#Enteric Disease Testing Industry
Explore tagged Tumblr posts
Text
Future of Enteric Disease Testing Market: Trends and Predictions
The global enteric disease testing market was valued at USD 3.81 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 2.7% from 2023 to 2030. The key drivers for this growth include technological advancements, increased government funding, and growing global demand for early diagnostic solutions. These factors are enhancing the applicability of enteric disease testing, contributing to the expansion of the market.
Technological advancements play a crucial role in accelerating the market's growth. One notable example is the development of gastrointestinal panel tests. These tests enable the rapid and simultaneous identification of multiple parasitic, bacterial, and viral nucleic acids in individuals who exhibit signs of gastroenteritis or infectious colitis. The tests, which are typically performed on stool or saliva samples, can identify up to 15 pathogens within just 6 hours, making them highly efficient. Additionally, these tests can be used to detect inherited food sensitivities or allergies caused by organisms such as Toxoplasma gondii and Entamoeba histolytica, which are known to affect the gastrointestinal system.
These advanced diagnostic tools have already been approved by regulatory bodies like the U.S. FDA, further boosting their credibility and adoption. For instance, the XTAG Gastrointestinal (GPP) test, approved in November 2019, is a multiplexed nucleic acid test designed to detect a range of viral, parasitic, and bacterial pathogens in stool samples. As clinical studies continue to advance and more diagnostic tests with higher accuracy and performance are developed, the market is expected to experience significant growth.
Gather more insights about the market drivers, restrains and growth of the Enteric Disease Testing market
Regional Insights
Europe
Europe held the dominant share of the global enteric disease testing market, accounting for 39.0% of the total revenue in 2022. The region's leadership can largely be attributed to Germany, which holds a significant portion of the market. Germany's large geriatric population plays a pivotal role in driving the demand for enteric disease testing. According to the United Nations population data, one in every 20 individuals in Germany is over the age of 80, and this proportion is expected to increase to one in every 6 people by 2050. Additionally, individuals over the age of 65 made up 22.4% of the total population in 2019, amounting to 90.4 million elderly people in Europe.
The aging population presents unique challenges, including an increased susceptibility to enteric diseases, due to the aging process and declining immunity in older adults. As these individuals face a higher risk of infection, the need for rapid diagnostic solutions like point-of-care diagnostic devices becomes crucial. These diagnostic devices allow for quicker detection and treatment, reducing the risks associated with delayed diagnosis. To meet this demand, major market players, such as Roche and Bio-Rad, are strengthening their presence in Germany, which is expected to further boost the market for enteric disease testing in Europe.
Asia Pacific
The Asia Pacific region is anticipated to grow at the fastest compound annual growth rate (CAGR) of 4.0% from 2023 to 2030. This growth is primarily fueled by increasing awareness about the importance of early testing and the rising incidence of enteric infections. Developing nations like China and India are expected to be key drivers of this growth due to their large populations, rapidly expanding healthcare sectors, and government efforts to increase access to diagnostic services.
In particular, Bangladesh reports the highest number of typhoid cases globally, with 252 cases per 100,000 people, highlighting the growing need for efficient enteric disease testing in the region. As the prevalence of these diseases rises, there is a significant demand for improved diagnostic tools and testing capabilities. The increasing awareness among the population about the benefits of early detection will likely contribute to the rapid growth of the market in Asia Pacific.
Brazil
Brazil presents significant opportunities for growth in the enteric disease testing market, supported by economic growth and political stability. Recent improvements in the Brazilian economy have led to better healthcare access, creating a favorable environment for the expansion of diagnostic services.
One key development in Brazil is the Unified Health System (SUS), which provides free healthcare services to all individuals, regardless of their financial status. The SUS system integrates public, private, and supplemental healthcare services, making healthcare more accessible to a larger portion of the population.
Additionally, government initiatives such as collaborations between the U.S. and Brazil in biomedical research are accelerating the research and development of diagnostic technologies for infectious diseases. These efforts are expected to further boost the demand for enteric disease testing in the country. However, challenges remain, as advanced diagnostics are currently more accessible in urban areas due to limited healthcare infrastructure in rural regions, which could potentially slow down overall market growth.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global medical simulation market size was estimated at USD 1.65 billion in 2024 and is expected to grow at a CAGR of 16.80% from 2025 to 2030.
• The global intraoperative neuromonitoring market size was estimated at USD 3.49 billion in 2024 and is anticipated to grow at a CAGR of 5.97% over the forecast period.
Key Companies & Market Share Insights
The enteric disease testing market is highly competitive, with key players focusing on technological advancements and new product development to enhance their market position and offer cost-effective solutions. Several major players have made strategic moves to strengthen their presence in this market.• BD (Becton, Dickinson and Company) launched an ultra-high-throughput molecular diagnostic platform in May 2022 for infectious diseases, specifically in the U.S. This fully automated system is designed to improve diagnostic accuracy and speed, catering to the growing demand for point-of-care testing.
• Thermo Fisher Scientific, Inc. made a significant move in February 2021 by acquiring Mesa Biotech, aiming to expand the reach of molecular diagnostics at the point of care. This acquisition aligns with the company's strategy to strengthen its position in the infectious disease diagnostic space.
Key Enteric Disease Testing Companies
Some of the major players contributing to the global enteric disease testing market include:
• Abbott
• BD (Becton, Dickinson and Company)
• Biomerica
• BIOMÉRIEUX
• Bio-Rad Laboratories, Inc.
• Cepheid
• Coris BioConcept
• DiaSorin S.p.A.
• Meridian Bioscience
• Quest Diagnostics Incorporated
Order a free sample PDF of the Enteric Disease Testing Market Intelligence Study, published by Grand View Research.
#Enteric Disease Testing Market#Enteric Disease Testing Market Analysis#Enteric Disease Testing Market Report#Enteric Disease Testing Industry#Enteric Disease Testing Market Regional Insights
0 notes
Text
The Business Research Company offers enteric disease testing market research report 2023 with industry size, share, segments and market growth
#enteric disease testing market#enteric disease testing market share#global enteric disease testing market#enteric disease testing market report#enteric disease testing market size#enteric disease testing market outlook#enteric disease testing market growth#global enteric disease testing market size#enteric disease testing market analysis#enteric disease testing market trends#enteric disease testing industry analysis#enteric disease testing industry report#enteric disease testing market insights
0 notes
Text
EXTREMELY IMPORTANT:
Democrats VOTED DOWN a bill that would have forced all meat products to clearly be label if injected mRNA vaccines “mRNA vaccines are entering our food chain in several species.
So currently all farmed shrimp are being vaccinated with mRNA. Currently pork is an unknown amount is being vaccinated with mRNA.”
“Some of the companies state on their advertising, we put the right gene in your livestock vaccine. So they have vaccines going into the swine industry. We don't have data on how much of it's being sold and where and who are the buyers.”
“A customized vaccines where they go on to a farm, they take samples of the of the diseases on the farm, then go into the lab and a few days later they produce an mRNA vaccine for your customized to your farm's diseases. There's no testing, no research, nothing.” - John Graff, a nutritionist, chemist, process engineer, agribusiness executive, and a board director of the National Citizen’s Inquiry Speaking of impact taking place in Canada right now.
Know Democrats shut down the bill requiring labeling here in America, so we are next. This is extremely, extremely dangerous.
15 notes
·
View notes
Text

The Ultimate At-Home Test That Will Make You Question Everything: Healthcare Practices that are Making Us Sicker?
If you have blackheads and/or acne, cover the area in some generic triple antibiotic ointment or neosporin from the store. Do this every night for at minimum at least a week.
You will notice: it’s actually one of the most effective antiacne treatments you’ve ever put on your face.
We have an entire huge multibillion dollar industry in the United States for skincare & one of the leading product sellers is antiacne products. If you’ve tried this at home test you’d know neosporin is probably the least harsh on your skin and most effective antiacne treatment you can get. Yet, billions are spent yearly to advertise less effective treatments.
Putting triple antibiotic cream inside of the nose (it doesn’t have to go up far into the nose, less than 1/4th of an inch) is also a relatively effective treatment for preventing yourself from getting sick from a lot of different diseases.
https://time.com/6971392/neosporin-covid-19-antiviral-study/
When bacteria gets on your skin, often times they are able to create groups of bacteria or colonies that are able to multiply and get stronger day after day they are able to survive ontop of the skin. Your skin has multiple protective mechanisms to prevent infection and colonization, but not in the same way the inside of your body does. Your immune system on the inside of your body is able to effectively mount a defense to prevent most bacteria from being able to stay alive and colonize or live on your skin. So, every day bacteria sit on your skin and multiply while the basic antibacterial defenses of the skin (like sweat) kill off only the weakest of the bacteria.
When colonies are not killed off fully and the weakest bacteria are wiped out from the gene pool, over time their genetic line will get stronger.
This is a major problem. One of the hardest advantages for bacteria to develop is surging the processes of going from the outside of the body to the inside. Pathogens often die during this process. This gives bacteria the ability to continually reproduce on your skin and everything weaker that attempts to enter the body will die. As the weak die off this will cause a process that eventually creates a stronger family of bacteria. As the stronger and stronger bacteria continue to breed ontop of the skin they will eventually develop advantages to survive and get inside of the body alive.
Although antiacne treatments available over the counter primarily use non antibiotic ingredients, our current practices heavily contribute to making pathogens stronger in the same way overuse of antibiotics does.
These antiacne products wipe out few bacteria and they are usually the least resistant bacteria on the skin.
Areas of the body with significant exposure to pathogens AND that experience colonization historically seem to be associated with the development of more serious and antibiotic resistant diseases like MRSA and CDIFF. This is in comparison to glandular organs near the entrance to the oropharynx (such as the various tonsils located throughout that area) that also experience a high exposure to pathogens, but due to the immune response of these organs they are able to kill off more & stronger bacteria. So, killing off stronger and higher amount of bacteria in the body does seem to have a corresponding relationship with an organ having a less likely chance of being colonized by an antibiotic resistant organism.
The ability to adapt to living outside the body to inside the body is a VERY challenging and resource intensive task for diseases. The ability to have this free & unlimited supply of skin to constantly reproduce & allow bacteria a free zone for trial and error until they develop the ideal characteristics to invade the body is a high risk activity.
This line of thinking also corresponds to why practices such as frequent hand washing & taking diluted bleach baths have been employed in the past to reduce disease in communities.
Ultimately, mindfulness & skepticism towards what we are told in all areas is key. Many practices commonly used even today will make us sicker over time if we do not recognize how important our role is in advocating for ourselves and our communities in all sectors of life.
#meds#medication#medical#doctor#nursing#hospital#chronic fatigue#chronically ill#chronic pain#chronic illness#sickness#sick#tired#exhausted
2 notes
·
View notes
Text
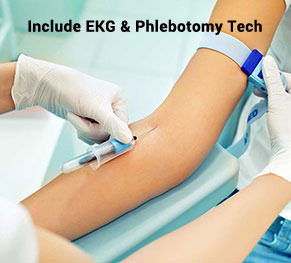
Encouraging Careers: EKG Tech Training in Queens and Islandia, NY
Encouraging Careers: EKG Tech Training in Queens and Islandia, NY
EKG technicians are vital to the ever-changing field of healthcare since they provide vital heart care services. Specialized training programs provided by Access Careers can help aspiring EKG technicians in Islandia, NY, and Queens launch their careers. We discuss the value of EKG technician training, Access Careers' life-changing experience, and how people can pursue a rewarding career in cardiac care in this blog article.
The Relevance of Training for EKG Technicians
1. Vital Cardiac Monitoring Skills:
EKG technicians are trained to perform electrocardiogram (EKG) tests, which are essential for diagnosing heart conditions.
Training equips individuals with the skills to accurately monitor and interpret cardiac activity.
2. Contribution to Patient Care:
EKG technicians play a vital role in patient care by assisting healthcare providers in diagnosing and treating cardiac conditions.
Their expertise ensures timely and accurate assessment of cardiac health, contributing to improved patient outcomes.
3. Career Stability and Growth:
The demand for EKG technicians is expected to grow as the population ages and cardiovascular diseases become more prevalent.
Training provides individuals with valuable skills that open doors to stable and rewarding career opportunities.
4. Specialized Training Programs:
EKG technician training programs are designed to provide specialized instruction in cardiac monitoring techniques.
Graduates are well-prepared to enter the workforce with the knowledge and confidence needed to excel in their roles.
The Training Experience at Access Careers
1. Comprehensive Curriculum:
Access Careers offers a comprehensive curriculum that covers both theoretical knowledge and practical skills training.
Students gain a thorough understanding of EKG procedures, cardiac anatomy, and interpretation of EKG results.
2. Hands-On Learning Opportunities:
Practical training is a key component of the program, with hands-on learning opportunities in simulated clinical settings.
Students practice performing EKG tests under the guidance of experienced instructors, ensuring proficiency in their skills.
3. Experienced Faculty:
Training is led by experienced faculty members who bring a wealth of knowledge and expertise to the classroom.
Instructors at Access Careers are committed to providing personalized instruction and support to each student.
4. Career Preparation and Placement Assistance:
Access Careers goes beyond training by offering career preparation and placement assistance to graduates.
Students receive guidance on resume building, interview skills, and job search strategies to help them launch successful careers.
Why Go for Access Careers for Training as an EKG Technician?
1. Industry-Driven Curriculum:
Access Careers designs its EKG technician training program based on industry standards and best practices.
Graduates are well-prepared to meet the demands of the healthcare job market.
2. State-of-the-Art Facilities:
Access Careers provides state-of-the-art facilities equipped with modern technology and resources.
Students have access to the latest EKG equipment and software to enhance their learning experience.
3. Supportive Learning Environment:
Access Careers fosters a supportive and collaborative learning environment where students can thrive.
The institution prioritizes student success and provides the resources and support needed to excel in training.
4. Convenient Locations:
With campuses in Islandia, NY, and Queens, Access Careers offers convenient locations for individuals seeking EKG technician training.
Students can choose the location that best fits their needs and preferences.
With Access Careers, Begin Your EKG Technician Career
Training as an EKG technician at Access Careers can be your first step toward a rewarding profession if you're driven to make a difference in the heart care industry. Examine the available training options and start your journey to become a knowledgeable and kind healthcare provider in Islandia, New York, or Queens.

Contact us
Hempstead Campus
🏢: 474 Fulton Avenue, Suite 201 Hempstead, NY 11550
📞: (516)-433-0034
Islandia Campus
🏢: 1930 Veterans Highway, Suite 10 Islandia, NY 11749
📞: (631)630-9410
🌐 : https://accesscareers.net/
#EKG Technician Training Islandia NY#EKG Technician Training Queens#Islandia NY Pharmacy Tech Training#Medical Assistant Training Islandia NY
2 notes
·
View notes
Text
COVID – 19 & ROBOTICS
Research
Robots in the Hospitality Industry
Part of the reason why robots have emerged as a popular technology trend within the hospitality industry is because ideas of automation and self-service are playing an increasingly vital role in the customer experience. The use of robots can lead to improvements in terms of speed, cost-effectiveness and even accuracy.
For example, chatbots allow a hotel or travel company to provide 24/7 support through online chat or instant messaging services, even when staff would be unavailable, delivering extremely swift response times. Meanwhile, a robot used during check-in can speed up the entire process, reducing congestion.
Research References
•https://www.revfine.com/robots-hospitality-industry/
•https://www.youtube.com/watch?v=GOO_wPI2J8o
•https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8675561/
•https://www.smithsonianmag.com/innovation/how-robots-are-on-front-lines-battle-against-covid-19-180974720/
•https://www.usnews.com/news/healthiest-communities/articles/2020-05-12/this-time-theyre-on-our-side-meet-the-robots-confronting-coronavirus
•https://www.weforum.org/agenda/2020/05/robots-coronavirus-crisis/
•https://spectrum.ieee.org/how-robots-became-essential-workers-in-the-covid19-response#toggle-gdpr
Story
A school student studying at James Albert College, when he entered the grade 10, the Covid 19 virus spread rapidly throughout the country. James was only able to move into the new grade for two weeks. Schools across the country began to close. Suddenly, James' father fell ill. Later it was discovered that the disease was the Covid 19 virus. Hospitals across the country were left full of people. James's father was unable to stay in the hospital. He cannot even come close to treating his father, so he creates a robot to treat his father.
Story Board

Test Short


Character Running
Challengers
• Krita is new for me so I'm still learning it while doing this project.
•Lack of knowledge about software to execute my ideas.
Final video
2 notes
·
View notes
Text
youtube
Bond University Physiotherapy Students Work with World Surf League
As the official higher education partner of the World Surf League (WSL), Bond University can offer its physiotherapy students unmatched opportunities in one of the most exciting industries.
Every semester, Bond students have access to internship opportunities across all facets of the organization, including sport management, sports science, event management, marketing and film and television. Bond academics and researchers also have opportunities to engage with the WSL Australia in the work and research.
For more than 20 years, the WSL hosts a yearly world surf league championship at Snapper Rocks on the Gold Coast, the world’s most famous right-hand sandy point break and a favourite location for the world’s best surfers. This event is so popular that it sees approximately 5,000–10,000 spectators on the beach—daily!
This year, the event will take place May 6–13. Surfers in this competition compete for a qualifying position on the 2024 WSL Championship Tour.
Bond Physiotherapy Practical Experience
This year, Bond physiotherapy students, academics, and Bond’s Water Based Research Unit (WBRU), will be holding surfer health checks for members of the public attending the event in May. These health checks will be available to surfers and spectators, and will provide as Bond DPT students with the opportunity to shadow their academics in the field and take in a world-class sports tournament.
The Bond physio students and academics will measure a participants’ blood pressure, ankle hip and shoulder strength, motion and flexibility, ear health, and body composition. The data collected from these checks will be entered into the WBRU database of both pro and recreational surfers, with participants receiving a report on how their results compare and outlining suggested areas they may like to work on or address with their health professionals of choice.
The fitness data collected will be compared to professional surfers such as Mick Fanning (Australian), Kelly Slater (American) and Stephanie Gilmore (Australian).
They will also measure strength and flexibility, and will conduct a bio-impedance analysis, which is great practical experience for physio students to get hands-on experience.
Assistant Professor of Physiotherapy James Furness overseas students during this practical experience. He has a background in musculoskeletal physiotherapy and he conducts innovative and novel research in the sports of surfing, stand-up paddle boarding, and swimming.
Since 2020, Bond University students in the Faculty of Health Sciences & Medicine and Bond Business School have enjoyed exciting internships with WSL.
Bond University Doctor of Physiotherapy
The two-year Doctor of Physiotherapy at Bond University is an accelerated degree—three semesters per year instead of just two. This means you can graduate a year ahead of the rest.
Boasting 36 weeks of clinical placements (well over 1200 hours), Bond has highest placement hours in Australia and a strong emphasis on clinical experiential learning. Placements are sought in a variety of settings across the full spectrum of clinical areas including pediatrics, orthopedics, cardiorespiratory, musculoskeletal, neurological, ortho-geriatric, rehabilitation, chronic disease, disability, occupational, and sports practice—like world surf league championship!
Practical experience is also built into theoretical learning, with classes primarily delivered at the Bond Institute of Health and Sport. At this world-class testing and training facility, you’ll work with elite athletes and professional sporting teams using the most advanced testing equipment available!
Program: Doctor of Physiotherapy (DPT) Location: Gold Coast, Queensland Intake: May each year Duration: 2 years
#bond university#bond dpt#doctor of physiotherapy#world surf league#physiotherapy students#study physiotherapy in australia#study in australia#Youtube
3 notes
·
View notes
Text
What is the Latest Technology in Optometry?
In the fast-evolving field of optometry, technology has become a game-changer, revolutionizing how eye care services are delivered and enhancing accuracy in diagnosis and treatment. From artificial intelligence (AI) to virtual vision care services, the latest advancements have brought convenience, precision, and accessibility to patients worldwide. If you're exploring ways to stay updated or improve your practice, this blog will delve into the top innovations in optometry that are shaping the industry.
1. AI-Based Eye Testing Tools
Artificial intelligence has made its way into optometry, enabling precise diagnosis and personalized treatment plans. AI-based eye testing tools analyze optical data with unmatched speed and accuracy. These tools are trained to detect early signs of eye diseases like glaucoma, diabetic retinopathy, and macular degeneration, ensuring timely intervention. Keywords: AI-based eye testing tools, Optical data analysis software
2. Vision Test Online with Snellen Chart
Traditional vision tests have taken a digital leap with the Snellen chart available for online vision testing. With just a few clicks, patients can now assess their vision from the comfort of their homes. These online tools maintain accuracy while being highly user-friendly, providing quick results that can help users decide whether to consult an optometrist. Keywords: Vision test online with Snellen chart, Eye chart test on phone
3. Eye Testing Apps for Smartphones
Smartphones have become powerful tools in modern optometry. Eye testing apps allow users to conduct basic vision tests, measure visual acuity, and track eye health over time. These apps are particularly beneficial for people in remote areas who might not have access to eye care clinics. Such innovations also allow optometrists to monitor patients' progress remotely. Keywords: Eye testing apps for smartphones, Virtual vision care services
4. Virtual Vision Care Services
Telemedicine has expanded into optometry with virtual vision care services, making consultations accessible regardless of location. Patients can now connect with certified optometrists via video calls for preliminary eye exams, follow-ups, and guidance. These services reduce wait times and improve accessibility, especially in rural or underserved areas. Keywords: Virtual vision care services, Eye care clinic near me
5. Optical Data Analysis Software
Advanced optical data analysis software plays a pivotal role in improving diagnosis and treatment plans. These tools help optometrists process large volumes of patient data, offering valuable insights that support clinical decisions. This technology reduces the margin of error, ultimately leading to better patient outcomes. Keywords: Optical data analysis software, Optical charting tools
The Future of Optometry is Here
The latest technologies in optometry are not just innovations; they are transforming the way optometrists and patients interact. Whether through AI-based tools, smartphone apps, or virtual consultations, these advancements make eye care more efficient, accessible, and reliable.
As optometry embraces the digital age, tools like Optic Chart (https://opticchart.com/) provide comprehensive solutions for professionals and patients alike. By integrating these cutting-edge technologies into your practice or daily life, you can stay ahead of the curve while ensuring the best care for your eyes.
Conclusion
Optometry has entered a golden age of innovation, with technology breaking barriers and offering solutions that were once unimaginable. Whether you're an eye care professional or a patient, understanding and utilizing these advancements can significantly improve outcomes.
Stay updated, stay connected, and let your eyes experience the future of optometry today!
#eye tester software#artificial intelligence#eye care#optical data analysis software#Eye care prescription chart#Eye sight check at home#Snellen chart vision test
0 notes
Link
0 notes
Text
Emerging Technologies in the Enteric Disease Testing Market
The global enteric disease testing market was valued at approximately USD 3.81 billion in 2022 and is projected to experience growth at a compound annual growth rate (CAGR) of 2.7% from 2023 to 2030. Key drivers behind this market expansion include ongoing technological advancements and supportive government funding, particularly in emerging economies. Furthermore, there is an increasing global demand for early diagnostic solutions, which is expected to enhance the relevance and applicability of this market, further fueling its growth.
During the COVID-19 pandemic, there was heightened awareness surrounding viral and bacterial infections, prompting individuals to prioritize the consumption of home-cooked and hygienic food to mitigate the risk of infections. As a result, there was a noticeable decline in the incidence of enteric diseases, which led to a subsequent decrease in the demand for enteric disease testing during 2020 and 2021. Additionally, testing for COVID-19 took precedence during the pandemic, overshadowing testing for other infectious diseases. This shift negatively impacted the demand for enteric disease testing products, which in turn hindered overall market growth.
In various regions, particularly Asia, Africa, and Latin America, the prevalence of enteric infections such as salmonella, campylobacter, Escherichia coli, and norovirus remains alarmingly high. According to the National Library of Medicine, as of 2018, enteric fever in these regions was associated with a case fatality rate of approximately 1%. Consequently, there is a growing demand for enteric disease testing to facilitate accurate and timely diagnoses.
Gather more insights about the market drivers, restrains and growth of the Enteric Disease Testing Market
Initial symptoms linked to enteric diseases often include abdominal cramps, vomiting, nausea, and anorexia. If left untreated, these symptoms can escalate into severe conditions characterized by significant fluid and nutrient loss from the body. Vulnerable populations, particularly children and the elderly, are particularly susceptible to these diseases due to their compromised immune systems. According to the Bill and Melinda Gates Foundation, approximately 500,000 young children under the age of 5 succumb to enteric and diarrheal diseases each year, primarily in developing countries. Furthermore, an estimated 25,000 children lose their lives to enteric fever annually, with the majority of cases occurring in regions lacking access to safe water and adequate sanitation facilities.
The mild initial symptoms of enteric diseases can often be overlooked, posing a significant barrier to effective market growth. These diseases have emerged as a leading cause of morbidity and mortality worldwide, especially in developing nations where drainage and sanitation infrastructure is insufficient. It is estimated that developing countries contribute to over 85% of total enteric infection cases in terms of incidence and epidemiology.
As the incidence of enteric diseases continues to rise, coupled with the cost-effectiveness of advanced diagnostic systems, the demand for early diagnosis is expected to increase. For example, according to the Centers for Disease Control and Prevention, salmonella bacteria are responsible for approximately 1.35 million infections, around 26,500 hospitalizations, and roughly 420 deaths each year in the United States. The increasing prevalence of enteric diseases in developing economies, combined with inadequate laboratory settings and limited public awareness, represents a largely untapped market. According to Foundation Merieux, enteric diseases rank as the second leading cause of death among children under 5 years old in Asian and African countries.
Order a free sample PDF of the Enteric Disease Testing Market Intelligence Study, published by Grand View Research.
#Enteric Disease Testing Market#Enteric Disease Testing Market Analysis#Enteric Disease Testing Market Report#Enteric Disease Testing Industry
0 notes
Text
Opening Career Opportunities: A Comprehensive Guide to Drawing Blood Jobs & Pathways in Healthcare
Unlocking Career opportunities: A Comprehensive Guide to Drawing Blood Jobs & Pathways in Healthcare
In today’s fast-paced healthcare environment, careers related to drawing blood are gaining popularity. These positions provide essential services that support diagnostic processes adn patient care. This guide aims to inform you about the various career paths available, the skills required, and practical tips to help you unlock these opportunities.
Understanding the Role of Phlebotomy in Healthcare
Phlebotomy is a critical procedure in the healthcare field involving the collection of blood samples for diagnostic testing. This process is vital for disease detection and managing patient care. In this vrey way, phlebotomists play a crucial role in the overall healthcare system.
Core Responsibilities of a Phlebotomist
Collect blood samples from patients using proper techniques.
Prepare and label blood samples for laboratory analysis.
ensure patient comfort and safety during the blood collection process.
Maintain and manage laboratory equipment and materials.
Keep accurate records of procedures and samples.
Educational Pathways to Becoming a Phlebotomist
To pursue a career in phlebotomy, you need the right education and training. Here are the typical steps along that pathway:
1. Obtain a High School Diploma or GED
Before entering a phlebotomy program, candidates should have a high school diploma or an equivalent qualification.
2. Enroll in a Phlebotomy Training Program
Phlebotomy training programs typically last from a few months to a year.These programs can be found in:
Community colleges
Vocational schools
Universities
3. Obtain Certification
While certification is not always mandatory, it is indeed highly recommended. Relevant certifications can be obtained from organizations like:
American Society for Clinical Pathology (ASCP)
National Phlebotomy Association (NPA)
American Medical Technologists (AMT)
4. Gain Practical Experience
Most programs include an externship component that provides hands-on experience under the supervision of experienced professionals.
Benefits of Working in Phlebotomy
Job Demand: The healthcare industry continues to grow, leading to high demand for skilled phlebotomists.
Flexible Hours: Many phlebotomy jobs offer flexible scheduling shifts, increasing work-life balance.
Career Advancement: Phlebotomists can advance to other medical technician roles or pursue specialties in laboratory work.
Personal Fulfillment: Contributing to patient care and impacting lives positively is among the most rewarding aspects of the job.
Practical Tips for Aspiring Phlebotomists
1. Stay Updated on Techniques
Continuously learn about new techniques and technologies in blood collection to maintain professionalism and efficiency.
2. develop Good Communication Skills
Building rapport with patients can enhance their experience, ensuring they feel comfortable during the procedure.
3.Practice Proper Hygiene
Following hygiene protocols is critical to ensure patient safety and prevent contamination.
Case Studies: Successful phlebotomists in Action
Phlebotomist
location
Success Story
Jane Doe
Community Hospital
Implemented a new blood collection protocol that reduced patient anxiety.
john Smith
City Clinics
Led a training program for new phlebotomists focusing on patient communication.
First-Hand Experience: A Day in the Life of a Phlebotomist
To illustrate the realities of working as a phlebotomist, here’s a brief account from Kelly, a certified phlebotomist with five years of experience:
“Every day is different! I start by reviewing the patient list and preparing my equipment. I ensure I create a comfortable atmosphere for my patients. Many are anxious about needles, so taking the time to reassure them makes a big difference.I love that I get to interact with people and play a key role in their healthcare journey. Though it can be challenging, seeing my work contribute to their diagnosis and treatment brings me great satisfaction.”
Career Outlook and Opportunities
the career outlook for phlebotomists is robust, with healthcare industry projections showing a growth rate of 17% from 2022 to 2032, which is much faster than average for all occupations. Below are some potential employers and job settings:
Hospitals
Private laboratories
Blood donation centers
Physician offices
Conclusion
Drawing blood jobs, especially phlebotomy, offer enriching career opportunities for those passionate about healthcare. By following the outlined pathways, obtaining necessary certifications, and continuously honing your skills, you can unlock a fulfilling career that contributes considerably to patient well-being. Whether you’re just starting or considering a career change, the phlebotomy field promises growth, stability, and the chance to make a real difference in the lives of others.
youtube
https://phlebotomyschoolsonline.org/opening-career-opportunities-a-comprehensive-guide-to-drawing-blood-jobs-pathways-in-healthcare/
0 notes
Text
Open Your Future: Exciting Career Opportunities in Phlebotomy
Unlock Your future: Exciting Career Opportunities in Phlebotomy
If you’re looking for a rewarding career in the healthcare field, phlebotomy might potentially be the perfect fit for you. With the growing demand for healthcare professionals, the role of a phlebotomist has become increasingly vital. This article will explore the exciting career opportunities in phlebotomy, benefits, practical tips for success, real-world experiences, and everything you need to know to unlock your future!
What is Phlebotomy?
Phlebotomy is the practice of drawing blood from patients for various diagnostic and therapeutic purposes. It plays a central role in healthcare by enabling medical professionals to conduct tests that are crucial for diagnosing diseases, monitoring health conditions, and conducting research.
Why Choose a Career in Phlebotomy?
There are numerous reasons to consider a career in phlebotomy:
Job Demand: The healthcare industry is expanding rapidly, increasing the demand for skilled phlebotomists.
Short Training Period: Phlebotomy training programs typically last just a few months, allowing you to enter the workforce quickly.
Variety of Work Settings: Phlebotomists can work in hospitals, laboratories, outpatient facilities, blood donation centers, and more.
Patient Interaction: If you enjoy working with people, phlebotomy offers plenty of opportunities to interact with patients.
Good Earning Potential: While starting salaries may vary, experienced phlebotomists can earn a competitive wage.
Career Opportunities in Phlebotomy
Phlebotomy offers a variety of career paths, including:
1. Clinical Phlebotomist
As a clinical phlebotomist, you’ll perform blood draws in hospitals and clinics, frequently enough working directly with patients and healthcare teams.
2.donor Center Phlebotomist
These phlebotomists work in blood donation centers, collecting blood donations from volunteers and ensuring donor safety.
3. Mobile Phlebotomist
Mobile phlebotomists visit patients at home or work, offering convenience and personalized service, particularly for those who may have difficulty traveling.
4.Phlebotomy Trainer
With experience, you coudl teach others the skills needed to become a accomplished phlebotomist, working in vocational schools or training centers.
5. Specialty Phlebotomist
Some phlebotomists specialize in areas like pediatric phlebotomy, working primarily with children, or geriatric phlebotomy, focusing on elderly patients.
Qualifications and Training Required
To become a phlebotomist, you typically need:
A high school diploma or equivalent.
Completion of a phlebotomy training program (often including clinical experience).
Optional certification from recognized organizations like the American Society of Phlebotomy Technicians (ASPT) or the National Phlebotomy Association (NPA).
Benefits of a Career in Phlebotomy
Beyond job opportunities and training, the phlebotomy profession offers various benefits:
Flexible Hours: Many phlebotomists enjoy varying shifts, which can accommodate different lifestyle needs.
Healthcare Benefits: Many full-time positions provide excellent healthcare benefits.
Personal Fulfillment: Contributing to patient care and improving health outcomes can be deeply rewarding.
Practical Tips for Success in Phlebotomy
To excel in a phlebotomy career, consider these practical tips:
Develop strong dialog skills to ease patient anxiety and explain procedures clearly.
practice your technical skills regularly to ensure precision during blood draws.
Stay updated on best practices and advancements in phlebotomy through continuous education.
Network with other healthcare professionals to create job opportunities and learn from their experiences.
Real-World Experiences: A Day in the life of a Phlebotomist
Understanding what a typical day looks like can provide crucial insights:
“Every day is different! I draw blood from a variety of patients, and I get to meet so many interesting people. It’s rewarding when patients say they feel comfortable with me. The best part is knowing that I’m helping with their healthcare journey.” – Jane Doe, Certified Phlebotomist
Case Studies: Success Stories in Phlebotomy
Name
Position
Journey
John Smith
Mobile Phlebotomist
Started as a clinical phlebotomist, transitioned to mobile services, improving patient access to care.
Emily Johnson
Phlebotomy Trainer
Began career in a hospital and now trains future phlebotomists, sharing her passion for the field.
Conclusion
Phlebotomy is more then just a job; it is a rewarding career that offers numerous opportunities and the ability to make a direct impact on patient care. Weather you are considering a career path in clinical settings, donor centers, or mobile phlebotomy, the demand for skilled professionals continues to grow. With flexible training programs and various opportunity pathways, now is the perfect time to unlock your future in phlebotomy!
youtube
https://phlebotomytechnicianschools.net/open-your-future-exciting-career-opportunities-in-phlebotomy/
0 notes
Text
Open Your Future: Top Phlebotomy Jobs in Omaha, NE - Your Guide to Starting a Rewarding Career
unlock Your future: Top Phlebotomy Jobs in Omaha, NE – Your Guide to Starting a Rewarding Career
Are you ready to dive into a rewarding health career that makes a real difference in people’s lives? Phlebotomy is an excellent choice for those looking to make their mark in the healthcare field. This article serves as your comprehensive guide to understanding phlebotomy jobs in Omaha, NE. With insights on job opportunities, training, benefits, and practical tips, you’ll be equipped to unlock your future!
What is Phlebotomy?
Phlebotomy is the process of drawing blood for medical testing, transfusions, donations, or research. As a crucial part of the healthcare system, phlebotomists help diagnose and treat diseases by ensuring that the proper blood tests are conducted accurately.
Why Choose a Career in Phlebotomy?
High Demand: The healthcare industry is rapidly growing, and phlebotomists are essential in various medical settings.
Short Training Period: Most phlebotomy programs take only a few months to complete, enabling you to enter the workforce quickly.
Job Satisfaction: Helping patients and being part of their healthcare journey can be incredibly rewarding.
Flexible Work Environments: Opportunities exist in hospitals, clinics, labs, and even mobile units.
Top Phlebotomy Jobs in omaha, NE
When considering a career in phlebotomy in Omaha, NE, you have several exciting opportunities. Below are some of the top job roles to explore:
Job Title
Average Salary
Typical Employers
Phlebotomist
$35,000 – $45,000
Hospitals, Clinics
Lead Phlebotomist
$40,000 - $55,000
Healthcare Facilities
Phlebotomy Trainer
$45,000 – $60,000
Training Programs, Colleges
Mobile Phlebotomist
$30,000 – $50,000
Home Health Services
Training and Certification to Become a Phlebotomist
To start your career in phlebotomy, it’s essential to complete training and certification. Here’s a step-by-step guide to get you started:
Enroll in a phlebotomy Program: Look for accredited programs in Omaha. These can be found at community colleges and vocational schools.
Complete Hands-on Training: Most programs include both classroom instruction and practical experience.
Obtain Certification: While certifications are not mandatory, they considerably enhance your job prospects. Consider organizations like the American society for Clinical Pathology (ASCP) or the National Phlebotomy Association (NPA).
Apply for Jobs: Start applying for entry-level positions in hospitals, clinics, and labs.
Benefits of Working in Phlebotomy
choosing a career as a phlebotomist comes with numerous benefits, including:
Flexible Hours: Many positions offer shifts outside the standard 9-to-5, allowing for better work-life balance.
opportunities for Advancement: Experience and additional training can lead to roles in management, education, or specialized areas.
Engagement with Patients: Working closely with individuals provides personal fulfillment and enhances your communication skills.
First-Hand Experience: A Day in the Life of a Phlebotomist
To better understand the role of a phlebotomist, let’s consider the experience of Jane, a certified phlebotomist in Omaha:
“My day starts early.I love the morning rush at the lab. I take pride in ensuring that patients feel comfortable; it’s about more than just drawing blood. I interact with many patients and help ease their fears. Each day is different, with new challenges and opportunities to learn.” – Jane Doe, Phlebotomist
Practical Tips for Aspiring Phlebotomists
If you’re considering a career in phlebotomy, here are some helpful tips:
Stay Calm Under Pressure: Learn stress-relief techniques; you’ll often work with anxious patients.
Practice Your Skills: Engage in as much hands-on training as possible before entering the workforce.
Network: Join local healthcare associations to connect with professionals in the field.
Keep Learning: The medical field is always evolving. Consider additional certifications for specializations.
Conclusion
Phlebotomy offers an exciting and rewarding career path for those looking to make a difference in healthcare. with a growing demand for skilled phlebotomists in Omaha, NE, there has never been a better time to jump in. By following the steps outlined in this guide, you can unlock your future and embark on a fulfilling career. don’t hesitate—start your phlebotomy journey today!
youtube
https://phlebotomyclassesonline.net/open-your-future-top-phlebotomy-jobs-in-omaha-ne-your-guide-to-starting-a-rewarding-career/
0 notes
Text
From Dollars to Data: Taggart McGurrin on Why Finance Leaders Are Flocking to Pharma Startups
America’s drug development pipeline needs help. As recent health scares like the COVID-19 pandemic have shown, the current model needs to be streamlined to create new, critically needed medicines quickly and efficiently.
Enter the finance pros.
While “Big Pharma” has long been dominated by MBAs, industry startups have traditionally been led by medical researchers or scientists. But, as new technologies like artificial intelligence and the pharmaceutical market become increasingly investor-driven, small companies need management at the top who understand how modern business is executed.
Now, financially trained professionals like Tagg McGurrin, CPA, MBA, JD, are leading some of the most promising and innovative pharmaceutical startups, helping steer them through the rough waters of investor demands, regulatory changes, and market dynamics.
The setup has created an exciting synergy. The biotech sector offers highly skilled business people the chance to use a wide portion of their skill sets in service of public good while freeing up medical specialists to concentrate on creating the very best solutions possible.
It’s also just good business.
“My background with finance, law, and accounting was very attractive to the team because they could get many people in one,” McGurrin said. “So, with capital being king in pharma, being able to pay one person what you otherwise have to pay three or four people to do is required when you're in that small but high-growth startup space.”
But does good business make for good drugs?
A New Kind of Leader
The idea of medical companies being led by financial wizards can feel wrong to the public. Not long ago, some so-called “pharma bros” gave the practice a bad rap, thanks to conspicuously greedy and amoral actors like Martin Shkreli, who famously raised the price of a lifesaving antiparasitic drug from $13.50 to $750 per pill, and Theranos founder Elizabeth Holmes, who defrauded investors out of billions for a blood-testing product that never worked. (Both individuals were sentenced to years in prison for their actions.)
But there’s an essential distinction between the pharma bros of the past and the new crop of professionals. The field has become more professionalized in light of those scandals, and investors have become more discerning. Today’s leaders aren’t solely drawn from a crop of young investment bankers but highly educated and passionate workers with motives beyond striking it rich and getting famous.
For McGurrin, the impetus to change careers was his family.
“I didn't plan to be in pharmaceuticals,” he said. “It was rather serendipitous that I could pursue something I felt very strongly about—the opioid epidemic. I had a family member who suffered from opioid use disorder. And so, going in there, I realized that not all that is complex has to be complicated, and there's no industry that I can think of that has all the various areas of risk and complexity that the pharmaceutical industry possesses. Think about financing, development, scientific, regulatory, and commercial risk. That's not even to mention the cultural risk you have with the teams you're building.”
Taggart McGurrin is not alone in his outlook. Other financial leaders have jumped to pharma in search of making a difference.
Renee Aguiar-Lucander, who left Lehman Brothers to become CEO of a company that develops drugs that treat often overlooked conditions, told Outsourcing Pharma: “I am driven by making a genuine difference in patients’ lives. At Calliditas, we are committed to helping people with rare diseases by identifying treatments that enable these patients to live longer, better lives.”
Do Financial Pros Make it Better?
It’s fair to wonder if even the most well-intentioned financial workers can deliver better patient outcomes than medical workers. After all, there’s a steep learning curve to medicine, and the values of the medical establishment can seem to be at odds with the ethos of startup culture.
For leaders like Taggart McGurrin, however, it’s not about a clash of values but a meshing of culture. When executives come from outside the medical field, they need to rely on the expertise of researchers and practitioners and the brilliance of visionaries.
“Something I've always strived to do is to empower the teams to inform executive decision-making,” he said. “You just don't make decisions in a silo. You go in with your teams, and you pressure test your assumptions. You encourage them to identify blind spots, raise issues not yet addressed, or challenge underlying assumptions. I want to hear all the negative things that could happen based on a particular action. And that's honestly what makes the team so critical.”
He added that even when hard decisions must be made, high-quality executives never lose sight of the company’s purpose.
In order to deliver for investors and patients, “You need to understand the amount of money that we need to raise or the capital we need to deploy to meet certain critical value inflection points for the company.
How do we make the company more valuable? How do we progress the drugs to the best of our ability, especially as a small startup biotech company where you always need more cash?” McGurrin said. “But the point I wanted to make about a company's purpose , and I think this is something that is often overlooked, is the bottom line starts and ends with the human impact the pharmaceutical industry has on patients’ health and well-being.”
###
0 notes
Text
Open Your Future: A Comprehensive Guide to Starting Your Phlebotomist Program
# Unlock Your Future: A Comprehensive Guide to Starting Your Phlebotomist Program
**Meta Title:** Unlock Your Future: A Complete Guide to Starting Your Phlebotomist Program
**Meta Description:** Discover how to kickstart your career as a phlebotomist with our comprehensive guide. Learn about requirements, benefits, tips, and what to expect in your phlebotomy program.
—
## Introduction
Are you ready to take the next step in your career? If you’re searching for a fulfilling job in the healthcare field, becoming a phlebotomist might be the right choice for you! Phlebotomists play a crucial role in hospitals and clinics by collecting blood samples for testing and surgeries. This comprehensive guide will equip you with everything you need to know about starting your phlebotomist program. From educational requirements to job outlook, we’ve got you covered. Read on to unlock your future!
## Why Become a Phlebotomist?
Before diving into the specifics of starting a phlebotomist program, let’s explore why this career could be an excellent fit for you.
### 1. **High Demand for Phlebotomists**
The healthcare industry continues to grow, and this field is no exception. According to the U.S. Bureau of Labor Statistics, employment for phlebotomists is expected to grow by 22% from 2020 to 2030, much faster than the average for all occupations.
### 2. **Short Training Period**
Most phlebotomy programs can be completed in a matter of months, allowing you to enter the workforce quickly compared to other healthcare roles that may require years of education.
### 3. **Opportunities for Advancement**
Many phlebotomists build a foundation to advance into roles such as laboratory technicians, nurses, or other medical professions.
## Steps to Starting Your Phlebotomist Program
Getting started in a phlebotomy program involves several key steps. Here’s a detailed breakdown:
### Step 1: Research Phlebotomy Programs
Phlebotomy programs can vary substantially, so it’s vital to do your research. Consider looking for:
– **Accredited Programs**: Ensure that the program is accredited by an organization like the National Accrediting Agency for Clinical Laboratory Sciences (NAACLS). – **Curriculum**: Look for hands-on training opportunities and comprehensive coursework that covers anatomy, safety protocols, and lab procedures. – **Duration**: Programs can last from a few weeks to several months depending on whether you attend full-time or part-time.
### Step 2: Understand the Requirements
Most phlebotomy programs will require you to meet specific prerequisites, including:
– **High School Diploma or GED**: This is typically the minimum educational requirement. – **Background Checks**: Be prepared for a background check, as most healthcare positions require it to ensure patient safety. – **Immunizations**: Some programs require proof of vaccinations, particularly for diseases such as Hepatitis B.
### Step 3: Enroll in a Program
Once you’ve thoroughly researched and aligned with a program, it’s time to apply. Be sure to:
– **Gather Required Documents**: This may include your high school transcript, identification, and proof of immunizations. - **Prepare Financially**: Investigate financial aid options, scholarships, and payment plans offered by the institution.
### Step 4: Complete Your Training
During your phlebotomy training, you will learn critical skills, including:
- **Blood Collection Techniques**: Learn the correct methods for drawing blood safely and efficiently. – **Patient Interaction**: Develop strong communication skills to help ease patient anxiety. – **Laboratory Safety**: Understand the importance of infection control and safety protocols.
### Step 5: Obtain Certification
While certification isn’t always necessary, many employers prefer or require it. Consider these organizations for certifications:
| Certification Organization | Description | Requirements | |—————————-|————————————————-|——————————————————–| | American Society for Clinical Pathology (ASCP) | One of the most widely recognized certifications | Completion of accredited program + exam | | National Center for Competency Testing (NCCT) | Offers a certification exam for phlebotomists | Education + clinical experience in phlebotomy | | National Phlebotomy Association (NPA) | Focuses on quality training and advocacy | Complete a training program + pass certification exam |
### Step 6: Apply for Jobs
Once you’ve completed your training and earned your certification, it’s time to look for phlebotomist positions. Here are some tips for landing your first job:
- **Build a Strong Resume**: Highlight your training, skills, and any volunteer experience in healthcare. - **Network**: Connect with instructors, attend job fairs, and engage in relevant online forums. – **Prepare for Interviews**: Be ready to discuss your training experience and demonstrate your understanding of blood collection protocols.
## Benefits of Choosing a Phlebotomy Career
Phlebotomy offers numerous benefits, including:
– **Flexibility**: Many phlebotomy jobs offer shifts that cater to various lifestyles, including weekends and evenings. – **Job Satisfaction**: Helping people and working in a critical healthcare role can provide immense job satisfaction. – **Transferable Skills**: The skills you acquire in phlebotomy can be beneficial in many other healthcare careers.
## Practical Tips for Successful Phlebotomy Training
To make the most of your phlebotomy training, consider these practical tips:
– **Practice, Practice, Practice**: Seek opportunities for hands-on practice, as proficiency in blood draws improves with experience. – **Stay Updated on Practices**: Follow industry trends and continue your education even after certification. – **Connect with Professionals**: Join phlebotomy associations or local groups to network and gain insights.
## First-Hand Experience: A Day in the Life of a Phlebotomist
To provide insight into the role, here’s a brief look at a typical day for a phlebotomist:
– **Morning Shift**: Arrive early to prepare equipment and review patient schedules. – **Blood Draws**: Take samples for various tests, ensuring patient comfort and safety. – **Record Keeping**: Maintain accurate records of blood samples and test orders. – **Collaboration**: Work alongside nurses and lab technicians to ensure samples are processed effectively.
## Case Study: Success Story of a Phlebotomy Graduate
Meet Sarah, a recent graduate of a phlebotomy program. After completing her training, she secured a position within three months:
– **Challenges**: Overcame initial anxiety about patient interactions with practice and mentorship. – **Achievements**: Acknowledged by her supervisor for excellent patient care and attention to detail. – **Future Plans**: As a result of her experience, Sarah is now pursuing further education to become a registered nurse.
## Conclusion
Embarking on a path to become a phlebotomist is a rewarding journey filled with opportunities for growth and advancement. Whether you are drawn to the healthcare field by a desire to help others or an interest in medical technology, phlebotomy can provide a solid foundation for your career. By investing in the right education, acquiring certifications, and networking effectively, you can position yourself for success in this growing field. Unlock your future today—your career as a phlebotomist awaits!
—
Utilizing the best SEO practices, this article provides valuable insights into starting your phlebotomy career, making it an excellent resource for aspiring professionals in the healthcare field.
youtube
https://phlebotomycertificationcourse.net/open-your-future-a-comprehensive-guide-to-starting-your-phlebotomist-program/
0 notes
Text
“Embark On Your Career With B.VoC Optometry And Ophthalmic Technology At Shri Ram Medical College”

The **B.Voc Optometry and Ophthalmic Technology** program at **Shri Ram Medical College**, located in Bankner, offers a cutting-edge educational experience designed to equip students with the essential skills needed to succeed in the growing field of **optometry** and **ophthalmic technology**. As a highly specialized course, it prepares graduates for hands-on roles in the healthcare industry, particularly focusing on **vision care** and **eye health**.
In this blog post, we’ll delve into the core features of the B.Voc Optometry and Ophthalmic Technology program at Shri Ram Medical College, exploring how this course stands out for aspiring eye care professionals.
## Specialized Ophthalmic Technology Courses at Shri Ram Medical College
The **B.Voc Optometry and Ophthalmic Technology** course at **Shri Ram Medical College** is specifically designed to give students specialized knowledge in **ophthalmic technology** and **vision care**. The curriculum covers a wide range of subjects such as **refraction**, **visual optics**, **ocular pathology**, and **contact lenses**. Students also gain an in-depth understanding of eye anatomy and disease management.
This specialized training ensures that students graduate with a clear understanding of optometry fundamentals and advanced practices, ready to contribute to the **eye care industry**. The curriculum is updated regularly to keep up with global standards, preparing students for a future in **modern ophthalmology**.
## Real-World Training in Labs
One of the highlights of the **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College is the **real-world training** students receive in specialized labs. These labs are equipped with the latest diagnostic tools and technology used in optometry practice. Students get to learn how to perform various eye tests and procedures on state-of-the-art equipment such as **retinoscopes**, **visual field analyzers**, and **tonometers**.
This practical training ensures that students not only learn theory but also apply their knowledge in real-world settings, helping them build a strong foundation in **ophthalmic care**. It also provides a unique opportunity for hands-on experience that is invaluable when entering the workforce.
## Advanced Ophthalmic Equipment Usage
In the **B.Voc Optometry and Ophthalmic Technology** program at Shriram Medical College, students are trained to use **advanced ophthalmic equipment** effectively. The college’s labs are fully equipped with the latest diagnostic instruments, including **fundus cameras**, **auto-refractors**, and **slit-lamp biomicroscopes**.
Being proficient in using such equipment is essential for a successful career in optometry. By using the most current technology, students gain a competitive edge in the industry, ensuring they are well-prepared to meet the challenges of modern eye care.
## Practical Skills in Eye Care
The **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College emphasizes the development of **practical skills** in eye care. Students are trained in the practical aspects of **eye exams**, **vision correction**, and **patient management**. The program also provides training in **ocular health assessment** and the **treatment of refractive errors**.
Students learn how to conduct detailed **visual acuity tests**, **retinal exams**, and **glaucoma screening**, which are essential components of primary eye care. This hands-on approach ensures that students are well-equipped to enter the workforce as **skilled optometrists** or **ophthalmic technicians**.
## Immersive Hands-On Learning Approach
Shri Ram Medical College believes in an **immersive learning approach**, where students are not just confined to classrooms. Instead, they are actively involved in hands-on activities that replicate real-life situations in **ophthalmic practice**. This approach includes **practical training** in hospitals, **optometry clinics**, and **ophthalmic centers**.
Students participate in live patient assessments, learning how to diagnose and treat common eye diseases. The immersive experience helps students develop **confidence** in their abilities and prepares them for the demands of the **optometry field**.
## Comprehensive Vision Technology Training
The **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College offers **comprehensive training** in the latest **vision technology**. The course covers the use of advanced **diagnostic instruments** and **treatment methods** used in modern optometry. Students gain experience with technologies like **digital refraction systems**, **ocular ultrasound**, and **wavefront analysis**.
The program ensures that students are well-versed in the **technological advancements** in optometry, giving them the expertise to handle the complexities of modern eye care. This specialized training opens up a range of career opportunities in **ophthalmic technology** and **optometry practice**.
## Cutting-Edge Ophthalmic Practice Sessions
The program also includes **cutting-edge ophthalmic practice sessions** where students apply their theoretical knowledge in a clinical environment. During these sessions, students get hands-on practice with **eye health assessments**, **contact lens fittings**, and **diagnostic procedures**.
These practice sessions are designed to simulate real-world scenarios, giving students the opportunity to refine their skills and gain valuable experience in **patient care**. By the time they graduate, students have mastered the clinical techniques required to succeed in **optometry practices** and **eye care facilities**.
## Focused Practical Experience Opportunities
Shri Ram Medical College offers **focused practical experience opportunities** as part of its **B.Voc Optometry and Ophthalmic Technology** program. This allows students to work alongside experienced professionals in **clinical rotations** and **internships** in real healthcare settings. Students gain invaluable exposure to the everyday operations of **optometry clinics** and **ophthalmic departments** in hospitals.
This practical experience is vital for building confidence and competence in patient care, eye diagnostics, and **treatment procedures**. It also gives students insight into the working of the **optometry industry** and prepares them for future job roles.
/media/ea7ca5016b829568771452fb5d10f757
## Frequently Asked Questions (FAQs)
**1. What are the eligibility criteria for the B.Voc Optometry and Ophthalmic Technology program at Shri Ram Medical College?**
To be eligible for the **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College, candidates should have completed their **higher secondary education (12th grade)** with a background in **science** (preferably with **Physics, Chemistry, and Biology** as subjects). Admission to the program may also require a **competitive entrance exam** or **personal interview**. Additionally, professionals with prior qualifications in **paramedical courses** like **B.Voc Paramedical** or **M.Voc Paramedical** are encouraged to apply.
**2. What kind of practical training do students receive in the B.Voc Optometry and Ophthalmic Technology program?**
Students enrolled in the **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College receive extensive **practical training** in **optometry clinics** and **ophthalmic labs**. The training includes using state-of-the-art diagnostic equipment, performing eye exams, and providing **vision care**. Students also participate in clinical rotations and internships, gaining hands-on experience with real patients, which prepares them for the challenges of working in the **optometry field**.
**3. Are there any job opportunities after completing the B.Voc Optometry and Ophthalmic Technology program?**
Yes, after completing the **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College, graduates have access to a wide range of job opportunities. They can work as **optometrists**, **ophthalmic technicians**, **vision therapists**, or **contact lens specialists** in hospitals, clinics, or private practices. The college also provides **career placement support** to help graduates find suitable positions in **optometry clinics**, **eye hospitals**, and **ophthalmic research centers**.
**4. What sets the B.Voc Optometry and Ophthalmic Technology program at Shri Ram Medical College apart from other institutions?**
The **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College is unique due to its **comprehensive curriculum**, which integrates both **theoretical knowledge** and **hands-on training**. The college boasts state-of-the-art **ophthalmic labs**, **advanced diagnostic equipment**, and **immersive clinical training** sessions, ensuring that students receive an industry-focused education. The program also offers strong **career placement assistance**, giving graduates a competitive edge in the job market.
**5. Can students pursue further studies after completing the B.Voc Optometry and Ophthalmic Technology program?**
Yes, after completing the **B.Voc Optometry and Ophthalmic Technology** program at Shri Ram Medical College, students have the option to pursue higher studies, such as **M.Voc Optometry**, **M.Sc in Optometry**, or other specialized postgraduate courses in **ophthalmic technology** or **eye care**. Further studies can enhance career prospects and provide opportunities for advanced roles in **optometry** and **ophthalmology**.
## Conclusion
The **B.Voc Optometry and Ophthalmic Technology** program at **Shri Ram Medical College** offers an excellent opportunity for students to embark on a successful career in the growing field of **vision care** and **ophthalmic technology**. With its **specialized curriculum**, **state-of-the-art labs**, and **practical experience** opportunities, the program provides students with the skills and confidence needed to excel in the industry. Whether you’re looking to work in **optometry clinics**, **hospitals**, or **eye care centers**, Shri Ram Medical College prepares you for a rewarding career in **eye health**.
Please Like, Share and Subscribe Shri Ram Medical College Youtube channel.
Stay connected with the latest updates in medical education and healthcare by subscribing to Shri Ram Medical College’s YouTube channel. Explore expert insights, student experiences, and in-depth videos on paramedical careers. Don’t miss out — like, share, and subscribe to stay informed about opportunities and advancements in the medical field. Join our growing community today!
0 notes