#Orthomyxoviridae
Explore tagged Tumblr posts
Text
#bird flu#covid19#Ebola#global pandemic#health threats#Mpox#nipah virus#norovirus#Orthomyxoviridae#paramyxoviridae#priority pathogen#public health crisis#vaccine#vaccines#Viruses#who#priority pathogen watch list for public health preparedness
0 notes
Note
I did a presentation on this exact topic for class once!
Viral reassortment (the mechanism that allows the influenza viruses to mix and match like that) is really fascinating - TLDR is that viruses have many varied ways of storing their genome, and in the Orthomyxoviridae, the family that includes the influenza viruses, the genome takes the form of segmented RNA strands. These strands can freely recombine while the new viral particles are built inside the host cells, and it takes just one host infected by two strains at once to potentially create a new strain. Plus, like any other living (citation pending) creature, they can also randomly mutate a lil' bit when they reproduce, so that adds even more variability.
Hi! This is kind of a weird question but how/why was influenza (and other diseases that we have vaccines for now) so deadly 100-200 years ago? Obviously vaccines help tremendously, and probably immunity over time, but are there other reasons that the flu was a much bigger deal a century ago? Sorry if this is oddly specific, but my current project is historical. Thank you!
This is a very interesting question and there are a couple of different ways of looking at it.
Let's start with influenza:
[Note: it's surprisingly difficult to get good worldwide flu data, so I'm going to use US numbers for the purposes of this post.]
I think the first thing to understand is that unlike many other infectious diseases, influenza is substantially different every year. That means that the immunity that you build in 2017 from either the flu or the flu shot won't necessarily help prevent you from getting the flu in 2023. By then it will be a different enough virus that your previous immunity won't be as helpful. Though it might make it a little milder. But keep reading, I'll give you some fun facts to share at parties:
We name flu (A) viruses based on two different proteins on the surface of the virus. The proteins are "H" and "N". There are 16 different "H" proteins, and 9 different "N" proteins that we currently know of. The combination of the two forms the "name" of a particular flu virus. Think H1N1, or H5N6, or any other combination. Each combination has their own attributes, which contributes to how infectious or deadly they are in any given year. And which ones circulate are different every year.
Just mathematically, that's a lot of substantially different flu viruses. Hundreds of them, in fact. And you have to build immunity to each one individually. You could, say, build immunity to H2N5, but that would do little to save you from next year's H4N3. And not only that, but within a single type there are many smaller variations. For example, say you got H5N3, but then it went and mutated. If you then got exposed again, you might have some immunity to new!H5N3, but it could also be just different enough that you still get sick.
Like I said above, different types of flu virus are deadlier or spread faster than others. H5N1 (a type of avian flu with a human mortality rate of 52%) is terrifyingly deadly but fortunately doesn't spread particularly well, while H1N1 (the star of both the 1918 and 2009 flu seasons) spreads rapidly and kills primarily young adults (weird, since flu usually kills babies and old people).
This is why in 2009 we did the whole "close the schools vaccinate the teens hide the president" routine. Because if it was *that* H1N1 we were all about to be screwed in ways we had never experienced before. Fortunately it wasn't, but thank goodness we did it. Also if you got vaccine #2 in 2009, you are also protected against the 1918 strain of H1N1. You're gonna be a hit at parties with that one.
Now, if you look at only deaths (not the best measure, but one with some emotional punch), within the last decade alone we have years where 12,000 people died of flu in the US (2011-2012) and years where that number is as high as 61,000 (2017-2018). These numbers are similar throughout recent history (relative to population), but then you get years like 1968 (where 100,000 people died in the US) and 1957 (where 116,000 died), and then sometimes you get these wild whopping years like 1918 where 675,000 died (equivalent to 1,750,000 people dying in today's US population). These fluctuations have happened since Hippocrates was around, and probably long before that, and there's really nothing to suggest it's getting any milder in any statistically significant way.
Now, outside of these natural fluctuations, we do have some ways of driving down these numbers. We do have a vaccine. It is different every year, based on our prediction of what the most likely or dangerous types of flus will be this year. Fortunately, you do get to keep this immunity for some time, so you can look at the flu vaccine as a personal collection of different flu viruses you have immunity to- you can collect 2-3 different ones every year in one shot and you didn't even have to catch them!! Yay! Unfortunately, since we never reach herd immunity with the flu vaccine, and we can't perfectly predict and incorporate all the strains that will circulate in a given year, while you do get some protection, it's not ever perfect. But it *is* still worth it.
We also have other feats of modern medicine as backup to the flu vaccine. We have oxygen, antiviral drugs like tamiflu, immune modulating drugs, and technology like ventilators to help keep people alive in ways we would not be able to in previous generations. So that's also an advantage. Unfortunately, these don't always work either, and we are still at the whim of those yearly fluctuations in influenza virus deaths.
And really, if you ask any epidemiologist, covid is just a little trial run for the next Big One. Which is both extremely likely to be a flu virus and which we're statistically overdue for.
TL;DR: The flu isn't getting milder so much as it varies wildly in severity every year. The next major flu pandemic is probably going to be in our lifetimes, so start collecting your flu immunity now if you haven't yet. New collections drop every August and are available until April. Get em' while they're hot. This year's included a 2009-like strain of H1N1 and a delightful H3N2 number from Hong Kong.
As for All the Other Vaccine Preventable Illnesses:
*ahem*
Yes, it's vaccines. It's obviously vaccines. Its basically only vaccines. Anyone who has ever told you it's not vaccines is lying. No other major discovery of modern medicine has ever saved as many lives, prevented as many disabilities, and created as many opportunities for a life well lived as vaccines have. No antiviral drug, no antibiotic, no ventilator can even hold a candle to vaccines. The answer is f*cking vaccines*.
I hope I have made myself clear.
Enjoy this table:
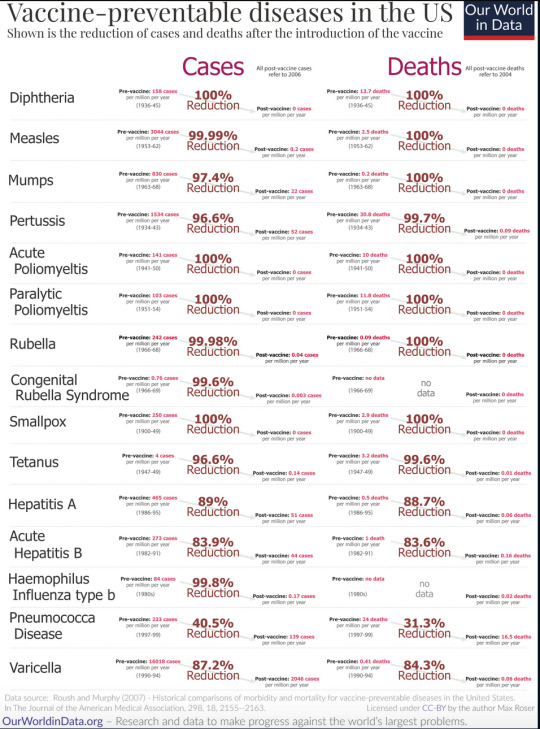
*Yes I do have a masters degree in public health and am a registered nurse that interacts with the public regularly, how did you know?
-Ross @macgyvermedical
#long post#also yes with viruses in particular you can't do better than vaccinate#bc they're so extremely simple there aren't many easily exploitable weak points compared to bacteria
7K notes
·
View notes
Text
Communicable Disease: Influenza Description of the Disease Influenza or "the flu" is a common illness in the winter months, all throughout the United States and many other countries. Both birds and all mammals can contract influenza (Brankston, et al., 2007). In recent years there have been scares regarding "bird flu" and "swine flu," both of which are simply different strains of influenza. The cause of the flu is an RNA virus in the family Orthomyxoviridae (Eccles, 2005). Once people contract the flu, they present with common symptoms such as chills, fever, a runny nose, muscle pains, a sore throat, and a headache. The headache is quite often severe, and flu sufferers may also have weakness, fatigue, severe bouts of coughing, and a general feeling of overall discomfort. People with the flu can also become nauseated and vomit, although that is more typical in children and not nearly as common in adults (Eccles, 2005). Many people also confuse influenza with the common cold, as many of the symptoms are similar. However, the flu is much more severe. The flu is transmitted person to person, and can also be transmitted to people from animals (Eccles, 2005). When a person who has the flu coughs or sneezes, the flu virus is transmitted through the air in the aerosol droplets that are produced. However, there are other ways to contract the flu. If a person touches nasal secretions from an infected person or droppings from an infected bird, he or she can catch influenza (Eccles, 2005). Additionally, viruses can live on various types of surfaces, sometimes for a long time. If a person touches a contaminated surface and then touches his or her nose, eyes, or mouth, the virus can be passed that way. That is why hand washing is discussed so much during flu season. Treatment of the flu is usually in the form of liquids and bed rest, as well as over the counter medications. Tamiflu is a popular option, but can be in short supply during widespread flu outbreaks. Demographics of Interest In most people, the flu is a troublesome and annoying illness that makes them feel bad for a few days to a week or longer. However, in very young children, the elderly, the immunocompromised, and people who have other medical conditions, the flu can be debilitating or even deadly. Seasonal epidemics are when the flu is most commonly seen, which translates to between three and five million cases of severe illness every year. Out of those severe illnesses, there are between 250,000 and 500,000 people who die either from the flu itself or from complications surrounding it (Ballinger & Standiford, 2010). In pandemic years, millions of people can die from influenza (Eccles, 2005). Three pandemics occurred in the 20th Century, and these generally occurred when a new strain appeared or the virus mutated in such a way that the current treatments and vaccines for it were not as effective (Harper, et al., 2005). There can be other reasons for pandemics to occur, but those are the most common causes. People who have preexisting conditions have both higher morbidity and higher mortality rates when it comes to the flu (Eccles, 2005). That is also true of the very young and the very old. The reason babies and the elderly have more trouble with influenza is due to the fact that they do not have the immunity levels seen in older children through middle-aged and senior adults. The same can be said of the people who have immune system issues. That can include people who have HIV or other immune suppressing diseases, but can also include people who have diseases that are not as serious (Brankston, et al., 2007). At times perfectly healthy people die from influenza, as well, and that can include people from any age group. While it is not as common to see this happen as it is to see people who are already sick pass away, there are times when the influenza virus hits someone particularly hard. In those cases, the flu often develops into viral and/or bacterial pneumonia, which can make breathing difficult (Eccles, 2005). Determinants of Health Determining a person's health is very important when it comes to judging how they may handle influenza. Of course, that does not mean health is the only factor. Stress, lack of sleep, lifestyle, proper eating habits, and other issues in a person's life can affect whether a person gets the flu and how he or she reacts to the illness (Eccles, 2005). For some people the flu is a minor annoyance for a few days, and for others it causes serious illness or death. Although age and preexisting conditions are both big factors for influenza problems, young people who are otherwise healthy can still experience complications that are completely unexpected. The flu can also be particularly hard on pregnant women and their developing fetuses, so they should always talk to their doctors about getting a flu shot and what other steps they can take in order to avoid contracting the flu (Ballinger & Standiford, 2010). Even people who do everything "right" can still get sick, but careful avoidance of those who are sick and proper hand washing can go a long way toward helping a person avoid the flu. Epidemiologic Triangle People who have weak immune systems and/or work extensively with the public are at the greatest risk for contracting the flu (Eccles, 2005). Both of those factors contribute to the development of the disease. The epidemiological triangle showcases this, as it is made up of the agent, the host, and the environment (Harper, et al., 2005). The agent is the virus itself, the host is the person or animal who harbors the virus, and the environment is the external factors that cause and/or allow for the transmission of the virus from one organism to another. The agent factors include the presence or absence of the virus, and the host factors include the person or animal that has the virus. The environmental factors are the most important, as they can be many and varied. It is the environmental factors that control how the virus spreads (Eccles, 2005). Role of the Community Health Nurse The community health nurse has an important role to play. He or she must focus on finding the cases of the flu in the area in order to accurately report them. The nurse must collect data from the patients, as well, and analyze that data in order to determine the severity of the level of flu being seen (Brankston, et al., 2007). If there are pandemic proportions, it is very important that be noted as soon as possible, so that people in the community can understand the seriousness of the issue. Additionally, it is vital that there are good follow-ups done in order to determine if the flu is peaking or if the number of cases is still building. By following up to see how ill people are getting and whether there are more or fewer deaths than would typically be seen, it is also possible to make a determination about the severity of the flu for that particular season (Harper, et al., 2005). This is highly valuable when it comes to making recommendations for people in the community and providing advice. National Agency or Organization One national agency that focuses on the flu and other diseases that fall into the communicable disease chain pattern is the Center for Disease Control (CDC) in Atlanta, Georgia (Ballinger & Standiford, 2010). The CDC is focused on controlling disease, but also on determining the severity of diseases in the United States and how big of a toll these diseases are taking on the public. Another main focus of the CDC is helping people learn how to break the communicable disease chain so that the disease does not get passed on from one person to another (Eccles, 2005). That is why the CDC advocates getting a flu shot, proper hand washing, staying home from work and other activities when one has influenza, and not sending children to school or daycare if they have the flu (Eccles, 2005). The more people do these types of things, the less likely they will be to get the flu and/or pass it along to others. References Ballinger, M.N. & Standiford, T.J. (2010). Postinfluenza bacterial pneumonia: Host defenses gone awry. Journal of Interferon Cytokine Research, 30(9): 643 -- 52. Brankston, G., Gitterman, L., Hirji, Z., Lemieux, C., & Gardam, M. (2007). Transmission of influenza A in human beings. Lancet Infectious Diseases, 7(4): 257 -- 65. Eccles, R. (2005). Understanding the symptoms of the common cold and influenza. Lancet Infectious Diseases, 5(11): 718 -- 25. Harper, S.A., Fukuda, K., Uyeki, T.M., Cox, N.J., & Bridges, C.B. (2005). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendation Report, 54(RR -- 8): https://www.paperdue.com/customer/paper/communicable-disease-180837#:~:text=Logout-,CommunicableDisease,-Length4pages Read the full article
0 notes
Text
Activitatea ingavirinului asupra virusurilor respiratorii umane în experimente in vivo
Citeste articolul pe https://consultatiiladomiciliu.ro/activitatea-ingavirinului-asupra-virusurilor-respiratorii-umane-in-experimente-in-vivo/
Activitatea ingavirinului asupra virusurilor respiratorii umane în experimente in vivo
Am mai scris despre Arbidol si Ingavirin, doua antivirale rusesti (descoperite si produse), fara licenta in alte state care emit astfel de licente, se pot cumpara din statele aflate in sfera de influienta a Rusiei. Profilul lor de siguranta pare a fi mai bun decat al antiviralelor produse de americani si europeni, desi studiile nu sunt cunoscute a fi la fel de riguroase. Din pacate nu putem face o comparatie pertinenta, asa ca luam datele asa cum sunt, tinand cont ca si in Rusia medicii s-au facut medici ca sa faca bine, si de faptul ca si in America, se castiga bani seriosi din dezvoltarea chimicalelor.
STUDIU Activitatea ingavirinului asupra virusurilor respiratorii umane în experimente in vivo
Vladimir V. Zarubaev 1,*,
Angelica V. Garshinina 1,
Nelly A. Kalinina 1,
Anna A. Shtro 1,
Svetlana V. Belyaevskaya 1,
Alexander V. Slita 1,
Vladimir E. Nebolsin 2 and
Oleg I. Kiselev 1
1Influenza Research Institute, 15/17 prof. Popova str., St. Petersburg 197376, Russia
2ValentaPharm, Ltd., 18 block 2, Gen. Dorokhova str., Moscow 119530, Russia*
Author to whom correspondence should be addressed.
Pharmaceuticals 2011, 4(12), 1518-1534; https://doi.org/10.3390/ph4121518
Submission received: 31 August 2011 / Revised: 16 November 2011 / Accepted: 18 November 2011 / Published: 25 November 2011
(This article belongs to the Special Issue Antivirals)
Rezumat
Infecțiile virale respiratorii constituie cel mai frecvent motiv de consultație medicală în lume. Ele pot fi asociate cu o gamă largă de manifestări clinice, de la infecții autolimitate ale tractului respirator superior până la afecțiuni mai devastatoare, cum ar fi pneumonia. În special, în cazurile grave, gripa A duce la pneumonie, care este fatală în special la pacienții cu boli cardiopulmonare, obezitate, copii mici și vârstnici. În studiul de față, arătăm un efect protector al compusului cu greutate moleculară mică Ingavirina (acid 6-[2-(1H-imidazol-4-il)etilamino]-5-oxohexanoic) împotriva virusului gripal A (H1N1), uman virusul parainfluenza și infecțiile cu adenovirus uman la animale. Mortalitatea, pierderea în greutate, titrul infecțios al virusului în țesuturi și morfologia țesuturilor au fost monitorizate în loturile experimentale de animale. Acțiunea protectoare a ingavirinei a fost observată ca o reducere a titrului infecțios al virusului în țesutul pulmonar, prelungirea vieții animalelor infectate, normalizarea dinamicii greutății pe parcursul bolii, scăderea mortalității animalelor tratate în comparație cu un controlul placebo și normalizarea structurii țesuturilor. În cazul infecției cu virusul gripal, activitatea protectoare a Ingavirinei a fost similară cu cea a compusului de referință Tamiflu. Pe baza rezultatelor obținute, ingavirina trebuie considerată o parte importantă a profilaxiei și terapiei antivirale.
Cuvinte cheie: gripă; virus paragripal; adenovirus; pandemic; Ingavirina; antiviral; model animal
1. Introducere Infecțiile virale respiratorii constituie cel mai frecvent motiv de consultație medicală în lume. Ele pot fi asociate cu o gamă largă de manifestări clinice, de la infecții autolimitate ale tractului respirator superior până la afecțiuni mai devastatoare, cum ar fi pneumonia. Prin urmare, prevenirea și controlul acestor infecții rămân obiective clinice majore. În prezent, există aproximativ 200 de viruși respiratori cunoscuți care pot fi grupați într-o familie de virusuri ADN (Adenoviridae) și patru familii de virusuri ARN (Orthomyxoviridae, Paramyxoviridae, Picornaviridae și Coronaviridae).
Virusul gripal A (IAV) este un agent extrem de infecțios care provoacă boli pulmonare acute. Focare de infecții cu virus gripal foarte patogen și apariția în 2009 a unei noi IAV pandemice au declanșat un reînnoit interes pentru cercetarea gripei. În mai 2010, peste 214 de țări și teritorii sau comunități de peste mări au raportat cazuri confirmate de laborator de IAV H1N1 2009, inclusiv peste 18.097 de decese [1]. Medicamentele antivirale ocupă o nișă importantă în managementul acestei boli [2,3]. Acestea vizează componente specifice virusului și sunt un tratament eficient atunci când sunt administrate în stadiul incipient al infecției sau imediat după expunerea la virus [3].
Două clase principale de medicamente antigripală sunt acceptate în prezent pentru chimioterapia IAV. Derivații adamantanului (amantadină și rimantadină) vizează canalul ionic M2 al IAV și nu sunt eficienți împotriva virusului gripal B [4]. Mai mult, apariția rapidă a rezistenței la medicamente în rândul virusurilor gripale de la mijlocul anilor 1990 a compromis mult eficacitatea acestor compuși [5]. Toți virusurile pandemice H1N1 testate până acum par, de asemenea, rezistente la medicamente [6].
Inhibitorii neuraminidazei (NAIs, oseltamivir, zanamivir și peramivir) au un spectru mai larg de activitate care include atât virusurile gripale A cât și virusurile B [7]. Cu toate acestea, din 2007 s-au observat apariția și transmiterea rapidă a virusurilor rezistente la medicamente [5,8,9]. Mai multe tulpini rezistente la NAI au fost, de asemenea, izolate de virusul pandemic H1N1 [10]. Există, prin urmare, o nevoie atât pentru identificarea de antivirale noi și eficiente, cât și pentru monitorizarea susceptibilității virusurilor circulante la compușii antivirali utilizați în clinici.
Paramixovirusurile includ virusuri importante asociate cu infecțiile tractului respirator superior și inferior la om. Printre acestea, trebuie remarcate virusul respirator respirator uman sincicial (RSV) și virusurile paragripale umane (HPIV).
RSV este o cauză majoră a bolii tractului respirator inferior la copiii prematuri (≤ 35 de luni de gestație), sugarii cu vârsta sub 6 luni și subiecții vârstnici instituționalizați. Rezultatul infecției cu RSV implică de obicei infecții ușoare ale tractului respirator superior; totuși, afecțiuni mai severe, cum ar fi pneumonia și bronșiolita, apar la 25-40% dintre copii. Aproximativ 1% dintre sugarii infectați cu RSV necesită spitalizare [11,12].
Au fost descrise patru serotipuri distincte de virusuri paragripale umane [13]. Acești virusuri pot provoca boli ale tractului respirator superior la indivizi de toate grupele de vârstă, deși copiii mici între 6 luni și 3 ani prezintă boli mai severe [13].
Adenovirusurile (AdV) sunt viruși ADN-genom fără anvelopă. În ciuda tropismului multiorganic, unele tipuri de AdV au un tropism preferențial pentru tractul respirator și pot provoca o gamă largă de simptome respiratorii, inclusiv coriza, faringita, amigdalita, bronșita și pneumonia. În general, infecțiile cu AdV sunt ușoare sau autolimitate și se rezolvă în două săptămâni fără complicații pe termen lung. Cu toate acestea, acești virusuri constituie o cauză importantă de mortalitate și morbiditate la pacienții imunocompromiși, inclusiv nou-născuții și primitorii de transplant de măduvă osoasă [11].
Mai mulți compuși pot fi utilizați pentru tratamentul infecției cu RSV, AdV și HPIV. Ribavirina inhibă replicarea virală prin mai multe mecanisme, inclusiv inhibarea polimerazei virale, inhibarea formării capacului 5′ a ARNm și inhibarea IMP dehidrogenazei conducând la o scădere a concentrațiilor intracelulare de GTP [14]. Nu există agenți terapeutici aprobați împotriva infecțiilor cu AdV. Cu toate acestea, unele antivirale cu spectru larg, cum ar fi ribavirina și analogul nucleozidic cidofoir, au fost utilizate în tratamentul infecțiilor severe cu Ad la gazdele imunodeprimate [11]. În plus, în acest scop au fost utilizați inhibitori de fuziune, oligonucleoide antisens și steroizi, niciunul dintre ei nu a fost aprobat pentru aplicare clinică.
Anterior, compusul cu greutate moleculară mică Ingavirina [6-[2-(1H-imidazol-4-il)etilamino]-5-oxohexanoic acid, cunoscut anterior și sub numele de Ingamine] s-a dovedit a avea activitate antigripală împotriva virusurilor gripale A(H3N2), A(H5N1) și B într-un model animal [15,16] și împotriva tulpinilor pandemice ale virusului gripal A/California/04/2009 și A/California/07/2009 [17–19]. În experimentele cu IAV H1N1, ingavirina a scăzut efectul citopatogen indus de virus în cultura celulară cu 50 până la 79% în comparație cu celulele martor. Șoarecii infectați cu IAV H3N2 sau H1N1 (2009) și tratați cu Ingavirină au demonstrat o mortalitate mai scăzută (aproximativ 40%) și o durată medie de viață crescută (aproximativ 4 zile) în comparație cu animalele tratate cu placebo. Luate împreună, aceste date sugerează că Ingavirina este un instrument prospectiv pentru tratamentul infecțiilor cu IAV, în special cele cauzate de virusurile pandemice. Mai mult, ingavirina a demonstrat activitate în experimente in vitro și in vivo împotriva adenovirusului uman și a virusului paragripal [20,21]. Cu toate acestea, mecanismul exact al eficacității sale clinice este departe de a fi înțeles complet.
Aici rezumăm rezultatele studiilor privind activitatea protectoare a Ingavirinei folosind modele de pneumonie gripală letală cauzată de virusul gripal pandemic A(H1N1)2009, pneumonie ușoară cauzată de HPIV și infecție diseminată indusă de adenovirus și prezentăm noi date despre aceasta. activitate antivirală privind virusurile respiratorii umane. Pe baza acestor rezultate se pot face concluzii cu privire la gama sa de activitate și aplicarea ulterioară împotriva bolilor specifice.
2. Materiale și Metode 2.1. Compuși Ingavirina (acid 6-[2-(1H-imidazol-4-il)etilamino]-5-oxohexanoic, Figura 1) a fost furnizată ca substanță pură de către producător (Valenta Pharmaceuticals, Moscova, Rusia). Tamiflu (fosfat de oseltamivir, LaRoche, Elveția), 6-azacitidina (Institutul de Biologie Moleculară și Genetică, Kiev, Ucraina) și ribavirina (ICN Biochemicals, SUA) au fost utilizate în experimente ca medicamente de referință.
Structura INGAVIRIN, un compus asemanator cu Arbidol
2.2. Viruși Virusul gripal A/California/07/09 (H1N1) a fost obținut din colecția de viruși de la Institutul de Cercetare a Gripei. Înainte de experimente, virusul a fost adaptat la șoareci prin trei pasaje în serie în țesutul pulmonar al șoarecilor, urmate de o trecere ulterioară prin cavitatea alantoică a embrionilor de pui de 10-12 zile și o trecere finală prin șoareci [22]. Omogenatul pulmonar în nouă volume de soluție salină sterilă tamponată cu fosfat a fost utilizat ca material infectant în experimente ulterioare.
Virusul paragripal uman (hPIV) de tip 3 (tulpina HA1) și adenovirusul uman (AdV) de tip 5 au fost obținute din colecția de viruși de la Institutul de Cercetare a Gripei și propagate în MA-104 (ATCC CRL-2378) sau HEp-2 (ATCC). celulele CCL-23), în mod corespunzător, la 36 °C în 5% CO2.
2.3. Animale În experimentele cu virusul gripal au fost utilizați femele de șoareci Balb/c, de 16–20 de grame, de la ferma de creștere a animalelor de laborator Rappolovo. Hamsterii sirieni crescuți în Institutul de Cercetare a Gripei au fost utilizați pentru experimente cu hPIV și AdV. Experimentele pe animale au fost planificate în conformitate cu principiile îngrijirii animalelor de laborator (Guide for the Care and Use of Laboratory Animals, National Academy Press: Washington, DC, USA, 1996) și aprobate de Comitetul de etică instituțională.
2.4. Titrarea virusului Înainte de studiile activității protectoare a ingavirinei la animale, virusul gripal adaptat la șoarece a fost titrat pentru efectul său letal. În acest scop, șoarecii (10 în fiecare grup experimental) au fost inoculați intranazal sub anestezie cu 50 μL de diluții zecimale în serie (10-1 până la 10-5) de omogenat pulmonar al șoarecilor infectați cu virus. Diluția care a provocat moartea a 50% dintre animale în 14 zile după infecție (DL50) a fost calculată așa cum a fost descris anterior [23] și utilizată pentru experimentele ulterioare.
2.5. Activitatea protectoare a INGAVIRIN Pentru evaluarea activității antigripală a Ingavirinei in vivo, șoarecii au fost infectați cu cinci LD50 (20 de șoareci) sau un LD50 (30 de șoareci) din virusul titrat anterior (vezi secțiunea „Titrarea virusului”). Ingavirina a fost diluată în ser fiziologic la doze de 15, 20 sau 30 mg/kg greutate corporală/zi și aplicată oral prin gavaj o dată pe zi în ziua unu, două, trei, patru și cinci după infecție (p.i.). Pentru a studia efectul programului de tratament asupra activității de protecție, grupuri separate de animale au fost tratate de două ori (zilele 1 și 2 p.i.) cu 30 mg/kg ingavirină urmate de trei doze de 15 mg/kg (zilele 3, 4 și 5 p.i.) . Cantitatea totală de ingavirină primită de animalele din acest grup a fost egală cu cea din grupul de 20 mg/kg. Medicamentul de referință Tamiflu (doza finală 20 mg/kg greutate corporală) a fost dizolvat în soluție salină și aplicat la 20 de șoareci (cinci DL50) sau 30 de șoareci (o DL50) pe cale orală într-un volum de 200 μL. Animalele martor au fost tratate cu ser fiziologic steril.
Animalele din toate loturile experimentale au fost cântărite zilnic. S-a calculat mortalitatea la fiecare grup de animale. Fiecare grup a fost verificat zilnic pentru animale moarte timp de două săptămâni după inoculare. Pe baza datelor primite, s-au calculat procentul de mortalitate, indicele de protecție (raportul mortalității în grupul de control față de mortalitatea din grupul experimental) și ziua medie a decesului (MDD).
În ziua a treia p.i., zece șoareci din fiecare grup infectat cu un DL50 al virusului au fost sacrificați, pieptul lor deschis și plămânii izolați. Cinci plămâni au fost utilizați pentru titrarea virusului și alți cinci pentru examinarea histologică (vezi secțiunea „Examinare histologică”).
Pentru a determina titrul infecțios al virusului în țesutul pulmonar, plămânii au fost omogenizați în zece volume de soluție salină sterilă tamponată cu fosfat. S-au preparat diluții în serie (10-1-10-7) din fiecare omogenat. Celulele MDCK crescute în plăci cu 96 de godeuri au fost inoculate cu 0,2 mL din fiecare diluție și incubate la 36 °C timp de 48 de ore în 5% CO2. După incubare, supernatantul a fost recoltat și testat pentru prezen��a virusului gripal prin amestecarea fluidului în godeuri cu fund rotund cu volume egale de suspensie 1% de eritrocite de pui în ser fiziologic. Titrul de virus în plămâni a fost considerat diluția finală atunci când a provocat o reacție de hemaglutinare pozitivă în godeu, iar titrul de virus este exprimat în log10EID50/20 mg țesut. Activitatea compușilor a fost evaluată prin capacitatea lor de a scădea titrul infecțios al virusului în țesutul pulmonar.
Pentru studiul activității anti-hPIV a ingavirinei, hamsterii sirieni în vârstă de patru până la cinci săptămâni au fost infectați cu 0,05 mL (104 TCID50) de virus parainfluenza uman (hPIV) intranazal, așa cum este descris în [24]. Ingavirina a fost utilizată așa cum este descris mai sus. În ziua 3 și 7 p.i. animalele au fost sacrificate, iar plămânii lor au fost utilizați pentru titrarea virusului în celulele MA-104 și, respectiv, analiza histologică (vezi mai jos). Titrul virusului a fost determinat prin ELISA utilizând anticorpi monoclonali anti-hPIV (PPDP Ltd., St. Petersburg, Rusia).
Activitatea anti-AdV a compusului a fost testată așa cum s-a descris anterior [25].
2.6. Examenul histologic Plămânii animalelor au fost fixați în formaldehidă tamponată cu PBS 4%, deshidratați în etanol gradat și încorporați în parafină. Secțiuni de patru micrometri au fost tăiate și colorate cu hemotoxilină-eozină.
În cazul infecției gripale, celulele epiteliului bronșic au fost împărțite în patru categorii distincte morfologic: (i) celule intacte fără semne de replicare a virusului; (ii) celule cu stadii inițiale de formare a incluziunilor specifice virusului; (iii) celule cu incluziuni avansate de virus; și (iv) celule moarte care arătau ca goluri între alte celule cu denudare a membranei bazale. Au fost calculate ratele fiecărei categorii de celule dintre celulele stratului epitelial bronșic. Valorile morfometrice au fost evaluate de doi observatori independenți.
3. Rezultate 3.1. Infecția gripală Inocularea animalelor cu un virus gripal adaptat a dus la dezvoltarea pneumoniei gripale. Semnele clinice ale bolii au fost tipice pentru infecția gripală severă și au inclus ataxie, tremor, respirație scurtă, precum și scăderea consumului de apă și alimente care duce la pierderea în greutate. În ziua 15 p.i. S-a observat moartea a 55–90% dintre animalele infectate, în funcție de doza infectantă a virusului.
Activitatea protectoare a ingavirinei a fost evaluată atunci când este aplicată o dată pe zi timp de cinci zile după inocularea virusului. Nu s-a observat nicio mortalitate nespecifică în grupurile de control de animale neinfectate tratate cu soluție salină și șoareci neinfectați tratați cu Ingavirin. Aplicarea ingavirinei a dus la o scădere a mortalității (la 18–67%), precum și la o creștere a zilei medii de deces (1,2–4,1 zile) comparativ cu animalele martor (în funcție de doza de virus și compus). Șoarecii tratați cu compusul de referință Tamiflu au demonstrat, de asemenea, o mortalitate semnificativ mai scăzută (indicele de protecție 80%) și prelungirea duratei medii de viață (până la 5,4 zile) în comparație cu valorile martor (Tabelul 1). În plus, tratamentul animalelor cu Ingavirina, în mod similar cu compusul de referință Tamiflu, a dus la normalizarea dinamicii greutății animalelor (Figura 2), conducând la profiluri de greutate similare în comparație cu animalele neinfectate.
Figura 2. Dinamica greutății corporale a șoarecilor în cursul pneumoniei cauzate de virusul gripal A/California/7/09 (H1N1)v. Tabelul 1. Activitatea protectoare a Ingavirinei împotriva gripei A (H1N1) 2009. Valorile p < 0,05 sunt indicate cu caractere aldine.
După cum se arată prin titrarea virusului din plămânii șoarecilor, în ziua a treia p.i. virusul s-a replicat în țesutul pulmonar până la 105,1 EID50/20 mg țesut. Aplicarea compusului de referință Tamiflu a scăzut titrul viral de aproximativ 320 de ori (102,6 EID50/20 mg țesut). Tratamentul animalelor cu Ingavirina a dus, de asemenea, la o scădere a titrurilor virusului (aproximativ 103,5 EID50/20 mg), care este statistic identică cu activitatea oseltamivirului (Tabelul 1).
Pentru a evalua efectul ingavirinei asupra structurii țesutului pulmonar, analiza morfologică a fost efectuată în ziua a treia p.i. Plămânii șoarecilor infectați cu virus au fost consolidați și edematoși. Toți șoarecii infectați au prezentat leziuni alveolare difuze exsudative cu edem interstițial, exsudate fibrinoase în alveolele lor, infiltrație inflamatorie, necroză epitelială bronșială și descuamare. Celulele epiteliului bronșic și bronșiolar au conținut incluziuni virale sau au fost absente cu denudarea membranei bazale [Figura 3(a)].
Figura 3. Patologii ale șoarecilor inoculați intranazal cu virusul gripal adaptat la șoarece A/California/7/09 (H1N1)v în ziua 3 p.i. (a) Plămânii unui șoarece tratat cu placebo. Suprafață mare de pneumonie cu exsudate intense în lumenul bronșic, alveolită severă neutrofilă până la histiocitară; (b) Peribronșită limfocitară ușoară cu alveolită ușoară la un șoarece tratat cu 30 mg/kg greutate corporală de ingavirină. Celulele epiteliului bronșic sunt intacte, exsudate ușoare în lumenul bronșic; (c) Peribronșită limfocitară ușoară cu alveolită la un șoarece tratat cu 20 mg/kg oseltamivir; (d) Plămânii șoarecilor intacți. Fără semne de inflamație sau distrugere celulară. Hematoxilin-eozină, ×200. Aplicarea Ingavirinei, similar cu compusul de referință Tamiflu, a dus la normalizarea structurii țesutului pulmonar, în special, la limitarea edemului și a leziunilor alveolare, scăderea cantității de reziduuri în lumenul bronșic și protecția epiteliului bronșic de moarte [Figura 3( b), Tabelul 1].
Pentru a studia mecanismul activității de protecție a ingavirinei, ne-am uitat mai atent la celulele epiteliului bronșic care sunt considerate ținte primare pentru virusul gripal. Analiza morfometrică a celulelor a arătat că aproape 100% din celulele epiteliale ale șoarecilor intacte au fost reprezentate de celule intacte.
Inocularea cu virusul gripal a condus la creșterea ratei celulelor din alte trei grupuri (adică celule cu incluziuni inițiale, incluziuni avansate și celule moarte), sugerând o acțiune citotoxică puternică a virusului. Aplicarea atât a Ingavirinei, cât și a compusului de referință oseltamivir a scăzut puternic numărul de celule moarte și a crescut rata celulelor intacte ale epiteliului bronșic. Din aceste rezultate sugerăm că acest compus este capabil să protejeze celulele împotriva daunelor induse de virus (Figura 4).
Figura 4. Activitatea citoprotectoare a ingavirinei în celulele epiteliului bronșic după infectarea cu virusul gripal adaptat la șoarece A/California/7/09 (H1N1)v (ziua 3 p.i.). 3.2. Infecția cu virusul parainfluenza Infectarea hamsterilor sirieni cu virusul parainfluenza umană (hPIV) are ca rezultat bronșită ușoară, bronșiolită și pneumonie neletale. Virusul poate fi recuperat din plămâni în ziua 3 p.i., iar afectarea specifică a țesuturilor poate fi observată în ziua 7.
După cum se arată prin titrarea virusului, aplicarea atât a ingavirinei, cât și a compusului de referință ribavirină a condus la reducerea titrului infecțios al hPIV în țesutul pulmonar. Ribavirina a demonstrat cea mai mare activitate în scăderea titrului, deși ambele doze de Ingavirina au condus, de asemenea, la o reducere semnificativă statistic (Tabelul 2).
Tabelul 2. Activitatea infecțioasă a hPIV în țesutul pulmonar al hamsterilor sirieni după aplicarea ingavirinei.
Hamsterii infectați cu hPIV nu au prezentat dovezi observabile de boală înainte de sacrificiu, dar din punct de vedere histologic a existat o producție constantă de pneumonie. Leziunile pulmonare constau din exsudate endobronșice împrăștiate compuse din celule mononucleare și polimorfonucleare, infiltrate de celule rotunde peribronșice și perivasculare și zone largi de pneumonie interstițială. Epiteliul bronșic avea un aspect specific care conținea grupuri de celule gigantice înalte care ieșeau în lumenul bronșic [Figurile 5 (a, b)]. Animalele tratate cu ingavirină și ribavirină au demonstrat o arhitectură a țesutului pulmonar aproape normal, cu puține celule infiltrante [Figurile 5 (c, d)].
Figura 5. Structura țesutului pulmonar al hamsterilor sirieni în ziua 7 după inocularea cu hPIV-3 cu (c,d) sau fără (a,b) tratament cu Ingavirină. a, c—Alveole, b, d—Epiteliu bronșic. Hematoxilină-eozină, ×100 (a), ×400 (b,c,d). 3.3. Infecția cu adenovirus uman Întrucât adenovirusurile umane nu provoacă infecții respiratorii în aer la animale, am studiat activitatea antivirală a ingavirinei folosind modelul dezvoltat anterior de infecție adenovirală diseminată a hamsterilor sirieni nou-născuți cauzată de adenovirusul uman de tip 5 [25]. Similar cu infecția cu hPIV, activitatea de protecție a fost evaluată prin titrarea virusului în organele țintă și analiza histologică a arhitecturii țesuturilor.
Infectarea subcutanată a hamsterilor sirieni nou-născuți a dus la replicarea virusului în plămânii și ficatul animalelor. Rezultatele titrarii virusului în celulele HEp-2 în ziua a treia p.i. sunt rezumate în tabelul 3.
Tabelul 3. Activitatea infecțioasă a AdV în ficat și plămâni la hamsterii sirieni după aplicarea ingavirinei.
După cum se poate observa, tratamentul infecției cu AdV cu compusul de referință 6-AC a dus la restricția replicării virusului atât în ficat, cât și în plămânii animalelor, ceea ce este în acord cu rezultatele noastre anterioare [25]. Aplicarea ingavirinei a condus la o reducere moderată (aproximativ o zecimală), dar semnificativă statistic, a titrului virusului atât în ficat, cât și în plămânii animalelor.
Investigarea histologică a ficatului animalelor infectate a evidențiat focare de necroză. Din punct de vedere morfologic, au fost zonele de distrugere a parenchimului cauzate de leziunea specifică a hepatocitelor și distrugerea nespecifică a țesutului din cauza unei reacții inflamatorii locale. Leziunile specifice celulelor hepatice s-au manifestat în creșterea nucleelor celulare, deformarea acestora și apariția corpurilor de incluziune nucleară eozinofili și bazofili specifici virusului. Alterările reactive ale țesutului s-au datorat distrugerii inflamatorii a hepatocitelor și infiltrației tisulare cu leucocite. Animalele infectate tratate cu ingavirina au avut focare mai mici de inflamație și hepatocite cu aspect intacte, spre deosebire de hepatocitele foarte vacuolizate la animalele de control (Figura 6).
Figura 6. Patologia hepatitei induse de AdV în ficatul hamsterilor sirieni nou-născuți în ziua 3 p.i. Focalizare mare de inflamație cu numeroase celule infectate cu AdV (vârfuri de săgeată), vacuolizarea hepatocitelor (a, b) la animalele de control, focar mic de inflamație și parenchim intact (c) la animalele tratate cu Ingavirina. Hematoxilin-eozină, ×400. Pentru a cuantifica efectul protector al ingavirinei, am măsurat dimensiunea focarelor de necroză în ficat și am numărat numărul de celule infectate cu AdV în fiecare focar (Tabelul 4).
Tabelul 4. Efectul ingavirinei asupra evoluției hepatitei induse de AdV la hamsterii sirieni nou-născuți. Masă După cum arată analiza morfometrică, aplicarea Ingavirinei a scăzut dimensiunea medie a focarelor de inflamație indusă de virus și a redus puternic numărul de celule infectate. Interesant, efectul unei doze mai mari de medicament (45 mg/kg/zi) a fost mai mic decât cel al dozei mai mici (30 mg/kg/zi), sugerând un mod specific de activitate a acestui compus.
4. Discuție În studiul de față, am arătat un efect protector al compusului cu greutate moleculară mică Ingavirina împotriva infecției letale cu virusul gripal cauzat de virusul gripal pandemic A (H1N1) la șoareci și a patologiilor nefatale ale hamsterilor sirieni cauzate de virusul paragripal uman și uman. adenovirus. Au fost investigate efectele dozei asupra activității de protecție a compusului și a replicării virusului în țesut. Acțiunea protectoare a ingavirinei a fost demonstrată ca o reducere a titrului infecțios al virusului în țesutul pulmonar, prelungirea vieții animalelor infectate, normalizarea dinamicii greutății în cursul bolii, scăderea mortalității animalelor tratate în comparație cu un control placebo și normalizarea structurii țesutului pulmonar și hepatic. În cazul infecției gripale, activitatea protectoare a Ingavirinei a apărut similară cu cea a compusului de referință Tamiflu.
În experimentele noastre, Ingavirina a demonstrat activitatea de protecție împotriva pneumoniei gripale letale la șoareci. La unele doze, efectul protector a fost egal cu activitatea oseltamivirului, care este un medicament acceptat la nivel internațional, dovedit a fi eficient împotriva acestui IAV. În general, rezultatele noastre sunt în acord cu datele obținute anterior [15-19] în care s-a demonstrat că Ingavirina are un efect protector împotriva virusurilor gripale A și B.
Krug și Aramini [26] au sugerat că două domenii posibile ale nucleoproteinei virusului gripal (NP), unul situat într-o buclă de coadă și altul într-un canal de legare a ARN găsit între domeniile capului și corpului la suprafața exterioară a trimerului NP, reprezintă potențialele ținte antivirale. Domeniul localizat în bucla de coadă este crucial pentru oligomerizarea NP care este, la rândul său, necesară pentru transcripția și replicarea eficientă a genomului viral. Prin urmare, inactivarea acestui domeniu ar putea fi eficientă pentru suprimarea replicării virusului. Într-adevăr, unele experimente sugerează că Ingavirina vizează nucleoproteina gripei (NP). Într-un studiu recent [27] s-a demonstrat că ingavirina interacționează cu virusul gripal NP, prevenind astfel oligomerizarea NP necesară pentru replicarea virală. În al treilea studiu, mai mulți analogi ai micalamidei A au fost identificați ca inhibitori direcționați de NP ai replicării virusului gripal [28]. S-a demonstrat că acești compuși se leagă de coada N-terminală de 13 aminoacizi, care mediază transportul nuclear al NP și legarea acestuia de ARN viral. Mai mult, Kao et al. [29] au raportat identificarea unui compus cu molecule mici, nucleozina, care declanșează agregarea NP și inhibă acumularea sa nucleară. Nucleozina a împiedicat replicarea virusului gripal A in vitro cu o concentrație nanomolară și a protejat șoarecii provocați cu doze letale de gripă aviară A H5N1. Aceste date sugerează că legarea compusului la această țintă poate inhiba replicarea virală prin inhibarea funcțiilor NP. Nici analogii micalamidei A și nici nucleozina nu demonstrează nicio asemănare structurală cu Ingavirina, ceea ce ne face să presupunem că alte domenii ale NP virale pot fi implicate în interacțiunea cu Ingavirina.
Dintr-o altă parte, s-a demonstrat că aplicarea Ingavirinei are ca rezultat modificarea morfologiei virionilor detectați în lavajul bronhoalveolar al șoarecilor infectați [30]. Animalele de control au produs în cea mai mare parte virioni sferici, în timp ce la animalele tratate cu ingavirină s-au format probabil particule filamentoase cu infectivitate redusă. Aceste rezultate sugerează că ingavirina ar putea interfera cu procesul de asamblare și/sau înmugurire a virusului, conducând la reducerea încărcăturii virale.
În studiul nostru, activitatea oseltamivirului a apărut mai mică decât în experimentele similare ale lui Smee și colab. [31]. Acest lucru ar putea fi legat de o doză mai mare de virus, virus diferit utilizat în experimentele noastre și program diferit de aplicare a medicamentului (o dată pe zi în loc de două ori pe zi în [31]). În același timp, efectele antivirale directe ale ingavirinei detectat printr-o scădere a titrului infecțios al virusului în țesutul pulmonar a apărut de zece ori mai puțin decât cel al Tamiflu, în ciuda nivelului similar de protecție împotriva letalității (Tabelul 1). Această contradicție ar putea indica faptul că alte mecanisme, în plus față de o activitate antivirală directă, ar putea contribui la protecția rezultată a animalelor împotriva letalității cauzate de IAV.
Infecția cu virusul gripal variază în severitate de la o infecție asimptomatică la o boală gravă cu caracteristici sistemice. Gripa severă se manifestă prin reacții specifice virusului cu dezvoltarea ulterioară a proceselor reactive. Aceste procese sunt induse de virusul care se replica în celulele țintă și realizate prin mecanisme gazdă, inclusiv reacții imune, stres oxidativ și alte procese de radicali liberi, activitate proteolitică îmbunătățită, creșterea bruscă a nivelului de citokine proinflamatorii și multe altele [32].
În clinici, unul dintre principalele motive pentru pneumonia gripală severă și complicată, inclusiv cazurile fatale, este tratamentul tardiv și/sau inadecvat [33,34]. În aceste cazuri, evoluția bolii este determinată de mecanisme care sunt inițial induse de virus, dar în cele din urmă realizate de gazdă, inclusiv, în special, inflamația severă („furtuna de citokine”) [35-37]. Experimentele folosind șoareci knockout infectați cu virusul gripal cu gene inactivate în căile inflamatorii, cum ar fi interleukina 1α/β, receptorii de chemokine macrofagice CCR5 și CCR2, ciclooxigenaza 1 și 2 [38–40] au demonstrat clar că, în plus față de nivelul de virus ‘ replicare în plămâni, intensitatea reacțiilor gazdei contribuie în mod semnificativ la cursul și rezultatul bolii. Prin urmare, în cazurile severe de gripă, atât medicamentele antivirale directe, cât și cele patogenetice ar trebui incluse în terapia complexă, în special acei compuși care limitează furtuna de citokine, edemele pulmonare, inflamația și afectarea țesuturilor [41]. De exemplu, recent a fost demonstrată activitatea de protecție ridicată a 7-hidroxicumarinei (7-HC) [42]. S-a demonstrat că 7-HC posedă proprietăți antivirale datorită capacității sale de a scădea nivelul de citokine proinflamatorii la animalele infectate, atenuând astfel infecția gripală severă. În același timp, 7-HC nu a redus nivelul de replicare a virusului în testele de reducere a plăcii, ceea ce sugerează că activitatea sa de protecție, inclusiv scăderea replicării virusului în plămânii de șoarece, este de natură complexă și poate fi mediată de semnalizarea celulară și căile reactive. S-ar putea sugera că, pe lângă capacitatea de a scădea în mod direct nivelul de replicare a virusului în plămâni (Tabelul 1), ingavirina ar putea avea proprietăți similare pe baza rezultatelor examinărilor de morfologie pulmonară la șoarece, care arată că tratamentul cu Ingavirina a redus semnificativ gradul de leziuni tisulare, inflamație și edem (Figura 3, Tabelul 1). Studii suplimentare asupra efectelor ingavirinei asupra diferitelor căi patogenetice ar fi utile pentru înțelegerea mecanismului său de activitate. Ingavirina trebuie considerată o parte importantă a profilaxiei și terapiei antigripală, în special în cazurile severe ale bolii.
În experimentele noastre, Ingavirina a demonstrat, de asemenea, activitate anti-virală împotriva altor două viruși utilizați, hPIV și AdV [19,20]. Deoarece acești viruși sunt filogenetic distincti unul de celălalt și nu există componente virale comune tuturor celor trei viruși utilizați în studiu, se poate concluziona că acest medicament vizează componentele și căile responsabile pentru dezvoltarea patologiei celulare și tisulare în timpul infecției virale. Într-adevăr, în toate cele trei cazuri a demonstrat un grad ridicat de citoprotecție și capacitatea de a normaliza arhitectura țesutului. Aplicarea sa a prevenit moartea celulelor epiteliului bronșic infectate cu virusul gripal, citopatologia indusă de hPIV în plămâni și vacuolizarea indusă de virus a hepatocitelor în timpul infecției cu AdV la hamsteri. Mai mult, în experimentele noastre anterioare [21] am demonstrat capacitatea ingavirinei de a preveni deteriorarea celulară indusă de AdV în cultură. În ciuda formării de incluziuni specifice virusului intranuclear tipic, celulele tratate cu Ingavirina nu au dezvoltat vacuole în citoplasmă și alte semne morfologice de citopatogenitate. Prin urmare, în plus față de alte mecanisme(e) de acțiune antiviră, Ingavirina posedă o activitate citoprotectoare care previne distrugerea celulelor infectate și menține funcția organului țintă minimizând astfel deteriorarea tisulară indusă de virus și simptomele de toxicitate în cursul bolii.
Trebuie remarcat faptul că nu a fost observată nicio toxicitate a ingavirinei la doze de până la doze de 3000 mg/kg. De asemenea, nu au fost observate semne de embriotoxicitate în studiile anterioare [43]. Pentru comparație, DL50 pentru Tamiflu a fost estimată la 100–250 mg/kg, în funcție de calea și programul de inoculare și speciile de animale [44]. Mai mult, în studiile clinice, ingavirina nu a demonstrat efecte secundare atunci când a fost aplicată la pacienții infectați cu gripă [45]. Prin urmare, ingavirina poate fi considerată un compus netoxic cu risc scăzut de supradozaj.
5. Concluzii Luate împreună, datele noastre sugerează că Ingavirina este un antiviral netoxic cu spectru larg, cu mecanism complex de acțiune. Studiul suplimentar al mecanismului fin al activității sale protectoare ar permite optimizarea structurii medicamentului și probabil dezvoltarea unei noi clase de compuși pentru profilaxia și tratamentul infecțiilor virale.
Mulțumiri Mulțumim Tatianei D. Smirnova de la Institutul de Cercetare a Gripei pentru furnizarea de linii celulare. De asemenea, mulțumim Lidiya Nosach de la Institutul de Microbiologie și Virologie, Kiev, Ucraina, și Innei Alexeeva de la Institutul de Biologie Moleculară și Genetică, Kiev, Ucraina, pentru că au furnizat cu amabilitate 6-azacitidină pentru experimente.
Conflict de interese Nu a fost detectat niciun conflict de interese în legătură cu această publicație.
Referințe și note
World Health Organization (WHO), CSR, Disease Outbreak News [abbreviated and edited]. Available online: http://www.who.int/csr/don/2010_05_21/en/index.html (accessed on 11 November 2011).
Moscona, A. Oseltamivir resistance—Disabling our influenza defenses. N. Engl. J. Med. 2005, 353, 2633–2636. [Google Scholar]
Moscona, A. Medical management of influenza infection. Annu. Rev. Med. 2008, 59, 397–413. [Google Scholar]
Hayden, F.G. Combination antiviral therapy for respiratory virus infections. Antivir. Res. 1996, 29, 45–48. [Google Scholar]
CDC. Influenza activity—United States and worldwide, May 18–September 19. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1046–1049. [Google Scholar]
Dawood, F.S.; Jain, S.; Finelli, L.; Shaw, M.W.; Lindstrom, S.; Garten, R.J.; Gubareva, L.V.; Xu, X.; Bridges, C.B.; Uyeki, T.M. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar]
Hayden, F. Developing new antiviral agents for influenza treatment: What does the future hold? Clin. Infect. Dis. 2009, 1, 3–13. [Google Scholar]
Hauge, S.H.; Dudman, S.; Borgen, K.; Lackenby, A.; Hungnes, O. Oseltamivir-resistant influenza viruses A (H1N1), Norway. Emerg. Infect. Dis. 2007, 15, 155–162. [Google Scholar]
Dharan, N.J.; Gubareva, L.V.; Meyer, J.J.; Okomo-Adhiambo, M.; McClinton, R.C.; Marshall, S.A.; St, G.K.; Epperson, S.; Brammer, L.; Klimov, A.I.; et al. Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. J. Am. Med. Assoc. 2009, 301, 1034–1041. [Google Scholar]
Chan, P.A.; Connell, N.T.; Gabonay, A.M.; Westley, B.; Larkin, J.M.; Larosa, S.P.; Chapin, K.; Mermel, L. Oseltamivir resistant 2009–2010 pandemic influenza A (H1N1) in an immunocompromised patient. Clin. Microbiol. Infect. 2010, 16, 1576–1578. [Google Scholar]
Abed, Y.; Boivin, G. Treatment of respiratory virus infections. Antivir. Res. 2006, 70, 1–16. [Google Scholar]
Greenough, A. Respiratory syncycial virus infections: Clinical features, management and prophylaxis. Curr. Opin. Pulm. Med. 2002, 8, 214–217. [Google Scholar]
Henrickson, K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003, 16, 242–264. [Google Scholar]
Balfour, H.H. Antiviral drugs. N. Engl. J. Med. 1999, 340, 1255–1268. [Google Scholar]
Loginova, Sia.; Borisevich, S.V.; Maksimov, V.A.; Bondarev, V.P.; Nebol’sin, V.E. Therapeutic efficacy of Ingavirin, a new domestic formulation against influenza A virus (H3N2). Antibiot. Khimioter. 2008, 53, 27–30. [Google Scholar]
Zarubaev, V.V.; Garshinina, A.V.; Kalinina, N.A.; Shtro, A.A.; Nebol’sin, V.E.; Kiselev, O.I. Antiviral activity of Ingavirin in experimental lethal influenza due to influenza virus B in albino mice. Antibiot. Khimioter. 2010, 55, 8–11. [Google Scholar]
Loginova, S.I.; Borisevich, S.V.; Lykov, M.V.; Vedenina, E.V.; Borisevich, G.V.; Bondarev, V.P.; Nebol’sin, V.E.; Chuchalin, A.G. In vitro efficacy of ingavirin against the Mexican pandemic subtype H1N1 of influenza A virus, strains A/California/04/2009 and A/California/07/2009. Antibiot. Khimioter. 2009, 54, 15–17. [Google Scholar]
Zarubaev, V.V.; Garshinina, A.V.; Kalinina, N.A.; Shtro, A.A.; Beliaevskaia, S.V.; Nebol’sin, V.E.; Kiselev, O.I. Protective activity of Ingavirin in experimental lethal influenza due to pandemic influenza virus A (H1N1)v in albino mice. Antibiot. Khimioter. 2010, 55, 24–31, Russian. [Google Scholar]
Shishkina, L.N.; Nebolsin, V.E.; Kabanov, A.S.; Skarnovich, M.O.; Mazurkova, N.A.; Sergeev, A.A.; Serova, O.A.; Stavsky, E.A.; Drozdov, L.G. In vitro and in vivo efficacy of Ingavirin against strains of pandemic influenza virus A(H1N1/09)V. Zh. Mikrobiol. Epidemiol. Immunobiol. 2011, 93–96. [Google Scholar]
Zarubaev, V.V.; Krivitskaia, V.Z.; Nebol’sin, V.E.; Kiselev, O.I. Experimental investigation of Ingavirin antiviral activity against human parainfluenza virus. Antibiot. Khimioter. 2010, 55, 13–6, (Russian). [Google Scholar]
Zarubaev, V.V.; Slita, A.V.; Sirotkin, A.C.; Nebol’sin, V.E.; Kiselev, O.I. Experimental investigation of Ingavirin antiviral activity against human adenovirus. Antibiot. Khimioter. 2010, 55, 19–24, (Russian). [Google Scholar]
Narasaraju, T.; Sim, M.K.; Ng, H.H.; Phoon, M.C.; Shanker, N.; Lal, S.K.; Chow, V.T. Adaptation of human influenza H3N2 virus in a mouse pneumonitis model: Insights into viral virulence, tissue tropism and host pathogenesis. Microbes Infect. 2009, 11, 2–11. [Google Scholar]
Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar]
Greer, C.E.; Zhou, F.; Goodsell, A.; Legg, H.S.; Tang, Z.; zur Megede, J.; Uematsu, Y.; Polo, J.M.; Vajdy, M. Long-term protection in hamsters against human parainfluenza virus type 3 following mucosal or combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles. Scand. J. Immunol. 2007, 66, 645–653. [Google Scholar]
Zarubaev, V.V.; Slita, A.V.; Sukhinin, V.P.; Nosach, L.N.; Dyachenko, N.S.; Povnitsa, O.Y.; Zhovnovataya, V.L.; Alexeeva, I.V.; Palchikovskaya, L.I. Effect of 6-azacytidine on the course of experimental adenoviral infection in newborn Syrian hamsters. J. Chemother. 2007, 19, 44–51. [Google Scholar]
Krug, R.M.; Aramini, J.M. Emerging antiviral targets for influenza A virus. Trends Pharmacol. Sci. 2009, 30, 269–277. [Google Scholar]
Semenova, N.P.; Prokudina, E.N.; L’vov, D.K.; Nebolsin, V.E. Effect of the antiviral drug Ingavirin on intracellular transformations and import into the nucleus of influenza A virus nucleocapsid protein. Vopr. Virusol. 2010, 55, 17–20. [Google Scholar]
Hagiwara, K.; Kondoh, Y.; Ueda, A.; Yamada, K.; Goto, H.; Watanabe, T.; Nakata, T.; Osada, H.; Aida, Y. Discovery of novel antiviral agents directed against the influenza A virus nucleoprotein using photo-cross-linked chemical arrays. Biochem. Biophys. Res. Commun. 2010, 394, 721–727. [Google Scholar]
Kao, R.Y.; Yang, D.; Lau, L.S.; Tsui, W.H.; Hu, L.; Dai, J.; Chan, M.P.; Chan, C.M.; Wang, P.; Zheng, B.J.; et al. Identification of influenza A nucleoprotein as an antiviral target. Nat. Biotechnol. 2010, 28, 600–605. [Google Scholar]
Zarubaev, V.V.; Belyaevskaya, S.V.; Sirotkin, A.C.; Anfimov, P.M.; Nebol’sin, V.E.; Kiselev, O.I.; Reikhart, D.V. Effect of Ingavirin on ultrastructure and infectivity of influenza virus in vitro and in vivo. Vopr. Virusol. 2011, 56, 21–25. [Google Scholar]
Smee, D.F.; Hurst, B.L.; Wong, M.H.; Tarbet, E.B.; Babu, Y.S.; Klumpp, K.; Morrey, J.D. Combinations of oseltamivir and peramivir for the treatment of influenza A (H1N1) virus infections in cell culture and in mice. Antivir. Res. 2010, 88, 38–44. [Google Scholar]
Zambon, M.C. Epidemiology and pathogenesis of influenza. J. Antimicrob. Chemother. 1999, 44S, 3–9. [Google Scholar]
Lapinsky, S.E. Epidemic viral pneumonia. Curr. Opin. Infect. Dis. 2010, 23, 139–144. [Google Scholar]
Rello, J.; Rodríguez, A.; Ibañez, P.; Socias, L.; Cebrian, J.; Marques, A.; Guerrero, J.; Ruiz-Santana, S.; Marquez, E.; Del Nogal-Saez, F.; et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit. Care 2009, 13, R148:1–R148:9. [Google Scholar]
Woo, P.C.; Tung, E.T.; Chan, K.H.; Lau, C.C.; Lau, S.K.; Yuen, K.Y. Cytokine profiles induced by the novel swine-origin influenza A/H1N1 virus: Implications for treatment strategies. J. Infect. Dis. 2010, 201, 346–353. [Google Scholar]
Garigliany, M.M.; Habyarimana, A.; Lambrecht, B.; van de Paar, E.; Cornet, A.; van den Berg, T.; Desmecht, D. Influenza A strain-dependent pathogenesis in fatal H1N1 and H5N1 subtype infections of mice. Emerg. Infect. Dis. 2010, 16, 595–603. [Google Scholar]
To, K.K.; Hung, I.F.; Li, I.W.; Lee, K.L.; Koo, C.K.; Yan, W.W.; Liu, R.; Ho, K.Y.; Chu, K.H.; Watt, C.L.; et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 2010, 50, 850–859. [Google Scholar]
Schmitz, N.; Kurrer, M.; Bachmann, M.F.; Kopf, M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005, 79, 6441–6448. [Google Scholar]
Dawson, T.C.; Beck, M.A.; Kuziel, W.A.; Henderson, F.; Maeda, N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 2000, 156, 1951–1959. [Google Scholar]
Carey, M.A.; Bradbury, J.A.; Seubert, J.M.; Langenbach, R.; Zeldin, D.C.; Germolec, D.R. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J. Immunol. 2005, 175, 6878–6884. [Google Scholar]
Alleva, L.M.; Cai, C.; Clark, I.A. Using Complementary and alternative medicines to target the host response during severe influenza. Evid. Based Complement. Alternat. Med. 2010, 7, 501–510. [Google Scholar]
Kurokawa, M.; Watanabe, W.; Shimizu, T.; Sawamura, R.; Shiraki, K. Modulation of cytokine production by 7-hydroxycoumarin in vitro and its efficacy against influenza infection in mice. Antivir. Res. 2010, 85, 373–380. [Google Scholar]
Loginova, S.Y.; Borisevich, S.V.; Maksimov, V.A.; Bondarev, V.P. Toxicity estimation of unspecific medicinal antiviral agents for prophylaxis and therapy of hazard and especially hazard viral infections. Antibiot. Khimioter. 2009, 54, 3–4. [Google Scholar]
Tamiflu safety data sheet. Available online: http://www.drugbank.ca/system/msds/DB00198.pdf?1265922744/ (accessed on 11 November 2011).
Kolobukhina, L.V.; Merkulova, L.N.; Shchelkanov, M.Iu.; Burtseva, E.I.; Isaeva, E.I.; Malyshev, N.A.; L’vov, D.K. Efficacy of ingavirin in adults with influenza. Ter. Arkh. 2009, 81, 51–54, Russian. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
0 notes
Text
Influenza A virus - Wikipedia
Influenza A virus (IAV) is the only species of the genus Alphainfluenzavirus of the virus family Orthomyxoviridae.[1] It is a pathogen with strains that infect birds and some mammals, as well as causing seasonal flu in humans.[2] Mammals in which different strains of IAV circulate with sustained transmission are bats, pigs, horses and dogs; other mammals can occasionally become infected.[3][4]
Symptoms of human seasonal flu usually include fever, cough, sore throat, muscle aches, conjunctivitis and, in severe cases, breathing problems and pneumonia that may be fatal.[11][2] Humans can rarely become infected with strains of avian or swine influenza, usually as a result of close contact with infected animals; symptoms range from mild to severe including death.[12][13] Bird-adapted strains of the virus can be asymptomatic in some aquatic birds but lethal if they spread to other species, such as chickens.[14]
0 notes
Text
Schweineinfluenza
!ZOONOSE!
Erreger: Influenza A Virus; RNA-Virus, Orthomyxoviridae Verschiedene Subtypen H1N1: Aviärer Typ, "Schweinegrippe" H3N2: Humaner Typ H1N2: Aviärer/humaner Typ Kreuzimmunität zw. Subtypen sehr schwach -> Schwein kann in kurzer Zeit mehrmals an Influenza erkranken
Schwein ist perfektes "mixing vessel" (zur Rekombination v. Influenzaviren), weil auf Schweinerespirationstrakt Rezeptoren f. menschliche u. aviäre Influenza A Viren sind
Übertragung:
aerogen
oronasal (Tröpfcheninfektion)
direkter Kontakt
!Tierarzt sollte sich Impfen um Eintrag zu verhindern!
Pathogenese:
Absorbtion an Zilien u. Membranen d. Respirationstraktes
Vermehrung in Epithel v. nasaler Mukosa, Tonsillen, Trachea, Bronchien, Bronchioli u. Alveolen
Maximale Virusmenge nach 24 Std., Replikation für 6-7 Tage
Symptome: gehäuftes Auftreten in Frühjahr u. Herbst betrifft alle Altersgruppen Verschlimmerung d. Symptome d. Sekundärerreger (meistens kommt Influenza gemeinsam mit PRRSV u. Mycoplasma hyopneumoniae vor)
bei Neuinfektion:
explosionsartiger Ausbruch (bis 100% betroffen), Mortalität aber gering
hohes Fieber
trockener Husten
Allgemeinverhalten vermindert (Apathie, vermehrt Liegen, hundesitzige Stellung)
Inappetenz
Anorexie
Nasenausfluss
Konjunktivitis
Aborte (wg. Fieber)
Endemischer Verlauf:
meist subklinisch
bei pandemischen Subtypen Symptome oft unspezifisch
Diagnose
Sektion:
Interstitielle Pneumonie Bronchiolitis -> später nekrotisierende Bronchitis
Direkter Erregernachweis:
mittels PCR aus Nasen-/Tonsillentupfer od. Lungengewebe !nur max. 5 Tage p.i. (=Akutphase) möglich!
Antikörpernachweis:
mittels Hämagglutinations-Inhibitions-Test paarige Beprobung (1. in Akutphase, 2. 3-4 Wo. später) -> 4-facher Titeranstieg ist beweisend mittels ELISA (nach 14-28 Tagen möglich)
Prophylaxe
Stallklima optimieren (Lüftungsanpassung an aktuelle Witterung)
Bekämpfung v. Sekundärerregern
Zukauf aus möglichst wenigen verschiedenen Betrieben
Abschirmung gegen Kontakt mit Vögeln
Rein-Raus-Verfahren
korrekte Reingung u. Desinfektion
Stress minimieren (Belegdichte, Transport, Fressplatzangebot)
Impfen: trivalent (H1N1, H3N2,H1N2) od. monovalent (H1N1) ab 56. Lebenstag Jungsauengrundimmunisierung (2x Abstand 2-4 Wo.) + 2-3x jährlich Auffrischen (am besten in Säugezeit)
Kolostrumaufnahme optimieren
korrekte Jungsaueneingliederung
Therapie
Symptomatisch
NSAIDs
Wasser- u. Wärmeversorgung sicherstellen
Antibiose gegen Sekundärerreger
0 notes
Link
Influenza A, a viral infection known for its seasonal outbreaks, remains a significant global health concern due to its potential for widespread transmission and varying degrees of severity. This respiratory illness is caused by the Influenza A virus, which belongs to the Orthomyxoviridae family and is characterized by its ability to mutate rapidly, giving rise to new strains that can evade immunity from previous infections or vaccinations. The symptoms of Influenza A typically include fever, cough, sore throat, body aches, fatigue, and respiratory symptoms, which can range from mild to severe. While most individuals recover within a week or two, certain populations, such as the elderly, young children, pregnant women, and those with underlying health conditions, are at higher risk of developing complications such as pneumonia. Understanding the transmission dynamics, seasonal patterns, vaccination strategies, and emerging antiviral treatments is crucial for effectively managing and mitigating the impact of Influenza A outbreaks on public health. This introduction aims to explore the key aspects of Influenza A, highlighting its significance, symptoms, and the ongoing efforts to combat its spread and associated complications. Types of Influenza VirusesSymptoms of Influenza ACauses of Influenza ADiagnosisTreatment for Influenza APreventComplicationsConclusion Types of Influenza Viruses Influenza viruses are classified into different types based on their surface proteins, specifically hemagglutinin (H) and neuraminidase (N). There are three main types of influenza viruses that infect humans: Influenza A, Influenza B, and Influenza C. Here's an overview of each type: 1. Influenza A Virus (IAV) Description: Influenza A viruses are the most diverse and can infect a wide range of hosts, including humans, birds, and other animals. Surface Proteins: They are classified into subtypes based on variations in hemagglutinin (H) and neuraminidase (N) proteins. For example, H1N1 and H3N2 are common subtypes that circulate in humans. Seasonal Outbreaks: Influenza A viruses cause seasonal epidemics and occasional pandemics when new strains emerge that can infect humans and spread efficiently. Symptoms: Symptoms are similar to other types of influenza and include fever, cough, sore throat, body aches, fatigue, and respiratory symptoms. 2. Influenza B Virus (IBV) Description: Influenza B viruses primarily infect humans and cause seasonal outbreaks of influenza. Surface Proteins: Unlike Influenza A viruses, Influenza B viruses do not have subtypes based on H and N proteins. Symptoms: Symptoms are similar to Influenza A but typically cause milder illness compared to Influenza A. Vaccination: Influenza B viruses are included in seasonal influenza vaccines along with Influenza A viruses to provide protection against circulating strains. 3. Influenza C Virus (ICV) Description: Influenza C viruses also infect humans but generally cause mild respiratory illness or are asymptomatic. Surface Proteins: Influenza C viruses have a single type of hemagglutinin-esterase-fusion (HEF) protein on their surface. Symptoms: Symptoms are generally mild and may include respiratory symptoms similar to other types of influenza. Epidemiology: Influenza C viruses are less common and less studied compared to Influenza A and B viruses. Key Differences and Similarities: Transmission: Influenza A and B viruses spread easily among humans through respiratory droplets, while Influenza C virus transmission is less well understood. Seasonal Patterns: Influenza A and B viruses cause seasonal epidemics, whereas Influenza C viruses circulate less frequently and do not cause widespread outbreaks. Public Health Impact: Influenza A viruses, due to their ability to cause pandemics and their diverse host range, pose a significant public health threat compared to Influenza B and C viruses. Understanding the differences between these influenza virus types is important for surveillance, vaccine development, and public health strategies aimed at preventing and controlling influenza outbreaks. Vaccination remains a key preventive measure against both Influenza A and B viruses, providing immunity against specific strains circulating each season. Symptoms of Influenza A Symptoms of Influenza A can vary in severity and presentation but typically include a combination of respiratory and systemic symptoms. Here are the common symptoms associated with Influenza A: Respiratory Symptoms: Fever: Often high, typically above 100.4°F (38°C), accompanied by chills and sweating. Cough: Dry or productive cough, which can be persistent and worsen over time. Sore Throat: Irritation or pain in the throat, often accompanied by discomfort when swallowing. Nasal Congestion: Stuffy or runny nose, with clear or sometimes thick nasal discharge. Systemic Symptoms: Body Aches: Generalized muscle aches and pains, often severe and affecting multiple muscle groups. Fatigue: Profound tiredness and weakness, sometimes lasting for several weeks after other symptoms resolve. Headache: A dull or throbbing headache, which can be severe in some cases. Chills: Feeling cold or experiencing sudden chills, especially during fever spikes. Gastrointestinal Symptoms (Less Common): Nausea: Feeling of queasiness or discomfort in the stomach. Vomiting: Episodes of throwing up, which can occur especially in children. Diarrhea: Loose or watery stools, though less common than other respiratory and systemic symptoms. Additional Symptoms: Shortness of Breath: Difficulty breathing or feeling breathless, especially in individuals with underlying respiratory conditions. Chest Discomfort: Pain or tightness in the chest, which may accompany coughing. Duration and Progression: Symptoms of Influenza A typically appear suddenly and can escalate rapidly within the first few days of infection. The illness usually resolves within 1 to 2 weeks, though fatigue and weakness may persist longer, especially in older adults or individuals with weakened immune systems. Understanding the symptoms of Influenza A is crucial for early detection and prompt medical intervention, especially during influenza season. Vaccination remains the most effective preventive measure against influenza, reducing the severity of illness and preventing complications in vaccinated individuals. Causes of Influenza A The primary cause of Influenza A is infection with the Influenza A virus. This virus belongs to the Orthomyxoviridae family and is characterized by its ability to infect humans, birds, and other animals. The virus spreads primarily through respiratory droplets when an infected person coughs, sneezes, or talks. Here are the key points about the causes of Influenza A: Influenza A Virus (IAV): The virus responsible for causing Influenza A is highly contagious and can mutate rapidly, leading to the emergence of new strains. Transmission: Influenza A spreads from person to person through the inhalation of airborne droplets containing the virus, direct contact with infected individuals, or touching surfaces contaminated with the virus and then touching the mouth, nose, or eyes. Seasonal Outbreaks: Influenza A viruses cause seasonal epidemics, typically during the fall and winter months in temperate regions, though outbreaks can occur year-round in some areas. Pandemics: Occasionally, novel strains of Influenza A virus emerge that have the potential to cause pandemics, such as the H1N1 pandemic in 2009. These pandemics occur when a new strain emerges to which most people have little or no immunity. Risk Factors: Factors that increase the risk of contracting Influenza A include close contact with infected individuals, crowded living conditions, compromised immune systems, and not being vaccinated against influenza. Zoonotic Transmission: Influenza A viruses can also infect animals, particularly birds and pigs, and occasionally jump from animals to humans, causing outbreaks or sporadic cases of avian or swine flu. Understanding the causes and modes of transmission of Influenza A virus is crucial for implementing preventive measures, such as vaccination, practicing good hygiene (like frequent handwashing), and avoiding close contact with sick individuals during influenza season. These measures help reduce the spread of the virus and protect individuals from contracting influenza. Diagnosis Diagnosing Influenza A typically involves a combination of clinical evaluation, patient history, and sometimes additional testing to confirm the presence of the virus and rule out other similar respiratory infections. Here's how Influenza A is diagnosed: 1. Clinical Evaluation Symptoms: A healthcare provider will assess symptoms typical of influenza, such as sudden onset of fever, cough, sore throat, body aches, and fatigue. Physical Examination: They will conduct a physical exam to check for signs of respiratory distress, fever, and other symptoms associated with influenza. 2. Patient History Exposure History: Inquiring about recent exposure to individuals known to have influenza or recent travel to areas experiencing influenza outbreaks. Vaccination Status: Asking about the individual's influenza vaccination history, as vaccination can affect the severity of symptoms and likelihood of infection. 3. Laboratory Tests Rapid Influenza Diagnostic Tests (RIDTs): These tests detect influenza viral antigens in respiratory specimens (e.g., nasal swab or throat swab) within minutes. RIDTs are commonly used in outpatient settings for rapid diagnosis. Reverse Transcription Polymerase Chain Reaction (RT-PCR): This molecular test detects the genetic material of the influenza virus and is considered the gold standard for diagnosing influenza. RT-PCR provides accurate results and can distinguish between different influenza virus types (A and B) and subtypes (e.g., H1N1, H3N2). 4. Viral Culture Cell Culture: In some cases, a viral culture may be performed to grow and identify the influenza virus from respiratory specimens collected via nasal or throat swabs. This method is less commonly used due to its longer turnaround time compared to rapid diagnostic tests. 5. Differential Diagnosis Exclusion of Other Causes: Since influenza shares symptoms with other respiratory infections (such as respiratory syncytial virus, adenovirus, and parainfluenza virus), differential diagnosis may be necessary to differentiate influenza from these conditions. 6. Timing and Testing Recommendations Early Testing: Testing for influenza should ideally be done within the first 2-3 days of symptom onset when viral shedding is highest. High-Risk Groups: Prompt diagnosis is especially important for high-risk individuals, such as young children, older adults, pregnant women, and those with underlying health conditions, to initiate early treatment and prevent complications. 7. Clinical Judgment Clinical Diagnosis: In some cases, especially during influenza outbreaks when testing resources may be limited, healthcare providers may diagnose influenza based on clinical symptoms and epidemiological factors. Diagnosing Influenza A involves a comprehensive approach to confirm the presence of the virus, guide appropriate treatment decisions, and implement preventive measures to reduce transmission and protect public health. Treatment for Influenza A Treatment for Influenza A focuses on alleviating symptoms, preventing complications, and reducing the duration of illness. Here are key aspects of treatment: 1. Antiviral Medications Early Initiation: Antiviral medications such as oseltamivir (Tamiflu), zanamivir (Relenza), and peramivir (Rapivab) are most effective when started within the first 48 hours of symptom onset. Mechanism: These medications inhibit the replication of the influenza virus, thereby reducing the severity and duration of symptoms. High-Risk Groups: Antiviral treatment is particularly recommended for individuals at higher risk of influenza complications, including young children, older adults, pregnant women, and those with underlying medical conditions. 2. Supportive Care Rest: Adequate rest is essential to help the body recover from influenza and reduce fatigue. Hydration: Drinking plenty of fluids helps prevent dehydration, especially if fever is present. Fever and Pain Relief: Over-the-counter medications such as acetaminophen (paracetamol) or ibuprofen can help reduce fever and alleviate body aches and headaches. 3. Symptomatic Relief Cough Suppressants: Medications to suppress coughing may be used if cough is severe or persistent. Decongestants: Nasal decongestants can help relieve nasal congestion and sinus pressure. Throat Lozenges: Lozenges or throat sprays can soothe a sore throat. 4. Prevention of Complications Monitoring: Close monitoring for signs of complications such as pneumonia, particularly in high-risk individuals. Prompt Medical Attention: Seek medical care if symptoms worsen or if there are signs of respiratory distress, chest pain, or confusion. 5. Infection Control Isolation: Infected individuals should stay home to prevent spreading the virus to others until they are fever-free for at least 24 hours without the use of fever-reducing medications. Hygiene: Practice good hand hygiene by washing hands frequently with soap and water or using alcohol-based hand sanitizers. 6. Vaccination Annual Vaccination: The best way to prevent influenza A and B infections is annual vaccination with influenza vaccines, which are updated each year to match circulating virus strains. 7. High-Risk Groups Special Considerations: High-risk individuals, including young children, older adults, pregnant women, and those with chronic medical conditions, should receive prompt medical evaluation and may require closer monitoring and more aggressive treatment. Considerations: Antibiotics: Influenza is caused by a virus, so antibiotics are not effective unless bacterial complications develop. Herbal Remedies: Use caution with herbal supplements and alternative treatments, as their efficacy and safety in treating influenza have not been extensively studied. Follow-Up: Recovery: Most people recover from influenza within 1-2 weeks with proper rest and supportive care. Complications: Monitor for complications, especially in high-risk individuals, and seek medical attention if symptoms worsen or new symptoms develop. Effective management of Influenza A involves a combination of antiviral medications, supportive care, prevention strategies, and monitoring for complications. Prompt diagnosis and treatment initiation are crucial for reducing the severity of illness and preventing transmission to others. Prevent Preventing Influenza A involves several key strategies aimed at reducing exposure to the virus and boosting immunity. Here are effective preventive measures: 1. Influenza Vaccination Annual Vaccination: Get vaccinated against influenza every year, ideally before the start of flu season (typically in the fall). Influenza vaccines are updated annually to protect against the most prevalent circulating strains of Influenza A and Influenza B viruses. Vaccine Effectiveness: Although vaccine effectiveness can vary each season, vaccination significantly reduces the risk of contracting influenza and its complications, especially in high-risk groups. 2. Hygiene Practices Hand Hygiene: Wash hands frequently with soap and water for at least 20 seconds, especially after coughing, sneezing, or touching potentially contaminated surfaces. If soap and water are not available, use alcohol-based hand sanitizers. Respiratory Etiquette: Cover your mouth and nose with a tissue or your elbow when coughing or sneezing to prevent the spread of respiratory droplets containing the influenza virus. Avoid Touching Face: Avoid touching your eyes, nose, and mouth, as these are entry points for the virus. 3. Avoidance of Exposure Stay Home When Sick: If you have symptoms of influenza (fever, cough, sore throat, body aches), stay home from work, school, and public places to avoid spreading the virus to others. Limit Close Contact: Avoid close contact with individuals who are sick with influenza or respiratory infections. 4. Environmental Measures Clean Surfaces: Regularly clean and disinfect frequently touched surfaces and objects, such as doorknobs, light switches, and electronic devices, to reduce the spread of germs. Ventilation: Ensure good ventilation in indoor spaces to reduce the concentration of respiratory droplets containing the virus. 5. Healthy Lifestyle Boost Immunity: Maintain a healthy lifestyle with balanced nutrition, regular exercise, adequate sleep, and managing stress, which can help strengthen your immune system and reduce susceptibility to infections. 6. High-Risk Groups Special Considerations: Individuals at higher risk of severe complications from influenza, such as young children, older adults (65 years and older), pregnant women, and those with chronic medical conditions (e.g., asthma, diabetes), should prioritize vaccination and take additional precautions to avoid exposure. 7. Travel Precautions Travel Advice: Check travel advisories and take precautions, especially during peak influenza seasons or when traveling to areas experiencing influenza outbreaks. 8. Educational Campaigns Public Awareness: Participate in public health campaigns promoting influenza vaccination and preventive measures to increase community immunity and reduce transmission rates. Preventing Influenza A involves a combination of vaccination, good hygiene practices, avoiding exposure to infected individuals, maintaining a healthy lifestyle, and taking specific precautions for high-risk groups. These strategies not only protect individuals from contracting influenza but also contribute to reducing the overall impact of influenza outbreaks on public health. Complications Influenza A, while typically causing mild to moderate illness in healthy individuals, can lead to complications, especially in high-risk groups or when the infection is severe. Here are some potential complications associated with Influenza A: 1. Pneumonia Viral Pneumonia: Influenza A can directly infect the lungs and cause viral pneumonia, which can be severe, particularly in older adults, young children, and individuals with weakened immune systems. Secondary Bacterial Pneumonia: Influenza A infection can weaken the immune system and make individuals more susceptible to secondary bacterial infections, such as Streptococcus pneumoniae or Staphylococcus aureus pneumonia. 2. Bronchitis and Respiratory Tract Infections Acute Bronchitis: Inflammation of the bronchial tubes, leading to persistent coughing and difficulty breathing. Sinusitis: Inflammation of the sinuses, causing facial pain, congestion, and nasal discharge. 3. Exacerbation of Chronic Medical Conditions Asthma: Influenza A infection can trigger asthma attacks or exacerbate existing asthma symptoms. Chronic Obstructive Pulmonary Disease (COPD): Individuals with COPD may experience worsening of their respiratory symptoms during and after influenza infection. 4. Cardiovascular Complications Myocarditis: Inflammation of the heart muscle, which can lead to chest pain, abnormal heart rhythms, and in severe cases, heart failure. Pericarditis: Inflammation of the lining around the heart (pericardium), causing chest pain and discomfort. 5. Neurological Complications Encephalitis: Inflammation of the brain tissue, leading to symptoms such as confusion, seizures, and altered mental status. Guillain-Barré Syndrome: A rare neurological disorder that can occur after influenza infection, causing muscle weakness and sometimes paralysis. 6. Secondary Infections Ear Infections: Particularly in children, influenza A can lead to middle ear infections (otitis media). Sinus Infections: Infection of the sinuses (sinusitis) may develop as a complication of influenza A. 7. Death Severe Cases: Influenza A can lead to severe illness and, in some cases, death, especially among high-risk individuals who develop complications such as pneumonia or exacerbation of underlying medical conditions. High-Risk Groups: Young Children: Especially those under 5 years old. Older Adults: Particularly those aged 65 years and older. Pregnant Women: Especially during the second and third trimesters. Individuals with Chronic Medical Conditions: Such as asthma, diabetes, heart disease, or weakened immune systems. Prevention of Complications: Vaccination: Annual influenza vaccination is the best way to prevent influenza A and reduce the risk of complications. Early Treatment: Prompt antiviral treatment within the first 48 hours of symptom onset can reduce the severity and duration of influenza illness, potentially reducing the risk of complications. Monitoring: High-risk individuals and those with severe symptoms should be closely monitored for signs of complications, and medical care should be sought promptly if complications are suspected. Understanding the potential complications of Influenza A underscores the importance of preventive measures, timely vaccination, and early medical intervention, especially for individuals at higher risk of severe illness. Conclusion In conclusion, Influenza A remains a significant respiratory illness with the potential for widespread transmission and varying degrees of severity. Characterized by symptoms such as fever, cough, body aches, and fatigue, this viral infection can affect individuals of all ages, though certain groups, such as young children, older adults, and those with underlying health conditions, are at higher risk of developing severe complications. Preventing influenza A involves vaccination, good hygiene practices, and early medical intervention when symptoms arise. Annual vaccination remains the cornerstone of prevention, providing immunity against circulating strains and reducing the likelihood of severe illness and complications. Timely antiviral treatment and supportive care are crucial for managing symptoms and preventing the spread of infection. Awareness of influenza A's potential complications, including pneumonia, exacerbation of chronic conditions, and in severe cases, death, highlights the importance of proactive health measures and community-wide efforts to limit transmission. By prioritizing vaccination, practicing good hygiene, staying home when sick, and seeking medical care when necessary, individuals can help mitigate the impact of influenza A on public health and ensure better outcomes for themselves and their communities. Continued research, education, and public health initiatives play essential roles in combating influenza A and enhancing overall influenza preparedness and response strategies. Search here
0 notes
Text
“Measles (susceptibility)”, Victor McKusick, Mendelian Inheritance in Man, 1966.
Here I present: “Measles (susceptibility)”, Victor McKusick, Mendelian Inheritance in Man’, 1966. INTRODUCTION. Hemagglutinins are receptor-binding membrane fusion glycoproteins produced by viruses in the Paramyxoviridae and Orthomyxoviridae families responsible for binding to receptors on host cells to initiate viral attachment and infection. The Measles virus uses a protein on its surface…
0 notes
Text
Influenza virus is a single-stranded RNA virus belonging to the Orthomyxoviridae family, with three types: A, B, and C.
0 notes
Text
Viruses, Vol. 15, Pages 2448: The Viromes of Six Ecosystem Service Provider Parasitoid Wasps
Parasitoid wasps are fundamental insects for the biological control of agricultural pests. Despite the importance of wasps as natural enemies for more sustainable and healthy agriculture, the factors that could impact their species richness, abundance, and fitness, such as viral diseases, remain almost unexplored. Parasitoid wasps have been studied with regard to the endogenization of viral elements and the transmission of endogenous viral proteins that facilitate parasitism. However, circulating viruses are poorly characterized. Here, #RNA viromes of six parasitoid wasp species are studied using public libraries of next-generation sequencing through an integrative bioinformatics pipeline. Our analyses led to the identification of 18 viruses classified into 10 families (Iflaviridae, Endornaviridae, Mitoviridae, Partitiviridae, Virgaviridae, Rhabdoviridae, Chuviridae, Orthomyxoviridae, Xinmoviridae, and Narnaviridae) and into the Bunyavirales order. Of these, 16 elements were described for the first time. We also found a known virus previously identified on a wasp prey which suggests viral transmission between the insects. Altogether, our results highlight the importance of virus surveillance in wasps as its service disruption can affect ecology, agriculture and pest management, impacting the economy and threatening human food security. https://www.mdpi.com/1999-4915/15/12/2448?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Grip Aşısı Geliştirilmesi
İnfluenza (grip) virüsü, memelilerde ve kuşlarda görülen bir solunum yolu enfeksiyonu virüsüdür. Virüslerin Orthomyxoviridae familyasındaki bir RNA virüsünden kaynaklanır. İnfluenza virüsü dört ana türe (İnfluenza A, İnfluenza B, İnfluenza C, İnfluenza D) ayrılır ve bunlar iki ana dahili proteindeki (hemaglutinin (HA) ve nöraminidaz (NA)) farklılıklarla ayırt edilir. Dört tip grip virüsünden üçü…

View On WordPress
0 notes
Text
Understanding The Influenza Virus
Influenza, commonly known as the flu, is a viral infection that has plagued humanity for centuries. Every year, during the flu season, millions of people around the world fall victim to this infectious disease. In this article, we will delve into the intricacies of the influenza virus, exploring its history, structure, transmission, and the science behind vaccines that aim to protect us from its grasp.
A Historical Perspective
The influenza virus has a long and storied history. Records of influenza-like illnesses date back as far as 412 BC in ancient Greece. However, it wasn't until the early 20th century that scientists first isolated and identified the influenza virus. In 1933, British scientists Wilson Smith, Christopher Andrewes, and Patrick Laidlaw made a groundbreaking discovery when they successfully isolated the influenza A virus, one of the three types of influenza viruses that affect humans.
The Anatomy of Influenza Virus
To understand the influenza virus, it's essential to comprehend its structure. The virus belongs to the Orthomyxoviridae family and comes in three main types: A, B, and C. Influenza A is the most diverse and virulent type, responsible for most seasonal flu outbreaks and the occasional pandemics that have left an indelible mark on human history. Read more

1 note
·
View note
Text
Influenza (Flu)

I. Introduction
A. Definition of Influenza (Flu)
Influenza (Flu) is a contagious diseases that causes respiratory disease caused by influenza virus(A, B, C). It spreads through respiratory droplets or contact with contaminated surfaces. A person might experience fever, cough, sore throat, muscle aches etc. The flu can lead to severe complications and is particularly dangerous for vulnerable groups. Annual flu vaccination and antiviral medications help mitigate its impact. Preventive measures, such as hand hygiene, respiratory etiquette, and staying home when sick, are essential to control its spread. Seasonal outbreaks and sporadic pandemics of influenza represent a hazard to global health, impacting people of all ages.
B. What are the various types of Influenza viruses (A, B, C). Seasonal nature and periodic pandemics

Types of Influenza Viruses:
1. Influenza A:
• Most common type responsible for seasonal flu outbreaks and pandemics.
• Can infect a wide range of animals, including birds and mammals.
• Its various Subtypes are classified as surface proteins: Hemagglutinin (H) and Neuraminidase (N), e.g., H1N1
2. Influenza B:
• Causes seasonal flu outbreaks but not pandemics.
• Mainly infects humans and can lead to similar symptoms as Influenza A but generally milder.
3. Influenza C:
• Causes mild respiratory illness and is less common than Influenza A and B.
• Usually does not cause seasonal outbreaks or pandemics.
II. Causes and Transmission

A. Orthomyxoviridae family
The Orthomyxoviridae family is a group of enveloped RNA viruses that includes several important pathogens, most notably the influenza viruses. Seasonal flu outbreaks and, in certain cases, pandemics are brought on by these viruses, which pose serious risks to the public’s health.
Orthomyxoviruses have a unique structure with a lipid envelope surrounding their helical nucleocapsid core. Hemagglutinin (HA) and neuraminidase (NA), two glycoproteins found in the envelope that are essential for viral attachment, entry, and release, are present.
The viral genome of Orthomyxoviridae family members consists of multiple segments of single-stranded negative-sense RNA. This segmented genome allows for genetic reassortment when two different influenza viruses infect the same host cell. As a result, new strains with different antigenic properties can emerge, leading to the potential for novel flu virus outbreaks.
The Orthomyxoviridae family consists of three genera: Influenza A, Influenza B, and Influenza C. A wide variety of hosts are susceptible to influenza A viruses, which can infect people, birds, pigs, horses, and other animals. Based on the interaction of the proteins hemagglutinin (H) and neuraminidase (N), they are further divided into subtypes. Influenza B viruses primarily infect humans and are less prone to genetic reassortment compared to Influenza A. Compared to influenza A and B viruses, influenza C infections in humans often result in less severe respiratory symptoms.
It is crucial to comprehend the genetics, structure, and behavior of viruses of the Orthomyxoviridae family in order to create vaccines and antiviral medications that will effectively combat influenza epidemics and potential pandemics. The ever-evolving nature of influenza viruses necessitates ongoing research and surveillance to keep ahead of new strains and safeguard public health globally.
B. Transmission through respiratory droplets
Transmission of respiratory infections through respiratory droplets is a common mode of spread for many contagious diseases, including influenza, COVID-19, and other respiratory viruses. A person who is infected releases respiratory droplets into the air when they talk, sneeze, cough, or even breathe. These droplets typically contain virus particles if the person is infected.
The size of respiratory droplets is greater, and they don’t hang in the air for very long. Instead, they quickly fall to the ground or nearby surfaces within a short distance from the infected person. The exact distance droplets can travel depends on factors like the force of the cough or sneeze and environmental conditions.
The virus can infect a person’s respiratory system if these droplets come into touch with their mucous membranes (such as their mouth, nose, or eyes). This direct transmission through respiratory droplets is a significant contributor to the rapid spread of respiratory illnesses, especially in crowded places or close-contact settings.
Public health precautions including using masks, according to respiratory etiquette (covering mouth and nose when coughing or sneezing), and keeping a physical distance from other people are essential in reducing the transmission of respiratory illnesses by respiratory droplets.
II. Signs and Symptoms
A. Incubation period
The interval between being exposed to a virus or other infectious agent and developing symptoms is referred to as the incubation period. For respiratory infections like the flu or COVID-19, the incubation period is typically around 1 to 14 days, depending on the specific virus. The virus may be reproducing inside the body throughout this time, and although the infected person may not yet be sick, they may still be contagious to others.
B. Common symptoms
Common symptoms of respiratory infections like the flu or COVID-19 include fever, cough, sore throat, runny or stuffy nose, muscle or body aches, fatigue, and headache. People may occasionally also have trouble breathing or feel short of breath. It’s crucial to remember that symptoms might differ in intensity and presentation from person to person, and some infected people may experience no symptoms at all or very minor ones.
C. Variability in severity
The severity of respiratory infections like the flu or COVID-19 can vary significantly from person to person. While some people might only have minor symptoms or even remain asymptomatic, others might get seriously ill and end up in the hospital or even pass away. This heterogeneity is influenced by a number of variables, including age, underlying medical problems, and immune response. It emphasizes the significance of taking public health precautions to safeguard vulnerable groups and stop the infection’s spread.
IV. High-Risk Groups
A. Vulnerable populations (young children, elderly, pregnant women, immunocompromised)
Children, the elderly, pregnant women, those with low immune systems, and people with certain underlying medical disorders are among the vulnerable groups who are more likely to experience serious complications from respiratory infections like the flu or COVID-19.
· Young Children: Children, especially those under five years old, have developing immune systems and may have difficulty fighting off infections effectively.
· Elderly People: Older people, especially those over 65, frequently have weakened immune systems and may suffer from chronic health issues that make them more prone to serious illness.
· Pregnant Women: Pregnancy can alter the immune response, potentially increasing the risk of severe complications from certain infections.
· Immunocompromised Individuals: Those with weakened immune systems are more prone to infections and may find it difficult to properly fight off the virus, including those undergoing chemotherapy, organ transplant recipients, and those with specific medical disorders.
A crucial public health objective is to protect these vulnerable groups, and preventative interventions including immunization, hand washing, and avoiding close contact with sick people are crucial to reducing their risk of exposure to respiratory infections. Additionally, healthcare providers may consider more aggressive management and monitoring of respiratory infections in these groups to prevent severe outcomes.
B. Increased risk of complications
Children, the elderly, pregnant women, those with compromised immune systems, and people with underlying medical disorders all have a higher risk of developing serious complications from respiratory infections like the flu or COVID-19. Pneumonia, respiratory failure, the deterioration of long-term medical issues, and, in the worst situations, death, are only a few examples of these complications. To safeguard these at-risk groups’ health and safety during infectious disease outbreaks, it is essential to give them special care and take preventive measures.
V. Prevention and Control
Prevention and control of respiratory infections like the flu or COVID-19 involve a combination of public health measures and individual actions. Regular handwashing, wearing masks in crowded or high-risk settings, and practicing respiratory etiquette (covering mouth and nose when coughing or sneezing) are essential in reducing transmission. Maintaining physical distance from others, especially when social distancing measures are recommended, helps limit exposure. Public health authorities play a significant role in surveillance, early detection, contact tracing, and implementing quarantine measures to contain outbreaks. Additionally, promoting awareness and providing accurate information to the public aids in fostering responsible behaviour and adherence to preventive measures.
VI. Treatment

A. Antiviral medications
Antiviral drugs are those used to stop viruses from replicating in the body. They are used to treat viral infections like influenza and COVID-19. These medications work by targeting specific viral enzymes or proteins, preventing the virus from multiplying and spreading. When given early in the course of the infection, antiviral medications such oseltamivir for influenza and remdesivir for COVID-19 can help decrease the intensity and length of the sickness. Proper medical guidance is essential for their use.
B. Effectiveness when administered early
Early on in the course of a viral infection is when antiviral drugs are most effective. Antiviral medications can prevent viral replication and stop the spread of the virus in the body when administered quickly after the onset of symptoms. This early management may lessen the intensity of the sickness, reduce the length of the symptomatic period, and maybe avert serious complications. However, their effectiveness may diminish if initiated later in the infection, underscoring the importance of prompt medical evaluation and treatment.
The . QMe hospital management system software’s comprehensive patient history and electronic health records ensure seamless access to critical medical information, enabling healthcare professionals to make informed decisions and provide personalized care.
C. Supportive care for symptom management
Supportive care for symptom management involves providing comfort and relief to individuals with viral infections through non-specific treatments. Adequate hydration is essential to prevent dehydration, especially during illnesses that involve fever and respiratory symptoms. Rest and sufficient sleep aid the body’s recovery process. For respiratory infections, maintaining a humid environment or using a humidifier can ease breathing. The goal of symptomatic therapy is to enhance the patient’s health as the body’s immune system battles the viral infection. QMe hospital management system is a cutting-edge hospital management software designed to revolutionized healthcare facilities worldwide.
VII. Differences between Influenza Types A. Influenza A B. Influenza B C. Influenza C
Here are the key differences between them:
1. Influenza A:
• Responsible for most flu outbreaks, including seasonal and pandemic flu.
• Can infect a wide range of animals, including birds, pigs, and humans.
• Classified into subtypes based on two surface proteins: Hemagglutinin (H) and Neuraminidase (N), e.g., H1N1, H3N2.
• Exhibits significant genetic variability and can undergo genetic reassortment, leading to new strains.
2. Influenza B:
• Causes seasonal flu outbreaks but not pandemics.
• Primarily infects humans and is less diverse than Influenza A.
• Not classified into subtypes based on H and N proteins.
3. Influenza C:
• Causes mild respiratory illness and is less common than Influenza A and B.
• Typically leads to mild symptoms and rarely causes outbreaks or epidemics.
• Not classified into subtypes and does not have the same impact as Influenza A and B.
Overall, Influenza A is the most significant in terms of its impact on human health due to its pandemic potential and ability to infect animals, while Influenza B is generally less severe and only affects humans. Influenza C is the mildest and least common among the three types.
VIII. Conclusion

In conclusion it poses a significant global health concern, leading to seasonal outbreaks and occasional pandemics. Children, the elderly, pregnant women, and people with impaired immune systems are vulnerable groups who are more likely to experience serious problems. Preventive measures like vaccination, hand hygiene, and respiratory etiquette are vital in controlling its spread. Early administration of antiviral medications can help reduce the severity of the illness. Supportive care for symptom management enhances patient comfort during the recovery process. Understanding the differences between influenza types is essential for effective management and control.
#influenzaVirus #cause #healthcareManagement #QMeHealthcare #medicalhealth #medicalhelp #treatment #prevention #healthcare
Healthcare
Influenza Virus
Influenza Treatment
Awareness
0 notes
Text
Communicable Disease: Influenza Description of the Disease Influenza or "the flu" is a common illness in the winter months, all throughout the United States and many other countries. Both birds and all mammals can contract influenza (Brankston, et al., 2007). In recent years there have been scares regarding "bird flu" and "swine flu," both of which are simply different strains of influenza. The cause of the flu is an RNA virus in the family Orthomyxoviridae (Eccles, 2005). Once people contract the flu, they present with common symptoms such as chills, fever, a runny nose, muscle pains, a sore throat, and a headache. The headache is quite often severe, and flu sufferers may also have weakness, fatigue, severe bouts of coughing, and a general feeling of overall discomfort. People with the flu can also become nauseated and vomit, although that is more typical in children and not nearly as common in adults (Eccles, 2005). Many people also confuse influenza with the common cold, as many of the symptoms are similar. However, the flu is much more severe. The flu is transmitted person to person, and can also be transmitted to people from animals (Eccles, 2005). When a person who has the flu coughs or sneezes, the flu virus is transmitted through the air in the aerosol droplets that are produced. However, there are other ways to contract the flu. If a person touches nasal secretions from an infected person or droppings from an infected bird, he or she can catch influenza (Eccles, 2005). Additionally, viruses can live on various types of surfaces, sometimes for a long time. If a person touches a contaminated surface and then touches his or her nose, eyes, or mouth, the virus can be passed that way. That is why hand washing is discussed so much during flu season. Treatment of the flu is usually in the form of liquids and bed rest, as well as over the counter medications. Tamiflu is a popular option, but can be in short supply during widespread flu outbreaks. Demographics of Interest In most people, the flu is a troublesome and annoying illness that makes them feel bad for a few days to a week or longer. However, in very young children, the elderly, the immunocompromised, and people who have other medical conditions, the flu can be debilitating or even deadly. Seasonal epidemics are when the flu is most commonly seen, which translates to between three and five million cases of severe illness every year. Out of those severe illnesses, there are between 250,000 and 500,000 people who die either from the flu itself or from complications surrounding it (Ballinger & Standiford, 2010). In pandemic years, millions of people can die from influenza (Eccles, 2005). Three pandemics occurred in the 20th Century, and these generally occurred when a new strain appeared or the virus mutated in such a way that the current treatments and vaccines for it were not as effective (Harper, et al., 2005). There can be other reasons for pandemics to occur, but those are the most common causes. People who have preexisting conditions have both higher morbidity and higher mortality rates when it comes to the flu (Eccles, 2005). That is also true of the very young and the very old. The reason babies and the elderly have more trouble with influenza is due to the fact that they do not have the immunity levels seen in older children through middle-aged and senior adults. The same can be said of the people who have immune system issues. That can include people who have HIV or other immune suppressing diseases, but can also include people who have diseases that are not as serious (Brankston, et al., 2007). At times perfectly healthy people die from influenza, as well, and that can include people from any age group. While it is not as common to see this happen as it is to see people who are already sick pass away, there are times when the influenza virus hits someone particularly hard. In those cases, the flu often develops into viral and/or bacterial pneumonia, which can make breathing difficult (Eccles, 2005). Determinants of Health Determining a person's health is very important when it comes to judging how they may handle influenza. Of course, that does not mean health is the only factor. Stress, lack of sleep, lifestyle, proper eating habits, and other issues in a person's life can affect whether a person gets the flu and how he or she reacts to the illness (Eccles, 2005). For some people the flu is a minor annoyance for a few days, and for others it causes serious illness or death. Although age and preexisting conditions are both big factors for influenza problems, young people who are otherwise healthy can still experience complications that are completely unexpected. The flu can also be particularly hard on pregnant women and their developing fetuses, so they should always talk to their doctors about getting a flu shot and what other steps they can take in order to avoid contracting the flu (Ballinger & Standiford, 2010). Even people who do everything "right" can still get sick, but careful avoidance of those who are sick and proper hand washing can go a long way toward helping a person avoid the flu. Epidemiologic Triangle People who have weak immune systems and/or work extensively with the public are at the greatest risk for contracting the flu (Eccles, 2005). Both of those factors contribute to the development of the disease. The epidemiological triangle showcases this, as it is made up of the agent, the host, and the environment (Harper, et al., 2005). The agent is the virus itself, the host is the person or animal who harbors the virus, and the environment is the external factors that cause and/or allow for the transmission of the virus from one organism to another. The agent factors include the presence or absence of the virus, and the host factors include the person or animal that has the virus. The environmental factors are the most important, as they can be many and varied. It is the environmental factors that control how the virus spreads (Eccles, 2005). Role of the Community Health Nurse The community health nurse has an important role to play. He or she must focus on finding the cases of the flu in the area in order to accurately report them. The nurse must collect data from the patients, as well, and analyze that data in order to determine the severity of the level of flu being seen (Brankston, et al., 2007). If there are pandemic proportions, it is very important that be noted as soon as possible, so that people in the community can understand the seriousness of the issue. Additionally, it is vital that there are good follow-ups done in order to determine if the flu is peaking or if the number of cases is still building. By following up to see how ill people are getting and whether there are more or fewer deaths than would typically be seen, it is also possible to make a determination about the severity of the flu for that particular season (Harper, et al., 2005). This is highly valuable when it comes to making recommendations for people in the community and providing advice. National Agency or Organization One national agency that focuses on the flu and other diseases that fall into the communicable disease chain pattern is the Center for Disease Control (CDC) in Atlanta, Georgia (Ballinger & Standiford, 2010). The CDC is focused on controlling disease, but also on determining the severity of diseases in the United States and how big of a toll these diseases are taking on the public. Another main focus of the CDC is helping people learn how to break the communicable disease chain so that the disease does not get passed on from one person to another (Eccles, 2005). That is why the CDC advocates getting a flu shot, proper hand washing, staying home from work and other activities when one has influenza, and not sending children to school or daycare if they have the flu (Eccles, 2005). The more people do these types of things, the less likely they will be to get the flu and/or pass it along to others. References Ballinger, M.N. & Standiford, T.J. (2010). Postinfluenza bacterial pneumonia: Host defenses gone awry. Journal of Interferon Cytokine Research, 30(9): 643 -- 52. Brankston, G., Gitterman, L., Hirji, Z., Lemieux, C., & Gardam, M. (2007). Transmission of influenza A in human beings. Lancet Infectious Diseases, 7(4): 257 -- 65. Eccles, R. (2005). Understanding the symptoms of the common cold and influenza. Lancet Infectious Diseases, 5(11): 718 -- 25. Harper, S.A., Fukuda, K., Uyeki, T.M., Cox, N.J., & Bridges, C.B. (2005). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendation Report, 54(RR -- 8): 1 -- 40. https://www.paperdue.com/customer/paper/diabetes-evidence-based-practice-diabetes-82045#:~:text=Logout-,DiabetesEvidenceBasedPracticeDiabetesisadisease,-Length13pages Read the full article
0 notes
Text
Avian Influenza Viruses
Avian Influenza, also known as bird flu, is a viral infection that affects birds, particularly poultry. The virus belongs to the family Orthomyxoviridae, which includes the human influenza virus. Although the virus primarily affects birds, it can also spread to humans and cause severe illness. In this article, we will discuss the different types of Avian Influenza, how it spreads, the diagnosis and treatment, prevention measures, and its impact on humans.READMORE
#Avian Influenza Viruses#What is Avian Influenza#avian influenza#How is Avian Influenza Transmitted#avian influenza symptoms#avian influenza a#avian influenza bird flu#avian influenza symptoms in chickens
0 notes
Note
I never saw you and Equine influenza in one room either. Does it mean you're orthomyxoviridae? If not, I have a gun and a treatment plan.

Hello, talking horse

I might be sleep-deprived
And do I fucking look lik3
11 notes
·
View notes