#Molnupiravir manufacturers
Explore tagged Tumblr posts
Text
Explore the journey of Molnupiravir from laboratory inception to its vital role in combating viral infections. Learn about its manufacturing process, the dedication of pharmaceutical innovators, and its significant impact on public health.
0 notes
Text
The Best News of Last Week
⚡ - Charging Towards a More Electrifying Future
1. The Kissimmee River has been brought back to life—and wildlife is thriving

The Kissimmee River in Florida was straightened in the 1960s, causing a sharp decline in wildlife and ecological problems. But in the 1990s, a $1 billion restoration project was initiated to restore the river's natural state.
Today, nearly half of the river has been restored, wetlands have been reestablished and rehydrated, and wildlife has returned, including rare and threatened species. Already the biological impact of the project has become clear. As the wetlands have come back, so have the birds.
2. Plastic wrap made from seaweed withstands heat and is compostable

A cling film made from an invasive seaweed can withstand high temperatures yet is still easily compostable. The material could eventually become a sustainable choice for food packaging.
Scientists started with a brown seaweed called sargassum. Sargassum contains long, chain-like molecules similar to those that make up conventional plastic, which made it a good raw material. The researchers mixed it with some acids and salts to get a solution full of these molecules, then blended in chemicals that thickened it and made it more flexible and pliable.
3. An Eagle Who Adopted a Rock Becomes a Real Dad to Orphaned Eaglet

Murphy, a bald eagle that had been showing fatherly instincts, has been sharing an enclosure with an eaglet that survived a fall from a tree during a storm in Ste. Genevieve. Murphy, his rock gone by then, took his role as foster parent seriously. He soon began responding to the chick’s peeps, and protecting it.
And when, as a test, the keepers placed two plates of food in front of the birds — one containing food cut into pieces that the chick could eat by itself, and another with a whole fish that only Murphy could handle — the older bird tore up the fish and fed it to the eaglet.
4. World's largest battery maker announces major breakthrough in energy density

In one of the most significant battery breakthroughs in recent years, the world’s largest battery manufacturer CATL has announced a new “condensed” battery with 500 Wh/kg which it says will go into mass production this year.
“The launch of condensed batteries will usher in an era of universal electrification of sea, land and air transportation, open up more possibilities of the development of the industry, and promote the achieving of the global carbon neutrality goals at an earlier date,” the company said in a presentation at Auto Shanghai on Thursday.
This could be huge. Electric jets and cargo ships become very possible at this point.
5. Cat with '100% fatal' feline coronavirus saved by human Covid-19 medicine

A beloved household cat has made an “astonishing” recovery from a usually fatal illness, thanks to a drug made to treat Covid-19 in humans – and a quick-thinking vet.
Anya, the 7-year-old birman cat, was suffering from feline infectious peritonitis (FIP), a “100% fatal” viral infection caused by feline coronavirus. That was, until Auckland vet Dr Habin Choi intervened, giving Anya an antiviral used to treat Covid-19 called molnupiravir.
6. Kelp forests capture nearly 5 million tonnes of CO2 annually

Kelp forests provide an estimated value of $500 billion to the world and capture 4.5 million tonnes of carbon dioxide from seawater each year. Most of kelp’s economic benefits come from creating habitat for fish and by sequestering nitrogen and phosphorus.
7. Medical Marijuana Improved Parkinson’s Disease Symptoms in 87% of Patients

Medical cannabis (MC) has recently garnered interest as a potential treatment for neurologic diseases, including Parkinson's disease (PD). 87% of patients were noted to exhibit an improvement in any PD symptom after starting medical cannabis. Symptoms with the highest incidence of improvement included cramping/dystonia, pain, spasticity, lack of appetite, dyskinesia, and tremor.
----
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation:
Buy me a coffee ❤️
Also don’t forget to reblog
817 notes
·
View notes
Text
Marty Makary is a covid contrarian, cozy with anti-vaxxers.
Marty Makary has been making the rounds for ages on everything from CSPAN and CBS to The Ralph Nader Radio Hour. But make no mistake, there's good reason he’s welcomed into the seedy MAGA ranks of Trumpy Trumpworld. He’s just another doctor in the infect-everyone club with people along the lines of Scott Atlas.
He was part of the symposium of covid contrarians celebrating the anniversary of The Great Barrington Declaration. In 2020 he was a Fox News contributor. A while back Makary was quoted in a far-right media outlet that sells supplements, an industry some say fuels the right-wing political world, in an article that was attacking Paxlovid, dangerously and wrongly telling people not to use it for covid, for reasons that didn’t make sense. Sure, many doctors sometimes get these things very wrong. But the strange part was that Marty Makary was confusing Paxlovid with Molnupiravir, an entirely different drug, and attributing the way Molnupiravir works to Paxlovid, which is not accurate. That’s a troubling mistake to make.
But then Makary is also a surgeon who actually mocked hand washing in a Congressional hearing, where he was brought in to testify by Republicans along with other Great Barrington Declaration natural herd immunity infect-everyone proponents. It’s odd how Trump has a reputation for germaphobia and yet two cabinet picks are actually notoriously known for anti handwashing. The other being Pete Hegseth who has such a laundry list of other things wrong with him you may have missed the handwashing thing - but that’s something that certainly stuck with me.
Marty Makary is apparently famous for the off-base factoid that medical errors are a leading cause of death even though his math doesn’t add up at all.
In August 2021 he co-authored an anti-mask op-ed with Trumpy doctor Cody Meissner who now chants anti-vax herd immunity crap at FDA vaccine meetings. In September 2021 he was pushing natural herd immunity, even after vaccines were available - which was essentially promoting needlessly letting babies get sick unvaccinated, a view shared with the notorious and sad Vinay Prasad, and Tracy Hoeg, someone with behaviour so unsettlingly right-wing that people only talk about some of it in private whispers.
So at some point you have to let go of the hopium that he’s some kind of moderate centrist who and both-sides things. This doctor shouldn't be normalized at all. He is NOT normal, no matter how much the media tries to manufacture mild on various and sundry Trumpy cabinet picks, or people wish-cast that nothing’s as bad as feared. Marty Markary is very much a Trumpy weirdo. And I wish people with platforms would stop being fooled, or somehow incentivized, into this truth-teller type nonsense when his math was all built on exaggerated fictions. And his opinions seem built on contrarianism.
I really wouldn’t trust this person for medical information, and I certainly don’t trust his politics.
Important Context - Everything You Need to Know About Donald Trump’s FDA Pick Johns Hopkins surgeon Marty Makary has spent years promoting fringe pandemic views and attacking the U.S. government. Walker Bragman Nov 20, 2024 He has authored multiple books on the subject including 2019’s “The Price We Pay,” which argued that costs were simply too high. With the pandemic, he has emerged as a popular figure on the political right for his particular brand of contrarianism, which has included falsely asserting in a February 2021 op-ed that the U.S. would achieve COVID herd immunity by April of that year. Even before COVID, Makary was controversial. In 2016, he was behind a widely rebuked study in the British Medical Journal purporting to find that medical error was the third leading cause of death in the U.S. Shortly after publication, the editors-in chief of BMJ Quality and Safety debunked the findings.
#marty makary#covid contrarians#covid deniers#great barrington declaration#government#pandemic#healthcare#infection control#public health#infectious diseases#anti-mask#anti-vax#anti-handwashing#trump administration#doctors#doctor pundits#politics#medical misinformation#misinformation#propaganda#trump world#trumpy#medical errors#factoids#Tracey Hoeg#cody meissner#vinay prasad#scott atlas#surgeon#maga
0 notes
Text
An antiviral drug widely used against COVID-19 is driving an unintended pattern of mutations in the SARS-CoV-2 virus that is increasing its genetic diversity.

This is the warning of an international team of researchers who studied some 15 million SARS-CoV-2 sequences to map out exactly how the coronavirus has mutated over time.
While viruses do naturally mutate, the analysis revealed mutational events that looked very different from the regular pattern of change - and nearly a third of these unusual shifts were associated with people who had taken the antiviral molnupiravir.
This drug - manufactured by Merck and Ridgeback Biotherapeutics - works by inducing mutations in the viral genome during replication, many of which either damage or kill the virus, helping to reduce the body's viral load.
However, the team found that some of the changes caused by molnupiravir aren't having the intended effect - and are causing enduring mutations instead.

The analysis revealed small clusters of these mutations, suggesting that they are being transmitted between patients. At present, the researchers said, no established variants of concern have been linked to these mutational signatures
1 note
·
View note
Text
0 notes
Text
Molnupiravir Manufacturers in India – 200mg/400mg Capsules

We are one of the leading molnupiravir manufacturers in India. Our effective anti-covid medicine is available in 200mg & 400mg pack at affordable prices. It is available for covid treatment at affordable prices under the brand name of Molnova. Molnupiravir capsule is well researched, safe and affordable medicine, it is one of the first antiviral medicine for effective treatment of corona.
#Molnupiravir manufacturers#helath#helathcare#pharmaceutical products#corona virus#corona infection#covidー19
1 note
·
View note
Link
Molnupiravir Capsules Manufacturers in India – Zoic Biotech is among the top prominent pharmaceutical manufacturers in Pharmaceutical Industry in India. Moreover, it is dealing with an extensive array of Molnupiravir Capsules in India and hence, our company is among the most trustworthy Molnupiravir Capsules Manufacturers in India.
0 notes
Text
Experts are predicting demand for life-saving antiviral drugs will rapidly outpace supply. Like the vaccine, the poorest countries will be left until last.
Covid-19 has quietly become the gift that keeps on giving for big pharma. The past two years has seen it reap huge profits from Covid vaccines, while simultaneously opposing wider sharing of the technology required to make them. And now there’s a new money-spinner on the rise: Covid antiviral treatment pills. Once again, we’re poised to fall into the same inequality traps we’re caught in with the global vaccine rollout.
Both Pfizer and Merck have new antiviral pills rapidly arriving on the market – Paxlovid and molnupiravir respectively. As with the vaccines that came before them, both corporations have made it their business to ultimately decide who gets to make generic versions through the medical patent system – a crucial, life-saving question for millions around the world.
And business certainly looks promising. Pfizer alone, freshly cemented as the global Covid-19 vaccine kingpin, expects to make as much as $22bn from its new pill this year, on top of $37bn it made in 2021 from the vaccine.
The new medication isn’t coming cheap. Pfizer’s Paxlovid currently costs about $530 for a five-day course of the treatment. Merck’s molnupiravir, now approved for use in the UK, costs about $700. Reportedly, the cost of production for molnupiravir stands at about $17.74.
Familiar alarm bells should be ringing. Experts across the board are predicting demand for antiviral drugs will rapidly outpace supply. A World Health Organization report produced in January warned of a “high risk of shortages” of Paxlovid for low- and lower-middle-income countries until generic versions became more widely available, which isn’t likely to be until the second half of 2022 at the earliest. Separate analysis from the data and analytics firm Airfinity suggests that could be as late as early 2023. After an uneven global vaccine rollout, lower-income nations are faced with the prospect of a “wild west” scenario for life-saving pills, too.
Pfizer and Merck have chosen to designate a select few generic manufacturers able to produce cheaper versions of their drugs, through the Medicines Patent Pool (MPP). But even with these deals in place, they remain firmly in control, and access to generic versions are within reach of only half the world’s population.
A number of countries including Argentina, Brazil, Thailand, Russia, Colombia, Peru, Turkey and Mexico have again been excluded from such licences and are left to try to cut deals for the most expensive products. With so many priced out of the market, global supply will again be prioritised to rich countries, while the companies refuse to make affordable generic antivirals available to everyone wherever they are needed.
This is a grim mirror of the dramatically uneven vaccine supply earlier in the pandemic, when rich nations bought up many more doses than they could use. The US, where almost two-thirds (65%) of the population is already fully vaccinated, has reportedly put up more than $10bn for Pfizer’s Paxlovid – more than twice the entire GDP of Sierra Leone, where just 9% of people have the same protections. For less wealthy nations, competition isn’t even a possibility.
Meanwhile, Merck continues its “evergreening” patent strategy to extend its monopoly on molnupiravir beyond the standard 20-year protection. Since developing the pill, it has sought at least 53 patent applications to tie it up in legal red tape and stay firmly in control of who gets to make it and where. It has already received emergency approval in the US and Japan, and has been given the green light in the UK.
Even in nations within the MPP, where the pills are allowed to be made by select manufacturers, a low cost is not guaranteed. Dr Reddy’s Laboratories in India has made a generic version of Merck’s pill that costs $18 for a course of treatment. However, these costs won’t necessarily be reflected everywhere. Across the border in Bangladesh, the generic version of Pfizer’s pill will cost more than $170 for a course of treatment – prohibitively expensive for a huge number of the population. By restricting which manufacturers may produce a generic version, firms maintain considerable control over the final price. In the past, Gilead’s treatment for hepatitis C, sofosbuvir, only dropped in price consistently when the number of manufacturers was increased without these limits.
There is an uncomfortable assumption those in the global north have tacitly begun to accept. When the demand is higher than supply, there is a pecking order: rich nations first, buying up more than they realistically need, while the poorest are forced to scramble to outbid each other over what is left, dramatically overpay, or just wait until they’re affordable and watch death tolls rise. But this supply crisis is entirely artificial. We could produce more – Pfizer and Merck’s drugs are not complex, and could be easily manufactured in a wide range of developing countries if they had access to the knowhow and could avoid the threat of legal action. We need patents and other intellectual property barriers on life-saving medicines to be waived – either voluntarily by companies, or by government decree – so we can quickly supply all countries of the world.
We’re doubling down on a two-tier world when it comes to Covid-19 – rich, highly vaccinated nations with easy access to both preventive measures and treatments, and poorer nations trying to get by without either. It’s vital that we don’t sleepwalk into giving corporations so much control over who gets to live and who gets to die, all balanced on what they deem an acceptable bottom line.
Source: Guardian
I want to cry.
Just as deliberately creating a food shortage and barriers to aid is a genocide, so is this.
The total worldwide death count from covid currently reported stands at 5.9 million. Data analysts who use excess deaths over the pandemic years in each country (comparing the number of deaths in pandemic years with the average pre-pandemic mortality rate) say that the real pandemic death count (that includes underreported deaths, fudged numbers, deaths from complications from covid, and otherwise preventable deaths that happened because the healthcare systems were at capacity) is anywhere from 12 million to 22 million. Most of those numbers are overrepresented and even outstripped in Global South countries, because our healthcare systems completely buckled and almost broke due to lack of resources and vaccine scarcity.
The percentage of covid patients who develop long-term disabilities is far higher in Global South countries because we are less likely to have adequate intervention and care resources. The pandemic has also been catastrophic for our economies, with millions of people plunging beneath the poverty line, entire industries capsizing and people left homeless and starving, in turn leading to dangerous political instability. Waiting another year for a generic drug means devastation on a truly incomprehensible scale.
And for what? So a handful of rich white assholes can get richer by magnitudes. All because Global North governments won't intervene.
#capitalism#covid-19#genocide#global north#global south#social justice#inequality#big pharma#white sociopathy#colonialism#neocolonialism#imperialism#ableism#medical abuse#disability#knee of huss
42 notes
·
View notes
Text
Here in this article, we have explained in detail the primary difference between Molnupirvir and Remdesivir, so read this article completely.
0 notes
Text
‘A pretty big deal’: U.S. makes COVID-19 technologies available for use in developing countries | Science

The U.S. government has agreed to put licenses for 11 medical technologies developed at the National Institutes of Health (NIH) into a so-called patent pool, a move that promises to make it easier for low- and middle-income countries to gain access to vaccines, drugs, and diagnostics for COVID-19. President Joe Biden made the announcement yesterday at the Global COVID-19 Summit.
The government cut a deal to provide the federally funded inventions with the COVID-19 Technology Access Pool, organized by the World Health Organization (WHO). WHO then turns over the licenses to a nonprofit, the Medicines Patent Pool (MPP), which negotiates with manufacturers interested in using the technologies to make products that can be sold worldwide. “It’s a pretty big deal,” says James Love, who directs Knowledge Ecology International, a nonprofit that advocates for sharing intellectual property to benefit the public.
The scheme is part of a broader push to make medicines developed in rich countries more broadly accessible that Love helped spark 2 decades ago by campaigning for the availability of HIV drugs in poor countries. That Biden himself made the announcement yesterday is a “significant” show of support, Love says.
Created in 2010, MPP today has patent agreements for several anti-HIV drugs and recently added two treatments for COVID-19, Pfizer’s Paxlovid and Merck & Co.’s molnupiravir. The new agreement also covers inventions used by companies that make existing COVID-19 vaccines, such as a modification that stabilizes spike, the surface protein of SARS-CoV-2. Companies could also use the technologies to make entirely new products. Research tools for drugmakers and diagnostic assays are also part of the agreement.
MPP forges deals with drugmakers that allow companies in the least developed countries to pay the lowest royalty fees—and some pay nothing at all. In many cases, however, the licenses in the NIH portfolio only remove one hurdle to making a vaccine or another product, which often require licensing agreements with several different patent holders.
Few developing countries manufacture vaccines—Pfizer and Moderna only recently began to help African countries make their COVID-19 vaccines—and the agreement could lead to more production plants in poorer regions of the world, says Ellen ’t Hoen, who founded MPP. “You can’t have sustainable vaccine manufacturing capacity if you’re only allowed to produce something when the world is on fire,” she says.
The agreement also could have a “symbolic and political” impact, ‘t Hoen says, on efforts underway to pressure companies and institutions to more quickly and broadly share intellectual property that is key to combating pressing diseases. It could signal to companies that have been reluctant to share patents that ���you should follow suit,” says ’t Hoen, who now directs Medicines Law & Policy, a nonprofit. “If companies now continue to give the COVID-19 Technology Access Pool the cold shoulder, I think the push on changing the rules of the game will only become stronger.” Both the World Trade Organization and the WHO-led Pandemic Preparedness Treaty have ongoing discussions about increasing access to intellectual property to share critical medicines more quickly in the future. But the issue is contentious, and the various parties have yet to reach a consensus on how to proceed.
New post published on: https://livescience.tech/2022/05/14/a-pretty-big-deal-u-s-makes-covid-19-technologies-available-for-use-in-developing-countries-science/
1 note
·
View note
Text

A five-day course of molnupiravir, the new medicine being hailed as a “huge advance” in the treatment of Covid-19, costs $17.74 to produce, according to a report issued last week by drug pricing experts at the Harvard School of Public Health and King’s College Hospital in London. Merck is charging the U.S. government $712 for the same amount of medicine, or 40 times the price.
Last Friday’s announcement that the new medicine cut the risk of hospitalization among clinical trial participants with moderate or mild illness in half could have huge implications for the course of the coronavirus pandemic. Because it’s a pill — as opposed to monoclonal antibodies, a comparable antiviral treatment that is administered intravenously — molnupiravir is expected to be more widely used and, hopefully, will cut the death rate. In the first 29 days of the trial, no deaths were reported among the 385 patients who received the drug, while eight of the people who received a placebo died, according to the statement put out by Merck and Ridgeback Biotherapeutics, the two companies that are jointly launching it.
In addition to having huge implications for health, the pill could bring staggering profits to both Merck and Ridgeback Biotherapeutics. A small Miami-based company, Ridgeback licensed the medicine from Emory University in 2020 and two months later sold the worldwide rights to the drug to Merck for an undisclosed sum. Although Ridgeback remains involved in the development of the drug, some have described the deal as “flipping.”
Like the vast majority of medicines on the market, molnupiravir — which was originally investigated as a possible treatment for Venezuelan equine encephalitis — was developed using government funds. The Defense Threat Reduction Agency, a division of the Department of Defense, provided more than $10 million of funding in 2013 and 2015 to Emory University, as research done by the nonprofit Knowledge Ecology International has revealed. The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, also provided Emory with more than $19 million in additional grants.
Yet only Merck and Ridgeback will reap the profits from the new antiviral, which according to Quartz could bring in as much as $7 billion by the end of this year. After the announcement of the encouraging clinical trial results on Friday, Merck’s stock price climbed, while stock prices of some vaccine makers sagged. Despite its initial investment, the U.S. government seems to be facing a steep markup in prices. In June, the government signed a $1.2 billion contract with Merck to supply 1.7 million courses of the medication at the $712 price. The transaction is due to take place as soon as molnupiravir receives emergency use authorization from the Food and Drug Administration.
Reasonable Terms
Good government advocates are pointing out that because federal agencies spent at least $29 million on the drug’s development, the government has the obligation to ensure that the medicine is affordable. “The public funded this drug, and therefore the public has some rights, including the rights you have it available under reasonable terms,” said Luis Gil Abinader, senior researcher at Knowledge Ecology International.
In an interview on CNBC, Ridgeback co-founder Wendy Holman noted that the company asked for but “never got government funding” to help manufacture molnupiravir. A whistleblower complaint filed by Rick Bright, the former director of the Biomedical Advanced Research and Development Authority, or BARDA, in May 2020, described Ridgeback’s unsuccessful efforts “to secure approximately $100 million” from BARDA to develop the drug as a Covid-19 treatment. The company’s press release about the study results also noted that “since licensed by Ridgeback, all funds used for the development of molnupiravir have been provided by Merck and by Wayne and Wendy Holman of Ridgeback.”
Abinader was critical of Ridgeback’s failure to acknowledge the government’s initial investment in the drug before the company acquired it. “What they want to do, apparently, is to shape the narrative about who paid for the development of this drug in order to avoid demands from the public to make it available at reasonable prices,” he said.
In an emailed response to questions submitted to Ridgeback Biotherapeutics for this article, Davidson Goldin wrote, “Ridgeback has never received any government funding for molnupiravir and self-funded the development of this medicine for treating SARS-CoV-2 when the government did not provide financial support.” Merck did not respond to inquiries about this article.
No Strings Attached
Merck has promised to make molnupiravir accessible around the world and has already entered into licensing agreements with five Indian companies that manufacture generic drugs. “Merck has committed to providing timely access to molnupiravir globally, if it is authorized or approved, and plans to implement a tiered pricing approach based on World Bank country income criteria to reflect countries’ relative ability to finance their health response to the pandemic,” the company said in its announcement of the trial results on Friday. Indian companies are planning to price the drug at less than $12 for a five-day course, according to recent reports.
In the U.S., and likely in many upper-middle-income and all high-income countries, the price will be determined by the market. Noting that the treatment may be offered to people who are not yet severely sick with Covid-19, health advocates fear that will mean some in these countries will not be able to afford the new drug. “Offering someone a $700 treatment when they don’t yet feel that ill is going to mean that a lot of people are not going to take it,” said Dzintars Gotham, a physician at King’s College Hospital in London and a co-author of the report on the pricing of molnupiravir. According to the report, pricing molnupiravir at $19.99 would allow a company a 10 percent profit margin.
Melissa Barber, a doctoral candidate at the Harvard School of Public Health and co-author of the report on molnupiravir, said that, while its pricing is not as extreme as that of some other drugs, it will likely still place the antiviral out of reach of some who could benefit from it. “If you can’t afford medicine because it’s 1,000 times more than you can afford, or because it’s 100 times more than you can afford, it doesn’t matter,” said Barber. “Those are both bad.”
Barber and Gotham acknowledge that the $17.74 cost of producing a five-day course of the antiviral pills is an estimate but said that the algorithm they used, and have employed to estimate the production costs for hundreds of drugs, tends to result in overestimates in the long run.
Meanwhile, the prices that private companies charge for drugs tend to go up rather than down. “For all these deals that have happened for therapeutics or vaccines, the price has only increased as uncertainty has decreased,” she said. “One price is given and then, for the next sale, the price goes up. The price went up for other drugs and vaccines, so I would be very surprised if this price didn’t go up, too.”
The pricing differential should be grounds to demand a better price under the Bayh-Dole Act, according to Knowledge Ecology International’s Abinader. Bayh-Dole, passed in 1980, regulates the transfer of federally funded inventions into commercial property and allows the government to “march in” and suspend the use of patents that were developed with government funding if it determines that the products are excessively priced.
“The pressure for march-in rights around this drug is going to be huge,” predicted Abinader, who suggested that the government could use the law to lower the price of molnupiravir. “When the Biden administration negotiates another supply agreement with Merck, they should probably leverage those rights in order to get a better price,” he said.
According to Gotham, who is based in London, the short story of molnupiravir already sums up the best and the worst of the U.S. pharmaceutical system. “It’s a great coup that the American government funded some scientists to develop antivirals,” he said. “The great tragedy is that, after their great success, they just gave it away to private industry with apparently no strings attached.”
5 notes
·
View notes
Text
Coronavirus Infection Market - Forecast, 2022-2027
The Coronavirus Infection Market Size is estimated to reach $191.8 billion by 2027 and it is poised to grow at a CAGR of 7.6% over the forecast period of 2022-2027. Corona Virus infection is caused by SARS -CoV-2 Virus from the largest category of coronavirus. It was discovered in December 2019 in Wuhan, China hence it is popularly known as COVID-19. The Covid 19 pandemic challenged the healthcare industry in terms of policy, risk management, supply chain management and healthcare infrastructure. Coronavirus infection shows symptoms like fever, cough, tiredness and difficulty in breathing which happens in chronic bronchiolitis, owing to common symptoms and changing nature of the coronavirus make it difficult to detect at the first stage of the pandemic. To diagnose coronavirus infection nucleic acid amplification tests like polymerase chain reaction (PCR) and antigen test are developed. Severe infection of coronavirus shows severe acute respiratory syndrome (SARS), with respiratory tract infections that cause pneumonia. At the end of May 20, 2022, coronavirus infection cases and deaths are rising in the United States and marked 1 million deaths. Such increasing prevalence of coronavirus infection helps to drive the Coronavirus Infection Market size over the forecast period 2022-2027.
Coronavirus Infection Market Report Coverage
The report: “Coronavirus Infection Market Forecast (2022-2027)", by Industry ARC covers an in-depth analysis of the following segments in the Coronavirus Infection Market. By Diagnosis: Serological test, Chest computed tomography (CT), Magnetic Resonance Imaging (MRI), Antigen Test, Polymerase Chain Reaction Test (PCR) and Others. By Drugs: Remdesivir (Veklury), Ritonavir, Paxlovid, Molnupiravir and Baricitinib.By Therapy: Immune-based Therapy, Anti-inflammatory Therapy and Others. By Healthcare Equipment: PPE kit (Personal Protective Equipment), Diagnostic Tests, Surgical-Mask, Sterilizers, Ventilators and Others.By Specimens: Nasal, Nasopharyngeal, Blood and Others.By Geography: North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia and the Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia and New Zealand and Rest of Asia Pacific), South America (Brazil, Argentina, Chile, Colombia and Rest of South America) and Rest of the World (the Middle East and Africa).
Key Takeaways
Geographically, North America held a dominant market share in the year 2021, owing to the increasing prevalence of coronavirus infection and increase in mortality owing to covid cases.
The Coronavirus Infection Market size is predicted to increase owing to the increasing prevalence of covid disease and increasing research and developments to provide new drugs and technologies by key market players to consumers and healthcare. However, the high cost of research and development may limit market growth over the forecast period 2022-2027.
A detailed analysis of strengths, weaknesses, opportunities and threats will be provided in the Coronavirus Infection Market Report.
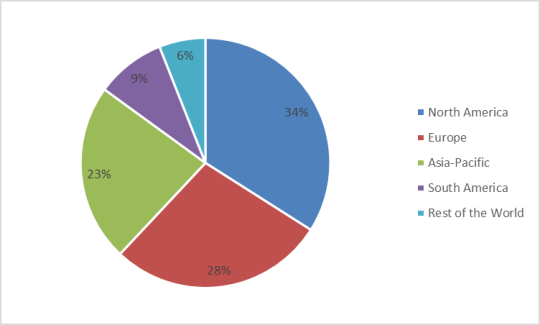
Coronavirus Infection Market: Market Share (%) by Region, 2021
For More Details on This Report - Request for Sample
Coronavirus Infection Market Segmentation Analysis- By Diagnosis
Coronavirus Infection Market based on diagnosis can be further segmented into Serological tests, Chest computed tomography (CT), Magnetic Resonance Imaging (MRI), Antigen tests, Polymerase Chain Reaction Test (PCR) and Others. The Polymerase Chain Reaction Test (PCR) segment held a dominant Coronavirus Infection market share in the year 2021, owing to the high efficiency and accurate result provided by the Rapid PCR test. According to the Indian Council of Medical Association (ICMR), the accuracy rate of PCR testing for Covid-19 is about 98.1%, of the positive nasal sample. Such high accuracy result of Covid-19 detection by PCR analysis develop rapid advancement in healthcare products manufactures company. However, the antigen test is estimated to grow with the fastest CAGR rate of 8.3% over the forecast period. Owing to the easy handling and quick results rapid antigen testing is popular among health care professionals. Rapid antigen testing is cost-cutting technology and helpful to perform in the mass population to quickly detect corona infection and enables to take further measures to limit and treat infections. Recently in May 2022, Nanomix obtained a CE mark for its covid-19 rapid point of care (POC) antigen panel. Also, key market players like Abbott, Access Bio, ACON laboratories and others are working on innovative product launches to diagnose corona infection; and several products have been approved by FDA. Such factors help to grow Coronavirus Infection Market size over the forecast period 2022-2027.
Coronavirus Infection Market Segmentation Analysis- By Drugs
The Coronavirus Infection Market based on drugs can be further segmented into Remdesivir (Veklury), Ritonavir, Paxlovid, Molnupiravir and Baricitinib. The Remdesivir (Veklury) segment held a dominant Coronavirus Infection market share in the year 2021. This is owing to the increasing use of Remdesivir to treat coronavirus infection in adults above age 12. Remdesivir act as a nucleoside analog that inhibits RNA-dependent RNA polymerase (RdRp) of coronavirus including SARS-CoV-2. It is approved by FDA and EUA to treat coronavirus infection, also Remdesivir with a combination of Baricitinib has been granted by FDA and EUA for clinical use. As a result of increasing hospitalization owing to a hike in coronavirus cases demand for Remdesivir is hike respectively. Such increasing use of Remdesivir helps to drive Coronavirus Infections Market. However, Baricitinib is estimated to grow with the fastest CAGR rate of 8.1% over the forecast period 2022-2027. The medicine Baricitinib is used to treat coronavirus infection in hospitalized patients aged 2 to less than 18 years of age with the FDA's approval and an Emergency Use Authorization (EUA). This drug is more helpful in changing the nature of coronavirus infection cases in children and teenagers by limiting pneumonia, bronchiolitis, respiratory tract infection, and Severe Acute Respiratory Syndrome. It is found that the use of Baricitinib and Remdesivir is cost-effective compared to using Remdesivir alone, as per a research article published in Springer. Such factors demand the use of Baricitinib for the treatment of coronavirus infection which helps to grow the coronavirus infection industry over the forecast period 2022-2027.
Coronavirus Infection Market Segmentation Analysis- By Geography
The Coronavirus Infection Market based on Geography can be further segmented into North America, Europe, Asia-Pacific, South America, and the Rest of the World. North America held a dominant Coronavirus Infection market share of 34% in the year 2021. This is owing to increasing cases of coronavirus infection and an increase in mortality by this disease. There are 82,459,419 cases reported of coronavirus infection in the U.S.A with 130,452 cases reported on a single day of May 25 2022, and a total number of deaths are 994,931 occurred to coronavirus infection as data published by World Health Organisation (WHO). Also, the cases of coronavirus infection are hiked by 13% in May 2022, according to Reutres Graphics report. Such increasing cases of coronavirus infections and high rate of mortality help to drive North America Coronavirus Infection Industry. Furthermore, the Asia-Pacific is estimated to grow with the fastest CAGR over the forecast period 2022-2027. This is the result of the recent outbreak of coronavirus in countries like China, on 7th March 2022, China reported 268 new cases of coronavirus infection within 24 hours. In India on 23 May 2022, 94 deaths by coronavirus infections were reported. According to Johns Hopkins University case fatality ratio in India is observed at 1.2%. According to the Health Minister of Maharashtra and Karnataka 4th wave of Covid-19 will hit between June 2022 to July 2022 and last till September 2022 as the increasing rate of coronavirus infection noted in these two states. Up to 26th May 2022, 192,820,355 doses of covid-19 vaccines were completed in India. Owing to such an increase in the number of coronavirus infections, mortality rate and vaccination help to grow the Asia-Pacific Corona Virus Infection Market over the forecast period 2022-2027.
Coronavirus Infection Market Drivers
Changing Nature of Corona Virus and Increasing Number of Deaths is Driving the Market Growth.
Since the emergence of Covid-19 the nature of the virus has been changing its nature as its genome is encoded in RNA like HIV and Influenza, a mutation that occurred in SARS-CoV-2 is the result of errors that occurred during the copy of RNA which leads to the production of different kind of enzymes. For instance, as per Lucy Van Dorp, a computational geneticist at University College London, a Sample of two SARS CoV-2 viruses collected from anywhere in the world shows a difference of 10 RNA letters out of 29903. As of May 2022, CDC listed only one variant of coronavirus as a variant of concern, that is the ‘Omicron’ variant. As per a research article published in WebMD, there are 99.9% cases of coronavirus reported in the United States and daily deaths crossed 2200 deaths in January 2022. Such an increasing number of deaths with new variants of coronavirus helps to drive the Coronavirus Infection industry over the forecast period.
Innovative Products Launched by Key Market Players and funding Provided by Governments and Organizations are Aiding the Market Growth.
Coronavirus infection spread over community-level with record break cases and deaths within a short period of time. There were 37 million Covid-19 cases and 1 million deaths were reported globally between October to December 2020. Nearly half of these cases (48%) and deaths (55%) continue to be reported in the Region of the Americas with the United States of America, Brazil and Argentina accounting for the greatest numbers of new cases and deaths in the region. Numerous academic institutions, governmental research facilities, and pharmaceutical firms have made significant financial investments in R&D to lessen the mortality rate and the effects of diseases including pneumonia, bronchiolitis, respiratory tract infections, and severe Acute Respiratory Syndrome. The International Monetary Fund $1 trillion for an unprecedented number of emergency financing requests for the Covid 19 relief fund. On 18 May 2022, WHO contribute a total fund of 3.35 billion for the vaccine, therapeutics, diagnostics, health systems and response. In February 2022, Cipla Health launched Naselin Anti-Viral Nasal Spray with Povidone-Iodine to protect against coronavirus and respiratory tract infection. Also in the same month, Glenmark Pharma and SaNotize Research launch Nasal Spray for Covid-19 treatment in India. Such a new launch of products and an increase in healthcare funding for diagnosis, treatment and prevention of covid-19 help to drive the market.
Coronavirus Infection Market Challenges
High Treatment Costs and Shortages of Essential Medical Supplies Limit Market Growth.
When a patient needs life support like a ventilator, the expense of hospitalization and medication is generally high. As coronavirus is a highly contagious illness that spreads through contact with infected people and the air, it has expanded quickly throughout the world and put a strain on the healthcare sector. The situation is made worse in nations like India where there are just 0.5 beds per 1,000 inhabitants, which is extremely low compared to other developing nations. The price inflation is also brought on by the increased demand for oxygen tanks and medications like Remdesivir as well as those products' limited supply. According to a January 2022 article in Down to Earth Organization, the average cost of Covid-19 treatment was INR112,179 in government hospitals and INR 297,577 in private hospitals. Prior to the pandemic, the cost for all symptoms combined, such as fever, respiratory infection, chest pain, and breathlessness, was only INR 4,622 in government hospitals and INR 28,932 in private hospitals. Therefore, the market is facing difficulties as a result of the inflation in drug prices. Over the projection period of 2022–2027, the Coronavirus Infection & treatment industry may be hampered by such high mortality and treatment costs.
Coronavirus Infection Industry Outlook
Product launches, mergers and acquisitions, joint ventures and geographical expansions are key strategies adopted by players in the Coronavirus Infection Market. The top 10- Coronavirus Infection Market companies are- 1. Altimune Inc.2. Moderna Inc.3. Gilead Sciences4. Novavax Inc.5. Inovio Pharmaceuticlas6. AbbVie7. Regeneron Pharmaceuticals8. GlaxoSmihKline Plc.9. Co-Diagnostics10. Steris Healthcare
Recent Developments
In March 2022, Moderna entered into a strategic partnership with the Australian Government to establish a state-of-art, domestic mRNA vaccine manufacturing facility in Australia. The new center provides access to a domestically manufactured portfolio of mRNA vaccines against respiratory viruses, including Covid-19, seasonal influenza, respiratory syncytial virus (RSV) and other potential respiratory viruses.
In March 2021, Altimmune Inc. collaborated with Lonza to expand the production of AdCOVID -a single-dose intranasal vaccine for COVID-19. AdCOVID activates systemic immunity (neutralizing antibodies and T cell responses) and mucosal immunity in the respiratory tract which has been proved in preclinical studies.
In March 2021, Gilead Sciences and Merck entered into an agreement to co-develop and co-commercialize long-acting treatment in HIC than combine Gilead’s investigational capsid inhibitor, lenacapavir and Merck’s investigational nucleoside reverse transcriptase translocation inhibitor, islatravir, into a two-drug regimen with the potential to provide new, meaningful treatment options for people living with HIV.
#Coronavirus Infection Market share#Coronavirus Infection Market size#Coronavirus Infection Market forecast
0 notes
Text
What is covid -19 ? what is Best medicine for covid ?
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. what is best Medicine for covid and how to detect it ? we will find all information about this here .
we all suffering from this disease since 2 year now still somewhere we found peoples are infected by this virus also no best medicine for covid has been found yet .
Most people infected with the virus will experience mild to moderate respiratory illness and recover without requiring special treatment. However, some will become seriously ill and require medical attention. Older people and those with underlying medical conditions like cardiovascular disease, diabetes, chronic respiratory disease, or cancer are more likely to develop serious illness. Anyone can get sick with COVID-19 and become seriously ill or die at any age.
What is symptoms of covid 19 ?
Most common symptoms:
fever
cough
tiredness
loss of taste or smell.
Less common symptoms:
sore throat
headache
aches and pains
diarrhoea
a rash on skin, or discolouration of fingers or toes
red or irritated eyes.
Serious symptoms:
difficulty breathing or shortness of breath
loss of speech or mobility, or confusion
chest pain.
Best medicine for covid
Before we jump to to get what is best medicine for covid you should always consult your doctor if you infected by covid don’t use this medicine without consulting your physician .
Now there are lots of medicine which helps to get overcome from covid . we are displaying best medicine for covid here which are selling most nowadays .
1 )
Moludac 200 mg / Molunat 200 ( Molnupiravir 200 mg )
Each capsule of Molunat 200 mg contains of Molnupiravir and comes 40 capsules per bottle. It is used to treat COVID-19 infected by SAR-CoV-2.
What is this drug used for?
It is used to treat COVID-19 in those infected by SARS-CoV-2.
Adverse Effects
1-10%
Diarrhea (2%)
Nausea (1%)
Dizziness (1%)
≤2%
Selected Grade 3 and 4 laboratory abnormalities in chemistry (ALT, AST, creatinine, and lipase) and hematology (hemoglobin, platelets, and leukocytes)
Cautions
There are limited clinical data available; serious and unexpected adverse events may occur that have yet to be reported.
May cause fetal harm when administered to pregnant females.
Read more
2 )
Covimectin ( Ivemectin 12 mg ) by singature pharma
covimectin 12 tablets You have got many options when it comes to buying covimectin 12 from online portals. Covimectin used to treat parasitic infections of your intestinal tract, skin, and eyes.
What is covimectin 12 tablets ?
Covimectin 12 tablet is mainly used to treat parasitic infections of your intestinal tract, skin, and eyes.
Generally, covimectin is taken on an empty stomach. You usually need to take it only once to get rid of your infection. However, if you do not feel better after taking it, talk to your doctor.
It’s only advisable to take the pills after consulting a doctor and upon examining certain parasitic infections on some specific body parts .
Covimectin 12 manufacturer
Product : Covimectin 12 mg
Manufacturer : Signature pharama
Covimectin 12 price : covimectin price starting from $15 for 8 tablets
Covimectin 12 signature is very trusted brand .
Ivermectin 12 mg tablet uses
Ivermectin mainly used fot parasitic infections of your intestinal tract, skin, and eyes. recently it founds effective in covid-19 treatments .
Read more
3 )
Iverjohn 12 mg ( ivermectin ) by jhonlee pharma
iverjohn 12 mg Online You have got many options when it comes to buying iverjhon 12 mg mg from online portals. With so many options given to you, the benefit is yours as you can choose the portal of your choice after checking in the expected delivery dates, offers, and discounts.
What Is Iverjohn 12 mg ?
Iverjohn 12 mg is a medication with generic Ivermectin in it that helps you cure various parasitic infections in the intestinal tract, skin, and eyes.
It’s only advisable to take the pills after consulting a doctor and upon examining certain parasitic infections on some specific body parts.
4 )
Ziverdo Kit (Zinc, Ivermectin, And Doxycycline)
ziverdo Kit Consists of Ivermectin 12 mg, Doxycycline 100 mg, and Zinc Acetate 50 mg. It is an effective medicine against primary contacts of Covid-19. Ziverdo Kit has been developed by Mankind compnay with dual purposes; majorly, it stops the coronavirus’s multiplication if entered into the human body.
These are four Best Medicne for covid nowdays you can buy it online any time it will require prescirption if you are buying it first time
Take Following prevention to stop covid
To prevent infection and to slow transmission of COVID-19, do the following:
Get vaccinated when a vaccine is available to you.
Stay at least 1 metre apart from others, even if they don’t appear to be sick.
Wear a properly fitted mask when physical distancing is not possible or when in poorly ventilated settings.
Choose open, well-ventilated spaces over closed ones. Open a window if indoors.
Wash your hands regularly with soap and water or clean them with alcohol-based hand rub.
Cover your mouth and nose when coughing or sneezing.
If you feel unwell, stay home and self-isolate until you recover.
0 notes
Text
Molnupiravir Manufacturers in India – 200mg/400mg Capsules
We are one of the leading molnupiravir manufacturers in India. Our effective anti-covid medicine is available in 200mg & 400mg pack at affordable prices. It is available for covid treatment at affordable prices under the brand name of Molnova. Molnupiravir capsule is well researched, safe and affordable medicine, it is one of the first antiviral medicine for effective treatment of corona.

#Molnupiravir Manufacturers in India – 200mg/400mg Capsules#Molnupiravir capsules manufacturer in india
1 note
·
View note
Text
Natco Pharma signs agreement with MPP to sell Molnupiravir capsules

Natco Pharma Ltd on Thursday said it has signed a non-exclusive license agreement with the Medicines Patent Pool (MPP), Switzerland to manufacture and sell Molnupiravir capsules 200 mg for treatment of COVID-19.
MPP had taken licence from Merck Sharp & Dohme Corp (MSD), USA for the same, the company said in a statement.
Natco said with this licence agreement it can manufacture and sell Molnupiravir capsules 200 mg for Indian market, which will be sold under brand name MOLNUNAT for treatment of COVID-19 infection in patients who have high risk of progression of the disease including hospitalisation or death.
0 notes
Text
India set to become hub for antiviral generics: Fitch
India set to become hub for antiviral generics: Fitch
India will become the largest global hub for COVID-19 antiviral generic drug production after the drug controller granted emergency-use authorisation to several pharmaceutical companies in the country to manufacture and market generic versions of molnupiravir, Fitch Solutions said on Friday. Increased access to molnupiravir will keep hospitalisations and deaths in India to a manageable level as…

View On WordPress
0 notes