#MHRA notice
Explore tagged Tumblr posts
Text
Blood pressure medication recalled over fears of 'microbial contamination'
A blood pressure medication has been recalled from suppliers due to possible “microbial contamination”. Amlodipine is a calcium channel blocker used to treat high blood pressure and can cut the risk of heart disease, heart attacks and strokes. It works by relaxing and widening blood vessels, making it easier for the heart to pump blood around the body. A notice issued by the Medicines and…
0 notes
Text
A newly leaked Pfizer report reveals that the hearts of vaccinated people are “rapidly decaying,” leading to various serious heart conditions.
According to a leaked MHRA report obtained by journalist Nick Hunt, vaccinated people are at significantly higher risk of dying from heart problems than their unvaccinated peers.

To recap: this is a report of a Post Authorisation Safety Study (PASS) of Pfizer’s Covid vaccine. National regulators routinely require pharmaceutical manufacturers to conduct PASS studies as a condition of authorisation of most new medicines. The regulators provide data to the manufacturer covering millions of patients registered in national healthcare systems. The manufacturer then conducts analysis, matched for things like age and sex, to determine whether the medicine has increased the risk of specified health conditions.
Let’s dive straight in. Below are some heart-related cumulative incidence graphs from Pfizer’s full ‘Interim Report 5’. You will immediately notice that the incidence for each type of condition is significantly greater in the Covid vaccinated (bad) – but we already knew that from the Hazard Ratios in the abstract. What’s worse is that the curves diverge over time, i.e., the relative incidence between vaccinated and unvaccinated increases over the time period of the data in the report (December 8th 2020 – March 21st 2022). I wonder what happened subsequently.
Acute cardiovascular injury (23% higher in the vaccinated and getting worse; page 130):
76 notes
·
View notes
Text
How the Saxenda Injection for Weight Loss Works: A Complete Guide
Losing weight isn’t just about looking good—it’s about feeling good, too. But for many, diet and exercise alone don’t bring the results they need. That’s where the Saxenda injection for weight loss comes in. This prescription medication has been clinically proven to help people lose weight and keep it off. But how does it actually work? In this guide, we’ll break down everything you need to know about the Saxenda weight loss pen, from how it affects your body to who it’s best suited for.
Saxenda (liraglutide) is an FDA and MHRA-approved prescription medication designed to help with weight loss. It’s an injectable treatment that mimics a natural hormone in your body called GLP-1 (glucagon-like peptide-1).
GLP-1 plays a crucial role in appetite regulation and digestion. Normally, when you eat, your body releases GLP-1 to slow down digestion and make you feel full. However, for those struggling with weight management, this process might not be as effective. Saxenda boosts GLP-1 levels, making you feel fuller for longer and reducing cravings.
Studies show that people using Saxenda alongside a calorie-controlled diet and regular exercise lose significantly more weight than those relying on lifestyle changes alone. In a 56-week clinical trial, participants lost an average of 8-10% of their body weight when using Saxenda.

The Science Behind Saxenda’s Effectiveness
Unlike other weight loss treatments that focus on burning fat or suppressing appetite artificially, Saxenda works with your body’s natural signals. Here’s how:
Slows down stomach emptying – You feel fuller for longer, which helps reduce calorie intake.
Reduces hunger signals in the brain – Saxenda interacts with the brain’s appetite control centre, making you feel satisfied with smaller portions.
Helps regulate blood sugar levels – While primarily a weight loss medication, Saxenda can also help stabilise blood sugar, making it beneficial for people at risk of type 2 diabetes.
Who Can Use Saxenda?
The Saxenda injection for weight loss isn’t for everyone. It’s typically prescribed to adults who meet one of these criteria:
A BMI of 30 or higher (classified as obese).
A BMI of 27 or higher with weight-related health conditions such as high blood pressure, type 2 diabetes, or sleep apnoea.
It’s important to note that Saxenda is not a quick fix. It’s most effective when combined with healthy eating habits and regular physical activity. If you’re unsure whether Saxenda is right for you, consulting a healthcare professional is essential.
What to Expect When Using Saxenda
When starting Saxenda, the dosage is gradually increased over several weeks to allow your body to adjust. Most people begin noticing a difference in appetite and portion sizes within a few weeks.
Common Side Effects
Like any medication, Saxenda can cause some side effects. The most common include:
Nausea (usually mild and temporary).
Diarrhoea or constipation.
Headaches.
These effects often subside as your body adapts. Drinking plenty of water and eating smaller meals can help reduce discomfort.
How Fast Will You See Results?
Weight loss varies from person to person, but studies suggest that most users lose at least 5% of their body weight within 12 weeks. If significant weight loss isn’t achieved within that time, a doctor may reassess the treatment plan.
How to Use the Saxenda Weight Loss Pen
The Saxenda weight loss pen is a pre-filled injection that is self-administered once daily. It’s injected just under the skin, usually in the abdomen, thigh, or upper arm.
For best results, follow these guidelines:
Inject Saxenda at the same time every day.
Rotate injection sites to prevent irritation.
Store the pen in the fridge before first use, then at room temperature for up to 30 days.
Missing a dose occasionally isn’t a major issue, but skipping multiple doses can reduce effectiveness.
Is Saxenda Worth It?
Saxenda has transformed the weight loss journey for thousands of people. Clinical trials show that 63% of users lost at least 5% of their body weight over a year, and 33% lost 10% or more.
However, it’s not just about the numbers. Many users report:
Reduced cravings and better portion control.
Higher energy levels.
Improved confidence and motivation to maintain a healthy lifestyle.
While Saxenda is effective, commitment to long-term lifestyle changes is key to keeping the weight off.
Take control of your journey today!
The Saxenda injection for weight loss is a scientifically backed tool for those struggling with obesity or weight-related health issues. By working with the body’s natural appetite signals, it helps control hunger, reduce calorie intake, and promote sustainable weight loss.If you’re considering Saxenda, consulting a healthcare provider is the best first step. With the right plan in place, you could be on your way to a healthier, happier you. Want to learn more? Explore our range of weight loss solutions at Your Medicals today!
0 notes
Text
Diazepam dealers: Pharmacists sentenced for ‘industrial’ illegal supply
Mandip Sidhu and Nabeil Nasr illegally supplied more than 55 million doses of controlled drugs, the MHRA has revealed.

Two pharmacists have been sentenced for the “industrial scale” illegal supply of class C controlled drugs (CDs), the Medicines and Healthcare products Regulatory Agency (MHRA) announced yesterday (May 16).
Mandip Sidhu of Littleover, Derby, and Nabeil Nasr of Cheadle, Greater Manchester, were found to have illegally supplied more than 55 million doses of class C CDs, including over 47m doses of diazepam, between May 2013 and June 2017, it said.
Both were registered pharmacists at the time of the offending, it added.
Sidhu, 47, was the director of Derby-based Pharmaceutical Health Limited (PHL) while Nasr, 42, owned “several pharmacies” in northwest England, according to the MHRA.
It revealed that PHL bought 4.27m tablets in August 2014 and 4.5m tablets in March 2015 but had not “legally dispensed” any prescribed medicines since July 2013.
The MHRA added “for perspective” that a total of “around five million” tablets of diazepam were legally dispensed in England during 2014 – figures “dwarfed by the quantities of drugs passing through the hands of Sidhu and Nasr”.
Neither Sidhu nor Nasr possessed a Home Office Controlled Drug Licence (HOCDL), it said.
Nasr pleaded guilty to two counts of supplying diazepam and zopiclone and two counts of wholesale dealing without a licence, while Sidhu pleaded guilty to five counts of supplying diazepam, zolpidem and zopiclone, it added.
Attempted deception
The watchdog revealed that Sidhu tried to dupe an MHRA inspector with a forged invoice claiming that the CDs had been “sold to a company outside the European Economic Area”.
For this attempted deception, she pleaded guilty to a charge of forgery, it said.
Sidhu and Nasr were both yesterday sentenced to two years’ imprisonment for their crimes at the Southwark Crown Court, suspended for two years, it added.
Sidhu also received an additional suspended sentence of four months’ imprisonment to run concurrently, while both of their suspended sentences are conditional on completing 200 hours of community service, according to the MHRA.
MHRA deputy director of criminal enforcement Andy Morling paid tribute to the “exceptional determination, skill and professionalism” shown by the authority’s Criminal Enforcement Unit in its investigation.
Morling said that the “successful prosecution” was a show of the MHRA’s “full range of powers and tools”.
Fraud-fighting pharmacists
In sharp contrast, C+D exclusively revealed the story of Noman Ahmed earlier this month, a Kent locum pharmacist who helped to uncover a years-long prescription fraud involving over 20,000 tramadol tablets after he noticed an “unusual” script.
Similarly, in July last year, C+D reported on a vigilant pharmacist who helped to nab another fraudster who was sentenced to 18 months in prison after he used faked prescriptions to acquire around £40,000-worth of drugs.
Also in July, a C+D investigation revealed that no fraud reports lodged against community pharmacy contractors had been converted into prosecutions by the NHS fraud office in over two years.
1 note
·
View note
Text
Diazepam dealers: Pharmacists sentenced for ‘industrial’ illegal supply
Mandip Sidhu and Nabeil Nasr illegally supplied more than 55 million doses of controlled drugs, the MHRA has revealed.

Two pharmacists have been sentenced for the “industrial scale” illegal supply of class C controlled drugs (CDs), the Medicines and Healthcare products Regulatory Agency (MHRA) announced yesterday (May 16).
Mandip Sidhu of Littleover, Derby, and Nabeil Nasr of Cheadle, Greater Manchester, were found to have illegally supplied more than 55 million doses of class C CDs, including over 47m doses of diazepam, between May 2013 and June 2017, it said.
Both were registered pharmacists at the time of the offending, it added.
Sidhu, 47, was the director of Derby-based Pharmaceutical Health Limited (PHL) while Nasr, 42, owned “several pharmacies” in northwest England, according to the MHRA.
It revealed that PHL bought 4.27m tablets in August 2014 and 4.5m tablets in March 2015 but had not “legally dispensed” any prescribed medicines since July 2013.
The MHRA added “for perspective” that a total of “around five million” tablets of diazepam were legally dispensed in England during 2014 – figures “dwarfed by the quantities of drugs passing through the hands of Sidhu and Nasr”.
Neither Sidhu nor Nasr possessed a Home Office Controlled Drug Licence (HOCDL), it said.
Nasr pleaded guilty to two counts of supplying diazepam and zopiclone and two counts of wholesale dealing without a licence, while Sidhu pleaded guilty to five counts of supplying diazepam, zolpidem and zopiclone, it added.
Attempted deception
The watchdog revealed that Sidhu tried to dupe an MHRA inspector with a forged invoice claiming that the CDs had been “sold to a company outside the European Economic Area”.
For this attempted deception, she pleaded guilty to a charge of forgery, it said.
Sidhu and Nasr were both yesterday sentenced to two years’ imprisonment for their crimes at the Southwark Crown Court, suspended for two years, it added.
Sidhu also received an additional suspended sentence of four months’ imprisonment to run concurrently, while both of their suspended sentences are conditional on completing 200 hours of community service, according to the MHRA.
MHRA deputy director of criminal enforcement Andy Morling paid tribute to the “exceptional determination, skill and professionalism” shown by the authority’s Criminal Enforcement Unit in its investigation.
Morling said that the “successful prosecution” was a show of the MHRA’s “full range of powers and tools”.
Fraud-fighting pharmacists
In sharp contrast, C+D exclusively revealed the story of Noman Ahmed earlier this month, a Kent locum pharmacist who helped to uncover a years-long prescription fraud involving over 20,000 tramadol tablets after he noticed an “unusual” script.
Similarly, in July last year, C+D reported on a vigilant pharmacist who helped to nab another fraudster who was sentenced to 18 months in prison after he used faked prescriptions to acquire around £40,000-worth of drugs.
Also in July, a C+D investigation revealed that no fraud reports lodged against community pharmacy contractors had been converted into prosecutions by the NHS fraud office in over two years.
#Pharmacists#industrial#illegal supply#breaking news#business news#news#newsies#public news#world news
0 notes
Text
Diazepam dealers: Pharmacists sentenced for ‘industrial’ illegal supply
Mandip Sidhu and Nabeil Nasr illegally supplied more than 55 million doses of controlled drugs, the MHRA has revealed.

Two pharmacists have been sentenced for the “industrial scale” illegal supply of class C controlled drugs (CDs), the Medicines and Healthcare products Regulatory Agency (MHRA) announced yesterday (May 16).
Mandip Sidhu of Littleover, Derby, and Nabeil Nasr of Cheadle, Greater Manchester, were found to have illegally supplied more than 55 million doses of class C CDs, including over 47m doses of diazepam, between May 2013 and June 2017, it said.
Both were registered pharmacists at the time of the offending, it added.
Sidhu, 47, was the director of Derby-based Pharmaceutical Health Limited (PHL) while Nasr, 42, owned “several pharmacies” in northwest England, according to the MHRA.
It revealed that PHL bought 4.27m tablets in August 2014 and 4.5m tablets in March 2015 but had not “legally dispensed” any prescribed medicines since July 2013.
The MHRA added “for perspective” that a total of “around five million” tablets of diazepam were legally dispensed in England during 2014 – figures “dwarfed by the quantities of drugs passing through the hands of Sidhu and Nasr”.
Neither Sidhu nor Nasr possessed a Home Office Controlled Drug Licence (HOCDL), it said.
Nasr pleaded guilty to two counts of supplying diazepam and zopiclone and two counts of wholesale dealing without a licence, while Sidhu pleaded guilty to five counts of supplying diazepam, zolpidem and zopiclone, it added.
Attempted deception
The watchdog revealed that Sidhu tried to dupe an MHRA inspector with a forged invoice claiming that the CDs had been “sold to a company outside the European Economic Area”.
For this attempted deception, she pleaded guilty to a charge of forgery, it said.
Sidhu and Nasr were both yesterday sentenced to two years’ imprisonment for their crimes at the Southwark Crown Court, suspended for two years, it added.
Sidhu also received an additional suspended sentence of four months’ imprisonment to run concurrently, while both of their suspended sentences are conditional on completing 200 hours of community service, according to the MHRA.
MHRA deputy director of criminal enforcement Andy Morling paid tribute to the “exceptional determination, skill and professionalism” shown by the authority’s Criminal Enforcement Unit in its investigation.
Morling said that the “successful prosecution” was a show of the MHRA’s “full range of powers and tools”.
Fraud-fighting pharmacists
In sharp contrast, C+D exclusively revealed the story of Noman Ahmed earlier this month, a Kent locum pharmacist who helped to uncover a years-long prescription fraud involving over 20,000 tramadol tablets after he noticed an “unusual” script.
Similarly, in July last year, C+D reported on a vigilant pharmacist who helped to nab another fraudster who was sentenced to 18 months in prison after he used faked prescriptions to acquire around £40,000-worth of drugs.
Also in July, a C+D investigation revealed that no fraud reports lodged against community pharmacy contractors had been converted into prosecutions by the NHS fraud office in over two years.
1 note
·
View note
Text
Diazepam dealers: Pharmacists sentenced for ‘industrial’ illegal supply
Mandip Sidhu and Nabeil Nasr illegally supplied more than 55 million doses of controlled drugs, the MHRA has revealed.

Two pharmacists have been sentenced for the “industrial scale” illegal supply of class C controlled drugs (CDs), the Medicines and Healthcare products Regulatory Agency (MHRA) announced yesterday (May 16).
Mandip Sidhu of Littleover, Derby, and Nabeil Nasr of Cheadle, Greater Manchester, were found to have illegally supplied more than 55 million doses of class C CDs, including over 47m doses of diazepam, between May 2013 and June 2017, it said.
Both were registered pharmacists at the time of the offending, it added.
Sidhu, 47, was the director of Derby-based Pharmaceutical Health Limited (PHL) while Nasr, 42, owned “several pharmacies” in northwest England, according to the MHRA.
It revealed that PHL bought 4.27m tablets in August 2014 and 4.5m tablets in March 2015 but had not “legally dispensed” any prescribed medicines since July 2013.
The MHRA added “for perspective” that a total of “around five million” tablets of diazepam were legally dispensed in England during 2014 – figures “dwarfed by the quantities of drugs passing through the hands of Sidhu and Nasr”.
Neither Sidhu nor Nasr possessed a Home Office Controlled Drug Licence (HOCDL), it said.
Nasr pleaded guilty to two counts of supplying diazepam and zopiclone and two counts of wholesale dealing without a licence, while Sidhu pleaded guilty to five counts of supplying diazepam, zolpidem and zopiclone, it added.
Attempted deception
The watchdog revealed that Sidhu tried to dupe an MHRA inspector with a forged invoice claiming that the CDs had been “sold to a company outside the European Economic Area”.
For this attempted deception, she pleaded guilty to a charge of forgery, it said.
Sidhu and Nasr were both yesterday sentenced to two years’ imprisonment for their crimes at the Southwark Crown Court, suspended for two years, it added.
Sidhu also received an additional suspended sentence of four months’ imprisonment to run concurrently, while both of their suspended sentences are conditional on completing 200 hours of community service, according to the MHRA.
MHRA deputy director of criminal enforcement Andy Morling paid tribute to the “exceptional determination, skill and professionalism” shown by the authority’s Criminal Enforcement Unit in its investigation.
Morling said that the “successful prosecution” was a show of the MHRA’s “full range of powers and tools”.
Fraud-fighting pharmacists
In sharp contrast, C+D exclusively revealed the story of Noman Ahmed earlier this month, a Kent locum pharmacist who helped to uncover a years-long prescription fraud involving over 20,000 tramadol tablets after he noticed an “unusual” script.
Similarly, in July last year, C+D reported on a vigilant pharmacist who helped to nab another fraudster who was sentenced to 18 months in prison after he used faked prescriptions to acquire around £40,000-worth of drugs.
Also in July, a C+D investigation revealed that no fraud reports lodged against community pharmacy contractors had been converted into prosecutions by the NHS fraud office in over two years.
1 note
·
View note
Text
my understanding of this is as follows:
the MHRA issued takedown notices to NiceNIC, which is a web hosting provider based in Hong Kong. NiceNIC received these on april 25th, before the guardian article dropped (per the supporting documents provided in the request to google pictured in the OP post above).
it's possible that the guardian's investigation prompted this, but there's no evidence to prove that (it could just as easily have been prompted by the cass review, for example).
NiceNIC seems to do a decent amount of business on the back of sketchy/legally dubious foreign companies, which would indicate that they're not in the business of cooperating with requests of this nature. a little independent googling shows that they come highly recommended as a hosting service among grey-market business owners for this exact reason.
furthermore, any kind of extradition between HK and the UK (as well as the US, Canada, Australia, New Zealand and most of Europe) has been suspended since 2020. even if there was an attempt to prosecute NiceNIC under UK law, there is no mechanism to do so.
it is my understanding that websites with this same provider who have received these same takedown notices in the past are still functioning.
certain websites (i'm not naming names out in the open, nor should anyone else) are currently inaccessible. this is not a result of any legal action. in the interests of opsec i can't say any more about this.
(sidenote: the question as to why the government would do this if it's functionally unenforceable is valid. what follows is pure speculation on my part, but i suspect that the MHRA may have been directed to issue the takedown request in order to be seen to be "doing something" about a hot-button issue. anyone familiar with the history of UK criminal legislation will know that this kind of thing is unfortunately common).
let me be absolutely clear: these are the facts as I understand them at the time of writing, based on discussion with people more knowledgeable than me. i'm not an expert in this or in anything else. i'm writing this because most of the reactions i've seen to this news are people (understandably) freaking out that this is the end of diy forever. i'm concerned that these reactions at a time when our community is already extremely on edge could get people hurt.
as a final note, i would like to encourage everyone (trans or otherwise) to maintain vigilance and discipline; keep diy discussion on a need-to-know basis. do not talk about it whatsoever with outsiders. if you don't need to know, don't ask about it. do not mention identifiable sources out in the open where anyone could see it (this includes tumblr, reddit, imageboards, forums and discord servers). if you're a moderator in any online community, remain vigilant for carelessness or possible intrusion. there's a lot of people who live to torment us and you never know how far your words will spread.
https://x.com/boymoderology/status/1787063776899903915

Hey by the fucking way (tweet linked)
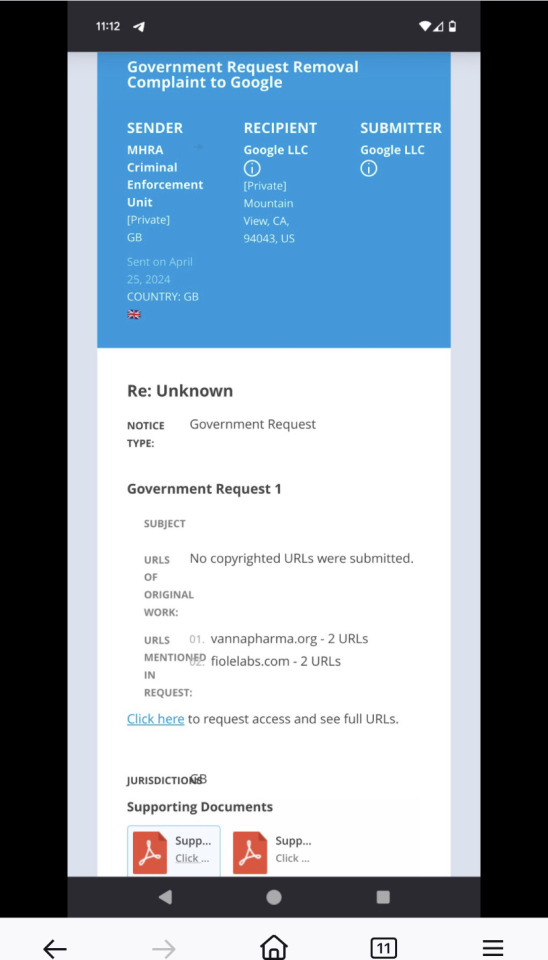
no clue if the guardian directly led to the first sc of course but … .
in lieu of me fearing spreading misinfo re: my other post im trying to be careful of wording
this is a bit alarming tho
4K notes
·
View notes
Text
Addressing Regulatory Observations in the UK
In the ever-evolving landscape of regulatory compliance, businesses in the United Kingdom often find themselves under the watchful eye of various regulatory bodies. Whether you operate in the pharmaceutical, financial, or any other sector, regulatory observations are something you may encounter. Understanding how to address these observations is crucial to maintaining your business's reputation and compliance standing.
What Are Regulatory Observations?
Regulatory observations are formal notices issued by regulatory agencies, such as the Medicines and Healthcare Products Regulatory Agency (MHRA) or the Financial Conduct Authority (FCA) in the UK. These observations are made when an agency identifies non-compliance or shortcomings in a business's operations, processes, or documentation during an inspection or audit.
Importance of Addressing Observations
Ignoring or neglecting regulatory observations can have serious consequences. These may include fines, legal actions, loss of licenses, damage to reputation, and even closure of the business. Therefore, addressing these observations promptly and effectively is paramount.
Steps to Address Regulatory Observations
Acknowledge and Document: The first step is to formally acknowledge and document the observation. This shows regulators that you take their findings seriously. Keep detailed records of the observation, including who was involved, the date, and any supporting evidence.
Root Cause Analysis: Once you have documented the observation, conduct a thorough root cause analysis to determine why the issue occurred. Identifying the underlying cause helps in implementing effective corrective actions.
Develop an Action Plan: Create a comprehensive action plan that outlines the steps you will take to address the observation. This plan should include specific tasks, responsible individuals, timelines, and resources required.
Implement Corrective Actions: Execute the action plan diligently. Ensure that the corrective actions address the root cause of the observation and are in line with regulatory requirements.
Communication: Maintain open and transparent communication with the regulatory agency. Inform them of your action plan and progress regularly. If there are delays or unforeseen challenges, communicate those as well.
Training and Education: Invest in training and education for your staff to prevent similar observations in the future. Ensure that your team is well-informed about the relevant regulations and compliance procedures.
Continuous Monitoring: After implementing corrective actions, continuously monitor and assess their effectiveness. This ongoing evaluation helps ensure that the issue is fully resolved and does not recur.
Documentation of Remediation: Keep thorough records of all remediation efforts. This documentation will be important in demonstrating your commitment to compliance during future regulatory interactions.
In conclusion, regulatory observations are a part of doing business in the UK, but they should not be viewed as insurmountable obstacles. Addressing these observations methodically, with a commitment to compliance, can resolve immediate issues and strengthen your organization's regulatory standing and reputation. By following these steps, businesses in the UK can navigate the regulatory landscape successfully while maintaining their integrity and trustworthiness in the eyes of both regulators and consumers.
Contact: +44(0)1903 366167
Email: [email protected]
Website: https://paulrpalmer.com/
0 notes
Text
Ensuring Authenticity and Quality When Buying Xanax Online in the UK
More people are using online stores to buy a variety of goods as the digital landscape develops, including drugs like Xanax, which is frequently prescribed to treat anxiety and panic disorders. However, purchasing medication online can be problematic, particularly given the possibility of receiving a fake or subpar product. It is critical to exercise caution while buying Xanax online in the UK in order to protect your health and wellbeing. Here are some vital recommendations to make sure your Xanax purchase from onlinesleepingpilluk or any other online source is genuine and of high quality
Check the Website's Legitimacy: Finding out if the website onlinesleepingpilluk is a reliable and trustworthy source is the first step. Look for a real address, a phone number, and client testimonials. Trusted internet pharmacies ought to make their necessary licenses and certificates readily available.

Before Buy Xanax UK or any other prescription drug online, speak with a healthcare professional. A qualified medical professional can evaluate your health and offer guidance on dosage and usage, ensuring that Xanax is safe and suited for you.
Seek Prescription Requirements: Genuine online pharmacies always demand a current prescription from a licensed physician or other competent healthcare provider before distributing Xanax. A website selling Xanax without a prescription should be avoided as this can be a sign of illicit activity.
Look for Quality Assurance: Authentic Xanax must adhere to strict quality requirements. Check to see if the online pharmacy provides information on the manufacturing and source of the drugs they sell. Reputable websites frequently obtain their merchandise from renowned pharmaceutical firms.
Beware of Unbelievable Offers: If a website offers Xanax at a price that is much cheaper than that of other providers, it can be a scam. Drugs that are counterfeit are frequently offered at discounts to entice naïve customers. Give quality and safety a higher priority than low pricing.
In order to protect your financial information, select online pharmacies that provide secure payment methods.
Report strange Activities: Contact the appropriate authorities, such as the UK's Medicines and Healthcare products Regulatory Agency (MHRA), if you notice any strange behavior or think a website is selling Xanax that isn't real.
In conclusion, purchasing Xanax online in the UK can be practical but calls for caution to assure quality and authenticity. Put your health first by checking the website's credibility, talking to a doctor, and learning what prescriptions are needed. You can get Xanax legally and responsibly from "onlinesleepingpilluk" or other online providers by adhering to these procedures.
0 notes
Text
How Much Nicotine is in a Geek Bar? Find Out Here!
After arriving on the scene, disposable vapes are all the rage right now with products such as the Geek Bar proving particularly popular. But after some alarming reports about ‘super strong' disposable vapes emerged in the media recently, lately we've noticed a lot of our customers asking questions like "are Geek Bars too strong?” and "how much nicotine is in a Geek Bar?”. If you're one of those vapers wondering if the Geek Bar nicotine content might be too much to handle, or you're just curious to know what your disposable vape is packing, fear not as we're on-hand with all the answers. What are Geek Bars? Geek Bars are disposable vaping devices that come pre-filled with e-liquid and a pre-charged battery. They are designed to be used once and then disposed of, eliminating the need for refilling or recharging. Geek Bars are available in a variety of flavors and nicotine strengths and are popular among vapers who want a convenient and hassle-free vaping experience. They are also small and discreet, making them easy to carry and use on-the-go. Geek Bars have divided opinion since they arrived on the scene, with a certain section of hardcore vapers unimpressed by a product they see as wasteful and restrictive. Nonetheless, it looks as though disposables are here to stay so let's clear up a few misconceptions about the amount of nicotine they contain. Rules on Nicotine in Vape Devices Without trying to bore you to death, before we get into exactly how much nicotine is in a Geek Bar, it's important to have a basic understanding of the rules and regulations around vape products. In 2016, the Tobacco Products Directive (TPD) was introduced for EU member states, and with that came a raft of brand-new regulation for the manufacturing and sale of tobacco products, as well as e-cigarettes and other vaping products. Since then, for a vape device or e-liquid to be sold in the UK, it must conform to the regulations set out by the TPD, which dictate the following: - How much a vape tank can hold - Maximum strength of nicotine in an e-liquid - All products must be submitted to MHRA before sale - All product must have relevant health warning and info How Much Nicotine is in a Geek Bar? As a result of the rules put in place by the TPD, vape products including disposables are only available in certain strengths and volumes in the UK. As such, in line with the TPD's regulations, the tank in each device must be capable of holding no more than 2ml of e-liquid, while the nicotine strength is also capped at 20mg/ml (20%). The Geek Bar comes in three different variations: the standard Geek Bar, the Geek Bar Lite and the Geek Bar Pro. When we take a look at the standard Geek Bar and the Geek Bar Lite, we can see both of these models are TPD-compliant. The Standard Geek Bar disposable vape contains 2ml of e-liquid with 40mg of nicotine, whilst the Geek Bar Lite comes with 1.8ml e-liquid with the same liquid:nicotine ratio. Why is geek bar so popular? So now we know the Geek Bar nicotine percentage and we can see these disposable devices conform to the rigorous standards of the EU, you might well be asking what all the fuss is about and why the media is printing these scare stories. "Super-strong Geek Bar vape craze causing health scare in kids” and "Health fears over new craze among young people for super-strength nicotine devices” are just some of the headlines published recently, but never ones to let the truth get in the way of a good story we can see the media has taken something out of context and tried to spin it into a juicier story. This is nothing new. Here in the vaping industry we're used to sensationalist publications printing outlandish stories to get clicks. Nevertheless, it's still disappointing to see the media take a product that doesn't comply with EU regulation and hold it up as an example of why Geek Bars are dangerous to people in the UK. Read the full article
0 notes
Text
By Nick Hunt 11 December 2024
I told you here about Pfizer’s abstract of its Interim Report 5, showing at least 23-40% higher risk of some heart-related conditions in the vaccinated, but that the MHRA, the U.K. medicines regulator, was withholding publication of the full report. As I said at the time : “In summary, if, as I suspect, MHRA is worried by the results in Pfizer’s ‘Interim Report 5’ then no wonder it is sitting on it.”
Well, MHRA is still sitting on the report but I’ve managed to obtain a copy. It looks like I was right – the detailed results in the full report are even more worrying than the Hazard Ratios in the abstract which I reported last time (below).
To recap: this is a report of a Post Authorisation Safety Study (PASS) of Pfizer’s Covid vaccine. National regulators routinely require pharmaceutical manufacturers to conduct PASS studies as a condition of authorisation of most new medicines. The regulators provide data to the manufacturer covering millions of patients registered in national healthcare systems. The manufacturer then conducts analysis, matched for things like age and sex, to determine whether the medicine has increased the risk of specified health conditions.
Let’s dive straight in. Below are some heart-related cumulative incidence graphs from Pfizer’s full ‘Interim Report 5’. You will immediately notice that the incidence for each type of condition is significantly greater in the Covid vaccinated (bad) – but we already knew that from the Hazard Ratios in the abstract. What’s worse is that the curves diverge over time, i.e., the relative incidence between vaccinated and unvaccinated increases over the time period of the data in the report (December 8th 2020 – March 21st 2022). I wonder what happened subsequently.
4 notes
·
View notes
Text
Pre-Clinical Research Development for Current Regulatory Requirements
Most likely, even before a medication could be endeavoured in a clinical basic, the improvement coordinated effort of a medication all around fuses three basic advances, including divulgence, preclinical unanticipated turn of events, and sometime later clinical starter that takes place by clinical research training. The headway from openness to preclinical improvement is a steady affiliation. The outcomes got from toxicology and primer pharmacology testing consistently add to competitor choice for a remedy. The account of an Investigational New Drug (IND) sets up a limit between preclinical new turn of events and clinical evaluation of a medication.
The going with generally speaking affiliations guarantee drug security and adequacy:
● World Health Organization (WHO),
● Pharmaceutical Inspection Co-development Scheme (PIC/S),
● International Organization for Standardization (ISO),
● International Conference on Harmonization (ICH),
● Parenteral Drug Association (PDA), and
● International Society for Pharmaceutical Engineering (ISPE).
A portion of the public definitive bodies giving rules for drug progress include:
● European Medicines Evaluation Agency (EMEA), Europe
● Food and Drug Administration (FDA), US
● Regulatory Operations and Enforcement Branch of Health Canada (ROEB), Canada
● Pharmaceuticals and Medical Devices Agency (PMDA), Japan
● Medicines and Healthcare things Regulatory Agency (MHRA), UK
● Brazilian Health Regulatory Agency (ANVISA), Brazil
● Therapeutic Goods Administration (TGA), Australia
● Turkish Medicines and Medical Devices Agency (TMMDA), Turkey
Should clinical groundwork’s be driven locally?
An ordinary solicitation that a significant part of the time rouses a passionate reaction is whether clinical basics are ought to have been composed locally as an imparted or certain condition to get propelling help. Most of nations, including India, require clinical essentials to be driven locally as a pre-condition for getting propelling help. It is done to show the thriving and reasonableness of a remedy or clinical gadget that fulfils the meaning of another medication and investigational clinical contraption, independently.
All things considered, if the investigational clinical gadget has been imported from the United States of America, the United Kingdom, Australia, Japan, Canada, or the European Union, by then the need to lead a clinical key locally can be conceded off if a free game plan affirmation is secured and submitted from the tremendous ward. Learn more about the groundwork’s of clinical research courses.
How are clinical essentials financed?
Despite where the partners are consolidated, clinical basic allies can straightforwardly back a clinical groundwork. The benefactors are in danger of picking a social affair of assessors and a genuine master who by then drives the get-together of trained professionals. The partner of a clinical starter can straightforwardly pay the auditors for their associations. The assistance is in like way allowed to make parcels for the site, additionally picked by the assistance, for the clinical essential for giving in-patient working environments, among different others. If there should arise an occasion of specialists being the site workers, the assistance can make direct bits to the site, which is likewise reallocated to the trained professionals.
Fundamentals for preclinical and clinical groundwork standards
Before fundamentals can be composed on creatures, explore express consent is needed from a selected notice social affair or authority. As composed by various laws supervising preclinical basics across the world, a touch of the central fundamentals unite appraisal thinking, study arrangement, subject capacity, study treatment, and foundation of the main trained professional. By and large, a morals driving assemblage of trustees is comparatively set up to audit and support the clinical principal going before its activities origin. The morals driving gathering of trustees is besides committed for studying and acknowledging any developments or updates made to the clinical preliminary shows going before their execution. Regardless, earlier endorsing from the morals notice gathering isn't needed when the execution of updates is key for patients' thriving and security.
Preclinical appraisal
Before a medication or treatment can be had a go at individuals, clinical specialists ought to guarantee whether it can cause expected risks, results, or confirmed damages, regardless called hurtfulness to the patients. The two sorts of preclinical examination get the going together with:
● In Vitro
● In Vivo
In the US, the FDA demonstrates that specialists should utilize unbelievable investigation office rehearses (GLP), as portrayed in clinical thing improvement rules for preclinical lab considers. The GLP rules set the base significant necessities for:
● study lead
● personnel
● facilities
● equipment
● written shows
● operating procedures
● study reports
It besides builds up a strategy of critical worth demand for each preclinical evaluation to guarantee the success and appropriateness of things upheld and composed by the FDA. By and large, preclinical assessments are not composed on an especially enormous expansion. Notwithstanding, these appraisals should give particular data on dosing and damaging tendency levels. Clinical analysts audit their outcomes after preclinical testing.
Preclinical assessments include different exercises that fill in as a relationship between drug divulgence and status and the commencement of a clinical introduction on people. The laws planning preclinical starter standards and necessities worldwide can bundle separates in any case by and large have some fundamental highlights. Rat and non-rat mammalian models are by and large used to present general success and perceive damaging tendency plans that reveal potential objective organs slanted to endure through the unsavoury impacts. Toxicology and security concentrates additionally see Therapeutic Index for picking the central beginning estimations in clinical groundwork’s. Past what in any occasion one animal social events can be utilized for picking the medication's mean home time in the body.
1 note
·
View note
Text
Addressing Regulatory Observations in the UK
In the ever-evolving landscape of regulatory compliance, businesses in the United Kingdom often find themselves under the watchful eye of various regulatory bodies. Whether you operate in the pharmaceutical, financial, or any other sector, regulatory observations are something you may encounter. Understanding how to address these observations is crucial to maintaining your business's reputation and compliance standing.

What Are Regulatory Observations?
Regulatory observations are formal notices issued by regulatory agencies, such as the Medicines and Healthcare Products Regulatory Agency (MHRA) or the Financial Conduct Authority (FCA) in the UK. These observations are made when an agency identifies non-compliance or shortcomings in a business's operations, processes, or documentation during an inspection or audit.
Importance of Addressing Observations
Ignoring or neglecting regulatory observations can have serious consequences. These may include fines, legal actions, loss of licenses, damage to reputation, and even closure of the business. Therefore, addressing these observations promptly and effectively is paramount.
Steps to Address Regulatory Observations
Acknowledge and Document: The first step is to formally acknowledge and document the observation. This shows regulators that you take their findings seriously. Keep detailed records of the observation, including who was involved, the date, and any supporting evidence.
Root Cause Analysis: Once you have documented the observation, conduct a thorough root cause analysis to determine why the issue occurred. Identifying the underlying cause helps in implementing effective corrective actions.
Develop an Action Plan: Create a comprehensive action plan that outlines the steps you will take to address the observation. This plan should include specific tasks, responsible individuals, timelines, and resources required.
Implement Corrective Actions: Execute the action plan diligently. Ensure that the corrective actions address the root cause of the observation and are in line with regulatory requirements.
Communication: Maintain open and transparent communication with the regulatory agency. Inform them of your action plan and progress regularly. If there are delays or unforeseen challenges, communicate those as well.
Training and Education: Invest in training and education for your staff to prevent similar observations in the future. Ensure that your team is well-informed about the relevant regulations and compliance procedures.
Continuous Monitoring: After implementing corrective actions, continuously monitor and assess their effectiveness. This ongoing evaluation helps ensure that the issue is fully resolved and does not recur.
Documentation of Remediation: Keep thorough records of all remediation efforts. This documentation will be important in demonstrating your commitment to compliance during future regulatory interactions.
In conclusion, regulatory observations are a part of doing business in the UK, but they should not be viewed as insurmountable obstacles. Addressing these observations methodically, with a commitment to compliance, can resolve immediate issues and strengthen your organization's regulatory standing and reputation. By following these steps, businesses in the UK can navigate the regulatory landscape successfully while maintaining their integrity and trustworthiness in the eyes of both regulators and consumers.
Contact: +44(0)1903 366167
Email: [email protected]
Website: https://paulrpalmer.com/
0 notes
Text
PUB’s Three Notice Process To Stop Employers Mandating Vaxxes.Notice of Conditional Acceptance
Dear Sir/Madam,
RE: EMPLOYER VACCINE POLICY.
In relation to UK Government COVID-19 ‘Vaccine’ Policy, under the protection of the People’s Union of Britain, you are hereby served notice that I conditionally accept that you have the right to mandate COVID-19 ‘vaccinations’ for all your staff, provided you deliver to me the following:
1) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that the COVID ‘vaccines’ are incapable of harming me. 2) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that the COVID ‘vaccines’ have undergone rigorous double-blind placebo safety studies. 3) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that I will not die, suffer or develop any adverse reactions including, but not limited to, neurological problems, blood clots, blindness, nerve damage, deafness, autoimmune disease, anaphylaxis, anaphylactoid reactions, allergies, fertility complications, Guillain-Barré Syndrome, etc and/or suffer any other form of harm, complication, or die as a result of or because of being injected with any COVID-19 ‘vaccine’. 4) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that the COVID ‘vaccines’ approved for emergency use by the MHRA provide immunity from either SARS-COV-2 or COVID-19. 5) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that the employer has sought legal advice on whether it is lawful to mandate the ‘vaccination’ of your staff and that you have performed an appropriate risk assessment. 6) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that you have employed the Precautionary Principle when deciding whether or not to ‘vaccinate’ staff at the employer. 7) Material evidence, not hearsay or opinion, which proves beyond reasonable doubt that you have informed your public indemnity insurers if there is any possibility that serious or fatal ‘vaccine’ adverse events might ensue upon ‘vaccination’ of myself or any other staff, in which case you would be liable for gross negligence and perhaps even manslaughter.
Please deliver to me these reasonably requested items within seven days of your receipt of this notice, given the seriousness of the matters raised and the apparent imminence of the employer adhering to the UK Government policy of mandating the COVID ‘vaccines’ for all UK employer staff.
I look forward to hearing from you without delay in signed writing.
In sincerity and honour, without ill will, frivolity or vexation,
NAME OF EMPLOYEE Trustee of People’s Union of Britain All Rights Reserved under the Treaty of Universal Community Trust Errors & Omissions Excepted
https://www.thebernician.net/pubs-three-notice-process-to-stop-employers-mandating-vaxxes/
0 notes
Text
Homerton Covid patient died after being left without oxygen for over 20 minutes
The death of a patient at Homerton Hospital has prompted a warning from a coroner that alarms on certain medical machines sound the same, making it harder for staff to discover what is wrong.
Inner North London’s senior coroner Mary Hassell sent a prevention of future deaths notice to the head of patient safety at NHS England and the Medicines and Healthcare products Regulatory Agency (MHRA) detailing her concerns.
She said alarms on a machine sound the same irrespective of whether the problem is serious or not. รับเงินฟรี
0 notes