#Neutrophil gelatinase-associated lipocalin (NGAL)
Explore tagged Tumblr posts
Text
Neutrophil gelatinase-associated Lipocalin
Neutrophil Gelatinase Associated Lipocalin is a protein that is primarily expressed in neutrophils, a type of white blood cell, as well as other cells such as kidney tubular cells.
One of the well-known applications of Neutrophil Gelatinase Associated Lipocalin is in the early diagnosis and monitoring of acute kidney injury (AKI). NGAL levels increase rapidly in response to kidney injury and can be detected in blood and urine. Measurement of uNGAL levels can aid in the early detection and prediction of AKI, facilitating prompt intervention and management. NGAL has also been studied in other conditions Neutrophil gelatinase-associated Lipocalin, such as cardiovascular diseases, sepsis, and various types of cancer. Its association with inflammation and tissue damage makes it a potential biomarker for assessing disease severity and prognosis. Research is ongoing to explore the full range of NGAL's functions and its potential as a diagnostic and therapeutic target in various medical conditions.
0 notes
Text
Neutrophil gelatinase-associated lipocalin (NGAL) and inflammatory markers in schizophrenia: A comparative analysis of drug-naive schizophrenia patients, remitted patients, and healthy controls - ScienceDirect
0 notes
Text
ไตวายป้องกันได้ นักวิจัย มข.คิดค้นแถบตรวจวัดโปรตีน NGAL คัดกรองความผิดปกติของไตด้วยตัวเอง ภายใน 15 นาที
#SootinClaimon.Com : ขอบคุณแหล่งข้อมูล : หนังสือพิมพ์แนวหน้า https://www.naewna.com/lady/775668 ไตวายป้องกันได้ นักวิจัย มข.คิดค้นแถบตรวจวัดโปรตีน NGAL คัดกรองความผิดปกติของไตด้วยตัวเอง ภายใน 15 นาที วันจันทร์ ที่ 18 ธันวาคม พ.ศ. 2566, 06.00 น. ทีมนักวิจัยมหาวิทยาลัยขอนแก่น นำโดย รศ.ดร.ทนพญ. จุรีรัตน์ ดาดวง ได้พัฒนา“แถบตรวจวัดโปรตีน NGAL (Neutrophil Gelatinase-associated Lipocalin) ในปัสสาวะ”…

View On WordPress
0 notes
Text
Urinary NGAL
Urinary NGAL (Neutrophil Gelatinase-Associated Lipocalin) refers to the measurement of NGAL levels in urine.
Healthcare professionals measure urinary NGAL levels using immunoassay techniques to detect and monitor kidney injury using NGAL. NGAL, a protein, is produced and released by various cells, including neutrophils (a type of white blood cell) and epithelial cells of the kidneys. NGAL serves as an early marking of kidney injury or insult, playing a role in the assessment and early detection diagnosis of acute kidney injury (AKI).When the kidneys are injured or under stress, NGAL levels increase rapidly in both blood and urine. Urinary NGAL Laboratory tests can measure levels to detecting and monitoring kidney injury. This will be especially valuable for early identification of. AKI, allowing for timely intervention and management.

0 notes
Link

1 note
·
View note
Text
Combination of berberine with pentoxifylline illustrated a synergistic effect in attenuation of diclofenac-induced acute kidney injury.
PMID: Int J Crit Illn Inj Sci. 2019 Apr-Jun;9(2):69-74. PMID: 31334048 Abstract Title: Synergistic effect of berberine and pentoxifylline in attenuation of acute kidney injury. Abstract: Objective: To evaluate the renoprotective effects of berberine and/or pentoxifylline in reduction of diclofenac-induced acute kidney injury (AKI) in rats.Material and Methods: Fifty male Sprague-Dawley rats were allocated into five groups, Group 1: Rats treated with distilled water plus normal saline for 12 days. Group 2: Rats treated with distilled water plus diclofenac for 12 days. Group 3: Rats treated with berberine plus diclofenac for 12 days. Group 4: Rats treated with pentoxifylline plus diclofenac for 12 days. Group 5: Rats treated with berberine + pentoxifylline plus diclofenac 15 mg/kg for 12 days. Blood urea, creatinine, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecules (KIM-1), and cystatin-c were used to measure the severity of AKI.Results: Diclofenac led to significant AKI by significant elevation of blood urea, serum creatinine, KIM-1, and NGAL. Treatment with berberine showed no significant effect on all biomarkers level compared to diclofenac group except on serum KIM-1 level which also seen in the pentoxifylline group whereas combination of berberine and pentoxifylline led to more significant effect in the reduction of all renal biomarkers.Conclusion: Combination of berberine with pentoxifylline illustrated a synergistic effect in attenuation of diclofenac-induced AKI.
read more
2 notes
·
View notes
Text
Kidney Function Test
Healthcare professionals use kidney function tests as diagnostic tools to evaluate the health and functioning of the kidneys.
These tests offer crucial insights into the kidneys' performance, facilitating the detection of abnormalities and aiding in the diagnosis and monitoring of kidney diseases or injuries. including chronic kidney disease (CKD) and acute kidney injury (AKI). Commonly used kidney function tests are: Blood Urea Nitrogen (BUN) Test, Serum Creatinine Test, Glomerular Filtration Rate (GFR) Calculation, Urinalysis involves analysing a urine sample for presence of abnormal levels of protein, blood cells, glucose, or bacteria, Albumin-to-Creatinine Ratio (ACR) and Imaging Tests like ultrasound, CT scan, or MRI. It is important to note that these tests are typically used in combination to provide a comprehensive assessment of kidney function.Healthcare professionals, typically nephrologists, interpret these tests by considering the results within the framework of an individual's medical history, symptoms, and other factors. This approach ensures precise diagnosis and the development of a suitable treatment plan. Additionally, various non-renal parameters such as age, gender, and muscle mass can influence the outcomes of these tests.

0 notes
Text
0 notes
Text
Neutrophil gelatinase-associated Lipocalin
It is a protein that is primarily expressed in neutrophils, a type of white blood cell, as well as other cells such as kidney tubular cells.
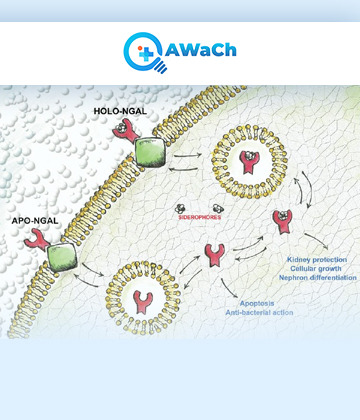
Neutrophil gelatinase-associated lipocalin (NGAL) is a protein that is primarily expressed in neutrophils, a type of white blood cell, as well as other cells such as kidney tubular cells. NGAL is involved in various physiological and pathological processes, particularly in the context of inflammation, infection, and kidney injury. NGAL is a member of the lipocalin protein family, which are small extracellular proteins that bind and transport small hydrophobic molecules. It can bind to a variety of ligands, including iron-containing siderophores, bacterial lipopolysaccharides, and certain matrix metalloproteinases (MMPs). This binding activity contributes to the role of NGAL in modulating the immune response and tissue remodelling.
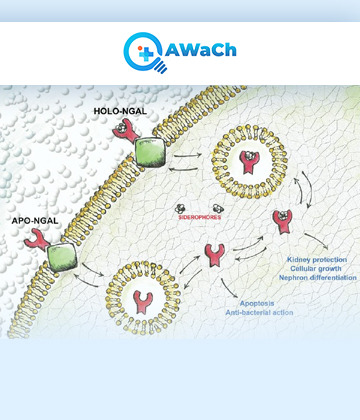
0 notes
Link
The International Journal of pediatric and Child Care purpose is to support enhance the event of medicine analysis and guide for clinical diagnosis within the reinforcement of kid care ethics. It aims to publish and support analysis for providing youngsters with state-of-art and and all clinical issues related to the child health.
This new issue includes the following open access articles:
1. Early Diagnostic and Prognostic Value of Urinary Neutrophil Gelatinase-Associated Lipocalin (Ngal) in Critically Ill Children With Septic Acute Kidney Injury
2. Townes-Brocks syndrome In Gaza Strip
3. VATER/VACTERL Association in Palestinian Children: A Case Report
4. Pregnancy, Maternal Unbound. Genesis of Filicide and Child Abuse
5. Prematurity in a Sample of Brazilian Twins: A Cross-Sectional Study
You can submit your manuscript here or Email to [email protected]

#pediatric care#diagnosis#pediatric medicine#kids care#child health#child care#pediatrics child care#malnutrition#child psychology#children#kids
2 notes
·
View notes
Text
Accentuated Risk of Tenofovir-Associated Nephrotoxicity and Hepatoxicity among HIV Patients in Nigeria: Empirical Evidence from a Cohort Study - BJSTR Journal

Accentuated Risk of Tenofovir-Associated Nephrotoxicity and Hepatoxicity among HIV Patients in Nigeria: Empirical Evidence from a Cohort Study by Irikefe P. Obiebi* in Biomedical Journal of Scientific & Technical Research https://biomedres.us/fulltexts/BJSTR.MS.ID.002627.php Background/Objectives: WHO recommends that all HIV patients have antiretroviral therapy (ART), however, ART has side-effects. This study assessed nephrotoxicity and hepatotoxicity among HIV patients on Tenofovir (TDF)-based ART in a tertiary facility in Nigeria. Methods: This cohort study was conducted among TDF-exposed and non-exposed HIV patients. Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Cystatin C (CC), as well as serum liver enzymes, lipid, and bilirubin levels, were estimated. Glomerular filtration rate was estimated using Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) Cystatin C formula. Results: Median values of CC and NGAL increased more rapidly among the TDF than the non-TDF group from the 16th to 24th week. TDF-group were almost three times as likely as non-TDF to have abnormal levels of CC at the 24th and 16th weeks; OR: 2.77 (1.17- 6.56); OR: 2.56 (1.15 - 5.66) respectively. Increased frequency of abnormal NGAL levels between visits did not differ across the two treatment groups, OR: 1.07 (0.51-6.67). The mean values of CC/creatinine ratio increased significantly between baseline and 16th week for both groups, however, the increase was significant only for the TDF group from 16th to 24th week (p=0.002). The pairwise mean increase in NGAL/creatinine ratio was significant only for the TDF group with at least one-fifth (20.6%, 24.2%) increase between visits. The prevalence chronic kidney disease among the TDF-group was twice as high as in others, (40.6% versus19.6%, p=0.019). Alkaline phosphatase and aspartate transaminase increased significantly between baseline, 16th and 24th weeks among the TDF group. Low-Density Lipoprotein at 24 weeks was higher among TDF group than the non-TDF (2.18 ± 0.41 and 1.44 ± 0.35 respectively; p<0.001). Conclusion: TDF-associated toxicity was more pronounced on the kidneys. Thus, patients on TDF require regular monitoring of their renal function. For more articles on Journals on Medical Drug and Therapeutics please click here bjstr Follow on Twitter : https://twitter.com/Biomedres01 Follow on Blogger :https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#Biomedical Open Access Journals#Open Access Journals on Surgery#Journal of Scientific and Technical Research#Biomedical Science Articles#Biomedical Research Articles
0 notes
Text
Levosimendan and Acute Kidney Injury by Uğur Koca in Open Access Journal of Biogeneric Science and Research

Mini Review Severe sepsis is one of the most common causes of death in intensive care units, while in the presence of septic shock, the mortality rate reaches approximately 70% despite the progress made in the care of critical patients [1]. The widespread inflammatory and procoagulant response caused by sepsis leads to diffuse endothelial dysfunction, endovascular damage, and eventually multiple organ failure. Sepsis, ischemia reperfusion damage, toxic nephropathy, hypovolemia and urinary system obstruction can cause acute renal failure (ARF). However, it was experimentally observed that medullary and cortical blood flow in septic ARF continued or even increased, and this was described as a completely different physiological event from ABY which was not due to sepsis [2]. Major systemic and local mediators, neutrophil-endothelial interactions, microvascular thromboses, renal hypoperfusion, and reperfusion damage have been blamed for the pathogenesis of acute renal failure. Norepinephrine, angiotensin II and vasopressin are important systemic mediators in sepsis. Local mediators, especially tumor necrosis factor (TNF) or interleukin 1 (IL-1), adhesion molecules, oxygen free radicals, catalyzes the if A2(TXA2), prostaglandin E2(PGE2), leukotrienes, platelet-induced growth factor, endothelin, nitric oxide(no) and adenosine include [3]. Nearly half of patients with acute kidney damage (AKD) have sepsis, while in intensive care units, AKD is accompanied by more septic shock. Mortality is higher in patients with sepsis-induced AKD. Adequate fluid replacement, early renal replacement therapy are useful for patients, but there is no method to treat septic AKD [4]. If hypotension cannot be corrected despite fluid resuscitation in sepsis treatment, vasopressor therapy is recommended [5]. Dopamine and norepinephrine were the first vasopressors to be selected in the treatment of Sepsis and septic shock [6]. Levosimendan is a new inotropic and vasodilator agent that has been proven to be beneficial, especially in patients with acute heart failure and acute coronary syndrome. [7]. It opens ATP - sensitive potassium channels in vascular smooth novellas cells, causing arteriolar-venous dilation. This mechanism of action is responsible for coronary, pulmonary, renal and systemic vasodilation [8,9]. In addition to blood urea nitrogen and creatinine, new and specific methods such as cystatin c, neutrophil gelatinase associated lipocalin (NGAL)], IL-8, kidney damage molecule [Kidney Injury Molecule(KIM)]-1 have been introduced in recent years to show acute kidney damage [10]. Neutrophil gelatinase associated lipocalin has been reported as the earliest and most reliable laboratory parameter showing renal ischemia or nephrotoxicity in humans, especially in kidney, lung, stomach and colon cells [11]. NGAL levels can be detected in both urine and plasma within 2-6 hours after AKD [12]. Law et al. [13] they showed that levosimendane reduces tubular necrosis and atrophy in experimental renal ischemia reperfusion damage. However, the study examining the effect of levosimendan on acute kidney damage in polymicrobial sepsis model induced by cecal ligation perforation method was not reached. To know more about open access Journal of Biogeneric Science and Research click on https://biogenericpublishers.com/ To know more about this article click on https://biogenericpublishers.com/pdf/JBGSR.MS.ID.00062.pdf https://biogenericpublishers.com/jbgsr.ms.id.00062.text/ For guidelines https://biogenericpublishers.com/author-guidelines/ For Online Submissions Click on https://biogenericpublishers.com/submit-manuscript/
0 notes
Text
Global Renal Biomarker Market Drivers, Restraints, Share and Growth Analysis by Top Leading Players
Global renal biomarker market is expected to rise by 2026 registering a substantial CAGR of 7.2% in the forecast period of 2019-2026. This evolution in the market can be attributed to the increase in research and development on new emerging drug delivery. The imminent market description contains data for historic years 2017, the base year of calculation is 2018 and the forecast period is 2019 to 2026.
Renal biomarkers are generally comes in use of treatment of renal diseases. It is introduced in the renal function to examine the function of the renal system. These biomarkers are applicable into research and clinical areas. Clinical applications of Cystatin C, β-trace protein (BTP), neutrophil gelatinase-associated lipocalin Kidney injury molecule are observed in the patients. Increasing geriatric population as well as pediatric, suffering from renal disease accelerates the market growth.

Get Exclusive Sample Report: @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-renal-biomarker-market
Competitive Analysis:
Global renal biomarker market is highly differentiated and the major companies have used various plans such as new product promotions, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes rehabilitation equipment market shares for Global, Europe, North America, Asia Pacific, and South America.
Key Market Competitors:
Few of the major competitors currently working in the global renal biomarker market are Abbott, F. Hoffmann-La Roche Ltd, Beckman Coulter Inc, Alere Inc., Siemens Healthcare, Randox Laboratories Ltd., Beckman Coulter, Inc., Thermo Fisher Scientific, Inc., BIOPORTO A/S, Astute Medical, Inc., Randox Laboratories Ltd., Alere, Inc., BioMérieux SA, QUIAGEN, Bio-Rad Laboratories, Enzo Biochem, Inc., PerkinElmer, Inc. and others
Segmentation: Global Renal Biomarker Market
Global Renal Biomarker Market By Marker Type (Creatinine, Blood Urea Nitrogen (BUN), Cystatin C, Neutrophil Gelatinase-Associated Lipocalin (NGAL), Others) Assay Platform Type (Enzyme Linked Immunosorbent Assay (ELISA), Enzymatic Assay, Turbidimetric Immunoassay, Others) Application (Diagnosis and Disease Progression Monitoring, Research) Geography (North America, Europe, Asia-Pacific, Europe, South America, Middle East and Africa – Industry Trends and Forecast to 2026
For More Inquiry Contact us at: @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-renal-biomarker-market
Market Drivers
Increasing in incidence of kidney-related diseases growth in biomarker approval boosts the market growth
Increasing geriatric patient as well as pediatric suffering from renal disease
Support from the governments are driving the market growth, by funding is boosting the market growth
Technological advancement in the field of genetics accelerate the market growth
Market Restraints
Market investigation for the global renal biomarker market, with area specific calculations and rivalry investigation on a global and county scale
Problem related to regulatory and reimbursement systems hinders the market growth
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market.
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
Related Reports:
Global Medical Document Management Systems Market
#Renal Biomarker Market#Renal Biomarker#Renal Biomarker Market Trends#Renal Biomarker Market Industry#Renal Biomarker Market News#Renal Biomarker Market Research#Renal Biomarker Market Analysis#Renal Biomarker Market Size#Renal Biomarker Market Share
0 notes
Text
Global Renal Biomarker Market Growth Is Driven By The Increasing Demands of Various Therapies and Geographical Regions- 2026

Data Bridge Market Research has recently added a concise research on Global Renal Biomarker Market to depict valuable insights related to significant market trends driving the industry. The report features analysis based on key opportunities and challenges confronted by market leaders while highlighting their competitive setting and corporate strategies for the estimated timeline. Some are the key & emerging players that are part of coverage and have being profiled are F. Hoffmann-La Roche Ltd, Beckman Coulter Inc, Alere Inc., Siemens Healthcare, Randox Laboratories Ltd., Beckman Coulter, Inc., Thermo Fisher Scientific, Inc., BIOPORTO A/S, Astute Medical, Inc., Randox Laboratories Ltd., Alere, Inc., BioMérieux SA, QUIAGEN, Bio-Rad Laboratories, Enzo Biochem, Inc., PerkinElmer, Inc. and others
Global renal biomarker market is expected to rise by 2026 registering a substantial CAGR of 7.2% in the forecast period of 2019-2026. This evolution in the market can be attributed to the increase in research and development on new emerging drug delivery. The imminent market description contains data for historic years 2017, the base year of calculation is 2018 and the forecast period is 2019 to 2026.
Renal biomarkers are generally comes in use of treatment of renal diseases. It is introduced in the renal function to examine the function of the renal system. These biomarkers are applicable into research and clinical areas. Clinical applications of Cystatin C, β-trace protein (BTP), neutrophil gelatinase-associated lipocalin Kidney injury molecule are observed in the patients. Increasing geriatric population as well as pediatric, suffering from renal disease accelerates the market growth.
Get Sample Copy of Report Here: @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-renal-biomarker-market
Market Drivers
· Increasing in incidence of kidney-related diseases growth in biomarker approval boosts the market growth
· Increasing geriatric patient as well as pediatric suffering from renal disease
· Support from the governments are driving the market growth, by funding is boosting the market growth
· Technological advancement in the field of genetics accelerate the market growth
Market Restraints
· Market investigation for the global renal biomarker market, with area specific calculations and rivalry investigation on a global and county scale
· Problem related to regulatory and reimbursement systems hinders the market growth
Leading Key players profiled in this report are:
Few of the major competitors currently working in the global renal biomarker market are Abbott, F. Hoffmann-La Roche Ltd, Beckman Coulter Inc, Alere Inc., Siemens Healthcare, Randox Laboratories Ltd., Beckman Coulter, Inc., Thermo Fisher Scientific, Inc., BIOPORTO A/S, Astute Medical, Inc., Randox Laboratories Ltd., Alere, Inc., BioMérieux SA, QUIAGEN, Bio-Rad Laboratories, Enzo Biochem, Inc., PerkinElmer, Inc. and others
Global Renal Biomarker Market Segmentation:
By Marker Type
· Creatinine
· Blood Urea Nitrogen (BUN)
· Cystatin C
· Neutrophil Gelatinase-Associated Lipocalin (NGAL)
· Others
o N-Acetyl-β-D-Glucosaminidase (NAG)
o Kidney Injury Molecule 1 (Kim1)
o TIMP-2 (TIMP Metallopeptidase Inhibitor 2)
o Liver-Type Fatty Acid-Binding Protein (L-FABP)
o Interleukin-18 (IL-18)
o Clusterin
By Assay Platform Type
· Enzyme Linked Immunosorbent Assay (ELISA)
· Enzymatic Assay
· Turbidimetric Immunoassay
o Particle-Enhanced Turbidimetric Immunoassay (PETIA)
· Others
o Chemiluminescent Immunoassay (CLIA)
o Fluorescence Immunoassay
By Application
· Diagnosis and Disease Progression Monitoring
· Research
By Geography
· North America
· South America
· Europe
· Asia-Pacific
Competitive Analysis:
Global renal biomarker market is highly differentiated and the major companies have used various plans such as new product promotions, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes rehabilitation equipment market shares for Global, Europe, North America, Asia Pacific, and South America.
Get 20% Extra Discount for Early Buyer, Know More @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-renal-biomarker-market
The Global Renal Biomarker Market report provides the global market size of the main players in each region. Moreover, the report provides knowledge of the leading markets players within the Global Renal Biomarker Market. The industry changing factors for the market segments are explored in this report. This analysis report covers the growth factors of the worldwide market based on end-users. Market opportunities and recommendations for new investments are also encompassed in this report.
Get Customization and Discount on Report by emailing [email protected] . We are content with our glorious 99.9 % client satisfying rate.
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge Market Research provides appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Data Bridge adepts in creating satisfied clients who reckon upon our services and rely on our hard work with certitude.
Contact:
Data Bridge Market Research
+1-888-387-2818
Find More Reports Related To This Category
Global Non-invasive Aesthetic Treatment Market
Global Sterilization Monitoring Market
0 notes
Text
Urinary NGAL
Urinary NGAL (Neutrophil Gelatinase-Associated Lipocalin) refers to the measurement of NGAL levels in urine.
Healthcare professionals measure urinary NGAL levels using immunoassay techniques to detect and monitor kidney injury using NGAL. NGAL, a protein, is produced and released by various cells, including neutrophils (a type of white blood cell) and epithelial cells of the kidneys. NGAL serves as an early marking of kidney injury or insult, playing a role in the assessment and early detection diagnosis of acute kidney injury (AKI).When the kidneys are injured or under stress, NGAL levels increase rapidly in both blood and urine. Urinary NGAL Laboratory tests can measure levels to detecting and monitoring kidney injury. This will be especially valuable for early identification of. AKI, allowing for timely intervention and management.

0 notes
Text
Biomed Grid | Vitamin A and its Derivatives- Retinoic Acid and Retinoid Pharmacology
Introduction
In vivo, the fat soluble Vitamin A (retinol) can be reversibly metabolised to the aldehyde (retinal) which can in turn, be further oxidised in a non-reversible manner to retinoic acid (RA). Enzymes that oxidize retinol to retinaldehyde belong to two classes: the cytosolic alcohol dehydrogenases (ADHs) belonging to the mediumchain dehydrogenases/ reductase family; and microsomal shortchain dehydrogenases/reductases (retinol dehydrogenases, RDHs [1]. The next step in RA synthesis is the oxidation of retinaldehyde to RA, which is carried out by three retinaldehyde dehydrogenases (RALDHs): RALDH1, RALDH4 and RALDH3 [1,2]. The orange pigment of carrots (beta-carotene) can be represented as two connected retinyl groups, which are used in the body to contribute to vitamin A levels [3]. The physiological and biological actions of this class of substances centre on vision, embryonic development and production, cellular growth and differentiation, skin health, and maintenance of immune function.
Initial studies had focused on vitamin A deficiency and its major consequences: night blindness and Xerophtalmia. Fridericia and Holm [4] investigated the influence of dietary A in the rhodopsin of the retina. Clearly, the rats lacking the fat-soluble vitamin A had a defect in the function of visual purple. Yudkin [5] achieved one of the earliest identifications of vitamin A as a component of the retina. Subsequently, Wald [6] determined the amount of vitamin A present in pig retinas. Wald G [7,8] was well established the visual cycle: light decomposed rhodopsin to retinal and opsin. Retinal could either recombine with opsin to reform rhodopsin or it converted to free retinol. Retinol could reform rhodopsin, but only in the presence of the RPE(Kuhne). The further structure and metabolism of retinoids implicated that retinaldehyde was the visual pigment.
Biochemistry of Vitamin A
There is now a well-developed medicinal chemistry of RA (Figure 1 & 3) [9]. The group of pharmacologically used retinoids include vitamin A (all-trans retinol), tretinoin (all-trans retinoic acid), isotretinoin (13-cis retinoic acid) and alitretinoin (9-cis retinoic acid). The monoaromatic retinoids include acitretin and etretinate. The third generation polyaromatic retinoids include bexarotene and tazarotene. In view of this broad spectrum of pharmacological activity, these substances provide useful to treat multifactorial dermatological disorders and other hematological disorders such as acute promyelocytic leukemias (APL) (Figure 1 & Figure 2) [8,10-12].
Figure 1
Figure 1 & 2: Discovery of the molecular basis of vitamin A derivative retinoic acid action (Figure data adapted from Zhu G,January1991,2013,at the top);and vitamin A in vision cycle (Figure data adapted from Wald G,1935;Wolf G,2001,at the bottom).
Function of Vitamin A and its Physiological Role Vision
Vitamin A is needed by the eye retina,11-cis-retinal (a derivative of vitamin A) is bound to the protein “opsin” to form rhodopsin (visual purple) in rods cells [8], the molecule necessary for both low light (scotopic vision). As light enters the eye, the 11-cis-retinal is isomerized to all-trans retinal in photoreceptor cells of the retina. This isomerization induces a nervous signal (a type of G regulatory protein) along the optic nerve to the visual center of the brain. After separating from opsin, the all-transretinal is recycled and converted back to the 11-cis-retinal form via a series of enzymatic reactions. The all-trans- retinal dissociates from opsin in a series of steps called photo-bleaching. The final stage is conversion of 11-cis-retinal rebind to opsin to reform rhodopsin in the retina [6-8] (Figure 2) vision cycle. Kuhne showed that rhodopsin in the retina is only regenerated when the retina is attached to retinal pimented epithelium (RPE) [8]. As the retinal component of rhodopsin is derived from vitamin A, a deficiency of vitamin A inhibit the reformation of rhodopsin and lead to night blindness. Within this cycle, all-trans retinal is reduced to all-trans retinol in photoreceptors via RDH8 and possible RDH12 in rods and transported to RPE. In the RPE, all-trans retinol is converted to 11- cis retinol, then 11-cis retinol is oxidized to 11-cis-retinal via RDH5 with possible RDH11 and RDH11 [1]. This represent each RDH for the roles in the visual cycle (Figure 2) .
Figure 3: A well developed medical chemistry of retinoic acid (RA). The all-trans and 13-cis forms of retinoic acid,two isomers of RA,are equally effective inhibiting proliferation. Retinyl acetate,and retinal(Vitamin A) are less potent inhibitor. Am80(Tamibarotene) is more potent inhibitor. The chemical structures of more potent analogues involved from labile flexible polyene structures to aromatic stable moieties are shown[9,10].
Embryonic Development
More recent, vitamin A and its metabolites play a key importance in embryo morphogenesis, development and differentiation in normal tissues. Retinoic acid (RA) is lipophilic molecule that act as ligand for nuclear RA receptors (RARs), converting them from transcriptional repressor to activators [2,11,12] in RA signaling pathway. It has been demonstrated that retinoic acid was identified as a morphogen(teratogen) responsible for the determination of the orientation of the limb outgrowth in chicken [13,14] and its retinoic acid receptors (RARs) appear at early stage of human embryonic development in certain types of tissues [15]. Vitamin A play a role in the differentiation of this cerebral nerve system in Xenopus laevi. The other molecules that interact with RA are FGF-8, Cdx and Hox genes, all participating in the development of various structures within fetus. For instance, this molecule plays an important role in hindbrain development. Both too little or too much vitamin A results in the embryo:defect in the central nervous system, various abnormalities in head and neck, the heart, the limb, and the urogenital system [15]. With an accumulation of these malformations, an individual can be diagnosed with DeGeorge syndrome [2].
Dermatology
Vitamin A, in the retinoic acid form, plays an important role in maintaining normal skin health through differentiating keratinocytes (immature skin cells) into immature epidermal cells. In earlier studies, Frazier and Hu (1931) [16] made the observation that both hypovitaminosis A and hypervitaminosis A provokes epithelial alterations together with decreased keratinization and hair loss. At present,13-cis retinoic acid (Isotretinoin) in clinical used to acne treatment. The mechanism was shown to reducing secretion of the sebaceous glands, triggering NGAL (neutrophil gelatinase-associated lipocalin) and other gene expression and selectively inducing apoptosis [17]. But precise action of retinoid therapeutic agents in dermatological diseases are being researched.
Hematopoiesis
vitamin A is important for the regulation of hematopoietic stem cell dormancy [18]. Mice maintained on a vitamin A-free diet loss HSCs (hematopoietic stem cells), showing a disrupted re-entry into dormancy after exposure to inflammatory stress stimuli. This condition highlight the impact of dietary vitamin A on the regulation of cell-cycle mediated stem cell plasticity [19]. In vitro, all-trans retinoic acid (ATRA) stimulates at least two-fold the clonal growth of normal human CFU-GM and early erythroid precursor BFU-E [20]. Cis-RA stimulates clonal growth of some myeloid leukemia cells. In suspension culture, there was an increase in cell number at day 5 in the presence of RA in half of 31 samples, which suggest that RA may play a role in the proliferation and survival of certain leukemia clones in vitro [21,22].
In contrast to the enhancement of normal hematopoietic proliferation, RA (10-6 - 10-9 mol/l) is capable of inducing differentiation of the F9 mouse teratocarcinoma, HL-60 cells [23,24] and some blasts from patients with promyelocytic leukemia [23]. Maximum HL-60 differentiation (90% of cells) occurs after a 6 day exposure to 10-6mol/l retinoic acid. Further in vitro studies found that retinoic acid induced differentiation of leukemic blast cells in only 2 of 21 patients with AML, both of these patients had promyelocytic variant [24]. These data suggest that retinoids may induce maturation of promyelocytes. Retinoic acid also inhibits the proliferation of other dermatological malignant cells (Myger,1975; Peck,1975).
Maintenance of Immune Homeostasis
There is a link between retinoid and immune homeostasis. RA is crucial for maintaining homeostasis at the intestinal barrier and equilibrating immunity and tolerance. de Mendonca Oliveira LM and colleagues [25] have in detail illustrated the impact of retinoic acid on immune cells and inflammatory diseases. After the absorption and metabolism of vitamin A and its precursor(β-carotene) into RA by alcohol dehydrogenase(ADH) and retinal dehydrogenase(RALDH) in CD103+ DC cells in gut, RA plays an important roles in mucosal immune response by promoting differentiation of Foxp3+ inducible regulatory T (Treg) cell and immunoglobulin(Ig) A production. In this process, RA promote dendritic cells to express CD103 and to produce RA. Vitamin A and zinc deficiency (VAD) lead to a decrease of serum IgA. Oral administration of RA in VAD mice can efficiently be reestablishing IgA production. These effects are mediated by an increase of the early B cell factor 1(EBF1) and paired box protein-5(pan-5) transcription factors, which are critical for B cell development. RA accelerates the maturation of human B cells and their differentiation into antibody-secreting plasma cells.
In addition, RA induces the homing of innate immune cells, such as innate lymphoid cells (ILCs) besides regulatory and effector T and B cells, to the gut. Among three ILCs, ILC3 depend on the transcription factor retinoic acid receptor-related orphan nuclear receptor gamma (RORrt) and secrete IL-17 and IL-22. During infections, RA can induce the production of proinflammatory cytokines by dendritic cells (DCs), promoting the generation of effector T cells and restoring the balance of Th17/Treg cells in the GALT (gut-associated lymphoid tissue), and the protection of the mucosa. Moreover, vitamin A is capable of inducing the IL-6- driven induction of proinflammatory T(H) 17 cells, promoting antiinflammatory T reg cells differentiation, regulating the balance between pro- and anti-inflammatory immunity [26,27].
Retinoid Acids in MDS Treatment
The geometric isomer of the naturally occurring retinoic acid is 13-cis retinoic acid (13-CRA). Based on in vitro and in vivo antineoplastic activity, this agent has entered clinical trials for a variety of neoplasms including MDS. Retinoic acid is one of the biological inducers of differentiation that has been preliminarily tested in patients with preleukemia. Myelodysplastic syndrome (MDS) are a group of hematopoietic disorders characterized by ui- or multilineage maturation defects of the bone marrow [28]. Differentiation induction therapy is used in MDS to improve this maturation defects and induce a multilineage clinical response in a subgroup of MDS patients.13-CRA may have moderate effect on 20-30% of patients with MDS [29]. A various of combination therapy with 13-cis RA and growth factors G-CSF or erythropoietin (EPO) improve impaired cytokine secretion (IL-1beta, IL-6, IL-8) from monocytes [30]. In a prospective multicenter study, EPO-beta- ATRA [31] or EPO-13-cis RA [32] combination appears to erythroid response reaching about 36%-60% of therapeutic efficacy in anemia of low/intermediate risk MDS(LDMDS) (marrow blasts < 10% or excluding RARBt). More data analysis, erythroid response maintained an independent positive impact on survival, particularly in non-RARE patients in the first 3 years from diagnosis (90% survival in EPO responders compared to 50% of non-responders) [33]. Zhu [34] successfully conducted a CR patient with refractory anemia with multilineage megaloblastic dysplasia following traditional medicine and erythropoiesis-stimulating agent vitamin B12 and folate growth factor. His peripheral parameters presented pancytopenia (hemoglobin 59g/l, red blood cell count 1.9x1012/l, leukocyte count 2.6x109/l, platelet value 11.8x109/l).
He remained well over 10 years. While another MDS had its unequivocal evidence of disease progression in response to phytohemagglutinin (PHA), inducing the generation of interleukin-2, accelerating the number recovery of CFU-S and initiating DNA synthesis of cells. She had 2.5% blast plus promyelocytes in ~70% cellular marrow before beginning PHA, and 20.7% blast plus promyelocytes in a 90% cellular marrow after ten days (total dosage 250mg) of PHA. Venditt etal [35] conduct that 23 patients with high-risk myelodysplastic syndrome (HRMDS) were treated with a 10 days course of oral ATRA (45mg/m2) and subcutaneous low-dose cytosine arabinoside (LDARAc) given at the dose of 20mg twice a day. In all cases (RAEB9, RAEBt9 and CMML4) [36] bone marrow blasts infiltration was greater than 10% (12-30%). Overall, 5(23%) of 22 patients achieved complete responder and 2(9%) as partial responders. The overall median survival was 8 months (range 1-27months), whereas the median survival of responders was 16months(8-27months), the median duration of response was 11months(2-21months). It seems that the combination of ATRA and LDARA-c may be effective in approximately 30% of HRMDS patients [35].
Valproic acid (VPA) has been used as an anticovulsant for decades. VPA is a potent inhibitor of histone deacetylases (HDAc). It can modify the structure of chromatin allowing recruitment of transcription factors to restore epigenetically suppressed genes. VPA has been shown to posses antiproliferative activity and to overcome the differentiation block in leukemia blast cells [37]. Some clinical trials with VPA monotherapy or in combination with ATRA have been reported in MDS. In a piloty study of Kuendgen and colleagues [38- 40] patients with MDS or AML secondary to MDS were treated with VPA monotherapy or with ATRA later resulting in a 44% of response rate. In the follow-up study of 43 patients, an even higher response rate of 52% was observed in those low-risk MDS patients, while for the patients with excess blasts (RAEB) and CMML response rates were 6% and 0% respectively, which implicate the difference of MDS subtypes. In another trials, Siitonen etal [41] reported that according to IWG criteria,3 patients(16%) of 19 MDS responded to treatment following VPA,13-cis RA and 1,25(OH)2D3 combination. All the responses were hematological improvement. One patient responded to the treatment with an increase in platelet value from 67x109/l to 105x109/l. His peripheral blood and bone marrow blast cells decreased from 4% to 0% and from 19% to 7%, respectively. Furthermore, the disease remained stable in 11 patients but progressed in 5 during treatment. This is encouraging results.
Table 1: Results of Retinoic acid therapy in MDS.
A series of these studies are summaried in (Table 1). While some patients experienced improvement in peripheral blood counts, complete responses were reported in only a small proportion of these studies [42-43]. The sole exception was a patient who presented with 29% marrow blasts and 90% abnormal metaphases with 13-cis RA. He obtained a complete clinical and cytogenetic remission therapy [44-49]. This clinical response to 13-cis RA drug was due to in vivo growth inhibition of malignant monocytoid clone [50]. Continued follow-up of this study in this field will be of interest [51-54] (Table 1).
Retinoic Acids in Skin Disease
Vitamin A is necessary for normal epithelial cell differentiation and maturation [55-57]. Retinoids influence on skin keratocyte proliferation, epidermal differentiation and kerintinisation. Those retinoids including natural and chemically synthesized vitamin A derivatives are common used as systemic and topical treatment of various skin disorders. At present there have well developed three generations: the naturally occurring retinoids (all-trans retinol, Aretinoin, Isotretinoin, Alitretinoin) the monoaromatic retinoid and the polyaromatic retinoid derivatives [58].
Table 2: Results of 13-cis RA in severe acne treatment.
Isotretinoin is an orally active retinoic acid derivative for the treatment of acne (papulo- pustular,nodulo-cystic, conglobata) [59],since it shows an excellent efficacy against severe refractory nodulocystic acne. Peck’s [60] original observation in 1978-79 of the effectives of 13-cis RA in cystic acne has been well supported. In double-blind studies using small doses of 13-cis RA regimen, Farrell [61] in 15 patients, Jones [62] in 76 patients, Plewig [63] in 79 patients and Rapini [64] 150 patients reporting have confirmed this results. A summary study of limited review on 365 affected persons are presented in (Table 2). The drug action involves an inhibition of sebum excretion rate(SER) in sebaceous glands and production rate of free fatty acids[60,61,65-68] through trigerring NGAL (neutrophil gelatinase-associated lipocalin) expression [17] normalise follicular keratinisation [69] and the decrease in colonisation of propionibacterium acnes and associated inflammation in skin surface microflora [70].This response, mediated by toll-like-receptor 2(TLR2), is increased in acne patients due to high expression of TLR2 [71] (Table 2).
Figure 4: An advanced squamous cell carcinoma of skin before(left) and after(right) isotretinoin [57].
Encouraging results have also been used 13-cis RA in small numbers of patients with rosacea, Gram-negative folliculitis, Darier’s disease, ichthyosis and pityriasis rubra pilaris [72,73]. In the treatment of rosacea, isotretinoins led to a significant reduction of erythemia, papules and pustules in several studies [72,73]. During treatment of rosacea,13-cis RA act as a potent anti-inflammatory and sebum-suppressive agent. Long-lasting remission can be reported for first patient over 12 months [72]. The use of low dose isotretinoin (0.15-0.3mg/kg bw daily) showed high efficacy and was well tolerated. Isotretinoin is only partially effective in psoriasis, in contrast etretinate which is effective in psoriasis but ineffective in severe acne. Promising, some trials have reported with isotretinoin in patients with squamous and basal cell carcinomas [74,75] cutaneous T-cell lymphoma [56] recurrent malignant glioma [76] malignant eccrine poroma [77] and keratoacanthomas [78,79] and xeroderma pigmentosum with squamous cell carcinoma [79]. In literature, there were at least 10 CR patients with squamous cell carcinoma (SCC). Skroza etal [74] reported a CR patient with well-differentiated SCC following the daily dosage of 0.5mg/kg/day for 5 months. Dring 1-year follow up, he remained all in normal range. Using combination chemotherapy and isotretinoin for 4 months, Zaman [80] reported a complete clinical remission of tumors in a case of 15 year old female of xeroderma pigmentosum with SCC. Another collection of four SCC of skin obtained CR through isotretinoin at daily dose of 1mg/ kg/day twice a day for 4 months (Figure 4) [57]. The mechanism may involve the modification of epidermal growth factor receptor (EGFR) and certain protein kinase. At present, It has clearly known the results that amplified (50-fold EGF receptor in SCC relative to normal skin keratinocytes) or mutant EGFR is oncogenic in origin of some SCC [81]. This oncogenic receptor EGFRvIII has also been found in malignant glioma and invasive breast carcinoma [82-89]. Zhu [90] conduct a short CR using chemotherapy and topical 5% Fu of retinoic acid ointment in a 75-year old patient with SCC. She had a 8x5cm rodent ulcer in her left ear and facial area. A shrinkage of irregular and harden marginal valgus converted to flat and superficial red and scar noted after one month treatment. These findings suggest that retinoids may be effective and well-tolerated therapy for advanced epidermoid SCCs in some studies [91-95] (Figure 4) .
ATRA in patients with gastric cancer (GC)
Recently [96], two cohorts of group presented ATRA trials on patients with GC. Jin etal presented a better benefits of gastric dysplasia with omeprazole and sucralfate and the addition of ATRA (68% vs 37%) compared to patients treated with omeprazole and sucralfate alone. Hu etal also showed that ATRA significantly prolong overall survival following the combination of conventional chemotherapy. ATRA anticancer mechanisms of action against GC cells included cell cycle blocking and differentiation initiation(p21WAF1/CIP1 induction, decreased ERK/MAPK pathway), decreased expression of HER2 oncogenic receptor in patient’s gastric mucosa, apoptosis initiation and inhibiting CSC(cancer stem cell) properties such as tumorspheres formation and patient derived xenografts(PDX) growth in mice. In GC cells, CD44+ stem/progenitor cells and a high ALDH (aldehyde dehydrogenase, R-ALDH, ALDH1A1 and ALDH1A3) activity could be considered as putative targets to inhibit tumor growth, to overcome resistance to cancer therapy and to improve GC prognosis.
The-Structure-of-Retinoic-Acid-Receptors-Molecular-Basis-of-Retinoic-Acid-Action-and-the-RAR-Gene-Transcription RARs structure
The retinoic acid receptors (RAR) belong to the large family of ligand responsive gene regulatory proteins that includes receptors for steroid and thyroid hormones [97]. There are three retinoic acid receptors (RAR), RARα, RARβ and RARγ which are conserved throughout vetebrates encoded by their different RAR (chr 17q21, chr 3p24 and chr12q13) gene, respectively. The RARA contains 462 amino acids(aa) [98,99] RARB consists of 455aa [100] and RARG contains 454aa [101] respectively. The RAR is a type of nuclear receptor which act as a transcription factor that is activated by both all-trans RA and 9-cis RA. The RARs have different functions and may activate distinct target genes. The RARa is expressed in a wide variety of different hematopoietic cells [98,99] the RARβ in a variety of epithelial cells [100] and the RARr in differentiation of squamous epithelia and human skin tissue [101,102]
All RARs contain a variable N-terminal region(A/B), a highly conserved cysteine-rich central domain(C) responsible for the DNA binding activity, and a relatively well-conserved C-terminal half(E) functionally its role in ligand binding and nuclear translocation. These three main domain are separated by a hinge region(D) [12,97,102].The central DNA binding domain(88-153aa) exhibits an array of cysteine residues compatible with the formation of two so-called zinc finger(Miller,1985).Each of them a zinc atom tetrahedrically coordinated to four cysteine and each of the hypothetical zinc finger is encoded by a separate exon of the receptor gene (Figure 5) Zinc finger 1, 88-108aa, Zinc finger 2, 124- 148aa] [97-103].The N-terminal zinc finger of the DNA binding domain confers hormone responsiveness to HREs, determing target gene specificity and responsible for functional discrimination between HREs whereas the C-terminal finger contains the sugarphosphamide backbone of the flanking sequences [103,104] (Figure 5) .
Figure 5:Amino acid sequence of the DNA binding domain of the hRARa into two putative zinc–binding finger (Figure from George Zhu a feeling for scientific drawing based on Evans RM, Science, 1988, 240:899- 895; Beato M,Cell,1989, 56: 335-344; Giguere V,Nature,1987;330:624-29; Petkovich M, Nature, 1987,330: 444).
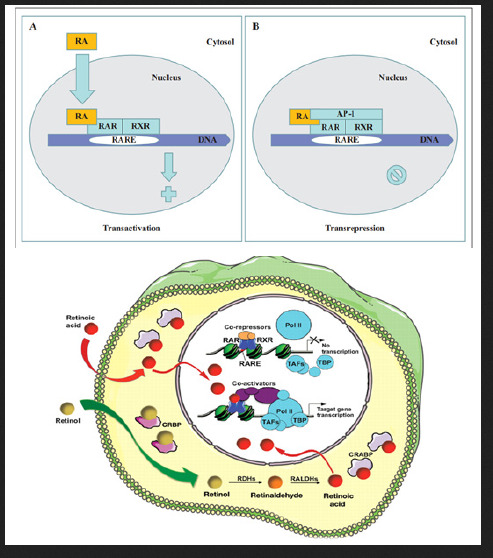
The molecular basis of retinoic acid action and the RAR gene transcription
Retinoic acid (RA) is a lipophilic signal molecule which is able to induce acute and direct activation of the expression of specific genes supports its molecular model of action that resembles that of steroid hormones [105]. The cellular retinoic acid-binding protein (CRABP) may be involved in this transfer [9,10]. In the nucleus, RA receptors (RAR) function as a heterodimer with retinoid X receptors (RXRs) [106-109]. RAR/RXR can bind to DNA motif at RA-response elements (RAREs, also HRE) in the regulatory sequences of target genes in the absence of ligand, thereby interacting with multiple protein complexes that include co-repressors N-CoR [110] SMRT [111] and histone deacetylases (HDACs), and maintaining gene repression. Here, RAREs consist of a direct repeat of a core hexameric sequence 5’ (A/G)G(G/T)TCA-3’ [112] or of the more relaxed 5’-(A/G)G(G/T) (G/T)(G/C)A-3’ motif, separated by 1,2,5 bp [113]. A corepressor represses expression of genes by binding to and activating a repressor transcription factor, the repressor in turn bind to target gene’s operator including RARE sequence, then blocking transcription of that gene (see corepressor-wikipedia). Transcriptional regulation thus drives from the binding of hormone-receptor complexes to RARE sites on target DNA [12,97,103]. In the presence of RA(all-trans RA,9- cis RA),binding of the RA ligand to RAR alter the conformation of the RAR, a conformational change in the DNA-bound receptor leads to the release of co-repressor complexes associated with the RAR/RXR dimer and the recruitment of co-acitivator complexes. These induce chromatin remodeling and facilitate assembly of the transcription pre-initiation complex including RNA polymerase II (Pol II) [114], TATA-binding protein (TBP) and TBP-associated factors (TAFs) [2,12,103,115,116] (Figure 6). Subsequently, transcription of target genes is initiated. This also represent liganddependent transcriptional activation which mediated by nuclear receptors. Like thyroid hormone receptor (THR) [117,118] retinoic acid act as ligand for RARs, converting RARa from transcriptional repressor to activators [2,12,119-122]. Numerous RAR target genes after RA induction have been identified including genes within retinoid pathway, such as RARB,Crbp1/2 (Rbp1/2),Crabp1/2 and CYP26a1.And also, several members of HOX gene family, including HOXa1,HOXb1,HOXb4 and HOXd4,and other genes Tshz1 and Cdx1 [123] the function of which has been demonstrated in vivo in the normal roles of retinoids in patterning vertebrate embryogenesis, early neurogenesis, cell growth and differentiation (Figure 6).
Figure 6:Retinoid receptor-dependent gene regulation [116] & (b): Gene regulation by retinoic acid signalling [2].
Molecular Model of the Gene Regulation of Retinoic Acid Action in APL
Acute promyelocytic leukemia (APL) is a clonal expansion of promyelocytic precursors . Retinoic acid(RA) (initial 13-cis RA, later ATRA and tamibarotene) plus chemotherapy is currently the standard of care [124-131]. APL has a very good prognosis, with long-term survival rates up to near 70%-90% [132]. Molecular analysis has uncovered the facts that approximately 98% of APL, RARa translocates and fuses with the PML gene on chromosome 15 [133-136]. The resulting RAR chimeric genes encode pml/RARa fusion protein, which is specifically expressed in the promyelocytic lineage [20]. In addition to oncogenic receptor derivative pml/RARa [108,137-139] the translocation involves oncogenic TBL1XR1- RARB [140] and NUP98/RARG [141] and oncogenic PML-RARG [142] which share high homolog (90%) of three RAR family that were also detected in APL rare cases.
Most studies have shown in APL that oncogenic pml/ RARa act as constitutive transcriptional repressor that blocks neutrophil differentiation at the promyelocyte stage. Without its ligand, retinoic acid (RA), PML-RARA functions as a constitutive transcriptional repressor of RARE-containing target genes, abnormally associating NcoR/HDACs complex and blocking hematopoietic differentiation. In the presence of pharmacological concentration of RA (about 350ng/ml), RA induce the corepressors NcoR/ HDACs dissociation from PML-RARA, thereby activates transcription and stimulate differentiation [11,12,108,139]. In vitro by using a dominant negative RAR construct transfected with interleukin 3(IL-3)-dependent multipotent hematopoietic cell line (FDCP mix A4) and normal mouse bone marrow cells, GM-CSF induced neutrophil differentiation was blocked at the promyelocyte stage. The blocked promyelocytes could be induced to terminally differentiate into neutrophils with supraphysiological concentration of ATRA [143]. Similarly, overexpression of normal RARa transduced cells displayed promyelocyte like morphology in semisolid culture,and immature RARa transduced cells differentiate into mature granulocytes under high dose of RA(10-6M) [144]. Moreover, mutation of the N-CoR binding site abolishes the ability of PML-RARa to block differentiation [145,146]. Therefore, ectopic expression of RAR fusion protein in hematopoietic precursor cells blocks their ability to undergo terminal differentiation via recruiting nuclear corepressor N-CoR/histone deactylase complex and histone methyltransferase SUV39H1 [147]. In vivo, transgenic mice expressing PML-RARA fusion can disrupt normal hematopoiesis, give sufficient time, develop acute leukemia with a differentiation block at the promyelocytic stage that closely mimics human APL (APL-like syndrome, even in its response to RA in many studies. These results are conclusive in vivo evidence that PML/ RARa is indeed oncogenic, and oncogenic pml/RARa is etiology of APL pathogenesis [148-150]. This also represent a steroid receptor in tumorigenesis (Figure 7).
Figure 7: pml/RARa fusion in differentiation block at promyelocytic stage in transgenic mice [149].
Moreover, in Rousselot’s group experiments, HL-60 cells transfected with 15-30ug of PML-RARa fusion in culture show no features of granulocytic differentiation after 7 days of incubation with 10-7,10-6 uM RA (5.5-9.5% of differentiated cells by the NBT test). At 5ug of PML-RARa plasmid concentration, the blockage of RA-dependent myeloid differentiation could be overcomes with high doses(10-6M) of RA (99% of differentiated cells by NBT test) (Figure 8) [151]. The results clearly indicate that PMLRARa mediated transcriptional repression, as well as PML-RARa oncoprotein blocks RA-mediate promyelocyte differentiation. (Figure 8).
Figure 8: Expression of pml-RARa in HL-60 cells blocks ATRA-induced promyelocytic differentiation a(in the presence of 10-7 M RA, top), and transcriptional repressive properties of pml-RARa in human myeloid cells as βRARE-luc assay(bottom) [151].
By using Xenopus oocyte system to uniquely the comparison of the transcriptional properties of RAR and PML-RAR is due to the lack of endogenous nuclear receptors and the opportunity to evaluate the role of chromatin in transcriptional regulation. The results shown in (Figure 9) demonstrated that, indeed, PML-RARA is a stronger transcriptional repressor that is able to impose its silencing effect on chromatin state even in the absence of RXR. Only pharmacological concentration of RA,pml/RARA become transcriptional activator function [139]. (Figure 9).
Figure 9: Shows pml/RARa as a constitutive transcriptional repressor in xenopus oocyte system, as measured by RARE3 CAT and GAL4 assay [139].
In vitro experiments, ATRA induce pml-RARA itself cleavage into a 85-97kd delta PML-RARA product (a truncated pml/RARA form) in RA sensitive NB4 [152-156] (Figure 10). Delta PML-RARa is not formed in ATRA differentiation resistant NB4 subclones [152,155] which indicate the loss of PML/RARa may be directly linked to ATRA-induced differentiation [152,155].This induction of of PML-RARa cleavage and degradation by RA(ATRA,9-cis RA,Am80) involve the proteasome-dependent [152-154] and caspase mediated pathway [155] or independent of proteasome and caspase cleavage[156] and possibly ubiquitin-activating enzyme EI-like(UBEIL) induction in NB4 cells. This is reason that proteasome inhibitor MG-132 and caspase inhibitor ZVAD do not block ATRA-induced pml/RARa cleavage and differentiation whereas this delta pml-RARA is blocked by RARA itself antagonist Ro-41-5253 [156].The proteasome-dependent pml/RARA degradation, by using proteasome inhibitor lactacystin test, allows APL cells to differentiation by relieving the differentiation block [153]. These data suggest a set of multiple molecular mechanisms for restoration by RA induced myeloid differentiation in APL cells. (Figure 10).
Figure 10: Shows delta pml/RARa cleavage products independent of proteasome and caspase in the presence of ATRA(a,b), and pml/RARa act as transcriptional repressor even in the presence of ATRA(0.01uM,1uM) in RARE-tu-luc assay while delta pml/ RARa is less potent activator of RARE-tk-leu activation than wild-type RARa(c) in NB4 cells [155].
Next we further examine the pml/RARa three region functions,in vitro deletion of the RARa DNA binding domain decreased the ability of pml/RARa to inhibit vitD3 and TGFinduced the myeloid precursor U937and TF-1 cell differentiation [145]. This is also supported by functional analysis of DNA binding domain mutation in vitro. The RARa zinc finger is a sequencespecific DNA binding through which RARa contacts the RA target genes. Moreover, deletion of PML coiled-coil region also blocked the differentiation capacity of TF-1 cells [145]. The coiled-coil region directs the formation of pml/RARa homodimers tightly interact with the N-CoR/HDACs complex, so that transcriptional derepression cannot occur at RARA target gene promoter even if the presence of ATRA [RA resistant, 12,157]. In vitro, using established subclones of NB4 resistant to both ATRA and 9-cis RA, they were significantly less able to stimulate transcription of a RARE driven CAT-reporter gene induction by ATRA and showed altered DNA binding activaty on a RARE [158]. In the resistant cases, mut PML stabilizes PML-RARa [159]. PML-RARA with ligand-binding domain (LBD) mutation, ligand RA binding with LBD is impaired. These results have clearly shown that PML protein dimerization and RARa DNA binding domain are indispensible for the myeloid precursors differentiation which was blocked by PML/RARA and eventually leukemic transformation.
In accordance,the pml/RARa/RXR target genes is found to block differentiation by consitutively silencing a set of RA-responsive genes in the control of hematopoietic precursor cells. Five major transcription factors, Ap-1 [160] C/EBPepsilon [161,162] Pu.1/ DAPK2 [163] PTEN [164] and p21WAF/CCKN1A [165] directly regulate genes important in myeloid differentiation. PML/RARA fusion is oncogenic transcriptional repressor of five genes. Inhibited expression or functions of these five transcription factors lead to a block in myeloid differentiation, which is a hallmark of APL.
In vitro cotransfection of pml/RARA with plasmid expressing AP-1 of c-Jun and c-fos proteins in MCF-7 cells, by using CAT assay, PML-RARa is a repressor of AP-1 transcriptional activity in the absence of RA while RA treatment converted the chimera into a strong activator [160]. Since high AP-1 activity is associated with differentiation of leukemic cells in several context [160] the stimulatory effects in the presence of RA could be relevance to its reversal by provoking differentiation. Another, in pml/RARacontaining cell lines, a close link exists between induction of differentiation and induction of C/EBP epsilon expression [161]. C/ EBPepsilon knockout mice had a block in myeloid differentiation [162]. In absence of retinoic acid (RA), induction of pml/RARa expression in U937PR9 cells stably transfected with zinc-inducible pml/RARa suppressed the expression of C/EBPepsilon. In contrast to its repression, in the presence of a pharmacologic concentration of RA, pml/RARa significantly increased the level of C/EBPepsilon expression in a time and dose-dependent manner [161]. The findings implicate that C/EBPepsilon is critical downstream target gene in RA-dependent granulocytic differentiation in the treatment of APL [163-165].
Phosphotase and Tensin homolog (PTEN) is a protein and lipid phosphatase, which plays a pivotal dual role in tumor suppression and self-renewal of hematopoietic stem cells as its promoting exhaustion of normal hematopoietic stem cells (HSCs) and generation of leukemia-initiating cells (LICs) [166,167]. PTEN expression is downregulated in APL, while ATRA treatment increases PTEN leves by inducing PU.1 transcriptional activity via pml/RARa degradation, allowing the binding of PU.1 in PTEN promoter, in turn promotes PTEN nuclear re-location and decreases expression of the PTEN target Aurora A kinases. Therefore, PTEN is one of the primary target gene of oncogenic pml/RARa in APL
Importantly, restoring DAPK2 expression in PU.1 knockdown APL cells partially rescued neutrophil differentiation [168]. In addition, DAPK2 interacts with other cyclin- dependent kinase inhibitors such as p15INK4b and p21WAF1/CIP, which is needed for the cell-cycle arrest in terminal differentiation of neutrophils. Moreover, DAPK2 can bind and activate the key autophagy gene beclin-1 [169]. DAPK phosphorylates beclin 1 on Thr 119 located at a crucial position within its BH3 domain and thus promotes the dissociation of beclin 1 from BCL-XL inhibitor and induction of autophagy [169]. Here, beclin 1 was initially identified as a BCL-2- binding protein, which is part of a class III PI3K (phosphatidylinositol -3-kinase) multiprotein complex that participate in autophagosome nucleation. Death- associated protein kinase (DAPK1) is a calcium/ calmodulin (CaM) serine/threonine kinase for mediator of cell death [170]. PU.1, an ETS transcription factor known to regulate myeloid differentiation. Silencing of PU.1 in the adult hematopoietic tissue produces dysfunctional stem cells and impaires granulopoiesis by inducing a maturation block. Overexpression of PU.1 overcomes the differentiation block in SCa 1+/Lin- HSC with transduction of PML/ RARa fusion, as measured by the Gr-1 and Mac-1 expression [171]. Thus, pml/RARa represses PAPK2/PU.1 - mediated transcription of myeloid genes in APL,linking a novel autophagy mechanism of pml/ RARA degradation [172].
Figure 11:Molecular model of the gene regulation of retinoic acid (RA) action (George Zhu, January 1991, revised in 2012, further revised in 2018 and in this paper). Schematic alignment of the receptor protein. The two highly conserved regions, identified as the putative DNA-binding (C) and hormone- binding (E), a hinge region (D) and the non-conserved variable NH4-terminus (A/B) as described above. CAT:CAAT box, CCAAT-enhancer binding proteins(or C/EBPs); GC:GC box; TATA:TATA box. Note: In APLcells, pml/RARa fusion point is located in the first 60 amino acids from the N-terminus(A/B) of RARa [12,182].
The elucidation of the molecular basis of retinoic acid and retinoid pharmacology in APL has been illustrated in several publications [157,173-176] the detail molecular model of gene regulation had also been proposed by Zhu in 1990s [12,177,178]. As an approach to APL treatment, one possible the action of retinoic acid, A consensus sequence (TCAGGTCA motif) has been postulated for thyroid hormone (TRE) and retinoic acid responsive element(RARE)-containing in the promoter region of target genes [112]. High dose of RA-RARE-PML/RARa complexes in intracellular localization appears to relieve repressors from DNA-bound receptor [11,12,117,145,158,179] including the dissociation of corepressor complexes N-CoR, SMRT and HDACs from PML- RARa or partially PML-RARa/RXR [11,12,146,157,179]. Also release PML/RARa -mediated transcription repression [175]. This transcriptional derepression occurs at RARa target gene promoter [12,157,180]. Consquentially, PML-RARa chimera converted receptor from a repressor to a RA-dependent activator of transcription [156,157,160]. Co-activator complexes containing histone acetyltransferase (e.g. p300/CBP) are recruited. The resulting pml-RARA oncoprotein proteolytic degradation occurs through the autophagy- lysosome pathway [172] and the ubiquitin SUMO-proteasome system (UPS) [152-155] as well as caspase 3 [155] or lysosomal protease (cathepsin D) enzyme or/and EI-like ubiquitin-activating enzyme (UBEIL) induction [181]. An effect is to relieve the blockade of pml/RARa-mediated RA dependent promyelocytic differentiation and retinoic acid (9-cid RA, ATRA, Am80) in APL therapy Zhu G, March 1990- January 1991, revised in 2012). Here, RA can overcome the transcriptional repressor activity of pml/RARa [11,12,156,179,182]. The oncogenic pml/ RARa uncover a pathogenic role in leukemogenesis of APL through blocking promyelocytic differentiation. This oncogenic receptor derivative pml/RARa chimera is locked in their “off” regular mode thereby constitutively repressing transcription of target genes or key enzymes (such as AP-1, PTEN, DAPK2, UP.1, p21WAF/CCKN1A) [160-165] that are critical for differentiation of hematopoietic cells. This is first described in eukaryotes (Figure 11).
Read More About this Article: https://biomedgrid.com/fulltext/volume3/vitamin-a-and-its-derivatives-retinoic-acid-and-retinoid-pharmacology.000656.php
For more about: Journals on Biomedical Science :Biomed Grid
#biomedgrid#medical and medicinal journal#Journals on Biomedical Imaging#Journals on Medical drug and theraputics#American medical journal#Journals on Biomedical Science
0 notes