#Severe Chronic Neutropenia
Explore tagged Tumblr posts
Text
Neutropenia Genetic Test Results 🧬
It’s just after midday, I’m finishing up with a member at work, and my Apple Watch pops up with a call. It’s from Melbourne. Who do I know that would be calling me from there right now? In my mind, it could only one person. The genetic councillor from VCGS who had my little girl’s genes looked at. My heart jumps up into my throat, and I answer the call out back. After weeks of waiting and…

View On WordPress
#Autoimmune neutropenia#Genetic Test#Health#Idiopathic neutropenia#Motherhood#Neutropenia#Neutrophils#Severe Chronic Neutropenia
0 notes
Text
Aplastic Anemia | Causes, Symptoms, Diagnosis and Treatments
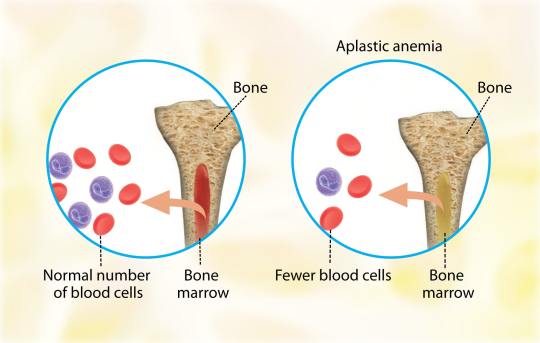
Aplastic anemia (AA) is the syndrome of chronic primary hematopoietic failure caused by injury, which results in decreased or absent hematopoietic precursors in the bone marrow and accompanying pancytopenia.
Aplastic anemia is classified as either moderate, severe (SAA), or very severe (vSAA).
Three primary mechanisms can result in the development of AA: –
1. Direct injury.
2. Immune-mediated.
3. Inherited or acquired bone marrow failure.
Usually, AA is idiopathic, however, it can be attributable to: –
- Radiation.
- Toxic chemicals (like Benzene, solvents, and glue vapors).
- Cytotoxic drugs (chloramphenicol, gold).
- Immune-related disorders (Eosinophilic fasciitis, SLE, Graft versus host disease).
- Thymoma.
- Viral infections (Epstein-Virus Infection, Parvovirus B19, Human immunodeficiency virus (HIV), and Hepatitis virus).
- Anorexia nervosa and paroxysmal nocturnal hemoglobinuria (PNH).
Patients typically present with non-specific symptoms caused by associated cytopenia, such as: –
- Low energy levels, pallor, and headaches with anemia.
- Mucosal bleeding.
- Bruising/petechiae.
- Menorrhagia with thrombocytopenia.
- Fever with or without evidence of infection with neutropenia.
The most common complications of aplastic anemia are bleeding, infections, and transformation to lymphoproliferative disorders.
Aplastic anemia has the following diagnostic criteria: –
The presence of bone marrow hypocellularity and two or more cytopenias (reticulopodia less than 1% or less than 40,000/microliter, neutropenia less than 500/microliter, or thrombocytopenia less than 20,000/microliter).
The bone marrow cellularity in moderate disease is less than 30%.
The severe disease has less than 25% cellularity or less than 50% cellularity with fewer than 30% hematopoietic cells.
Very severe meets the severe criteria in addition to neutropenia less than 200/µL.
Aplastic anemia treatment focuses on the underlying cause.
If possible, remove the offending agent(s).
Treatment is dependent on the patient’s age, disease severity, donor availability, and performance status when there is no distinctive reversible cause.
Young patients (less than 50 years old) with severe disease who are in good health should receive an allogeneic hematopoietic cell transplant (HCT) before starting immunosuppressive therapy.
Older patients (50 years and older) in good health, as well as young patients without an HCT donor, are given full-dose immunosuppressive therapy using: –
- Eltrombopag.
- Horse/rabbit anti-thymocyte globulin (ATG).
- Cyclosporine A.
- Prednisone.
Read more at: https://medicaregate.com/aplastic-anemia-causes-symptoms-diagnosis-and-treatments/
2 notes
·
View notes
Text
Colony-Stimulating Factor Therapy Market
The Colony-Stimulating Factor (CSF) Therapy Market has seen significant growth and transformation in recent years, driven by advancements in biotechnology, an increasing prevalence of cancer, and rising demand for targeted therapies. Colony-Stimulating Factors are crucial in the treatment of various hematologic conditions as they stimulate the bone marrow to produce white blood cells, which are essential in fighting infections, especially in immunocompromised patients, such as those undergoing chemotherapy.
Request To Download Sample of This Strategic Report - https://univdatos.com/report/colony-stimulating-factor-therapy-market/get-a-free-sample-form.php?product_id=58550
Market Overview
The global CSF therapy market is poised for robust growth, with a projected compound annual growth rate (CAGR) of 6.5% from 2023 to 2030. This growth is attributed to the increasing incidence of cancer and other conditions that necessitate chemotherapy, which in turn leads to a higher demand for CSF therapies to mitigate the side effects associated with cancer treatments.
Key Drivers
Rising Cancer Prevalence: With cancer cases on the rise globally, the demand for effective treatment protocols, including supportive care like CSF therapy, has surged. Chemotherapy-induced neutropenia is a common side effect that necessitates CSF therapy to prevent infections and maintain treatment schedules.
Technological Advancements: Innovations in biotechnology have led to the development of more effective and targeted CSF therapies. These advancements include pegylated formulations, which offer longer half-life and reduced dosing frequency, improving patient compliance and outcomes.
Aging Population: The increasing elderly population is more susceptible to cancer and other chronic diseases, thereby driving the demand for supportive therapies like CSF.
Government Initiatives and Funding: Increased government funding and initiatives aimed at improving cancer care infrastructure have significantly bolstered the CSF therapy market. Programs to enhance healthcare access and affordability further support market growth.
Market Segmentation
The CSF therapy market can be segmented based on product type, application, distribution channel, and region.
By Product Type: The market is divided into granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and others. G-CSF dominates the market due to its widespread use in treating neutropenia.
By Application: The primary applications include oncology, hematology, and others. Oncology is the leading segment, driven by the high incidence of cancer and the necessity for supportive treatments.
By Distribution Channel: The market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies hold the largest share due to the administration of CSF therapies in clinical settings.
By Region: North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa are key regions. North America leads the market owing to advanced healthcare infrastructure and high cancer prevalence.
𝐓𝐨 𝐆𝐞𝐭 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐟𝐮𝐥 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡, 𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐏𝐃�� 𝐂𝐨𝐩𝐲 - https://univdatos.com/report/colony-stimulating-factor-therapy-market/get-a-free-sample-form.php?product_id=58550
Recent Developments
FDA Approvals and Expansions: In recent years, there have been several FDA approvals for new CSF formulations. For instance, in 2023, the FDA approved a new biosimilar for pegfilgrastim, which is expected to provide a cost-effective alternative to existing therapies.
Strategic Collaborations and Acquisitions: Companies are increasingly entering into partnerships and acquisitions to expand their CSF therapy portfolios. In 2022, Amgen acquired Five Prime Therapeutics, enhancing its oncology and immunotherapy pipeline, which includes CSF therapies.
Research and Development: Significant investments in R&D are leading to the development of novel CSF therapies. Ongoing clinical trials are exploring new indications and more efficient formulations, such as oral CSF therapies that could revolutionize the market.
Biosimilars Market Expansion: The introduction and acceptance of biosimilars are transforming the CSF therapy landscape. Biosimilars provide more affordable treatment options, increasing accessibility and driving market growth.
Challenges
Despite the positive outlook, the CSF therapy market faces several challenges. High treatment costs, stringent regulatory requirements, and the potential for adverse effects limit market growth. Additionally, the competitive landscape, with numerous companies vying for market share, necessitates continuous innovation and cost management.
Future Prospects
The future of the CSF therapy market looks promising, with ongoing research and technological advancements paving the way for more effective and accessible treatments. Personalized medicine, where treatments are tailored to individual patient profiles, is an emerging trend that could significantly impact the market. Additionally, increasing awareness about the importance of supportive care in cancer treatment will continue to drive demand for CSF therapies.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐭𝐫𝐚𝐭𝐞𝐠𝐢𝐜 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐇𝐞𝐫𝐞 - https://univdatos.com/report/colony-stimulating-factor-therapy-market/get-a-free-sample-form.php?product_id=58550
Conclusion
The Colony-Stimulating Factor Therapy Market is on a growth trajectory, propelled by rising cancer incidence, technological advancements, and strategic industry initiatives. While challenges persist, ongoing innovations and increasing demand for effective supportive therapies promise a dynamic and evolving market landscape. Stakeholders, including healthcare providers, patients, and investors, can expect continued advancements and opportunities in this crucial segment of cancer care.
#healthcare#market analysis#market insights#market report#market research#market trends#univdatos#health#Colony-Stimulating Factor Therapy Market
0 notes
Text
Continuing Education Activity
Esophagogastroduodenoscopy (EGD) is a diagnostic endoscopic procedure used to visualize the oropharynx, esophagus, stomach, and proximal duodenum.
It is one of the most common procedures for gastroenterologists.
This activity describes the diagnostic and therapeutic capabilities of upper endoscopy and highlights the role of the interprofessional team in looking after patients with pathology of the upper digestive tract.
Objectives :
Identify the indications for esophagogastroduodenoscopy.
Describe the technique for performing upper endoscopy.
Review the complications associated with esophagpgastroduodenoscopy.
Explain interprofessional team strategies for improving care coordination and communication to advance the appropriate and safe use of esophagogastroduodenoscopy to improve patient outcomes.
Introduction
Esophagogastroduodenoscopy (EGD) is a diagnostic endoscopic procedure that includes visualization of the oropharynx, esophagus, stomach, and proximal duodenum.
It is one of the most common procedures that a gastroenterologist performs.
Anatomy and Physiology
Esophagus
The esophagus is located posterior to the trachea and begins distal to the cricoid cartilage and ends at the cardiac orifice of the stomach.
It ranges in diameter from 4 to 6 mm and in length from 9 to 10 cm in the term infant to approximately 25 cm in the adult.
The change in the mucosa color from pale- to reddish-pink marks the transition from the esophagus and gastric epithelium (Z line).
Stomach
The stomach is usually located beneath the diaphragm and is approximately 40 cm distal to the incisors in an adult.
The area of the stomach where the esophagus enters is known as gastric cardia.
The portion of the stomach above the junction of the esophagus and stomach is known as fundus. It is visible in a retroflexed endoscopic view.
The majority of the stomach is known as stomach body.
Along the lesser curvature of the stomach is the incisura which divides the gastric body from the antrum. Endoscopically, the transition from the body to the antrum is from rugae to flat mucosa. The pylorus is the muscular opening between the lower end of the stomach and duodenum bulb.
Duodenum
The duodenum extends from the pylorus to the duodenojejunal angle.
The duodenum bulb is an expanded region immediately distal to the pylorus.
The duodenum then forms a C-shaped loop and endoscopically turns posteriorly and to the right for 2.5 cm, then inferiorly for 7.5 to 10 cm (descending portion), then anteriorly and to the left for approximately 2.5 cm, and finally connects to the jejunum at the level of ligament of Treitz.
Indications
Diagnostic
Persistent upper abdominal pain or pain associated with alarming symptoms such as weight loss or anorexia.
Dysphagia, odynophagia or feeding problems.
Intractable or chronic symptoms of GERD.
Unexplained irritability in a child.
Persistent vomiting of unknown etiology or hematemesis.
Iron deficiency anemia with presumed chronic blood loss when clinically an upper gastrointestinal (GI) source is suspected or when colonoscopy is normal.
Chronic diarrhea or malabsorption.
Assessment of acute injury after caustic ingestion.
Surveillance for malignancy in patients with premalignant conditions such as polyposis syndromes, previous caustic ingestion, or Barrett esophagus.
Therapeutic
Foreign body removal.
Dilation or stenting of strictures.
Esophageal variceal ligation.
Upper GI bleeding control.
Placement of feeding or draining tubes.
Management of achalasia (botulinum toxin or balloon dilation).
Contraindications
Absolute Contraindications
Perforated bowel.
Peritonitis.
Toxic megacolon in an unstable patient.
Relative Contraindications
Severe neutropenia.
Coagulopathy.
Severe thrombocytopenia or impaired platelet function.
Increased risk of perforation including connective tissue disorders, recent bowel surgery or bowel obstruction.
Aneurysm of the abdominal and iliac aorta.
Equipment
Gastroscopes
The standard gastroscopes have a diameter of 10 mm with an instrument channel of 2.8 mm. In children weighing less than 10 kg, endoscopes smaller than 6 mm in diameter for routine endoscopy should be used.
A gastroscope with a large operating channel measuring 3.8 to 4.2 mm is useful in severe acute upper GI bleeding.
High-definition gastroscopes with optical zoom should be available to screen for pre-malignant gastric or duodenal lesions.
Accessories
The biopsy forceps (standard and jumbo) are needed for tissue sampling. For retrieval of a foreign body during esophagogastroduodenoscopy (EGD), rat tooth forceps, alligator forceps, retrieval net, polypectomy snare, overtubes of esophageal and gastric lengths, and a foreign body protector hood should be available. Additional equipment may be required if therapeutic procedures are anticipated.
Preparation
Routine endoscopy in children and adults is usually performed in an outpatient setting using parenteral or general anesthesia.
Occasionally, endoscopy is necessary at the hospital bedside or in an operating room.
Diet :
Preparation for elective upper endoscopy procedure involves a period of fasting.
As per American Society for Anesthesiologists (ASA) guidelines, patients should fast a minimum of 2 hours after ingestion of clear liquids and 6 hours after ingestion of light meals.
In emergency situations or in conditions where gastric emptying is impaired, the potential for pulmonary aspiration of gastric contents must be considered to determine (1) level of sedation, (2) whether endotracheal intubation should be considered to protect the airway or (3) whether the procedure should be delayed.
Medications :
Most medications can be continued and are usually taken with a small sip of water before endoscopy, although diabetes medications need to be adjusted due to the period of fasting before the procedure.
American Society for Gastrointestinal Endoscopy (ASGE) guidelines should be followed for decisions regarding the management of anti-thrombotic agents or for the use of antibiotic prophylaxis in at-risk patients before the endoscopy.
Sedation and Monitoring
Sedation is used in most patients not only to minimize discomfort but also to provide amnesia for the procedure.
All patients undergoing upper endoscopy require pre-procedural evaluation to assess their risk for sedation and to manage potential problems related to pre-existing health conditions.
The choice of sedation varies from conscious sedation delivered by the proceduralist or monitored anesthesia care provided by an anesthesiologist, and preferences for one type of sedation over another are largely based on training and available local resources.
For routine upper endoscopy, many endoscopists utilize intravenous sedation using propofol.
For therapeutic endoscopic procedures such as foreign body removal or in patients in whom cooperation is not anticipated, including very young patients, general anesthesia may be required.
ASGE guidelines recommend routine monitoring of vital signs in addition to clinical observation for changes in cardiopulmonary status during all endoscopic procedures performed under sedation.
Informed consent
Patients, parents, or legal guardians should provide informed consents before the Esophagogastroduodenoscopy (EGD) and for the administration of sedation.
Technique or Treatment
Handling the Endoscope
The endoscope is mostly held in the left hand.
The control section of the endoscope should rest comfortably in the palm of the left hand.
The thumb controls up or down movement of the tip of the endoscope using a large wheel.
The index finger and, at times, the middle finger control the suction, air, and water valves.
The right hand is used to advance and withdraw the endoscope and its axial rotation.
The right hand is also used to insert instruments such as biopsy forceps, cytology brushes, needles for injection, hemostatic clips, polypectomy snares, foreign body retrieval instruments, and syringes for irrigation via the biopsy channel.
Esophageal Intubation
For esophagogastroduodenoscopy (EGD), patients are typically placed in left lateral decubitus with neck flexed forward.
A bite block is placed in the mouth before the endoscope is inserted into the oral cavity.
The endoscope is introduced into the mouth and to the base of the tongue under direct visualization.
The tip of the scope is then gently angulated downward until the vocal cords, epiglottis, both piriform sinuses, and cricoarytenoid cartilages are visualized.
The scope is then passed behind and to the right of the arytenoid cartilage towards the upper esophageal sphincter.
The upper esophageal sphincter is passed under direct visualization, often with application of gentle pressure while insufflating air.
Esophagus and Esophagogastric junction
After intubating the esophagus, the scope is advanced down the esophagus lumen while simultaneously examining the mucosa for any inflammation, ulcerations, furrowing, varices, narrowing or strictures.
The location of the esophagogastric junction should be noted.
The squamocolumnar junction, also referred as Z-line, is the area where the squamous epithelial lining of the esophagus (pale pink colored) meets the columnar lining mucosa of the stomach (salmon-colored).
The level of the Z-line should also be noted. If the Z-line is displaced proximal to the gastroesophageal junction, biopsies should be taken to evaluate for Barrett esophagus.
Stomach
The stomach is entered after passing the esophagogastric junction.
Once the stomach is entered, any residual gastric secretions should be suctioned, and air is insufflated to improve visualization.
The endoscope is then advanced while torquing to the right. The endoscope is advanced along the lesser curvature towards the pylorus, but to fill the greater curvature with the endoscope is usually necessary before the cannulation of the pyloric canal.
The pylorus is a small opening with radiating folds around it.
To pass through the pylorus, the endoscope is positioned in front of the pylorus, and a little air and gentle pressure should be applied against the orifice.
Duodenum
After passing through the pylorus, the endoscope enters the duodenum bulb.
The duodenum bulb should be examined on endoscope insertion rather than during withdrawal as passage of the instrument can cause possible mucosal changes.
After all four quadrants of the bulb are inspected the scope is advanced to the posterior aspect of the bulb; here the duodenum turns right sharply and takes downward turn. To pass the superior flexure of the duodenum and enter the second part of the duodenum, the instrument is advanced using the dials and shaft torque, usually down and to the right followed by an upward spin of the dial.
The superior flexure of the duodenum is often passed blindly and examined on the way back.
The lower part of the second portion of the duodenum is reached by straightening the endoscope, in other words, pulling the endoscope slowly backward while maintaining the view of the lumen. This maneuver reduces the loop along the greater curvature of the stomach and, paradoxically, advances the endoscope into the distal duodenum.
The duodenum distal to the bulb has distinctive circular rings called valvulae conniventes.
The ampulla of Vater is found in the second portion of the duodenum and examined while withdrawing the endoscope.
After careful examination of the duodenum, pylorus, and antrum, the endoscope is retroflexed to visualize the gastric cardia and fundus.
The endoscope is then returned to a neutral position.
Once the stomach has been fully inspected, and biopsies, if necessary, are obtained, the endoscope is then withdrawn.
Before leaving the stomach, air should be suctioned.
The esophagus is again examined on withdrawal of the endoscope.
The average duration of a diagnostic EGD is 5 to 10 minutes under optimal sedation conditions.
Tissue sampling is obtained from suspicious lesions during EGD, although many gastroenterologists perform routine biopsies from designated sites, as a clinically significant disease may be present in an apparently normal looking mucosa.
Specimens obtained include biopsies, brushings of mucosal surface, and polypectomy.
Specimens are sent for histological, cytological, or microbiologic analysis based upon the type of the sample and clinical situation.
Complications
Complications following esophagogastroduodenoscopy (EGD) are rare, occurring in less than 2% of patients.
These could be related to sedation, endoscopy, and complications related to diagnostic or therapeutic maneuvers.
The most frequent and serious complications of sedation are cardiopulmonary.
Adverse events from over sedation include hypoxemia, hypoventilation, hypotension, airway obstruction, arrhythmias, and aspiration.
The complications following diagnostic EGD include infection, bleeding, duodenal hematoma, and bowel perforation.
The risk of bleeding following EGD with biopsy is 0.3%. Post mucosal biopsy bleeding can occur as intraluminal hemorrhage or intraluminal hematoma.
A duodenal hematoma is a rare complication of EGD with an unknown incidence and seems to occur more often in children than adults.
Bowel perforation occurs in less than 0.3 % of cases, and infection is rarely reported.
Complications typically are identified in the first 24 hours after the procedure.
Bleeding presents with hematemesis or bloody output from the gastrostomy tube.
Perforation is identified due to fever, tachycardia, abdominal pain or discomfort.
An abdominal x-ray should be done to reveal extra-luminal air.
Conservative therapy with bowel rest and antibiotics is the typical treatment, although some patients might require surgical repair.
Clinical Significance
Esophagogastroduodenoscopy (EGD) has become a key element in the diagnosis and treatment of esophageal, gastric, and small-bowel disorders.
The many accepted indications for EGD include evaluation of dysphagia, GI bleeding, peptic ulcer disease, medically refractory GERD, esophageal strictures, celiac disease, and unexplained diarrhea.
During EGD evaluation, diagnostic biopsies can be performed as well as therapies to achieve hemostasis and dilation for significant strictures.
If properly performed, it is generally a safe and well-tolerated procedure. EGD's availability and use in the pediatric population have increased.
Decisions surrounding the conditions and time for EGD use in children remain more of an art than a science, and additional critical review of this tool's use is needed to maximize results and minimize risk.
Enhancing Healthcare Team Outcomes
In the pediatric population, endoscopy is typically performed by a pediatric endoscopist with the medical knowledge and technical competency specific to perform safe and effective GI procedures in this population.
The American Society for Gastrointestinal Endoscopy (ASGE) published practice modification guidelines to provide guidance regarding performing endoscopy in infants and children.
If it is not possible for a pediatric-trained endoscopist to perform the procedure, an adult-trained endoscopist should perform endoscopic procedures in children in coordination with a pediatrician and pediatric specialists.
During endoscopic procedures, procedural and resuscitative equipment appropriate for pediatric use should be readily available.
If sedation is needed for the procedure, personnel trained specifically in pediatric life support and airway management should also be readily available.
In symptomatic children with known or suspected caustic ingestion, endoscopy should be performed within 24 hours.
It is recommended to perform emergent foreign body removal of esophageal button batteries as well as two or more rare-earth neodymium magnets.
0 notes
Text
Blasts shouldn't be seen in peripheral blood.
Rule out leukemia, myelodysplastic syndrome, chronic myeloid leukemia.
Blasts may be other immature hematopoeitic cells. High monocyte count might actually be blasts, not monocytes.
If auto differential is abnormal, get a manual one.
Blasts newly elevated is more suggestive of acute leukemia. Pancytopenia suggests bone marrow failure. Febrile neutropenia is also concerning.
Make sure pt is stable with vitals, oxygen if needed, fluids.
Rule out emergencies and then contact heme/once.
APL is a subtype of AML. AML has 7 morphological categories using a system called fab. APL is the third subtype, M3. It has unique history and treatment. It presents with DIC and has early mortality. People hemorrhage and die. You can prevent it by giving all trans retinoic acid (ATRA). It helps APL cells differentiate into normal cells. APL is curable. Give all trans retinoic acid (ATRA). Diagnose by looking at source of the tumor cells. Look at peripheral blood smear. It separates ALL from AML. Auer rods are seen in AML. APL cells have multiple auer rods and folded nuclei that are very granulated.
CML has maturation of all the white cells, mature granulocytes, basophilia.
MDS (myelodysplastic syndrome) = elderly pts; hypogranular neutrophils, bilobed neutrophils, red cell shape/size changes (anisopoikilocytosis)*.
Do the peripheral blood smear first. Next do a peripheral blood flow cytometry or cell markers. It classifies cells based on the markers they express. Blasts express CD34. If CD34 is elevated, then the pt has increased blasts.
The definitive test to diagnose acute leukemia is a bone marrow biopsy. AML = more than 20% myeloblasts on bone marrow biopsy. ALL = more than 20% lymphoblasts on bone marrow biopsy.
APL has a characteristic genetic translocation--chromosome 15 and 17.
Before talking to heme/onc, also get bloodwork to rule out DIC (fibrinogen level, INR, PTT, d-dimer). If pt is febrile, work up includes blood culture, chest X-ray, urine culture for febrile neutropenia.
Also get CMP, phosphorus, magnesium, calcium, uric acid level to look for tumor lysis syndrome.
Transfuse platelets if platelets less than 10,000 and bleeding. If symptomatic anemia, transfuse RBCs.
Leukostasis = elevated WBCs such that blood flow to organs is impeded. Immature cells clog up the vasculature. Causes hypoxia, pulm infiltrates, SOB, AMS, HA, dizziness. WBC can be 50 to 100 or even less than that in leukostasis.
Other hematologic emergencies: cauda equina (spinal cord compression) is seen in lymphoproliferative disorders (lymphoma-> mass effect on spinal cord); myeloma (alters bone integrity-> compression fractures). Sometimes myeloma deposits can compress the spinal cord. So assess lower extremity reflexes, tone, Babinski sign, saddle anesthesia, rectal tone, bowel or bladder incontinence.
Top 5 clinical pearls
1) blasts are worrisome in peripheral blood, should not be in peripheral blood. Get heme/onc on board fast.
2) get a manual differential, review the film manually
3) acute leukemia can present with pancytopenia and no circulating blasts, OR with just circulating blasts. So either of those should be explored.
4) if you think the pt has acute leukemia, suspect APL, which is a medical emergency that can be treated with all trans retinoic acid immediately.
5) hematologic emergencies: severe cytopenia, febrile neutropenia, DIC, tumor lysis syndrome, leukostasis, cord compression. So screen for these.
*Anisopoikilocytosis is when you have red blood cells that are of different sizes and shapes.
The term anisopoikilocytosis is actually made up of two different terms: anisocytosis and poikilocytosis. Anisocytosis means that there are red blood cells of varying sizes on your blood smear. Poikilocytosis means that there are red blood cells of varying shapes on your blood smear.
So basically: get a peripheral blood smear, which let's you look at the cells. Flow cytometry can further differentiate the specific cell type that is elevated. Bone marrow biopsy is the definitive way to diagnose.
#heme#AML#APL#CML#febrile neutropenia#blasts#leukostasis#monocytosis#M3#leukemia#all trans retinoic acid#ATRA#Auer rods#Auer rod#hemeonc
3 notes
·
View notes
Text
Next in the Cat Series: Hyperthyroidism!

One of my favorite cat diseases, because unlike other chronic health issues, it’s not only easy to diagnose, but curable too! (phew, not all of them are just supportive care)
Who gets it? The typical hyperthyroid cat is older (mean age of 13 years), with females being predisposed and with no breed predilection beyond “mixed breed.” It can be diagnosed in cats from 4 to 22 years old, though it’s very uncommon to see it in cats younger than 8 years.
What’s it look like? These cats are usually thin or losing weight, despite a ravenous appetite (polyphagic). They can also be PU/PD (polyuric/polyphagic, ei, they drink and pee waaay more than is normal). They can become more active, literally putting the hyper in hyperthyroid, or more aggressive than they used to be. Other signs that aren’t very specific to hyperthyroidism are vomiting and diarrhea.
So what is it? Hyperthyroidism is a condition where a tumor in the thyroid gland, located in the neck on each side of the trachea, causes excess production of thyroid hormones, especially notable is T4. Thyroid hormone is responsible for a variety of metabolic functions, and this increase in metabolism is what causes many of the presenting signs: hyperactivity, weight loss despite appetite, aggression.
How do you diagnose it? The most definitive diagnosis of hyperthyroidism is through checking blood concentrations of T4. It’s increased? Congrats, you’ve diagnosed hyperthyroidism! Though a lot of feline specialty practices have it always on the normal blood chemistry, it may be an add-on at other general practices, and that’s when it may get a bit trickier to diagnose originally.
The history should help clue you in a lot, but on physical exam you’ll also potentially see:
Thin/decreased weight from last visit
Dehydration
A palpable “thyroid slip” - the thyroid gets large enough you can feel it when you run your fingers along the side of the trachea. Not all hyperthyroid cats have it, and it can be hard to get used to finding the slip
Poor hair coat
Cardiac abnormalities: tachycardia (>220 bpm), murmur, arrhythmias, a gallop rhythm
Hypertension, which can cause ocular or neurologic issues
Besides increased T4 on bloodwork, you may also see an increased ALT and/or ALP, sometimes insanely high while all the other liver enzyme values are normal. A fifth will also have azotemia (increased BUN), either due to dehydration or concurrent renal disease.
And since they’re older cats, there may be other chronic illnesses like CKD, diabetes, or GI disease.
You said this one’s not just treatable, but curable?? Yes! The treatment of choice is radioactive iodine therapy. Iodine is taken up by the thyroids to create thyroid hormone, so once the radioactive iodine is injected it’s selectively picked up by the thyroid. And since the tumor cells are working overtime, the radioactive iodine (I-131) is picked up by the tumor cells, killing them, and leaving the currently-dormant normal thyroid cells intact.
Pros:
It works rapidly (improvement within 2 weeks, back to full normal in 3 months)
Very effective, with an over 90% cure rate
No pills in angry old cats
Permanent, one time correction
Cons:
Expensive, usually costing over $1000 (~1400)
Requires special facilities, so it’s not available everywhere
Using radioactive materials, so requires containment of your cat at the hospital from anywhere from 2 days to 2 weeks, and usually the shorter time periods require special at home distancing from your cat
May need retreatment if it’s a thyroid carcinoma (2% of cases), so more $$$
What if I can’t afford that? Any other options? Yep, there’s methimazole, which come in a pill form or a transdermal cream. It works by blocking thyroid hormone synthesis, so while it doesn’t cure hyperthyroidism, it will lower T4 levels and mitigate clinical signs.
Pros:
Not as expensive up front, though can cost more over the course of the disease
No hospitalization
Available anywhere cats are seen
Cons:
Pilling angry cats sucks, some don’t like the cream either
Twice daily, nonpermanent medication
Manages, doesn’t treat the disease. The thyroid tumor can grow, requiring more medication, or transform into a carcinoma instead of a benign tumor
Side effects! - GI upset, neutropenia/thrombocytopenia, GI upset (pill), and the worst: facial excoriation, where the cats’ faces are so itchy they scratch off their skin. Reversible with discontinuation.
There’s also surgical removal of the thyroids, which has fallen out of favor, and Hill’s Y/D hyperthyroidism food, which is iodine restricted and not highly recommended by endocrinologists.
I’ve heard hyperthyroid treatment causes renal disease, is that true? No, but it often unmasks kidney disease. These two diseases, hyperthyroidism and CKD, have a very common demographic in older cats. Hyperthyroidism can increase the filtration rate of the kidneys, which makes the bloodwork levels, BUN and creatinine, that we use to diagnose CKD, decrease. Once the hyperthyroidism is cured, the filtration rate decreases, and those values jump up.
If the kidneys already look bad before treatment, the vet may want to do a “methimazole trial” before committing to radioiodine. This will allow the T4 to drop and uncover the true extent of renal disease, and since methimazole isn’t permanent it can be discontinued if the renal disease is too bad.
Most feline specialists still recommend treating the hyperthyroidism even if there is severe underlying kidney disease, but this can help us prepare for what to expect after radioiodine.
What’s the prognosis, almost-doc? Well, it depends. These cats are already fairly old, so part of the shorter lifespan can be due to other concurrent old age changes/diseases. If properly managed, the median survival time is 2-4 years, though if cured with iodine at 10 years old they could truly live into their 20s. I heard of a cat treated with radioiodine in their 20s and is now in their 30s!
33 notes
·
View notes
Text
It’s been a while
I’m not on here much anymore, almost everything I do now goes straight to Instagram.
If you want an update, here ya go.
2019 was wild.
I never talked about it much aside from my immediate support system, as they were experiencing it all with me.
I moved in with my boyfriend, and finally experiencing the healthiest living situation I’ve had for years.
Despite this, I also had the largest health scare. We fully thought I had cancer for a while, thankfully that isn’t the case. I do have hypothyroidism, though, as well as Severe Chronic Idiopathic Neutropenia. Which means, in short, that I’m tired all of the time, my hormones are fucked up, on a good day I’m still missing about 80% of my white blood cells (specifically the neutrophils) and have to live life like a germaphobe. I try sticking to an anti-microbial diet, so I don’t eat things like raw sushi, raw fruit.. really anything raw. Everything I eat needs to be cooked. Sometimes I stray from this if I’m feeling good, just for the quality of life’s sake, but I have to be careful.
Not everyone in my life seems to believe me.
Some people think it’s just going to magically clear up one day, that I’m being paranoid, or that it’s something new. The diagnosis was new, but I’ve been experiencing the symptoms of it all since 2009. It just happened to be getting dangerously worse, which is when I finally got my blood work done.
Anyway, I’m going to try going to school for coding in a couple of months. Hopefully it all works out and I can get a career where I can be the quarantined hermit I need to be.
That’s all for now I guess, later.
4 notes
·
View notes
Text
About Revlimid and where to buy
Revlimid® (lenalidomide), an immunomodulatory drug (IMiD®), is the first oral medication that was developed for treatment of multiple myeloma. It is used in the newly diagnosed, maintenance therapy, and relapsed and/or refractory settings.
It is a very expensive drug with a four month supply costing as much as $20,150 and this is for those with Insurance. The high cost of the drug has made many to now buy Revlimid online from oversea pharmacies.
How Does It Work?
Revlimid has multiple actions, including both anti-cancer and anti-inflammatory activities. It induces immune responses, prevents inflammation, and enhances the activity of immune cell—specialized white blood cells known as T cells and natural killer (NK) cells. Revlimid also prevents the formation of new blood vessels that cancer cells depend upon for sustenance and growth.
Possible Side Effects
Embroyo-fetal toxicitiy
Animal studies have shown that Revlimid can cause severe birth defects. To prevent severe birth defects from occurring as a result of pregnancy during treatment, the FDA required that a risk management program be established. The goals of the Revlimid Risk Evaluation and Mitigation Strategy (known as Revlimid REMS™) are as follows:
To prevent the risk of embryo-fetal exposure to the drug.
To inform prescribers, patients, and pharmacists on the serious risks and safe-use conditions for Revlimid.
Constipation
Prevention is the key to managing constipation, which is defined as having fewer than three bowel movements a week. Chronic constipation is defined as infrequent bowel movements or difficult passage of stools that persists for several weeks or longer.
Neutropenia
Patients taking Revlimid may experience neutropenia, or low levels of our most abundant type of white blood cell, the neutrophil. White blood cells make up your immune system. Your immune system defends against viral and bacterial illnesses. Therefore, having too few of white blood cells can lead to infection. Fever is the most common sign of having too few neutrophils, and is a sign that you need immediate medical attention. Other common symptoms associated with a low neutrophil count include sore throat and mouth sores. For more information on neutropenia, visit Myelosuppression.
Thrombocytopenia (decreased platelet levels)
Patients taking Revlimid may experience thrombocytopenia, a lowered level of platelets in the blood. Platelets help blood to clot after an injury; fewer platelets can lead to bruising, bleeding, and slower healing. For more information on thrombocytopenia, visit Myelosuppression.
Fatigue
Fatigue is commonly associated with Revlimid therapy. Although fatigue is generally not severe, caution is advised if you are operating machinery, including automobiles.
Deep-vein thrombosis
Deep-vein thrombosis (DVT) is a serious condition and is potentially life threatening. DVT is a blood clot in a deep vein of the lower extremities, usually occurring in the leg or thigh, and very occasionally in the neck or upper arm. A blood clot from a DVT can break loose (embolize) and travel to the heart or lungs. An embolus is very dangerous. If you start taking Revlimid and experience difficulty breathing, warmth, swelling, redness, and/or pain in an extremity, notify your doctor immediately. For more information on DVT, visit Heart and Lung Complications.
Rash
Rash is a serious concern. It is potentially dangerous, as a rash may be mild initially and then escalate in severity. Drug rashes vary in severity from mild redness with tiny bumps over a small area to peeling of the entire skin. Rashes may appear suddenly within minutes after a person takes a drug, or they may be delayed for hours or days
Additional Information
Revlimid prescribing information, including updated renal dosing guidelines
Dosing guidelines from the FDA and the EMA.
Patient Assistance
Celgene's patient support program
Celgene's Patient Support Coordinators assist patients and healthcare providers to navigate the challenges of reimbursement, provide information about co-pay assistance, and answer general questions about Revlimid.
Fast Track for First Prescription™
Celgene has developed a new program called Fast Track for First Prescription™ to help eligible patients receive their first prescriptions for a Celgene product quickly and efficiently. Fast Track is an optional service of Celgene Patient Support that facilitates communication between doctors, patients, insurance providers, risk management programs, pharmacies, and co-pay foundations. A Celgene Patient Support coordinator will ensure that “all the ducks are in a row” as quickly as possible, and remove the hurdles to accessing that first cycle of medication. To be eligible, patients must be receiving their first prescription of either Thalomid or Revlimid, must have documented proof of insurance, and must be registered in a Celgene risk management program and have a valid authorization number. For more information on the program, call the IMF Hotline at 800-452-2873.
1 note
·
View note
Text
[streptococci notes]
Streptococcus Group A (S. pyogenes)
Mild strep throat and skin infections (impetigo, cellulitis)
rapid, streaky cellulitis
erysipelas – cellulitis with fever & toxicity
scarlet fever – raised tongue papule and pharyngitis
Invasion to sterile sites with mucosal barrier breakage » severe disease
necrotizing fasciitis
streptococcal toxic shock syndrome
Immune hypersensitivity develops after infection
rheumatic fever: strep antibodies attack cardiac, joint, and neural tissue » incidence is going down now
rheumatic heart disease occurs as chronic sickness
acute glomerulonephritis: antibody-strep complexes cluster and deposit in kidney
Risk factors
symptomatic transmission only, peaks in winter and spring
immunocompromised/chronic illness
age
skin lesions
substance users
Treatment
penicillin-, erythromycin-, and clindamycin-susceptible
severe infections: penicillin and clindamycin that interrupts protein synthesis (lowers TOXIN synthesis)
amputate necrotizing fasciitis sites
Streptococcus Group B (S. agalactiae)
Pneumonia, sepsis, and meningitis in newborns
vaginal colonies in women that are passed to newborn lungs via placental penetration
OR vaginal colonies passed to infant in birth canal
infants have poor immune protection and develop severe symptoms above
Treatment
prophylaxis for pregnant women who test positive
treatment for pregnant women who present with symptoms
ampicillin + aminoglycosides for infected newborns
Streptococcus pneumoniae
Gram-positive, lancet-shaped diplococcus with lance-tip, alternative-pathway-blocking capsule
Pneumonia, bacteremia, sepsis, and meningitis in adults
abrupt onset pneumonia with coughing and rusty sputum
pleuritic chest pain
Most common cause of otitis media in children
Risk factors:
transmission from person to person in the winter
age
crowding
acute viral infections
chronic respiratory diseases (asthma, COPD, sickle-cell anemia, spleen loss)
aspiration-causing events
neutropenia, lymphopenia
Treatment
[archaic] horse serum antibodies when alternative pathway isn't responding due to S. pneumoniae capsule
PCV15/20 vaccination with capsule in adults
PCV13 vaccination with conjugated carrier + capsule in toddlers
0 notes
Text
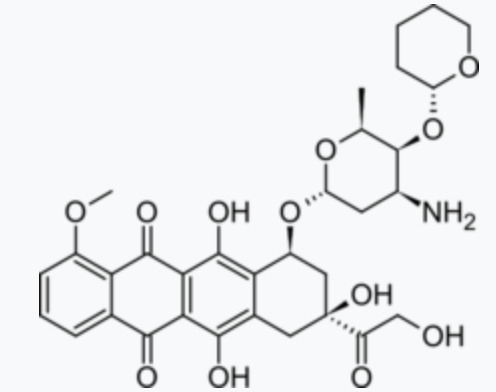
Dosage, adverse reactions, and contraindications of pirarubicin
Pirarubicinis an anthracycline cell cycle non-specific antineoplastic drug synthesized from doxorubicin and dihydropyran. It is suitable for the treatment of breast cancer. It is also effective in patients with drug resistance.
The mechanism of action of the drug is that it can directly intercalate between DNA double strands, inhibit DNA polymerase, prevent nucleic acid synthesis, and prevent cells from dividing in the G2 phase, resulting in tumor cell death.
Dosage
Dissolve this product in 10ml of 5% glucose injection or water for injection. Intravenous, arterial, and intravesical infusion.
Intravenous administration: generally 25-40 mg/m2 per body surface area. For breast cancer, the recommended combination is 40-50 mg/m2 each time. It is administered on the first day of each course of treatment and can be repeated at 21-day intervals according to the patient's blood picture. For acute leukemia, the adult dose is 25 mg/m2 once based on body surface area.
Arterial administration: For head and neck cancer, 7-20 mg/m2 per body surface area, once a day, for 5-7 days, or 14-25 mg/m2 each time, once a week.
Intravesical administration: for the prevention of postoperative recurrence of superficial bladder cancer. According to the body surface area, 15~30mg/m2 once, diluted to 500~1000μg/ml concentration, injected into the bladder cavity for 0.5h, once a week, 4~8 times in a row; then once a month, a total of 1 year.
Doctors can adjust the time, dosage, and frequency of administration according to the patient's condition.
Adverse reactions
1. Myelosuppression is dose-limiting toxicity, mainly neutropenia, with an average minimum value of 14 days and recovery on the 21st day, anemia and thrombocytopenia are rare;
2. Cardiotoxicity is lower than doxorubicin, acute cardiotoxicity is mainly reversible ECG changes, such as arrhythmia or non-specific ST-T abnormalities, and chronic cardiotoxicity is dose-accumulating. The incidence of acute and chronic cardiotoxicity of this product is about 1/7 and 1/4 of doxorubicin.
3. Hair loss: The overall incidence of hair loss with this product is about 40%, which is significantly lower than that of doxorubicin (80%); the incidence of severe hair loss is about 20%, which is significantly lower than that of doxorubicin (60%).
4. Gastrointestinal reactions: nausea, vomiting, loss of appetite, oral mucositis, and sometimes diarrhea;
5. Others: abnormal liver and kidney function, skin pigmentation, etc., occasional rash. The intravesical injection can cause bladder irritation symptoms such as frequent urination, dysuria, occasional hematuria, and rarely bladder atrophy.
Taboo
1. Patients with obvious bone marrow suppression due to chemotherapy or radiotherapy are contraindicated;
2. Patients with severe structural heart disease or abnormal cardiac function and those who are allergic to this product are prohibited;
3. Patients who have used high-dose anthracyclines (such as doxorubicin or daunorubicin) are contraindicated;
4. Women of pregnancy, lactation, and childbearing age are prohibited.
Minbiotech is a Sirolimus supplier and factory, Minbiotech CAS 53123-88-9 sale, Sirolimus supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Where to buy sirolimus cheap
Introduction to Biosimilars
0 notes
Text
Straight Outta Neutrophils
Back in July, when I was dealing with a sick 9 month baby, I had never heard of neutrophils. Three months on, my learner plates have come off, so to speak, as I’ve learned a whole lot more about them. I’m currently coming to terms with the fact that, Kaydence has got a rare blood disorder called Severe Chronic Neutropenia (SCN). Which is also a rare type of primary immunodeficiency. And what I…

View On WordPress
0 notes
Text
Distribution of Hematological Parameters Counts for Children with Leukemia in Children’s Cancer Units at Al-Kuwait Hospital, Sana’a City: A Cross-Sectional Study
Abstract Introduction and aims: Leukemia is a heterogeneous group of hematological disorders that is made up of several diverse and biologically distinct subgroups. Leukemia is the eleventh and tenth most common cause of cancer morbidity and mortality worldwide, respectively. There are insufficient data on the hematological parameters counts of leukemia in Yemen. This cross-sectional study aims to determine the hematological parameters counts for children with leukemia. Materials and methods: A cross-sectional study was conducted on children with leukemia who were treated selectively in the pediatric leukemia units of Kuwait University Hospital in Sana’a. Group diagnostics and histopathological diagnoses were formed in line with the French, American and British classifications of leukemia in children in the pediatric leukemia units, over a period of 5 years from January 1, 2014 to December 31, 2018. Hematological Parameters between Types of Leukemia. The distribution of some hematological parameters with respect to leukemia types with reference values of WBC count (3.31-11.62×109 /L), platelet counts (145.5-444.5×109 /L), neutrophil count (1.01-7.22×109 /L), eosinophil count (0.05-1.21×109 /L), and basophil count (0.01–0.05 ×109 /L) was analyzed and compared with results of non-malignant diseases. Results: 241 leukemia patients were diagnosed, treated and followed up; there was association of leukemia with younger age group; 50% were in the age group 1-5 years and with mean ± SD age= 6.44 ± 3.7 years. There was significant association with male (66.7%). In Acute leukemia, neutropenia (59.1%) and thrombocytopenia (75.7%) were found, while in chronic leukemia, neutrophilia (0%), basophilia (38.3%), and eosinophilia (5.7%) were recorded. Leukocytosis was observed in all types of leukemia. In other non-malignant diseases: leukopenia 12.2%, leukocytosis 53%, thrombocytopenia (50%), thrombocytosis (9.9%), neutropenia (28%), and neutrophilia (29.3%), basopenia (28.6%), Basophilia (27.9%) and eosinopenia in 39.6% of the patients. Conclusion: There is significant differences between acute, chronic leukemia and with non-malignant patients. Thus, laboratory professionals, the first who encounter the patients’ results, should perform more laboratory investigation as ,immunophenotyping , cytogenetic and molecular diagnostic for peripheral and bone marrow morphology assessment as a reflex test for those who have abnormal hematological parameters.
Read More about this article: https://irispublishers.com/acrci/pdf/ACRCI.MS.ID.000560.pdf
Read More about Iris Publishers Google scholar Articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=d1PdB38AAAAJ&authuser=1&citation_for_view=d1PdB38AAAAJ:hMod-77fHWUC
1 note
·
View note
Text
Best pulmonary doctor in gurgaon

Neonatal Pneumonia: Causes, Symptoms, Treatment
If your newborn is suffering from neonatal pneumonia, check out below its causes, symptoms and treatment.
Causes
Neonatal pneumonia occurs when germs (bacteria, viruses, fungi or parasites) enter the neonate from the nursery or the maternal genital tract. Such bacteria include gram-positive cocci like Staphylococcus aureus, and gram-negative bacilli like Escherichia coli, Proteus sp., Klebsiella sp.
Other bacteria like Pseudomonas, Bacillus, Citrobacter and Serratia may enter infants through broad-spectrum antibiotics.
Responsible viruses include influenza virus (flu), adenoviruses, respiratory syncytial virus (RSV), rhinovirus and parainfluenza virus.
Symptoms
Very fast breathing (with wheezing or grunting sounds) and breathing difficulties are sometimes, the only symptom.
Neonates with pneumonia caused by bacteria commonly fall sick rapidly, beginning with a sudden high fever and extremely fast breathing.
Neonates with pneumonia caused by viruses develop the symptoms more slowly with less severity but wheezing may be more common.
Other possible symptoms include:
Stuffy nose
Cough
Fever
Shaking chills
Chest pain
Vomiting
Abdominal pain (due to coughing and breathing difficulties)
Poor feeding that may cause dehydration
Less activity
In severe cases, greyish or bluish colour of the fingernails and lips
Late-onset hospital-acquired pneumonia leads to unusual worsening of the neonate’s respiratory condition and, more quantities with changed quality of respiratory secretions (such as thick and brown). Newborns may fall severely ill, with neutropenia and unstable temperature.
Pneumonia because of chlamydia can cause ‘pink-eye’ (conjunctivitis) with no fever and mild weakness.
Pneumonia because of whooping cough (pertussis) may cause long coughing spells, blueness due to lack of air, or ‘whoop’ sounds on trying to breathe.
Treatment
Neonatal pneumonia usually requires hospital treatment if it causes breathing problems and a long-lasting high fever, or if the neonates:
Require oxygen therapy
Get a lung infection which might have spread to the bloodstream
Vomit too much to take medicine by mouth
Suffer a chronic disease which affects the immune system
Develop whooping cough
Keep getting pneumonia
Hospital treatment may include:
Intravenous (IV) antibiotics (administered into the vein through a needle)
Respiratory therapy (breathing treatments)
Treatment in the Intensive Care Unit (ICU) in more critical cases
Neonatal pneumonia caused by viruses
Here, antibiotics are not required. Antiviral medicines are now available but are reserved for the flu in early-onset pneumonia.
Neonatal pneumonia caused by bacteria
Its treatment includes:
In early-onset pneumonia, antibiotics are given orally at home. This antimicrobial therapy is similar to that for neonatal sepsis.
For most hospital-acquired late-onset pneumonia, the primary treatment of choice includes Vancomycin along with a broad-spectrum beta-lactam drug, like tazobactam/piperacillin, meropenem, or cefepime. This regimen treats pneumonia (and sepsis too) with the typical hospital-acquired pathogens like P. aeruginosa.
Local patterns of bacterial resistance and infection must always be employed to help conduct empiric choices of antimicrobials. After receiving the sensitivity results, more definite antibiotics are substituted.
Chlamydial pneumonia
It’s treated with either of the following:
Erythromycin (for 14 days)
Azithromycin (for 3 days)
A newborn treated with erythromycin or azithromycin must be monitored for signs of Hypertrophic Pyloric Stenosis (HPS).
0 notes
Text
Analysis of Inflammatory Cytokines in Pre-Diabetic Subjects

Authored by Saket Gupta
Abstract
Objective: Pre-diabetes is defined as either impaired fasting glucose ((IFG) between 5.6 and 6.9mmol/l) or impaired glucose tolerance (IGT) wherein fasting or post-prandial plasma glucose levels are above normal levels, but below that of clinical Type-2-Diabetes Mellitus (T2DM). Both IFG and IGT, risk factors for T2DM and macrovascular diseases, have previously been linked with inflammation. With this in mind, we sought to comparatively evaluate the levels of inflammatory markers in pre-diabetics relative to normal healthy individuals.
Methods: We determined the levels of serum cytokines in a cohort of 9 patients with pre-diabetes and thirty four individuals with normal glucose control using a 96-Well Multi-array 7-plex assay or 1-plex IFN-β and Rantes 96-well plates (Meso Scale Discovery, Gaithersburg, Maryland, USA).
Results: Our study demonstrated that the patient group with pre-diabetes exhibited a non-significant trend towards elevated IL-6, TNFα, IFN-β, IL-12, Rantes, IL-10 and IL-8 when compared with the healthy controls group. After adjustment for age, sex, BMI and WHR, in the study population, there was no difference in the levels of cytokines between the pre-diabetes and normal groups.
Conclusion: Measurement of serum cytokine levels alone is unlikely to be a predictor of clinical disease activity in individuals with pre-diabetes.
Keywords: Pre-diabetes; Inflammation; Pro-inflammatory cytokine
Introduction
Pre-diabetes may be defined as either an impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) wherein fasting or post-prandial plasma glucose levels are above normal levels, but below that of clinical Type-2-Diabetes Mellitus (T2DM) [1]. Elevated haemoglobin A1c, or glycosylated haemoglobin (HbA1c) of 5.7% to 6.4%, as per American Diabetes Association is also denoted as an indicator of pre-diabetes [2]. Together with obesity, both IFG and IGT are risk factors for T2DM and macrovascular diseases. Given this, it is arguable whether screening for pre-diabetes should be undertaken on the premise that the health benefits outweigh the cost implications.
Studies have reported that inflammatory cytokines (IL-1β, IL-6) and C-reactive protein are elevated in patients with T2DM [3,4]. Following on from this, several studies have investigated and reported that pro-inflammatory cytokines are elevated in patients with IGT [5,6] and predicted the progression to T2DM [4]. However, there is paucity of data exploring whether perturbations occur in the level of other cytokines, such as IL-12 and IFN-β and chemokines in the context of pre-diabetes. With this in mind, we analysed a cohort of 43 healthy Irish subjects to evaluate the relationship between pre-diabetes and circulating cytokine levels.
Materials and Methods
Study subjects and design
Thirty four healthy control participants with normal glucose control (NGT) were recruited to the study, as described in our previous study [7]. The healthy control participants were screened for diabetes using the standard oral glucose tolerance test (American Diabetes Association (ADA)) and individuals with abnormal blood results (diabetes or pre-diabetes) and individuals with a history of chronic illness and/or individuals taking medication were excluded from the NGT group. Nine prediabetes participants were recruited to the study. Of the nine pre-diabetes subjects, eight subjects had IFG and one subject had IGT (Table 1) and were diagnosed as having pre-diabetes based on the ADA criteria following an OGTT test (Table 2 [8]). Participants with pre-diabetes were healthy volunteers and were not on any medications. All participants were >18 years old and were not pregnant. Exclusion criteria included the following: Evidence of current infection (white cell count >11 or significantly elevated CRP), current treatment with antibiotics, neutropenia (a leucocyte count of less than 2000 per cubic millimetre), pregnancy or breast-feeding, non diabetic renal disease or liver disease (a level of asparate aminotransferase or alanine aminotransferase of more than three times the upper limit of the normal range), ongoing or previous cancer and the use of oral/inhaled glucocorticoids, immunosuppressive treatment or immunodeficiency. Participants with abnormal results were excluded from the study. Personal and medical data were obtained by patient interview, by using hospital medical notes, and from using hospital blood test results. The demographic information was obtained from research participants and their weight, height, blood pressure & waist and hip circumferences were determined. BMI was calculated as body weight (in kilograms) divided by body height (in meters) squared. Waist-to-hip ratio (WHR) was calculated as waist divided by hip circumference. Informed consent was obtained from all participants and the protocol was approved by the Midlands Research Ethics Committee, Health Service Executive, Ireland, and by the Ethical Review Board, Maynooth University, Maynooth, Ireland.
Biochemical analysis
Following the collection of 40ml of peripheral blood from consenting fasting study participants between 8 and 10am (to minimise the impact of diurnal variation), various biochemical parameters and cytokines were measured. HbA1C was measured using a haemoglobin analyser HA-8160 (Menarini Pharmaceuticals, Ireland). Whole blood counts were measured using an Advia analyser. Lipid profile, urea, creatinine, sodium, potassium, asparate aminotransferase, alanine aminotransferase, bilirubin, alkaline phosphatase, gamma glutamyl transferase, CRP, ferritin, coagulation screen, thyroid profile and plasma glucose levels were measured using a Roche Modular 1800 analyser. An early morning urine sample was also collected for the measurement of ACR.
Cytokine measurements
For cytokine analysis, the blood sample was placed on ice and then centrifuged at 3000rpm for 5min at 4 oC within 1 hour of venesection. Serum was collected and stored at -80 °C until required. Levels of serum TNFα, IL-6, IL-1β, IL-10, IL-8, IL-12p70 and IFN-γ were determined using a 96-Well Multiarray 7-Multiplex Assay (Meso Scale Discovery, Gaithersburg, Maryland 20877, USA). Levels of serum IFN-β and Rantes were determined using single-plex 96-well plates (Meso Scale Discovery, Gaithersburg, Maryland, USA).
Statistical analysis
Data are expressed as median (interquartile range) and represented as box-and-whiskers plots wherein the dark midline in the box represents the distribution’s median value. The top and bottom edges of the box respectively represent the 75th and 25th percentile values. The top and bottom of the vertical lines, the whisker respectively represent the upper and lower maximum value. χ2 test or Fisher exact test (as appropriate) were used to compare proportions. The Mann-Whitney test was used in case of non-normally distributed parameters to compare median between two groups. Data were transformed to log normal format and multifactorial ANOVA was applied to evaluate the effect of variables (age, sex, BMI, WHR and duration of diabetes) on different inflammatory markers. Correlations between values were examined by calculating Spearman correlation coefficients. All the statistical analysis was performed using the Prism 6.0 computer program (GraphPad, La Jolla, CA) and p values less than 0.05 were considered significant.
Results
Participant characteristics
The median age and smoking status was comparable in the pre-diabetes and the normal glucose control study groups. In the NGT group, 20.5% were smokers and 30.7% were ex-smoker, whereas in the pre-diabetes group, 22% were smokers and 28.6% were ex-smoker (Table 1). The pre-diabetes study group exhibited a significantly higher BMI when compared to the NGT control group (p<0.05). However, no significant difference was seen in the WHR and blood pressure between the two groups. The fasting glucose and HbA1c was significantly higher in the prediabetes study group when compared to the NGT study control group (p<0.05). Interestingly, levels of CRP were significantly elevated in the pre-diabetes study group when compared to the NGT study group (p<0.05). However, no significant differences in the lipid profile were observed between the two groups (Table 2).
Analysis of cytokine levels in patients with NGT and IGT
Our data shows a trend towards elevated serum levels of IL- 6, TNFα and IFN-β in the pre-diabetic study study group when compared with healthy control group (Table 3); however, the differences were not statistically significant (Figure 1A & 1B). Further, serum levels of IL-12 were marginally higher in the prediabetic patient group when compared with the normal glucose control study group (Table 3 and Figure 1C). In contrast, a trend towards lower levels of IFN-γ among the pre-diabetic study group when compared with the healthy control group was seen (Figure 1C). Levels of Rantes were marginally higher in the prediabetic study group when compared to the group with normal glucose control (Figure 2A and Table 3). Similarly, serum levels of the chemokines IL-10 and IL-8 were marginally higher in the pre-diabetes participant group when compared with healthy control group (Figure 2B & 2C). Notably, the differences in cytokine levels did not reach statistical significance.
Following multifactorial ANOVA, applied on all cytokines measurements using lognormal values and adjustment for age, sex, BMI and WHR, it was found that there were no significant differences in cytokine levels between the pre-diabetes study group and the group of individuals with normal glucose control.
Discussion
Herein, we sought to establish whether perturbations in inflammatory markers were evident among individuals with pre-diabetes and whether measurement of serum cytokine levels alone may be a predictor of clinical disease activity in individuals with pre-diabetes. To do this, thirty four healthy control participants were recruited to the study and are further described in another study that we undertook [7]. Concurrently, nine pre-diabetes participants were recruited to the study and are described herein. While the prevalence of pre-diabetes is approximately 30% in the general population (UK/USA) [9,10], it was noted that the aforementioned group were generally not captured as a study population presenting to the Endocrinology clinic, Midlands Regional Hospital, Mullingar. Therefore, in terms of the recruitment of pre-diabetes study participants at this location, while eight of the individuals were identified during the course of our efforts to recruit healthy individuals, as described in our previous study [7], one individual was identified following a GP referral. Despite our best efforts and in the context of our strict inclusion/exclusion criteria and ethical approval, it was only possible to recruit a total of nine pre-diabetes individuals to the current study. Notably, treatment-naïve pre-diabetes subjects were required for the study and therefore limited the number of individuals that could be recruited to the pre-diabetes study group. Following recruitment of the study participants, we comparatively assessed the inflammatory milieu of pre-diabetes study participants and healthy individuals. The BMI of the prediabetic study group was significantly higher than that of the healthy control group, as was the fasting glucose and HbA1c levels of the pre-diabetic group. In contrast, the lipid profile of the pre-diabetic study group was comparable to that of the healthy control group.
Regarding the pro-inflammatory cytokine levels, we found that the pre-diabetes study group tended to have elevated levels of the pro-inflammatory cytokines, IL-6, TNFα and IFN-β, when compared with the normal glucose control group. Further, while IL-12 levels was slightly higher in the pre-diabetes study group, IFN-γ levels were lower in the pre-diabetic group when compared to individuals with normal glucose control. In addition, there was a trend towards elevated levels of Rantes, IL-10 and IL-8 in the group with pre-diabetes when compared with the healthy control group. Notably, the differences in cytokine levels among the groups failed to reach statistical significance. Further, after adjustment for age, sex, BMI and WHR, there were no differences in the levels of cytokines between the pre-diabetic and normal study groups
Our findings are in contrast to that previously reported by Spranger et al. [3] who showed that levels of plasma IL-6 and TNFα, but not IL-1β, were associated with future T2DM. Furthermore, the study reported that following adjustment for age, sex, BMI, alcohol consumption, waist-to-hip ratio (WHR), smoking status and HbA1c, IL-6, but not TNFα or IL-1β, was identified as an independent predictor for T2DM [3]. Another study reported an association between elevated levels of IL-6 and CRP and the risk of developing T2DM in the future and the link remained significant following adjustment for BMI, family history of diabetes, smoking, exercise and alcohol use using multivariate analysis [4]. More recently, studies have shown that HbA1c and IL-6 levels were significantly higher in IFG and IGT patients when compared to healthy individuals [11,12].
Conclusion
In summary, our data suggests that the pre-diabetic patient group do not exhibit statistical significant differences in circulating cytokine levels when compared to the normal healthy group. Further, it is noted that multivariate analysis confirmed that there were no significant differences following adjustment for age, sex, BMI and WHR. However, though non-significant, it is noted that our study showed a trend towards elevated levels of serum IL-6, TNFα, IFN-β and IL-8 among the pre-diabetes group. It is plausible to speculate that the robustness of our study would be enhanced by the recruitment and inclusion of additional study participants to the pre-diabetes group. However, as previously indicated, the inclusion of additional pre-diabetes participants was beyond the scope of the current research due to the confined nature of our study criteria.
Importantly, our study shows that the measurement of serum cytokine levels alone in individuals with pre-diabetes may not serve as a robust readout of pre-diabetes state. In conclusion, our study shows that our pre-diabetic group present with a nonsignificant trend towards elevated levels of serum cytokines that may be linked with confounding factors such as BMI, WHR, age and gender. Based on these results, serum cytokine levels alone is unlikely to be a predictor of clinical disease in individuals with pre-diabetes.
To More articles in Current Research in Diabetes & Obesity Journal Please click on: https://juniperpublishers.com/crdoj/index.php
For more about Juniper Publishers please click on: https://juniperpublishers.com/video-articles.php
0 notes
Text
A Rare Cause of Gastrointestinal Bleeding: A Jejunal Dieulafoy’s Lesion- Juniper Publishers
One in a thousand people have an acute gastrointestinal (GI) hemorrhage per year [1]. There are around 300,000 hospitalizations for GI bleeds, costing an estimated $2 billion per year [2-4]. Compared to lower gastrointestinal bleeding (LGIB), upper gastrointestinal bleeding (UGIB) is associated with a much higher mortality rate, with some studies suggesting a 30-day mortality rate of up to 14% [2,3]. A majority of these UGIB (67 - 80%) are attributed to gastric erosions/ulcers 6,17,18. However, of this morbid group of bleeds, a rare (1% or less), yet more serious cause is a Dieulafoy’s lesion (DFL). DFL, is an obscure type of bleeding that can cause life threatening hemorrhages with a mortality rate ranging from 28-67% [5,6].
DFL was first described by MT Gallard [7] in 1884 as a type of aneurysm and later clarified by P. G. Dieulafoy in 1898 who believed this was an early stage of ulceration [7-9]. DFL’s are a collection of large tortuous arterioles of the gastrointestinal vessels. These are often compared to aneurysms, however, DFL’s are caused by genetic malformations rather than degeneration. While the exact mechanism of rupture and subsequent hemorrhage is still poorly understood, several studies have suggested mucosal erosion and ischemic injury, related to aging and/or cardiovascular disease, as possible causes [10]. DFLs are predominantly seen in elderly patients (mean age 69.7 years) though they can be seen in younger patients as well. A vast majority of patients also will have underlying comorbidities such as renal failure, diabetes, or coronary artery disease. Additionally, there have been a few isolated cases associated with chronic immunosuppression whether from underlying malignancies or medication induced [11].
More than 70% of these rare lesions are found in the stomach, usually near the lesser curvature. The discovery of extragastric DFL’s are infrequent, with the duodenum (14%) and colon (5%) being the most common locations [12-14]. The most unusual site is the jejunum, which accounts for 1% of all DFL’s [12-14]. Historically, there have been a few case reports worldwide of jejunal DFL’s, however, of these reported cases, the lesions were found by advanced imaging (CT angiogram or Bleeding scan). We present a case of a jejunal DFL that was unable to be found by advanced imaging but was diagnosed on push enteroscopy.
Assessment
The patient was a 79-year-old African American female with a known history of end stage renal disease and large granular lymphocytic leukemia. Over the course of 8 months, the patient had 3 admissions for gastrointestinal bleeds. She received a total of 16 units of blood with 4 EGDs, 2 colonoscopies, 1 bleeding scan and 1 CT angiogram. Of the listed procedures performed, all were negative for active bleeding and there was no solid evidence of a bleeding source. She was presumed to have bled twice from erosive gastritis and the most recent admission was from an unknown etiology. An outpatient small bowel capsule study was done after these admissions showing no findings.
She subsequently presented to the ER 24 days after her most recent admission for melena and orthostasis, where she was found to have a hemoglobin of 4.4 g/dL (previous hemoglobin was 10.2 g/dL). EGD and colonoscopy did not show any findings or source of bleeding. The patient continued to have melena and required 8 units of blood. A CT angiogram and bleeding scan were again inconclusive. A push enteroscopy was performed on day 6 of admission, showing an actively oozing area in the jejunum with no surrounding ulceration or malformations (Figure 1). Bipolar cauterization and 2 hemoclips were placed ceasing the actively bleeding Dieulafoy’s lesion. The patient’s hemoglobin stabilized, and the melena resolved 1 day after the enteroscopy.
Management
Our patient had a history of large granulocytic lymphocytic leukemia (LGL) and end stage renal disease. Though the usual course of LGL presents with neutropenia and anemia, thrombocytopenia can be seen in up to 20% of cases [15]. Thrombocytopenia combined with immunosuppression from underlying malignancy and ESRD put this patient at an increased risk of developing hemorrhagic complications such as DFLs. On multiple admissions, our patient was found to pancytopenia, likely as a result of her bleeding and immunosuppression. To date, there have been a few case reports citing immunosuppression, immunotherapy, and thrombocytopenia all being associated with GI bleeds due to a DFL [16,17]. While the mechanism is not yet established, it is thought to be related to impaired tissue remodeling and repair.
The current endoscopic modalities to manage a DFL include mechanical treatment (with endoclips or band ligators), injection therapy (with diluted epinephrine), thermal coagulation therapy, or in some cases, a combination of different modalities. Two controlled trials suggested that mechanical hemostasis with endoclips can control acute bleeding and may reduce recurrent bleeds compared to injection therapy alone [18]. There have been no studies comparing the efficacy of thermal coagulation alone or in combination with other methods. A second trial comparing endoclips to injection therapy with epinephrine showed equal rates of initial hemostasis but significantly lower rates of rebreeding in the endoclip arm (0%) vs epinephrine arm (35%) [19].
Post endoscopic management depends on whether the patient is at high risk or low risk for rebleeding. One method used to calculate risk of recurrence is the Glasgow - Blatchford bleeding score (GBS score). A GBS score of 0-1 is considered low risk for rebleeding, and in this case the patient may be discharged from inpatient care with plans for outpatient endoscopic intervention. Inpatient treatment is recommended for patients with GBS scores of 2 or greater [20]. The rate of recurrence of bleed for a Dieulafoy’s lesion ranges from 9-40% [21]. There is a higher rate of recurrence with endoscopic monotherapy compared to combined endoscopic interventions [22]. The rate of rebleeding is not associated with gender or location of DFL or past medical history [23]. Inpatient treatment recommendation is to treat with IV pantoprazole for 72 hours in order to keep gastric pH above 6 in order to maintain intact coagulation process, followed by oral pantoprazole therapy. While mortality is lowest in patients with no significant medical history or comorbidities, overall longterm prognosis of a DFL is favorable once primary hemostasis is achieved [24]. Following push enteroscopy our patient did well without any further complications or signs of rebleeding [25-30].
Conflict of Interest
All authors have read and approved the submission of this manuscript. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part. The authors declare that there is no conflict of interests regarding the publication of this paper
To read more articles in
Journal of Surgery
Please Click on:
https://juniperpublishers.com/oajs/index.php
For More
Open Access Journals
in
Juniper Publishers
Click on: https://juniperpublishers.com/journals.php
#surgery journals#plastic surgery#JuniperPublishers#openacessjournals#Cardiac Surgery Orthopedic Surgery
0 notes
Link
https://www.theauric.com/blogs/news/benefits-of-copper-for-a-human-body
0 notes