#Sulphur Polymer Production
Explore tagged Tumblr posts
Text
#Consulting Engineering#Consulting and Engineering Services#engineering consultants#Consulting Services#Systems Integration Services#Sulphur Polymer Production
0 notes
Text
Calcium sulphur batteries (uwu)
Okay, so, i've become interested in z-pinch studies for aerospace purposes (i'm really excited about the prospects, everything works on paper, but i naturally want to actually witness p+N14 fusion for above 0.01% of available protons before i go trying to get the materials to build a real liquid fueled SSTO fusion rocket, especially since there are thousands of folks way smarter than me who have presumably thought of this before and we don't have it yet, so yeah). Anyways, if i want the extremely large electricity input without making my electricity bill higher than a whole month's rent and getting my roommates mad at me, i'll need to collect solar or wind in a battery bank. Since lithium batteries are just about all immoral and expensive (yes i am writing this on a device powered by lithium batteries, it would be lovely if capitalists would take a hint and switch to things that just objectively perform better and are cheaper, but whatever), i figured this would be a nice excuse to experiment around with some new battery designs. Since all of them will require sulphur, i won't be able to really get into it before mid may due to some concerns about the smell and risks of getting sulphur powder everywhere (it's very yellow and hard to clean out), but i felt i might as well share my preliminary ideas. First off, in order to make the organic sulphur polymer, i'm looking to explore mostly citrate based polymers, perhaps with phenylalanine mixed in in order to both give more bulk as well as providing nitrogens for sulphenamides to form. Since i'll need urea later, i was also considering partially polymerizing urea with citric acid and adding that into the molten sulphur mix, but i'm less confident in the stability of that and a bit concerned about the potential noxious fumes produced. Regardless, that's the short of the sulphur cathode, details will definitely change after i refind that paper which went over a great way of preventing insoluble polysulphide production. I'm also gonna experiment with anode material and even the ions i use. I know i said "calcium sulphur batteries" in the title, but due to how common aluminium is and how much easier magnesium is to work with (and the fact that their specific energies are higher), i'll also be considering those two. Even beyond that, there are so many potential anode materials, including even amorphous carbon and carbon nitrides which i'd love to test since there's just so much to improve on and i'd rather do a lot of experiments with cheap to make materials and potentially land on a great solution than accept something subpar because it took less effort. Anyways, of the materials i plan on using, there's magnesium sulphate, aluminium sulphate, calcium chloride, potentially other calcium salts (is the salt with taurine soluble in water? IDK, can't find an answer so i'll test it), charcoal, vegetable oil, urea, and phenylalanine. Those may seem like an unrelated hodgepodge of compounds, but they've been chosen because they're what i have/will soon have and they're also all extremely cheap. If the urea works out well in the battery, i may have to make this project a meme and attempt to make a z-pinch device with as much urine as possible (use it to make ammonia for the plasma, to make the batteries, and i'm sure there's some way to use urine in a capacitor (maybe just distilling off the water to use as a dielectric? idk, it's been a while since i tried making a capacitor)).
Anyway, i really didn't expect this long trainwreck of a post to end with discussions of urine, but what can you do? This is all probably nonsensical, even by my standards, but basically i want batteries and i think i can make them cheaper per megajoule of stored energy than the ones i could buy, even accounting for the inevitable failed experiments.
#utter nonsense#chemistry?#batteries#calcium sulphur batteries are cool i guess#z-pinch shit#almost certainly the beginning of a ton of failures#fortunately i should be able to afford all the chemicals with less than 1 month of income (after rent and utilities and whatnot)#sulphur is so cheap#so am i lol#idk if i want to attempt to make my own solar or wind farm or just buy some turbines or solar cells#turbines are pretty easy so i might build some myself#magnets are relatively cheap and i can use them for other things#and if you know where to look (trashcans behind the college) wire is free#and to make the turbine blades i can just take some sheet metal from the same dumpsters as the wire#alternatively i could just try charging the batteries during off-hours when electricity is super cheap#or making a simple biofuel engine#i should also look into making the capacitors#good bye!
2 notes
·
View notes
Text
What are the various purposes, Zinc Stearate is used for?
To keep water at bay, use Zinc Stearate. Common applications for zinc stearate include those of a releasing agent, heat stabiliser, and lubricant in the production of polymers like polyolefin, polystyrene, and rubber. When applied to wood, zinc stearate acts as a sanding additive for lacquers. Zinc stearate acts as a lubricant and thickener in cosmetics to get the desired effect. The commercial stearic acid used to make the insoluble salt is a moderate fatty acid that serves as a lubricant as well as an emollient. Mixtures using zinc oxide are common. On its alone, zinc has been shown to be effective against bacteria and viruses. If you are looking for the best Zinc Stearate supplier in Ecuador, Palvi Chemicals is the right place for you.

Appearance:
The powdered form of zinc stearate is incredibly fine, mild, and colourless. Extremely fine particle size is one of the hallmarks of this high-quality substance. A faint fatty acid aroma may be detectable due to the presence of long-chain fatty acids, although the odour is not overpowering. When heated, zinc stearates start to get dissolved in chlorinated hydrocarbons as well as aromatic compounds, but they don't dissolve in alcohol or ethers.
Safety:
The Cosmetics Database classifies Zinc Stearate as a low-to-moderate danger ingredient, depending on the grade used, and it has received approval from the FDA and CIR. A higher incidence of cancer, allergies, and organ damage are only some of the issues brought forth. Of course, the animals in this research were given quite large doses of the chemical. Zinc stearate is an irritant to humans but has been shown to be safe in animal studies when used as directed, according to the Occupational Safety and Health Administration.
When stearic acid is hydrolyzed, it yields zinc stearate, which is used in paints and varnishes. It has the following qualities: It is fine, white, and smooth to the touch
● Under high-temperature conditions, it maintains a high degree of stability.
● It is impervious to water, giving it a repellent quality.
● It has excellent water resistance and repellent qualities.
● It works wonderfully as a separator.
● It's useful in some operations because it provides zinc.
Applications:
Because of its many desirable qualities, zinc stearate finds application in numerous fields. As an example, zinc stearate is widely utilised in the rubber and plastics industries as a lubricant, release agent, as well as a heat stabiliser. It's an essential component of wood finishes that helps smooth the wood once it's been coated. You can use zinc stearate as a thickening and lubricant in your makeup. Zinc stearate manufactured and supplied by the most distinguished Zinc Stearate distributor in Ecuador can be used in a wide variety of applications due to its lubricating properties, water repellency, gelling capacity, and non-stick texture.
- Vulcanized Rubber:
Since zinc enhances the interaction of sulphur with polyolefin, zinc stearate is referred to as a "vulcanization activator." Because of its high solubility in the polyolefins’ apolar region, zinc stearate greatly aids in dispersion. It acts as a polyolefin antacid, helping to maintain colour and protecting against rust.
- Cosmetics:
The liquid and greasy components of cosmetics are held together by the lubricant and thickening zinc stearate. The product's overall aesthetic quality is enhanced as a result as well.
It is commonly found in cosmetic products like eyeliner, eye shadows, face masks, lipsticks, face powders, and foundation creams. The addition of zinc stearate makes the mixture more fluid and smooth.
- Metallurgy’s Separating Agent:
It works well as a detaching agent for metal and plastic machine parts that are particularly stubborn to disassemble. As a result, the finished product is not ruined by the components sticking to the mould.
- Paints and Varnishes:
Zinc stearate, in its purest form, is used to make varnishes and paints due to its transparency.
It's a thickening, sealant for surface flaws, and excellent dispersant all in one. It also has water-repellent characteristics, so it can keep the paint dry.
- Powder Metallurgy:
The metal powder is combined with zinc stearate, which is a lubrication ingredient exported by Palvi Chemicals, an excellent Zinc Stearate exporter in Ecuador. The lubricants might make up anything between 0.5% and 5.0% of the total mass.
Lubricants have the opposite effect of binders during the pressing phase of compacting, improving flow and compressibility. To varying degrees, the material's ultimate characteristics and porosity are affected by the proportions of the two additions. Compared to other lubricants, zinc stearate is superior because it contributes zinc to the alloy, which can improve the efficiency of the process.
After being compacted in a press, the "green" section is sent to a sintering oven, where its additives are burned off.
- Colour and Plastics Additives:
Similar to other variants of stearates, this has many uses in the plastics sector.
● When making PVC, it acts as a stabiliser as well as a lubricant.
● When used in Masterbatch, it helps to disperse the various colours.
● Transparent polymers for glass and others are employed in impact glass applications because of their durability and safety.
● Used in polyurethane as a metal deactivator.
- Lubricant in Extruders:
Calcium stearate is often used to enhance output in manufacturing processes like plastic extrusion and metal lamination. When added to the feed hopper, the calcium stearate acts as a solid lubricant as well as an additive. Dosages range from 0.3% to 1.0%. This maintains its form even when heated to high temperatures, and it enhances the following aspects of production:
● Product homogeneity
● Hot spots
● Energy consumption
● Saving raw materials
#Zinc Stearate supplier in Ecuador#Zinc Stearate distributor in Ecuador#Zinc Stearate exporter in Ecuador#palvichemical
3 notes
·
View notes
Text
Global Top 15 Companies Accounted for 58% of total Potassium Sulphate (SOP) market (QYResearch, 2021)
Potassium sulphate (K2SO4, commonly referred to as sulfate of potash or SOP) is a water soluble, white and crystalline salt. Potassium sulphate is the world's most popular low-chloride fertilizer.
The main resources for potassium sulphate are found in combination with other mineral sulfates, such as magnesium, sodium and calcium. Another way of producing potassium sulphate is a combination of potassium chloride and kieserite. Most common forms of manufacture are from potassium chloride through a synthetic reaction with sulfuric acid or sulfur dioxide.
Potassium sulphate contains between 50-52% of K2O and approx. 18% of sulphur which is a needed element in plant growth and deficient in many soils. In the commercial market, combining potassium (50% K2O) and sulphur (18%) potassium sulfate is the majority commercial product due to the high concentration of nutrients readily available to plants. Otherwise than potassium chloride (SOP) does not contain any chlorides, which can be harmful to some crops like tobacco, fruits and vegetable.

According to the new market research report “Global Potassium Sulphate (SOP) Market Report 2023-2029”, published by QYResearch, the global Potassium Sulphate (SOP) market size is projected to reach USD 4.57 billion by 2029, at a CAGR of 2.3% during the forecast period.
Figure. Global Potassium Sulphate (SOP) Market Size (US$ Million), 2018-2029
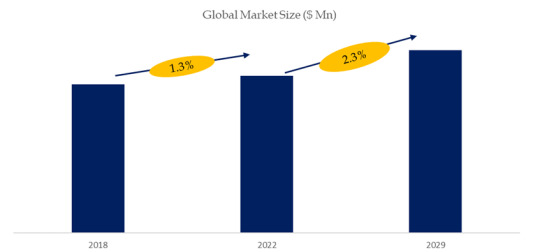
Figure. Global Potassium Sulphate (SOP) Top 15 Players Ranking and Market Share(Based on data of 2021, Continually updated)

The global key manufacturers of Potassium Sulphate (SOP) include K+S Group, Guotou Xinjiang LuoBuPo Potassium Salt, Tessenderlo Group, Qing Shang Chemical, Compass Minerals, SQM, Migao Group, YARA, Qinghai CITIC Guoan Technology, Rusal, etc. In 2021, the global top five players had a share approximately 58.0% in terms of revenue.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
What is a vulcanizing machine?
What is a vulcanizing machine?
Principles of Vulcanization
Polymer chains in rubber or plastic are bonded through vulcanization, which is one way chemical process. The latter’s proceeds under the action of sulphur or other vulcanizing agents. The machine allows for uniform application of heat and pressure that leads to effective vulcanization reaction on materials hence enhancing their elasticity, tensile strength against heat and chemicals among others.Get more news about vulcanizing machine,you can vist our website!
Components and Structure of the Vulcanizing Machine.
A typical vulcanizing machine comprises a heated platen, a system of applying force, as well as controls. The heating plates constitute the main working surface on which materials are placed for curing/gingerly. Electrical elements or alternative heating methods are employed in achieving desired temperatures at this location. The force application unit may be hydraulic or pneumatic thereby facilitating uniform distribution during pressurized processing while control unit enables setting up as well as monitoring temperature levels, amount of pressure exerted together with timing required for particular products during adsorption/vulcanization cycles.
Applications of Vulcanizing Machine
The use of vulcanizer is widespread across different sectors throughout various industries. In automobile industry, it helps produce tires, seals and gaskets out of rubber material. Footwears such as shoes comprise parts made from materials that have passed through curing processes using this critical industrial equipment. Apart from these applications in footwear manufacture over there is also usage in electrical insulation materials production like hoses cables belts etcetera where they get integrated by blending their basic making components with several additives including curatives accelerators stabilizers retarders modifiers fillers plasticizers cross linking agents and colourants for achieving the desired properties depending on specific functional requirements of each product group.
Advantages of Vulcanization
Vulcanizing machine offers several benefits in the manufacturing process. Firstly, vulcanization enhances the physical characteristics of rubber and plastics to ensure they are stronger and more resilient. These qualities result in products that last longer even under tough environmental conditions and when under heavy use. Secondly, vulcanization allows forming complex shapes and structures which could not be formed using un-vulcanized materials. This consistent strength is achieved through uniform application of heat and pressure across all sections of molding plates during manufacture.
In summary, a vulcanizing machine is an important equipment used in manufacturing industries for producing long lasting rubber or plastic goods of high quality. The effective cure carried out by this machine converts raw materials into functional parts with increased physical properties. As such, it can be deployed in different sectors from motor industry to footwear because it has been designed with versatile features that make it useful across various fields. The vulcanizing machine has experienced significant technological advancements that have made them more accurate as well as efficient within production processes unlike before
0 notes
Text
Best External Gear Pump Manufacturer in Ahmedabad
Being External Gear Pump manufacturer we provide excellent quality range. These pumps are manufactured using a premium quality material, it is light weighted and self priming gear pumps. Dev Engineers are leading manufacturer and supplier of External Gear Pump in Ahmedabad.
What is External Gear pump?
External Gear Pump are mechanical devices designed for the efficient transfer of fluids in various industrial applications.
What are Advantages of External Gear Pumps?
As External Gear Pump are compactin design and consists only two gears. Its structure is small, simple and light weight . External gear pumps are cheaper because of its simple structure and lower manufacturing cost. It is smaller in weight and easy to transport, which saves transportation costs too.
What are Disadvantages of External Gear Pump?
External Gear Pump is not easy to repair. If the parts of pump are worn or damaged the entire gear pumps is almost impossible to repair. It has the characteristics of radial force imbalance and large flow artery, so it generates very loud noise.
What are Industrial Applications of External Gear Pump?
Used for pumping water, light oils, chemical additives, resins or solvents.
For Accurate dosing or high pressure output is required
External gear pumps are capable of sustaining high pressures.
Used for handling the corrosive liquids such as sulphuric acid, sodium hypochlorite, ferric chloride and sodium hydroxide.
Applications involving polymers, fuels, and chemical additives
Our Products
Rotary Gear Pumps
External Gear Pumps
Internal Gear Pumps
Rotary Lobe Pumps
Rotary Shuttle Block Pumps
Rotary Screw Pumps
Twin Screw Pumps
Triple Screw Pumps
For More Details about Click here : https://www.rotarygearpumpsindia.com
#Rotary Gear Pumps#External Gear Pumps#Advantages of External Gear Pumps#Applications of External Gear Pump
0 notes
Text
What is Acetic Acid
What is Acetic Acid
An organic substance having the chemical formula CH3COOH is acetic acid. It is an opaque liquid with a strong smell and sour flavour. It is commonly known as the main component of vinegar, which is a diluted form of acetic acid.
Acetic acid occurs naturally in various sources, including fruits, vegetables, and fermented products. It is produced through the fermentation of ethanol by acetic acid bacteria or through chemical synthesis methods.
In terms of its chemical structure, acetic acid belongs to the carboxylic acid group, characterized by the presence of a carboxyl group (-COOH). This functional group gives acetic acid its acidic properties.
Overall, acetic acid is a versatile compound that plays a significant role in various domains, including food, chemical, and health industries. It is an important substance in our daily lives because of its unique characteristics and diverse applications. The biological approach is typically used to produce acetic acid for industrial use using microorganisms like yeast and bacteria.
For large-scale production, two techniques—surface and submerged fermentation processes—are frequently used. Acetic acid can be fermented either anaerobically or aerobically. Utilizing Clostridium sp., anaerobic fermentation is carried out.
of bacteria (in the absence of oxygen). Aerobic fermentation is primarily carried out by the yeasts (Saccharomyces cerevisiae) and secondly by the bacteria (Acetobacter acetic).
There is no need to add extra nutrients when using substrates with low alcohol concentration, such as malt, whey, etc. For the best growth of the bacteria and the formation of acetic acid, fertilisers must be provided if substrates like potato and grain spirits are utilised.
What are the uses of Acetic Acid?
Acetate, cellulose fibers, and plastics: Numerous compounds, such as vinyl acetate, acetic anhydride, and acetate esters, are produced using acetic acid. Vinyl acetate is converted into polyvinyl acetate, a polymer that is used in paints, adhesives, plastics, and textile treatments. Acetic anhydride is needed to make the cellulose acetate fibers and plastics that are used in photographic film, clothing, and coatings. Acetic acid is also used in the chemical process that produces pure terephthalic acid (PTA), which is used to make the PET plastic resin used in synthetic fibers and food.
Solvents: Like ethanol, acetic acid is a hydrophilic solvent. It dissolves materials like oils, sulphur, and iodine by mixing with water, chloroform, and hexane.
Acidizing oil and gas: In oil and gas well applications, acetic acid can aid in lowering metal corrosion and scale build-up. Additionally, it is used in oil well stimulation to improve flow and increase both gas and oil production.
Pharmaceuticals and vitamins: The use of acetic acid in the pharmaceutical and drug industries is significant and encompasses various applications.
Drug Formulation and Manufacturing: Acetic acid is employed in the formulation and manufacturing of pharmaceutical drugs. It is an essential part of the process used to create intermediates and active pharmaceutical ingredients (APIs). Acetic acid can be used as a solvent, catalyst, or reactant in various chemical reactions during drug synthesis.
pH Adjustment and Buffering: Acetic acid is utilized for pH adjustment and buffering in pharmaceutical formulations. It acts as an acidic component to maintain the desired pH range of drug solutions, suspensions, and emulsions. The pH of a formulation plays a critical role in drug stability, solubility, and bioavailability.
Food Industry: The use of acetic acid in the food industry is extensive, with a wide range of applications in various processes and products.
Vinegar Production: One of the most well-known uses of acetic acid in the food industry is its role in vinegar production. Vinegar, a diluted form of acetic acid, is a popular condiment and ingredient in many cuisines worldwide. Acetic acid bacteria convert ethanol into acetic acid through fermentation, resulting in the production of vinegar with its characteristic sour taste and aroma.
Flavor Enhancer and Acidifier: Acetic acid serves as a natural flavor enhancer and acidifier in food products. It contributes a tangy and sour taste, which is particularly desirable in dressings, sauces, pickles, and marinades. Acetic acid helps balance and enhance flavors by providing acidity to various dishes, giving them a pleasant and refreshing taste.
Food Preservation: Acetic acid plays a role in food preservation due to its antimicrobial properties. It creates an acidic environment that inhibits the growth of bacteria, yeast, and mold, helping to extend the shelf life of certain food products. Pickling, a preservation method that involves immersing food items in vinegar or acetic acid solutions, is a popular application of acetic acid in preserving fruits, vegetables, and other perishable foods.
Other uses: Salts of acetic acid and various rubber and photographic chemicals are made from acetic acid. As a food preservative, acetic acid and its sodium counterpart are frequently employed.
Textiles & Dye Industries: The use of acetic acid in the textile and dye industries is significant, with diverse applications in various processes.
Dyeing and Printing: Acetic acid is commonly used in the dyeing and printing of textiles. It acts as a fixing agent and mordant, helping to enhance color fastness and improve dye penetration into the fabric fibers. Acetic acid aids in the fixation of dyes to the textile material, ensuring vibrant and long-lasting colors.
Textile Fiber Production: In the production of textile fibers such as acetate, triacetate, and cellulose acetate, acetic acid is a key component. It is used in the acetylation process, where cellulose fibers are chemically modified by reacting with acetic acid or acetic anhydride. This process imparts desirable properties to the fibers, including improved strength, durability, and resistance to wrinkles.
Ingredients & Specifications
Properties
Product Specifications
Acetic Acid - 99.85 % min. by wt.
Water - 00.15% max.
Colour - 10 APHA max.
Formic Acid - 0.05 % max. by wt.
Acetaldehyde - 0.05% max. by wt.
Heavy Metals as Pb - Less than 2 ppm
Iodides - 40 ppb max.
Permanganate - 2.00 hrs. min.
Freezing Point - 16.4 deg C
Specific Gravity - 1.049 at 25 deg C
CAS No. - 64-19-7
UN No. - 2789
Have a look at our Prakash Chemical Agencies Pvt. Ltd for more information on Acetic Acid: https://www.pcaplindia.com/Product-acetic-acid.aspx
GNFC Glacial Acetic Acid
An example of water-free (anhydrous) acetic acid is glacial acetic acid. It has the appearance of a colorless, clear liquid that is entirely miscible with water. It is commonly used as a solvent for recrystallization to purify organic molecules since it is a superb polar protic solvent. When performing processes that include carbocations, such as the Friedel-Crafts alkylation, acetic acid is frequently employed as a solvent. It functions like vinegar. Metals and tissue are both corroded by glacial acetic acid. On touch, it might cause skin damage. used to make petroleum and other chemicals and as a food additive.
Ethanoic acid, also referred to as acetic acid, is a liquid organic chemical that is colorless. It is the most significant carboxylic acid. Vinegar is a diluted (5% by volume solution) form of acetic acid created by the oxidation and fermentation of natural carbohydrates. The acyl salt of acetic acid is known as acetate. Industrial acetic acid production involves both synthetic and bacterial fermentation. The chemical industry uses over 75% of the acetic acid produced by the carbonylation of methanol. This synthetic carboxylic acid possesses antifungal and antibacterial properties.
To get more information & get in touch with us please do share your inquiry with us https://www.pcaplindia.com/inquiry.aspx
Safety Precautions
The purest form of acetic acid is glacial acetic acid, which can be exposed at work by inhalation, skin contact, or eye contact. Acetic acid corrodes the skin and eyes. The Occupational Safety and Health Administration (OSHA) has created regulations for those who are exposed to acetic acid.
Acetic acid has an allowed exposure limit (PEL) of 10 parts per million (ppm) over an 8-hour work shift. Exposure to acetic acid vapours at such concentrations may cause eye, nose, and throat irritation. At 100 ppm, there may be significant pulmonary irritation as well as potential lung, eye, and skin damage. Additionally, pharyngeal edoema and chronic bronchitis can be brought on by acetic acid exposure. Acetic acid should normally not be exposed in amounts greater than those found in commercial products and preparations since it can irritate the skin and eyes even at relatively high doses.
Conclusion
Acetic acid stands tall as a vital compound in the chemical industry, offering a multitude of applications and playing a crucial role in the production of various chemicals and intermediates. Its versatility as a chemical feedstock, solvent, catalyst, and cleaning agent highlights its significance in diverse manufacturing processes.
As the chemical industry progresses toward sustainability and innovation, acetic acid continues to pave the way for greener and more efficient practices. With ongoing research and technological advancements, the potential of acetic acid in the chemical industry is poised for further exploration and growth.
To have a deeper insight into what sectors visit our website https://www.pcaplindia.com/
1 note
·
View note
Text
Let’s Know Karl Fischer Titration In Detail
Karl Fischer titration is a volumetric or coulometric titration method for estimating the amount of water in a given analyte. Karl Fischer, a German scientist, devised this method for quantitative chemical analysis in 1935; today, similar titrations can be performed using specialist titrators (known as Karl Fischer titrators) by lab chemical suppliers.
The Principle Of Karl Fischer Titration
Karl Fischer reagent titration is based on the oxidation reaction between iodine and sulphur dioxide. Water combines with iodine and sulphur dioxide to form sulphur trioxide and hydrogen iodide, and the process is complete when all of the water has been consumed.
The following is the chemical equation for the reaction of sulphur dioxide, iodine, and water (as used in Karl Fischer titration):
I2 + SO2 + H2O → 2HI + SO3
The Process Of Karl Fischer Titration
There are two ways to conduct the Karl Fischer titration experiment. These are their names:
Volumetric determination- This approach can determine water content to 1% accuracy. After the sample has been dissolved in KF methanol, iodine is added to KF Reagent. The endpoint is detected potentiometrically.
Coulometric determination - The endpoint is sensed electrochemically in this experiment. Iodine for the KF reaction is produced via anodic oxidation of iodide from solution.
The International Standards Organization (ISO) has recognised it as an American Standard Testing Method (ASTM) in the United States. It is also included in the German DIN and BS standards. Japanese standards, such as the Japanese Industrial Standards (JIS) and the Japanese Agricultural Standards (JAS), have also adopted the method (JAS).
Applications Of Karl Fischer Titration
It is utilized in multiple products, including polymers, oils, and gases.
It's found in pharmaceutical items.
It's found in cosmetic items.
Advantages Of Karl Fischer Titration
It can be used to indicate the concentration of water in gases, liquids, and solids.
The coulometric titrator helps identify free, dissolved, and emulsified water.
It is a quick and accurate method that requires little sample preparation.
Finar, one of India's leading laboratory chemical manufacturers in India, has a robust distribution network and sales team that allows us to service customers in over 50 countries globally. Their R&D department has created in-house technology that is suited for both manual and automatic equipment, ensuring a consistent factor.
0 notes
Text
Glass Additives Market In-Depth Analysis, Growth Strategies and Comprehensive Forecast to 2032
Glass additives demand was estimated at US$ 1.3 billion in 2021 and is expected to reach US$ 1.4 billion by the end of 2022. In the long run, the market is predicted to grow at a 4.2% CAGR from 2022 to 2032, reaching US$ 2.1 billion.
The extensive usage of glass in fibre optics, electronics, and consumer goods drives the global glass additives industry. As a result, there is a growing demand for additives that help identify and enhance glass materials.
Aside from that, glass is widely utilised as a packaging material in the food and beverage industries, and has been the most chosen packaging material for soft drinks and edible semi-fluids, gaining new ground for glass additives globally.
Why is Asia-Pacific Emerging as a Market for Opportunistic Glass Additives?
The Asia Pacific region is expected to expand the fastest among the world's important geographical regions for glass additives. The APAC region's high glass manufacturing capacity makes it attractive for the expansion of the glass additives industry.
The region's demand for glass dinnerware is increasing as the hospitality business expands and pub and bar culture grows. Furthermore, many province residents are switching from traditional stainless steel, plastic, and melamine dinnerware to glass tableware, which is fueling the region's glass tableware industry. As a result, the growing popularity of glass furniture is exerting pressure on the worldwide glass additives industry.
Get a Sample Copy of the Report @ https://www.futuremarketinsights.com/reports/sample/rep-gb-374
Glass Additives Market: Key Segmentation
Extensive study of the geographical regions offers detailed insights on the market performance across major regions along with descriptive info graphics, datasets, and list of tables.
Product:
Metal Oxide
Nanoparticles
Polymers
Rare Earth Metals
Application:
Silicate Glass Manufacturing
3D Printing
Aerodynamic Levitation
Glass Transition
Customization of Tableware
Lamps & Eyeglasses
Chemical Elements:
Iron
Manganese
Sulphur
Nickel
Titanium
Chromium
Uranium
Others
Glass Additives Market: Competitive Evaluation
The FMI’s Glass Additives market report provides a comprehensive analysis on key players operating in the Glass Additives market. Some of the key players are:
Air Products and Chemicals Inc.,
BASF SE
DuPont
Torrecid Group
Bayer Material Science
Nanobase
With a detailed analysis on positioning of top companies across the globe, emerging players, strategic players and innovators, the FMI’s study presents the strengths, weaknesses, growth prospects and challenges of key players over the forecast period.
Browse latest Market Reports@ https://www.futuremarketinsights.com/category/chemicals-and-materials
0 notes
Text
Centrifuge tube material introduction-GST centrifuge tube automatic production line
GST centrifugal tube automatic production line is used for automatic feeding, automatic printing, automatic drying, automatic capping, automatic detection, automatic bagging, automatic packaging and automatic coding of centrifugal tubes.GST centrifugal tube automatic production line has the characteristics of accurate feeding; good screen printing; adjustable capping; neat bagging and adjustable packaging quantity. Here is a look at the material introduction of centrifugal tubes following GST Technology!
Glass centrifuge tubes: cannot be used in high and ultra high speed centrifuges, only for low speed centrifuges.
PP (polypropylene): translucent, good chemical and temperature stability, but will become brittle at low temperatures, so do not centrifuge below 4°C.
PC (polycarbonate): better transparency, hardness, can be sterilised at high temperatures, but not resistant to strong acids and bases and some organic solvents such as alcohol. It is mainly used for ultra-high speed centrifugation above 50,000 rpm.
PE (polyethylene): opaque. It does not react with acetone, acetic acid, hydrochloric acid, etc. It is more stable and easily becomes soft at high temperatures.
PA (polyamide): this material is a polymer of PP and PE material, translucent, chemically very stable, single not resistant to high temperatures.
PS (polystyrene): transparent, hard, stable to most aqueous solutions, but can be corroded by a variety of organic substances, mostly used for low-speed centrifugation, and is generally a one-time use.
PF (polyfluoro): translucent, can be used at low temperatures, if it is an experimental environment at -100°C - 140°C, you can use centrifuge tubes made of this material.
CAB (cellulose butyl acetate): transparent, can be used for more dilute acids, bases and salts, as well as for gradient determination of alcohols and sucrose.
CN tubes: softer and transparent, but not resistant to strong acids and bases and certain organic solvents, and cannot be autoclaved. Suitable for density gradient centrifugation of sucrose, glycerol, etc. Should be transparent and easy to collect.
Steel centrifuge tubes: strong, not deformed, resistant to heat, frost and chemical corrosion, widely used, but avoid contact with strong corrosive chemicals when used.
Cautions.
1. The use of organic solvents in plastic tubes must comply with the compatibility regulations with the corresponding materials. Polypropylene (PP) centrifuge tubes (bottles) cannot be used in contact with petrol, paraffin, etc. Polycarbonate (PC) centrifuge tube (bottle) can not be used in contact with gasoline, acetaldehyde, acetone, ethanol, isobutanol, cresol, etc. Polyethylene (PE) centrifuge tubes (bottles) should not be used in contact with sulphuric acid (50%, 75%), benzene, petrol, paraffin, etc.

2. Centrifuge tubes should be disinfected under high temperature and pressure, and the caps should be separated from the tubes.
https://www.gst-automation.com/News/208.html
0 notes
Photo

Transforming sulphur dioxide from harmful to useful
Scientists have created molecular cages within a polymer to trap harmful sulphur dioxide pollution in order to transform it into useful compounds and reduce waste and emissions.
A unique new material developed by an international collaboration of scientists has proved that it can help reduce sulphur dioxide (SO2) emissions in the environment by selectively catching the molecules in minutely engineered cages. The captured toxic gas can then be safely released for conversion into useful industrial products and processes.
Around 87% of sulphur dioxide emissions are the result of human activity, typically produced by power plants, other industrial facilities, trains, ships, and heavy equipment, and can be harmful to human health and the environment. The international team developed porous, cage-like, stable copper-containing molecules known as molecular organic frameworks (MOFs) that are designed to separate sulphur dioxide (SO2) gas from other gases more efficiently than existing systems.
Professor Martin Schröder, Vice-President and Dean of the Faculty of Science and Engineering at the University of Manchester, and Dr. Sihai Yang, a Senior Lecturer in Department of Chemistry at the University of Manchester, led an international research team from UK and U.S. on this work.
Read more.
#Materials Science#Science#Sulfur dioxide#Sulfur#Oxides#Pollutants#Molecules#Metal Organic Framework#MOF#University of Manchester
49 notes
·
View notes
Text
Marine Scrubber, Global Market Size Forecast, Top 17 Players Rank and Market Share
Marine Scrubber Market Summary
The overwhelming majority of ships around the world operate using engines and boilers powered by heavy fuel oil. The resulting combustion exhaust gases that are emitted contain soot and sulfur oxides that pollute the environment. Ship Exhaust Gas Scrubber exhaust gas cleaning reduces sulphur (SO2) and particulate emissions from ship engines, generators, and boilers. Ship Exhaust Gas Scrubber should enable ships to meet sulphur emission limits as required by IMO MARPOL Annex VI regulations without switching to low-sulphur fuel. This report mainly covers the Ship Exhaust Gas Scrubber product type: Open Loop Scrubbers, Closed Loop Scrubbers, and Hybrid scrubbers.
According to the new market research report “Global Marine Scrubber Market Report 2024-2030”, published by QYResearch, the global Marine Scrubber market size is projected to reach USD 5.72 billion by 2030, at a CAGR of 3.9% during the forecast period.
Figure. Global Marine Scrubber Market Size (US$ Million), 2019-2030

Figure. Global Marine Scrubber Top 17 Players Ranking and Market Share (Ranking is based on the revenue of 2023, continually updated)

According to QYResearch Top Players Research Center, the global key manufacturers of Marine Scrubber include Wartsila, Yara Marine Technologies, HHI Scrubbers, Alfa Laval, Puyier, Panasia, Valmet, Bilfinger, ME Production, Clean Marine, etc. In 2023, the global top 10 players had a share approximately 77% in terms of revenue.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
TAFAKKUR: Part 126
Spider Silks: Part 2
Analysis of silk
The silk itself is a material identified as a “scleroprotein.” When created in the glands it is a fluid; only when dragged outside the body does it solidify into thread. Once it was believed that contact with air produced the toughening, but it currently looks that the drawing-out activity alone is accountable for the change.
To carry out the exertion done by the glands, a spider is armed with spinnerets, usually six in number. These are as accommodating as fingers; they can be prolonged, compacted, and overall be applied like human hands. In the “spinning field,” where the spinnerets are congregated, single threads are joined into numerous compound threads, and some of the dehydrated threads may be covered with a gluey substance. Thus, a completed thread may be thin or thick, dry or sticky. It may also have the look of a bead-trimmed necklace. For the last kind, the spider spins rather unhurriedly and, drawing out the gluey thread, lets it go with a jolt. The liquid thus is organized in beads spread out lengthwise across the completed line.
The strand known as the dragline may be understood as a spider's “life line” because it performs as a lifeguard in all kinds of situations. The dragline goes along with the spider, no matter where or how far it journeys, winding out from spinnerets at the back of the body. It forms a portion of the building of webs, it grips its tiny builder firmly in problematic places, and it helps in absconding from adversaries. When a spider is inactive in a web, the dragline enables a rapid descent and escape. It allows energetic chasing spiders to jump from buildings, cliffs, or any tall position with absolute security.
Benefits of spider silk to us
The silk of the silkworm could be very profitable and marketable. There are, however, challenges. One is the changing thickness of a spider’s strand; the other is that it doesn’t well endure the interweaving process. Housing and feeding large numbers of silkworms is not difficult. But housing and feeding large numbers of spiders? There are enormous difficulties.
Native inhabitants of New Guinea have used spider silk in a variety of conditions. They make fishing nets, traps, and such objects as bags, headdresses that will keep away rain, and caps. These are not formed from single threads but from tangled, warped threads. The aboriginals of North Queensland, Australia, look to spiders for their angling supplies.
Spider silk has been valuable to the manufacturers of such complex instruments as astronomical telescopes, guns, and engineers’ levels. The threads, being exceedingly fine but nonetheless robust, are outstanding for sighting marks. Throughout the Second World War, there was a significant demand for spider thread for surveying and laboratory instruments. Black widow spiders were utilized for the manufacture of this silk.
One drawback to the use of spider silk in industry is that it might slump in a moist environment. To overcome this problem, strands of platinum or etching on glass plates take its place in such instruments as periscopes and bombsights.
Spider’s silk also might have healing properties. Due to its antibacterial properties and because the silk is abundant in vitamin K, it may be efficient at clotting blood. Because of the problems in obtaining and handling extensive amounts of spider silk, the largest known piece of cloth made of spider silk is an 11 by 4-foot (3.4 by 1.2 m) fabric made in Madagascar in 2009. Eighty-two persons labored for a period of four years to gather over one million golden orb spiders and extract silk from them.
Applications of spider silk
As mentioned, human beings have been using spider silk for thousands of years.
The manufacture of contemporary synthetic super-fibers such as Kevlar (bulletproof material) includes petrochemicals, which adds to pollution. Kevlar is also strained from concentrated sulphuric acid. In comparison, the manufacture of spider silk is totally ecologically sustainable. It is created by spiders at ambient temperature and pressure and is strained from water. Furthermore, silk is totally biodegradable. If the manufacture of spider silk ever becomes industrially practical, it could be a substitute for Kevlar and be used to create a varied extent of articles such as: bulletproof vests, wear-resistant lightweight clothing, ropes, nets, seat belts, parachutes, rust-free boards on motor vehicles or boats, biodegradable bottles, bandages, surgical thread, artificial tendons or ligaments, and backings for weak blood vessels.
Synthetic spider silk
Duplicating the multifaceted settings needed to make threads that are similar to spider silk has been difficult to both research and manufacture. Through genetic engineering, Escherichia coli bacteria, yeasts, plants, silkworms, and animals have been utilized to produce spider silk proteins. Yet, these synthetic threads have diverse, simpler features than those of a spider. Manmade spider silks have lesser and unsophisticated proteins than natural dragline silk, and have subsequently half the diameter, strength, and flexibility.
One tactic is to remove the spider silk gene and utilize additional life forms to generate the spider silk. Canadian biotechnology company Nexia effectively produced spider silk protein in transgenic goats that passed the gene for it; the milk made by the goats comprised noteworthy amounts of the protein: 1-2 grams of silk proteins per liter of milk. To make spider silk, Nexia utilized damp whirling and pressed the silk protein across minor extrusion cavities in order to mimic the performance of the spinneret, but this process was not adequate to duplicate the sturdier characteristics of innate spider silk.
In March 2010, investigators from the Korea Advanced Institute of Science and Technology was able to produce spider silk by means of the bacteria E. coli, altered with definite genes of the spider Nephila clavipes. This tactic removes the necessity of milking spiders.
It should be noted that the manufacture of spider silk is not easy and there are intrinsic difficulties. First of all, spiders cannot be cultivated like silkworms since they are flesh-eaters and will merely eat each other if in proximity to each other. The silk produced is very slight, so 400 spiders would be required to make only one square yard of cloth. The other problem is, silk also toughens when subjected to air, which makes working with it problematic.
A different tactic is to study how spiders whirl silk and then replicate this process to make artificial spider silk. The silk itself would also have to be synthetically produced. Chemical production of spider silk is not feasible at present due to the absence of information about the makeup of silk. Randolph V. Lewis, Professor of Molecular Biology at the University of Wyoming in Laramie, has introduced silk genes into Escherichia coli bacteria so that the recurring sections of spidroin 1 and spidroin 2 efficaciously come to form. Others theorize about the likely gene introduction into fungi and soya plants. It may also be possible to modify the silk genes for precise intentions.
Why a spider’s house is the frailest of houses
Spider silk is stronger than steel, but the Qur’an (29:41) states that the flimsiest of houses is the spider’s house. The per unit weight of the dragline silk of the golden orb spider is one of the world’s hardest fibers. Webs are combinations of many kinds of spider silk, all able to be produced by the same spider. The web radials are strong, but the somewhat feebler circumferential (quasi-circular concentric) fibers are flexible and gluey to absorb the energy of a flying insect and hold it in place. The strongest of all is the fiber, which the spider uses for transport, the dragline silk. In summary, the spider fabricates both sturdy as well as feeble fibers and the web it weaves to catch flying insects is weaker; this may be the reason why it is referred to in the Qur’an as the “frailest” of houses.
Conclusions
Scientists are foreseeing many potential uses for biosilk. Textile usages are noticeable one. The flexibility and potency of prevailing merchandises such as spandex and nylon have to be improved. Since it is lightweight, hardy and flexible, biosilk may also have uses in satellites and aircraft. More prominently, the new group of progressive things that spider silk investigation may cause has the prospective to alter our lives in innumerable manners that we can barely imagine. More than 72 years have passed since the inventions of Wallace and Carothers that gave the world nylon that led us into the age of polymers. Artificial spider silk may help produce super-performing clothes of the future. Earthquake resistant suspension bridges hung from cables of synthetic spider silk fibers may someday be a reality.
#allah#god#muhammad#prohet#sunnah#hadith#quran#ayah#islam#revert#muslimah#hijab#help#convert#religion#muslim#reminder#dua#salah#pray#prayer#welcome to islam#how to convert to islam#new muslim#new revert#new convert#revert help#convert help#islam help
1 note
·
View note
Text
Textile Dyeing and Printing
Dyeing and printing processes are employed in the conversion of raw textile fibers into finished goods that add much to the appearance of textile fabrics. DYEING Dyeing is the application of dyes or pigments on textile products such as fibers, yarns, and textiles with the goal of accomplishing color with preferred color fastness. Dyeing is usually done in a special option consisting of dyes and particular chemical product. Dye particles are fixed to the fiber by absorption, diffusion, or bonding with temperature level and time being vital managing elements. The bond between dye particle and fiber may be solid or weak, depending on the dye made use of. Dyeing and printing are different applications; in printing, color is applied to a local location with desired patterns. In dyeing, it is related to the whole textile. The main source of dye, historically, has been nature, with the dyes being drawn out from animals or plants. Considering that the mid-19th century, however, people have actually created synthetic dyes to accomplish a wider range of colors and to render the dyes extra stable to washing and general use. Various classes of dyes are utilized for various sorts of fiber and at different phases of the textile manufacturing procedure, from loose fibers via yarn and towel to complete garments.

Polymer fibers are dyed with fundamental dyes, while nylon and protein fibers such as wool and silk dye with acid dyes, and polyester thread is colored with disperse dyes. Cotton is dyed with a series of dye types, consisting of barrel dyes, and contemporary synthetic reactive and direct dyes. THERE ARE THREE CATEGORIES: •Cellulose fiber dye. •Protein fibers dye. •Synthetic fibers dye. THERE ARE MANY CLASS OF DYE IN THE FOLLOWING: •Reactive dye: •Disperse Dye •Direct Dye •Basic dye •Acid dye •Sulphur dye. •Vat dye •Azoic Dye •Oxidation dye •Optical dye or Fluorescent •Solvent Dye METHODS OF DYEING •Direct Dyeing •Stock Dyeing •Top Dyeing •Yarn Dyeing •Skein Dyeing •Package Dyeing •Warp Beam Dyeing •Garment Dyeing ADVANCES TECHNOLOGIES OF DYEING •Advanced DENIM concept •Supercritical fluid dyeing (SFD) •Ultrasound technology •Powder dyes from textile fibers •DyeCoo PRINTING Printing is a process of decorating textile fabrics by application of pigments, dyes, or various other associated materials in the form of patterns. Although apparently developed from the hand paint of materials, such approaches are also of great antiquity. There is evidence of printing being carried out in India throughout the 4th century BCE, and a printing block dated at about 300 CE has been discovered in the burial grounds of Akhmīn in Upper Egypt. Textile printing has actually ended up being highly sophisticated and has involved the skills of several musicians and designers.

The four major methods of textile printing are block, roller, display, and heat transfer printing. In each of these approaches, the application of the color, usually as a thick paste, is followed by fixation, usually by steaming or home heating, and afterwards removal of excess colour by cleaning. Printing styles are classified as direct, discharge, or stand up to. In straight printing, coloured pastes are published straight on the cloth. For discharge printing, the cloth is first dyed with a background color, which is ruined by reagents, or lowering representatives, lugged in a print paste. This activity may leave the released design white on a tinted history, although print pastes might additionally consist of tinting matters not destroyed by the discharging representative, generating a colored design. In the resist process, the cloth is first published with a compound called a withstand, safeguarding these published locations from accepting color. When the cloth is colored or pigment cushioned only those components not published with the withstand are colored. A special application of this strategy, passing on plissé results, is the printing of the fabric with a resist, complied with by therapy with caustic soda. TYPES OF TEXTILE PRINTING: •Block printing •Roller printing •Heat transfer printing •Digital Printing •Screen Printing •Flexography printing •Litho Printing •Engraving •Embossing •Embroider Printing ADVANCES TECHNOLOGIES OF PRINTING •Nanotechnology for printing •Water-based printing •Smart Cutting for B1 and B2 formats •Biodegradable printer inks •Conductive inks •3D Printing Thanks for reading the post, Please visit our website to know more about textile dyeing and printing.
1 note
·
View note
Text
Zajok a nappaliból – Grand Traxelektor 2019 / 2. Move!
Kétezertizenkilenc elektronikus zenei undergroundjának legtöbbet hallgatott és legérdekesebbnek ítélt művei hármas tematika szerint rendezve. (második rész)
Spotify playlist:
https://open.spotify.com/playlist/3cCgKZ3autPSLenlyyER5w 1800HaightStreet – Infestation [Confess, EP, Lobster Theremin] 2000 And One - The Needs Of The Many [The Needs Of The Many, EP, OFF] Anthony Rother – Hyperbolic [Hyperbolic, EP, Psi49net] Anthony Rother - Inner Space Odyssey [Hyperbolic, EP, Psi49net] Anunaku - Bronze Age [Whities 024, EP, Whities] Anunaku – Temples [Whities 024, EP, Whities] ASC - Black Rooms [Realm Of The Infinite, LP, Auxiliary] Black Merlin - Psych 73 [SFORMATOR 1, EP, Pinkman] Blawan – Gadget [Many Many Pings, EP, Ternesc] Blawan - Many Many Pings [Many Many Pings, EP, Ternesc] Broken English Club - The Modern Desire [White Rats II, LP, L.I.E.S.] Chants - Assiah Dance [Seven Spheres, LP, Astral Plane] Clouds - Another Day [Sharp Like A Razor, EP, Headstrong] Clouds - Arkhangelsk Nightmare [Sharp Like A Razor, EP, Headstrong] Cop Envy - Rat Break [Cotton, EP, Hypercolour] De Bons en Pierre - Frog Stoemp [No. 2, EP, Dark Entries] Desert Sound Colony - Can Can Wingspan [Can Can Wingspan, EP, On Loop] Developer – Buffalo [Four Corridor's, EP, Coincidence] Djedjotronic feat. Lokier - Are Friends Electric (Lokier Remix) [Are Friends Electric, EP, Boysnoise] Download - GUI goats [Unknown Room, LP, Sub-Conscious Communications] Echologist – Resistance [Resistance, EP, Anemone] Eomac - Being, Not Object (Eomac VIP) [Reconnect, EP, Eotrax] Eric Copeland - Beat It [Trogg Modal Vol. 2, LP, DFA] E-Saggila - My World My Way [My World My Way , LP, Northern Electronics] Exium – Unemotional [XX Part 1, LP, Nheoma] Franck Vigroux – Carre [Théorème, EP, D'Autres Cordes] Giant Swan - 55 Year Old Daughter [Giant Swan, LP, Keck] Giant Swan – YFPHNT [Giant Swan, LP, Keck] Goth-Trad - Bloody Dice [Knights Of The Black Table, LP, Daymare] GusGus - Fireworks (C.P.I. Remix) [Remixes Are More Flexible, Pt. 1, EP, Oroom] Headless Horseman - The Distress Subsided [Headless Horseman 008, EP, Headless Horseman] Homemade Weapons - Svalsat (Donato Dozzy Remix) [Gravity Remixed, EP, Samurai Music] Jensen Interceptor - EM Damage [SAFE, EP, Who's Susan] JK Flesh & Orphx - Mutagen (Live) [Light Bringer, LP, Hospital Productions] Kangding Ray - Polygon [Azores, EP, Figure] Karenn - Rek [Kind Of Green, EP, Voam] Kris Baha - Non For The Sane [Palais, LP, CockTail d'Amore Music] Luke Vibert - I Will Always Hate You [Valvable, LP, I Love Acid] Machine Woman - East Midlands Rave Tune [When A Machine Cries, You Get Petrol, EP, Take Away Jazz] Matrixxman & Physical Therapy - The Survivors [Threads, EP, Nonplus] Meat Beat Manifesto - Pin Drop [Opaque Couché, LP, Flexidisc] Om Unit & Kid Drama - Untitled 3 [Untitled Works, EP, Apollo] Pfirter - A Different Reality [The Empty Space, LP, MindTrip Music] Phil Kieran, Douglas McCarthy - Fall Rise (Druggs Mix) [Fall Rise, EP, Optimo Music] Pinch - Fortune Tellers [Border Control, EP, Berceuse Heroique] Plaid - Meds Fade [Polymer, LP, Warp] Pris – Aquarius [New Babylon, EP, Empathy Corp] Pris – Discovery [Sulphur City, EP, Empathy Corp] Red Axes - Kookoo Papa (Original Mix) [Sound Test, EP, Phantasy Sound] Redshape – Bishop [Android Malfunction, EP, Delsin] Retina.it - Memory Sensation [Formant / Neural Map, EP, Nonplus] Rex The Dog - Experimental Housing [Experimental Housing, EP, Soft Computing] Sam KDC - Trial by Fire [Omen Rising, LP, Horo] Soren Roi - Bishop Ford [Retrograde Amnesia, LP, BANK Records NYC] Soren Roi - More About Myself [Retrograde Amnesia, LP, BANK Records NYC] Stanley Schmidt - Critical History [Smart Replies, EP, Vienna] Stanley Schmidt - Smart Replies [Smart Replies, EP, Vienna] Test Dept - Full Spectrum Dominance (JD Twitch Remix) [Disturbance Disordered, LP, One Little Indian] Test Dept - Gatekeeper (Wrangler Remix) [Disturbance Disordered, LP, One Little Indian] Test Dept - GBH84 (Angst78 DDR mix) [Disturbance Disordered, LP, One Little Indian] Test Dept - Landlord (Living Totem Remix) [Disturbance Disordered, LP, One Little Indian] Thighpaulsandra - The Goat Owl [Practical Electronics With Thighpaulsandra, EP, Editions Mego] Tom Of England - Sniffin' at the Griffin [Sex Monk Blues, LP, L.I.E.S.] Tommy Four Seven - Aphelion (Silent Servant Remix) [Veer Remixed, LP, 47] UVB76 – Extend [Session Extend, EP, Tabernacle] UVB76 – Hajime [S A N, LP, Teenage Menopause] UVB76 - Itaewon [S A N, LP, Teenage Menopause] UVB76 – Nox [S A N, LP, Teenage Menopause] Vatican Shadow - The House Of The Followers (JK Flesh Remix) [American Flesh For Violence, LP, Hospital Productions] Zaliva-D – Calling [Calling, EP, SVBKVLT]
3 notes
·
View notes
Text
How and Why is Karl Fischer Reagent Used?
Laboratory chemicals manufacturers in India are producing tonnes of chemicals every year. Some of these chemicals and reagents are used to determine water content.
To determine the amount of moisture in pharmaceutical, petroleum, cosmetic, chemical, and food samples, Karl Fischer titration (Volumetric and Coulometric) is utilised. Karl Fischer Reagents are used to assess moisture content by quantitatively and selectively reacting with water. Iodine, sulphur dioxide, a base, and a solvent, such as alcohol, make up the Karl Fischer reagent.
The water content in raw materials and finished products in lab chemicals are analysed using Karl Fischer reagents. Finar's R&D has created an in-house technique appropriate for manual and automatic equipment, assuring a consistent factor to answer the growing demand for stable Volumetric Karl Fischer reagents. We additionally provide Coulometric Karl Fischer Reagents from Japan's Aquamicron.
The Karl Fischer (KF) titration is a redox reaction that gauges the amount of water in a sample by using the water consumed during the reaction. Because of its specificity, precision, and measuring speed, it is the go-to method for determining the amount of water. In an organic solvent, it happens.
Because different samples are difficult to dissolve, other solvents are required. A gradual discharge of water with a floating endpoint result from the presence of an incompletely dissolved sample. Physical methods like grinding the material and reheating the titration cell occasionally achieve complete dissolving.
Karl Fischer's titration principles:
The KF reaction is based on water consumption in a buffered solution, while sulphur dioxide is oxidised by iodine.
FORMULA: I2 + 2H2O + SO2 -> 2HI + H2SO4
When the titrating agent has reached a large enough volume to react with all the water in the sample, the titration has reached its endpoint. Iodine and water are depleted in an equimolar ratio.
The most typical sample kinds where Karl Fischer Reagents are used are as follows:
Alkali and alkali earth salts, among other inorganic substances, do not dissolve well in methanol; other mixes, such as 50% formamide and 50% methanol, may be employed instead. If no suitable organic solvent is available, the water is evaporated in a KF oven and titrated directly using the KF reaction. Because they consume the reagent, substantial reducing and oxidising agents are inappropriate for this titration.
Water analysis of the finished product will reveal whether it has the appropriate physical properties and shelf life. Due to the poor solubility and effervescence of many formulations, KF titration is challenging. Liquids, creams, and suppositories are appropriate since they may be added directly and, if necessary, dissolved in propanol or chloroform in a warmed cell.
Oils and lubricants made from petroleum are not advised for KF titration since they often only contain trace amounts of water and do not readily dissolve in the standard reagent. However, coulometric KF titration can be performed using a unique reagent with chloroform or long-chain alcohols created for this purpose.
Plastics can be found as fibres, granules, or solutions, frequently containing minuscule amounts of water. Therefore, coulometry is the preferable technique, with the addition of chloroform or methyl pyrrolidone to thoroughly dissolve them. Fine polymeric polymer powders can be titrated voluminously.
Finar is one of the best lab chemicals suppliers in India, keeping a close eye on the quality. If you are looking for an expert in industrial chemical solutions, then look no further and connect with Finar today.
0 notes