tissues performing a function, such as adipose tissue. An organ is a group of tissues working together to perform a similar function. For example, the brain.
Organisms making up the phylum Porifera, like sponges are made of a loose confederation of cells. Since those cells are not specialized, they are not considered tissue. These cells can react to stimuli, however, lack muscle or nerve tissue.
Organisms making up the phylum cnidaria possess tissue, however the most primitive and simple form of tissue. However, no organs. Flatworms do have organs, however, lack an organ system. Annelids, however, possess a full organ system.
Germ Layers
Germ layers make up the tissues and organs of the body. They form early in embryonic development. There are 3 kinds, however, not all organisms have all 3.
Ectoderm- outermost layer, makes up skin and nervous system
Mesoderm- middle layer, becomes blood, muscles, and bones
Endoderm- innermost layer, makes up the viscera (guts)
Porifera and cnidarians only have 2 layers. They lack mesoderm and instead have mesoglea or middle glue which holds the 2 layers together. Organisms that have 3 true germ layers are called triploblastic.
Body Symmetry
Most primitive animals exhibit radial symmetry. More complex animals exhibit bilateral symmetry. This is displayed in the drawings below. Echinoderms are a key exception to this rule. They develop with bilateral symmetry, however, as an adult, they exhibit radial symmetry. In bilateral symmetry, the body mirrors itself along the left and right on the longitudinal axis.
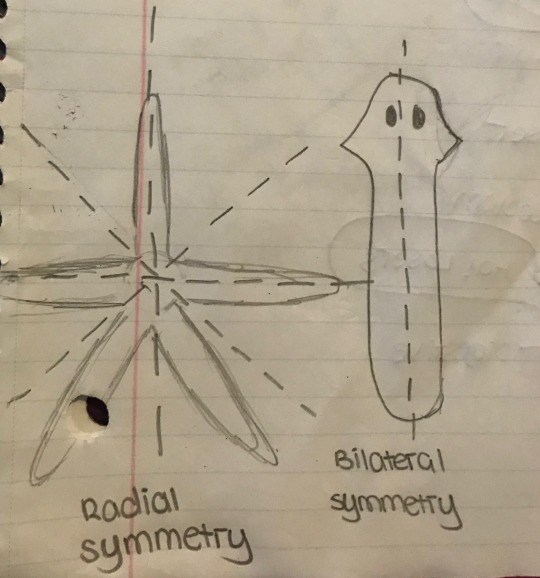
This also means that Patrick Star is not drawn biologically accurate. Shame.

Body Cavity Formation
The coelom is a fluid-filled body cavity, completely surrounded by mesoderm tissue. It is found only in more evolutionarily advanced organisms. Organisms like flatworms, who lack a coelom are known as acoelomates. Organisms, like nematodes or roundworms, who have a fluid-filled tube between the endoderm and mesoderm, functioning as a hydrostatic skeleton, are known as pseudocoelomates. Coelomates are organisms with a true coelom. Annelida, Mollusca, Arthropoda, and Chordata are all phyla that have this structure.
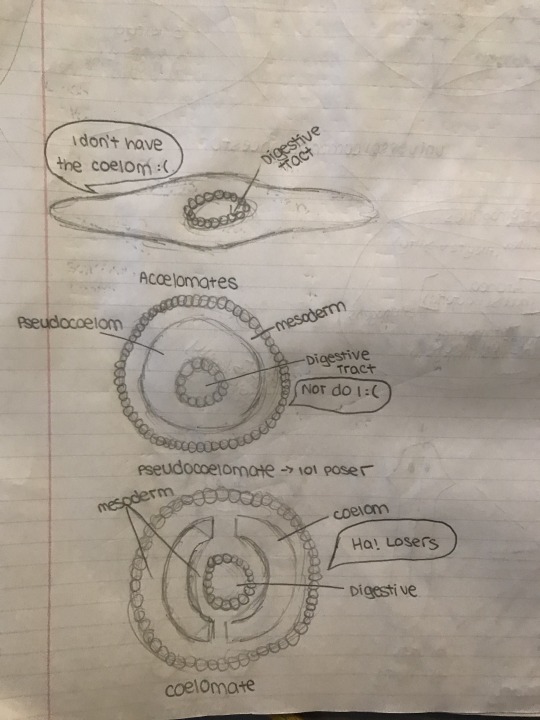
Development of a Head (Cephilization)
Organisms that developed bilateral symmetry also have an anterior and posterior end. (The head and rear end). The sensory apparatus and brain, or ganglia in less developed organisms are organized on the anterior end, while digestion, excretion, and reproduction all keep their organs on the posterior end. Cephilization began with flatworms.
Here is a cladogram to help visualize when different traits developed.
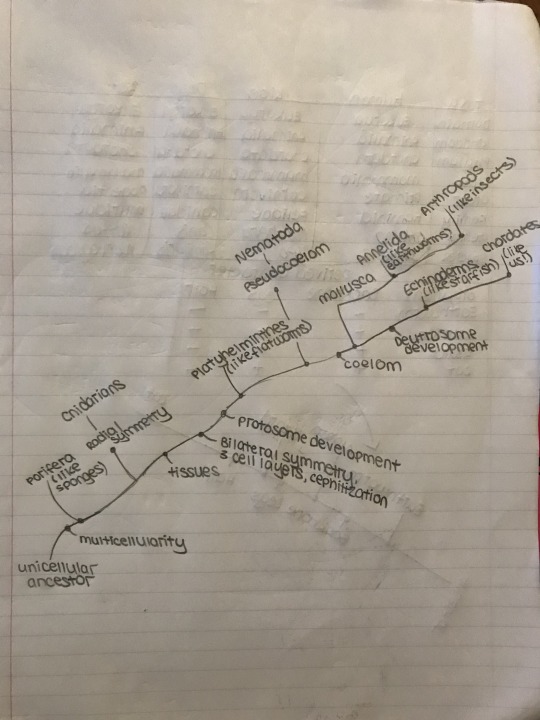
Traits of 9 different phyla:
Porifera:
No symmetry at all
No nerve or muscle tissue, sessile (nonmotile)
Filter nutrients from water drawn into a central cavity
Like many other primitive organisms, they only have 2 cell layers, ectoderm and endoderm, with the noncellular mesoglea holding them together
They have specialized cells, however, there is no organization to the cells, therefore they do not have tissue or organs.
Evolved from colonial organisms: fun fact, you can push a sponge through a cheesecloth, which will separate into individual cells, all and become a sponge. This is related to how a sponge reproduces
They reproduce asexually via fragmentation, meaning each piece that is separated has the necessary cells to become an individual organism. This means that technically,

Spongebob is reproducing here. Good on him.
They also reproduce sexually. They are hermaphrodites, meaning that they have characteristics of both males and females.
Cnidarians
Include organisms like hydra and jellyfish
Radial symmetry
Body plan is a polyp (vase-shaped, like hydra) which is mostly sessile or medusa (upside-down bowl-shaped, like jellyfish) which is mostly motile.
Life cycle- although there are exceptions, some go through a planula larva (free-swimming) stage, then proceed to their reproductive stage, that being asexual (polyps, ) or sexual (medusas)
Only have ectoderm and endoderm cell layers
Have a gastrovascular cavity where extracellular digestion occurs. They only have one opening to this cavity, so waste and food both go through the mouth.
Have lysosomes where intracellular digestion occurs.
No transport system, since each cell is in contact with the outside environment.
All have stinging cells (cnidocytes) for protection, with nematocysts, which are stingers.
Platyhelminthes
Include organisms like flatworms like tapeworms
These are the most simple organisms with bilateral symmetry, an anterior end, and 3 distinct cell layers (ectoderm, endoderm, and mesoderm... yay bones muscle and blood!)
The digestive cavity has only 1 opening for egestion and ingestion, like cnidaria, so food can’t be continuously processed.
Their body is solid and has no room for a true digestive and respiratory system to circulate food or oxygen. The solution to this problem was to develop an extremely flat and thin body that allowed most of their body cells to have contact with the outside and thus exchange nutrients and waste via diffusion.
Nematodes
Include roundworms like pinworms
Unsegmented worms with bilateral symmetry, but very little sensory apparatus.
A large majority of them are parasitic. Trichinosis is caused by the worm Trichinella, which is often found in uncooked pork.
C. elegans is often used as an animal model when studying genes and embryonic development.
Digestive tract is two way, meaning they have a mouth and an anus
Annelids
Include earthworms and leeches
Segmented worms with bilateral symmetry, and very little sensory apparatus.
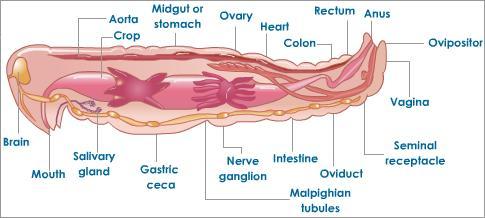
Two-way digestive tract, and a tube within a tube, consisting of a crop, gizzard, and intestine.
They have a nephridium, which is a tubule responsible for the excretion of nitrogen waste, urea.
They have a closed circulatory system and a heart with 5 pairs of aortic arches
Diffuse oxygen and carbon dioxide through their moist skin
Hermaphrodites
Mollusca
Include squids, octopi, slugs, clams and snails.
Have soft bodies, protected by hard calcium shells
They have open circulatory systems. This means they don’t have capillaries, however, have blood-filled spaces called hemocoels, or sinuses.
Have bilateral symmetry and 3 distinct body zones: The head-foot, with sensory and motor organs, Visceral mass, with organs of digestion, excretion, and reproduction, and the mantle, a specialized tissue that surrounds the visceral mass and produces the shell.
They have something known as a radula, which is moveable and has teeth, that behaves like a tongue.
Many have gills and nephridia
Arthropods
Include insecta (like grasshoppers), crustacea (like shrimp and crabs), and arachnida (like spiders and scorpions
Have jointed appendages
Segmented into head, thorax, and abdomen
Contain more sensory apparatuses than annelids which means they can move much more freely
Have an exoskeleton made of a polysaccharide known as chitin.
They also have an open circulatory system, with a tubular hard and hemocoels
For excretion, they have structures known as Malpighian tubules, which remove the nitrogenous waste; uric acid.
They have air ducts known as trachea which bring air from the environment into hemocoels.
Echinoderms
Include sea stars and sea urchins.
Most are sessile, or slow-moving (so stop judging Patrick. It’s just how he was born)
They are an exception to the bilateral symmetry rule. As embryos, they have bilateral symmetry, however, as they develop, they develop radial symmetry. This evolved for their sedentary lifestyle.
They have a water vascular system, which creates hydrostatic support for their tube feet which allow for locomotion
They reproduce sexually via external fertilization
They also have the ability to reproduce asexually via fragmentation, and regeneration. As long as the new sea star has part of the central canal, it will become a new organism.
They have an endoskeleton with calcium plates. Endoskeletons grow with the body, as opposed to exoskeletons that have to be shed
Chordates
Include vertebrae (like us!)
Chordates have a notochord which is a rod that extends the length of the body and is a flexible axis.
They have a dorsal, hollow nerve cord
The tail is responsible for movement and balance. We, humans, have a coccyx, which is a vestige of what was once our tail. Hence the name, tailbone.
Birds and mammals are homeotherms, meaning they are able to maintain consistent body temperature. The other chordates, like fish, reptiles, and amphibians are cold-blooded.
Let’s get specific, with mammals (because mammals are a superior class of animals. I would know, I am one.)
Mammals are named after their mammary glands. These glands let mothers provide milk to their babies.
They all have hair or fur
They are endotherms, meaning they generate their hair from within
Most are placental mammals, also known as eutherians. The embryo develops internally in a uterus connected to the mother via a placenta. Since the embryo is unable to perform essential functions such as digestion and excretion by itself, until late into the pregnancy, the placenta diffuses nutrients in and waste out for the baby.
Marsupials are an interesting class of animals. Their babies are born extremely early in development (after about 36 days), however, the mother has a pouch, where the baby will nurse until around 9 months.
Most mammals give birth to live young. There are exceptions to this rule, as our favourite egg-laying mammal of action’s theme explained to us. (Dooby dooby dooa dooby dooby dooaa AGENT P!)
Platypi and spiny anteaters derive their nutrients from a shelled egg.

Getting even more specific, let’s talk about primates. These are the least superior mammals. I should know. I am one.
Primates were descendants of insectivores. They have dexterous hands, and opposable thumbs, which allow their hands to perform fine motor tasks. Instead of claws, they have nails
Their hands contain many nerve endings, making them very sensitive (which is why papercuts are so agonizingly painful.) Their eyes are forward-facing and close together. This allows face to face communication. Close eyes allow for overlapping fields of vision, increasing depth perception and hand-eye coordination.)
Primates engage in the most intensive parenting out of any mammal. They tend to have single births and build strong bonds with their young.
The book organized 3 different organisms based on their taxonomy. I put that down and added rats because rats are cool. Don’t @ me.
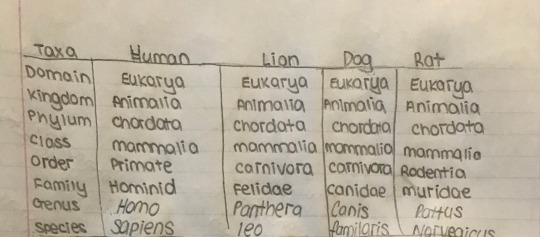
Cladograms
Cladograms are an extremely useful tool to show how organisms evolved different traits over time. There is a more complicated one above, however, the book included an extremely simplified one also that helped me understand how these graphs are made, so I will include that here as well.
First, like any graph, a table is made detailing the data that will be graphed. In this case, this data will be the specific organisms (cats, lizards, salmons, and earthworms) and the existence of specific traits (backbone, legs, and hair.)
Then a line is drawn, showing each trait as it developed, following by the organism with that trait.
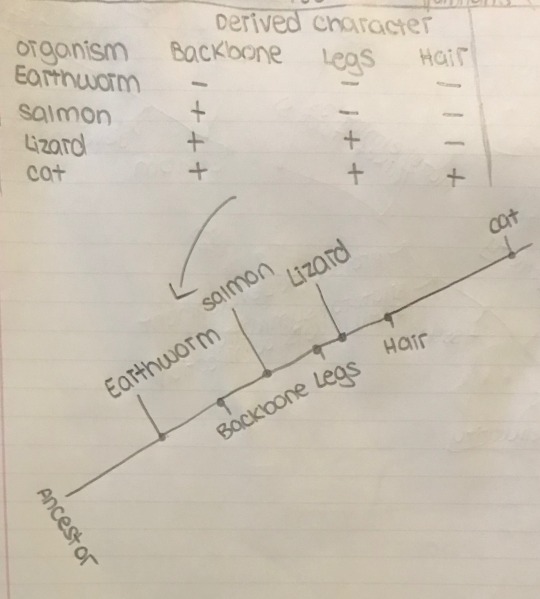
What this graph shows is that cats and lizards are more related than lizards and earthworms, etc. Tldr; a cladogram/phylogenetic tree draws distinctions between shared traits (traits different organisms have in common) and derived traits (traits that the ancestor did not have) displayed in such a way so as to show the evolutionary history of a group of organisms.
So what qualifies an animal? Animals are multicellular eukaryotes. They are all heterotrophs, meaning they acquire nutrients via ingestion. (Unlike plants, which manage to get nutrients through photosynthesis, such as the Calvin Cycle which produces a plants sugar.) All animals can move in some form.
Movement is a broad term. Beating cilia, and waving tentacles both count as movement. The movement that often comes to peoples minds, however, is locomotion, which is the movement from place to place. Some animals are sessile, which means they lack the capability to move from place to place. Hydra can still wave their tentacles (in the air like they just don’t care). Sponges are an interesting case, as many legitimately, cannot move.
Above, I mentioned terms like endoskeletons, exoskeletons, and hydrostatic skeletons. Hydrostatic skeletons are closed body compartments filled with fluid, that provide support. Exoskeletons are external, nongrowing skeletons, made of chitin (which also makes up the cell walls of fungi). Endoskeletons are internal skeletons made of bone and cartilage that grow with the organism. They are connected to each other at joints via ligaments, and to skeletal muscles (voluntary muscles) via the tendons.
All life has the ability to maintain homeostasis. Life survives within a narrow temperature range, from around 0 degrees Celsius to around 50 degrees celsius. In the ocean, this was not a massive problem, as it is the most stable environment temperature-wise, as water is able to absorb a lot of heat. However, the land is a lot more crazy. Different organisms found different ways to adapt and survive.
For example, a jackrabbit's ears are a major tell about what climate they live in. Jackrabbits that survive in the cold have small ears to minimize heat loss. Jackrabbits living in the heat have large ears that allow heat to dissipate, filled with small capillaries making the ears appear pink.
Huddling, basking, panting and sweating, swarming, and shivering are all examples of adaptations different organisms use to survive in extreme temperature. Depending on whether an organism is an ectotherm or endotherm, their temperature regulation will be different. An ectotherm is heated from the outside. For example, crocodiles bask in the warm sun to heat their bodies up. Endotherms or homeotherms generate their heat from the inside by using large quantities of energy. For example, a litter of cold puppies will huddle together and with their mother, as their warmth, and their mothers warmth help heat them up.
Excretion refers to the removal of metabolic waste, such as excess water, carbon dioxide, and nitrogenous waste. There are 3 different kinds produced by different organisms
Ammonia
Ammonia is soluble in water and extremely toxic. Anybody who takes proper care of a fish tank is aware that cleaning the ammonia from their tank is essential in keeping their fish healthy.
Excreted mainly by marine life, like hydra and fish.
Urea
Not as toxic as ammonia
Excreted by earthworms and humans (urine contains urea and water)
In mammals, the liver is responsible for turning ammonia into urea.
Uric Acid
A paste-like substance that isn’t soluble, and not very toxic
Excreted by insects, many reptiles, and birds, and allow for the preservation of water.
Different organisms have different structures that allow for excretion.
Hydra excretes ammonia with no aid from any excess structure.
Platyhelminthes have flame cells that help them excrete ammonia
Earthworms have nephridia (metanephridia) to excrete Urea
Insects have Malpighian tubules to excrete uric acid
Humans have nephrons to excrete urea.
Following up, let's look at 3 different organisms and the characteristics that make them unique!
Hydra (from Cnidaria)
Hyrda digest their food in the gastrovascular cavity. They, unfortunately only have one hole, where food goes in and waste comes out. The gastrodermis (gastrovascular cavity lining, or gastrocoel) secrete digestive enzymes to help extracellular digestion progress. Lysosomes, which are found in animal cells are responsible for intracellular digestion.
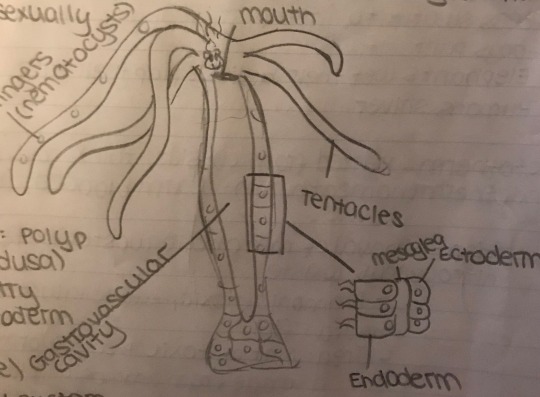
Hydra reproduces asexually by budding. A bud is a genetically identical, but tiny little hydra that grows within or on the parent.
Earthworms (From Annelida)
The digestive system of earthworms is much more complex than that of the hydra. Luckily, they have a mouth and an anus. The mouth ingests decaying organic matter along with the soil. The food travels down the oesophagus into the crop. The crop stores the food until it is ready to be digested. The food then moves into the gizzard, with thick muscular walls that digest the food mechanically, with the aid of the ingested sand and soil. The food moves into the intestines, where chemical digestion occurs. The intestine has a large fold, called the typhlosole, which increases the surface area.
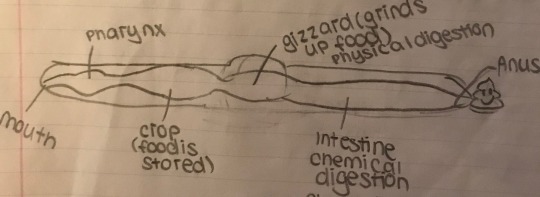
Worms don’t have a traditional respiratory system. Instead, gas is exchanged by diffusion, through moist skin. This type of respiratory system is called an external respiratory system. Their hearts have 5 aortic arches that pump blood. Worms have capillaries, giving them a closed circulatory system. Their blood contains haemoglobin, making it red. Earthworms have nephridia, excreting urea, and are hermaphrodites. A worms brain is made of two dorsal, solid, fused ganglia, with a solid, ventral, nerve cord.
Grasshoppers (From Arthropoda)
Grasshoppers also have a digestive tract consisting of a crop and gizzard. They also have mouthparts specialized for tasting, biting, and crushing food, and their gizzard has chitin plates that aid in mechanical digestion. Their digestive tract contains Malpighian tubules that remove nitrogenous waste in the form of uric acid. (No, I did not draw a grasshopper. I know when I am defeated.)
Grasshoppers have a similar nervous system to worms, however, they have an open circulatory system. They lack capillaries, and blood moves through hemocoels instead. Arthropod blood has no haemoglobin. They have an internal respiratory surface because gas exchange occurs on the inside. They have a system of tracheal tubes that lead to the hemocoels. Oxygen is carried by hemocyanin, with copper as the core atom. This is why molluscs and insects have blue blood.
#animals#animal physiology#physiology#biology#animal biology#bacteria#fungi#protists#archea#domains#phylum#taxonomy#platyhelminthes#annelids#porifera#nematodes#cnidaria#echinoderms#chordates#mollusca#arthropods#grasshopper#grasshopper biology#grasshopper physiology#hydra#hydra physiology#earthworms#earthworm physiology#SAT Bio#SAT biology
27 notes
·
View notes
Text
Climate-friendly microbes chomp dead plants without releasing heat-trapping methane
https://sciencespies.com/nature/climate-friendly-microbes-chomp-dead-plants-without-releasing-heat-trapping-methane/
Climate-friendly microbes chomp dead plants without releasing heat-trapping methane
The tree of life just got a little bigger: A team of scientists from the U.S. and China has identified an entirely new group of microbes quietly living in hot springs, geothermal systems and hydrothermal sediments around the world. The microbes appear to be playing an important role in the global carbon cycle by helping break down decaying plants without producing the greenhouse gas methane.
“Climate scientists should take these new microbes into account in their models to more accurately understand how they will impact climate change,” said Brett Baker, assistant professor at The University of Texas at Austin’s Marine Science Institute who led the research published April 23 in Nature Communications.
The new group, which biologists call a phylum, is named Brockarchaeota after Thomas Brock, a pioneer in the study of microbes that live in extreme environments such as the hot springs of Yellowstone National Park. Sadly, Brock died April 4. His research led to a powerful biotech tool called PCR, which is used, among other things, in gene sequencing and COVID-19 tests.
“The description of these new microbes from hot springs is a fitting tribute to Tom’s legacy in microbiology,” Baker added.
So far, Brockarchaeota have not been successfully grown in a laboratory or imaged under a microscope. Instead, they were identified by painstakingly reconstructing their genomes from bits of genetic material collected in samples from hot springs in China and hydrothermal sediments in the Gulf of California. Baker and the team used high-throughput DNA sequencing and innovative computational approaches to piece together the genomes of the newly described organisms. The scientists also identified genes that suggest how they consume nutrients, produce energy and generate waste.
“When we looked in public genetic databases, we saw that they had been collected all around the world but described as ‘uncultured microorganisms,'” said Valerie De Anda, first author of the new paper, referring to specimens collected by other researchers from hot springs in South Africa and Wyoming’s Yellowstone, and from lake sediments in Indonesia and Rwanda. “There were genetic sequences going back decades, but none of them were complete. So, we reconstructed the first genomes in this phylum and then we realized, wow, they are around the world and have been completely overlooked.”
The Brockarchaeota are part of a larger, poorly studied group of microbes called archaea. Until now, scientists thought that the only archaea involved in breaking down methylated compounds — that is, decaying plants, phytoplankton and other organic matter — were those that also produced the greenhouse gas methane.
“They are using a novel metabolism that we didn’t know existed in archaea,” said De Anda. “And this is very important because marine sediments are the biggest reservoir of organic carbon on Earth. These archaea are recycling carbon without producing methane. This gives them a unique ecological position in nature.”
A phylum is a broad group of related organisms. To get a sense of just how large and diverse phyla are, consider that the phylum Chordata alone includes fish, amphibians, reptiles, birds, mammals and sea squirts. The phylum Arthropoda, which accounts for about 80% of all animals, includes insects, arachnids (such as spiders, scorpions and ticks) and crustaceans (crabs, lobsters, shrimp, and other tasty sea denizens).
In July 2020, Baker, De Anda and others suggested the possible existence of several new phyla among the archaea, including Brockarchaeota, in a review article in Nature Microbiology. This latest study adds more than a dozen new species to Brockarchaeota, describes their metabolism and demonstrates that they are indeed a distinctly new phylum.
In addition to breaking down organic matter, these newly described microbes have other metabolic pathways that De Anda speculates might someday be useful in applications ranging from biotechnology to agriculture to biofuels.
#Nature
0 notes
Text
For the past 3 weeks from May to June I embarked on a journey to the undermined country of Cambodia. The Magellan Program, through my alma mater of Washington & Jefferson college has given me an experience I will never forget. I have learned in depth about the rich ecotourism in Cambodia while walking among some of the worlds most mysterious archeological sites which are rich with history, bathing some of the worlds largest animals, pachyderms, and living with some of the poorest families who have the biggest hearts and kindest hospitality. I cannot help to reflect on my short time here and think of all the things I have learned about this developing third-world country but here it goes...
1.Cambodia is HOT! 2.Try not to act like a tourist 3.Tuk tuks are the way to travel 4.Learn some Khmer 5.SMILE and say hello 6.Try frog at least twice 7.Introduce the people to Snapchat filters 8.Play with babies 9.Make the kids laugh, usually if you make the kids laugh the parents laugh too 10.Give people little gifts when taking up their time, time is money 11.Learn some weird tricks to entertain with, poorly done magic tricks work, also whistling with grass 12.Whatever you do, do NOT tell a bunch of school kids “yum yam ching cha” or in English, I eat geckos. They will think you’re a weird white guy and run to their boats to go home 13.Children mean everything to their families 14.Pimp babies wear gold chains with crocodile teeth to protect them from spirits and apparently it keeps them from crying 15.Children are their own bus to school, they actually want to learn! 16.OUR FIRST WORLD PROBLEMS ARE PETTY COMPARED TO CAMBODIAS PROBLEMS 17.Families have to worry everyday if they’re going to eat or catch enough to eat 18.Be very thankful for your hosts at home stays, they give you everything 19.Don’t worry about the 7 foot crocodile that is right outside while you’re sleeping, no problem, the rotting wood and chicken wire will save you 20.Siamese Crocodiles are nearly extinct in the wild but are very plentiful in captivity where they are bred for their hides 21.If you catch a tokay gecko the natives will think you’re awesome! 22.People are afraid of geckos 23.Water hyacinth is very very invasive, thank you French for introducing it 24.Don’t move in the boat 25.Be so thankful for what you have! 26.A pile of ashes from the deceased still smokes after being burned 27.Monks are bald 28.If the guide says the treehouse is safe to climb, climb it 29.Seeing several internationally protect birds is awesome, they are very graceful 30.1,000 lingas represent sexual organs for a God but once the water passes over them it’s like holy water 31.On top of the mountain there is usually a very big Buddha 32.Go swimming in the waterfall and don’t be afraid if the little fish bite your nipples or feet, they’re just eating the dead skin 33.People climb trees to collect honey from honey bees without protective clothing, very gutsy 34.Go see Angkor Wat, definitely not small and a archeological world wonder 35.See some other temples too, they’re all unique 36.There’s over 300 temples in Cambodia 37.The little frog has a big sound 38.The monkeys may be cute, but don’t trust them, them try to steal your stuff 39.If the tree wants to grow, it will grow wherever it wants to 40.Eat some bugs 41.Giant African Rats save lives by sniffing out bombs and land mines 42.Night buses are cool, definitely a efficient way of traveling. Bed on a bus, who knew? 43.You will most likely see dead people at the killing fields 44.The Khmer Rouge caused a Cambodian Holocaust that killed over 3 million people in the most inhumane and horrific ways 45.Phnom Penh is the capital city 46.Everything can be found at the markets 47.If you’re staying at a hotel and the front desk man calls to tell you someone is in the lobby for you it’s most likely a hooker 48.Don’t sleep with hookers! 49.Drivers don’t use their breaks when going around turns so mountain roads can be scary 50.Laying in a hammock watching the sun go down is great in Mondulkiri👌, don’t say “chemoy” or you’ll fall out of the hammock 51.Elephant riding is bad for the animals but feeding them bananas is alright 52.Elephants like mud so if you go to visit them dress accordingly 53.Bathing an elephant under a waterfall sounds like a fantasy but it’s actually a reality in Cambodia, steer clear of floaters! 54.Cashews are extremely poisonous until they are processed and are attached to an apple 55.Old women smoke pipes while gardening 56.Rice wine and go fish- enough said... 57.There’s a really cool French couple in Banlung, Author and Claire are awesome, and Author is a great cook 58.A muddy road and motorcycle don’t mix, stay alert, don’t freak out, don’t put your feet down, and don’t move! 59.If you go into the jungle and don’t loose blood you’re doing it wrong 60.All the Arthropoda is out to get you and everything is bigger in the jungle 61.Mountain stream bamboo showers is recommended and exposing 62.I hate fire ants- a lot 63.Seeing endangered gibbons in the wild swinging from the trees for 20 seconds is well worth it 64.Jungle trekking is the hardest hiking I’ve ever done 65.Stay hydrated! 66.Do not stick your hand in your bag without checking it when out of the jungle, scorpion stings hurt like you wouldn’t believe and make you question if you’re going to die 67.Cambodia is a great weight loss program 68.Cambodians are never on time, your bus could leave early or late and you stop at least once an hour for a 12 hour night bus 69.Most of Cambodia’s big wildlife is threatened or endangered because of the illegal animal trade and poaching 70.Bears don’t make good pets and shouldn’t be used to show a person’s social class 71.Cambodian circuses are hilarious and done by kids sometimes 72.Master-bating monkeys will try to bite you, don’t trust monkeys, and always keep a Cambodian boy with a stick close by 73.Nuns shoot monkeys with slingshots 74.Cambodian spelunking or caving is self-led and usually involves a lot of bats 75.Parents want you to take their kids to America, this is very tempting 76.Kids in Cambodia still play with sticks because they don’t have phones 77.If you’re attractive and speak a little Khmer you will most likely be offered a wife on the daily 78.Hello Kitty! Helmets and pink bikes are stylish 79.Apparently, two white men on the same bike is very funny 80.All the dogs are all bark and no bite 81.Roadside crickets and silkworms are sold at the bus station 82.Entomology is very understudied 83.Every part of the palm tree can be used 84.Movies are $3 85.PCVs are cool people 86.Lady boys try to give you a massage and then some, just don’t say yes to the massage and run away from them 87.Whatever happens, just roll with it, God’s Plan! 88.Cambodia’s ecotourism is amazing, and so is the country
Magellan 2018- Siem Reap, Cambodia
“Don’t cry because it’s over, be happy because it happened” -Dr. Seuss
0 notes
Text
I’m not a birder, well, not really. If I was to call myself one, I would then have to call myself a planter, rocker, crabber, beacher, insecter, spider-er, general bugger,….er, yeah, some of them are mis-informative and downright impolite 😀 I’m interested in so many things in the natural environment, I can’t really narrow it down to one animal class. It is concurrent with my art practice – I have my fingers in all the pies.
But over the years, despite photographing many living things, I have neglected birds. Why? Mostly technology. Until about four years ago, my camera, which was fantastic for macro, only had a 4x zoom, which kinda sucks when trying to capture wildlife outside of Arthropoda. Four years ago I discovered the compact zoom camera and grabbed myself a 42x zoom – well, suddenly birds became a viable topic to photograph 😀 And just as I was started to get used to the idea, the stupid camera broke – twice ::glares at it menacingly as it sits ornamentally beside my desk because that is all it can currently do::
So a few weeks back I finally replaced the ornament with my new 65x optical zoom compact (had to drop 2Mps down to 16Mps to do it though as I needed a mic input ::pouts::) and then promptly went for a week’s holiday on the Yorke Peninsula.
When our family goes on holiday, we usually do a wildlife count of what we came across. This time we saw the following: Edithburgh/Sultana Point (including a drive to Corny Point and along the coast to Hardwicke Bay), Yorke Peninsula, South Australia (in a little more detail than normal)
mammals (2 wild species) – a seal feeding (Corny Point), a fox, farm animals including alpacas
reptiles (2 species) – two skinks in the sandhills (there will be a separate post about that!) + several stumpy tails on the dirt roads
fish (approx. 2 live species) – gobi, juvenile fish in the shallows + 2 dead ringed toad fish and a dead shovel-nosed ray
crustaceans (approx 4 or 5 live species) – several species of pebble crab, and little under-rock crabs + dead blue swimmer crabs left over by fishermen
insects – ladybirds (several species), a wasp, a bee fly, painted lady butterflies, a large weevil, shield bugs, and a green vegetable bug nymph + the usual mozzies (ugh, lots of those), flies, ants, cabbage butterflies, and moths.
molluscs – a whole ecosystem of sea snails, including hundreds of Conincal Sand Snail eggs (the jelly blobs on the seashore the kids love to collect) + one largish Fimbriate Helmet Shell and many Razor shells.
echinoderms (2 live species) – starfish (Parvulasta exigua) and a See-Through Sea Cucumber (Paracaudina australis)
…plus a worm and a couple of living sponges.
This is a pretty low count for us as we didn’t get out as much as we could have since stupid me sunburnt my feet on day three and hobbled around for three days at least.
Missing from this list is at least 25 species of bird 😀
Because I’m new to birds, my sighted bird list includes mostly common species and pretty much every bird I could find at Sultana Point, and I photographed as many of them as I could.
Photo Gallery of birds at Sultana Point, Yorke Peninsula, SA, 1 – 10 December 2017
Silver gulls
Australian pelican
Pacific gull
Flying Pacilic Gull
Red-necked stint?
Red-necked stint?
Red-necked stints?
Red-capped Plover (female)
Pied cormorant
Black-faced cormorant
Juvenile pied cormorant?
Cormorant parade – pick the odd one out.
Whiskered tern?
Crested tern?
Crested tern?
Fairy tern?
Sooty oystercatcher posse
Pied oystercatchers
Singing honeyeater
Singing honeyeater eating dianella berries
Common starling
Unknown wader
Same unknown wader
White-faced heron
Unknown egret
As for the total holiday bird score, added to the above list – Willie Wagtails, House Sparrows, Pigeons (Ardrossan cliffs), various birds of prey that always appear while I’m driving, magpies, peewees, little ravens, currawongs and several black swans.
I’m a strong believer in taking many shots to get good shots, so these are only a portion of the over 1500 photos I took in 10 days and don’t include the non-avian shots. I think there may be further posts about several of these birds in the future as I got some good shots of some of their activities, particularly the red-capped plover. Plus some videos I have yet to sort through (it has taken me most of today to sort these few out).
Also, if I have mis-identified any of these, don’t hesitate to let me know, and any advice on the unidentifed wader would be wonderful. Unfortunately I was not in the right spot to get a good shot and it flew away before I could get into one.
I’m not really a birder, but I am interested in them and am planning to photograph as many as I can find 😀 In any case, these are all stockshots for artwork in the future 😀
I’m incredibly late for Saturday Critters this week, but I’m adding this post there anyway, so go over there and gaze at the wonders of our world.
Art Always!
Best wishes, Liz
Not a birder I'm not a birder, well, not really. If I was to call myself one, I would then have to call myself a planter, rocker, crabber, beacher, insecter, spider-er, general bugger,....er, yeah, some of them are mis-informative and downright impolite 😀 I'm interested in so many things in the natural environment, I can't really narrow it down to one animal class.
0 notes
Text
DNA Sequencing is a BLAST!
Introduction/Initial Hypothesis
This lab introduces the value of DNA sequence analytics to group organisms based on phylogenetics rather than morphological features or arbitrary taxonomy. Since DNA serves as a physical heritable factor that can be traced and sequenced, biologists use it as the most effective tool to classify organisms. As father of modern binomial nomenclature, Carl Linnaeus (1707-1778) set the foundation of classifying organisms based on common similarities and thus established a hierarchy to place any living creature depending on their outward appearance. However, only after Charles Darwin (1809-1882) introduced his theory of evolution in On the Origin of Species did biologists seek to classify species based on their lineage of inheritance, spurring the field of phylogeny. Phenotypic traits originally seemed to be shared between evolutionarily linked species were sometimes instead seen to possess analogous structures, which arose from twin adaptations to combat similar environments. The emergence of DNA analysis, especially nucleotide sequencing also gave biologists a concrete method to determine similar descent. Suddenly the age of Linnaean classification crumbled as previously thought monophyletic taxons (groups with all descendants sharing common ancestry) instead became paraphyletic (missing a descendant) or polyphyletic (containing species with no evolutionary descent). In addition, the discovery of parallel gene transfer in some bacteria has led some to question vertical evolutionary charts in favor of circular ones.

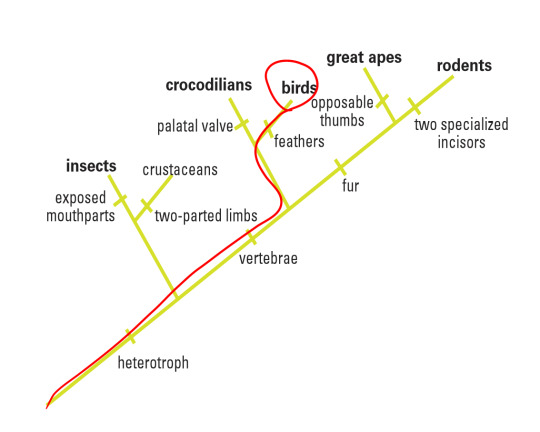
In this particular laboratory activity, I was given a picture of a fossil specimen collected near Lioning Province in China. Based on its morphological features shown in the amber, I was able to make some initial references that would contribute to my hypothesis. For instance, I could spot some very thin sprouting along the backbone of the animal (easily classifying it as a vertebrate, or part of the Chordata phylum) and assumed it was a group of feathers, thus placing this animal perhaps in the Aves category (birds). In addition, I could not discern any temple openings in its skull, thus classifying it as an Anapsid and part of the Reptilia class. Finally, I could make out that this organism did not have any opposable thumbs, disqualifying it as part of the Primate order in Mammalia and noticed that it was a tetrapod. Thus, I tentatively placed it in the Aves class. By comparing four different gene sequencing found in this sample with the BLAST site database, this experimentation would either corroborate or question my hypothesis. My proposed class the organisms would sit within is circled in red on the following cladogram:
Methods/Materials
I utilized my computer and the four ASN files given by the lab handout (the gene sequencing) as well as the Basic Local Alignment Search Tool (BLAST website) in order to obtain DNA sequencing evidence. I entered all four ASN files into the “Saved Strategies” page and both observed the alignment distributions of the top 100 subject sequences and the “Distance tree of results” page, which gave a prediction of where the targeted organism would fall within. Using all eight pages, I formulated a qualitative analysis of the data.
Data
In order to group the data systematically, I first listed the two most similar species based on their DNA sequencing score, which combined the Identification percentage (the proportion of nucleotides that were identical between the two species, the target and the compared species) and the Query cover (the percentage of the identical nucleotides aligned correctly). As independent identifiers, a low Query cover but a high Identification score could indicate a good nucleotide match but with one containing more introns or having insertions and deletions rather than substitutions between the identical groups of nucleotides.
I listed the most similar species with their most identical protein scores with the convention of the numbers in the parentheses being (Query cover, Identification percent).
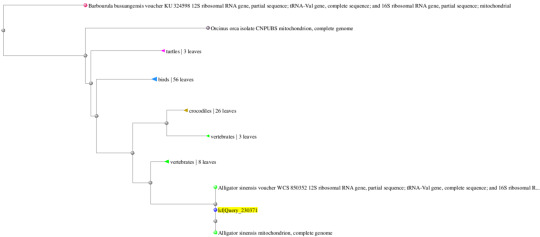
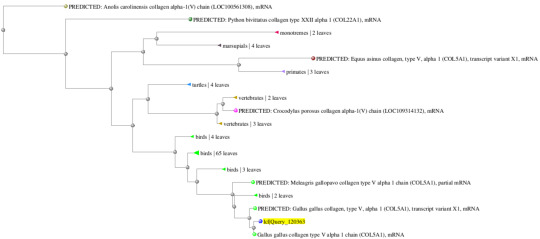
Gene 1: Gallus gallus (100%/99%) and Coturnix japonica (100%/97%)
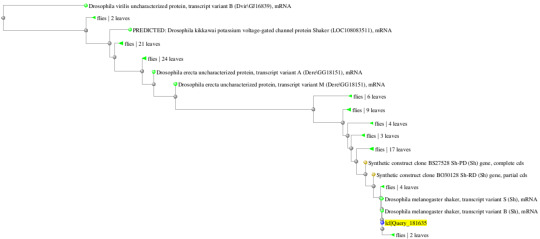
Gene 2: Drosophilia melanogaster (92%/99%) and Drosophilia yakuba (95%/97%)
Gene 3: Taeniopygia guttate (95%/100%) and Corvus brachyrhynchos (95%/99%)
Gene 4: Alligator sinesis (100%/99%) and Alligator mississippiensis (91%/83%)
In addition, the following are the predicted phylogenetic tree containing the most immediate evolutionary ancestors of the target organism. Using these trees, I was able to quickly discern which class or family or organisms the compared species were originated.

Analysis/Conclusions
Overall, I believe that my hypothesis that the target organism fell underneath the Aves class was adequately supported. Thus, my conclusive cladogram would be the same as my hypothesis:
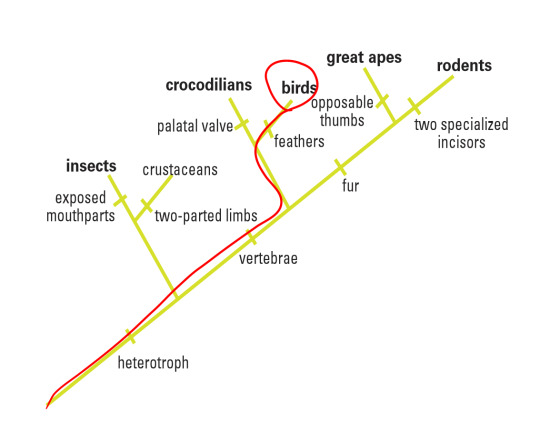
There are several reasons for my reasoning. First of all, looking at the data, the closest relative species based on both nucleotide similarity and alignment happened to be included in groups chickens (aves), fruit flies (arthopoda), finches (aves), and alligators/crocodiles (reptilia), it seems fair to group the target organism in aves as two of the four closely related species belonged to that group. Why not arthropoda? Looking at the morphological data, the organism possessed a backbone (thus it is part of the Chordata phylum) and as a basal group, the arthropods are farther than aves than reptilia. It is interesting to note that the Query cover for the arthropod species are the lowest (92% and 95%) of all the other species even though the Identification score is high, alluding that perhaps while the nucleotide bases might have been preserved through evolution, many insertions and deletions between them could have caused wildly different protein to be synthesized.
In addition, the Aves class is less inclusive than the Reptilia group, and especially to be noted is that birds may be thought as a subset of reptiles because they are reptiles’ evolutionary descendants. Although the Reptilia class could be an alternate supported hypothesis, I would put a higher precedent on the Gallus gallus because it has a higher Query cover with a near perfect Identification percent, meaning that of the 99% nucleotides that are identical, they are all aligned the same as well.
Finally through interesting research outside this laboratory, I found a species of dinosaur named Sinosauropteryx that bears close resemblance to this laboratory’s fossil specimen, and even may be the same species. It was the first dinosaur recorded that possessed feathers, and may have been the bridge species between reptiles and birds.

0 notes
Text
Mapping the Different Planktonic Groups at One of the Egyptian Bays along Mediterranean Coast- Juniper publishers
Abstract
The abundance and community composition of zooplankton is spatially and temporally variable so it requires sampling over space and time. Quantitative assessment of biomass, community composition, and abundance is sensitive for sampling methodology, including the location and seasonal timing of sampling, as well as mesh size and gear type. Therefore, repeated and consistent sampling is essential to determine the changes in zooplankton distribution, abundance, community composition and seasonal timing at time scales that have impacts on higher trophic levels. In the present study, El-Mex Bay is highly diversified (204 forms) but low standing crop (annual average 8935 organisms/m3). Using GIS and the other mapping applications give an easy and clear image about the distribution of aquatic fauna especially microscopic forms which help in understanding the dynamic of biological ecosystem.
Introduction
Zooplankton species are important lower trophic level members of marine ecosystem. They are a good indicator of ecosystem status since their populations respond relatively rapid to environmental variability and they are usually not fished [1].
In aquatic environment zooplankton is considered as one of the most important biotic components, particularly in the pelagic habitat. Zooplankton play a key role in the pelagic food web by controlling phytoplankton production and shaping pelagic ecosystem. In addition it has a critical role as a food source for larval and juvenile fish and consequently the dynamics of its populations have a great influence on recruitment to fish stocks. Such intermediate role of zooplankton makes it as a regulator of the biological productivity in the pelagic habitat, since it has a detrimental effect on phytoplankton by grazing and beneficial effect to the water fertility through nutrient recycling [2]. Zooplankton community demonstrates variable structure in the different aquatic habitats, relative to differences in the ecological conditions. Shallow marine coastal areas present an interesting subject of biological studies because of their productivity and the diversity of organisms which occur there [3]. Coastal marine areas are ecologically and economically important and of social interest [4]. They are extremely variable systems, where changes in the water circulation patterns and fluctuations of land influences (e.g. rivers, sewage flow) induce high temporal variability on scales ranging from hours to seasons. This variability may be reflected the dynamics of the populations, particularly planktonic ones, thriving in coastal systems and can hide the underlying seasonal patterns of organisms' abundance and biomass [4].
A fundamental challenge in Earth System science is the response of the marine ecosystem to changes in climatic forcing. In particular how will it affect ecosystem functioning and the sustainability of bio-resources. Datasets which allow us to map the distribution of marine biota are sparse. Satellite remote sensing can provide detailed information on a large scale for several bio-physical parameters, such as temperature and chlorophyll [5]. The challenge is to combine satellite data with the sparse in-situ datasets to generate distributions of higher trophic levels. To monitor the aquatic ecosystem and integrated of water, zooplankton has been recently used as bioindicator [6-12].
Abo-Taleb [13], Abdel Aziz et al. [14], Abo-Taleb et al. [12] they were found that fluctuation in the domination of copepods rotifers, Chromista and Protozoa resulted from the unstable environmental conditions of the bays and estuaries along the Egyptian Mediterranean coast. In the Egyptian part of the Mediterranean Sea, Zooplankton has attracted more attention particularly in the neritic waters. The first study has been conducted by [15]. Steuer [16,17] as preliminary reports on plankton hauls collected off Alexandria and Rosetta Coasts. Later one more detailed and comprehensive studies were carried out on zooplankton in different parts of the Egyptian Mediterranean waters. Some of these studies concern with the total zooplankton community off Alexandria Coast and Nile Delta Region Dowidar [18] studied the distribution and ecology of both phytoplankton and zooplankton in Alexandria region. El- Maghraby & Halim [19] investigated quantitatively and qualitatively the phytoplankton and zooplankton off the Eastern Harbor. Halim et al. [20] gave brief notes on the distribution of plankton organisms off the Egyptian Mediterranean Coast during the last normal Nile flood of 1964. Guerguess [21] and Dowidar & El- Maghraby [22], studied the distribution and ecology of the neritic zooplankton of the area surrounding Alexandria with special reference to Copepoda. Hendy [23] and El Raey et al.[24] , assess quantitatively the vulnerability to sea level rise of El Mex Bay and determine areas at risk of flooding and erosion.
El-Mex Bay has attracted little attention for the biological studies, although it lies under stress of huge and different types of waters. So, it is necessary to study zooplankton community in the bay. Hence, the present study is designed to study the dynamics of zooplankton community at El- Mex Bay, Using GIS (ARCMAP 10) to produce maps illustrating plankton abundance and diversity.
Material and Methods
Study area
El- Mex Bay is located west of Alexandria City, at longitude 29° 45' and 29° 54' E and latitude 31° 07' and 31° 15' N, It extends for about 15Km from Agami headland to the west to the Western Harbor to the east. The bay has a mean depth of 10m. Its surface area is about 19.4Km2, and its volume 190.3 x 106m3[25] . The shoreline of El- Mex Bay is rocky with narrow sandy beaches. It receives a heavy load of wastewater (7*109 m3/year) both directly from industrial outfalls (El- Umoum Drain) and indirectly from Lake Mariut via El- Mex Pumping Station [26]. El- Umoum Drain is mainly agricultural water. Lake Mariut receives also wastewater from the four sources in its eastern section, consisting of domestic, industrial and agricultural wastes, this liquid wastes discharged to the harbor via El- Mex Pumping Station.
Samples were collected seasonally during 5 seasons from autumn 2011 to autumn 2012 from eight stations. The stations were selected to cover all possible environmental changes of the study area. The locations of the sampling stations are shown in (Figure 1).
Zooplankton samples
Zooplankton samples were collected at each station by standard plankton net (No. 25) of 55μm mesh size which lowered vertically till near the bottom then pushed up to the water surface. The zooplankton organisms which retained in the net were then transferred into smaU bottle and preserved in 5% neutralized formalin solution and the sample volume was then adjusted to 100ml. The samples were examined under a binocular research microscope. The identification was undertaken to species levels. For estimation of standing crop, sub samples of 5ml were transferred to a counting chamber (Bogorov chamber) using a plunger pipette this operation performed three times and the average of the three counts was taken, and the standing crop was calculated and estimated as organisms per cubic meter according to the following formula [27]
N= (n * v) / (V * 5)
V= πr2.d
Where;
N: Total number of zooplankton per cubic meter.
n: Average number of zooplankton in 5ml of the sample.
v: Volume of concentrated sample (100ml).
V: Volume of total water filtered (m3).
d: Length of the traction by the net.
Identification of the different species of zooplankton species was carried out according to Sars [28,29] (Copepoda), Rose [30] (Mediterranean copepods), Tregouboff & Rose [31] (Mediterranean plankton), Pontin [32] (Freshwater plankton), Guerguess [33], Hutchinson [34] (Plankton as a general), Marshall [35], Bick [36], Paulmier [37], Jorgensen [38] (Protozoa), Gurney [39-41] and Hardig & Smith [42] (freshwater Copepoda), Wilson & Yeatman [43], Edmondson [44], Gurney [45] (Copepoda and Cladocera), Berzins [46], Berzins & Pejler [47] (Rotifera), Sars [48] (Ostracoda), Sars [49] (Entomostraca) and using WORMS database [50].
Data analysis
The remotely sensed satellite imagery (LAND SAT 7) was found to be the most appropriate one for this study, as with its regional coverage, all necessary map features were obviously clear and interpreted. A raster depending software was used for the purpose of digitizing the map features. ARC-GIS 10 and Envi software was used for this purpose for its high digitization capabilities, also in finalizing and visualizing data. The analysis and interpretation of different zooplankton groups was done by ARC-GIS 10.
Diversity index was estimated according to Shannon & Weaver [51] as follows:
H = - Σ Pi ln Pi
Where;
Pi = n/N
Pi: is the proportion of a species number (n) to the total number of zooplankton (N).
Results
Diversity of zooplankton groups in the bay
Zooplankton community in the El-Mex Bay was represented by 204 species belonging to 12 groups. Protozoa was the most diversified group in the bay 69 species, followed by Copepoda which represented by 50 species. Rotifera ranked the third diversified group in the bay represented by 38 species, Chordata represented by 9 forms while, Cnidaria, Mollusca and other Arthropoda were represented by 6 forms for each one of them, Cypredina, Cladocera and Annelida were represented by 12 forms, four to every group. Two cirripedian forms and two Chaetognathans were recorded, two Radiolaria, one Pteropoda and Porifera residues (Figure 2).
The number of species varied temporary where the highest number of species were recorded during winter (151 species), decreased during spring to reach 123 species and still decreased during summer and autumn 2012 to reach (114 and 113 species) respectively. Number of species differed from one station to another with its maximum at station I and VI (130 and 131 species respectively) reaches 126 species at station III and ranged from 105 to 116 species at the other stations, with the most diversity at station IV (100 species) as recorded in (Table 1) .
Relative contribution (%) of different groups
As shown in Table 2, Copepoda was the most important group, contributing 55% of the total zooplankton. Protozoa occupied the 2nd order of abundance forming 15.6% of the total zooplankton. Rotifera formed 11.7% of the total zooplankton. Other groups were frequently encountered such as Larvacea, Chaetognatha, Cladocera, Cirripedia, Ostracoda, Chordata, Mollusca, Annelida, Nematoda and Cnidaria.
Dynamics of zooplankton groups in the bay (Zooplankton groups distributions)
Zooplankton communities showed wide seasonal variations at El- Mex Bay, the following data described these fluctuations (Figure 3-7).
Copepoda: Appeared as the predominant component of zooplankton in El- Mex Bay. The adult copepods formed 63% of total copepod counts with an average of 3218 individuals/m3 while, the rest 37% were represented by copepodite stages and nauplii with an average of 1866 individuals/m3. The maximum density was recorded during autumn 2011 and gradually decreased to their minimum value during spring 2012.
Protozoa: Ranked the 2nd dominant group in the bay with annual average of 1440 organisms/m3. Generally, winter and spring seasons showed maximum densities (average of 2272 and 2045 individuals/m3), while minimum average counts were recorded at the beginning of the study during autumn 2011. Their counts ranged between maximum of 5860 individuals/m3 at station VII during spring and minimum of 212 individuals/m3 at station V during autumn 2011.
Rotifera: Ranked the 3rd group and recorded annual average 1077 individuals/m3. 38 rotifer species were identified under 16 genera within 12 families, 3 orders and one class. The maximum counts were recorded at station VII (4161 individuals/m3) during autumn 2012 and minimum of 170 individuals/m3 at station V during autumn 2011. The highest rotifer average was recorded at station VII (2508 individuals/m3), while stations III and V showed minimum average counts of rotifers (596 and 558 individuals/m3)
Annelida: Ranked as the 4th dominant group at the El- Mex Bay during the study period, they constituted 3.9% of the total zooplankton groups with average total counts of 332 individuals/m3. Their minimum values were recorded during autumn 2011 (188 individuals/m3) and flourished during winter 2012 with a maximum of 556 individuals/m3. Annelids were represented by some adult polychaete species, polychaete larvae and different stages of Spionid larvae included several stages of trochophore larvae (late trochophore, early trochophore, and mid. trochophore). According to spatial distribution they recorded their maximum values (1137 individuals/m3) at station II during winter 2012 and minimum of 42 individuals/ m3 at station V during autumn 2011(Table 3).
Chordata: Constituted 2.9% of the total zooplankton groups and occupied the 5th rank in the bay after annelids with average numbers were 259 individuals/m3. During winter and spring 2012 chordates flourished and recorded their maximum average counts (424 and 329 individuals/m3) respectively while it showed lower values during summer (105 individuals/m3). They recorded the maximum density (1035 individuals/m3) at station I during winter 2012, while it was absent at station IV during autumn 2011 and at station VII during summer 2012. Chordata in the El-Mex Bay were represented by Euchordata and Urochordata. Euchordata expressed as fish eggs and larvae. While Urochordata represented by two classes; Appendicularia and Ascidiacea, the first class included five species were Folia sp., Oikopleura dioica, O. parva, O. fusiformis and O. longicauda. On the other hand the second class was represented by Tadpole larvae of tunicate (Phallusia mammillata and Ciona intestinalis), this class was recorded by small counts (Table 3).
Cirripedia: Ranked the 6th order of abundant and was represented by nauplii larvae and cypris larvae of cirripeds. They formed collectively 2.9% with total average counts 264 organisms/m3. It was recorded throughout all the year except at stations IV and V during summer 2012. They recorded their maximum counts (589 individuals/m3) during autumn 2012 and decreased to 77 individuals/m3 during summer 2012. Cirriped larvae were the dominant Cirripeda (Table 3).
Mollusca: Ranked the 7th order of abundant with total average of 196 individuals/m3 during the investigated period; they constituted about 2.1% to total zooplankton groups. Mollusca were recorded in all the study seasons, their maximum values were during summer and autumn 2012 (268 and 285 individuals/m3), while they became the lowest (133 individuals/ m3) during spring. Mollusca in the bay were represented by gastropods, pteropods and the lamellibranch veliger (Table 3).
Cnidaria: was represented by 1.2% of total zooplankton groups with total average was 94 individuals/m3. Generally, Cnidaria recorded their maximum average values (165 individuals/m3) during spring; on the other hand the minimum values were recorded during the two autumn seasons (42 individuals/m3). Cnidaria recorded their maximum values at the stations which located at the sea side (stations III with average 95 individuals/m3 and station VIII with average 125 individuals/ m3) and total average of 140 individuals/m3 at station IV due to the relatively high salinity values at this station (Table 3).
stracoda: represented 0.9% of total zooplankton with average was 59 individuals/m3. Their maximum densities were recorded during autumn 2011 and winter (74 and 77 individuals/m3), while minimum densities (21 individuals/ m3) were recorded during summer 2012. Ostracoda at El-Mex Bay dominated by Cyclocypris sp., Cypridina mediterrianea, Cytheridea punctillata and Xestoleberis depressa (Table 3).
Other arthropoda: In this study was represented by Insect species and their larvae, water mites, Mysis relicta, Gammarus marinus and Zoea of Decapoda. They formed collectively 0.9% of total groups with average numbers were 41 individuals/m3. During winter and spring seasons they flourished to be 87 and 48 individuals/m3 respectively, while their minimum values were recorded during the two autumn seasons (6 and 25 individuals/ m3 respectively). Through the whole study period these forms were absent at station V. While the maximum values (average 74 individuals/m3) were recorded at station VII and station IV (average of 54 individuals/m3).
Nematoda: Were represented by free living nematodes which formed only 0.7% of the total zooplankton groups, with total average 30 individuals/m3. They recorded their maximum density during autumn 2011 (44 individuals/m3) and minimum of 11 individuals/m3 during autumn 2012. According to spatial distribution, nematodes were completely absent from station IV all the study period. On the other hand they were dominated at stations I with total average of 58 organisms/m3 and decreased to minimum at station V (9 individuals/m3) (Table 3).
Cladocera: Was dominated by Evadne spinifera, Podon polyphemoides and P. leuckarti and Moina micrura. Cladocera represented by 0.6% of total zooplankton with total average number were 23 individuals/m3. Cladocera flourished during two seasons, winter and spring (26 and 63 individuals/m3) but their minimum value (5 individuals/m3) was recorded during summer. They were absent at station II. On the other hand they recorded their maximum average counts at stations III (35 individuals/m3) at the first section, VII and VIII (34 and 36 organisms/m3 respectively) which located at the right side of El Umoum Drain inlet (Table 3).
Chaetognatha: In the El- Mex Bay constituted 0.5% and averaged 11 individuals/m3. They disappeared from the bay during spring but recorded their maximum densities (average, 19 individuals/m3) during winter. According to spatial distribution Cheatognatha was absent from stations I, V and VI through the whole study period, while they recorded their maximum values (average, 28 individuals/m3) at stations III and VIII during winter and station IV during autumn 2012 (Table 3).
Diversity index
Shannon’s diversity index of the zooplankton assemblages fluctuated between a minimum of 2.77±0.23 during autumn 2012 to maximum of 3.39±0.18 during spring (Figure 8) Station VII showed maximum diversity index (3.63) during spring season and also the minimum diversity index (2.48) during autumn 2012. According to spatial distributions the maximum diversity index was recorded for stations II and VI where there diversity index values were 3.25±0.28 and 3.22±0.18 respectively (Figure 9).
Discussion
The observed spatial and temporal variations of the zooplankton abundance might be traced to the effect of El- Umoum Drain discharge into El- Mex Bay. With increasing phytoplankton biomass, the abundance of phytoplankton cause herbivorous zooplankton species to increase [52]. Due to pollution and eutrophication the copepod Acartia clausi was favored, while rare species became extinct [53]. Zhenbin et al. [54] reported that zooplankton community structure changed from eutrophic-indicator genera (Brachionus, Polyarthra and Keratella) to genera more characteristic of oligotrophic conditions (Tintinnopsis and Acanthocyclops). Li et al. [55] also found that the dominant species Brachionus spp. and Keratella spp. were replaced by Tintinnopsis spp. in Xihu Lake.
Hussein [56] recorded 121 zooplankton species, while El- Sherif [57] found that zooplankton community in El- Mex Bay was represented by 130 taxa. The present study revealed that zooplankton of El-Mex Bay is highly diversified (204 forms) with low standing crop (annual average 8935 organisms/m3). The species composition of zooplankton community reflects clearly the effect of the land based effluents, whereas the high salinity stations were comparatively lesser diversified than the low salinity stations. On the other hand, effect of El-Umoum Drain and El-Mex Pump Station resulted also in clear temporal variation of species richness at each station relative to variations in values of discharged water.
The high zooplankton diversity during the present study is attributed to the intrusion of the freshwater ciliates and rotifers. Day et al. [58] reported that most estuarine zooplankton organisms have evolved to broad physiological tolerance in order to ensure their survival into unstable environmental conditions and consequently results in high species composition. The temporal distribution of zooplankton composition demonstrated the highest diversified community (151 species) appeared in winter and the lowest (113 species) in autumn 2012, these variations could be attributed to temporal changes in the number of freshwater species enter into the bay through the discharged waste waters also due to the environmental variables like temperature , salinity, nutrients and phytoplankton biomass. These observations are in agreement with Morques et al. [59,60].
The temporal variations of diversity provide useful information on succession of community structure and it may be used as an index for assessing the degree of environmental stress [61]. Day et al. [58] whereas the pollution causes the loss of some sensitive species and led to the occurrence of few of the most tolerant species in great numbers.
The temporal zooplankton abundance showed peaks during winter and autumn 2012. The high water dynamics in these two seasons may play clear role in temporal variations of zooplankton abundance. This contradicts with the observation in other areas, where temperature was the essential element in the seasonal dynamics of zooplankton [62].
The copepods were the dominant component of zooplankton in El-Mex Bay. This observation is agreed with other studies on the marine ecosystem [63-68]. El- Sherif [57] found that Copepoda was represented in the study area by 22 species, only one of them (Acanthocyclops americanus) belongs to the freshwater forms. Other recorded species, Acartia clausi, A. latisetosa, Paracalanus parvus, Oithona nana and Euterpina acutifrons are eurythermal and euryhaline species. They are common at the near shore waters west of Alexandria [56,69].
In El- Mex Bay, the freshwater rotifers are more diversified and the predominant zooplankton component in the water mass is directly stressed by El- Umoum Drain. Rotifers are usually known as the major zooplankton component in the freshwater habitate [70]. The high species number of rotifers at the low salinity stations compared to those at stations (I and IV) indicates the role of discharged freshwater. The rotifers' diversity in El- Mex Bay were rich (38 species), perhaps due to the effect of the mixture of freshwater and marine species and the high trophic level of the system. This agreed with [53] and [9] whom mentioned that the bay subjected to high trophic conditions. The majority of species in this study were euryhaline forms (21 species) and the rest species were freshwater forms (17 species). Throughout the present investigation, the percentage of genus Synchaeta was 51.3% of the total rotifer abundance followed by Brachionus with 19.1% and Keratella 4.5 % [9].
[71] stated that rotifer was the leading group at the mixed land drainage water type constituted 85.75 % of the total zooplankton community in the bay. Protozoa occupied the 2nd order of abundance among zooplankton groups in El-Mex Bay contribution 15.6 % of the total zooplankton counts (averaged 1440 organisms/m3), predominated by ciliates. Protozoa are characterized by many specific structural and functional features, present an important ecological assemblage in aquatic ecosystem and play a crucial role in the function of microbial food webs in addition to their role as indicators of water quality [72]. Protozoa community in El- Mex Bay is pronounced affected by the dispersion pattern of discharged waters. Higher values were particularly observed during winter 2012 (2272 organisms/m3) while autumn 2011 displayed lower densities (519 organisms/m3). Protozoa reached the maximum density at station VII during spring 2012 (5860 organisms/m3) due to the predominance of Centropyxis aculeate, Difflugia oblonga, Favella azorica, Tintinnopsis beroidea, T. campanula, T. cylindrical, and T. lobiancoi.
El- Mex Bay has the highest tintinnid densities during the study period which was dominated by Tintinnopsis beroidea, this agreed with [73] while [57] stated that Protozoa was the highly diversified group in the Western part of Alexandria. It was represented by 63 species (48.46% to the total number of the recorded species). Out of them, 40 tintinnid species, 11 Foraminifera species and 12 species of fresh water ciliates. All tintinnid species are marine forms while some of Foraminifera species are belonging to freshwater forms. Zakaria et al. [71] stated that Protozoa was the second important group after rotifers in the bay. Pteropoda appeared very rare in El-Mex Bay due to the acidification of the bay during sometimes, absence of this group is considerable evidence of the high acidity of any water body, This agree with [74]. Pteropods are the most sensitive Planktonic group because their shell is composed of aragonite, which will be subject to increased dissolution under more acidic conditions. Pteropods would not be able to adapt quickly enough to live in under saturated conditions. Pteropods, with their aragonite shells, are highly vulnerable, while, foraminifera and some crustaceans, with their calcite shells and liths, are less vulnerable. Pteropods are likely to decline and may eventually disappear in response to ocean acidification [75].
Nematods represented by 0.7 % of the total zooplankton with an average of 65 organisms/m3. Nemeth-Katona [76] considered that the presence of nematodes is an indication of the final stage of contamination with sewage, the ultimate putrefaction of water: hydrogen sulphide indicator. Cladocera are considered to be important in the economy of the area because of their relation to pelagic fisheries and are believed to play an important role in the phosphorus regenerate [77]. during the study period Cladocera represented by 0.6% of the total zooplankton with total average number were 23 organisms/ m3 dominated by Evadne spinifera, Podon polyphemoides and P leuckarti and Moina micruraflourished during winter and spring, their minimum values during summer, occurred intermittently at the sampled stations. Although salinity seems to impact the spatial distribution of Cladocera at El-Mex Bay the freshwater species Moina micrura was recorded at some sampled stations indication it is tolerance of wide salinity. Moina micrura is a common species in eutrophic water and can be indicator of eutrophication [78]. Food concentration may affect growth and reproduction of cladocerans [79].
Cirripedia contributed 2.9 % of the total zooplankton (averaged 264 organisms/m3), exhibited comparatively high abundance at high salinity, and disappeared at low salinity stations. This showed by significant correlation between cirripeds and salinity. Jeffries [80] reported that the overabundance phytoplankton as food is associated factors may have been responsible for delay reproduction of adult cirripeds. Polychaetes and larvae were found during the study period with a percentage of 3.9 % and covered the whole area at wide salinity range. These larvae are described as estuarine zooplankton component, being restricted to low salinities and an aerobic conditions or high pollution levels [81] and they play crucial role in meroplankton in the Egyptian Mediterranean coastal water [82].
The presence of freshwater species (Copepoda, Cladocera, Protozoa and so on) in marine coastal areas are considered a biomarkers on the presence of fresh water discharge into these areas, and according to types of this species can determine the source of water discharged neither rivers, lakes, drainage or sewage Froneman [83].
Conclusion
From the obtained results it concluded that El-Umoum Drain discharge, maritime activities and shipping movements in the study area were reflected on the hydrographic conditions and the dynamics of zooplankton community in the El-Mex Bay. The zooplankton showed great numbers of freshwater species which considered as bioindicator to the huge amount of freshwater entering the bay, also the great numbers of rotifers and the presence of other forms like nematodes and some ciliated protozoa serve as bioindicators of pollution and declining water quality.
To Know More About Journal of Oceanography Please Click on: https://juniperpublishers.com/ofoaj/index.php
0 notes