#Peptide Receptor Radionuclide Therapy (PRRT)
Explore tagged Tumblr posts
Text
PRRT is a molecular technique in which a radioisotope which is labeled with a small body that actually targets a particular receptor which is known as the somatostatin receptors is used to treat a specific kind of tumor known as a Neuroendocrine Tumor.
#prrt therapy#Actinium Ac 225 Alpha PRRT#PRRT in India#Peptide Receptor Radionuclide Therapy in India#Nuclear Medicine Expert in India#PRRT Treatment for Neuroendocrine Tumors#PRRT Therapy Side Effects#PRRT Therapy#PRRT Treatment#PRRT in Neuroendocrine Tumors#Peptide Receptor Radionuclide Therapy#PRRT
0 notes
Text
Peptide Receptor Radionuclide Therapy (PRRT) Market end-user demand, trend, new innovations, global forecast to 2032

This Peptide Receptor Radionuclide Therapy market study offers a comprehensive analysis of the business models, key strategies, and respective market shares of some of the most prominent players in this landscape. Along with an in-depth commentary on the key influencing factors, market statistics in terms of revenues, segment-wise data, region-wise data, and country-wise data are offered in the full study. This study is one of the most comprehensive documentation that captures all the facets of the evolving Peptide Receptor Radionuclide Therapy Prrt market.
Advancement and growing researches in the medical industry leads to a dramatic surge in the availability of new cancer treatment options. Radiation therapies and targeted therapy is showing significant promise in cancer treatment. For example, peptide receptor radionuclide therapy (PRRT) had recently approved the neuroendocrine tumors and projecting significant growth in the cancer treatment market. Peptide receptor radionuclide therapy (PRRT) is radioisotope or molecular therapy used to treat neuroendocrine tumors (NETs).
Get Sample Report@ https://www.futuremarketinsights.com/reports/sample/rep-gb-10376
Peptide receptor radionuclide therapy (PRRT) is recommended for the somatostatin receptor-positive gastroenteropancreatic NETs affected patients. Peptide receptor radionuclide therapy (PRRT) is a targeted therapy designed to slow the progression of gastroenteropancreatic NET and limiting radiation exposure to healthy tissue. Lutathera (lutetium Lu 177 Oxodotreotide) was the first drug approved for the peptide receptor radionuclide therapy (PRRT) for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). In September 2017, the European Commission approved Lutathera peptide receptor radionuclide therapy (PRRT) drug manufactured by Advanced Accelerator Applications S.A. Although, the U.S. Food and Drug Administration (FDA) was approved LUTATHERA in January 2018. Growing clinical trials and development activities to create therapeutic radiopharmaceuticals expected to surge the growth of the peptide receptor radionuclide therapy (PRRT) market over the forecast period.
Peptide Receptor Radionuclide Therapy (PRRT) Market: Drivers and Restraints-
Increasing prevalence of the neuroendocrine tumors expected to impel the demand for peptide receptor radionuclide therapy (PRRT) as an increasing number of the patient pool. Establishment of reimbursement policies for Lutathera favors the demand for peptide receptor radionuclide therapy (PRRT). A temporary insurance/billing code was used for the peptide receptor radionuclide therapy (PRRT) till 2018. Moreover, increasing clinical trials for the new radiopharmaceuticals drugs approval for cancer therapy expected to surge the growth of the peptide receptor radionuclide therapy (PRRT) market.
Increasing government and private organizations funding for cancer drugs and therapy development is another major factor expected to propel the growth of the peptide receptor radionuclide therapy (PRRT) market. Moreover, growing manufacturer’s interest in the radiopharmaceutical and cancer market flourish the growth of the peptide receptor radionuclide therapy (PRRT) market. Side effects such as the transient decrease in blood counts, nausea and others associated with PRRT expected to hamper the growth of the peptide receptor radionuclide therapy (PRRT) market.
Peptide Receptor Radionuclide Therapy (PRRT) Market: Overview
Peptide receptor radionuclide therapy (PRRT) a special type of radiopharmaceutical which injected into the patients’ bloodstream. This radiopeptide travels and binds to neuroendocrine tumor cells and provide a high dose of radiation directly to the cancer cell. Big pharmaceutical market players are focusing to enter in the radiopharmaceutical market. For instance, In October 2017, Novartis AG acquired Advanced Accelerator Applications (AAA) for $3.9 billion to expand oncology portfolio. This acquisition was valuable for Lutathera radiopharmaceutical candidates which were under FDA review in 2018.
Peptide Receptor Radionuclide Therapy (PRRT) Market: Region-wise Outlook
North America and Europe region are expected to grebe more than half of the market share for peptide receptor radionuclide therapy. Increasing clinical trials for radiopharmaceuticals and growing demand for the targeted therapy for cancer are the major factors driving the growth of the peptide receptor radionuclide therapy market in the U.S. and European countries. The Asia pacific peptide receptor radionuclide therapy market expected to grow with significant growth rate as growing demand for advance treatment option and comparatively high prevalence of cancer in India and China.
Peptide Receptor Radionuclide Therapy (PRRT) Market: Key Market Participants
Example of some market players participants in global peptide receptor radionuclide therapy (PRRT) market find across the value chain are Advanced Accelerator Applications (AAA) (Novartis AG) and others.
The research report presents a comprehensive assessment of the market and contains thoughtful insights, facts, historical data, and statistically supported and industry-validated market data. It also contains projections using a suitable set of assumptions and methodologies. The research report provides analysis and information according to market segments such as geographies, application, and industry.
The report covers exhaust analysis on:
Market Segments
Market Dynamics
Market Size
Supply & Demand
Current Trends/Issues/Challenges
Competition & Companies involved
Technology
Value Chain
Regional analysis includes:
North America (U.S., Canada)
Latin America (Mexico. Brazil)
Western Europe (Germany, Italy, France, U.K, Spain)
Eastern Europe (Poland, Russia)
Asia Pacific (India, China ASEAN, Australia & New Zealand)
Japan
Middle East and Africa (GCC Countries, S. Africa, Northern Africa)
The report is a compilation of first-hand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry participants across the value chain. The report provides in-depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
Peptide Receptor Radionuclide Therapy (PRRT) Market: Segmentation
On the basis of indication, peptide receptor radionuclide therapy (PRRT) market can be segmented as:
Foregut Neuroendocrine Tumors
Midgut Neuroendocrine Tumors
Hindgut Neuroendocrine Tumors
On the basis of end user, peptide receptor radionuclide therapy (PRRT) market can be segmented as:
Hospitals
Ambulatory Surgical Centers
Cancer Care Centers
For in-depth insights, Download a PDF Brochure – https://www.futuremarketinsights.com/reports/brochure/rep-gb-10376
Report highlights:
Detailed overview of parent market
Changing market dynamics in the industry
In-depth market segmentation
Historical, current and projected market size in terms of volume and value
Recent industry trends and developments
Competitive landscape
Strategies of key players and products offered
Potential and niche segments, geographical regions exhibiting promising growth
A neutral perspective on market performance
Must-have information for market players to sustain and enhance their market footprint
#Peptide Receptor Radionuclide Therapy (PRRT) Market Market#Peptide Receptor Radionuclide Therapy (PRRT) Market Market Size#Peptide Receptor Radionuclide Therapy (PRRT) Market Market Growth
0 notes
Text
Carcinoid Tumor Drug Pipeline Analysis Report 2024
Carcinoid Tumor Market Outlook
Carcinoid tumors are uncommon, representing roughly 0.5% of all cancers. Typically diagnosed in individuals around their early 60s, these tumors mainly occur in the gastrointestinal system. In the United States, approximately 8,000 new cases of carcinoid tumors are reported annually. Treatment options include surgical intervention, chemotherapy, targeted therapies, and the development of new immunotherapies. These innovative treatments focus on slowing tumour growth and enhancing patient prognosis.
Carcinoid Tumor: Introduction
Carcinoid tumours are rare, slow-growing neuroendocrine tumours that typically develop in the gastrointestinal tract, lungs, or other endocrine organs. These tumours secrete hormones, such as serotonin, which can lead to a condition called carcinoid syndrome, characterised by symptoms like diarrhoea, flushing, and wheezing. Diagnosis often occurs late due to the insidious nature of symptoms, making treatment challenging. The development of new therapies focuses on controlling tumour growth, managing hormone secretion, and improving patient quality of life. Ongoing clinical trials and drug innovations are crucial to addressing the unmet needs in the treatment of these rare tumours.
Get a Free Sample Report with Table of Contents: https://www.expertmarketresearch.com/clinical-trials/carcinoid-tumor-drug-pipeline-analysis/requestsample
Carcinoid Tumor Treatment Overview
Carcinoid tumours are typically treated with surgical resection if possible, alongside therapies that control tumour growth and hormone secretion. The main treatment approaches include somatostatin analogues to reduce symptoms, chemotherapy for advanced disease, and targeted therapies. Early-stage disease may be managed with surgery alone, while more advanced stages require a combination of therapies, including systemic treatments and novel therapies under investigation.
Treatment options include somatostatin analogues such as octreotide and lanreotide to manage symptoms. Other approaches include chemotherapy, targeted therapies, and peptide receptor radionuclide therapy (PRRT). Targeted drugs, such as mTOR inhibitors and tyrosine kinase inhibitors, are emerging as promising options in advanced or resistant cases.
Drug Pipeline Therapeutic Assessment
Analysis by Route of Administration
Oral
Parenteral
Others
Analysis by Phase
Preclinical Phase
Phase I
Phase II
Phase III
Phase IV
Analysis by Drug Class
Monoclonal Antibody
Peptides
Polymer
Small Molecule
Gene Therapy
Carcinoid Tumor Drug Classes
Carcinoid tumor treatments utilise a range of drug classes, each designed to target specific pathways and mechanisms involved in cancer growth and survival. These diverse classes enhance the effectiveness of therapy and contribute to personalised treatment strategies. Understanding these drug classes is essential for optimising patient outcomes.
Monoclonal Antibody
Monoclonal antibodies are laboratory-engineered molecules designed to target specific antigens on the surface of tumour cells, which helps to block their growth and enhances the immune system's ability to destroy them. In carcinoid tumours, these antibodies may target receptors that drive hormone secretion or tumour proliferation, improving disease control. By blocking key growth signals or immune evasion mechanisms, monoclonal antibodies offer an effective and precise treatment strategy for managing advanced or metastatic carcinoid tumours.
Peptides
Peptide-based therapies consist of short chains of amino acids that can interfere with the processes responsible for tumour growth. In carcinoid tumours, somatostatin analogues are commonly used, which bind to specific receptors on the tumour cells, inhibiting the release of excess hormones such as serotonin. These therapies help to manage symptoms like flushing and diarrhoea associated with carcinoid syndrome, and stabilise tumour growth, offering symptom relief and improving patients’ quality of life.
Polymer
Polymers play an important role in cancer therapy by improving the delivery and stability of drugs. In carcinoid tumours, polymer-based drug delivery systems are used to target therapies more efficiently to the tumour site. This enhances the effectiveness of drugs by increasing their concentration at the tumor while reducing side effects on healthy tissues. Such advanced delivery mechanisms help to improve therapeutic outcomes, particularly for patients with advanced, resistant, or metastatic disease.
Small Molecule
Small molecules are drugs that are small enough to enter cells and interfere with cellular pathways responsible for tumour growth and survival. In carcinoid tumours, small molecules target specific signalling pathways involved in tumour proliferation and hormone production. By modulating these pathways, small molecules can help slow down tumour growth, manage symptoms, and provide an effective option for treating progressive or metastatic carcinoid tumours, where traditional therapies may be less effective.
Gene Therapy
Gene therapy aims to address the underlying genetic causes of cancer by modifying or replacing defective genes in tumour cells. For carcinoid tumours, gene therapy is being investigated to either restore normal cellular function or enhance the immune system’s ability to recognise and destroy tumour cells. This approach holds promise for offering long-term solutions in treating hard-to-treat, resistant cases of carcinoid tumours by targeting the molecular drivers of tumour growth.
Carcinoid Tumor- Pipeline Drug Profiles
This section provides an overview of the various drugs used to treat carcinoid tumor. It covers their classifications, mechanisms of action, and methods of administration, offering essential insights for effective treatment strategies.
40 mg Paltusotine
Paltusotine is a selective somatostatin receptor type 2 agonist that is being investigated for its potential to control hormone secretion in patients with carcinoid syndrome. By reducing the serum levels of serotonin, it helps to manage the symptoms of diarrhoea, flushing, and wheezing associated with the syndrome. Clinical trials have shown promising results, especially for patients who have not responded to other standard therapies, making it a potential new option for long-term management.
Pasireotide (SOM230)
Pasireotide is a multireceptor-targeted somatostatin analogue used to manage the symptoms associated with neuroendocrine tumours, including carcinoid syndrome. It works by binding to somatostatin receptors and inhibiting the release of hormones like serotonin, which causes symptoms like diarrhoea and flushing. Pasireotide has shown efficacy in clinical trials, particularly in patients whose symptoms are not adequately controlled by first-line treatments. It offers an alternative therapeutic option for patients with advanced or resistant cases.
Quarfloxin
Quarfloxin is an investigational small molecule that targets the mitochondrial DNA replication machinery in cancer cells, causing tumour cell death. In carcinoid tumours, quarfloxin is being evaluated for its potential to inhibit tumour growth, particularly in patients with advanced or metastatic disease. Early clinical trials are assessing the drug’s safety and efficacy, and its combination with other therapies is being explored to improve overall treatment outcomes and manage resistant cases of carcinoid tumour.
Carcinoid Tumor: Competitor Landscape
The key features of the report include patent analysis, clinical trials, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
Novartis Pharmaceuticals
Headquartered in Basel, Switzerland, Novartis is a global leader in pharmaceutical innovation, with a strong presence in the oncology space. The company is known for its contributions to neuroendocrine tumour treatments, including the development of Pasireotide (SOM230) for managing carcinoid syndrome. Novartis continues to advance its research into targeted therapies and combination treatments, aiming to improve survival and quality of life for patients with rare, difficult-to-treat cancers such as carcinoid tumours.
CASI Pharmaceuticals, Inc.
CASI Pharmaceuticals, located in Rockville, Maryland, USA, is a biotechnology company focused on the development of novel therapies for rare and difficult-to-treat cancers, including carcinoid tumours. The company’s pipeline includes therapies designed to target tumour growth and hormone secretion in neuroendocrine cancers. By developing precision medicine approaches, CASI aims to provide more effective treatment options for patients with advanced or refractory cases of carcinoid tumours, addressing an unmet need in this area of oncology.
Crinetics Pharmaceuticals Inc.
Crinetics Pharmaceuticals, headquartered in San Diego, California, USA, is a leading biotechnology firm focused on the development of innovative treatments for endocrine diseases, including carcinoid tumours. The company is advancing several drug candidates that aim to modulate hormonal pathways and inhibit tumour growth. Crinetics’ approach to precision medicine aims to provide more personalised and effective treatments for patients with neuroendocrine cancers, and their research is expected to significantly improve treatment outcomes for carcinoid tumour patients.
Other key players in the landscape include Molecular Insight Pharmaceuticals, Inc., Neotropix, Ipsen, Cylene Pharmaceuticals, Lexicon Pharmaceuticals, TerSera Therapeutics LLC, Trio Medicines Ltd., and Endo Pharmaceuticals.
We at Expert Market Research always strive to provide you with the latest information. The numbers in the article are only indicative and may be different from the actual report..
0 notes
Text
Applications of Radioisotopes in Cancer Treatment

Did you know in 2022, there were an estimated 20 million new cancer cases and about 9 million cancer-related deaths globally? What’s more staggering is cancer is one of the leading causes of death worldwide and by 2040, the number of new cancer cases annually is forecasted to rise to 29.9 million! Despite being such a fatal condition for humankind, the treatment of cancer is still a dilemma. When you think about cutting-edge treatments for cancer, all we can imagine is complex procedures or advanced technology. However, only a few people know about medical radioisotopes because although they are effective, their production has been a challenge.
Medical radioisotopes are tiny but powerful elements that play a substantial role in the fight against cancer. How? Well, here’s a guide on how these medical marvels work and what their transformative impact on cancer treatment is.
What are Medical Radioisotopes?
Medical radioisotopes are radioactive isotopes used in diagnostic imaging and therapy. Their ability to emit radiation makes them invaluable in pinpointing and treating cancer cells or oncogenic cells. These isotopes are carefully packed based on their properties, such as half-life and type of radiation emitted, so healthcare professionals can target cancerous tissues precisely without damaging any healthy cells.
Lutetium-177: A Game Changer in Therapy
Lutetium 177 production has revolutionized targeted cancer therapy. The isotope is used in therapies like peptide receptor radionuclide therapy (PRRT), where it is attached to a molecule that binds specifically to cancer cells and delivers targeted radiation directly to the cancerous cells.
The beta radiation emitted by this isotope is perfect for this cause because it penetrates tissues just enough to destroy the tumor without excessively harming any surrounding tissues that might be healthy.
Actinium-225
Ac-225 is another promising radioisotope used in cancer treatment, part of a newer class of targeted alpha therapy agents. Unlike beta radiation, Ac-225 emits alpha particles, which have a higher energy and a shorter range. This is beneficial for targeting small clusters of cancer cells or even individual cells that might be resistant to other forms of radiation.
Challenges in Producing Radioisotopes
Yes, medical isotopes can save lives, but their production process is no cakewalk because it requires specialized facilities and complex technology, which is expensive and time-consuming. Besides, the production of these isotopes requires a consistent and reliable supply, which is tough due to the short half-lives of the isotopes and limited production sites. Stringent regulations and quality control are not easy either and the high production costs can also limit the accessibility and affordability for clinicians, researchers, as well as healthcare giants.
Conclusion:
Although the medical application of radioisotopes has been a game-changer in the field of oncology, their production has been limited by technical limitations, waste steam concerns, and supply outages. To combat these issues, Nusano has come up with a wide variety of isotopes for researchers, drugmakers, and clinicians while stabilizing supply chains. Assisting the fight against Cancer, Nusano is helping healthcare leaders meet the increasing need for medical radioisotopes and helping patients worldwide save lives with super-efficient technology. Our technology can produce up to 12 different radioisotopes simultaneously, and this is a major stepping stone needed to advance cancer care. Source: https://nusanous.blogspot.com/2024/09/applications-of-radioisotopes-in-cancer.html
1 note
·
View note
Text
0 notes
Text
youtube
What is Neuroendocrine Tumours & It’s Symptoms and Treatment by Dr. Anil Kamath Surgical Oncologist
Neuroendocrine tumors (NETs) are a rare and complex group of cancers that arise from neuroendocrine cells, which have traits of both nerve cells and hormone-producing cells. Early detection and treatment are crucial for better outcomes. In Bangalore, Dr. Anil Kamath, a highly experienced surgical oncologist, specializes in treating these challenging tumors. This article delves into the nature of NETs, their symptoms, causes, diagnosis, and the treatment options available, with a spotlight on Dr. Kamath’s expertise.
Understanding Neuroendocrine Tumors (NETs)
What are Neuroendocrine Tumors?
Neuroendocrine tumors develop from neuroendocrine cells found throughout the body. These cells produce hormones that regulate various bodily functions. NETs can be benign or malignant, and their behavior can vary significantly.
How NETs Differ from Other Tumors
NETs are distinct due to their ability to produce hormones, leading to unique symptoms based on the hormones they release. This characteristic often makes them different from other types of tumors.
Symptoms of Neuroendocrine Tumors
Common Symptoms
NETs can cause a wide range of symptoms, often depending on the tumor’s location and the hormones it secretes. General symptoms include unexplained weight loss, fatigue, and changes in bowel habits.
Causes and Risk Factors
Genetic Factors
Some NETs are associated with genetic conditions such as Multiple Endocrine Neoplasia (MEN). Family history can play a role in the likelihood of developing these tumors.
Environmental and Lifestyle Factors
While the exact causes are often unknown, factors like smoking, exposure to certain chemicals, and chronic conditions like diabetes can increase the risk of NETs.
Diagnosis of Neuroendocrine Tumors
Initial Medical Consultation
Diagnosis usually begins with a thorough medical history and physical examination. Symptoms and risk factors are evaluated to guide further testing.
Diagnostic Tests and Procedures
Blood and Urine Tests
These tests can detect abnormal hormone levels indicative of NETs.
Imaging Studies
Techniques such as CT scans, MRIs, and PET scans help visualize the tumor and assess its spread.
Biopsy
A tissue sample is taken from the tumor to confirm the diagnosis and determine its type and grade.
Treatment Options for Neuroendocrine Tumors
Overview of Treatment Approaches
Treatment depends on the tumor type, location, stage, and grade. Options include surgery, chemotherapy, radiotherapy, and targeted therapies.
Non-surgical Treatments
Chemotherapy
Uses drugs to kill cancer cells or stop them from growing. It’s often used for advanced or aggressive NETs.
Radiotherapy
High-energy radiation targets and destroys cancer cells. It’s typically used when surgery isn’t an option.
Targeted Therapy
Drugs like everolimus and sunitinib specifically target cancer cell mechanisms, sparing normal cells.
Peptide Receptor Radionuclide Therapy (PRRT)
This innovative treatment delivers radiation directly to the tumor cells via hormone receptors, offering a targeted approach with fewer side effects.
Choosing the Right Oncologist
Importance of Specialized Care
NETs require specialized knowledge for effective treatment. Choosing an experienced oncologist can significantly impact outcomes.
About Dr. Anil Kamath
Expertise and Experience
Dr. Anil Kamath is a leading oncologist in Bangalore with extensive experience in treating NETs. His advanced training and commitment to patient care make him a top choice for NET treatment.
Patient Testimonials
Many patients have shared their positive experiences with Dr. Kamath, praising his expertise, compassionate care, and successful treatment outcomes.
Recovery and Follow-Up Care
Post-treatment Recovery
Recovery times vary based on the treatment type and the patient’s overall health. Post-treatment care focuses on managing side effects and regaining strength.
Long-term Follow-Up
Regular follow-up appointments are essential to monitor for recurrence and manage any long-term effects of treatment.
Managing Side Effects
Side effects like fatigue, digestive issues, and hormonal imbalances are common. Effective management strategies can improve the patient’s quality of life.
Living with Neuroendocrine Tumors
Lifestyle Adjustments
Adopting a healthy lifestyle, including a balanced diet and regular exercise, can support recovery and overall well-being.
Support Systems and Resources
Access to support groups and resources can provide emotional and practical support for patients and their families.
Monitoring and Preventing Recurrence
Ongoing monitoring and lifestyle adjustments can help detect any recurrence early and prevent further complications.
Conclusion
Neuroendocrine tumors are complex and require specialized care for effective treatment. With the expertise of Dr. Anil Kamath, patients in Bangalore have access to comprehensive and cutting-edge treatment options. Early detection, appropriate treatment, and continuous support are crucial for managing NETs and improving patient outcomes.
0 notes
Text
High Grade Neuroendocrine Neoplasms Market - Size, Trends & Competition Analysis 2030 | Credence Research
High Grade Neuroendocrine Neoplasms Market
The latest market report published by Credence Research, Inc. “Global High Grade Neuroendocrine Neoplasms Market: Growth, Future Prospects, and Competitive Analysis, 2023 – 2030. The global High Grade Neuroendocrine Neoplasms Market has been steadily growing in recent years and is predicted to grow at a 8.50% CAGR between 2023 and 2030. The market is anticipated to be worth USD XX million by 2030, up from USD XX million in 2022.
The term "High Grade Neuroendocrine Neoplasms Market" refers to the market for medical treatments, therapies, and related products and services that are specifically focused on high-grade neuroendocrine neoplasms (NENs). High-grade neuroendocrine neoplasms (NENs) are a group of rare and aggressive cancers that originate in neuroendocrine cells. These cells are found throughout the body, particularly in organs like the pancreas, lungs, gastrointestinal tract, and other endocrine glands. Neuroendocrine cells release hormones into the bloodstream and play a role in regulating various bodily functions.
High-grade NENs are characterized by their rapid growth and tendency to metastasize (spread to other parts of the body). They are considered more aggressive and have a poorer prognosis compared to low-grade NENs. These tumors are often classified as neuroendocrine carcinomas and can be further categorized based on their tissue of origin, such as small cell or large cell neuroendocrine carcinoma.
Diagnosis typically involves a combination of imaging tests (such as CT scans, MRI, or PET scans) and biopsies to confirm the presence of high-grade NENs. Treatment options may include surgery to remove the tumor, chemotherapy, radiation therapy, and targeted therapies. The specific treatment plan depends on factors like the tumor's location, stage, and whether it has spread to other parts of the body.
Because high-grade neuroendocrine neoplasms are aggressive, early diagnosis and prompt treatment are essential for improving outcomes and extending survival. Patients with these tumors often require close monitoring and ongoing medical care. The prognosis for high-grade NENs can vary widely depending on the individual case and the success of treatment. It is important for patients to work closely with a medical team specializing in neuroendocrine cancers to develop an appropriate treatment plan and receive the necessary support.
The high-grade neuroendocrine neoplasms (NENs) market, like any other medical field, faces several major challenges and risks. These challenges can impact patient care, research, and the development of new therapies. Here are some of the key challenges and risks associated with the high-grade NENs market:
Limited Understanding: High-grade NENs are relatively rare and complex tumors, which makes them less understood compared to more common cancers. Limited knowledge about the disease's biology, progression, and treatment options hampers effective management.
Late Diagnosis: High-grade NENs are often diagnosed at an advanced stage, leading to poorer outcomes. Lack of specific symptoms and low awareness among healthcare providers can contribute to delayed diagnosis.
Treatment Options: The treatment landscape for high-grade NENs is limited compared to other cancers. Standardized treatment protocols are lacking, and therapeutic options may include surgery, chemotherapy, targeted therapies, and peptide receptor radionuclide therapy (PRRT). Determining the most appropriate treatment can be challenging.
Tumor Heterogeneity: High-grade NENs are characterized by tumor heterogeneity, meaning that different parts of the tumor may have different characteristics and respond differently to treatment. This complicates treatment decisions and may lead to treatment resistance.
Some of the major players in the market and their market share are as follows:
Brain Storm Cell Therapeutics
Holostem Terapie Avanzate S.R.L
Pharmicell Co. Inc
Opexa Therapeutics
Caladrius Biosciences Inc
U.S. Stem Cell Inc
Lonza
Bristol Myers Squibb
Novartis
Autolus therapeutics
Browse 247 pages report High Grade Neuroendocrine Neoplasms Market By Primary Site of Origin (Pulmonary Neuroendocrine Neoplasms (Lung), Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs), Other Primary Sites (e.g., Neuroendocrine Neoplasms of the Thymus), By Tumor Grade and Stage (Grade 3 Neuroendocrine Neoplasms (Well-Differentiated), Small Cell Neuroendocrine Carcinomas (Poorly Differentiated) – Size, Share, Growth, Trends and Segment Forecasts to 2023-2030- https://www.credenceresearch.com/report/high-grade-neuroendocrine-neoplasms-market
High Grade Neuroendocrine Neoplasms Market Growth Factor Worldwide
The High Grade Neuroendocrine Neoplasms Market is experiencing significant growth worldwide, driven by several key factors. With an increasing prevalence of neuroendocrine tumors and a growing awareness among healthcare professionals regarding the diagnosis and treatment options for these rare malignancies, the market is poised to expand in the coming years. Furthermore, advancements in medical research have led to a better understanding of high-grade neuroendocrine neoplasms and the development of targeted therapies that can effectively combat these aggressive tumor types.
Additionally, favorable reimbursement policies for novel therapeutics and the availability of government funding for cancer research are further fueling market growth. Moreover, technological innovations in diagnostic imaging techniques such as positron emission tomography (PET) scans and molecular testing methods enable more accurate detection and staging of high-grade neuroendocrine neoplasms, facilitating timely intervention. The emergence of personalized medicine approaches tailored specifically to individual patients based on their genomic profile holds immense promise for improving overall survival rates in this patient population.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global High Grade Neuroendocrine Neoplasms Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global High Grade Neuroendocrine Neoplasms Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Complete Report- https://www.credenceresearch.com/report/high-grade-neuroendocrine-neoplasms-market
Visit our Website- https://www.credenceresearch.com
Related Reports- https://www.credenceresearch.com/report/bone-grafts-and-substitutes-market
https://www.credenceresearch.com/report/autologous-cell-therapy-market
Browse Our Blog- https://www.linkedin.com/pulse/high-grade-neuroendocrine-neoplasms-market-key-players-shukla
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Neuroendocrine Tumors Pipeline Drugs Analysis : An Overview
Neuroendocrine tumors (NETs) represent a complex and diverse group of rare neoplasms that arise from neuroendocrine cells. These tumors can occur in various organs, including the gastrointestinal tract, pancreas, lungs, and other parts of the body. In recent years, significant advancements have been made in understanding and treating NETs, with a growing focus on innovative pipeline drugs. This article delves into the promising developments in the neuroendocrine tumors pipeline, shedding light on potential breakthroughs for patients and healthcare providers.
Introduction
Neuroendocrine tumors, often referred to as NETs, are a heterogeneous group of malignancies that originate from neuroendocrine cells, which have characteristics of both nerve cells and hormone-producing cells. These tumors can exhibit varying degrees of aggressiveness and can be challenging to diagnose and treat effectively. Over the years, researchers and pharmaceutical companies have been diligently working to develop innovative treatments that address the unique complexities of NETs.
Understanding Neuroendocrine Tumors
Neuroendocrine tumors can develop in numerous organs throughout the body, primarily in the gastrointestinal tract and the lungs. These tumors can be functional, producing hormones that lead to distinct clinical syndromes, or non-functional, causing symptoms due to their size or location. Diagnosing NETs often involves a combination of imaging studies, biomarker measurements, and histopathological examination.
Challenges in Neuroendocrine Tumors Treatment
Treating NETs presents several challenges. One major hurdle is the potential for late-stage diagnosis, as symptoms can be nonspecific and mistaken for other conditions. Additionally, the slow-growing nature of some NETs can lead to delayed interventions. Surgical resection remains a cornerstone of treatment, but it may not always be feasible, particularly in cases of advanced disease. Thus, there is a critical need for effective medical therapies to manage unresectable or metastatic NETs.
Emerging Therapeutic Approaches
4.1 Targeted Therapy
Targeted therapies aim to exploit specific molecular alterations in tumor cells. These therapies can interfere with pathways that promote tumor growth, leading to cell death or slowed progression. In NETs, targeted therapies may focus on receptors and enzymes involved in neuroendocrine cell function and signaling.
4.2 Immunotherapy
Immunotherapy has revolutionized cancer treatment by harnessing the body's immune system to recognize and attack tumor cells. Immune checkpoint inhibitors are being explored as potential options for certain types of NETs, enabling immune cells to mount a stronger anti-tumor response.
4.3 Peptide Receptor Radionuclide Therapy (PRRT)
PRRT involves delivering radioactive particles directly to tumor cells. This approach utilizes peptide receptors on the surface of NET cells to guide the delivery of radiation, minimizing damage to healthy tissues.
4.4 Combination Therapies
Combination therapies, which involve administering two or more drugs with complementary mechanisms of action, are being investigated to enhance treatment efficacy. These combinations may include targeted therapies, immunotherapies, or conventional chemotherapy.

Key Pipeline Drugs
5.1 Drug A: Mechanism of Action and Potential Benefits
[Details about Drug A and its mechanism of action, potential benefits, and ongoing clinical trials.]
5.2 Drug B: Clinical Trials and Efficacy
[Information on Drug B's clinical trial progress, efficacy data, and its impact on patients.]
5.3 Drug C: Addressing Treatment Resistance
[Insights into Drug C's unique approach to overcoming treatment resistance and its potential implications for NET patients.]
Clinical Trial Progress and Results
[Discuss recent advancements in clinical trials, highlighting notable findings and their implications for NET treatment.]
Patient Perspectives and Quality of Life
[Explore the impact of NETs on patients' lives, emphasizing the importance of therapies that enhance quality of life.]
The Role of Genetic Research
[Examine the role of genetic studies in understanding NETs and tailoring personalized treatment strategies.]
Future Outlook and Implications
[Discuss the future of NET treatment, potential challenges, and how pipeline drugs could transform patient outcomes.]
Conclusion
In conclusion, the landscape of neuroendocrine tumors is rapidly evolving, with a promising array of pipeline drugs offering hope for improved therapies. As researchers delve deeper into the intricacies of NETs and their underlying mechanisms, new avenues for treatment are being explored. Patients and healthcare providers alike anticipate a brighter future in the fight against neuroendocrine tumors.
For more region insights on Neuroendocrine Tumors Pipeline Drugs Analysis report, download a free report sample
0 notes
Text
Radionucleotide Therapy In Mumbai – Medcare Daignostic
Exploring the Advancements of Radionuclide Therapy in Mumbai
Introduction
In recent years, the field of medical science has witnessed remarkable advancements, with innovative approaches to treating various diseases. One such groundbreaking development is Radionuclide Therapy, a targeted treatment method that utilizes radioactive substances to target and destroy cancerous cells while minimizing damage to healthy tissues. Mumbai, India's bustling metropolis, has emerged as a hub for cutting-edge medical treatments, including Radionuclide Therapy. In this blog, we will delve into the world of Radionuclide Therapy, its applications, benefits, and how Mumbai is at the forefront of providing this revolutionary treatment. Radionucleotide Therapy Specialist in Mumbai and be part of a transformative journey towards a healthier and brighter future.

Understanding Radionuclide Therapy
Radionuclide Therapy, also known as targeted molecular therapy or molecular radiotherapy, involves the use of radioactive substances, known as radionuclides, to treat various medical conditions, primarily cancer. These radioactive substances emit radiation that damages the DNA within cancer cells, leading to their destruction. What sets Radionuclide Therapy apart is its precision; it specifically targets cancer cells while sparing healthy tissues, reducing the side effects commonly associated with traditional treatments like chemotherapy and radiation therapy.
Applications of Radionuclide Therapy
Cancer Treatment: Radionuclide Therapy is commonly used to treat certain types of cancer, such as thyroid cancer, neuroendocrine tumors, and bone metastases. The therapy can effectively target cancer cells that have spread to bones, offering relief from pain and improving the patient's quality of life.
Pain Management: In cases where cancer has metastasized to the bones, Radionuclide Therapy can provide significant pain relief by targeting the cancerous cells causing discomfort. This can greatly enhance the patient's overall well-being.
Hyperthyroidism Treatment: Radionuclide Therapy is employed to treat hyperthyroidism by administering a radioactive iodine isotope. This isotope selectively accumulates in the thyroid gland, helping to regulate its activity and treat the condition.
Peptide Receptor Radionuclide Therapy (PRRT): PRRT is an innovative approach used for treating neuroendocrine tumors. It involves attaching a radioactive substance to a peptide that binds to specific receptors on cancer cells, delivering radiation directly to the tumor.
Mumbai's Role in Advancing Radionuclide Therapy
Mumbai, a city renowned for its medical expertise and state-of-the-art facilities, has been a pioneer in the field of Radionuclide Therapy in India. The city boasts several leading healthcare institutions that offer advanced treatment options for cancer patients, including Radionuclide Therapy.
Cutting-edge Facilities: Mumbai is home to world-class medical facilities equipped with the latest technology for diagnosis, treatment, and research in the field of nuclear medicine and Radionuclide Therapy.
Expert Medical Professionals: The city attracts top-notch medical professionals, including nuclear medicine physicians, radiation oncologists, and radiologists, who are well-versed in the intricacies of Radionuclide Therapy.
Research and Innovation: Mumbai's healthcare institutions actively engage in research and innovation related to Radionuclide Therapy. Clinical trials and studies are conducted to continually enhance the effectiveness of this treatment approach.
Benefits of Radionuclide Therapy
Precise Targeting: Radionuclide Therapy's precision targeting ensures that cancer cells are destroyed while healthy tissues remain unharmed, minimizing side effects.
Reduced Pain and Discomfort: For patients with bone metastases or certain types of cancer, Radionuclide Therapy can alleviate pain and discomfort, significantly improving their quality of life.
Minimized Invasive Procedures: In some cases, Radionuclide Therapy can be administered through oral or intravenous methods, reducing the need for invasive surgeries.
Conclusion
Radionuclide Therapy has revolutionized the landscape of cancer treatment, offering a more targeted and effective approach while reducing the adverse effects commonly associated with conventional treatments. Mumbai's prominent role in advancing this field highlights the city's dedication to providing cutting-edge medical care to its residents and patients from around the world. With its state-of-the-art facilities, expert medical professionals Pathology Lab Near Me, Mumbai and commitment to research, Mumbai is poised to continue making significant contributions to the field of Radionuclide Therapy, providing hope and healing to countless individuals battling cancer and other medical conditions.
#thyroid cancer specialist in mumbai#best diagnostic center in mumbai#best pathology lab in mumbai#neuroendocrine tumor specialist in mumbai#pathology lab near me
0 notes
Text
0 notes
Text
Neuroendocrine Tumors often present with large volume Liver Metastases and because these are relatively Indolent Tumors the patient often does not know that he has a Neuroendocrine Tumors specially those who have non-functioning Neuroendocrine Tumors.
#Transarterial Radioembolisation#Transarterial Radioembolisation in India#Side Effects of TARE#TACE vs TARE#PRRT#PRRT in India#PRRT in Neuroendocrine Tumors#PRRT Therapy#PRRT Therapy Side Effects#PRRT Treatment#PRRT Treatment Cost in India#PRRT Treatment for Neuroendocrine Tumors#PRRT Treatment in India#Peptide Receptor Radionuclide Therapy#Peptide Receptor Radionuclide Therapy in India#Nuclear Medicine Expert in India#Nuclear Medicine Therapy#Nuclear Medicine Therapy in India#Dr. Ishita B. Sen
0 notes
Text
Italy Peptide Receptor Radionuclide Therapy (PRRT) Market Analysis - 2027
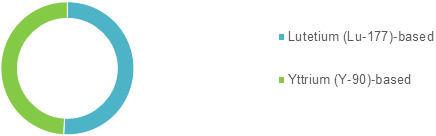
Italy Peptide Receptor Radionuclide Therapy (PRRT) Market, by Drug Type (Lutetium (Lu-177)-based, and Yttrium (Y-90)-based), by Indication (Gastroenteropancreatic Neuroendocrine Tumor), and by Distribution Channel (Hospital Pharmacies and Retail Pharmacies), was valued at US$ 3.6 million in 2019 and is expected to exhibit a CAGR of 9.2%over the forecast period (2019-2027), as highlighted in a new report published by.
Increasing drug approvals by regulatory bodies are expected to drive the Italy peptide receptor radionuclide therapy market growth over the forecast period. For instance, in 2018, Novartis received U.S FDA approval for lutetium (177Lu) oxodotreotide (Lutathera) for treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (NETs) (GEP-NETs). Furthermore, rising number of cancer cases in Italy is a major factor driving growth of the peptide receptor radionuclide therapy (PRRT) market in the country. For instance, according to European Union statistics related to cancer, in 2015, about 33,851 number of deaths were recorded due to lung cancer in Italy.
* The sample copy includes: Report Summary, Table of Contents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/3139
This contributed to around 8% in total Europe region, in which the share of all deaths attributed to lung cancer was 7.2 % among men, more than double the share (3.4%) recorded for women in Europe region.
Moreover, according to the WHO, in 2018, a total number of 40,9,808 cancer cases were recorded in Italy, which included prostate cancer, lung cancer, bladder cancer, colorectal cancer, breast cancer, and other type of cancers.
Browse 9 Market Data Tables and 12 Figures spread through 64 Pages and in-depth TOC on 'Italy Peptide Receptor Radionuclide Therapy (PRRT) Market, by Drug Type (Lutetium (Lu-177)-based, and Yttrium (Y-90)-based), by Indication (Gastroenteropancreatic Neuroendocrine Tumor), and by Distribution Channel (Hospital Pharmacies and Retail Pharmacies) – Italy Forecast to 2027'
Browse Research Report: https://www.coherentmarketinsights.com/market-insight/italy-peptide-receptor-radionuclide-therapy-market-3139
Key Takeaways of the Italy Peptide Receptor Radionuclide Therapy (PRRT) Market:
The Italy Peptide Receptor Radionuclide Therapy (PRRT) Market is expected to exhibit a CAGR of 9.2% over the forecast period (2019–2027) owing to increasing regulatory approvals and incidence of cancer
Among drug type, the Lutetium (Lu-177)-based segment is expected to account for a major revenue share by 2027, owing to increasing strategic acquisitions by key players for expanding their drug portfolio. For instance, in 2017, Novartis acquired Advanced Accelerator Applications. With this acquisition, Novartis has expanded its product offering in the neuroendocrine business, through which the company offers the Lutetium (Lu-177)-based drug.
Major player operating in the Italy Peptide Receptor Radionuclide Therapy (PRRT) Market is Novartis International AG.
Buy-Now this research report: https://www.coherentmarketinsights.com/insight/buy-now/3139
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
mailto:[email protected]
U.S. Office:
Name: Mr. Shah
Coherent Market Insights 1001 4th Ave,
# 3200 Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737
#Italy Peptide Receptor Radionuclide Therapy (PRRT) Market Size#Italy Peptide Receptor Radionuclide Therapy (PRRT) Market
0 notes
Text
PEPTIDE RECEPTOR RADIONUCLIDE THERAPY (PRRT) MARKET ANALYSIS
Peptide receptor radionuclide therapy (PRRT) is considered as targeted radionuclide therapy, as it provides radiation to destroy cancer cells via radiolabeled peptides. There are three types of radiations including β−particles (electrons), α particles, and Auger electrons that are used in the peptide receptor radionuclide therapy (PRRT). Among these radiations, β−particles (electrons) are widely used in PRRT, as it has a long range to penetrate in target tissues (0.05–12 mm), and neighboring cells around the targeted cell/tissue. Peptide receptor radionuclide therapy (PRRT) is majorly used in treatment of pancreatic & gastrointestinal tract - neuroendocrine tumor.
Rising incidence of pancreatic neuroendocrine tumor and gastrointestinal tract neuroendocrine tumor is expected to drive the market growth over the forecast period. According to the American Society of Clinical Oncology’s (ASCO) 2019 statistics, around 1,000 people are diagnosed with pancreatic neuroendocrine tumor each year in the U.S. and it accounts for 7% of pancreatic cancer cases. According to the same source, the number of patients diagnosed with pancreatic neuroendocrine tumor has been increasing by around 5% per year in the U.S.
The global peptide receptor radionuclide therapy (PRRT) market was valued at US$ 231.5 million in 2018 and is expected to exhibit a CAGR of 27.3% during the forecast period (2019–2027).
Figure 1. Global Peptide Receptor Radionuclide Therapy (PRRT) Market Share (%), By Disease Indication

Source: Coherent Market Insights Analysis (2019)
Increasing research and development activities, in order to develop novel treatment approach for cancer and regulatory approval for peptide receptor radionuclide therapy are expected to be major factors driving growth of the global peptide receptor radionuclide therapy (PRRT) market over the forecast period
Increasing pipeline studies to develop novel radiopeptide therapy is expected to drive growth of the global peptide receptor radionuclide therapy (PRRT) market over the forecast period. For instance, in May 2018, Merck Sharp & Dome Corp, in collaboration with BTG International Inc., initiated phase II clinical trial on peptide receptor radionuclide therapy for patients with well-differentiated neuroendocrine tumors and symptomatic and/or progressive metastases. The study is expected to be completed by November 2020.
Moreover, increasing product approvals by regulatory authority is expected to drive growth of the global peptide receptor radionuclide therapy (PRRT) market. For instance, in June 2019, Advanced Accelerator Applications S.A. (AAA), a Novartis company, received State of Israel Ministry of Health approval for its Lutathera, for treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). It is the first PRRT therapy approved in Israel. Lutathera is a lutetium Lu 177-labeled somatostatin analog peptide.
High cost associated with cancer treatment, which includes diagnosis and therapeutic treatment, is a major factor that is expected to restrain growth of the market
According to the World Health Organization’s 2018 data, the economic impact of cancer is increasing significantly. The total annual economic cost of cancer treatment in 2010 was estimated at US$ 1.16 trillion, globally. Moreover, till 2018, only one PRRT drug was approved in the U.S. and Europe based on peptide receptor radionuclide therapy (PRRT) and used in the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) including foregut, midgut, and hindgut neuroendocrine tumors in adults. Therefore, lack of drugs is another major factor restraining growth of this market. Novartis company’s drug named Lutathera is priced at US$ 47,500 for a full course of treatment, which comprises four infusions (cost for total 4-doses is US$ 190,000). Therefore, such high cost of drugs is expected to restrain growth of this market.
Global Peptide Receptor Radionuclide Therapy (PRRT) Market- Regional Analysis
On the basis of region, the global peptide receptor radionuclide therapy (PRRT) market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to hold dominant position in the market, owing to increasing product approval by regional drug regulatory authorities such as the U.S. FDA and Health Canada, to launch the products in the region. For instance, in January 2018, Advanced Accelerator Applications (AAA), a subsidiary of Novartis AG, received the U.S. Food and Drug Administration approval for its Lutetium (177Lu) Oxodotreotide (Lutathera) drug, which is indicated for gastroenteropancreatic neuroendocrine tumor.
Moreover, Asia Pacific peptide receptor radionuclide therapy (PRRT) market is expected to hold a significant market share during the forecast period, owing to increasing incidence of neuroendocrine tumor and rising adoption of expansion strategies by major manufacturers. For instance, in June 2015, Advance Accelerator Application, a subsidiary of Novartis AG, entered into a distribution agreement for Lutathera with FUJIFILM RI Pharma, Co., LTD. According to the agreement, FUJIFILM will be marketing and distributing Lutathera in the Japan market.
Figure 2: Global Peptide Receptor Radionuclide Therapy (PRRT) Market Share (%), By Region

Source: Coherent Market Insights Analysis (2019)
Novartis AG (Advanced Accelerator Applications, S.A.) is the major player operating in the global peptide receptor radionuclide therapy (PRRT) market.
About Us- Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. What we provide: Customized Market Research Services Industry Analysis Services Business Consulting Services Market Intelligence Services Long term Engagement Model Country Specific Analysis Mr. Shah
Coherent Market Insights Pvt.Ltd. Address: 1001 4th Ave, #3200 Seattle, WA 98154, U.S. Phone: +1–206–701–6702 Email: [email protected]
#PEPTIDE RECEPTOR RADIONUCLIDE THERAPY (PRRT) MARKET ANALYSIS#GLOBAL PEPTIDE RECEPTOR RADIONUCLIDE THERAPY (PRRT) MARKET ANALYSIS#PRRT MARKET ANALYSIS
0 notes
Text
I’ll have what she’s having.
Yesterday we had a consult with Dr. Ursina Teitelbaum at Penn. Aside from the issue of it taking an hour and a half to get there and then not being able to find the Abramson building or the correct parking garage, the Penn experience was a good one. (We get it, Abramson family, you’re super rich and love donating buildings). First, we met with Dr. Scully, who reviewed my history and diagnosis, and then she brought Dr. Teitelbaum in.
Dr. Teitelbaum started the appointment by saying while she didn’t have a plan yet, she hopes to have one by Friday. There is a monthly meeting at Penn where the cancer team gets together and discusses things and I happen to be one of those things tomorrow. She said there are three head honchos, who oversee the three main divisions of the cancer center. One, the head of “weird cancers” will be helping her work on a plan. She introduced what she called a "menu" of treatment options. It was not a menu in the traditional sense, where I could choose an entree, but more like a if/then flow chart with lots of catch-22 situations mixed in.
One of the biggest things I took away from this appointment was that I am a medical quandary to doctors, not because of the unusual nature of having two cancers, or even the rarity of my current cancer, but rather that I was young when I received the treatment for Hodgkins, and that chemotherapy has a lasting effect on my body, one that will unfortunately alter the plan for treatment now. Since I'm young and otherwise healthy, the "best" course of action would be to aggressively treat my liver, in hopes to remove the tumors or at least place them into a "hibernation." The issue is that all of the aggressive forms of treatment lead to future cancer, and being 37 years old, means it could certainly have negative implications before I am too old for it to matter. The primary concern is bone marrow cancer.
When I bluntly asked about my prognosis, or life expectancy, Dr. Teitelbaum said she REALLY didn’t like what she saw in my liver. She said there is a lot of unknown since many of the treatments available now didn’t even exist the first time I had this cancer, and so the future may provide more options, and my prognosis may be an ever changing thing, versus something definitive. This is hard. While I don’t want to hear that I have five years to live (or something relatively as morbid) it’s nice to know, and we just don’t.
Regarding treatment, everyone agrees, the first step is the Lanreotide. It's a simple decision because it has proven to be effective in many cases, it has minor side effects, and few long term effects on the body. Although, for the first time ever - Dr. Teitelbaum mentioned Lanreotide causing hair loss. Fun.
She mentioned debulking surgeries (taking large portions of the liver in hopes it will grow back healthy - sounds like multiple resection surgeries) but it’s a pretty intense process with the possibility that the cancer comes back. So onto other options...
Apparently there are three types of embolizations possible. These involve a tube going into the femoral artery in the groin, snaking it through the body to the tumor, and shooting beads into it to stop the blood supply and thus rendering the tumor inactive. The first, which is what we’ve always discussed, is bland embolization. That, is the most simple (or maybe blandest, bu-dum-dum) version. Then there is chemoembolization and radioembolization - which, to the best of my knowledge, is the same thing PLUS chemo or radiation in that targeted area. This would be more effective and also more dangerous (because of long term effects).
One of the other treatment options, based on the amount of cancer going on in my liver is Peptide Receptor Radionuclide Therapy or PRRT, which is a newer treatment, that was happening in Europe more often than the states (people were actually going to Europe to receive this therapy when it was not happening here) and it was historically reserved to treat tumors that were not receptive to somatostatin drugs alone, like Lanreotide, as a next step. Recently, according to Teitelbaum, it has been use as a more preventative (or earlier) measure as well. This treatment involves a peptide (cell-targeting protein) combined with a small amount of radiation to be injected into the bloodstream. The radiopeptide travels to and binds to the neuroendocrine tumor cells and delivers a high dose of radiation, thus "killing" the tumors. I don't know anything about the side effects of this, other than exhaustion and I have no idea how or when it would occur and how it would interfere with general life things.
The final additional treatment options were oral chemotherapy and targeted oral treatment. Both of these have more negative side effects and possible long term implications.
Lastly, the team wants me to do some genetic counseling. I have a second cousin with a similar condition (pancreatic and liver NETs) and my grandfather also had tumors - so it’s worth checking out. And this is something we can get ahead of with the kids if it turns out to be genetic after all. I can’t even begin to think about that - but I do need to know.
Dr. Teitelbaum recommends getting scanned every three months - probably an MRI, rather than a PET or CT scan to reduce the amount of radiation I am exposed to. This should be enough to monitor the liver and ensure we are making smart decisions about treatment.
I hope to have some more information tomorrow and will update accordingly! I was told my ears may be ringing between 7:30-8:30 a.m. — so I hope I can sleep!
* Dark side: My strengths (youth, health, beating cancer once before) are also my weaknesses.
* Bright side: Olive is totally on board the formula train!

* Next steps:
3/13/20 - phone call with Dr. Teitelbaum
3/25/20 - genetic counseling at Penn
* Irony GIF:

2 notes
·
View notes
Photo

Peptide receptor radionuclide therapy (PRRT) is an emerging modality for treating metastatic cancer. The current paradigm is to deliver a beta-emitting radionuclide to tumor sites via molecular specificity of the chelating agent.
The PRRT approach has shown efficacy in somatostatin-receptor positive cancers. The FDA has approved PRRT for treatment of metastatic midgut neuroendocrine tumors. The somatostatin analog octreotate with an attached “DOTA” chelator is known as DOTA-TATE, with (DOTA chelates the radionuclide). Imaging with gallium-68 (Ga-68) DOTA-TATE PET shows distribution of the cancer and subsequent therapy is accomplished with lutetium-177 (Lu-177) DOTA-TATE. Lu-177 is a beta emitter and the high energy electrons ablate the tumor from within.
The image below shows that this approach can be used for other somatosttin receptor positive tumors. The patient has widely metastatic Merkel cell carcinoma. A) Ga-68 DOTA-TATE PET and B) Lu-177 DOTA-TATE SPECT show the distribution of the cancer, with similar distribution of the two tracers. C) Post-treatment Ga-68 DOTA-TATE PET demonstrates the marked efficacy of the Lu-177 therapy. Image credit: Case Reports in Oncology 12 (1):98-103, January 2019.
#TeachingRounds #FOAMEd #FOAMRad #NuclearMedicine #PRRT #Nuclear #Therapy #Radiology #Oncology
1 note
·
View note
Text
Netspot pet

Netspot pet full#
As part of our commitment to care, we also offer same-day scheduling for your peace of mind.Ĭontact us to find out more about NetSpot in Santa Fe. PET imaging with NETSPOT has 2 to 3 times higher spatial resolution than somatostatin receptor scintigraphy with. We’re proud to be leaders in the field, working in 6 clinics located throughout the state. Since then, we’ve been bringing New Mexico patients leading-edge technology like NetSpot. Newer imaging agents targeting SSTR labeled with 68Ga have subsequently been developed, namely, DOTATATE and DOTATOC (7). XRANM has been in the vanguard of diagnostic radiology for over 70 years. following PET scans: FDG F18 Fluorodeoxyglucose NETSPOT - Ga68 Dotatate. These will be shared with your doctor and then, with you. CT Scan, Pet Scan / CT Scan, all offered at locations close to your home. Movement can impact the quality of captured images.įollowing the procedure, your technician and others will review your results. You’ll be asked to lie as still as possible during the scan. FDA-approved in 2016, NETSPOT is used in imaging to locate somatostatin receptor positive neuroendocrine tumors in adult and pediatric patients. NETSPOT ( 68 Ga-Dotatate) is a radioactive diagnostic agent used for PET imaging that is gaining ground among practices. Once the scan is started, the procedure will take 30 – 60 minutes. The Clinical Impact of Utilizing NETSPOT. Once you’re injected with NetSpot, your technician will ask that you wait between 40 and 90 minutes to proceed with the scan. Those taking short-acting versions of this medication can use them up to 24 hours prior to their procedure. If you’re taking a long-acting somatostatin analog, your scan will be scheduled before your next dose. If you’re taking Somatostatin analogs, it’s important that your doctor know. How to prepareīefore your NetSpot PET scan, you’ll need to advise your doctor of any and all medications, whether over the counter, prescription, herbal supplements or vitamins. This is a type of radiation therapy which uses hormones as part of the radiation to get to and destroy cancer cells. Guides therapeutic response: Due to the quality of captures obtained with this technology, oncologists can respond with more accurately targeted therapies.Īnd when this diagnostic is chosen, patients can source a new treatment for NETs – Peptide Receptor Radionuclide Therapy (PRRT).Higher quality images: The images produced by the PET scan used in combination with NetSpot are of a much higher resolution, which makes abnormalities easier to spot – even miniscule ones.Faster: NetSpot takes only 2 hours, whereas the octreoscan traditionally used as a diagnostic is a three-day process.With NetSpot, tumor detection is enhanced is 3 key ways: NETs absorb glucose very slowly in comparison to other cancers but contain somatostatin receptors. NetSpot deploys Ga-68 dotatate, a radioactive clone of a hormone which serves to bind somatostatin receptors, making tumors visible. NetSpot can identify even the smallest abnormalities with more success than other type of imaging agent currently in use. Previously, PET scans relied on tracers which identified tumors using radiolabeled glucose. PET scans on their own tend not to be effective in detecting these types of tumors, due to their slower uptake of glucose (unlike other cancer tumors).īut now, NetSpot has been developed. Their size makes them difficult to detect early.Īpproved by the FDA in 2016, NetSpot is a new tool which has revolutionized the standard positron emission tomography (PET). They can occur anywhere in the body, from the intestines to the lungs to the stomach and pancreas.
Netspot pet full#
Voiding is allowed a full bladder is not necessary.Neuroendocrine tumors tend to be quite small.For example: A 12:00 appointment would have a person drinking one glass of water at 9:00, 10:00, and 11:00.Beginning 3 hours before your scheduled exam time, drink 1 glass of water every hour, for a total of 3 glasses.Reading and phone use is allowed while in uptake.Wear comfortable clothing without metal clasps or zippers.If on a somatostatin analog such as Octreotide – please talk to your doctor as there are restrictions Anyone that accompanies the patient will be limited to the waiting area and may be allowed in patient areas at the sole discretion of the staff To ensure the safety of our patients and staff, only the patient will be allowed into the scan area. Appointment Instructions (Allow 1 ½ – 2 hours for this examination) Patients that do not show or cancel less than 24 hours prior to their appointment may be charged. Late arrivals may result in rescheduling of your scan to a later date. Please arrive 30 minutes prior to your scan time. Examination Instructions Arrival time for appointment

0 notes