#Challenges With Mesenchymal Stem Cells
Explore tagged Tumblr posts
Text
Why GMP Compliance Is Paramount For High-Quality Mesenchymal Stem Cell?
The past twenty years have witnessed a fascinating unboxing of the mesenchymal stem cells. These microscopic marvels hold the potential to revolutionize how we approach disease. These mesenchymal stem cells are extracted from adult cells, and hence, they rarely receive any ethical backlash. Unlike most stem cells, mesenchymal ones boast remarkable versatility and can morph into diverse cell types, from bone to blood vessels. These cells carry several regenerative and anti-inflammatory prowess, which has propelled them to the forefront of stem cell therapy. The use in therapeutic applications has ignited a surge in demand that outpaces our current production capabilities.
Given the surge in demand for MSCs for research, providing researchers with high-quality stem cells for reproducible research is necessary. Enter the realm of Good Manufacturing Practices (GMP), our roadmap towards building factories for these cellular powerhouses, ensuring not just quantity but unparalleled quality and safety. Buckle up, science researchers, for we’re about to delve into the intricate dance of scaling up MSC production while upholding the highest standards, paving the way for a future where these microscopic maestros weave their magic on a grand scale.
#Mesenchymal Stem Cells#MSCs#GMP Compliance Is Paramount For High-Quality Mesenchymal Stem Cell#Challenges With Mesenchymal Stem Cells#Cell culture#customized primary cells#primary cells#biotech company#stem cells#exosomes#stem cell research center#regenerative medicine#bioengineering#Kosheeka
0 notes
Text

I don't know much about 'skin things' but I do have a little bit of information on it for those who are interested! (I hope you don't mind me using your comment). I'll be supplementing my knowledge with some research under the read more.
Skin grafting is a dermatological procedure utilized to facilitate wound closure.
We'll talk about some commonly used techniques:
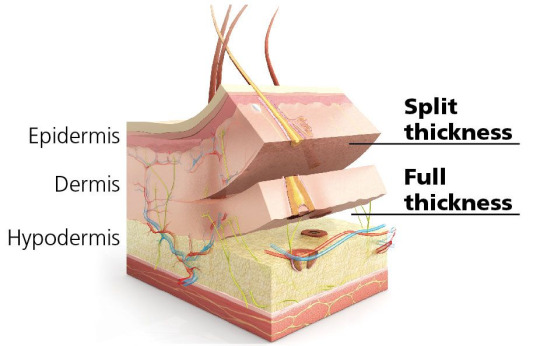
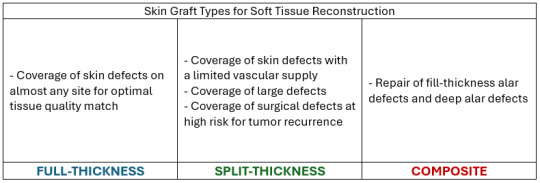
1. Split-Thickness Skin Grafts (STSG) are composed of the epidermic and a superficial part of the dermis. These grafts are commonly used to cover large wounds, burns, and areas of skin loss. They are thinner than full-thickness grafts, which allows them to cover larger areas.
2. Full-Thickness Skin Grafts (FTSG) contain both the full epidermis and the dermis. These grafts are typically used for smaller wounds in areas where aesthetics and durability are essential, such as the face, hands, or neck. Since FTSGs retain the full dermal layer, they offer better cosmetic outcomes, including improved texture, pigmentation, and reduced scarring compared to split-thickness grafts. They also tend to resist contracture better, making them ideal for regions requiring flexibility. - FTSGs are more complex because they require a well-vascularized wound bed to survive and heal. - FTSGs are the most commonly used graft.
3. Composite Skin Grafts are usually small and include a combination of skin and underlying tissues, such as fat, cartilage, or muscle. These grafts are used to reconstruct areas where multiple tissue types are needed to restore both form and function, such as the nose, ears, or fingertips. - Composite skin grafts which combine allogeneic dermis and an expanded autologous epidermis can effect rapid wound closure.
It is further broken down by the following:
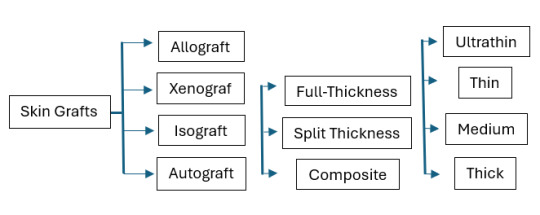
Permanent Skin Grafts
1. Autografts (autologous graft): skin collected from the patient 2. Isograft (syngeneic graft): skin collected from a genetically identical donor (twin)
Temporary Skin Grafts
1. Allocrafts (heterologous graft): skin from a cadaver (living donors are possible) 2. Xenograft (heterograft): skin from another species
Can be Temporary OR Permanent
1. Synthetic skin substitutes: use of manufactured skin - The only technique that is permanent is cultured epithelial autograft (CEA), which is essentially a skin graft grown from a patient's own skin cells.
NO NON-SELF TISSUE IS GUARANTEED TO COMPLETELY AVOID DEATH OR REJECTION.
Transplant Rejection: a patients immune system identifies the graft as a foreign body, which triggers an immune response to get "rid" of the tissue.
Skin implant compatibility is based on three highly polymorphic MHC genes (HLA-A, HLA-B, and HLA-C) that encode proteins and are a part of the Human Leukocyte Antigen (HLA) system. This system identifies foreign bodies.
Knowing this, the use of modified donor animals, such as pigs, to provide transplantable organs, is gaining some renewed research. It involves excising the genes in the pig that are most responsible for the rejection reaction after transplantation. However, finding these genes and effectively removing them is a challenge.
The use of autologous skin grafts is the most common approach in the treatment of chronic wounds. However, in the case of deep and/or large wounds or with extensive severe burns, the use of autografts is limited, and either allogeneic (from cadaver) or xenogeneic skin grafts are used for transplantation.
The use of allogenic/xenogenic tissue carries a high risk of graft rejection, limiting their clinical applications.
Tissue Engineering
Advanced therapies for chronic wounds involve application of bioengineered artificial skin substitutes to overcome graft rejection as well as topical delivery of mesenchymal stem cells to reduce inflammation and accelerate the healing process.
Photo shows potentially ideal artificial skin graft:
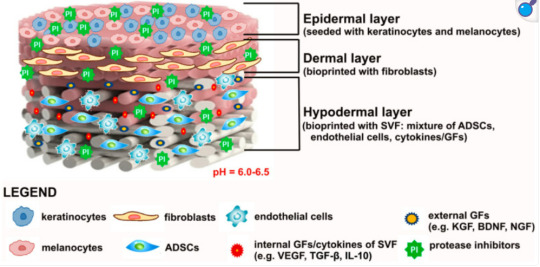
Modern treatment includes skin tissue engineering aiming to produce bioengineered biomaterial-based artificial skin grafts. Therefore, the main roles of bioengineered skin grafts is to supply oxygen (by being oxygen permeable), keep the wound from dehydration, promote healing, and prevent infections. - Depending on the type of biomaterial used for the production of artificial skin grafts, they may function as skin equivalents providing temporary wound covers or permanent skin substitutes. - When the biomaterials are pre-seeded or have cells incorporated within their matrix, they are classified as cellular artificial skin grafts, whereas biomaterials without or deprived of cells are defined as acellular artificial skin grafts.
Here are some current commercially available synthetic skin grafts I found applicable to Curly's injuries:
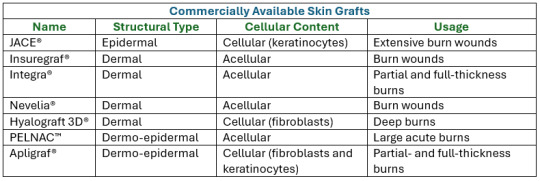
Definitions for Clarity: 1. Epidermal: Pertaining to the outermost layer of the skin. 2. Cellular Content: the complex structures and biomolecules that make up cells, the smallest units of life. 3. Acellular: not consisting of, divided into, or containing cells. 4. Fibroblasts: a cell in connective tissue which produces collagen and other fibers. 5. Keratinocytes: an epidermal cell which produces keratin (a fibrous protein forming the main structural constituent of hair).
Articles to Reference
Organ Transplantation and Rejection by Libretexts biology. LINK
(CW: Images) Skin Grafting by Joseph Prohaska and Christopher Cook. LINK
A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? by Agata Przekora LINK
Composite skin graft: frozen dermal allografts support the engraftment and expansion of autologous epidermis by E L Heck, P R Bergstresser, C R Baxter LINK
#curly mouthwashing#mouthwashing#mouthwashing theories#leo vomits medical jargon#i didnt proof read this if there are errors you dont see them
26 notes
·
View notes
Text
Can This Fast-Growing Medical Trend Finally End Lower Back Pain for Good?

Can This Fast-Growing Medical Trend Finally End Lower Back Pain for Good?
Is it possible we’re nearing a real solution to one of the most common and stubborn health problems in the world? If you’ve ever dealt with back pain that just won’t quit, you’re not alone—and that’s exactly why the Chronic Lower Back Pain Treatment Market is seeing explosive growth right now.
Chronic lower back pain affects millions globally, impacting everything from work productivity to mental health. Unlike short-term pain that disappears in days or weeks, chronic pain can last months—or even years—causing frustration, disability, and an endless cycle of treatment attempts.
Why Are More People Struggling with Chronic Back Pain?
The causes are everywhere in modern life. Poor posture, sedentary jobs, obesity, injuries, and aging populations are all contributing to a rising number of people living with chronic back pain. Add to that high stress levels and a lack of preventive care, and it’s no wonder that this issue has become a global health crisis.
As a result, the demand for effective and long-lasting treatments has never been higher. From advanced pain management therapies to surgical innovations and regenerative medicine, the race is on to deliver better solutions with fewer side effects and improved quality of life.
What’s Driving the Rapid Growth of This Market?
Several key trends are fueling expansion. First is the shift toward non-invasive and minimally invasive treatment options. Patients are increasingly opting for therapies that provide relief without surgery, including spinal injections, radiofrequency ablation, nerve stimulation, and even stem cell therapy.
Second, there’s a strong push for multimodal treatment approaches. This means combining medication, physical therapy, psychological support, and lifestyle changes into one holistic treatment plan. Healthcare providers are now recognizing that no single solution fits all.
The Chronic Lower Back Pain Treatment Market is also benefiting from technological innovation. Wearable devices that track posture and movement, virtual physical therapy platforms, and mobile health apps are making treatment more accessible and personalized than ever before.
Which Treatments Are Gaining the Most Popularity?
Among the fastest-growing segments are neuromodulation devices, such as spinal cord stimulators. These are especially promising for patients who haven’t responded to other treatments. Non-opioid pain relief alternatives are also on the rise as concerns over addiction and side effects from long-term medication use continue to grow.
Regenerative medicine is another exciting area. Treatments like platelet-rich plasma (PRP) and mesenchymal stem cell injections are being explored for their potential to actually repair damaged tissues rather than just manage symptoms.
Where Is Growth Happening the Fastest?
North America leads in innovation and adoption due to strong healthcare systems, high awareness levels, and significant investment in R&D. Europe follows closely, especially in countries with aging populations and advanced pain management centers.
However, Asia-Pacific is becoming a major player. Rising incomes, urbanization, and an increase in lifestyle-related back pain cases are prompting higher demand for both traditional and advanced treatment options in countries like China, Japan, and India.
What Challenges Are Limiting Access and Effectiveness?
While the market is growing, it still faces significant hurdles. High treatment costs, limited insurance coverage, and varying effectiveness between patients make it difficult to create one-size-fits-all solutions.
Additionally, there’s often a gap between diagnosis and appropriate treatment. Many patients go through years of trial-and-error before finding lasting relief. That’s why early intervention, better education, and individualized treatment plans are crucial moving forward.
What’s Next for the Future of Back Pain Treatment?
The future of the Chronic Lower Back Pain Treatment Market looks both technologically advanced and more human-centered. As AI-driven diagnostics and virtual therapy tools evolve, patients can expect faster access to customized care.
There’s also a growing emphasis on preventive strategies—helping people correct posture, strengthen core muscles, and reduce lifestyle risks before pain becomes chronic. The industry is shifting from reactive care to proactive management.
For the millions still suffering in silence, relief may finally be within reach. The next generation of back pain treatments is smarter, safer, and more targeted than ever—and this booming market is just getting started.
0 notes
Text
The Science of Hope: Exploring Newborn Stem Cell Banking Sunrise Benefits
The birth of a child heralds a future filled with dreams, and increasingly, with incredible medical possibilities. Among the most promising advancements is newborn stem cell banking sunrise, a practice that offers a unique biological resource for potential future medical needs. This isn't just about storing cells; it's about preserving a powerful, regenerative toolkit derived from your baby's umbilical cord. These remarkable cells, harvested at the moment of birth, hold the potential to treat a growing list of diseases, offering a proactive approach to family health. At American Cell Technology, we are at the forefront of medical research, dedicated to illuminating the profound benefits and scientific promise behind newborn stem cell banking sunrise.
Newborn Stem Cell Banking Sunrise Fundamentals
At its core, newborn stem cell banking sunrise involves collecting and preserving hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) from your baby's umbilical cord blood and tissue shortly after birth. These cells are unique because they are "naïve," meaning they haven't been exposed to environmental toxins or diseases, making them highly versatile and potent for therapeutic use. Unlike adult stem cells, newborn stem cells have a higher proliferative capacity and are less likely to be rejected if used for a family member. This fundamental understanding is crucial for appreciating the long-term health advantages inherent in newborn stem cell banking sunrise.
Therapeutic Applications of Newborn Stem Cell Banking Sunrise
The therapeutic applications of newborn stem cell banking sunrise are continually expanding, offering hope for various medical conditions. HSCs, found in cord blood, are already used in the treatment of over 80 diseases, including certain cancers like leukemia and lymphomas, as well as blood disorders such as anemia and thalassemia. MSCs, found in cord tissue, are being investigated in clinical trials for their potential in regenerative medicine, including treating neurological disorders, autoimmune diseases, and even orthopedic injuries. This broad spectrum of potential uses highlights the remarkable versatility that newborn stem cell banking sunrise provides for future health challenges.
Future Medical Discoveries via Newborn Stem Cell Banking Sunrise
The landscape of medicine is constantly evolving, and newborn stem cell banking sunrise positions families at the cutting edge of future therapeutic breakthroughs. Researchers are actively exploring the use of these powerful cells in areas such as spinal cord injuries, diabetes, heart disease, and even Alzheimer's disease. The unique regenerative properties of newborn stem cells make them prime candidates for repairing damaged tissues and organs, or even for growing new ones. As scientific understanding deepens, the scope of what newborn stem cell banking sunrise can address is expected to grow exponentially, offering unprecedented possibilities for medical intervention.
Personalized Medicine through Newborn Stem Cell Banking Sunrise
One of the most compelling aspects of newborn stem cell banking sunrise is its role in personalized medicine. Stem cells collected from your child are a perfect genetic match for them, eliminating the risk of rejection if needed for their own treatment. Furthermore, there's a significant chance of a partial or full match for siblings and other family members, potentially providing a life-saving resource without the complexities of finding a compatible donor. This individualized approach to healthcare, made possible by newborn stem cell banking sunrise, underscores its value as a tailored medical resource for your family.
Safety and Collection of Newborn Stem Cell Banking Sunrise
The process of newborn stem cell banking sunrise is remarkably safe, posing no risk to either the mother or the baby. The collection of umbilical cord blood and tissue occurs immediately after birth, a time when these materials are typically discarded. The procedure is non-invasive, painless, and takes only a few minutes, seamlessly integrating into the delivery room experience. Reputable banking facilities, like those working with American Cell Technology, adhere to stringent collection, processing, and storage protocols to ensure the viability and integrity of these precious cells for decades, making newborn stem cell banking sunrise a secure investment.
Considering Long-Term Value in Newborn Stem Cell Banking Sunrise
While the initial decision to pursue newborn stem cell banking sunrise involves a financial consideration, it's essential to view it as a long-term investment in your family's health security. The potential to access these unique cells for unforeseen medical needs, without the lengthy and often uncertain search for a donor, can be priceless. Comparing the costs to the potential benefits—including the peace of mind and the availability of a readily accessible, perfectly matched biological resource reveals the profound long-term value that newborn stem cell banking sunrise offers as a proactive health measure.
Ethical Considerations for Newborn Stem Cell Banking Sunrise
As with any advanced medical technology, newborn stem cell banking sunrise involves important ethical considerations. Discussions often revolve around the equitable access to this technology, the distinction between private and public banking, and the informed consent process. Reputable institutions prioritize transparency, ensuring parents fully understand the benefits, limitations, and future implications of their decision. American Cell Technology is committed to upholding the highest ethical standards in all aspects of our research and services related to newborn stem cell banking sunrise, ensuring that hope is balanced with responsibility and integrity.
Conclusion
The decision to embark on newborn stem cell banking sunrise is a profound one, reflecting a forward-thinking approach to family health and well-being. It represents more than just a medical procedure; it's an investment in a future brimming with potential medical advancements and personalized treatment options. By preserving your baby's unique stem cells, you are securing a precious biological resource that could one day offer life-saving therapies for a range of conditions. At American Cell Technology, we believe in empowering families with knowledge and cutting-edge science, offering the promise that newborn stem cell banking sunrise embodies. It is a testament to the power of proactive health planning, giving families a tangible asset in the face of tomorrow's uncertainties.
0 notes
Text
Stem Cells Market Investment Trends, Partnerships, and Competitive Landscape 2032
The global stem cells market was valued at USD 17.02 billion in 2024 and is projected to grow from USD 19.34 billion in 2025 to USD 55.75 billion by 2032, registering a robust compound annual growth rate (CAGR) of 16.3% over the forecast period.
The stem cells market is experiencing substantial growth driven by advancements in regenerative medicine, increasing research investments, and the rising prevalence of chronic and degenerative diseases. Stem cells possess the unique ability to develop into various specialized cell types, making them highly valuable for therapeutic applications, including tissue repair, organ regeneration, and the treatment of conditions such as cancer, diabetes, and neurological disorders. Additionally, growing interest in personalized medicine and stem cell banking is further propelling market demand. As clinical trials progress and regulatory frameworks evolve, the stem cells market is poised to play a transformative role in the future of healthcare and biomedical innovation.
Continue reading for more details:
Market Segmentation:
The stem cells market is primarily driven by the prominence of Mesenchymal Stem Cells (MSCs), which continue to see high demand in regenerative medicine research. Induced Pluripotent Stem Cells (iPSCs) are also gaining significant momentum, particularly for their applications in disease modeling. The research segment currently dominates the market, propelled by the growing use of stem cells in disease-related studies. Meanwhile, the clinical segment is poised for rapid expansion, fueled by ongoing advancements in regenerative therapies. Pharmaceutical and biotechnology companies account for the largest share of the market, backed by their extensive involvement in clinical trials. However, academic and research institutions are expected to register the fastest growth, supported by increasing collaborations with industry players, favorable government initiatives, and rising private sector investments aimed at advancing stem cell research.
List Of Key Companies Profiled In Stem Cells Market:
PromoCell GmbH (Germany)
AcceGen (U.S.)
Bio-Techne (U.S.)
Cellular Engineering Technologies (U.S.)
Merck KgaA (Germany)
Thermo Fisher Scientific Inc. (U.S.)
Lonza (Switzerland)
Miltenyi Biotec B.V. & Co. KG (Germany)
STEMCELL Technologies (Canada)

Driving Factors:
Stem cell products are increasingly gaining momentum in both research and therapeutic applications, especially in regenerative medicine, which plays a vital role in restoring normal function in patients with chronic diseases and severe injuries. Rising research initiatives and clinical trials underscore their potential—for example, a 2023 study published in JACC Journals highlighted the effectiveness of Mesenchymal Precursor Cells (MPCs) in treating chronic heart failure. Strategic acquisitions and collaborations are further accelerating market growth. Notably, Bio-Techne’s acquisition of Namocell in 2022 and Pluristyx’s partnership with Stem Genomics in 2023 are driving innovation across the sector. Additionally, increasing government funding and the growing prevalence of neurological disorders, diabetes, and cancer are set to propel the market forward. The expanding focus of companies on induced pluripotent stem cells (iPSCs) further enhances the industry’s growth prospects.
Restraining Factors:
Despite its promise, stem cell research faces significant ethical and regulatory challenges. Human embryonic stem cell (hESC) research, for instance, remains controversial due to the ethical concerns surrounding embryo destruction, raising debates over federal funding, research ethics, and informed consent. Safety concerns have also emerged around non-FDA-approved stem cell treatments. A 2019 report from the California Department of Public Health identified bacterial infections linked to unregulated umbilical cord blood-derived products. These ethical complexities and regulatory barriers could hinder the market’s expansion.
Regional Analysis:
In 2024, North America dominated the stem cells market, reaching a value of USD 9.04 billion, supported by robust R&D activities, rising approvals of stem cell therapies, and frequent product launches. For example, in May 2023, the Maryland Stem Cell Research Commission committed over USD 14.1 million to advance stem cell research. Europe is expected to register notable growth, driven by increasing R&D investments targeting rare disease treatments. While Latin America and the Middle East & Africa currently hold smaller market shares due to limited research infrastructure, Asia Pacific is anticipated to post the highest CAGR. This growth is fueled by industrial expansion, a surge in clinical trials, advanced biomedicine infrastructure, and increasing investment inflows.
Contact Us:
Fortune Business Insights™ Pvt. Ltd.
9th Floor, Icon Tower,
Baner - Mahalunge Road,
Baner, Pune-411045, Maharashtra, India.
Phone: U.S.: +1 424 253 0390
U.K.: +44 2071 939123
APAC: +91 744 740 1245
Email: [email protected]
0 notes
Text
Cell Freezing Media for Cell Therapy Market Growth Analysis, Market Dynamics, Key Players and Innova
The global cell freezing media for cell therapy market size was valued at USD 78.3 million in 2024. The market is projected to grow from USD 89.7 million in 2025 to USD 195 million by 2032, exhibiting a CAGR of 14.3% during the forecast period.
Cell freezing media are specialized formulations designed to preserve cell viability during cryopreservation, a critical process for cell therapy applications. These media typically contain cryoprotectants such as dimethyl sulfoxide (DMSO) to prevent ice crystal formation and cellular damage during freezing and thawing cycles. However, DMSO-free formulations are gaining traction due to safety concerns, particularly for clinical applications where regulatory compliance is essential. Advanced formulations now include serum-free and xeno-free options that meet good manufacturing practice (GMP) standards for therapeutic use.
Get free sample of this report at : https://www.intelmarketresearch.com/download-free-sample/984/cell-freezing-media-for-cell-therapy-2025-2032-317
List of Key Cell Freezing Media Companies Profiled
BioLife Solutions (U.S.)
Thermo Fisher Scientific (U.S.)
Merck KGaA (Germany)
Cytiva (U.S.)
Zenoaq (Japan)
WAK-Chemie Medical (Germany)
Sartorius (Germany)
Akron Biotechnology (U.S.)
Sansheng Bio (China)
Yoon Bio (South Korea)
Segment Analysis:
By Type
DMSO-based Formulations Lead Due to Widespread Adoption in Conventional Cryopreservation
The market is segmented based on type into:
DMSO-based formulations
Subtypes: Standard DMSO media, Serum-containing DMSO media
DMSO-free formulations
Subtypes: Synthetic cryoprotectant media, Glycerol-based media
Serum-free formulations
Animal component-free formulations
GMP-grade formulations
By Application
CAR-T Cell Therapy Segment Shows Strong Growth Potential Due to Rising Immunotherapy Adoption
The market is segmented based on application into:
CAR-T cell therapy
Mesenchymal stem cell therapy
Hematopoietic stem cell transplantation
Regenerative medicine research
Biobanking
By End User
Pharmaceutical & Biotechnology Companies Dominate as Major Consumers of Cell Freezing Media
The market is segmented based on end user into:
Pharmaceutical & biotechnology companies
Research institutions
Cell therapy centers
Diagnostic laboratories
Blood banks
Report Scope
This market research report offers a holistic overview of global and regional markets for the forecast period 2025–2032. It presents accurate and actionable insights based on a blend of primary and secondary research.
Key Coverage Areas:
✅ Market Overview
Global and regional market size (historical & forecast)
Growth trends and value/volume projections
✅ Segmentation Analysis
By product type or category
By application or usage area
By end-user industry
By distribution channel (if applicable)
✅ Regional Insights
North America, Europe, Asia-Pacific, Latin America, Middle East & Africa
Country-level data for key markets
✅ Competitive Landscape
Company profiles and market share analysis
Key strategies: M&A, partnerships, expansions
Product portfolio and pricing strategies
✅ Technology & Innovation
Emerging technologies and R&D trends
Automation, digitalization, sustainability initiatives
Impact of AI, IoT, or other disruptors (where applicable)
✅ Market Dynamics
Key drivers supporting market growth
Restraints and potential risk factors
Supply chain trends and challenges
✅ Opportunities & Recommendations
High-growth segments
Investment hotspots
Strategic suggestions for stakeholders
✅ Stakeholder Insights
Target audience includes manufacturers, suppliers, distributors, investors, regulators, and policymakers
FREQUENTLY ASKED QUESTIONS:
▶ What is the current market size of Global Cell Freezing Media for Cell Therapy Market?
The global cell freezing media for cell therapy market was valued at USD 78.3 million in 2024 and is projected to reach USD 195 million by 2032.
▶ Which key companies operate in Global Cell Freezing Media for Cell Therapy Market?
Key players include BioLife Solutions, Thermo Fisher Scientific, Merck, Cytiva, and Zenoaq, collectively holding 87% market share in 2023.
▶ What are the key growth drivers?
Key growth drivers include rising demand for cell-based therapies, expansion of clinical trials, and advancements in cryopreservation technologies.
▶ Which region dominates the market?
North America leads the market with 42% share, while Asia-Pacific shows the highest CAGR of 16.1% during 2024-2032.
▶ What are the emerging trends?
Emerging trends include DMSO-free formulations, GMP-compliant media development, and automation in cryopreservation processes.
Get free sample of this report at : https://www.intelmarketresearch.com/download-free-sample/984/cell-freezing-media-for-cell-therapy-2025-2032-317
0 notes
Text
Addressing Unmet Needs: How Allogeneic Cell Therapy Market is Providing New Hope
Allogeneic Cell Therapy Market Growth & Trends
The global Allogeneic Cell Therapy Market is poised for significant expansion, with an expected valuation of USD 1.72 billion by 2030. This growth represents a remarkable CAGR of 27.40% from 2023 to 2030. This upward trend is driven by several factors, including the inherent advantages of allogeneic therapies and a surge in related clinical development and regulatory approvals.
Key Drivers of Market Growth:
Benefits Over Autologous Therapies: Allogeneic cell therapies involve transferring cells from healthy donors to patients, offering key advantages over autologous therapies (which use a patient's own cells). These benefits include lower cost due to scalable production from different donor tissues (like bone marrow) and availability as an "off-the-shelf" product, simplifying logistics and access.
Increasing Clinical Trials and Research: There has been a significant surge in allogeneic cell-based therapy clinical trials. For example, the number of mesenchymal stem cell (MSC)-based clinical trials has doubled over the last five years, with 1014 registered as completed or in process as of July 14th, 2021, according to the US National Institutes of Health. This growth, coupled with advancements in precision medicine and an increase in cell therapy production facilities, is a major market driver.
Rising Regulatory Approvals: Regulatory bodies are increasingly approving allogeneic cell-based therapies. A notable instance is the FDA's approval of Enzyvant's RETHYMIC in October 2021, a single-dose regenerative tissue-based therapy for pediatric patients with congenital athymia. Such approvals validate the efficacy and safety of these therapies and open doors for broader market adoption.
Expansion of Manufacturing Capabilities: The approval of manufacturing facilities, such as Charles River Laboratories' Memphis cell therapy manufacturing facility by the European Medicines Agency (EMA) in August 2022 for commercial production of allogeneic cell therapy, signifies a crucial milestone. This expands the capacity for large-scale production, addressing the growing demand.
Strategic Collaborations and Novel Offerings: Key market players are actively pursuing strategic initiatives to develop new therapies. An example is the February 2022 collaboration between ONK Therapeutics and Intellia Therapeutics to develop five allogeneic NK cell therapies using Intellia's genome editing platform. Such partnerships accelerate innovation and contribute to market growth.
Impact of COVID-19:
The COVID-19 pandemic initially had an unfavorable impact on the allogeneic stem cell therapy industry, causing disruptions in research and development activities, clinical trials, manufacturing, and logistics. This made it challenging to evaluate the full treatment benefits and economic impacts of cell and gene therapies.
However, the pandemic also paradoxically raised awareness about the potential of cell therapy as a treatment option for COVID-19. This increased awareness is expected to have a positive long-term impact on the market. For instance, in May 2020, Lineage Cell Therapeutics received emergency funding to develop a potential vaccine against SARS-CoV-2 using their allogeneic dendritic cell therapy (VAC).
Curious about the Allogeneic Cell Therapy Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Allogeneic Cell Therapy Market Report Highlights
The stem cell therapies segment held the largest share of 77.00% in 2022. Allogeneic stem cell therapy is most frequently used to treat chronic diseases like blood cancers, leukemia, and lymphoma as well as specialized blood or autoimmune disorders
By therapeutic area, the hematological disorders segments accounted for the largest share of 58.22% in the global allogeneic cell therapy industry in 2022. Since leading market players are implementing several strategic plans into action to create cutting-edge allogeneic cell therapies for hematological disease treatment
North America held the largest market share of 80.42% in 2022. This can be attributed to the presence of the major global players in the market, and growing investments from public and private organizations for proteomic and life science research in the region
Allogeneic Cell Therapy Market Segmentation
Grand View Research has segmented the global allogeneic cell therapy market based on therapy type, therapeutic area, and region:
Allogeneic Cell Therapy Type Outlook (Revenue, USD Million, 2018 - 2030)
Stem Cell Therapies
Hematopoietic Stem Cell Therapies
Mesenchymal Stem Cell Therapies
Non-stem Cell Therapies
Keratinocytes & Fibroblast-based Therapies
Others
Allogeneic Cell Therapy Therapeutic Area Outlook (Revenue, USD Million, 2018 - 2030)
Hematological Disorders
Dermatological Disorders
Others
Download your FREE sample PDF copy of the Allogeneic Cell Therapy Market today and explore key data and trends.
0 notes
Text
The Role of Innovation at Regenerative Medicine Conferences
Innovation is the heartbeat of progress in healthcare, and nowhere is this more evident than at a regenerative medicine conference. Events like the ones hosted by Cell Surgical Conference bring together leading minds from clinical, scientific, and technological sectors to explore groundbreaking ideas that redefine what’s possible in medicine. From stem cell advancements to AI-powered diagnostics, regenerative medicine conferences serve as incubators for discovery and dialogue.
Emerging Therapies Spotlighted at Regenerative Medicine Conference
One of the biggest draws of any regenerative medicine conference is the unveiling of new therapies with the potential to change lives. Attendees gain early access to clinical research breakthroughs and therapeutic protocols that focus on reversing tissue damage and restoring function. From mesenchymal stem cells to exosome therapy, the innovation on display often leads to more targeted, effective treatments. These discoveries are not theoretical—they are evidence-based and ready to be translated into practice.
Technology Integration Driving Clinical Improvements
Technological advancements are seamlessly woven into every regenerative medicine conference agenda. Whether it’s AI-driven imaging tools, 3D bioprinting, or remote diagnostics, technology enhances how medicine is delivered and how outcomes are measured. Cell Surgical Conference often features sessions that dive into how these digital tools are improving patient engagement, procedural accuracy, and long-term recovery. Innovation here is not about replacing practitioners—it’s about empowering them with more precise instruments and data.
Collaborative Learning at Regenerative Medicine Conference Events
A regenerative medicine conference isn’t just about sitting through lectures—it’s about engaging in two-way learning and collaborative exchange. The Cell Surgical Conference emphasizes workshops, case reviews, and expert roundtables that promote shared insights and collective problem-solving. Innovation thrives in this environment. Doctors, researchers, and medical entrepreneurs from around the world bring different perspectives that challenge traditional thinking.
Ethical Innovation and Responsible Advancements in Focus
While innovation is exciting, regenerative medicine conference organizers like Cell Surgical Conference place strong emphasis on ethical considerations and regulatory compliance. Panels frequently address the importance of responsible research, informed consent, and transparency in patient care. As new therapies emerge, so do questions around accessibility, long-term safety, and societal impact.
Inspiring Next-Gen Leaders in Regenerative Medicine
One of the most exciting roles of innovation at a regenerative medicine conference is its impact on future generations of medical professionals. Cell Surgical Conference provides educational content that appeals to emerging clinicians, researchers, and students eager to be part of the regenerative revolution. Exposure to innovation early in their careers helps shape a mindset of creativity and adaptability. This not only builds stronger medical teams but also ensures that the legacy of discovery continues.
Real-Time Application of Breakthrough Techniques
Innovation loses impact if it’s not applicable. That’s why a regenerative medicine conference like Cell Surgical Conference places great importance on real-time demonstrations, live case studies, and protocol walk-throughs. These practical sessions allow attendees to see exactly how groundbreaking therapies are implemented in clinical settings. From PRP injections to minimally invasive cell therapy applications, participants witness innovation in action.
Shaping Global Healthcare Trends Through Conferences
What happens at a regenerative medicine conference doesn’t stay there—it often sets the tone for global medical trends. Cell Surgical Conference acts as a catalyst for change by identifying emerging needs and facilitating innovation that addresses them. Topics like aging reversal, immune modulation, and tissue regeneration are not only explored—they’re mapped out for future research and commercialization. In this way, the innovation shared at these events extends far beyond the venue.
Conclusion
Innovation is more than a buzzword at regenerative medicine conferences—it is the driving force behind every presentation, partnership, and practice change. Through conferences like those hosted by Cell Surgical Conference, healthcare professionals access the knowledge, tools, and community they need to stay ahead. The blending of advanced science, ethical rigor, and hands-on application ensures that innovation remains grounded in real-world healing. As the field of regenerative medicine continues to grow, these conferences will play a pivotal role in shaping a healthier, more responsive future for patients and providers alike.
0 notes
Text
Healing the Brain After Stroke: Dr. David Greene Explains the Power of R3 Stem Cells
Stroke recovery has long been a challenge, with traditional treatments offering limited restoration of brain function. In this insightful article, Dr. David Greene, founder of R3 Stem Cell, sheds light on how regenerative medicine—particularly stem cell therapy—is revolutionizing stroke rehabilitation. By harnessing the power of mesenchymal stem cells, R3 Stem Cell aims to promote brain repair, reduce inflammation, and improve quality of life for stroke survivors. Discover how this cutting-edge therapy offers new hope for healing the brain and restoring independence, even months or years after a stroke. Explore the science, success stories, and future of recovery.
#drdavidgreeneorthopedicsurgeon#drdavidgreenearizona#drdavidgreener3stemcell#drdavidgreene#r3stemcell#davidgreeneorthopedicsurgeon#davidgreeneorthopedic#drdavidgreenemd#davidgreenorthopedic
0 notes
Text
Exploring the Future of Healing: Advancements in Therapeutics Stem Cell Therapy
In recent years, therapeutics stem cell therapy has emerged as one of the most promising innovations in regenerative medicine, offering new hope for patients suffering from chronic conditions, degenerative diseases, and injuries that were once considered untreatable. This approach, especially when involving fat derived stem cell therapy, is revolutionizing the way medical professionals think about healing and cellular repair.
The fundamental concept behind therapeutics stem cell therapy is the use of stem cells to replace, repair, or regenerate damaged or diseased tissues within the body. These cells have the remarkable ability to transform into various types of cells and facilitate natural healing processes. While stem cells can be harvested from several sources, fat derived stem cell therapy has gained significant traction due to the abundance and ease of accessibility of adipose tissue. This makes it a less invasive and more patient-friendly option compared to traditional sources such as bone marrow.
One of the defining benefits of fat derived stem cell therapy is the high concentration of mesenchymal stem cells found in adipose tissue. These stem cells possess strong anti-inflammatory properties and the capability to promote tissue regeneration. Moreover, adipose-derived cells are more readily available and do not require the extensive procedures often associated with other sources, which enhances the overall appeal and practicality of this therapeutic strategy.
The application of therapeutics stem cell therapy extends across various medical fields. In orthopedics, it is used to treat joint injuries, osteoarthritis, and tendon damage. In cosmetic medicine, it aids in tissue reconstruction and skin rejuvenation. Even in cardiology and neurology, where treatment options are limited, early research has shown potential for improving heart function and slowing the progression of neurodegenerative diseases. With such diverse applications, it’s evident that this innovative approach is reshaping modern medicine.
Despite the growing popularity and promising outcomes, stem cell therapy—particularly when derived from fat—still faces regulatory and scientific challenges. Rigorous clinical trials and standardization are needed to ensure its safety and efficacy across different patient populations. This underscores the importance of choosing reputable medical providers and staying informed about ongoing research.
rmrmco.com, a brand committed to excellence in regenerative health, recognizes the transformative power of stem cell science. With a focus on integrity, innovation, and patient outcomes, they support education and advancements in this field. As awareness grows and technology evolves, more individuals are seeking alternative therapies that are natural, minimally invasive, and scientifically backed.
Moreover, as interest in personalized medicine increases, fat derived stem cell therapy is positioned to play an even larger role. By using a patient’s own cells, the risk of rejection or adverse immune response is significantly reduced. This compatibility enhances the therapy’s effectiveness and safety profile, providing patients with more confidence in pursuing regenerative treatment options.
The future of therapeutics stem cell therapy is closely tied to our growing understanding of cellular biology and regenerative mechanisms. New delivery methods, improved harvesting techniques, and deeper clinical insights are on the horizon, which will likely lead to more widespread adoption and refined protocols. The more we understand the natural regenerative capacities of the human body, the more we can harness these capabilities to combat illness and enhance quality of life.
As with any emerging medical technology, education and cautious optimism are key. Patients are encouraged to consult with qualified professionals and consider clinical evidence before pursuing any treatment. rmrmco.com advises individuals to explore all available options and to consider stem cell therapy as part of a comprehensive health plan.
In conclusion, therapeutics stem cell therapy and fat derived stem cell therapy represent a bold step forward in the quest for better, more effective healthcare solutions. As science continues to evolve, these regenerative approaches will likely become a cornerstone of modern medicine, offering renewed hope and healing possibilities to countless patients around the world.
0 notes
Text
Human Platelet Lysate (HPL) Market Size, Growth Drivers and Analysis 2037
The global Human Platelet Lysate (HPL) Market has emerged as a vital component in the field of advanced cell culture and regenerative therapies. Valued at USD 1.2 billion in 2024, the market is expected to grow robustly, reaching an estimated USD 2.7 billion by 2037. This growth represents a Compound Annual Growth Rate (CAGR) of 12.5% during the forecast period. This upward trajectory reflects increasing adoption across clinical, pharmaceutical, and research environments, driven by the shift toward animal-free culture supplements and growing demand in regenerative medicine.
Human Platelet Lysate (HPL) Industry Demand
Human Platelet Lysate (HPL) is a cell culture supplement derived from human platelets, replacing traditional animal-derived products like fetal bovine serum (FBS). Rich in growth factors and cytokines, HPL provides an optimal environment for the proliferation and expansion of human cells, making it highly suitable for clinical-grade applications.
Key Demand Drivers:
Cost-Effectiveness: HPL provides a more economical option in the long run by reducing batch-to-batch variability and minimizing contamination risks often associated with animal serums.
Ease of Use and Integration: HPL is compatible with a broad range of cell types and is easily incorporated into existing laboratory workflows.
Extended Shelf Life and Stability: Cryopreserved and freeze-dried HPL variants ensure long-term usability without compromising bioactivity, contributing to consistent research outcomes and logistics efficiency.
The growing focus on personalized medicine, stem cell-based research, and biopharmaceutical innovations continues to fuel demand for high-quality, GMP-compliant cell culture supplements like HPL.
Human Platelet Lysate (HPL) Market: Growth Drivers & Key Restraint
Growth Drivers –
Shift Toward Animal-Free Culture Systems: Regulatory pressures and ethical concerns are pushing researchers and manufacturers away from animal-derived components. HPL, as a human-origin alternative, is gaining traction in clinical and pharmaceutical-grade applications.
Expansion of Regenerative Medicine and Cell Therapy: With stem cell therapy and tissue regeneration seeing exponential growth, demand for xeno-free culture mediums like HPL is rapidly accelerating. Its compatibility with mesenchymal stem cells (MSCs) makes it a preferred choice in clinical research.
Technological Advancements and Automation in HPL Production: Modern production technologies now enable consistent batch quality, scalable volumes, and enhanced sterility, which collectively support wider market adoption and reliability in high-end research.
Restraint –
Regulatory and Ethical Challenges in Sourcing Human Platelets: Despite its advantages, HPL production depends on human blood donations, raising ethical and supply chain concerns. Regulatory compliance, donor variability, and limited donor pools can hinder large-scale manufacturing and standardization.
Human Platelet Lysate (HPL) Market: Segment Analysis
Segment Analysis by Product Type (Heparin-Free HPL, Heparin-Added HPL):
Heparin-Free HPL: Gaining popularity for its compatibility with serum-free and GMP-grade applications. Eliminating heparin reduces the risk of unwanted cellular responses, making it ideal for clinical research and therapeutic use.
Heparin-Added HPL: Traditionally used to prevent clotting during cell culture, this variant still finds demand in legacy systems and non-clinical research settings, although its use is gradually being replaced by heparin-free options.
Request Report Sample@ https://www.kennethresearch.com/sample-request-10352539
Segment Analysis by Application (Cell Therapy, Regenerative Medicine, Tissue Engineering, Vaccine Development, Drug Development):
Cell Therapy: HPL plays a pivotal role in expanding stem cells for therapeutic applications, offering a safer and more consistent alternative to animal-based supplements.
Regenerative Medicine: Used in wound healing, cartilage repair, and organ regeneration studies, HPL supports cell viability and proliferation in tissue repair settings.
Tissue Engineering: Facilitates the development of complex tissue constructs by providing the necessary growth factors for cell differentiation and matrix development.
Vaccine Development: HPL serves as a supportive medium for the growth of virus-producing cell lines, enabling more efficient vaccine production protocols.
Drug Development: Assists in cytotoxicity testing, pharmacokinetics modeling, and high-throughput screening processes that require a human-relevant cellular environment.
Segment Analysis by End‑User (Biopharmaceutical Companies, Academic Research Institutes, Academic Research Institutes):
Biopharmaceutical Companies: Leading adopters of HPL, these firms utilize it in clinical trials, vaccine development, and manufacturing processes under GMP regulations.
Academic Research Institutes: Universities and research centers depend on HPL for a range of studies, including stem cell behavior, regenerative techniques, and in vitro modeling.
c: As outsourcing grows, CROs are leveraging HPL to support pharmaceutical companies with scalable, human-relevant testing environments.
Human Platelet Lysate (HPL) Market: Regional Insights
North America:
North America leads the global market, supported by advanced healthcare infrastructure, significant investments in regenerative medicine, and a strong regulatory framework promoting the use of human-origin materials. The U.S. in particular hosts numerous biotech firms and research initiatives focused on stem cell-based therapies and vaccine innovation, driving continuous demand for HPL.
Europe:
Europe remains a prominent region for HPL utilization, fueled by favorable government policies, collaborative research projects, and a rising emphasis on xeno-free biological products. Countries such as Germany, the UK, and France are at the forefront of clinical trials using cell-based therapies, further elevating HPL demand across academic and biopharmaceutical sectors.
Asia-Pacific (APAC):
The APAC region is poised for rapid growth, driven by increasing government funding for life sciences, expanding pharmaceutical production, and growing awareness of regenerative medicine. Nations like China, India, and Japan are investing heavily in biotechnology and cell therapy infrastructure, presenting strong opportunities for HPL adoption, particularly as cost-effective and scalable solutions gain attention.
Access our detailed report link:https://www.kennethresearch.com/report-details/human-platelet-lysate-market/10352539
Top Players in the Human Platelet Lysate (HPL) Market
Thermo Fisher Scientific (USA),Merck KGaA (Germany),STEMCELL Technologies (Canada),Cook Medical (USA),Lonza Group (Switzerland),ZenBio (USA),PromoCell (Germany),Macopharma (France),Biological Industries (Israel),Cellular Engineering Technologies (USA),Japan Blood Products Organization (Japan),CryoLife (USA),BioIVT (USA),RegenMed (South Korea),HiMedia Laboratories (India),CellGenix (Germany),AventaCell (Australia),TC Biopharm (UK),StemBioSys (USA),StemX (Malaysia)
0 notes
Text
Bone Regeneration Market Growth Driven by Advancements in Biomaterials, Stem Cell Therapy, and 3D Printing
The global bone regeneration market is experiencing remarkable growth, fueled by significant advancements in biomaterials, stem cell therapy, and 3D printing. As the prevalence of bone-related disorders such as osteoporosis, fractures, and bone defects rises, the demand for innovative and effective bone regeneration solutions continues to increase. In parallel, the bone densitometer market is expanding due to the growing need for precise bone density assessment, further driving advancements in regenerative treatments.

Advancements in Biomaterials Enhancing Bone Regeneration
Biomaterials play a crucial role in bone regeneration, providing the necessary scaffolding to support bone growth and healing. Over the years, researchers have developed advanced biomaterials that mimic the natural extracellular matrix, promoting faster and more efficient bone healing. Some of the most promising materials include:
Bioceramics: These include calcium phosphate-based materials such as hydroxyapatite and tricalcium phosphate, which closely resemble the mineral composition of natural bone, making them highly effective in bone grafting procedures.
Biodegradable Polymers: Materials such as polylactic acid (PLA) and polycaprolactone (PCL) are widely used in scaffolds as they provide temporary support and degrade over time as new bone forms.
Bioactive Glass: This innovative material has been shown to stimulate bone cell activity and promote regeneration by releasing beneficial ions into the surrounding tissue.
The continuous improvements in biomaterials have led to better biocompatibility, enhanced mechanical properties, and increased effectiveness in treating bone defects.
Stem Cell Therapy: A Breakthrough in Bone Regeneration
Stem cell therapy is emerging as a game-changing technology in bone regeneration. Mesenchymal stem cells (MSCs), derived from sources such as bone marrow, adipose tissue, and umbilical cord blood, have demonstrated the ability to differentiate into osteoblasts, the cells responsible for bone formation. Stem cell-based approaches offer several advantages, including:
Faster Healing: Stem cells accelerate the bone repair process by directly contributing to new bone formation and releasing growth factors that enhance tissue regeneration.
Reduced Risk of Rejection: Since stem cells can be harvested from the patient’s own body (autologous transplantation), the likelihood of immune rejection is significantly minimized.
Minimally Invasive Procedures: Advances in stem cell therapy have led to less invasive techniques for delivering cells to the affected area, reducing recovery time and post-surgical complications.
Despite its potential, challenges such as scalability, ethical concerns, and regulatory approvals need to be addressed for stem cell therapy to become a mainstream treatment in bone regeneration.
3D Printing Revolutionizing Bone Reconstruction
3D printing technology is reshaping the landscape of bone regeneration by enabling the production of patient-specific bone grafts and implants. With the ability to create highly customized and complex structures, 3D printing offers numerous benefits, including:
Personalized Implants: Using patient imaging data, 3D printers can produce implants that perfectly match the size, shape, and structure of the missing bone, leading to better integration and functionality.
Enhanced Biocompatibility: Advanced 3D printing techniques allow the incorporation of bioactive materials and growth factors, improving the body’s natural healing response.
Cost-Effective Solutions: Traditional bone grafting methods can be expensive and time-consuming. 3D printing streamlines the process, reducing costs and improving accessibility to cutting-edge treatments.
With ongoing research and technological refinements, 3D printing is expected to further revolutionize bone regeneration, making treatments more effective and widely available.
Market Outlook and Future Trends
The bone regeneration market is poised for substantial growth, driven by an aging population, increasing incidences of bone disorders, and continuous advancements in regenerative medicine. Companies and research institutions are investing heavily in R&D to develop next-generation biomaterials, enhance stem cell therapies, and refine 3D printing technologies.
Moreover, the integration of artificial intelligence (AI) and digital health solutions is expected to further optimize bone regeneration procedures, enabling better diagnosis, treatment planning, and patient monitoring. As these technologies continue to evolve, the bone regeneration market will witness significant expansion, providing patients with more efficient, minimally invasive, and highly effective treatment options.
Conclusion
The convergence of biomaterials, stem cell therapy, and 3D printing is revolutionizing the bone regeneration market. These cutting-edge advancements are improving patient outcomes, reducing recovery times, and offering innovative solutions for bone repair and reconstruction. With continued research and technological progress, the future of bone regeneration looks promising, offering hope to millions of individuals suffering from bone-related ailments worldwide.
0 notes
Text

Stem Cell Biotechnology
Stem Cell Biotechnology is a rapidly advancing field at the intersection of stem cell research and biotechnology. It involves the use of stem cells for therapeutic, diagnostic, and research purposes, with applications that span regenerative medicine, drug discovery, disease modeling, and tissue engineering. Stem cells are unique due to their ability to self-renew and differentiate into various specialized cell types, making them a powerful tool for understanding developmental biology and treating degenerative diseases.
Types of Stem Cells:
Embryonic Stem Cells (ESCs) – Pluripotent cells derived from early-stage embryos, capable of developing into any cell type in the body.
Adult Stem Cells (ASCs) – Found in various tissues like bone marrow, blood, and the brain, these multipotent cells help in tissue repair and maintenance.
Induced Pluripotent Stem Cells (iPSCs) – Reprogrammed adult cells that behave like embryonic stem cells, offering an ethical alternative to ESCs.
Mesenchymal Stem Cells (MSCs) – Found in bone marrow and adipose tissues, these cells are widely studied for their potential in regenerative therapies.
Applications of Stem Cell Biotechnology:
Regenerative Medicine: Developing cell-based therapies to repair damaged tissues and organs (e.g., for spinal cord injuries, heart disease, and diabetes).
Drug Discovery and Toxicology Testing: Using stem cells to test drug efficacy and safety in a lab environment.
Disease Modeling: Creating in vitro models of diseases like Parkinson’s, Alzheimer’s, and cancer to study their progression and develop targeted treatments.
Tissue Engineering: Developing bioengineered tissues and organoids for transplantation and research.
Gene Editing and CRISPR Applications: Modifying the genetic makeup of stem cells to correct mutations or enhance therapeutic properties.
Challenges and Ethical Considerations: Despite its immense potential, stem cell biotechnology faces challenges related to immune rejection, ethical concerns (especially regarding embryonic stem cells), regulatory hurdles, and the high cost of research and therapy development.
Future Trends: The future of stem cell biotechnology is focused on advancing personalized medicine, improving 3D bioprinting of tissues and organs, enhancing stem cell storage techniques (cryopreservation), and integrating AI and bioinformatics for better stem cell characterization and application.
Biotechnology Scientist Awards
Visit Our Website : http://biotechnologyscientist.com
Contact Us : [email protected]
Nomination Link : https://biotechnologyscientist.com/member-submission/?ecategory=Membership&rcategory=Member…
#sciencefather#researchawards#Scientist#Scholar#Researcher#StemCellResearch#RegenerativeMedicine#StemCellBiotech#CellTherapy#BiotechnologyInnovation#TissueEngineering#GeneTherapy#PluripotentStemCells#MesenchymalStemCells#StemCellTherapies#EmbryonicStemCells#iPSCResearch#Organoids#BiotechRevolution#Bioethics#PersonalizedMedicine#StemCellApplications#DrugDiscovery#Biomanufacturing#CRISPRGeneEditing#DiseaseModeling#ClinicalTrials#StemCellScience#BiomedicalResearch#FutureOfMedicine
👉 Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Facebook : https://www.facebooFk.com/profile.php?id=61572562140976
Twitter : https://x.com/DiyaLyra34020
Tumblr : https://www.tumblr.com/blog/biotechscientist
Blogger: https://www.blogger.com/u/1/blog/posts/3420909576767698629
Linked in : https://www.linkedin.com/in/biotechnology-scientist-117866349/
Pinterest : https://in.pinterest.com/biotechnologyscientist/
0 notes
Text
The Newest FDA-Approved Drugs of 2025
The first quarter of 2025 has already witnessed significant advancements in pharmaceutical innovation, with the U.S. Food and Drug Administration (FDA) approving several groundbreaking therapies addressing critical unmet medical needs. These novel medications span diverse therapeutic areas including oncology, rare genetic disorders, and pain management, offering new hope for patients with previously limited treatment options. This comprehensive analysis examines the newest FDA-approved drugs of 2025 thus far, exploring their mechanisms of action, approved indications, and potential impact on patient care. Ryoncil (remestemcel-L): A New Hope for Graft Versus Host Disease On January 7, 2025, the FDA granted approval to Ryoncil (remestemcel-L), developed by Mesoblast Limited, for steroid-refractory acute graft versus host disease. This approval represents a significant milestone for patients undergoing allogeneic stem cell transplantation who subsequently develop this potentially life-threatening complication. Ryoncil utilizes mesenchymal stem cells to modulate the inflammatory response associated with graft versus host disease, offering an alternative therapeutic approach for patients who do not respond adequately to conventional steroid therapy. The approval provides transplant physicians with an important additional treatment option for managing this challenging complication that can significantly impact post-transplant outcomes and quality of life.

Datroway (datopotamab deruxtecan-dlnk): Advanced Therapy for Metastatic Breast Cancer AstraZeneca and Daiichi Sankyo received FDA approval for Datroway (datopotamab deruxtecan-dlnk) on January 17, 2025, indicated for hormone receptor-positive, HER2-negative metastatic breast cancer. This antibody-drug conjugate (ADC) specifically targets TROP2, a protein frequently overexpressed in various epithelial tumors. Datroway represents an important addition to the breast cancer treatment armamentarium, especially for patients who have progressed on previous therapies. The targeted delivery mechanism of this ADC allows for precise delivery of cytotoxic agents to cancer cells while minimizing damage to healthy tissues, potentially improving both efficacy and tolerability compared to conventional chemotherapy approaches. Lumakras and Vectibix Combination: Targeted Therapy for Colorectal Cancer The FDA approved the combination of Lumakras (sotorasib) with Vectibix (panitumumab) on January 16, 2025, for adult patients with KRAS G12C-mutated metastatic colorectal cancer. This approval represents a significant advancement in precision oncology, specifically addressing a patient population with historically poor treatment outcomes. Lumakras, a KRAS G12C inhibitor, works synergistically with Vectibix, an EGFR inhibitor, to simultaneously block two critical signaling pathways driving tumor growth. This dual-targeting approach demonstrates how combinatorial strategies can overcome resistance mechanisms that often limit the efficacy of single-agent therapies in advanced cancer treatment paradigms. Journavx (suzetrigine): Non-Opioid Solution for Acute Pain Vertex Pharmaceuticals received FDA approval for Journavx (suzetrigine) on January 30, 2025, for the management of moderate to severe acute pain. This approval addresses the critical need for effective non-opioid pain management options amidst the ongoing opioid crisis. Journavx works by selectively targeting the NaV1.8 sodium channel, which plays a key role in pain signaling. The development and approval of Journavx aligns with broader public health efforts to reduce reliance on opioid medications while still providing effective pain relief options for patients. This non-addictive alternative represents an important step forward in addressing both pain management needs and substance use disorder concerns. Grafapex (treosulfan): Advancing Hematopoietic Stem Cell Transplantation January 2025 also saw the approval of Grafapex (treosulfan) as a preparative regimen for allogeneic hematopoietic stem cell transplantation in adult and pediatric patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). This alkylating agent offers a potentially less toxic conditioning regimen compared to traditional myeloablative approaches, expanding transplant options for patients who might not tolerate more intensive preparative regimens. The approval of Grafapex demonstrates ongoing innovation in transplant medicine, with particular focus on optimizing the risk-benefit profile of these potentially curative but intense therapeutic approaches. February 2025 FDA Approvals Romvimza (vimseltinib): Targeted Therapy for Rare Joint Tumor On February 14, 2025, the FDA approved Romvimza (vimseltinib), developed by Deciphera Pharmaceuticals, for adult patients with symptomatic tenosynovial giant cell tumor (TGCT) where surgical resection would potentially cause worsening functional limitation or severe morbidity. Romvimza is a kinase inhibitor that targets the colony-stimulating factor 1 receptor (CSF1R) pathway implicated in TGCT pathogenesis. This approval provides a non-surgical therapeutic option for patients with this rare benign tumor that can cause significant joint destruction and functional impairment. The development of targeted therapies for such rare conditions highlights the pharmaceutical industry's increasing focus on addressing orphan diseases with significant unmet needs. Adcetris Combination: Novel Approach for Refractory Lymphoma The FDA granted approval on February 11, 2025, for brentuximab vedotin (Adcetris) in combination with lenalidomide and rituximab for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy who are ineligible for autologous hematopoietic stem cell transplantation or CAR T-cell therapy. This novel combination regimen addresses an important therapeutic gap for patients who have exhausted standard treatment options but cannot receive more intensive cellular therapies. The approval reflects growing recognition of the value of rational drug combinations that leverage complementary mechanisms of action to overcome treatment resistance in aggressive hematologic malignancies. Gomekli (mirdametinib): Breakthrough for Neurofibromatosis Type 1 SpringWorks Therapeutics received FDA approval for Gomekli (mirdametinib) on February 11, 2025, for adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas not amenable to complete surgical resection. This kinase inhibitor targets the MEK enzyme within the MAPK pathway that is dysregulated in NF1. Gomekli represents a significant advance in the management of this genetic disorder, offering a targeted approach to addressing one of its most challenging manifestations. The pediatric indication is particularly noteworthy, as it provides an important new therapeutic option for young patients who previously had limited treatment alternatives beyond complex surgical interventions. Ctexli (chenodiol): Treatment for Rare Metabolic Disorder On February 21, 2025, Mirum Therapeutics secured FDA approval for Ctexli (chenodiol) for the treatment of cerebrotendinous xanthomatosis (CTX). This rare genetic disorder affects bile acid synthesis and leads to abnormal deposits of cholesterol and lipids throughout the body, resulting in progressive neurological damage if untreated. Ctexli works by suppressing bile acid synthesis and facilitating elimination of accumulated sterols. The approval highlights the critical importance of early intervention for metabolic disorders and demonstrates industry commitment to developing therapies for ultra-rare conditions affecting small patient populations but with devastating consequences if left untreated. The Significance of 2025's Novel Drug Approvals The novel drug approvals in early 2025 demonstrate several important trends in pharmaceutical development and regulatory science. First, there is continued momentum in precision medicine approaches, with multiple therapies targeting specific molecular pathways or genetic alterations. Second, the approval of several orphan drugs for rare diseases reflects sustained investment in addressing conditions with limited treatment options despite small patient populations. Third, the development of non-opioid pain management solutions like Journavx exemplifies how industry innovation is responding to pressing public health challenges. These approvals also highlight the FDA's commitment to accelerating review of potentially transformative therapies. Several of these medications received expedited review designations, including breakthrough therapy and fast track status, underscoring the agency's focus on facilitating patient access to innovative treatments that address significant unmet medical needs. The diversity of modalities represented in these approvals—from small molecules to antibody-drug conjugates and cellular therapies—illustrates the expanding technological toolkit available to modern drug developers. Upcoming FDA Decisions in 2025 The remainder of 2025 promises to be equally productive for novel therapeutics, with numerous important regulatory decisions anticipated in the coming months. In March 2025, the FDA is expected to rule on several significant applications, including rivoceranib/camrelizumab for unresectable or metastatic hepatocellular carcinoma, vutrisiran for ATTR-CM, diazoxide choline for Prader-Willi syndrome, and fitusiran for hemophilia A or B. These pending decisions span multiple therapeutic areas and could potentially address significant unmet needs. Later in 2025, additional highly anticipated regulatory decisions include semaglutide for reducing risks related to chronic kidney disease in adults with type 2 diabetes, as well as for treating metabolic dysfunction-associated steatohepatitis with moderate to advanced liver fibrosis. The potential approval of fam-trastuzumab deruxtecan-nxki for HER2-low or HER2-ultralow metastatic breast cancer in patients who have received at least one line of endocrine therapy could significantly expand treatment options for a broader population of breast cancer patients. These upcoming decisions underscore the robust innovation pipeline that continues to transform treatment landscapes across multiple disease areas. The Impact of Novel Therapies on Healthcare in 2025 The novel drugs approved in early 2025 collectively represent significant advances in medical treatment that will likely transform care paradigms across multiple therapeutic areas. For oncology patients, the approval of targeted therapies like Romvimza, Datroway, and the Lumakras-Vectibix combination offers new precision medicine approaches that may improve outcomes while potentially reducing adverse effects compared to conventional treatments. For patients with rare genetic disorders like NF1 and CTX, medications such as Gomekli and Ctexli provide disease-modifying therapies for conditions that previously had limited treatment options. These approvals also reflect the evolving regulatory landscape, with increased emphasis on accelerated approval pathways for therapies addressing serious conditions with unmet medical needs. Such regulatory flexibility enables faster patient access to innovative treatments while manufacturers continue to gather long-term efficacy and safety data. The continued development and approval of these breakthrough therapies demonstrates the pharmaceutical industry's ongoing commitment to addressing medical challenges through scientific innovation, ultimately improving patient outcomes and quality of life across a spectrum of diseases. Read the full article
0 notes
Text
North America Stem Cell Therapy Market Segments, Opportunities, Regional Forecast To 2028
The North America stem cell therapy market was valued at US$ 1,299.39 million in 2022 and is projected to reach US$ 3,582.26 million by 2028; it is expected to grow at a CAGR of 18.4% from 2022 to 2028.
Increasing Research Activities Related to Stem Cell Therapy for Effective Disease Management is Driving the North America Stem Cell Therapy Market
Stem cell therapy has been widely investigated across the world. Stem cells are mainly used to replace dying cells and reconstruct damaged tissues. Based on the results of extensive stem cell research conducted so far, many scientists have claimed that these cells could probably be utilized to generate cures and treatments for diseases such as cancer and cardiovascular disease. Newly developed stem cell therapies involve replacing disease-causing cells with stem cells. Many potential treatments involving stem cells are in different phases of clinical trials. The FDA has also approved a few stem cell therapies involved in treating complications related to stem cell transplants. In September 2021, the US Food and Drug Administration (FDA) announced approval for "Ruxolitinib" for treating "graft-versus-host-disease" (GVHD) in patients aged 12 and above. Ruxolitinib provides a patient new hope that suffers from fatal complications associated with stem cell transplants.
📚𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲@ https://www.businessmarketinsights.com/sample/BMIRE00028486
Researchers are further investigating the use of stem cell therapy in treating autoimmune disorders. For example, stem cells can turn into the cells of damaged organs and are used in treating autoimmune diseases. Treatment is carried out using mesenchymal stem cells or fetal stem cells. Hematopoietic stem cells are currently used for treating more than 80 medicals conditions, including immune system diseases, blood disorders, neurological disorders, metabolic disorders, genetic disorders, and cancer types such as leukemia and lymphoma.
𝐓𝐡𝐞 𝐋𝐢𝐬𝐭 𝐨𝐟 𝐂𝐨𝐦𝐩𝐚𝐧𝐢𝐞𝐬
MEDIPOST
RichSource
BioTime, Inc.
Mesoblast Limited
U.S. Stem Cell, Inc.
TiGenix NV
AlloSource
NuVasive Inc
📚𝐅𝐮𝐥𝐥 𝐑𝐞𝐩𝐨𝐫𝐭 𝐋𝐢𝐧𝐤 @ https://www.businessmarketinsights.com/reports/north-america-stem-cell-therapy-market
Segments Covered
By Type
Adult Stem Cell Therapy
Embryonic Stem Cell Therapy
Induced Pluripotent Stem Cell Therapy
Other Stem Cell Therapy
By Treatment
Allogeneic
Autologous
By Application
Musculoskeletal
Dermatology
Cardiology
Drug Discovery & Development
Other Applications
By End User
Hospitals & Specialty Clinics
Academic & Research Institutes
The Future Outlook:
The North American stem cell therapy market is poised for significant growth in the coming years. The increasing prevalence of chronic diseases, the aging population, and the growing demand for innovative treatment options are driving market expansion. Advances in stem cell research, coupled with regulatory clarity and improved reimbursement policies, will further accelerate market growth.
The market is expected to witness a shift towards personalized stem cell therapies, with iPSCs playing a prominent role. The development of off-the-shelf stem cell products, derived from allogeneic sources, will also contribute to market growth.
The future of stem cell therapy in North America is bright, with the potential to revolutionize the treatment of a wide range of diseases and improve the lives of millions of patients. However, navigating the complex regulatory landscape, addressing ethical concerns, and overcoming reimbursement challenges will be crucial for realizing the full potential of this transformative field. The continued investment in research and development, coupled with strong collaborations and a focus on patient safety, will pave the way for a future where stem cell therapies are a mainstream treatment option.
𝐀𝐛𝐨𝐮𝐭 𝐔𝐬: Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
0 notes
Text
North America Stem Cell Therapy Market - Key Players, Size, Trends, Opportunities, Growth Analysis to 2028
The North America stem cell therapy market was valued at US$ 1,299.39 million in 2022 and is projected to reach US$ 3,582.26 million by 2028; it is expected to grow at a CAGR of 18.4% from 2022 to 2028.
Increasing Research Activities Related to Stem Cell Therapy for Effective Disease Management is Driving the North America Stem Cell Therapy Market
Stem cell therapy has been widely investigated across the world. Stem cells are mainly used to replace dying cells and reconstruct damaged tissues. Based on the results of extensive stem cell research conducted so far, many scientists have claimed that these cells could probably be utilized to generate cures and treatments for diseases such as cancer and cardiovascular disease. Newly developed stem cell therapies involve replacing disease-causing cells with stem cells. Many potential treatments involving stem cells are in different phases of clinical trials. The FDA has also approved a few stem cell therapies involved in treating complications related to stem cell transplants. In September 2021, the US Food and Drug Administration (FDA) announced approval for "Ruxolitinib" for treating "graft-versus-host-disease" (GVHD) in patients aged 12 and above. Ruxolitinib provides a patient new hope that suffers from fatal complications associated with stem cell transplants.
📚𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲@ https://www.businessmarketinsights.com/sample/BMIRE00028486
Researchers are further investigating the use of stem cell therapy in treating autoimmune disorders. For example, stem cells can turn into the cells of damaged organs and are used in treating autoimmune diseases. Treatment is carried out using mesenchymal stem cells or fetal stem cells. Hematopoietic stem cells are currently used for treating more than 80 medicals conditions, including immune system diseases, blood disorders, neurological disorders, metabolic disorders, genetic disorders, and cancer types such as leukemia and lymphoma.
𝐓𝐡𝐞 𝐋𝐢𝐬𝐭 𝐨𝐟 𝐂𝐨𝐦𝐩𝐚𝐧𝐢𝐞𝐬 MEDIPOST
RichSource
BioTime, Inc.
Mesoblast Limited
U.S. Stem Cell, Inc.
TiGenix NV
AlloSource
NuVasive Inc
📚𝐅𝐮𝐥𝐥 𝐑𝐞𝐩𝐨𝐫𝐭 𝐋𝐢𝐧𝐤 @ https://www.businessmarketinsights.com/reports/north-america-stem-cell-therapy-market
Segments Covered
By Type
Adult Stem Cell Therapy
Embryonic Stem Cell Therapy
Induced Pluripotent Stem Cell Therapy
Other Stem Cell Therapy
By Treatment
Allogeneic
Autologous
By Application
Musculoskeletal
Dermatology
Cardiology
Drug Discovery & Development
Other Applications
By End User
Hospitals & Specialty Clinics
Academic & Research Institutes
The Future Outlook:
The North American stem cell therapy market is poised for significant growth in the coming years. The increasing prevalence of chronic diseases, the aging population, and the growing demand for innovative treatment options are driving market expansion. Advances in stem cell research, coupled with regulatory clarity and improved reimbursement policies, will further accelerate market growth.
The market is expected to witness a shift towards personalized stem cell therapies, with iPSCs playing a prominent role. The development of off-the-shelf stem cell products, derived from allogeneic sources, will also contribute to market growth.
The future of stem cell therapy in North America is bright, with the potential to revolutionize the treatment of a wide range of diseases and improve the lives of millions of patients. However, navigating the complex regulatory landscape, addressing ethical concerns, and overcoming reimbursement challenges will be crucial for realizing the full potential of this transformative field. The continued investment in research and development, coupled with strong collaborations and a focus on patient safety, will pave the way for a future where stem cell therapies are a mainstream treatment option.
11111111111111
Unlocking Autoimmune Mysteries: Stem Cell Therapy's Expanding Role in Disease Management
The burgeoning field of stem cell therapy is increasingly focused on addressing the complexities of autoimmune disorders, offering a potential path to remission and improved quality of life for millions. Researchers are delving deeper into the mechanisms by which stem cells can modulate the immune system and regenerate damaged tissues, paving the way for innovative treatments.
Autoimmune Disorders: A Challenge for Conventional Medicine:
Autoimmune diseases, characterized by the immune system mistakenly attacking the body's own tissues, pose a significant challenge for conventional medicine. Conditions like multiple sclerosis, rheumatoid arthritis, lupus, and type 1 diabetes often require lifelong management with immunosuppressant drugs, which can have significant side effects.
𝐀𝐛𝐨𝐮𝐭 𝐔𝐬: Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
0 notes