#Microbial Limit
Explore tagged Tumblr posts
Text
Microbial Limit Testing of Effervescent Tube Caps
Microbial Limit Testing of Effervescent Tube Caps
Effervescent tube is a kind of packaging form with special structure design. It consists of two parts: a cover and a tube body. It is mostly used for tablets, capsules and other drugs that need moisture-proof preservation. In the quality standard of packaging, the microbial limit is an important test item. 144mm*29mm effervescent tube packaging caps for effervescent tube The quality standard…

View On WordPress
#144mm effervescent tube#effervecant packaging#effervescent cap#effervescent tabket tubes#Microbial Limit#Microbial Limit Testing
0 notes
Text
How do people write short things. Like 1000k word total stories. Less even. How do you do tjat. I have shit to say
#I've been able to do it in classes with word limits and etc but being concise is not a strong point of mine#'Short' for me is passing at least 8k words#'Microbial' is around 2k
4 notes
·
View notes
Text

Unraveling the Depths of Bioaugmentation and Biostimulation: A Comprehensive Comparison
Introduction:
If you've ever delved into the field of environmental biotechnology, you've likely stumbled across the terms "bioaugmentation" and "biostimulation". These sophisticated approaches to environmental remediation are both aimed at enhancing natural processes to treat contamination. But what exactly distinguishes one from the other? In this article, we will delve into the specifics of Bioaugmentation vs Biostimulation, breaking down their definitions, applications, and key differences.
Understanding Bioaugmentation:
Bioaugmentation, in its simplest form, is the introduction of a group of natural microbial strains or a genetically engineered variant into an environment to enhance the rate of pollutant degradation. These microbial strains, often referred to as 'augments', are known for their specialized ability to degrade contaminants that the existing microbial community cannot effectively handle. This technique is frequently employed to address the contamination of soil and water bodies with organic pollutants such as oil spills and certain types of industrial waste.
Diving into Biostimulation:
On the other hand, biostimulation involves the stimulation of indigenous microbial communities already present in the environment by providing nutrients, electron acceptors, or substrates that enhance their activity. Unlike bioaugmentation that adds new organisms to an ecosystem, biostimulation works with the existing microbial population, encouraging their growth and pollutant degradation capabilities. Often used in environmental cleanup efforts, biostimulation can enhance the breakdown of a broad range of pollutants, including petroleum hydrocarbons and heavy metals.
Bioaugmentation Vs Biostimulation: A Comparative Study:
Now that we understand the fundamentals of both processes, it's time to compare them head-to-head.
Techniques Involved: While bioaugmentation is about introducing specific microbial strains to boost pollutant degradation, biostimulation works by providing necessary nutrients or substrates to stimulate the indigenous microbial population.
Scope of Application: Both techniques are used in environmental remediation, especially for soil and groundwater. Bioaugmentation has a slight edge in cases where specific contaminants require particular microbial strains for degradation. Biostimulation, however, is often favored for broader applications, given that it enhances the overall microbial activity and not just that of specific strains.
Economic Aspects: Bioaugmentation requires the cultivation and addition of specific microbial strains, which can be costly and technically demanding. On the contrary, biostimulation usually involves adding relatively inexpensive nutrients or substrates, making it a more economically feasible option in many cases.
Environmental Impact: Bioaugmentation involves adding new organisms, which raises concerns about the impact on the existing ecosystem and the potential for creating imbalances. Biostimulation, working with existing microbial communities, is generally viewed as having a less disruptive impact on ecosystem balance.
Effectiveness: Both techniques have proven effective in various scenarios, but their success heavily depends on site-specific conditions. For instance, bioaugmentation's effectiveness might be hindered by the inability of the added microbes to survive in the new environment. Biostimulation's success, on the other hand, could be limited by the potential growth of undesired microbial communities.
Conclusion:
Bioaugmentation and biostimulation, though conceptually distinct, share a common goal: to utilize biological processes for environmental remediation. Choosing between them demands a clear understanding of the contamination at hand, the existing microbial community, and the economic and environmental implications of each approach. As scientists continue to explore these fascinating techniques, our ability to heal the environment using nature's own tools will only continue to improve.
#Bioaugmentation#Biostimulation#Bioremediation technologies#Environmental Remediation#Pollution control methods#Bioaugmentation vs Biostimulation#Benefits of bioaugmentation#Benefits of biostimulation#Bioaugmentation in habitat restoration#Biostimulation for pollution control#Limitations of bioaugmentation#Limitations of biostimulation#Bioaugmentation-assisted biostimulation#Microbial bioremediation#Sustainable environmental practices#Choosing between bioaugmentation and biostimulation#Understanding bioaugmentation#Understanding biostimulation#Industrial waste management#Oil spill cleanup techniques
5 notes
·
View notes
Text
"BOPP Tape Manufacturer: High-Quality Tapes for Secure Packaging"
https://jesons.net/about-us/
"Premium BOPP tape manufacturer offering high-strength tapes for secure and efficient packaging. Ideal for sealing cartons and protecting shipments."
#jesons industries limited#tapes manufacturer#anti-microbial#packaging#coating emulsions#construction chemicals
0 notes
Text
Chef WK, lead charcuterie specialist in Alberta Canada
Table of contents
1. Control Program Requirements for Fermented Meat Products
2. Facility and Equipment Requirements
3. Starter Culture
4. Chemical Acidification
5. Water Activity Critical Limits
6. Time and Temperature for Fermented Products
7. Fermentation Done at a Constant Temperature
8. Examples of Degree-hours at constant room temperatures
9. Fermentation Done at Different Temperatures
10. Fermentation done at Different temperatures
11. What happens if fermentation fails to hit critical limit?
12. E. coli and Salmonella Control in Fermented Sausages
13. Options for E. coli validation
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
Control Program Requirements for Fermented Meat Products
The producer must have a program in place to assess the incoming product. This program should outline specifications for the incoming ingredients. This may include criteria including receiving temperature, farm/ supplier, lot code or packed on date, species/cut etc.
2. Facility and Equipment Requirements
Equipment used in the fermentation process must be included in the operator's prerequisite control programs. These must include the following elements:
Temperature in the fermentation, drying and smoking chambers must be uniform and controlled to prevent any fluctuation that could impact on the safety of the final product.
Fermentation, drying and smoking chambers must be equipped with a shatter resistant indicating thermometer, (or equivalent), with graduations of 1°C or less. If mercury thermometers are used, their mercury columns must be free from separations. All thermometers must be located such that they can be easily read.
Fermentation and smoking chambers must be equipped with a recording thermometer for determining degree-hours calculations in a reliable manner. Recording thermometers are also preferable in drying and aging rooms but, in these rooms, it may be sufficient to read and record the temperatures 2 times a day.
Drying and aging rooms must be equipped with humidity recorders in order to prevent uncontrolled fluctuations of the relative humidity. The only alternative to an automatic humidity recorder in these rooms would be for the company to manually monitor and record ambient humidity twice a day (morning and afternoon) every day with a properly calibrated portable humidity recorder.
For routine monitoring, accurate measurement electronic pH meters (± 0.05 units) should be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument as well as recommended sample preparation and testing be followed.
When the aw of a product is a critical limit set out in the HACCP plan for a meat product, accurate measurement devices must be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument be followed.
3. Starter Culture
The operator must use a CFIA approved starter culture. This includes Freeze-dried commercially available culture as well as back-slopping (use of previously successful fermented meat used to inoculate a new batch). When performing back-slopping, the operator must have a control program in place to prevent the transmission of pathogens from when using the inoculum from a previous batch to initiate the fermentation process of a new batch. These must include:
The storage temperature must be maintained at 4°C or less and a pH of 5.3 or less.
Samples for microbiological analysis must be taken to ensure that the process is in line with the specifications.
The frequency of sampling is to be adjusted according to compliance to specifications.
Any batch of inoculum which has a pH greater than 5.3 must be analysed to detect at least Staphylococcus aureus. Only upon satisfactory results will this inoculum be permitted for use in back slopping.
This can be an expensive and a time exhaustive process and is generally avoided due to food safety concerns. AHS does not allow back-slopping.
[Chef WK was in communication with the U of A to get his method, a starter mix, studied.]
4. Chemical Acidification
If product is chemically acidified by addition of citric acid, glucono-delta-lactone or another chemical agent approved for this purpose, controls must be in place and records kept to ensure that a pH of 5.3 or lower is achieved by the end of the fermentation process. These acids are encapsulated in different coatings that melt at specific temperatures, which then release the powdered acids into the meat batter and directly chemically acidulate the protein.
Summer sausage is a very common chemically acidified product. The flavor profile tends to be monotone and lacking depth.
5. Water Activity Critical Limits
The aw may be reduced by adding solutes (salt, sugar) or removing moisture.
Approximate minimum levels of aw (if considered alone) for the growth of:
molds: 0.61 to 0.96
yeasts: 0.62 to 0.90
bacteria: 0.86 to 0.97
Clostridium botulinum: 0.95 to 0.97
Clostridium perfringens: 0.95
Enterobacteriaceae: 0.94 to 0.97
Pseudomonas fluorescens: 0.97
Salmonella: 0.92 - 0.95
Staphylococcus aureus: 0.86
parasites: Trichinella spiralis will survive at an aw of 0.93 but is destroyed at an aw of 0.85 or less.
The above levels are based on the absence of other inhibitory effects such as nitrite, competitive growth, sub-optimum temperatures, etc., which may be present in meat products. In normal conditions, Staphylococcus aureus enterotoxins are not produced below aw 0.86, although in vacuum packed products this is unlikely below aw 0.89.
6. Time and Temperature for Fermented Products
Certain strains of the bacteria Staphylococcus aureus are capable of producing a highly heat stable toxin that causes illness in humans. Above a critical temperature of 15.6°C, Staphylococcus aureus multiplication and toxin production can take place. Once a pH of 5.3 is reached, Staphylococcus aureus multiplication and toxin production are stopped.
Degree-hours are the product of time as measured in hours at a particular temperature multiplied by the "degrees" measured in excess of 15.6°C (the critical temperature for growth of Staphylococcus aureus). Degree-hours are calculated for each temperature used in the process. The limitation of the number of degree-hours depends upon the highest temperature in the fermentation process prior to the time that a pH of 5.3 or less is attained.
The operator is encouraged to measure temperatures at the surface of the product. Where this is not possible, the operator should utilize fermentation room temperatures. The degree hour calculations are based on fermentation room temperatures. Temperature and humidity should be uniform throughout the fermentation room.
A process can be judged as acceptable provided the product consistently reaches a pH of 5.3 using:
fewer than 665 degree-hours when the highest fermentation temperature is less than 33°C;
fewer than 555 degree-hours when the highest fermentation temperature is between 33° and 37°C; and
fewer than 500 degree-hours when the highest fermentation temperature is greater than 37°C.
This means that as the temperature increases, the amount of time that you have available to reach 5.3 or under is shorter. The warmer the temperature, the sharper the log growth phase of bacteria, which equates to more overshoot in lactic acid production, faster.
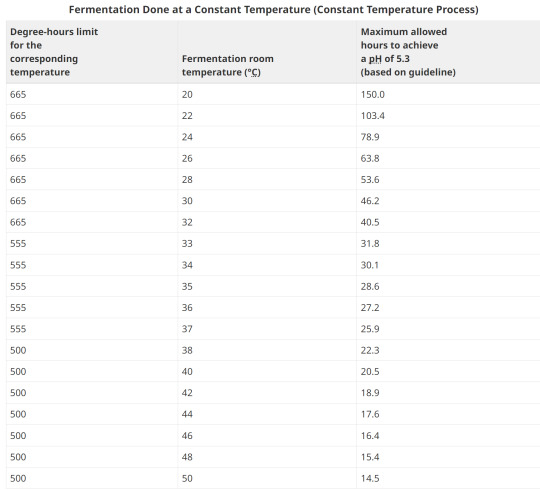
8. Examples of Degree-hours at constant room temperatures
Example 1:
Fermentation room temperature is a constant 26°C. It takes 55 hours for the pH to reach 5.3.
Degrees above 15.6°C: 26°C - 15.6°C = 10.4°C Hours to reach pH of 5.3: 55 Degree-hours calculation: (10.4°C) x (55) = 572 degree-hours
The corresponding degree-hours limit (less than 33°C) is 665 degree-hours.
Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
Example 2:
Fermentation room temperature is a constant 35°C. It takes 40 hours for the pH to reach 5.3.
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C Hours to reach pH of 5.3: 40 Degree-hours calculation: (19.4°C) x (40) = 776 degree-hours
The corresponding degree-hours limit (between 33 and 37°C) is 555 degree-hours.
Conclusion: Example 2 does not meet the guideline because its degree-hours exceed the limit
9. Fermentation Done at Different Temperatures
When the fermentation takes place at various temperatures, each temperature step in the process is analyzed for the number of degree-hours it contributes. The degree-hours limit for the entire fermentation process is based on the highest temperature reached during fermentation.
Example 1:
It takes 35 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for second 10 hours and 35°C for the final 15 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C Hours to reach pH of 5.3: 15 Degree-hours calculation: (19.4°C) x (15) = 291 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 291 = 519
The highest temperature reached = 35°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
10. Fermentation done at Different temperatures
Example 2:
It takes 38 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for the second 10 hours and 37°C for the final 18 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 37°C - 15.6°C = 21.4°C Hours to reach pH of 5.3: 18 Degree-hours calculation: (21.4°C) x (18) = 385.2 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 385.2 = 613.2
The highest temperature reached = 37°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)
Conclusion: Example 2 does not meet the guidelines because its degree-hours exceed the limit.
11. What happens if fermentation fails to hit critical limit?
What happens if the batch takes longer than degree-hours allows? For restaurant level production, it's always safer to discard the product. The toxin that Staph. Aureus produces is heat stable and cannot be cooked to deactivate. In large facilities that produce substantial batches, the operator must notify the CFIA of each case where degree-hours limits have been exceeded. Such lots must be held and samples of product submitted for microbiological laboratory examination after the drying period has been completed. Analyses should be done for Staphylococcus aureus and its enterotoxin, and for principal pathogens, such as E. coli O157:H7, Salmonella, and Clostridium botulinum and Listeria monocytogenes.
If the bacteriological evaluation proves that there are fewer than 104 Staphylococcus aureus per gram and that no enterotoxin or other pathogens are detected, then the product may be sold provided that it is labelled as requiring refrigeration.
In the case of a Staphylococcus aureus level higher than 104 per gram with no enterotoxin present the product may be used in the production of a cooked product but only if the heating process achieves full lethality applicable to the meat product.
In the case where Staphylococcus aureus enterotoxin is detected in the product the product must be destroyed.
12. E. coli and Salmonella Control in Fermented Sausages
Business' that manufacture fermented sausages are required to control for verotoxinogenic E. coli including E. coli O157:H7 and Salmonella when they make this type of product. This includes:
establishments which use beef as an ingredient in a dry or semi-dry fermented meat sausage;
establishments which store or handle uncooked beef on site;
Establishments which do not use beef and do not obtain meat ingredients from establishments which handle beef are not currently required to use one of the five options for the control of E. coli O157:H7 in dry/semi-dry fermented sausages.
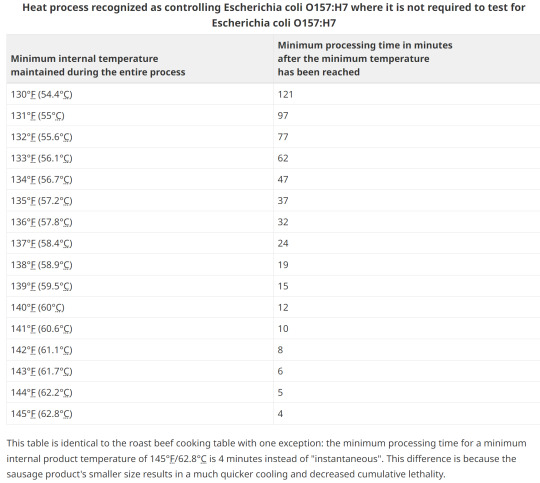
Any processed RTE product containing beef or processed in a facility that also processed beef, must be subjected to a heat treatment step to control E. coli O157:H7. Heating to an internal temperature of 71°C for 15 seconds or other treatment to achieve a 5D reduction is necessary. This is a CFIA requirement and is not negotiable.
Uncooked air dried products produced as RTE, must meet shelf stable requirements as detailed for Fermented-Dry products.
13. Options for E. coli validation
Without lab testing, the two main methods of validation are with heat treating by either low temp and a long duration, or various hotter processing temperatures for a shorter timeframe.
A challenge study to validate a process can take 1 year and over $100,000!
14. Option1; Heating
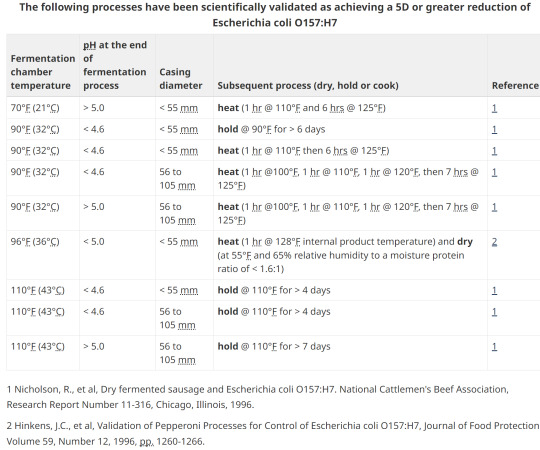
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
The aw and pH values are critical in the control of pathogens as well as to ensure shelf-stability in all semi-dry and dry fermented meat products. Each batch must be tested for aw and/or pH in order to verify that the critical limits are met.
Although aw measurement is mandatory only for shelf stable products, it is strongly recommended that the producer determine the aw values achieved for each product type they manufacture and for each product. Once this has been established, frequent regular checks should be made to ensure consistency. In the U.S., they rely on moisture to protein ratio and have set targets. This lab-tested value is a direct correlation of the % water to % meat protein and not aw. This gives more consistency to common names. For example, to legally call a product "jerky" it must have a MPR of 0.75:1 or lower. Remember your ABCs:
Always be compliant.
-AND-
Documentation or it didn't happen.
(tags)
Charcuterie,Fermented Meat,Food Safety,Starter Culture,Chemical Acidification,Water Activity,Fermentation Process,Degree-Hours Method,Foodborne Pathogens,Meat Processing Guidelines,Chef WK Alberta Canada,Food Industry Standards,pH Critical Limits,Thermal Processing,Food Preservation,Food Microbiology,Sausage Fermentation,Charcuterie Expertise,Fermented Meats ,Food Safety Standards,Food Processing Guidelines,Starter Cultures,Chemical Acidification,Water Activity (a_w),Critical Limits,Degree-Hours Method,Foodborne Pathogens,Meat Processing Equipment,Processing Facility Requirements,Hazard Analysis and Critical Control Points (HACCP),Food Preservation Techniques,Temperature Control,Pathogen Reduction,Food Industry Compliance,Documentation Practices,Heat Treatment,pH Control,Food Stability,Consistency in Production,Microbial Testing,Real-time Monitoring,Process Validation,Regulatory Requirements,Verotoxigenic E. coli,Lethality Standards,Product Labelling,Spoilage Prevention,Enterotoxin Detection,Shelf-Stable Products,Moisture to Protein Ratio (MPR)
#Charcuterie#Fermented Meat#Food Safety#Starter Culture#Chemical Acidification#Water Activity#Fermentation Process#Degree-Hours#Meat Processing Guidelines#Thermal Processing#Food Preservation#Food Microbiology#Sausage Fermentation#Starter Cultures#Critical Limits#Meat Processing#Food Preservation Techniques#Temperature Control#Pathogen Reduction#Food Industry#Heat Treatment#pH Control#Food Stability#Microbial Testing#Real-time Monitoring#Process Validation#Spoilage Prevention#Enterotoxin Detection#Shelf-Stable Products#Moisture to Protein Ratio (MPR)
1 note
·
View note
Text
Scientists are very serious.
This is a post about science. And soup.
Dr. Elinne Becket, a microbiologist from Cal State University, is in the middle of one of those Fridge Experiments that happens to us all - except in this case, she is uniquely placed to unravel the science down to the microbial level.
While cleaning out her fridge, Dr. Becket found that a tub of family-recipe beef vegetable soup had turned bright blue. “Ok I'm outing myself here,” she tweeted, “but there was forgotten beef soup in our fridge we just cleaned it out and it was BLUE?!?!? Wtf contam would make it blue??? Like BRIGHT blue!! Even w/ all my years in micro I'm not handling this well.“
Read on for a breathless and ongoing saga of Soup and Science, and the wonderful international community that is Academic Twitter.
Academic Twitter quickly reminded her of her Responsibilities to Scientific Inquiry. (Cue the chanting from around the world of “CLONE THE SOUP! CLONE THE SOUP!”)
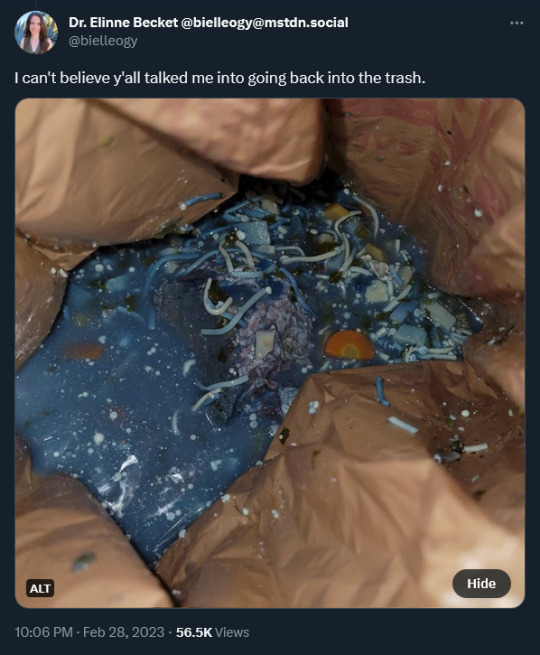
“I can’t believe y’all talked me into going back into the trash.” she tweeted in response, over a photo of a puddle of beautiful Mediterranean-sea blue soup in the trash bin, with bits of veg and noodles arising from the depths.
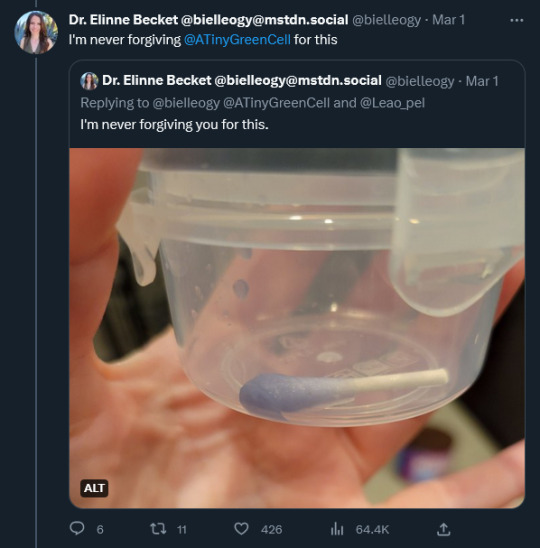
Scientists being scientists, Dr. Becket agreed to take a sample and send it to colleagues for cloning and microbial analysis.This involved getting arms-deep into the trash bin of Old Soup. “I’m never forviging @ATinyGreenCell (genomic biologist Sebastian Cocioba) for this.” Dr. Becket tweeted, with a photo of a properly dipped and snipped and VERY blue q-tip in a small clear plastic tub.
Diving into decomposing soup was not the only hazard. She writes: “My mom (who made the soup for my birthday) came across this thread and now 1) I have to answer for letting her soup spoil and 2) she's worried @ATinyGreenCell will figure out her secret recipe.“
Dr. Becket and Sebastian were able to culture the Blue Goo!
Becket posted a photo of three petri plates of streaked beef bouillon agar at 72 hours incubation, at 37C, room temp and 4C. She writes: “Left the plates where they were for another 2 days, except the 37°C one was brought to RT, which then grew white stuff over the yellow stuff and stinks to high heaven. RT looked the same. 4°C had impressive growth. Restreaked them all onto TECH agar, awaiting results!”

Sebastian, from his lab, tweeted a photo of three more covered petri dishes, with early results: “Great progress on isolating the glowy microbe from our #BlueSoup! It's so fluorescent the streak is GREEN. Still needs another restreak as it seems there is a straggler but should clear up in the next plate. Exciting!”
Then yesterday, Sebastian tweeted out an updated photo of his plates under daylight and blacklight. “Whatever grew on the #BlueSoup colony plates overnight glows under UV, but only on King's Agar B! That particular media is used to tease out fluorescein expression in pseudomonads. What are the chances that the same cell line expresses fluorescent AND blue pigments?“

“Looking closer, there definitely is a handful of different microbes showing distinct phenotypes. Could be that the blue producer and the fluorescent microbes are totally different microbes!”
At which point, Professor Cynthia Whitchurch of Norwich, England, responded: “Consistent with P. fluorescens being at least part of the #BlueSoup community. The fluorescence is due to production of the siderophore pyoverdine which is up-regulated when iron availability is limited. P. aeruginosa produced this too but my guess is you have blue Pf.”
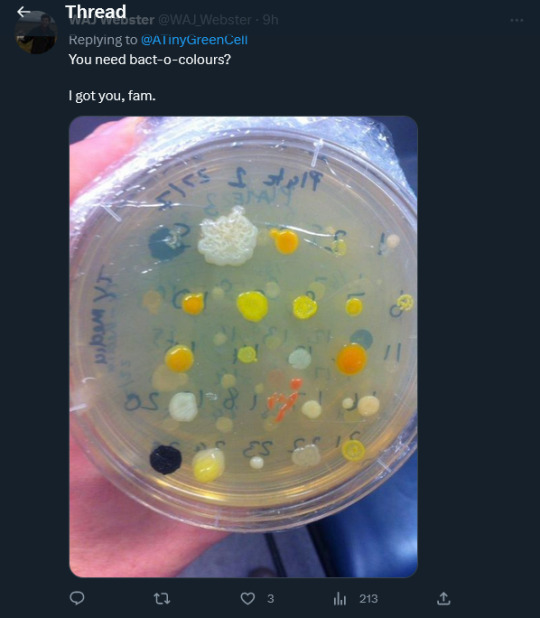
And Australian agricultural researcher @WAJWebster helpfully tweeted a petri dish of ALL KINDS of colourful bacterial colonies from white to yellow to orange to stark black, with a cheerful: “You need bact-o--colours? I got you, fam.”
The best part is that as of today, March 9, 2023, THE BLUE SOUP MYSTERY CONTINUES. WE ARE WATCHING SCIENCE HAPPENING!
A paper is being written. And Dr. Becket’s mum is getting an author credit as the proprietary owner of the #BlueSoup recipe.

Dr. Becket’s Twitter is here: https://twitter.com/bielleogy
Sebastian Cocioba’s Twitter is here: https://twitter.com/ATinyGreenCell
Fun IFLS story is here: https://www.iflscience.com/microbiologist-investigates-after-her-beef-soup-turned-blue-in-the-freezer-67894?fbclid=IwAR0H27KqVZhzzrosnjzzKkxuKASZ-0L0Lt6hGwCRDJK8xvFbbSlyS4JvwlM
15K notes
·
View notes
Text
Clean Room Validation in Pharmaceutical Industry how to do
Clean Room Validation how it’s done different requirements of clean Room Validation from particle Count to Microbial Count Limits methods to do it? Validation is an important activity in Pharmaceutical Industry, it impart trust on the process and inturn the product manufactured. For doing validation of clean room, unless all the installed equipment are validated, the Clean Room validation…
View On WordPress
#Clean Room Validation#Clean Room Validation how it&039;s done different requirements of clean Room Validation particle Count to Microbial Count Limits
0 notes
Text
Microbial Limits Testing Market to Develop New Growth 2022
Microbial Limits Testing Market to Develop New Growth 2022
Market Overview | Microbial Limits Testing Market The report gives an outline of the Microbial Limits Testing Market and methodically discusses the present and future market prospects at length. It provides a synopsis that comprises of the definition, the manufacturing technique used, along with the key application and a detailed market summary that provides clarity concerning what the market is…

View On WordPress
#Microbial Limits Testing Market#Microbial Limits Testing Market News#Microbial Limits Testing Market News Industry
0 notes
Text
Soil stores large amounts of organic carbon, whose dynamic changes can cause a huge impact on the global climate system. Forest succession is a long-term ecological process that can exert powerful effects on soil organic carbon (SOC) due to variations in plant community composition, microenvironment, plant debris stock, soil nutrient availability and microbial community composition among different successional stages. Clarifying the dynamic changes of SOC across the forest successional series will provide insights into the global soil carbon cycle and improve the accuracy of terrestrial carbon cycle models. However, existing literature mostly focuses on the changes in SOC in the surface soil across forest succession, while SOC buried in deep soil is often neglected. Moreover, how SOC quality changes across the forest successional series remains unclear. Despite these limitations, there is a lack of comprehensive investigation of the changes in SOC stock and quality across the forest succession.
Continue Reading.
95 notes
·
View notes
Note
Hi! I really love your metas and they’ve cleared up a lot of what I missed when I read the books. I was wondering if I could ask you a question. (Totally fine if not!)
So we know that John killed the entire Solar System because he was angry the trillionaires got to escape a dying Earth and leave of the rest of its inhabitants behind. He details killing Earth, the Sun, the other planets. My question is, what happened to the moons? Did he overlook them? Did the Lyctors get to them later?
I ask because I’m wondering if the moons have anything to do with the Resurrection Beasts and/or their Heralds. Frankly, I’m just curious about what happened to them.
I get that killing a planet and everyone and everything on it would create a Resurrection Beast. But I’m wondering if size matters. Pluto is a dwarf planet. I’m sure there are moons that are larger. I’m not sure whether life-supporting environments mattered either. I can’t recall if any of the planets had stations that could maintain human life before the Resurrection.
John mentioned killing the Sun and later resurrecting it into Dominicus. I thought that was interesting because there is no way humans could have lived near or on the surface of the Sun, so its thalergenic potential would be incredibly limited if not zero, right? But it is now a thanergetic celestial body. Dominicus is remarked upon in the books and seems to have some significance because it is tied to John’s life. (Or was that a lie he told the Lyctors? I don’t remember.)
In comparison, moons get almost no mention. I’m wondering if that’s a choice or if there are just some thalergenic moons in the Dominican System. That seems unlikely to me and I’m wondering at the significance.
Perhaps I’m overthinking it lol. What are your thoughts?
I'm honestly so confused about moons.
There's only two references to moons that I can think of:
Mercymorn says to Harrow "I'm going to go do the moonlet next door. It'll be covered in reflected thalergy." But what is a moonlet, and is this a real phenomenon, or just one she made up as an excuse for Harrow to encounter the BoE crew?
And of course, there's Pyrra's "half-flipped moon", which is again rather confusing - what does it mean to be 'half-flipped'?
John doesn't mention them in his Very Hungry Caterpillar adventures, and there's no direct references to moons from any of the other Housers we meet - with the exception of Gideon using 'moonspeak' to mean something like technobabble or nonsense. There's no specifically moon-based pre-res stations mentioned that I can recall - there's some kind of city on Mars, the Kuiper Platform (perhaps near Neptune), and the shell being built around Uranus. So we can't assume RBs for Jupiter and Saturn came solely from humans living near them.
The Glossary in GTN goes out of its way to specify that "planets and gaseous bodies in space usually produce thalergenic radiation." It further says, "thalergy is produced by cellular growth and reproduction. Most planets, even ones without a biological mass of life, are thalergenic." So that's clear... The waters are further muddied by the explanation we get in HTN, where Harrow says thalergy "comes from the accumulation of microbial life" and John talks about how this produces a "communal soul". From the way John describes it, a revenant seems to the result from any swift planetary murder.
So thalergy is caused by an accumulation of microbial life which creates a communal planetary soul...but gaseous bodies also radiate thalergy?
I think there's two possibilities here.
The first is that we try to treat this as a properly sci fi endeavour to create a fantastical yet scientifically cohesive system, and we assume that perhaps that radiated thalergy Mercymorn describes relates to the idea that anything within the planet's gravitational orbit is part of its communal self. Perhaps the gas giants draw their thalergy from as-yet-undiscovered microbial life on their moons? Perhaps the sun is the sum of the solar system in some way?
The second is to assume that while this story is fiction that portrays scientific understandings in its fictional world, it is fundamentally a story about metaphysics. So the value of a moon is not determined by having a particular amount of microbial life or a specific relationship with another planet, but some mythological or metaphysical purpose - for the most part, moons aren't symbolically important to the story as it's being told.
So perhaps the moons of the solar system are part and parcel with the RBs of their planets.
60 notes
·
View notes
Text
DIY AGE-OF-SAIL INSPIRED FOULIES
part III: the process
it’s been a couple weeks since i finished making the alterations i wanted to make to the bibs before waxing, but we finally had an open shop day at school where i'm not bothering my buddies over at the Lady Washinton (though let’s be honest, the only reason i’m not there now is because they’re in anacortes and i dont wanna do the whole drive-ferry-drive thing). HOWEVER, that means i got to spend 4 hours painting my overalls with hot toxic soup. as far as the soup recipe goes, I did actually end up changing it again. in my first post i said i’d do varnish, and the second post i said black paint. i was going to measure everything out nice and had oz quantities i was going to adhere to, but i forgot my measuring cup… lets be real though, it’s probably more historically accurate to just throw shit in a pot and go. I’M MEASURING BY VIBES FOR SCIENCE!! the final recipe went something like this:
1lb microcrystalline wax
~1 cup mineral spirits
~3/4 cup tung oil
~1/2 cup rust-oleum oil based enamel paint (black)
~2 tbsp pine tar
I probably could have done more pine tar but the class bucket was basically empty and i didn’t want to walk down the hill to get more. I also know that pine tar takes fucking forever to cure, and even a small amount smells incredibly strong (though i certainly don’t mind, i actually prefer to be covered in the stuff most times- it’s more a courtesy to the non-tall shippers who aren’t used to the incredibly concentrated stink of 10 campfires burning directly into your nostrils). the reason i added the pine tar is because of it’s anti-bacterial and anti-microbial properties, since once the bibs are cured i really won’t be able to wash them. also, from my (limited and haphazard) research, you don’t need a lot to reap those benefits.
i put the wax in a double boiler, and once melted, added the oil, thinner, and paint/pine tar all at once. once it was all sufficiently combined, i started painting it on, let it cool a little bit, and then went back in with a heat gun and brush to help the solution impregnate the fibers of the cloth. oh also. make sure you are in a well ventilated space AND WEAR A RESPIRATOR (see the i-learned section below). i did 2 coats all over in this manner, and then a third over the knees, butt, and ankles for good measure.
oils and tar over any kind of fibrous material can take weeks to fully cure (as i have learned well from rigging), so i am expecting to leave my garment and it’s accoutrements hanging in the shop for about 3 weeks before they reach any kind of wearable or testable condition. everything seemed to soak in pretty well, but i left the shop before everything fully cooled so i’ll do another update at the beginning of next week- i’m anticipating that i over-waxed and there will be some residue i will have to deal with (though in what way is to be decided).
cleanup was pretty easy, considering my proclivity for giant messes with any project i engage in- lots of mineral spirits and several rags seemed to do the trick.
some things i learned/would do differently:
oh my god this recipe makes so much. like. so much. i had like 2 cups leftover and i did 2 coats on my overalls, pockets, AND a 1’x3’ piece of spare canvas. if you were just waxing a pair of pants, halving the recipe would still probably be more than enough
putting the cold liquids into the hot wax makes it congeal a little bit, but you can’t tell when the black paint makes the entire contents of the pot turn, well, BLACK. id put the transparent stuff in first, let it all melt together, and then add the black paint so that there wouldn’t suddenly be so many solid particles all at once
MIX FREQUENTLY. photo 3 shows the difference. i had mixed it really well at the beginning, but once it was all (presumably) a single solution, i stopped worrying about mixing it. the thing about paint/varnish/buildable coatings is that the reason they are buildable or have any sort of pigment is because of the suspended solids within it. this means that over time, the solids will coagulate at the bottom of the container, which is why you have to shake nail polish or stir paint before using it. this also means that i should have been mixing every couple minutes as i was painting it onto the bibs, so i ended up with a very pigmented mixture at the end, and a relatively translucent mix at the beginning. up until a certain point, i was getting a pigment that was not opaque but i was happy with, so i didn’t think too much of it until i was putting on coats that looked more brown than grey or black. anyways, mix your shit.
so… cotton burns. i was painting one leg at a time and then heat gunning it before moving on to the next leg. the wax/oil solution seems to make the fabric more resistant to burning, so the painted bits can take more heat than the untreated cotton next to it. if you, say, for example, (i definitely DID NOT DO THIS) get distracted by a particularly riveting tiktok your friend sent you of a snail vibing on a car windshield while your heat gun is blasting on high 2 inches from your pants, the raw canvas may or may not start smoking. i switched up to painting the Entire back or Entire front before heat gunning, and that seemed to solve the problem (also no more snail tiktoks)
respirators are kind of important. i was in a giant shop with vaulted ceilings next to a wide open garage door and i still had a bit of a headache after 4 hours of standing unprotected next to a pot of hot poison.
photo descriptions:
setup
setup part 2: electric boogaloo
pant ass- upper section 1 coat unmixed, lower section 1 coat mixed
spare canvas in the midst of coat 2
back of spare canvas after coat 1
back of spare canvas after coat 2
waterproof test!
finished garments and spare canvas, ready to cure
cleanup









#tall ship#sailor#historic sailing#boat building#sewing#oilskins#bibs#foulies#tin cloth#waxed canvas#age of sail#rain gear#fibre arts
62 notes
·
View notes
Text
Monopolizing turds

Update 31 May 2023: an earlier edition of this article identified the price of Rebyota as $20,000; this was the rumored price prior to Rebyota’s release in December 2022, when Stephen Skolnick wrote the article I referenced. When Rebyota was actually released in 2023, the average wholesale price (AWP) was $10,800. Thanks to Benjamin Jolley for catching this error, and to Stephen Skolnick for getting to the bottom of it.
It’s been ten years — to the day! — since I first started writing about the bizarre, amazing world of turd transplants, in which a sick person receives a microbiotic infusion in the form of some processed poop from a healthy person:
https://web.archive.org/web/20130608030455/http://blogs.plos.org/publichealth/2013/05/29/why-diy-fecal-transplants-are-a-thing-and-the-fda-is-only-part-of-the-reason/
Gut biomes are one of those understudied, poorly understood medical areas that are both very promising and also full of sketchy medical claims from “supplement” companies, influencers, quacks and grifters. But in the decade since I first started tracking turd transplants (formally called “Fecal Microbiota Transplants” or FMTs), a growing body of sound science has emerged on the subject.
One thing that’s increasingly undeniable is that the composition of your microbial nation is related in significant ways to both your physical and mental health. What’s more, as antibiotic resistant “super bugs” proliferate, FMTs are becoming increasingly central to treating dangerous gut infections that otherwise stand a high chance of killing you.
“Eat Shit and Prosper” is Stephen Skolnick’s delightfully named newsletter about poop and health science. Skolnick is a physicist by training, but has a long history of collaboration with Openbiome, a nonprofit that coordinates between doctors, patients and donors to provide safe FMTs:
https://stephenskolnick.substack.com/
In an edition of Eat Shit from last December, Skolnick recounts the amazing history and dismaying future of FMTs. In 2013, the FDA announced it would regulate FMTs as “Investigational New Drugs,” which could only be administered as part of a registered clinical trial:
https://stephenskolnick.substack.com/p/a-monopoly-on-poop
At that point, FMTs were already in widespread use by docs to treat otherwise untreatable cases of Clostridioides difficile (C. diff), an antibiotic resistant bacterial infection that literally makes you shit yourself to death. These doctors were in no position to run registered clinical trials, which meant that they would have to stop using the most effective therapy they had for a potentially lethal infection.
Doctors and patients kicked up a fuss, and the FDA walked back its guidance, announcing that it would exercise “discretion” in enforcing its Investigational New Drug rule, giving a pass to docs who were treating C. diff with FMTs:
https://www.federalregister.gov/documents/2013/07/18/2013-17223/guidance-for-industry-enforcement-policy-regarding-investigational-new-drug-requirements-for-use-of
That’s where things have stood for the past decade or so. The “discretion” rule means that patients could still get FMTs, but their insurance wouldn’t cover it. But even if you had cash to pay for an FMT, your doc probably wouldn’t administer it for anything except a C. diff infection, despite the promising signs that FMT can help treat other conditions, and despite the generally safe nature of FMTs.
If your doc did give you an FMT, chances are good that they sourced their poop from Openbiome. Openbiome recruits very healthy people, gets them to poop in a bag, then processes the poop — removing nonbacterial solids, testing it for pathogens, freezing it, portioning it, and sending it to docs. All this is done at cost, and it’s not cheap: $1–2k/treatment, mostly due to cold-chain logistics (the poop is shipped at -80C).
Despite the cost, and despite the limitations on treatment, the Openbiome method has proved very reliable. Indeed, FMTs as a whole are pretty darned safe, with the most common side-effects being transient gas and bloating. In the past decade, there’ve been a total of six “adverse effects” associated with Openbiome’s 5,000+ procedures, all in severely immunocompromised people, and none conclusively linked to the treatment:
https://www.sciencedirect.com/science/article/pii/S0016508522003511/pdf
A decade into this system, the FDA has taken the next step forward — only it’s actually a step backwards.
During this intervening decade, a pharma company called Ferring has conducted clinical trials on FMTs and received approval for an FMT product called Rebyota. The process for making Rebyota is effectively identical to the process used by Openbiome: collect poop, remove solids, test for pathogens, add glycerol, freeze and ship.
The main difference between Rebyota and Openbiome’s poop is price. While Openbiome charges $1–2k per treatment, Rebyota charges $10,800
That’s some expensive shit!
Fine. Getting Rebyota through clinical trials means that insurers might start covering it, and perhaps some patients will prefer brand-name poop to open-source poop. But as part of the FDA’s approval of Rebyota, the agency also rescinded its “discretionary enforcement” guidance, making it illegal for docs to source their poop from Openbiome:
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-regarding-investigational-new-drug-requirements-use-fecal-microbiota
For Ferring, this is a monopoly on shit, one that lets them charge patients $10.8k for poop that costs $1–2k to process. The FDA does not claim that this is being done in the name of safety. Instead, an FDA official told Skonick that the goal was to “incentivize innovation without creating an access crisis.”
That is, the FDA changed its guidance and put nonprofit stool banks out of business because it wants to incentivize pharma companies to perform expensive clinical trials, and it believes that these companies won’t pay for trials if they have to compete with the likes of Openbiome, which would make it impossible to charge 900% markups on poop.
Trials are important! Evidence-based medicine is important! But Ferring’s clinical trials didn’t tell us anything we didn’t already know. FMTs were already the best therapy we had for C. diff. Testing Rebyota against a placebo didn’t tell us anything new — unlike testing Rebyota against the existing therapies, e.g. product from open stool banks.
Such a trial might have given rise to a very different regulatory outcome, because the cure rate reported by Rebyota is much lower than the cure rate from Openbiome’s own interventions:
https://link.springer.com/article/10.1007/s40265-022-01797-x
That is, using the $1k poop from Openbiome seems to be much more effective than using the $10.8k poop from Ferring. But Openbiome, a nonprofit, hasn’t been able to perform the kind of rigorous — and expensive — clinical trial that Ferring funded.
This points to a significant problem with the FDA’s model. The agency wants good clinical data for the medicines it regulates, as it should, It presumes that the only way to get that data is through granting commercial exclusivity to a for-profit, which ends up costing patients vast sums, and locking many patients out altogether.
This creates all kinds of new dangers. 150,000 people/year in the US contract Recurrent Clostridium difficile Infection (RCdI). FMT increases the cure rate by 20% relative to antibiotics alone. That means that if everyone with RCdI gets a poop transplant, 30,000 extra people will get better. That’s a big number!
For well insured people, Rebyota probably represents a cash-savings — if your insurance covers the $10,800 procedure, you might pay $500 out of pocket, which is far less than the $1–2K you’d pay to get an Openbiome poop transplant. But if you’re uninsured or underinsured, the FDA’s new enforcement rules mean that you’re now on the hook for $10,800.
The FDA did carve out a loophole: if your doc or their hospital are willing to prepare the poop transplant themselves, they can administer that. On the one hand, preparing a poop transplant isn’t that hard — some people do them at home, on their own:
https://web.archive.org/web/20211015060558/https://thepowerofpoop.com/epatients/fecal-transplant-instructions/
But on the other hand, there’s been exactly one death conclusively linked to FMT, and it was from one of these hospital-prepared transplants (the patient had just had a marrow transplant for cancer that wiped out their immune system, and the donor had a novel pathogen that the hospital failed to test for).
So the FDA has created a situation where, if you can’t afford a $10,800 proprietary formulation, your only option is to convince your doc or hospital to prepare their own poop transplant, which will cost less than the $10.8k for Rebyota, but more than the $1–2k from Openbiome, which has all kinds of economies of scale. And if you do manage it, you’ll be getting a procedure that has a much worse safety track-record than the Openbiome process that the FDA just killed.
The FDA has an important role to play here, but as with so many policy questions, how the FDA plays that role depends on things that are far upstream from the agency and its decisions. The choice to fund medical trials through the promise of exclusivity — and with it, extremely high margins — puts the FDA in the position of choosing winners in the marketplace: Ferring wins, Openbiome loses.
Ironically, this is the thing that exclusivity is supposed to prevent. By using profit to incentivize medical research, the FDA is supposed to be recruiting the Invisible Hand as its partner in regulation. But exclusivity is incompatible with the idea of medicine as a public good. The tens (hundreds) of millions that Americans will pay for $10.8k poop transplants from Ferring will add up to far more than it would cost to underwrite clinical trials for an open process like Openbiome’s.
The result: both Americans’ wallets and Americans’ guts suffer.

Catch me on tour with Red Team Blues in Hay-on-Wye, Oxford, Manchester, Nottingham, London, and Berlin!

If you’d like an essay-formatted version of this post to read or share, here’s a link to it on pluralistic.net, my surveillance-free, ad-free, tracker-free blog:
https://pluralistic.net/2023/05/29/oh-shit/#rebyota

[Image ID: A poop emoji wearing a top hat and a monocle, posed against a backdrop of e coli bacteria seen through a high-resolution microscope.]
#pluralistic#stool bank#eat shit and live#pharma#fda#regulatory capture#fecal transplants#microbiomes#rebyota#openbiome#c diff#fmt#fecal microbiota transplant
272 notes
·
View notes
Text
i think the green planet did a rly good job showing how plants are as interesting and,, "alive" as animals so.... what if they did that w microbes
i self indulgently wish that sir david attenborough made a documentary series about microbes.
7 notes
·
View notes
Text
Packaging Tape Manufacturer: The Backbone of Secure Shipping Solutions
https://jesons.net/
In today’s global marketplace, secure packaging is more important than ever. One of the essential components in ensuring the safe transit of goods is packaging tape. Traditionally, various types of tape were used in shipping and storage, but with increasing demands for quality and reliability, the role of packaging tape manufacturers has become critical. These manufacturers are now focused on producing high-performance tapes that not only protect products but also enhance branding.
The Importance of Quality Packaging Tape
Quality packaging tape is crucial for several reasons. First and foremost, it provides a secure seal that keeps packages intact during transportation. This is especially vital in industries such as e-commerce, where product integrity is paramount. Additionally, quality packaging tape can withstand various environmental conditions, including moisture and temperature fluctuations, ensuring that the contents remain protected.
Packaging tape manufacturers are now utilizing advanced materials and technologies to produce tapes that offer superior adhesion and durability. Options like reinforced tapes and custom-printed designs allow businesses to enhance their packaging while ensuring safety.
Mumbai: A Hub for Packaging Tape Manufacturers
Mumbai, as a major industrial center in India, is home to some of the leading packaging tape manufacturers who are setting the standard for quality and innovation. These manufacturers are equipped to cater to a diverse range of industries, including retail, logistics, and manufacturing. By leveraging local resources and expertise, Mumbai-based companies are producing tapes that not only meet but exceed industry standards.
Benefits of Partnering with a Packaging Tape Manufacturer
Reliability: High-quality packaging tape ensures that products are securely sealed, minimizing the risk of damage during shipping.
Customization: Many manufacturers offer customizable options, allowing businesses to print their logos or branding on the tape, which can enhance brand visibility.
Variety: From standard packing tapes to specialty tapes, manufacturers provide a range of products to meet diverse needs.
Cost Efficiency: Investing in quality tape reduces the likelihood of product returns due to damage, ultimately saving businesses money.
Conclusion
As the demand for secure packaging solutions continues to grow, the role of packaging tape manufacturers becomes increasingly vital. By offering high-quality, reliable products, these manufacturers help businesses protect their goods and enhance their brand presence. Choosing the right packaging tape not only safeguards products during transit but also aligns with the growing emphasis on quality and sustainability in the marketplace.
0 notes
Text

Meet the Townies: ᴏʀɪᴏɴ
Orion, otherwise known as the Operations for Research, Intelligence, and Optimization Network, was composed of a specialized group of Sixonians tasked to study and understand other planets and lifeforms. Their primary mission was to gather intelligence, conduct biological research, and develop new technologies to enhance the capabilities and knowledge of their race. The team consisted of (from left to right) Jorlan Vex, Lieutenant Zerath Veylor, Doctor Velana Krynn, and Zyri Solith. Before being offered a position on the ORION team, Jorlan attended the prestigious Korthis Institute of Technology, where he specialized in advanced systems engineering and quantum mechanics. After graduating with honors, Jorlan was employed by the Orbitex Industries, where he worked on developing cutting-edge technology for space travel. He led multiple successful projects that secured him a spot within ORION. As the team's systems engineer, Jorlan was responsible for maintaining and enhancing the team's technological equipment. Following his graduation from the Kharis Military Academy, Zerath was a member of the SPECTREs (Special Planetary Exploration and Combat Tactical Response Experts) where he served with distinction for several decades. He participated in numerous high-risk missions. During one such mission, Zerath sustained a severe injury to his lower limbs. The injury left him with limited mobility, forcing him into early retirement from active military service. While adjusting to his new life away from the battlefield, Zerath received an unexpected offer from in search of security officer. Despite his injury, Zerath immediately jumped at the chance. As the security officer, his primary responsibility was to ensure the safety of the team during their missions, handling any security threats and leading defensive operations if necessary. Velana attended the Valtara Academy of Sciences, where she majored in xenobiology. Her exceptional performance and passion for extraterrestrial life earned her a scholarship to the Sixonian Institute of Extraterrestrial Research (SIER), the leading institution for space biology and alien ecology studies. At SIER, she conducted research on extremophiles and their potential to survive on other planets. Her doctoral thesis on the adaptability of alien microbial life forms received widespread acclaim and set the stage for her future career. The Sixonian Institute of Extraterrestrial Research, recognizing the growing need for a dedicated team to explore and study new planets and alien life forms, greenlit the creation of ORION with the intent to combine the best minds in various scientific and technological fields to conduct in-depth research and ensure Sixam's continued dominance of exploration. Dr. Velana Krynn was selected as the team's xenobiologist due to her unparalleled expertise and proven track record in alien biology. Her role in ORION involved studying the physiology, behavior, and ecosystems of extraterrestrial species. Zyri was an employee at the Sixonian Intergalactic Communication Bureau where she specialized in xenolinguistics, semiotics, and interspecies communication. Her groundbreaking research on deciphering alien languages and developing universal translation algorithms garnered the attention of the Sixonian Institute of Extraterrestrial Research, earning her a place on the team. As the team's Communication and Linguistics Specialist, Zyri is responsible for establishing and maintaining communication with alien species. Her tasks included decoding and interpreting alien languages, developing translation protocols, and ensuring clear and effective communication during missions. The team, once celebrated for their discoveries and technological advancements, are now largely seen as the catalyst for Sixam's downfall.
43 notes
·
View notes
Text
Parasites take an enormous toll on human and veterinary health. But researchers may have found a way for patients with brain disorders and a common brain parasite to become frenemies.
A new study published in Nature Microbiology has pioneered the use of a single-cell parasite, Toxoplasma gondii, to inject therapeutic proteins into brain cells. The brain is very picky about what it lets in, including many drugs, which limits treatment options for neurological conditions.
As a professor of microbiology, I’ve dedicated my career to finding ways to kill dangerous parasites such as Toxoplasma. I’m fascinated by the prospect that we may be able to use their weaponry to instead treat other maladies.
Microbes as Medicine
Ever since scientists realized that microscopic organisms can cause illness—what’s called the 19th-century germ theory of disease—humanity has been on a quest to keep infectious agents out of our bodies. Many people’s understandable aversion to germs may make the idea of adapting these microbial adversaries for therapeutic purposes seem counterintuitive.
But preventing and treating disease by co-opting the very microbes that threaten us has a history that long predates germ theory. As early as the 1500s, people in the Middle East and Asia noted that those lucky enough to survive smallpox never got infected again. These observations led to the practice of purposefully exposing an uninfected person to the material from an infected person’s pus-filled sores—which unbeknownst to them contained weakened smallpox virus—to protect them from severe disease.
This concept of inoculation has yielded a plethora of vaccines that have saved countless lives.
Viruses, bacteria, and parasites have also evolved many tricks to penetrate organs such as the brain and could be retooled to deliver drugs into the body. Such uses could include viruses for gene therapy and intestinal bacteria to treat a gut infection known as C. diff.
Why Can’t We Just Take a Pill for Brain Diseases?
Pills offer a convenient and effective way to get medicine into the body. Chemical drugs such as aspirin or penicillin are small and easily absorbed from the gut into the bloodstream.
Biologic drugs such as insulin or semaglutide, on the other hand, are large and complex molecules that are vulnerable to breaking down in the stomach before they can be absorbed. They are also too big to pass through the intestinal wall into the bloodstream.
All drugs, especially biologics, have great difficulty penetrating the brain due to the blood-brain barrier. The blood-brain barrier is a layer of cells lining the brain’s blood vessels that acts like a gatekeeper to block germs and other unwanted substances from gaining access to neurons.
Toxoplasma Offers Delivery Service to Brain Cells
Toxoplasma parasites infect all animals, including humans. Infection can occur in multiple ways, including ingesting spores released in the stool of infected cats or consuming contaminated meat or water. Toxoplasmosis in otherwise healthy people produces only mild symptoms but can be serious in immunocompromised people and to gestating fetuses.
Unlike most pathogens, Toxoplasma can cross the blood-brain barrier and invade brain cells. Once inside neurons, the parasite releases a suite of proteins that alter gene expression in its host, which may be a factor in the behavioral changes it causes in infected animals and people.
In a new study, a global team of researchers hijacked the system Toxoplasma uses to secrete proteins into its host cell. The team genetically engineered Toxoplasma to make a hybrid protein, fusing one of its secreted proteins to a protein called MECP2, which regulates gene activity in the brain—in effect, giving the MECP2 a piggyback ride into neurons. Researchers found that the parasites secreted the MECP2 protein hybrid into neurons grown in a petri dish as well as in the brains of infected mice.
A genetic deficiency in MECP2 causes a rare brain development disorder called Rett syndrome. Gene therapy trials using viruses to deliver the MECP2 protein to treat Rett syndrome are underway. If Toxoplasma can deliver a form of MECP2 protein into brain cells, it may provide another option to treat this currently incurable condition. It also may offer another treatment option for other neurological problems that arise from errant proteins, such as Alzheimer’s and Parkinson’s disease.
The Long Road Ahead
The road from laboratory bench to bedside is long and filled with obstacles, so don’t expect to see engineered Toxoplasma in the clinic anytime soon.
The obvious complication in using Toxoplasma for medical purposes is that it can produce a serious, lifelong infection that is currently incurable. Infecting someone with Toxoplasma can damage critical organ systems, including the brain, eyes, and heart.
However, up to one-third of people worldwide currently carry Toxoplasma in their brain, apparently without incident. Emerging studies have correlated infection with increased risk of schizophrenia, rage disorder, and recklessness, hinting that this quiet infection may be predisposing some people to serious neurological problems.
The widespread prevalence of Toxoplasma infections may also be another complication, as it disqualifies many people from using it for treatment. Since the billions of people who already carry the parasite have developed immunity against future infection, therapeutic forms of Toxoplasma would be rapidly destroyed by their immune systems once injected.
In some cases, the benefits of using Toxoplasma as a drug delivery system may outweigh the risks. Engineering benign forms of this parasite could produce the proteins patients need without harming the organ—the brain—that defines who we are.
17 notes
·
View notes