#Pre-Exposure Prophylaxis (PrEP) Market
Explore tagged Tumblr posts
Text
1. Au revoir les trottinettes: Paris ban on shared e-scooters takes effect tomorrow
Banned from Paris by popular vote, shared e-scooters will roll for the last time in France's capital on Thursday 31 August, marking the end of five years of their controversial presence. Read more.
2. Tackling Belgium’s drug problem: Legalising cannabis is ‘common sense,’ says Economy Minister
A solution to the drug and security problem in Belgium's bigger cities, such as Brussels and Antwerp, could be legalising the sale and use of cannabis, according to Federal Economy and Employment Minister Pierre-Yves Dermagne. Read more.
3. Cinema tickets for €1 in Brussels and Wallonia in September
From 1-30 September, 21,000 independent cinema tickets will be available for the price of €1 across 34 cinemas in Brussels and Wallonia, as part of this year's edition of J’peux pas, j’ai cinéma. Read more.
4. Will the Belgian property market cool down enough to make buying more affordable?
As interest rates have reached record-high levels, the property market in Belgium has cooled down. The Brussels Times asked Bart van Opstal of the Federation of Notaries what this means for people looking to buy property at the moment. Read more.
5. Harder to trick: New alcohol check procedure to increase chance of being caught
The alcohol breath test procedure currently in place for checks on the road in Belgium will be adapted so that testing is quicker and more efficient for catching offenders. It is expected that the changes will increase the number of people caught. Read more.
6. The price of a healthy life: How to access PrEP in Belgium as an expat
PrEP (pre-exposure prophylaxis) can be a powerful tool in reducing the chances of HIV infection in at-risk groups. When taken as prescribed, it is up to 99% effective at reducing infection. Read more.
7. Open-air party at Rogier denounced as 'a commodification of public space'
The news of a 10-hour marathon electronic dance open-air being organised in the centre of Brussels will be music to the ears of party-goers, but one action group has heavily criticised it. Read more.
2 notes
·
View notes
Text
A federal appeals court panel appeared skeptical on Tuesday of calls to impose a nationwide freeze on Obamacare’s rules for no-cost coverage of preventive care while litigation continues — a move the Biden administration warned would threaten access to a range of services for millions of people on employer-sponsored insurance and Obamacare’s individual market.
Both sides in the case agreed that the individual Texas businesses that sued over the mandate should be shielded from it while the case makes its way through the courts. But they split on whether more harm would be caused by keeping the current coverage rules intact for everyone else in the country or by suspending them nationwide.
Attorneys representing the Texas employers and individual workers challenging the policy argued that because
the United States Preventive Services Task Force is made up of outside experts who are not Senate-confirmed or overseen by Senate-confirmed government employees, their recommendations of what preventive services should be covered by insurance — from syphilis tests to depression screenings — must be “set aside” and can’t be enforced.
Their suit also claims the Obamacare requirement for insurance to cover the pre-exposure prophylaxis pill used to prevent transmission of HIV — known as PrEP — violates the religious rights of the challengers. In their legal briefs, they equated covering the highly effective medication with encouraging homosexuality and promiscuity.
The merits of those legal arguments didn’t come up in Tuesday’s hearing before the 5th U.S. Circuit Court of Appeals in New Orleans, which focused squarely on whether the nationwide freeze of the Obamacare mandate a lower court ordered in March went too far.
That ruling, Justice Department attorney Alisa Klein told the court, was a “legal error” that “extinguished the rights of about 150 million people who are not parties to the case.”
Klein urged the appeals court judges to consider the “balance of equities,” arguing that there would be no harm done to the already-protected plaintiffs by putting the nationwide injunction on hold, but great harm done to everyone else if they failed to do so.
“It can’t be overstated how important the guarantee of cost-free access is when patients go to get their mammograms and colonoscopies,” she said. “We’re talking about 50 different types of care.”
The attorney for the conservative challengers, Jonathan Mitchell, responded that the nationwide ruling was appropriate because “agency actions must be set aside if they are unlawful.”
Mitchell — the architect of the six-week abortion ban Texas imposed before Roe was overturned — attempted to reassure the judges that imposing a nationwide injunction wouldn’t cause harm because insurers are unlikely to drop coverage of preventive care services while the case is still in process.
The judges on the appeals court panel seemed unconvinced.
Leslie Southwick — an appointee of former President George W. Bush — called that assertion “speculative” and said it was “very unusual” to be asked to rule on “our sense of how insurance companies would react.”
“I’m not sure what we have to go on,” he said.
The judges also grilled Mitchell on whether a win for his side would solve his clients’ problem — a legal threshold known as “redressability.” When Mitchell argued that individual workers he represents who are refusing to buy insurance because of the PrEP coverage requirement would be able to get covered if the mandate were lifted, Judge Stephen Higginson noted that the workers’ own affidavits “don’t say that.”
Higginson — an appointee of former President Barack Obama — pointed out that only one of the four workers came anywhere close to making that claim, and that he expressed “a desire to buy insurance, not a specific intent.”
Mitchell acknowledged that there’s no “iron-clad guarantee” his clients would buy health insurance if courts blocked the Obamacare mandate.
In closing, the judges urged both him and the DOJ to try to broker a compromise that would more narrowly tailor the nationwide ruling without infringing on the rights of the plaintiffs.
Tuesday’s hearing was the latest in a months-long saga over the preventive care mandate that’s been in place for more than a decade.
Texas District Court Judge Reed O’Connor — the author of several rulings against pieces of Obamacare — issued a nationwide ruling in March for the challengers, striking down all of the decisions made by the United States Preventive Services Task Force since 2010 about what insurers must cover without cost sharing.
In May, the 5th Circuit Court issued an administrative stay of that lower court ruling — keeping the current coverage rules in place while the case proceeds.
Public health groups warn of serious consequences if O’Connor’s ruling is upheld — citing research showing that even small out-of-pocket costs deter many people from seeking preventive care, leading to sicker patients and more costly treatments. Medical experts are particularly worried that coverage rollbacks would exacerbate already record rates of sexually transmitted diseases by making testing and treatment services unaffordable for vulnerable populations.
Many major insurers have pledged to maintain preventive care at no cost to patients for the time being no matter what courts decide, but experts fear that patients could eventually be hit with out-of-pocket charges should the 5th Circuit and Supreme Court side with the challengers.
The case also throws more than two-dozen new recommendations the federal task force is currently weighing in jeopardy, rules that could expand coverage of everything from prenatal care to speech therapy to osteoporosis.
3 notes
·
View notes
Text
0 notes
Text
Gilead’s Breakthrough HIV Prevention Shot Shows Promise
Source – PinkNews
Successful Trial Results
Gilead Sciences announced a groundbreaking achievement on Thursday: its experimental lenacapavir shot, administered twice-yearly, demonstrated 100% effectiveness in HIV Prevention in a late-stage clinical trial. The interim analysis revealed that none of the approximately 2,000 women who received the shot contracted HIV, prompting the trial’s independent data monitoring committee to recommend unblinding the study and offering lenacapavir to all participants. Those in the control group received standard daily pills.
Market and Investor Response
Following the promising results, Gilead’s stock surged by approximately 7% on Thursday. The pharmaceutical giant views these findings as a significant advancement towards expanding its HIV treatment portfolio and introducing a new form of pre-exposure prophylaxis (PrEP) to the market.
Future Prospects and FDA Approval
Before seeking approval from the FDA, Gilead plans to replicate these results in ongoing Phase 3 studies, including one involving men who have sex with men, with data expected later this year or early next year. If successful, Gilead aims to launch lenacapavir for PrEP as early as late 2025, pending regulatory approval.
Impact on HIV Prevention Landscape
Gilead’s Truvada, introduced over a decade ago, was the first FDA-approved PrEP, revolutionizing HIV prevention with daily pills. However, the market is now shifting towards longer-acting alternatives like injectable shots, which offer convenience and potentially higher adherence rates among users.
PrEP has been shown to reduce the risk of HIV transmission significantly—from sex by 99% and from injected drug use by 74% when taken correctly. Despite its efficacy, adoption rates in the U.S. remain low, with just over one-third of eligible individuals utilizing PrEP, according to the CDC.
Broader Implications and Access Challenges
Health policymakers and advocates welcome longer-acting PrEP options as potential game-changers in combating HIV globally. These alternatives are seen as crucial for individuals who find daily pills impractical or undesirable due to lifestyle factors. Activists urge Gilead to ensure equitable access to lenacapavir, especially in regions with high HIV incidence and limited resources.
Gilead has faced scrutiny over the pricing of its HIV medications, including Descovy, which costs $26,000 annually. In response, the company plans to address access concerns for lenacapavir in affected countries, as part of its commitment to global health equity.
The success of lenacapavir represents a significant milestone in HIV prevention, potentially expanding treatment options and improving outcomes for individuals at risk worldwide. As Gilead advances towards regulatory approval, stakeholders remain optimistic about the prospect of transforming HIV prevention strategies with innovative, long-acting therapies.
Curious to learn more? Explore our articles on Enterprise Wired
0 notes
Photo
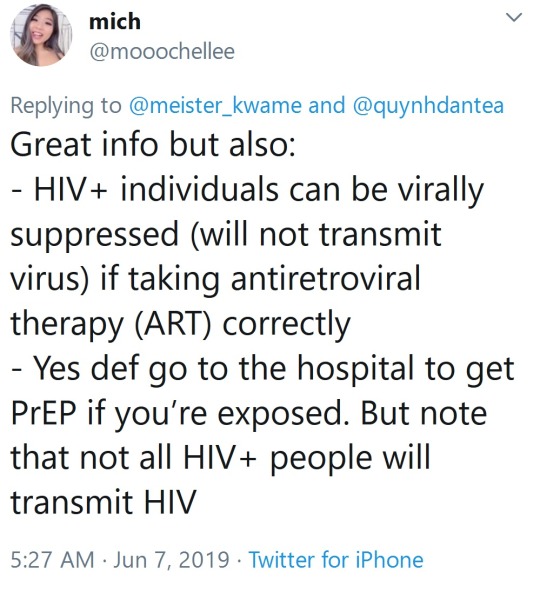
Very important distinction/clarification on the last screenshot:
PrEP is short for Pre-Exposure Prophylaxis and when taken as directed, *before* exposure to HIV, is upwards of 99% effective at preventing you from contracting HIV on its own. There are other methods that can be used in combination to prevent contracting HIV. There are multiple drugs on the market that do this. In the US the only approved dosage is as a once-a-day pill for PrEP. There is a dosage that allows for on-demand PrEP but it is not approved in the US.
PEP (what the thread was initially about) is short for Post-Exposure Prophylaxis and this is what you would take *after* being exposed to HIV.



312K notes
·
View notes
Text
Bridging the Gap: How the Global Prophylactic HIV Drugs Market is Expanding Access to Care
The prophylactic HIV drugs market is witnessing significant expansion, with robust growth projected in the coming decade. In 2023, the market size reached an estimated USD 32,516.1 million, driven by increased investment in HIV research and development, rising drug approvals, and a surge in HIV incidence rates.
According to Future Market Insights, the market is forecasted to expand at a Compound Annual Growth Rate (CAGR) of 4.1% between 2023 and 2033, reaching a valuation of approximately USD 48,822.4 million by 2033. This growth trajectory underscores the critical importance of prophylactic HIV drugs in combating the global HIV/AIDS epidemic.
Request Your Detailed Report Sample With Your Work Email: https://www.futuremarketinsights.com/reports/sample/rep-gb-9582
Global sales of HIV prophylaxis drugs are projected to reach a value of about USD30 billion by 2021. Owing to growing awareness of HIV and the rising prevalence of HIV infection worldwide, As for AIDS prevention, between 2022 and 2032, the market for preventative HIV drugs grew at a compound annual growth rate (CAGR) of 4%, reaching USD 40 billion in 2028.
HIV has been a major global cause of death for many years, impacting millions of people. People whose immune systems are compromised by the virus are more susceptible to a range of ailments and cancers. HIV can be controlled even when there isn’t a long-term cure by expanding access to adequate care, diagnosis, medication, and prevention.
Prophylactic HIV Drugs: A Powerful Prevention Tool
Effective management techniques are available for HIV, even though a long-term cure is still unattainable. One of the most important aspects of fighting the virus is expanding access to care, diagnosis, treatment, and prevention services. HIV prevention medications are becoming an essential weapon in the fight against HIV infection. These cutting-edge treatments work especially well at preventing HIV transmission through drug injection and sexual contact.
Focus on Pre-Exposure Prophylaxis (PrEP):
Pre-Exposure Prophylaxis (PrEP), one of the preventive HIV medications, is becoming increasingly popular because of its great effectiveness. The risk of HIV infection from sex and injectable drug use can be considerably decreased with PrEP, according to the Centers for Disease Control and Prevention (CDC), by 74% and 99%, respectively. The extraordinary efficacy of PrEP is spearheading a global movement for its expanded usage.
Market Competition:
Some of the prominent players operating in the global market are-
Gilead Sciences, Inc
Merck Sharp & Dohme Corp.
Merck & Co. Inc.
Mylan NV
Cipla Inc.
Genentech Inc.
Bristol-Myers Squibb Company
Johnson & Johnson Health Care Systems Inc
Pfizer Inc.
GalaxoSmithKline PLC
Notable Developments of the Key Players in the Market
In April 2023, Merck & Co (MRK.N) confirmed that it is going to buy Prometheus Biosciences Inc (RXDX.O) for about $10.8 billion, by picking up a promising experimental treatment for ulcerative colitis and Crohn’s disease and building up its presence in immunology.
In November 2022, Merck, known as MSD outside the United States and Canada, and Imago BioSciences, Inc. (“Imago”) announced that the companies have entered into a definitive agreement under which Merck, through a subsidiary, might acquire Imago for US$ 36.00 per share in cash for around total equity value of US$ 1.35 billion.
Key Companies Profiled:
Gilead Sciences, Inc
Merck Sharp & Dohme Corp.
Merck & Co. Inc.
Mylan NV
Cipla Inc.
Genentech Inc.
Bristol-Myers Squibb Company
Johnson & Johnson Health Care Systems Inc
Pfizer Inc.
GalaxoSmithKline PLC
Key Segments Profiled in the Prophylactic HIV Drugs Industry Survey:
By Drug Class:
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTI)
Integrase Inhibitor
By Distributional Channel:
Hospital Pharmacies
Retail Pharmacies
Mail Order Pharmacies
Drug Stores
By Region:
North America
Latin America
Western Europe
Eastern Europe
Asia Pacific Excluding Japan
Japan
The Middle East and Africa
0 notes
Text
Breaking Barriers: The Ongoing Fight Against HIV Type-1 - A Look into the Market for Hope and Progress! 🌈💪
Hello, Tumblr community! Let's discuss the ongoing efforts in the battle against HIV Type-1 and the incredible progress in the market dedicated to providing hope, treatment, and support for those affected. 🌍🏥
💡 What's the buzz all about? The HIV Type-1 Market is all about advancing research, treatment, and awareness to combat one of the most significant public health challenges of our time. It's a market with a mission! 💊🔬
Here's why it's essential to stay informed:
1️⃣ Lifesaving Medications: The market offers antiretroviral drugs that help manage HIV, enabling those affected to lead healthy lives. 2️⃣ Prevention Strategies: Ongoing research focuses on developing new prevention methods, including vaccines and PrEP. 3️⃣ Community Support: Advocacy and awareness campaigns work tirelessly to break down stigmas and offer support.
🌟 Leading Innovations: 🔍 Antiretroviral Therapy (ART): These drugs suppress the virus and help prevent the progression of HIV. 💉 Pre-Exposure Prophylaxis (PrEP): An innovative approach for high-risk individuals to prevent HIV transmission. 🌈 Supportive Communities: Non-profits, clinics, and organizations offer vital support to those affected by HIV.
💖 The future is filled with hope! As technology and research advance, the HIV Type-1 Market is poised to make even greater strides in the fight against this virus, bringing us closer to a world without HIV. Let's celebrate the tireless work of healthcare professionals, researchers, and advocates dedicated to this mission. 🌈💕💪
Join the conversation, raise awareness, and show your support for the ongoing battle against HIV Type-1. Together, we can make a difference and stand with those affected by this virus. 🏳️🌈❤️🔬
0 notes
Text
Exploring HIV Treatment: Revealing Antiretroviral Medications, Obstacles, and Emerging Directions
Antiretroviral therapies (ART) have revolutionized the landscape of HIV treatment, offering a beacon of hope for individuals living with the virus. These medications effectively inhibit HIV replication, allowing for longer, healthier lives. Continuous advancements in ART have made managing HIV a journey marked by hope and progress.
Write to us at [email protected] Learn how GRG Health is helping clients gather more in-depth market-level information on such topics
Achieving Effective Viral Suppression: A Cornerstone of ART
The primary objective of ART is to attain and sustain viral suppression. By reducing the viral load to undetectable levels, ART not only enhances the quality of life for those with HIV but also prevents viral transmission to others. This dual benefit has spurred global efforts to expand ART accessibility.
Navigating Challenges: Adherence and Resistance
Despite the transformative impact of ART, challenges persist. Strict adherence to the medication regimen is crucial for success. Skipping doses or inconsistent use can lead to viral resistance, wherein the virus mutates and becomes resistant to the drugs. Overcoming these hurdles demands ongoing support, education, and personalized treatment plans.
Stigma and Access: Barriers to Receiving Care
The stigma surrounding HIV remains a significant obstacle to treatment access. Fear of discrimination and a lack of awareness can deter individuals from seeking diagnosis and treatment. Furthermore, unequal access to ART in various regions underscores the need for global initiatives to improve availability and affordability.
Synergistic Approaches: Augmenting Effectiveness
Combination therapies, also known as antiretroviral regimens, are central to contemporary HIV treatment. These regimens integrate multiple antiretroviral drugs to target the virus at various stages of its life cycle. This strategy enhances treatment effectiveness, minimizes the risk of resistance, and offers more flexible dosing options.
Future Prospects: A Beacon of Hope
The future of HIV treatment is characterized by promising trends that inspire optimism for enhanced care and outcomes. Long-acting injectable ART, requiring fewer doses while maintaining viral suppression, is poised to be a game-changer. Additionally, advancements in prevention strategies, such as pre-exposure prophylaxis (PrEP), contribute to global efforts to reduce new infections.
Holistic Care: Beyond Medications
While antiretroviral therapies are pivotal, comprehensive care is essential for individuals with HIV. This encompasses regular medical monitoring, mental health support, nutritional guidance, and addressing co-existing conditions. A holistic approach ensures that individuals attain optimal health and well-being.
Conclusion: A Journey of Resilience, Progress, and Hope
The journey of navigating HIV treatment is one marked by resilience, progress, and hope. Antiretroviral therapies have transformed the lives of those affected by HIV, providing effective viral suppression and prevention. Although challenges persist, advancements in treatment and prevention strategies, coupled with a growing commitment to education and awareness, pave the way for a future where HIV can be managed confidently and optimistically.
0 notes
Text
In its activism and agitation, ACT UP was often fighting against inherent biological limits as much as governmental policies. No amount of rage and public awareness could by themselves cure a disease. To this day, despite decades of well-funded research, there is still no vaccine for HIV-AIDS. The drugs have gotten more sophisticated, but in principle, the treatment remains the same as it was with AZT: AIDS is still treated as a chronic condition to be managed with antiretrovirals.
With the appearance of Pre-Exposure Prophylaxis, or PrEP, in 2012, it became common to prescribe antiretrovirals not just for AIDS patients, but also as a daily preventive measure for healthy sexually active gay men. This trend of medicalizing the healthy hasn’t been unique to homosexuals, of course. As the physician and critic of medicine Seamus O’Mahony has written, “Pharma’s single greatest idea was to move its focus from the sick to the well, thus creating vast new markets of ‘patients’ requiring lifetime treatment with drugs.” For example, healthy people can be redefined as being at risk of future disease based on measures like blood pressure or cholesterol and then prescribed medication they must take for the rest of their lives to keep the risk at bay.
The increased medicalization of everyday life in recent decades hasn’t been a purely top-down process. However unwittingly, AIDS activists played a part in bringing it about. ACT UP provided a model for patients organizing themselves into a political constituency to raise awareness and demand drugs for their condition. Pharma is an enormously powerful industry, but by funding patient-support groups, it can present itself as the voice of the powerless.
During the Covid pandemic, many activists demanded that medicine and public health be accorded massive power over our daily lives. Notable among them was Yale epidemiologist and public intellectual Gregg Gonsalves, a veteran of ACT UP. As a young man, Gonsalves spoke of the need for activists to prod science in the right direction on AIDS research. More recently, he became a vociferous advocate of mask and vaccine mandates to fight Covid.
But nowhere is ACT UP’s legacy more apparent than in the relationship between contemporary transgender activism and transgender medicine. In this politically charged domain, the division between science and activism has broken down almost completely. Activists have managed to get the medical establishment to adopt the so-called affirmative model for gender dysphoria. According to this model, if patients experience distress about their secondary sex characteristics, the role of medical professionals is to affirm them as having a transgender identity and put them on path to hormones and surgeries.
In their rhetoric and style, trans activists bear a close resemblance to ACT UP. Both groups have argued that withholding access to experimental drugs amounts to deliberate murder, and the ubiquitous talk of “trans genocide” echoes ACT UP’s rhetoric from decades ago. Trans activists, like AIDS activists in their day, see themselves as a radically oppositional force, but their demands happen to coincide with the interests of a pharmaceutical industry always seeking out new groups of lifelong customers and novel rationales for loosening regulatory oversight.
In one crucial respect, trans activism goes well beyond ACT UP’s work. While Kramer and others exaggerated the dangers of AIDS to the population at large, it was certainly true that many people were dying or desperately sick of the disease in the group’s heyday. By contrast, trans activists’ claims about looming death are almost entirely a rhetorical tactic. We see this, for example, in the routine claim that dysphoric youth will all kill themselves if they aren’t allowed to transition. As a statistic, this is a fabrication, but suicidal ideation is contagious; by endlessly repeating it, activists make it more likely to be true. The assertion is better understood as a threat than a statement of fact.
0 notes
Text
United States Pre-Exposure Prophylaxis Market to Grow with an Impressive CAGR until 2026 | TechSci Research
Increasing awareness about HIV/AIDS is driving the growth of United States Pre-Exposure Prophylaxis (PrEP) Market, in the forecast period, 2022-2026.

According to TechSci report on, “United States Pre-Exposure Prophylaxis (PrEP) Market By Drug Type (Truvada v/s Descovy), By Route of Administration (Oral v/s Topical), By Region, Competition Forecast & Opportunities, 2026”, United States pre-exposure prophylaxis (PrEP) market has shown promising growth in historical years until 2020 and is expected to continue its growth in upcoming forecast years 2021 to 2026. The United States pre-exposure prophylaxis (PrEP) market owes its growth to the factors like growing awareness about precaution against HIV viral infection and preventive therapeutics.
The surging demand for the effective precautionary methods or therapeutic products like PrEP drugs for the patients under the risk of contracting HIV infection in future is driving the growth of the United States pre-exposure prophylaxis (PrEP) market in the upcoming five years.
Also, rapidly increasing instances of HIV among the MSM population of the country have scared the population and the government of the country to a large extent thus the population is inclined toward regular checkups and diagnostic tests that can help in deciding the population for the preventive treatment thus supporting the growth of the United States pre-exposure prophylaxis (PrEP) market in the next five years.
Browse over XX market data Figures spread through 70 Pages and an in-depth TOC on "United States Pre-exposure prophylaxis (PrEP) Market"
https://www.techsciresearch.com/report/united-states-pre-exposure-prophylaxis-market/8124.html
Additionally, increasing MSM population of the country is further substantiating the growth of the United States pre-exposure prophylaxis (PrEP) market in the future five years. The MSM population is highly susceptible to HIV infectious diseases and are supporting the market growth in the future. Private fundings, and governmental aids with favorable schemes are supporting the robust growth of the United States pre-exposure prophylaxis (PrEP) market in the forecast period.
The United States pre-exposure prophylaxis (PrEP) market segmentation is based on drug type, route of administration, competitional landscape, and regional distribution. Based on drug type, the market is further bifurcated into Truvada and Descovy. Truvada is anticipated to hold the largest revenue shares of the market and dominate the market segment in the upcoming five years on the account of its high adoption and increased demand since the drug is marketed since early 2019.
Also, higher efficiency and reduced risk of acquiring HIV/AIDS infection comparatively is anticipated to support the growth of the Descovy drug type and thus support the growth of the United States pre-exposure prophylaxis (PrEP) market in the next five years. Truvada is a combination of two drugs, tenofovir disoproxil and emtricitabine. Truvada is a fixed dose of combination of antiretroviral medication that has proven effect on the treatment and prevention of HIV virus.
Download Sample Report @ https://www.techsciresearch.com/sample-report.aspx?cid=8124
Customers can also request for 10% free customization on this report.
The drug is administered along with the recommendation of following safer sex practices thus increasing awareness among the population. Tenofovir alafenamide is available in the market under the brand name of Descovy. It is a Hepatitis B virus inhibitor that also acts against HIV viral infection.
On the basis of route of administration, the market segments are defined as oral, and topical. Oral administration of the drugs in anticipated to hold the largest revenue shares of the market and dominate the market segment in the next five years on the account of availability of the drug in pill form.
Also, oral administration is more convenient for daily/ regular administration. Maintaining the dosage is also favorable for oral route of administration and thus supports the growth of the sub-segment and drives the growth of the United States pre-exposure prophylaxis (PrEP) market in the upcoming five years.
A partial list of major market players of the United States pre-exposure prophylaxis (PrEP) market includes:
Gilead Sciences, Inc.
Teva Pharmaceuticals USA, Inc.
Merck & Co., Inc.
Cipla Inc
Bristol-Myers Squibb Company
Genentech, Inc
These market players along with new market entrants, are focused on extensive research and bringing innovative and advanced products. Technologically advanced manufacturing of the pharmaceutical products and therapeutic processes would aid in decreasing the cost of the production and aid the players in managing their investments. Moreover, initiatives from the government and private fundings would support the United States pre-exposure prophylaxis (PrEP) market growth indirectly.
Press Release: https://www.techsciresearch.com/news/6705-united-states-pre-exposure-prophylaxis-market.html
“Young population in the United States is getting highly aware of the increasing instances of HIV virus and their awareness is boosting the growth of the United States pre-exposure prophylaxis (PrEP) market in the upcoming five years. Increasing research and innovative therapeutic product development is further driving the growth of the United States pre-exposure prophylaxis (PrEP) market in the future five years. Through researched studies and surveys authoritative bodies are creating awareness among black and Hispanic population about them being more susceptible to HIV infection and further aiding the market growth in next five years,” said Mr. Karan Chechi, Research Director with TechSci Research, a research based global management consulting firm.
“United States Pre-Exposure Prophylaxis (PrEP) Market By Drug Type (Truvada v/s Descovy), By Route of Administration (Oral v/s Topical), By Region, Competition Forecast & Opportunities, 2026” has evaluated the future growth potential of United States pre-exposure prophylaxis (PrEP) market and provides statistics & information on market size, structure and future market growth. The report intends to provide cutting-edge market intelligence and help decision makers take sound investment decisions. Besides, the report also identifies and analyzes the emerging trends along with essential drivers, challenges, and opportunities in United States pre-exposure prophylaxis (PrEP) market.
Browse Related Projects:
United States Smart Hospitals Market By Component (Hardware, Software System, Services) By Technology (Cloud Computing, Artificial Intelligence, Wearable Technologies, Others) By Connectivity (Wired v/s Wireless) By Application (Remote Medicine Management, Medical Connected Imaging, Medical Assistance, Electronic Health Record, Others) By Services Offered (General Services, Specialty, Super Specialty), By Region, Company Forecast & Opportunities, 2026
https://www.techsciresearch.com/report/united-states-smart-hospitals-market/8037.html
Vietnam Telemedicine Market By Component (Services, Software, Hardware), By Deployment Mode (Cloud v/s On-Premises), By Type (Tele-Hospitals, mHealth, Tele-Homes), By Technology (Store & Forward, Real Time, Others), By Application (Tele-Psychiatry, General Consultations, Tele-Radiology, Tele-Pathology, Others), By End User (Payer, Provider, Patients), By Region, Competition Forecast & Opportunities, 2026
https://www.techsciresearch.com/report/vietnam-telemedicine-market/7899.html
Contact
Mr. Ken Mathews
708 Third Avenue,
Manhattan, NY,
New York – 10017
Tel: +1-646-360-1656
Email: [email protected]
Website: https://www.techsciresearch.com/
For More Market Research Blogs Visit: https://techsciblog.com/
#TechSci#United States Pre-Exposure Prophylaxis (PrEP) Market#Healthcare#Market Research Reports#Pre-Exposure Prophylaxis (PrEP) Market#US Pre-Exposure Prophylaxis (PrEP) Market Size#Medical Devices#Consumer Healthcare#US Pre-Exposure Prophylaxis (PrEP) Market Forecast
0 notes
Link
When PrEP—or pre-exposure prophylaxis, a daily pill that protects against HIV—came on the U.S. market in 2012, it captured the attention primarily of men who have sex with men. According to the Food and Drug Administration, the first PrEP pill, Truvada, provides over 90 percent protection from sexually acquiring HIV when taken daily. Logically, this is a boon to women who may not be able to insist on condoms or prefer not to use them. So why did the FDA recently approve a new PrEP pill without approving it for cisgender women’s use?
Truvada isn’t cheap. Without insurance or other cost breaks, one month’s worth costs about $1,800 in the United States. The price is much lower in countries where essential medicines’ prices are capped by government.
Given its cost and the fact that Truvada was marketed primarily to gay men, it is not surprising that 95 percent of Americans using PrEP in 2016 (the last year data was available) were men. More than two thirds (69 percent) of U.S. PrEP users were white, over half (56 percent) lived in a Western or Northeastern state, and 81 percent of all PrEP prescriptions were covered by commercial insurance. In other words, PrEP was (and still is) mostly used by affluent white men.
When PrEP was being developed more than a decade ago, two major clinical trials were conducted to assess its effectiveness: one enrolling 4,758 heterosexual couples and one enrolling 2,499 HIV-negative gay men and transgender women. High levels of protection were achieved in both trials. Findings confirmed that Truvada is highly effective for cis women, even though it may be slightly “less forgiving” than for cis men if doses are missed. Research shows that one of the two components in Truvada is not absorbed by vaginal tissue as readily as by rectal tissue. Missing doses can cause the level of this drug to drop in the vagina, possibly reducing the user’s protection. So consistent, daily use of Truvada is more important for cis women to ensure full protection.
read more
47 notes
·
View notes
Link
There’s a very successful little blue pill on the market. It’s highly effective, it benefits thousands of people, and it can transform your love life. No, it’s not Viagra. It’s Truvada, also known as pre-exposure prophylaxis (PrEP), and it is 99 percent effective at preventing HIV infections.
PrEP is so good it has almost eradicated new HIV infections in Australia and helped New York achieve historic lows in HIV diagnoses. Now, doctors and policy makers are working to expand access to the drug and to refine delivery methods that are easier and more personalized for users’ needs. “There’s a lot of interest in what we can do with PrEP to meet consumers where they are, find the right formulation for each type of consumer,” says John Brooks, a senior medical adviser at the Centers for Disease Control and Prevention’s Division of HIV/AIDS Prevention...
61 notes
·
View notes
Link
Pharmacies at Walmart stores, Sam’s Clubs and Albertsons Companies will donate their dispensing services to people in the federal HIV prevention program “Ready, Set, PrEP,” according to an announcement by the Department of Health & Human Services (HHS), which oversees the program. This means the pharmacies will refill participants’ prescriptions for free.
Currently, the Food and Drug administration has approved two forms of pre-exposure prophylaxis, or PrEP: Truvada and Descovy. Both are highly effective regimens of daily tablets and are manufactured by Gilead Sciences. (For more details, see “What’s the Difference Between Truvada and Descovy for PrEP?”)
Walmart stores and Sam’s Clubs operate over 5,000 pharmacies, and Albertsons Companies includes pharmacies in the supermarkets Albertsons, Safeway, Vons, Jewel-Osco, Shaw’s, Acme, Tom Thumb, Randalls, United Supermarkets, Pavilions, Star Market, Haggen, Amigos United, Market Street United and Carrs.
#Department of Health and Human Services#HIV#Gilead Sciences#Descovy#PrEP#Truvada#Walmart#prevention#health
0 notes
Photo

"Pre-exposure prophylaxis, also known as PrEP, is a treatment for those who have a high risk of getting HIV by using intravenous drugs or intercourse. The Centers for Disease Control (CDC) recommends this as part of their HIV preventive approach. It is available only as a cure in several places. The FDA-approved PrEP drug, which is marketed under the trade names Truvada and Descovy, combines the drugs tenofovir and emtricitabine and must be properly administered to prevent HIV from taking root in your body. Click here (https://asphealth.co/blogs/prep-for-hiv-prevention-and-how-it-works-and-its-side-effects/) to read more about it in detail."
0 notes
Text
HIV & AIDS & QUEER POLITICS
Queerness is now global. Many arising economies are experiencing queer mobilization and sexual identity politics raising fundamental issues of citizenship and human rights on the one hand. Communist countries like China has its own queer movement or queer underground movement as we had in America (Stone Wall Movement). Along with this change, there are also people wanting cultural identity, family values and traditions to be kept as is. While some researchers contend that with economic globalization in the developing world, a Western, hegemonic concept of lesbian, gay, bisexual and transgender (LGBT) has played a huge factor in mobilizing queer society onto less popular culture through media, and other platforms. Now, there is global queer mobilization and sexual identity politics. Queerness was not broadcasted in the 1950s as openly, or as proudly. It was always cast through agenda and propaganda to shape and reconstruct peoples perception. Now, Queerness is being shared in broadcasting, advertising, shows, Broadway, film, performing arts, the internet or the political debates of human rights in emerging governments, images of LGBTQ position, experiences, practices, and opinions now circulate around the world.
When it comes to the culture phenomenon of MTVs hit show, we are moving toward an inclusive environment. The award winning show WildN'Out casted many members from the LGBQT community as of season 12. Lauren Flan, Jason Lee, Jaywill, Kandie. According to the producer Nick Cannon, this was in demand by the viewers. This political, cultural, and media changed happened because of the request of the audience. This goes to show, unlike the 1950's we have the remote in our hands. We have the power to control, and we have the power to change.
However, when it comes to such epidemic like HIV & AIDS, there is prevention method taking place, opposed to treatment since HIV & AIDS are still incurable. According to the CDC, "Pre-exposure prophylaxis (or PrEP) is when people at very high risk for HIV take HIV medicines daily to lower their chances of getting infected." This medication contains tenofovir and emtricitabine which are used to treat HIV's. But instead of treating it, this can also be used to prevent the disease. This is being administered and marketed to the LGBQT community because this creates antibodies in the body to fight off, and to prevent such diseases from harboring. This measure is crucial because gay and bi-sexual men are at high risk when it comes to HIV & AIDS.
According to www.hiv.org in 2016
"Gay and bisexual men are the population most affected by HIV.
Gay and bisexual men accounted for 67% (26,570) of all diagnoses and 83% of HIV diagnoses among males.
Black/African American gay and bisexual men accounted for the largest number of HIV diagnoses (10,223), followed by Hispanic/Latino (7,425) and white (7,390) gay and bisexual men.
Among all gay and bisexual men, trends have varied by race and over time. From 2011 to 2015:
Among white gay and bisexual men, diagnoses decreased 0%.
Among Hispanic/Latino gay and bisexual men, diagnoses increased 14%.
Among African American gay and bisexual men, diagnoses increased 4%.
After years of sharp increases, diagnoses among young African American gay and bisexual men (aged 13-24) stayed about the same."
3 notes
·
View notes
Text
United States PrEP Market Gaining Momentum—Projected to Grow at a CAGR of 15.4% by 2027
Over the forecast period (2021-2027), United States PrEP (Pre-Exposure Prophylaxis) market is expected to be driven by the increasing rate of generic drug approvals and decreasing treatment costs…
A study recently conducted by the strategic consulting and market research firm, BlueWeave Consulting, revealed that United States PrEP (Pre-Exposure Prophylaxis) market was worth USD 5,774.0 Million in 2020. According to the report, the market is projected to grow at a CAGR of 15.40% during the forecast period (2021-2027) to reach the valuation of USD 15,515.2 Million by 2027.The growth of the market is attributable to the innovations taking in this field. Lupin, for example, announced the launch of Emtricitabine with Tenofovir Disoproxil Fumarate Tablets in July 2021. Gilead Sciences, Inc.'s Truvada Tablets 200 mg/300 mg generic equivalents are Emtricitabine and Tenofovir Disoproxil Fumarate Tablets 200 mg/300 mg. Furthermore, many generic versions of PrEP drugs have been approved, which has proliferated its sale in the United States, thereby, driving the United States PrEP (Pre-Exposure Prophylaxis) market.
Growing Government Initiatives for Free Medication of HIV is Driving the Market Growth
Since the WHO recognizes HIV as a global pandemic that has caused many deaths in the United States, it has compelled the government of the United States to update medical infrastructure and improve HIV regulations. The US Department of Health and Human Services' Ending the HIV Epidemic (EHE) in the United States mission aims to reduce new HIV infections by 90% by 2030, with a target of fewer than 3,000 per year.
For instance, the US Health Resources and Services Administration (HRSA) announced USD 99 million in 2021 for implementing the EHE campaign in the U.S. with Ryan White's HIV programs to improve the healthcare infrastructure, including HIV care, treatment, medication, and essential support services. In 2019, Gilead Sciences announced that they are likely to donate Truvada for PrEP to the US CDC to support the government initiative for End the HIV Epidemic. Gilead Sciences announced that they would provide 2.4 million bottles of Truvada to the CDC annually for uninsured citizens of the U.S. Thus, growing government initiatives for the free medication of HIV have propelled the growth of the United States PrEP market.
Increasing Population of Lesbian Gay Bisexual Transgender (LGBT) and Adoption by Society Represent a Lucrative Opportunity
Nearly 70% of the people living with HIV are bisexual men or homosexuals. This is generally because contracting HIV through anal sex is ten times more likely than through vaginal contact. Thus, anal sex has a higher transmission rate, which means that the chances of contracting HIV increase in bisexual men or homosexuals.
LGBT awareness has grown to a point where the younger generation is more likely to identify as LGBT than heterosexual. The latest update on gay, lesbian, transgender, bisexual finds that 5.6% of the U.S. population (mainly adults) identifies as LGBT in 2020. This includes one in six adult members of generation Z.
Thus, the rising population of LGBT and their acceptance by society has led key players to develop more effective drugs.
Request For Free Sample Report @ Click here
Impact of COVID-19 on United States PrEP (Pre-Exposure Prophylaxis) Market
As a result of the COVID-19 pandemic, the U.S. and global economies experienced substantial volatility and uncertainty in the early months of 2020, increasing operational risks. The manufacturing industry has been profoundly affected in multiple industries. Clinical trials have been adversely affected by the pandemic, including their ability to be completed on time. Some clinical trial sites had to implement restrictions on patient visits during ongoing trials to reduce the risk of exposure to the deadly virus. Additionally, the companies found it difficult to maintain participant adherence to the clinical trial schedules because of quarantines, travel restrictions, and healthcare disruptions. As a consequence of the clinical trial sites remaining open, there was also a risk of biased data collection. In light of these challenges, manufacturers failed to meet predicted filing and marketing timelines for certain products.
United States PrEP (Pre-Exposure Prophylaxis) Market: Competitive Landscape
The United States PrEP market is highly consolidated. The rising prevalence of HIV cases in men and women, regulatory approvals, and the global HIV pandemic has aided the firms in launching new medications and clinical studies. Gilead Science Inc., Teva Pharmaceuticals Industries Ltd., and others are among the leading market competitors in the US PrEP industry.
In June 2021, Lupin announced the launch of Emtricitabine and Tenofovir Disoproxil Fumarate Tablets after receiving approval from the United States Food and Drug Administration (FDA). Emtricitabine and Tenofovir Disoproxil Fumarate Tablets 200 mg/300 mg are the generic equivalents of Gilead Sciences, Inc.'s Truvada Tablets 200 mg/300 mg. They are used to treat HIV-1 infection in combination with other antiretroviral drugs. These are also used for pre-exposure prophylaxis (PrEP), which lowers the risk of HIV-1 infection acquired through sexual contact. In the United States, Emtricitabine and Tenofovir Disoproxil Fumarate Tablets (RLD: Truvada) had yearly sales of USD 2.1 billion. (IQVIA MAT March 2021).
Don’t miss the business opportunity of the United States PrEP market. Consult our analysts to gain crucial insights and facilitate your business growth.
The report's in-depth analysis provides information about growth potential, upcoming trends, and the United States PrEP market statistics. It also highlights the factors driving forecasts of total market size. Furthermore, the report also analyzes the growth drivers, challenges, and competitive dynamics of the market. The report promises to provide recent technology trends in theUnited States PrEP market, along with industry insights, to help decision-makers make sound strategic decisions.
About Us
BlueWeave Consulting provides all-inclusive Market Intelligence (MI) Solutions to businesses regarding various products and services online & offline. We offer comprehensive market research reports by analyzing both qualitative and quantitative data to boost up the performance of your business solution. BWC has built its reputation from the scratches by delivering quality inputs and nourishing long-lasting relationships with its clients. We are a promising digital MI solutions company providing agile assistance to make your business endeavors successful.
0 notes