#Petroleum Testing manufacturer
Explore tagged Tumblr posts
Text
Color Tester
Labtron Color Tester typically features measurement of Lab, RGB, and HEX values, an LED light source, a touchscreen display, USB/Bluetooth connectivity, and a portable, battery-operated design. Specifications include an accuracy of ΔE < 1.0, a measurement range of 0-100 for L, -128 to +127 for a* and b*, and repeatability of ΔE < 0.2.

1 note
·
View note
Text
Acid Number and Acidity Tester
Labtron Acid Number and Acidity Tester is a compliant for precise acidity testing of petroleum products. With dimensions of 830 x 290 x 370 mm, it fits compactly into any lab space. Its adjustable heating power ensures accurate results in ambient temperatures up to 35°C and humidity ≤ 85%. Easy to install and operate, ideal for streamlined testing processes.

#Petroleum Testing#Buy Petroleum Testing#Petroleum Testing Suppliers#Petroleum Testing Manufacturers#Automatic Kinematic Viscometer
1 note
·
View note
Text



Adventuresses We Love – Bertha Benz Adventuress Bertha Benz was a woman with a vision, one she shared with her husband, Carl – to invent a practical “horseless carriage.” She believed in this vision so much that two years before their wedding, she used her dowry to bail out his failing company and invest in its future. The two of them would collaborate on the design and engineering of the car’s components, including its two-stroke engine, throughout the vehicle’s development. Progress was slow, but steady, and on New Year’s Eve1879 they finally got their engine to work. They continued to make improvements to the vehicle, until finally, in early 1886, Carl obtained a patent for their “motor car with gas engine operation.” The car made its public debut in Mannheim that summer, where… …nobody wanted it. There had been a few cars built before the Benz’s, enough to make everyone really, really nervous about them. Even the Vatican had spoken out against them, declaring the automobile to be a devil’s or witch’s carriage. Some localities in Germany had already outlawed the use of such vehicles, threatening to fine anyone operating them. Now, Carl was an engineering marvel, but a complete dunce at marketing. It would fall to Bertha to win people’s hearts and minds, and change the view of the automobile in their eyes. To accomplish this, she knew exactly what she had to do. She had to go visit her mom. At dawn on August 5, 1888, Bertha and her sons, Robert and Eugen, left Mannheim and headed towards Pforzheim, about 60 miles away. Today, we might not think of that as any big deal – that’s not much more than my commute to work – but in 1888, it was an Epic Road Trip. The first of its kind! The trip was fraught with challenges, not the least of which was that the car got 25mpg – but only carried about 1.3 gallons of fuel. To resolve this, Bertha bought the entire supply of ligroin – a petroleum-based cleaner – from a chemist in Wiesloch and used that to power the car. (The chemist’s in Wiesloch is still today recognized as the world’s first service station.) Bertha also found innovative solutions for some of the mechanical failures the car ran into on the way. For example, she used her hat pin to clear a clogged fuel line, and her garter to insulate a frayed spark plug wire. When the wooden blocks used in the brakes started to wear out, she stopped at a cobbler’s shop and had leather added to them – thereby inventing the world’s first brake pads. Another challenge – hills. The car had two gears – not quite enough to summit some of the hills on the route. Robert and Eugen got out and pushed it up a couple of them. Finally, after 13 hours on the road, Bertha and her boys arrived in Pforzheim. She telegraphed Carl to let him know, then enjoyed a few days with her mom before driving home. Bertha’s road trip started to change public opinion about the car, and led to her and Carl’s company being the automotive giant we know today. She also showed the importance of test drives – innovations were added to the design to overcome the issues she’d found on this trip (including adding a third gear which made hills much easier). Test drives are standard, essential practice for automobile manufacturers today, but had never been done before Bertha’s trip in 1888. Adventuress Bertha Benz died on May 5, 1944, two days after her 95th birthday. On May 3, 2024, Bertha’s 175th birthday, the German government issued a postage stamp honoring her and her contributions to automotive history.
18 notes
·
View notes
Note
What would you consider to be an ethically sourced tail? Where might somebody purchase an ethically sourced tail?
Thank you so much for asking!!! 🐾
Personally I believe It should either: Be sourced as a by-product of the food industry, Transform a waste product to give it value, such as the reclaiming fur from animals culled for environmental management, Minimise waste by re-manufacturing vintage pieces or using surplus manufacturing material, instead of only using new material. There are other options such as taxidermy from ranched animals which were stillborn or died from illness or other natural causes. Byproducts of roadkill, pest management, and wildlife population control which are done in a sustainable manner that keeps the natural population at a healthy and maintainable level. Also if there is no unnecessary pain or cruelty that’s inflicted and killing of said animal involves minimal waste and has a purpose other than simply their fur.
And I know there is the argument that we don’t need to kill animals to make clothing because of course there are other materials to keep us warm, but the best of them (wool, down, leather) also come from animals. Meanwhile, most synthetic fibers (including fake or “faux” fur) are derived from petroleum, a non-renewable resource, the extraction and transformation of which entails serious environmental risks.
In many regions, wildlife populations must be culled annually to maintain healthy and stable populations, to preserve habitat, to protect endangered species (e.g., by culling predators that attack ground-nesting birds or sea turtle eggs), and to safe-guard human health, livestock and property. If furbearer populations must be culled, surely it is more ethical to use these animals than to discard them?
Farmed minks manure, soiled straw bedding and carcasses are composted to produce organic fertilizers, to enrich the soil and produce more food, completing the agricultural nutrient cycle. Biofuels made from mink remains now power buses in Aarhus, Denmark, the world’s largest producer of farmed mink. Similar projects are being tested in North America.
Now after all that here are some options for furs/tails. Though please do your own research into each small business or company you buy from.

https://www.etsy.com/shop/SterlingFoxTaxidermy
https://www.etsy.com/shop/ChimeraTaxidermyAU
#wolf#therian#wolf therian#wolfkin#wolves#canine therian#therianthropy#canine#theriotype#canis lupus irremotus#belgian malinois therian#belgian malinois#dog therian#dog theriotype#alterhuman#nonhuman#fox therian#coyote therian#bear therian#deer therian#cat therian#mouse therian#bird therian#therian gear#therian tail
25 notes
·
View notes
Text

On the eastern edge of Girard Cemetery, you’ll find an unusual headstone featuring a century-and-a-half-old consumer tip, of sorts. And talk about tragic irony.
The epitaph reads: “In memory of Ellen Shannon, age 26 years, who was fatally burned Mar. 21, 1870 by the explosion of a lamp filled with R.E. Danforth’s non explosive burning fluid.”he fire occurred at Shannon’s workplace, the Girard Hotel, which stood on the northeast corner of Rice Avenue and East Main Street.In the 1960s an older, broken stone with the same wording was replaced by the current one by Girard historian Hazel Kibler, who died in 1973 at age 89, said Stephanie Wincik, past president of the West County Historical Society.Wincik said Kibler wanted to preserve Girard’s past, even the strange stuff. “She was very interested in all these weird things in history,” Wincik said. “She would think (the epitaph) was cool.”The Shannon headstone is interesting, but R.E. Danforth’s non-explosive burning fuel might have been flat-out dangerous.According to the La Crosse (Wisconsin) Tribune, there is evidence that R.E. Danforth’s stuff might have been the cause of a fire — also in 1870 — that destroyed the War Eagle steamship. At least six died when the vessel burned and sunk where it was docked just north of La Crosse on the Black River.From a 2015 Tribune article:“In spring 1870, Danforth’s oil was a relatively new product in an unregulated marketplace. Without safety testing, manufacturers could experiment with and sell highly flammable, unstable oils. New York City’s Board of Health conducted a review of Danforth’s Non-Explosive Petroleum Fluid the same year that the War Eagle burned and concluded that the New York-based product was no less than a ‘murderous oil.'”Ellen Shannon of Girard and the people of La Crosse would have agreed. {Read}
5 notes
·
View notes
Text

Producing JP-7, the fuel that powered the SR-71 Blackbird, caused a nationwide shortage of bug spray. Here’s why.
The SR-71 Blackbird
In the 1960’s, the US Air Force (USAF) developed the SR-71 Blackbird, a plane that could travel more than 3 times as fast as the sound produced by its own engines.
SR-71 T-Shirts

CLICK HERE to see The Aviation Geek Club contributor Linda Sheffield’s T-shirt designs! Linda has a personal relationship with the SR-71 because her father Butch Sheffield flew the Blackbird from test flight in 1965 until 1973. Butch’s Granddaughter’s Lisa Burroughs and Susan Miller are graphic designers. They designed most of the merchandise that is for sale on Threadless. A percentage of the profits go to Flight Test Museum at Edwards Air Force Base. This nonprofit charity is personal to the Sheffield family because they are raising money to house SR-71, #955. This was the first Blackbird that Butch Sheffield flew on Oct. 4, 1965.
Throughout its nearly 24-year career, the SR-71 spy plane remained the world’s fastest and highest-flying operational aircraft. Flying at Mach 3+ from 80,000 feet, it could survey 100,000 square miles of Earth’s surface per hour. And in the off chance an enemy tried to shoot it down with a missile, all the Blackbird had to do was speed up and outrun it.
Its engineering was so cutting edge that even the tools to build the SR-71 needed to be designed from scratch.
JP-7, the SR-71 Blackbird fuel
In fact, given that the Blackbird became so hot because it cruised at a speed of Mach 3.2 conventional jet fuel could not be used in it. A jet fuel with a high flash point, and high thermal stability was required. To satisfy this requirement Shell produced a special blend of fuel called JP-7.
Specifically, JP-7 fuel (referred to as Jet Propellant 7 prior to MIL-DTL-38219) was developed for the Blackbird’s Pratt & Whitney J58 (JT11D-20) turbojet engine. During flight, the SR-71 could attain speeds in excess of Mach 3+, which was the most efficient cruising speed for the J58 engines. However, very high skin temperatures were generated at this speed due to friction with the air. A new jet fuel was needed that was not affected by the heat, so JP-7 jet fuel, with a high flash point and high thermal stability, was developed for this purpose.
JP-7 fuel production caused a nationwide shortage of bug spray
According to the SR-71A Flight Manual, “The operating envelope of the [J58] JT11D-20 engine requires special fuel. The fuel is not only the source of energy but is also used in the engine hydraulic system. During high Mach flight, the fuel is also a heat sink for the various aircraft and engine accessories which would otherwise overheat at the high temperatures encountered. This requires a fuel having high thermal stability so that it will not break down and deposit coke and varnishes in the fuel system passages. A high luminometer number (brightness of flame index) is required to minimize transfer of heat to the burner parts. Other items are also significant, such as the amount of sulfur impurities tolerated. Advanced fuels, JP-7 (PWA 535) and PWA 523E, were developed to meet the above requirements.”
Producing JP-7, the fuel that powered the SR-71 Blackbird, caused a nationwide shortage of bug spray. Here’s why.

This print is available in multiple sizes from AircraftProfilePrints.com – CLICK HERE TO GET YOURS. SR-71A Blackbird 61-7972 “Skunkworks”
Flit mosquito repellant
Noteworthy, JP-7 production caused a nationwide shortage of bug spray.
Shell Oil developed JP-7 in 1955. Manufacturing several hundred thousand gallons of the new fuel required the petroleum byproducts Shell normally used to make its Flit insecticide, causing a nationwide shortage of that product!
One of the ingredients in JP-7 just so happened to be a crucial part of Flit mosquito repellant. Bearing in mind the huge amount of fuel we’re talking about here, Shell didn’t exactly have enough supply to meet the newly increased demand, so mosquitos everywhere caught a lucky break!
JP-7 had a high flashpoint. It was not flammable and every time an SR-71 needed fuel, tankers were always there, they were terrific and deserve high praise.
SR-71 Blackbird JP-7 fuel used by the Boeing X-51 Waverider
Today the JP-7 fuel is used by the Boeing X-51 Waverider in its Pratt & Whitney SJY61 scramjet engine, with fuel capacity of some 270 pounds (120 kg). As with the SR-71, the X-51A design super-cools this fuel (cooled by extended subsonic flight in the stratosphere; prior to acceleration to supersonic speeds); then, when in supersonic flight, the fuel is heated by its circulation through heat exchangers which transfer to it the heat load of the interior spaces of the airframe. The fuel is then pumped through rotating mechanical parts of the engines and auxiliary mechanical equipment, providing both lubrication and cooling. Finally, at a temperature of nearly 550 °F (290 °C), it is pumped into the fuel nozzles of the engines.
Be sure to check out Linda Sheffield Miller (Col Richard (Butch) Sheffield’s daughter, Col. Sheffield was an SR-71 Reconnaissance Systems Officer) Twitter X Page Habubrats SR-71 and Facebook Page Born into the Wilde Blue Yonder for awesome Blackbird’s photos and stories.
Photo credit: U.S. Air Force
@Habubrats71 via X
11 notes
·
View notes
Text
Discover the Strength and Versatility of GI Flange
Q1: What are the key processes involved in the manufacture of GI flanges?
A: The key processes in the manufacture of GI flanges include:
Material Preparation: Raw materials like steel plates or sheets are cut to size.
Forming: The material is shaped into the flange design using processes like stamping or forging.
Welding: If needed, flanges may be welded to create specific designs.
Galvanization: The flanges are coated with zinc through hot-dip galvanization or electro-galvanization to enhance corrosion resistance.
Finishing: Final finishing processes include cleaning, inspection, and surface treatment to ensure quality.
Q2: Which materials are typically used in the production of GI flanges?
A: The primary material used in the production of GI flanges is carbon steel, which is coated with zinc to provide corrosion resistance. Depending on specific requirements, other materials such as stainless steel may also be used.
Q3: How do manufacturing standards affect the quality of GI flanges?
A: Manufacturing standards, such as ASTM, ANSI, and ISO, provide guidelines for the design, material specifications, and testing of GI flanges. Adhering to these standards ensures consistency, reliability, and safety in the final product, significantly affecting its overall quality.
Q4: What industries commonly utilize GI flanges?
A: GI flanges are commonly used in various industries, including:
Construction
Oil and gas
Water and wastewater treatment
HVAC (Heating, Ventilation, and Air Conditioning)
Shipbuilding
Q5: Can you customize GI flanges according to specific project requirements?
A: Yes, GI flanges can be customized based on specific project requirements, including size, thickness, pressure rating, and design specifications, to meet the needs of different applications.
Q6: What certifications should a manufacturer of GI flanges have?
A: A manufacturer of GI flanges should ideally have certifications such as:
ISO 9001 (Quality Management System)
ISO 14001 (Environmental Management)
API (American Petroleum Institute) certifications for oil and gas applications
Other industry-specific certifications as required.
Q7: How does the manufacturing process ensure corrosion resistance in GI flanges?
A: Corrosion resistance is ensured through the galvanization process, where flanges are coated with zinc, which serves as a sacrificial anode. This protects the underlying steel from rust and corrosion when exposed to moisture and other corrosive elements.
Q8: What are the advantages of using GI flanges over other types of flanges?
A: The advantages of using GI flanges include:
Cost-Effectiveness: Generally less expensive than stainless steel flanges.
Corrosion Resistance: The zinc coating provides excellent protection against rust.
Versatility: Suitable for a wide range of applications and environments.
Strength and Durability: Good mechanical properties make them reliable under pressure.
Q9: How does the cost of manufacturing GI flanges compare to other flange types?
A: The cost of manufacturing GI flanges is typically lower than that of stainless steel or other specialty flanges, primarily due to the lower cost of raw materials and the manufacturing processes involved.
Q10: What quality control measures are in place during the manufacture of GI flanges?
A: Quality control measures during the manufacture of GI flanges may include:
Regular inspections and testing of raw materials.
In-process inspections to ensure dimensional accuracy and adherence to specifications.
Testing for corrosion resistance, mechanical strength, and other relevant properties.
Final product inspections before shipment to ensure compliance with standards and customer specifications.
#GIFlanges#GalvanizedIron#IndustrialFlanges#SteelManufacturing#CorrosionResistance#FlangeManufacturing#ConstructionMaterials#MSMEs#QualityControl#B2BIndustry#PipeFittings#FlangeDesign#ManufacturingStandards#EngineeringSolutions#CustomFlanges
2 notes
·
View notes
Note
With regards to modern coal tar dye: is the production of it safe for the factory workers? What about the environment, in terms of waste water and petroleum processing, etc? Just curious.
i think this is a really good question, and one that deserves an answer. it's also not a question that i can answer comprehensively.
i want to be up front here that—again—i'm not a chemist or a scientist. i'm just a guy on the internet who is super interested in food processing, food history, and dye. in real life, i'm mostly an editor and sometimes a writer and researcher. because of my specific life, i'm arguably more comfortable reading government regulations and material safety data sheets than most people are, but i have no expertise here. if someone does have that expertise and feel that i'm misrepresenting things in this post, they should please 100% correct me.
but so: is the production of modern coal tar dye safe the factory workers? the first and biggest problem with this question is that these dyes are made in a lot of factories. there are a ton of chemical manufacturers out there, and many of them make food dyes. for the dyes to by approved, they have to be batch tested to meet quality parameters, so we can be pretty sure that the end products are all approximately the same. does that mean that the working conditions in the factory are good? no, it doesn't. i assume that working conditions vary as much from one chemical manufacturer to another as they do between other factories. as far as i'm aware, there's no way for an end consumer to tell what factory made the dye they're eating or putting in their hair or whatever.
i'm guessing that's not what you meant by safety, though, and you're asking more about the petroleum-specific aspects. and again, honestly, i don't know this about dye specifically. however, we as a global society are incredibly dependent on petroleum products—and there are a lot of them. (more on that in a sec.) but petroleum is used in so, so many things. it's used in asphalt and aspirin, dish soap and deodorant, mascara and mineral oil, plastic and paint, toothpaste and tires. it's what we make plastic from. it's genuinely almost impossible to overstate how many things in our lives are created with petroleum products. but we don't think of most of those things as threatening or toxic, and we often don't really worry that the materials themselves are going to be hazardous to the workers who are making them.
i don't know enough about the specific bits of petroleum used to say which, if any, of these products are more or less hazardous to the people making them. hopefully none of them are; probably some of them are. given how incredibly broad the overarching category of 'made at least in part from petroleum' is, though, i'm going to guess that dyes, specifically, are at least not hugely better or worse than the other things on that list.
part of why i'm comfortable saying that is because most of these dyes have actually been around for quite a long time! by the 1930s, six of the seven coal-tar dyes currently in use in the united states were already being made and regulated by the fda. (source) so we've had at least a hundred years of these specific dyes, which is generally plenty of time to realise if they're going to give everyone who works with them cancer. a hundred years of non-controversial production and usage is, in my opinion, a pretty decent safety assessment.
but let's back up some more. we know that they're ok for the end consumers, and we know that they're probably ok for the people who are making products with them. but 'petroleum' is kind of a weird word that encompasses a lot of things, ranging from crude oil fresh out of the ground all the way on down to some of the products that are made with it. mineral oil is petroleum. gasoline is petroleum. but they don't come out of the ground like that.
here's another place where my knowledge falls apart a little. my understanding is that crude oil is split into different parts through a process called fractional distillation. the oil is heated until it vapourises, and then components of the vapour condense at different temperatures and are split out like that. i have to reiterate: this is my very baby-level understanding of it. i've fact-checked myself and it seems that at an incredibly basic level, this is correct, but feel free to google 'fractional distillation' or 'fractionation' to read about it yourself.
i have no idea how bad this process actually is for the environment, and there are—obviously—strong incentives for corporations to conceal this. there's also a human urge to blame things on 'that new plant'. i used to live by a cracker plant, which both fractionates and processes the ethane created by the fractionation process. i had neighbours who worked there and seemed happy enough about it. i also had neighbours who swore up and down that it's the sole cause of pollution in that area, that the smells and air quality are terrible. (worth noting that because of the nature of this kind of plant, i can't differentiate between 'pollution etc that is directly related to fractionation' and 'pollution etc that is from the cracking process', though my understanding of the matter is that the latter is a bigger problem than the former.)
having lived there, i know full well that the plant wasn't the sole reason for the bad smells and air quality—there were other, different plants that definitely helped. did the cracker plant make it worse? in my opinion, yeah, it did. but did it have to make things worse? was it worse in part because of lax regulations and regulatory enforcement? was it worse in part because the company was given waivers and legally permitted to release pollution over the normal legal limit? (this part i know—it was.)
could regulations negate any pollution from the plant? i have no idea; i honestly don't know enough about the process to say. could stronger regulations (and stronger enforcement of the regulations, and not giving companies regulatory waivers because they ask nice) make that plant pollute a lot less? it could. is there the political will to do that? certainly not where i was living, but possibly in other places.
so the plants aren't great, but they could be made better, at least. i'm not aware of any unique health and safety concerns for workers at those plants, assuming that things are up to code and they're wearing appropriate ppe, but my source here is a couple guys i know who worked at one. would someone who's studied chemicals say differently? i don't know.
that's a little ambiguous! how safe is it for the workers? safe enough that the factories exist and are able to be in compliance with the health and safety requirements of many countries. could they be safer? probably. are they meaningfully less safe than working in factories that create other types of chemical? i don't have enough data to say for sure, and i don't really know how to get it, either.
ok we're almost done, but let's back up one more step. coal-tar dyes were originally made from coal, and are now mostly made from petroleum. but coal and petroleum have to be extracted, so what about the people who are extracting them?
and here we're into a whole different kind of difficult topic. how safe is mining? how safe is working on an oil rig? not very. they're both industries that are notorious for being ruthlessly profit-focused, no matter what the human cost of that is. they both have high mortality rates, and depending on what list you're looking at, they're consistently considered very dangerous.
but a lot of people take those jobs anyway, either because they're the best option on offer or because they hope the payoff will be worth it. i have an uncle who's worked his whole life in the mines and has the physical damage to show for it. his son turned eighteen and went over to the same mine to see if he could get a job. (he did, eventually.) i don't especially like this uncle or this cousin, but they're not stupid men. they looked at their options and figured this was the best one. i've known people who worked on offshore oil rigs, people who chose that job on purpose, who felt that it was worth the tradeoffs and risk. and maybe for them it was! i wish no one was making the choice of 'is the increased risk of death worth the increased rate of pay', but i wish a lot of things.
how safe is resource extraction for people who don't work in it directly? again, i think that most of us will agree that it's not very safe. fracking isn't good! our horrifying single-minded reliance on extracted resources is slowly killing us all! i don't think anyone's ever shouted for joy because the local mining company bought all that forest outside of town and they're gonna raze it. no one's thought that it would just be really cool to have an oil well in the back pasture just for the aesthetic value.
on the other hand, what's the alternative right now? i don't mean in an ideal future where we've done sensible things like converted everything to renewable fuel sources—what's the alternative right now? short of some sort of global cataclysm, i don't think there is a viable alternative right now. i think that a lot of very smart people are working very hard to create alternatives; i think that a lot of very wealthy oil executives are working very hard to make sure that those alternatives will fail before most of us ever hear so much as a whisper about them.
so ultimately, are coal-tar dyes safe? yes and no! yes in the sense that consuming them isn't going to hurt you. yes in the sense that i can't find any particular reason to think that they're riskier to make than any other product being made. yes in the sense that their production is in compliance with governmental health and safety regulations. and also no in the sense that resource extraction is inherently damaging to the land, water, and people of the world. no in the sense that it's not environmentally sustainable.
this is a really long answer to say, essentially, 'it's complicated'. but also: it's complicated. the closer to the consumer end of the chain you get, the safer things generally become. farming is more dangerous than cooking is more dangerous than eating; using hair dye is less dangerous than making hair dye is less dangerous than extracting the resources from which the hair dye will be made.
i don't know if this answers your question. it's a hard thing to wrestle with, for me. how safe does something have to be for it to be safe enough? where's the cutoff? is there anything where enough is known about the entire supply chain that we can accurately say that nothing and no one is harmed by its production?
honestly, probably not, at least right now, and i don't really think that it's mentally healthy for us as individuals to dwell on that for too long. the world is imperfect, and we make the best choices we can with the information we can get. in the specific instance of coal-tar dyes, i think we can probably say that they're not causing an unusual amount of harm, or more harm than most products do, and that's probably about as good as it'll get.
#dye#this ended up being ten billion words long and i'm so sorry#but this is genuinely a moral question that's almost impossible to answer#but also one that i've spent a lot of time thinking about#just by virtue of where i've lived and stuff like that#i honestly think that the hardest thing about trying to make the world better is that you have to keep living in it while things improve#smartest raccoon i know
12 notes
·
View notes
Text
What is ESG Investing? What is the Best Way to Get Started?
ESG is the next big thing in investing. It offers real-world performance factors that help investors consider how companies impact the regional community when making investment decisions. They also develop strategic thinking to work toward sustainable development goals (SDGs). This post will discuss what matters in ESG investing and to get started.
What Is ESG Investing?
ESG investing means investors utilize the three types of compliance metrics of corporate impact metrics to screen the target companies’ stocks or funds. Moreover, corporations seek to attract such investments through responsible and sustainable business practices.
If investors want data on the beneficial effect of a company’s operations on the local community, they can use ESG services. They can get reports from a data-driven survey concerning the environmental, social, and governance (ESG) compliance standards.
ESG audits enable informed investment decisions and portfolio management strategies. Investors can monitor whether a firm delivers its promised SDG metrics using such inspections. Likewise, consider the investors who invest their capital into the businesses that provide their employees with fair wages and respect.
How to Get Started with ESG Investing?
1| Specify Which Metrics Matter the Most to You
Investors must identify the ESG metrics, like forest preservation or tax transparency, before selecting a stock or asset class. They must also consider how all metrics have a unique significance in several industries. For example, carbon and greenhouse gas (GHG) emission risks will differ across data centers, agricultural businesses, and construction firms.
If an organization wants to attract investors using sustainability performance, it can benefit from ESG consulting. Consultants understand the investors’ conceptualization of an ESG-first enterprise of investors and how companies can work towards improving their operations to fulfill them.
2| Determine Realistic Goals
Depending on the scope of the energy transition, adopting greener resources and production technologies can financially burden a business at the initial stage. So, investors, regulators, and entrepreneurs must use real-world data to estimate the progress rate of compliance improvement initiatives.
An organization or exchange-traded fund (ETF) can fail to retain investors if the compliance milestones remain distant. Accordingly, administrators involved in regulatory policy changes that can impact an industry’s ESG dynamics must consider how long the corporate world will need to modify its operations.
3| Mitigate Greenwashing Risks
Companies might advertise their brand as “eco-friendly” or socially responsible. However, investors must watch out for the greenwashing attempts. Greenwashing refers to magnifying a company’s sustainability commitments with no on-ground implementation.
An enterprise might declare it opposes discriminatory practices while showing inaction when an employee experiences workplace harassment. Another example can be an energy distributor not reducing its usage of coal and petroleum derivatives as fuel.
Therefore, investors and fund managers must cross-verify the “green claims” that a target company makes during press releases or marketing campaigns.
4| Get ESG Ratings Using Multiple Frameworks
To test the legitimacy of a corporation’s SDG commitments, a rating mechanism based on multi-variate performance analytics can help in ESG investing. Today, many sustainability accounting frameworks exist. For example, the global reporting initiative (GRI) allows sectorial modules.
Each GRI criterion addresses a family of interdependent services and products. So, an agricultural business will use a separate GRI standard, differing from the modules used in technology, finance, and manufacturing firms.
How can investors get started with ESG score comparisons? Some online databases offer preliminary insights into how different brands and ETFs compete in this space. However, more extensive data becomes available through paid platforms or experienced consultants.
Conclusion
ESG criteria will empower investors to evaluate the ecological or social risks associated with how an enterprise handles its operations. Fund managers and similar financial institutions can gain a more objective outlook on stock screening using industry-relevant assistance.
Furthermore, combating the greenwashing risks will be challenging if you are a sustainability investor, but extensive analytical models will come to your rescue. Finally, investors must refer to multiple sustainability accounting frameworks or databases to check a firm’s compliance ratings. This approach is how you get started with ESG investing.
Nevertheless, manual inspection is time-consuming, and ESG ratings keep changing due to mergers and new projects. So, collaborating with data partners capable of automating compliance tracking, controversy analytics, and carbon credit assessments is vital.
3 notes
·
View notes
Text
For two decades, researchers worked to solve a mystery in West Coast streams. Why, when it rained, were large numbers of spawning coho salmon dying? As part of an effort to find out, scientists placed fish in water that contained particles of new and old tires. The salmon died, and the researchers then began testing the hundreds of chemicals that had leached into the water.
A 2020 paper revealed the cause of mortality: a chemical called 6PPD that is added to tires to prevent their cracking and degradation. When 6PPD, which occurs in tire dust, is exposed to ground-level ozone, it’s transformed into multiple other chemicals, including 6PPD-quinone, or 6PPD-q. The compound is acutely toxic to four of 11 tested fish species, including coho salmon.
Mystery solved, but not the problem, for the chemical continues to be used by all major tire manufacturers and is found on roads and in waterways around the world. Though no one has studied the impact of 6PPD-q on human health, it’s also been detected in the urine of children, adults, and pregnant women in South China. The pathways and significance of that contamination are, so far, unknown.
Still, there are now calls for regulatory action. Last month, the legal nonprofit Earthjustice, on behalf of the fishing industry, filed a notice of intent to sue tire manufacturers for violating the Endangered Species Act by using 6PPD. And a coalition of Indian tribes recently called on the EPA to ban use of the chemical. “We have witnessed firsthand the devastation to the salmon species we have always relied upon to nourish our people,” the Puyallup Tribal Council said in a statement. “We have watched as the species have declined to the point of almost certain extinction if nothing is done to protect them.”
The painstaking parsing of 6PPD and 6PPD-q was just the beginning of a global campaign to understand the toxic cocktail of organic chemicals, tiny particles, and heavy metals hiding in tires and, to a lesser extent, brakes. While the acute toxicity of 6PPD-q and its source have strong scientific consensus, tire rubber contains more than 400 chemicals and compounds, many of them carcinogenic, and research is only beginning to show how widespread the problems from tire dust may be.
While the rubber rings beneath your car may seem benign — one advertising campaign used to feature babies cradled in tires — they are, experts say, a significant source of air, soil, and water pollution that may affect humans as well as fish, wildlife, and other organisms. That’s a problem because some 2 billion tires globally are sold each year — enough to reach the moon if stacked on their sides — with the market expected to reach 3.4 billion a year by 2030.

(Researchers weigh a salmon that died after four hours in a tank filled with road runoff.)
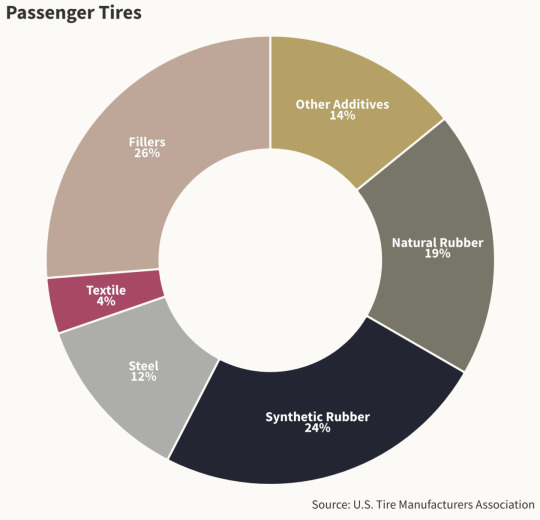
Tires are made from about 20 percent natural rubber and 24 percent synthetic rubber, which requires five gallons of petroleum per tire. Hundreds of other ingredients, including steel, fillers, and heavy metals — including copper, cadmium, lead, and zinc — make up the rest, many of them added to enhance performance, improve durability, and reduce the possibility of fires.
Both natural and synthetic rubber break down in the environment, but synthetic fragments last a lot longer. Seventy-eight percent of ocean microplastics are synthetic tire rubber, according to a report by the Pew Charitable Trust. These fragments are ingested by marine animals — particles have been found in gills and stomachs — and can cause a range of effects, from neurotoxicity to growth retardation and behavioral abnormalities.
“We found extremely high levels of microplastics in our stormwater,” said Rebecca Sutton, an environmental scientist with the San Francisco Estuary Institute who studied runoff. “Our estimated annual discharge of microplastics into San Francisco Bay from stormwater was 7 trillion particles, and half of that was suspected tire particles.”
Tire wear particles, or TWP as they are sometimes known, are emitted continually as vehicles travel. They range in size from visible pieces of rubber or plastic to microparticles, and they comprise one of the products’ most significant environmental impacts, according to the British firm Emissions Analytics, which has spent three years studying tire emissions. The company found that a car’s four tires collectively emit 1 trillion ultrafine particles — of less than 100 nanometers — per kilometer driven. These particles, a growing number of experts say, pose a unique health risk: They are so small they can pass through lung tissue into the bloodstream and cross the blood-brain barrier or be breathed in and travel directly to the brain, causing a range of problems.
According to a recent report issued by researchers at Imperial College London, “There is emerging evidence that tyre wear particles and other particulate matter may contribute to a range of negative health impacts including heart, lung, developmental, reproductive, and cancer outcomes.”
The report says that tires generate 6 million tons of particles a year, globally, of which 200,000 tons end up in oceans. According to Emissions Analytics, cars in the U.S. emit, on average, 5 pounds of tire particles a year, while cars in Europe, where fewer miles are driven, shed 2.5 pounds per year. Moreover, tire emissions from electric vehicles are 20 percent higher than those from fossil-fuel vehicles. EVs weigh more and have greater torque, which wears out tires faster.
Unlike tailpipe exhaust, which has long been studied and regulated, emissions from tires and brakes — which emit significant amounts of metallic particles in addition to organic chemicals — are far harder to measure and control and have therefore escaped regulation. It’s only in the last several years, with the development of new technologies capable of measuring tire emissions and the alarming discovery of 6PPD-q, that the subject is receiving much needed scrutiny.
Recent studies show that the mass of PM 2.5 and PM 10 emissions — which are, along with ozone and ultrafine particles, the world’s primary air pollutants — from tires and brakes far exceeds the mass of emissions from tailpipes, at least in places that have significantly reduced those emissions.
The problem isn’t just rubber in its synthetic and natural form. Government and academic researchers are investigating the transformations produced by tires’ many other ingredients, which could — like 6PPD — form substances more toxic than their parent chemicals as they break down with exposure to sunlight and rain.
“You’ve got a chemical cocktail in these tires that no one really understands and is kept highly confidential by the tire manufacturers,” said Nick Molden, the CEO of Emissions Analytics. “We struggle to think of another consumer product that is so prevalent in the world, and used by virtually everyone, where there is so little known of what is in them.”
“We have known that tires contribute significantly to environmental pollution, but only recently have we begun to uncover the extent of that,” said Cassandra Johannessen, a researcher at Montreal’s Concordia University who is quantifying levels of tire chemicals in urban watersheds and studying how they transform in the environment. The discovery of 6PPD-q has surprised a lot of researchers, she said, because they have learned that “it’s one of the most toxic substances known, and it seems to be everywhere in the world.”
Regulators are playing catch up. In Europe, a standard to be implemented in 2025, known as Euro 7, will regulate not only tailpipe emissions but also emissions from tires and brakes. The California Environmental Protection Agency has passed a rule requiring tire makers to declare an alternative to 6PPD-q by 2024.

(A worker takes apart a tire at a recycling shop in Mit al-Harun, Egypt.)
Tire companies are conducting their own studies of 6PPD, which they have long considered critical for tire safety, and seeking alternatives. In response to new regulations and the emerging research on tire emissions, 10 of the world’s large tire manufacturers have formed the Tire Industry Project to “develop a holistic approach to better understand and promote action on the mitigation” of tire pollution, according to a statement by the project. The group has committed to search for ways to redesign tires to reduce or eliminate emissions.
One critical area of research is how long tire waste, and its breakdown products, persist in the environment. “A five-micron piece of rubber shears off the tire and settles on the soil and sits there a while,” said Molden. “What, over time, is the release of those chemicals, how quickly do they make their way into the water, and are they diluted? At the system level, how big of a problem is this? It is the single biggest knowledge gap.”
Another area of research centers on the impacts of aromatic hydrocarbons — including benzene and naphthalene — off-gassed by synthetic rubber or emitted when discarded tires are burned in incinerators for energy recovery. Even at low concentrations, these compounds are toxic to humans. They also react with sunlight to form ozone, or ground-level smog, which causes respiratory harm. “We have shown that the amount of off-gassing volatile organic compounds is 100 times greater than that coming out of a modern tailpipe,” said Molden. “This is from the tire just sitting there.”
When tires reach their end of life, they’re either sent to landfills, incinerated, burned in an energy-intensive process called pyrolysis, or shredded and repurposed for use in artificial turf or in playgrounds or for other surfaces. But as concern about tire pollutants grows, so do concerns about these recycled products and the hydrocarbons they may off-gas. There is ongoing debate over whether crumb rubber, made from tire scraps, poses a health threat when used to fill gaps in artificial turf. Based on several peer-reviewed studies, the European Union is instituting stricter limits on the use of this material. Other studies, however, have shown no health impact.
Besides California’s requirement to study alternatives to 6PPD, there are a number of efforts worldwide to redesign tires to counter the problems they pose. More than a decade ago, tire makers hoped that dandelions, which produce a form of rubber, and soy oil could provide a steady and sustainable supply of rubber. But tires made from those alternatives didn’t live up to expectations: they still required additives. The Continental Tire Company, based in Hanover, Germany, markets a bicycle tire made of dandelion roots. Tested by Emission Analytics, it emitted 25 percent fewer carcinogenic aromatics than conventionally made bike tires, but the plant-powered tire still contained ingredients of concern.

(Rubber made from dandelions.)
Other companies are searching for ways to address the problem of tire emissions. The Tyre Collective, a clean-tech startup based in the U.K., has developed an electrostatic plate that affixes to each of a car’s tires: The plates remove up to 60 percent of particles emitted by both tires and brakes, storing them in a cartridge attached to the device. The particles can be reused in numerous other applications, including in new tires.
In San Francisco, scientists studying the pollutants in storm runoff found a potential solution: Rain gardens, installed in yards to capture stormwater, were also trapping 96 percent of street litter and 100 percent of black rubbery fragments. In Vancouver, B.C. researchers found that rain gardens could prevent more than 90 percent of 6PPD-q from running off roads and entering salmon-bearing streams.
Tire waste particles, says Molden, of Emissions Analytics, are finally getting the attention they deserve, thanks in part to California’s rule requiring a search for alternatives to 6PPD. The legislation “is groundbreaking,” he says, “because it puts the chemical composition [of tires] on the regulatory agenda.” For the first time, he adds, “Tire manufacturers are being exposed to the same regulatory scrutiny that car manufacturers have been for 50 years.”
8 notes
·
View notes
Text
Coulometric Karl Fischer Titrator
Labtron Coulometric Karl Fischer Titrator features precise moisture analysis with a sensitivity down to microgram levels, an integrated reagent exchange system, and a user-friendly interface with digital display. Specifications include titration cell volume, measurement range (ppm to percent), accuracy, compliance with ASTM and ISO standards, and compatibility with various sample types.
0 notes
Text

Plastic Devil
A rubbery, pliable surface of surprising density. Tests show the material is a variety of petroleum-derived plastic polymers, all entirely known to modern science and used commonly in manufacturing. The plasticizing agent, however, is of a unique variety which is yet to be artificially synthesized.
This substance is presumed to account for the stability of the materials used, given radiological dating places the item in the 1.75-1.9 MYO range.
This does not explain the whispering.
The image(s) above in this post were made using an autogenerated prompt and/or have not been modified/iterated extensively. As such, they do not meet the minimum expression threshold, and are in the public domain.
Prompt: an image from the book hodgepodge of hordes and a beast, in the style of steelpunk, yellow and blue, precision painting, dark azure and green, ps1 graphics, extreme angle, sharp attention to detail :: a toy with horns and a mask, in the style of bold saturation innovator, shodo, rangercore, dau al set, high resolution, eastern and western fusion, majismo
#ai artwork#deepSCREAM nights#halloween#unreality#generative art#midjourney v5#midjourney#free art#public domain#public domain art#monster
6 notes
·
View notes
Text
Petrochemical Solutions – Optimizing Performance and Sustainability in Oilfield Operations
Introduction
In the dynamic and demanding landscape of oilfield operations, finding innovative and sustainable solutions is paramount. As a leading player in the petrochemical sector, Imperial Chemical (ICPL) is at the forefront of providing cutting-edge petrochemical solutions that not only optimize performance but also contribute to the sustainability of oilfield operations. This blog will delve into the significance of petrochemical products, the role of petrochemical companies in Gujarat, and ICPL's commitment to being a premier petrochemical solutions provider in India.

The Petrochemical Industry in India
India's petrochemical industry plays a pivotal role in the country's economic growth. It encompasses the production of a wide range of chemicals derived from petroleum and natural gas, serving as the backbone for various sectors, including agriculture, manufacturing, and energy. As one of the fastest-growing economies globally, India relies heavily on petrochemical products to meet its ever-expanding industrial demands.
Petrochemical companies in Gujarat, with its strategic location and robust infrastructure, have emerged as key contributors to India's petrochemical landscape. Gujarat's proactive policies, state-of-the-art facilities, and access to key resources position it as a hub for petrochemical manufacturing. Imperial Chemical, headquartered in Gujarat, takes pride in being a frontrunner among petrochemical companies, offering comprehensive solutions to cater to the diverse needs of the industry.
Imperial Chemical: A Petrochemical Solutions Provider in India
Imperial Chemical (ICPL) stands tall as a leading petrochemical solutions provider in India. With a commitment to innovation, sustainability, and customer satisfaction, ICPL plays a vital role in shaping the future of the petrochemical sector. Let us explore how ICPL's petrochemical products and solutions contribute to optimizing performance and promoting sustainability in oilfield operations.
Diverse Range of Petrochemical Products:
ICPL boasts a diverse portfolio of petrochemical products designed to meet the specific requirements of oilfield operations. From specialty chemicals to essential components used in extraction and refining processes, our product range is tailored to enhance efficiency and performance.
Advanced Technology and Research:
Innovation is the driving force behind ICPL's success. Our state-of-the-art research and development facilities are dedicated to exploring new technologies and formulations. This commitment to innovation ensures that our petrochemical solutions remain at the cutting edge, providing our clients with the latest advancements in the industry.
Sustainability at the Core:
Recognizing the global shift towards sustainable practices, ICPL integrates environmental responsibility into every aspect of our operations. Our petrochemical solutions are designed to minimize environmental impact while maximizing operational efficiency. This includes the development of eco-friendly additives, cleaner extraction processes, and sustainable packaging solutions.
Customized Solutions for Oilfield Challenges:
Oilfield operations are multifaceted, with unique challenges requiring tailored solutions. ICPL collaborates closely with clients to understand their specific needs and challenges. Our team of experts then develops customized petrochemical solutions that address these challenges effectively, ensuring optimal performance and resource utilization.
Stringent Quality Control:
Quality is non-negotiable at ICPL. Our petrochemical products undergo rigorous testing and quality control measures to meet and exceed industry standards. This commitment to quality ensures that our clients receive reliable and high-performance solutions for their oilfield operations.
Petrochemical Solutions in Gujarat: A Regional Perspective
Gujarat has emerged as a key player in India's petrochemical sector, housing some of the most significant petrochemical companies in the country. The state's strategic location, well-established infrastructure, and business-friendly policies have attracted investments, making it a vibrant hub for petrochemical manufacturing.
Imperial Chemical, with its headquarters in Gujarat, is proud to contribute to the state's reputation as a petrochemical powerhouse. Our presence in Gujarat allows us to leverage the region's resources, collaborate with local talent, and actively participate in the state's economic growth. As a responsible corporate citizen, ICPL is committed to upholding the highest standards of environmental stewardship and community engagement in Gujarat.
Sustainable Practices in Petrochemical Operations
Sustainability is a cornerstone of ICPL's philosophy. As a responsible petrochemical solutions provider in India, we are dedicated to incorporating sustainable practices into every aspect of our operations. Here's how ICPL contributes to sustainability in oilfield operations:
Reduced Environmental Impact:
ICPL focuses on developing petrochemical products and solutions that minimize environmental impact. This includes the reduction of emissions, efficient use of resources, and the development of sustainable alternatives to traditional petrochemical products.
Energy-Efficient Processes:
Our manufacturing processes prioritize energy efficiency, reducing the carbon footprint associated with our operations. By adopting advanced technologies and energy-efficient practices, ICPL strives to contribute to the overall sustainability of the petrochemical sector.
Waste Minimization and Recycling:
ICPL implements waste minimization and recycling initiatives to reduce the generation of waste and promote a circular economy. By reusing and recycling materials, we aim to minimize the environmental footprint of our petrochemical operations.
Community and Stakeholder Engagement:
ICPL actively engages with local communities and stakeholders to foster a collaborative approach to sustainability. Through community outreach programs, education initiatives, and transparent communication, we aim to build lasting relationships that benefit both the industry and the communities we serve.
Conclusion
In the ever-evolving landscape of oilfield operations, the role of petrochemical solutions cannot be overstated. Imperial Chemical (ICPL), as a leading petrochemical solutions provider in India, is dedicated to optimizing performance and promoting sustainability in the oilfield sector. With a diverse range of petrochemical products, advanced technology, and a commitment to sustainable practices, ICPL is poised to shape the future of the petrochemical industry in Gujarat and beyond. As the industry continues to grow and adapt, ICPL remains at the forefront, delivering innovative solutions that drive efficiency, performance, and environmental responsibility. Contact us today to explore how ICPL's petrochemical solutions can elevate your oilfield operations to new heights of success.
#Petrochemical products#Petrochemical companies in Gujarat#Petrochemical industry in India#Petrochemical solutions provider in India#Petrochemical sector#Oil and gas industry#Oil and gas companies in Vadodara#oil and gas pipelines#oil and gas product solution provider in India#Oil and gas sector#oil and gas services companies in india#India#Gujarat#Tamilnadu#Kerala#Andhrapradesh#ICPL
6 notes
·
View notes
Text






























#career #education #ndt #qa qc #oil and gas
RAZI INSTITUTE OF PETROLEUM ENGINEERING is a dynamic, multi-dimensional, training & placement organization in the field of oil and gas, petroleum sector. We have evolved a unique blend of training programs, which are structured to provide effective career solutions to the youth. Our training programs includes oil and gas, quality assurance and quality control (QAQC), nondestructive testing (NDT), health, safety & environment (HSE),welding, piping, pressure vessel manufacturing, storage tank construction ,heat exchangers ,boilers, ship building ,offshore & onshore drilling ,pipeline construction , structural, painting ,coating, Insulation and more. RAZI INSTITUTE have direct contact with MNCs and private organizations for which we place our students. This not only helps us place students, but also structure the training programs to suit the needs of the corporate. RAZI have an eminent Advisory Board, which provides direction and guidance on the course design and developmental delivery process. Our primary strength is our ability to understand the aspirations, needs and potential of the youth and also committed to young generation.
2 notes
·
View notes
Text
Lyocell, milk fibre and pineapple leather: New textile fibres advertise sustainability. Few can keep their promises

Initiatives are many, the textile industry is in a frenetic state
Polyester causes microplastics, and the natural fibre cotton also has environmental damage in its luggage. No wonder some manufacturers are looking for alternatives. But they can at best be part of the solution.
When someone buries his/her underpants in the garden, it's not necessarily a sign of outlandish or disturbing preferences. It can also be just a slightly more entertaining test of soil quality, which the University of Zurich has even used scientifically. However, most of our clothes would probably emerge from the earth relatively unchanged after a few months, even if the soil in question contains enough beneficial organisms. Because most of our clothes are not biodegradable in the environment - often not even those made of supposedly more sustainable fibres.
“First of all, you have to differentiate between natural fibres that grow on bushes, stalks or trees and are already in fibre form, and man-made fibres,” says Anett Matthäi, who works on sustainable textiles at the engineering faculty of the Hof University of Applied Sciences[1] in Bavaria.
Up until the turn of the 19th and 20th centuries, clothing was always made of natural fibres, cotton, linen, wool or silk. Then, at the beginning of the 20th century, the first synthetic fibre was commercially manufactured: wood became viscose. In 1940, nylon was the first completely synthetic fibre to appear on the market. In the 1950s, the first items of clothing made of polyester were found in stores. Today, polyester is by far the most common material for clothing, accounting for 52 percent of global fibre production[2].
Polyester, cotton and viscose are harmful to the environment
Polyester[3], nylon[4], and other man-made fibres like polyamide[5], acrylic[6], and elastane[7] have advantages—they're cheap, and they use relatively little water to make. But their starting material is the finite raw material petroleum. And they contribute to plastic and microplastic pollution when they enter the environment.
And they do that on a significant scale: clothing loses fibres, during manufacture, during wear and during washing; In Switzerland, 650 tons of microplastics from textiles end up in the environment every year. And in countries like Chile or Ghana[8], old clothes collected abroad rot in huge landfills; some of them are washed into the sea. They will not rot in the ground or in salt water. Instead, like other plastic waste, they break down into smaller and smaller particles over time.
The second most important fibre on the market does not have this problem: cotton[9] is a natural fibre and biodegradable. But growing them requires a lot of fertilizer, pesticides and water. An estimated 3,700 or 4,700 litres of water are needed to produce one pair of jeans.
And viscose[10], which is made from renewable raw materials, also has its pitfalls. The cellulose from the wood of beech or eucalyptus is dissolved and the resulting so-called dope is pressed through nozzles to create the fibre. "You can imagine the process as making spaghetti from dough," says Matthäi, "only much finer, and the 'spaghetti' doesn't tear as quickly."
In this process, however, large amounts of toxic carbon disulfide and caustic soda are sometimes used. The same applies to modal, which is also made from cellulose. Bamboo viscose has therefore also fallen into disrepute. At first it was considered particularly sustainable because of the fast and pesticide-free growing raw material.
The production of lyocell does not require any toxic chemicals
Because neither viscose nor polyester nor cotton are really sustainable, manufacturers are looking for alternative fibres. And so there are now clothes made of materials with names like Lyocell[11] or PLA[12], bamboo viscose or soy silk, pineapple leather or milk fibre.[13] A lot of it sounds like nature. But the raw material alone does not make a fibre sustainable.
Lyocell is considered to be comparatively environmentally friendly, and the label often includes the brand name Tencel. It is also a so-called regenerated fiber that is chemically synthesized from renewable raw materials. But unlike classic viscose, the solvent used in lyocell is not toxic.
And lyocell is – just like classic viscose – biodegradable, i.e. it is broken down by microorganisms into CO2, water and minerals. A recently published study showed that this not only works in the garden, but also in the sea.
"Compostable" does not mean "biodegradable"
Scientists hung samples of different textiles in the sea for more than a year. The cotton samples then dissolved – no surprise, even from the cotton underpants buried in the garden only the seams and the elastic band are left after two months if the soil is healthy. The lyocell had also disappeared after months in the sea.
The sample made of PLA, a bioplastic that is also processed into textiles, was almost unchanged. PLA is the abbreviation of Polylactic Acid. It is made from fermented starch from sugar beets or corn, advertised as particularly sustainable and is officially compostable. But that does not mean that it is degraded in the environment.
Because in order to be able to call itself "compostable", the material only has to decompose within three months in an industrial composting plant. In this, however, there are completely different conditions of temperature, humidity and oxygen supply than in the garden - and even more so than in the sea.
“You cannot draw any direct conclusions from results on the compostability of a material as to whether it can also be decomposed by microorganisms in a different environment,” says Matthäi. "The conditions and the composition of the microorganisms are completely different." The study shows that bioplastics also contribute to the littering of the oceans with plastic.[14]
Soy silk, milk fibre and pineapple leather: new fibres have their pitfalls
It might be different with soy silk.[15] According to Matthäi, it is in principle biodegradable, but like viscose, manufacturing processes and chemical additives could impair its degradability. The material is often featured in reports on sustainable clothing, but only a few raw fabrics and yarns are available to knit yourself. According to the information provided, they are made from waste from tofu production in a closed cycle.
While soy silk[16] does not seem to play a role on the market so far, an Austrian underwear manufacturer has released the first models with another new material this spring: milk fibre[17]. It should be very comfortable to wear. How good it is for the environment depends on whether only dairy waste that is no longer suitable for consumption is processed. And it depends on which additives are needed to spin a fibre from the milk protein. However, the underwear manufacturer does not provide this information – and does not provide it later on request either.
Pineapple leather[18] is just one particularly exotic-sounding example of a non-animal and therefore supposedly sustainable alternative to leather. Other manufacturers use cork, mushrooms[19], apples[20], coffee, grapes, cacti or bananas as raw materials. But the naturalness usually doesn't go any further than that. Plastic is always involved, as a carrier material, adhesive or coating, and then often polyurethane[21].
Not all fibres can be recycled
The same applies to all fibres: even if a material is sustainable and biodegradable in itself, this is by no means necessarily the case with the finished garment. "A chemical change caused by dyeing or functionalisation - for example to make the clothing easy to iron or water-repellent - can impair biodegradability," says Matthäi. A small percentage of elastane, which many cotton dresses have, also has this effect.
And recyclability also suffers as a result. Pure natural fibres can be shredded mechanically, and the resulting shorter fibres can be spun again. This is often not possible with synthetics; recycled polyester is not made from reused clothing, but from PET bottles.
It's certainly better than oil, but – contrary to what the advertising suggests – it shouldn't be seen as a solution to the packaging waste problem. All the more so since PET is particularly easy to recycle and should serve better as a raw material for a new bottle than for a fleece jacket.
For Anett Matthäi, all these fibres alone cannot be the solution anyway. There is probably not enough cultivable land to produce the quantities of clothing currently made from polyester with clothing made from fibres from renewable raw materials. "In my opinion, the most important thing is that the consumption of materials is reduced overall," she sums up. Then, she believes, it would also be possible to produce enough materials from renewable raw materials or by recycling waste.
So before you bury your underpants in the garden, you should ask yourself whether you could still wear them for a while.
Source
Esther Widmann, Lyocell, Milchfaser und Ananasleder: Neue Textilfasern werben mit Nachhaltigkeit. Ihre Versprechen halten können die wenigsten, in Neue Zürcher Zeitung, 29-08-2023, https://www.nzz.ch/wissenschaftnachhaltige-textilfasern-wie-gut-sind-lyocell-co-wirklich-ld.1745536
[1] Hof University, German: Hochschule Hof, full name Hochschule für Angewandte Wissenschaften Hof, is a public non-profit business, media and technical vocational university founded in 1994 in Upper Franconia, Bavaria, Germany.
[2] Read also: https://www.tumblr.com/earaercircular/725160672165543936/scientists-develop-simple-way-to-recycle-polyester?source=share
[3] Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
[4] Nylon is a generic designation for a family of synthetic polymers composed of polyamides (repeating units linked by amide links). Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes]: 2 Nylon polymers can be mixed with a wide variety of additives to achieve many property variations. Nylon polymers have found significant commercial applications in fabric and fibers (apparel, flooring and rubber reinforcement), in shapes (molded parts for cars, electrical equipment, etc.), and in films (mostly for food packaging)
[5] A polyamide is a polymer with repeating units linked by amide bonds. Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis yielding materials such as nylons, aramids, and sodium polyaspartate. Synthetic polyamides are commonly used in textiles, automotive industry, carpets, kitchen utensils and sportswear due to their high durability and strength. The transportation manufacturing industry is the major consumer, accounting for 35% of polyamide (PA) consumption
[6] Acrylic fabric is made with plastic threads. The plastic threads are made of a manmade polymer fiber created from fossil fuels through a chemical process. Acrylic fabric is made in a way similar to the production of polyamide fabric (or nylon fabric) and polyester fabric.
[7] Spandex, Lycra, or elastane is a synthetic fibre known for its exceptional elasticity. It is a polyether-polyurea copolymer that was invented in 1958 by chemist Joseph Shivers at DuPont.
[8] Read also: https://www.tumblr.com/earaercircular/720260226679488512/hms-answer-about-the-dumped-clothes-article?source=share
[9] Read also https://www.tumblr.com/earaercircular/715379082096951296/the-type-of-cotton-matters-betting-on-more?source=share
[10] Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. It is also called viscose. Many types and grades of viscose fibers and films exist. Some imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk.
[11] Lyocell is a semi-synthetic fiber used to make textiles for clothing and other purposes. It is a form of regenerated cellulose made by dissolving pulp and dry jet-wet spinning. Unlike rayon made by some of the more common viscose processes, Lyocell production does not use carbon disulfide, which is toxic to workers and the environment. Lyocell was originally trademarked as Tencel in 1982.
[12] Polylactic acid, also known as polylactic acid or polylactide (PLA), is a thermoplastic polyester.
[13] Read also: https://www.tumblr.com/earaercircular/721296904220196864/joline-jolink-makes-biodegradable-fashion?source=share
[14] Read also: https://www.tumblr.com/earaercircular/656486012918333440/fashion-brands-are-diving-into-ocean-plastic-but?source=share
[15] With the softness of silk, soy fabric or “vegetable cashmere” is one of the world’s most eco-friendly fabrics. Produced using soy protein derived from the hulls of soybeans, this intriguing textile takes a waste product and transforms it into a usable textile with minimal use of toxic chemicals and limited processing. Soy fabric has excellent drape, and it is highly elastic. While this textile dyes well, colors sometimes bleed during the first few washings. Though reasonably prone to pilling, soy fabric does not wrinkle, and it doesn’t shrink. https://sewport.com/fabrics-directory/soy-fabric
[16] Soy silk has similar properties to animal silk: it has a smooth, soft structure, a shimmering shine, it is temperature regulating has high moisture absorption. Unlike conventional silk, it hardly creases and is completely biodegradable… https://www.glore.de/Materiallexikon/Sojaseide/
[17] Milk protein fibers are synthetic fibers made from the milk protein casein. In 2011, the new fiber made headlines as a particularly ecological alternative to cotton. Casein fibers have been known since the 1930s. Designer Anke Domaske developed the new milk fiber Qmilk together with the Fiber Institute Bremen. For the production, casein powder is heated together with other natural ingredients and drawn into threads through a nozzle. Only 2 liters of water are needed to produce 1 kg of milk fibre. On the other hand, in the production of cotton textiles, 10,000-25,000 liters are used for 1 kg of fabric. Every year in Germany alone, 1.9 million tons of milk have to be disposed of because it is no longer suitable for consumption. It still contains valuable ingredients and offers great potential for technical purposes. https://www.glore.de/Materiallexikon/Milchfaser/
[18] Piñatex is a non-biodegradable leather alternative made from cellulose fibres extracted from pineapple leaves, PLA (polylactic acid), and petroleum-based resin. Piñatex was developed by Dr Carmen Hijosa and first presented at the PhD graduate exhibition at the Royal College of Art, London. Piñatex is manufactured and distributed by Hijosa's company Ananas Anam Ltd.
[19] Read also: https://www.tumblr.com/earaercircular/667314088734507008/mushrooms-as-raw-material-for-leather-accessories?source=share
[20] Read alsop: https://www.tumblr.com/earaercircular/677442405046321152/we-make-a-sneaker-out-of-apples?source=share
[21] Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibres such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.
2 notes
·
View notes
Text
The significance of hazardous area lighting
Ensuring the safety of workers is paramount in every industry. Certain industries, including gas and oil, pose particular challenges and high risks of explosions. Improperly classified lighting fixtures can ignite a buildup of flammable gases and materials from a stray spark, resulting in an explosion. Therefore, it is crucial to have appropriately designed lighting fixtures in hazardous locations.

Hazardous area lighting is designed to be explosion-proof, containing any sparks or fire that may arise within the device. It plays a vital role in promoting the well-being of a business and, most importantly, its employees.
Facilities:
Numerous facilities and industries fall under one of the three risk levels of hazardous locations that necessitate adequately designed lighting fixtures.
Some of the most common facilities that fall under hazardous locations and require properly designed lighting fixtures include:
Class 1 Flammable Vapor Environment:
Petroleum and Gasoline Refineries
Dry Cleaning Plants
Spray Paint Booths
Paint Shops and Facilities
Aircraft Hangars
Chemical and Utility Gas Plants
Detergent Manufacturing Plants
Alcohol Production Facilities
Textile Dyeing and Printing Plants
Wastewater Treatment Plants
Class 2 Ignitable Dust Environment:
Plastic Production Plants
Pharmaceutical Factories
Firework Factories
Coal Mines
Flour and Feed Mills
Grain Elevators
Class 3 Combustible Fibers Environment:
Textile Mills and Cotton Gins
Cotton Seed Mills
Flax Seed Processing Plants
Leather Goods Workshops
Shoe Manufacturing Plants
Alcohol Distilleries
Choosing Factors:
A safer lighting fixture is one that lasts longer. When selecting or designing lighting fixtures for hazardous locations, durability must be a top consideration. This includes examining the following characteristics of hazardous area lighting:
Operating temperature
IP Rating
Vibration and impact resistance
Surge resistance
Long-lasting LED technology.
An adequately designed and manufactured hazardous location light should be able to withstand constant vibrations and unintended impacts or shocks. Failure to test lighting fixtures for such extreme conditions could result in premature failure of the entire fixture. Therefore, hazardous area lighting must pass a 35-hour vibration test at 2,000 cycles per minute.
In certain hazardous locations, lightning strikes and current surges can pose a costly threat to a facility's lighting systems. To mitigate this risk, hazardous area lighting must be designed with built-in surge suppression, eliminating the need for additional protective devices.
A longer-lasting light is a safer one, especially in hazardous locations that require sturdier lighting fixtures capable of withstanding hazardous materials. As discussed in our previous blog, hazardous location LED lighting enables you to save on maintenance costs while providing a longer-lasting light without compromising the safety of workers. Hazardous location LED lights have fewer components that, when exposed to flammable gases or vapors, could cause or ignite a fire.
Types:
A variety of hazardous area lighting fixtures are available to provide widespread illumination while minimizing risk. Please find below a list of our explosion-proof lighting fixtures and their corresponding applications.
LED high bay lights are ideal lighting fixtures for buildings or areas with high ceilings. They are perfect for hazardous locations as they enhance visibility.
Area lights are primarily used for outdoor lighting and are typically explosion-proof. Area lights can be effectively used in hazardous outdoor areas such as gasoline and oil loading docks, gas stations, oil refineries, and distilleries.
Flood lights are also used for outdoor lighting and provide illumination for large areas while offering superior protection from the elements. Flood lights can be effectively applied in parking lots, warehouses, and loading dock driveways.
Inadequate lighting in hazardous locations can lead to three consequences:
Safety and security risks
Penalties for noncompliance
Diminished durability
Conclusion:
Properly classifying a hazardous environment and understanding its potential dangers is essential for safely lighting such areas. Before selecting any lighting fixtures, it is important to know the facility and identify flammable vapors, ignitable dust, or combustible fibers. This enables us to design lighting that offers a safe and productive working environment.
2 notes
·
View notes