#Cell & Gene Therapy Manufacturing Services Market Trends
Explore tagged Tumblr posts
Text
Cell & Gene Therapy Manufacturing Services Market: Growth Opportunities for New Entrants
The global Cell & Gene Therapy Manufacturing Services Market is experiencing significant growth, driven by advancements in therapeutic approaches for life-threatening and rare diseases. Valued at USD 11.4 billion in 2023, the market is projected to reach USD 70.7 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 22.4% over the forecast period 2024-2032.
Market Segmentation:
The market is segmented based on therapy type, manufacturing scale, manufacturing mode, workflow, and region.
By Therapy Type:
Cell Therapy Manufacturing
Gene Therapy Manufacturing
By Manufacturing Scale:
Preclinical
Clinical
Commercial
By Manufacturing Mode:
In-House Manufacturing
Contract Manufacturing
By Workflow:
Vector Production
Cell Banking
Process Development
Fill & Finish Operations
Analytical & Quality Testing
Get Free Sample Report @ https://www.snsinsider.com/sample-request/1187
Regional Analysis:
North America: Leading the market due to substantial investments in gene therapy companies and a high number of ongoing clinical trials.
Europe: Experiencing growth driven by increased R&D activities and supportive regulatory frameworks.
Asia-Pacific: Anticipated to witness rapid growth owing to rising prevalence of target diseases and expanding healthcare infrastructure.
Key Players
Cellular Therapeutics
Lonza
Bluebird Bio Inc.
Thermo Fisher Scientific
Samsung Biologics
Boehringer Ingelheim
Hitachi Chemical Co., Ltd.
Takara Bio Inc.
Catalent Inc.
Miltenyi Biotec
F. Hoffmann-La Roche Ltd
Novartis AG
Merck KGaA
Wuxi Advanced Therapies and others.
Key Highlights:
As of May 2022, there were 329 cell and gene therapies undergoing clinical trials, with numbers expected to rise due to improved scientific understanding and clinical practices.
Approximately USD 2.3 billion has been invested in gene therapy companies over the past decade, indicating strong commitment from global pharmaceutical and biotechnology firms.
The increasing prevalence of cancer and other target diseases, along with heightened R&D spending by pharmaceutical companies, is propelling market growth.
Future Outlook:
The Cell & Gene Therapy Manufacturing Services Market is poised for substantial expansion, driven by the rising incidence of diseases such as cancer and orthopedic disorders, which necessitate innovative treatment solutions. The establishment of new manufacturing facilities and advancements in therapeutic approaches are expected to further fuel market growth. However, challenges such as high operational costs and the need for specialized infrastructure may impact the pace of expansion.
Conclusion:
The global Cell & Gene Therapy Manufacturing Services Market is on a robust growth trajectory, with significant developments across various segments and regions. Stakeholders, including manufacturers, healthcare providers, and investors, are well-positioned to benefit from the evolving landscape of cell and gene therapy manufacturing services.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Medical Display Market Size
Medical Waste Containers Market Size
IoT Medical Devices Market Size
eClinical Solutions Market
#Cell & Gene Therapy Manufacturing Services Market#Cell & Gene Therapy Manufacturing Services Market Share#Cell & Gene Therapy Manufacturing Services Market Size#Cell & Gene Therapy Manufacturing Services Market Trends#Cell & Gene Therapy Manufacturing Services Market Growth
0 notes
Text
The Cell and Gene Therapy Manufacturing Services Market in 2023 is US$ 6 billion, and is expected to reach US$ 22.14 billion by 2031 at a CAGR of 17.70%.
#Cell and Gene Therapy Manufacturing Services Market#Cell and Gene Therapy Manufacturing Services Market Growth#Cell and Gene Therapy Manufacturing Services Market Trends
0 notes
Text
Life Sciences BPO Market: Key Trends and Growth Drivers
The global life sciences BPO market size is expected to reach USD 827.5 billion by 2030, registering a CAGR of 9.3% over the forecast years, according to a new report by Grand View Research, Inc. The rising costs of drug development; pre-clinical and clinical trials; and post-marketing surveillance; a rise in the patent cliff; a growing product pipeline; and regulatory constraints are some of the major factors driving the market. The pharmaceutical and biotech industries invest heavily in the R&D sector to continuously introduce new molecules, devices, and treatments. The different stages of drug development, such as drug discovery, pre-clinical studies, and clinical trials, require huge financial, technological, and human resources.
To cater to the growing needs of the industry, the outsourcing vendors are expanding their product and service offerings and they are targeting niche areas for long-term growth and profitability. Altogether, these trends are expected to boost the life sciences outsourcing market over the forecast period. During the COVID-19 pandemic, a significant number of outsourcing providers expanded their existing manufacturing and research facilities to meet the growing demand for COVID-19 vaccines and diagnostics. Even post-pandemic, CDMOs are practicing similar strategies to support the future demand for pharmaceuticals. For instance, in April 2022, Aenova Group developed a new facility for manufacturing highly potent drugs with an investment of EUR 10 million.
The adoption of such strategies by the market players is expected to be profitable for the market. There has been a rising demand to reduce the cost of manufacturing and development of drugs and medical devices. Outsourcing manufacturing, research, and marketing services provide pharmaceutical and medical device companies with cost- and time-saving benefits. This is expected to have a positive impact on the market. Over the years, mergers and acquisition deals between CROs and CDMOs have increased. The rising demand for clinical services and the growing need for specialized service providers to improve the focus on their core competencies are some of the factors that are expected to drive the incidence of M&A deals.
Gather more insights about the market drivers, restrains and growth of the Life Sciences BPO Market
Life Sciences BPOMarket Report Highlights
• The increasing number of M&A transactions has broadened the global reach and improved the capabilities of CROs and CDMOs to provide end-to-end services; a continuation of this trend is expected to benefit the market significantly
• COVID-19 incidence has decreased significantly as a result of a growing global vaccination campaign
• Owing to this, the CRO and CDMO are now refocusing on developing drugs for oncology and other diseases owing to their high burden
• For instance, in April 2022, Labcorp collaborated with Xcell Biosciences to support the company in developing cell and gene therapies for treating cancer, Parkinson’s, and other rare diseases
• Such initiatives by the CDMOs are likely to profit the market owing to the high effectiveness of gene therapy in treating cancer and other rare diseases
• The medical device segment is expected to register the fastest CAGR from 2023 to 2030 due to the complexities associated with medical device designing
• The strict regulatory framework for medical device approval globally has further contributed to the demand for medical device outsourcing services
• Asia Pacific held the largest revenue share in 2022 due to the presence of a significant number of CROs providing cost-effective BPO services
Life Sciences BPO Market Segmentation
Grand View Research has segmented the global life sciences BPO market based on service and region:
Life Sciences BPO Services Outlook (Revenue, USD Billion, 2018 - 2030)
• Pharmaceutical outsourcing
o Contract Manufacturing Market
o API
o Finished Dose Form
o Packaging
o Contract Research Organizations
o Drug Discovery
o Pre-clinical Studies
o Clinical Trial Studies
o Regulatory Services
o Pharmacovigilance
• Medical Devices Outsourcing
o Contract Manufacturing Market
o Electronic Manufacturing Services
o Finished Goods
o Raw Materials/ Components
o Contract Research Organizations
o Regulatory Consulting Services
o Product Design and Development Services
o Product Testing Services
o Product Implementation Services
o Product Upgrade Services
o Product Maintenance Services
• Contract sales and marketing outsourcing
• Others
Life Sciences BPO Regional Outlook (Revenue, USD Billion, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o Japan
o China
o India
o Australia
o Thailand
o South Korea
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the Life Sciences BPO Market Intelligence Study, published by Grand View Research.
#Life Sciences BPO Market#Life Sciences BPO Market Size#Life Sciences BPO Market Share#Life Sciences BPO Market Analysis#Life Sciences BPO Market Growth
0 notes
Text
Leading Countries in the Global Pharmaceutical Market
The global pharmaceutical industry is among the largest and most dynamic sectors worldwide, influenced by healthcare systems, innovation in drug development, pricing strategies, and government regulations. Certain nations lead the market due to their substantial healthcare investments, advanced R&D capabilities, and supportive regulatory policies.
United States: A Global Leader in Healthcare and R&D
The United States stands at the forefront of the global pharmaceutical market, driven by its significant healthcare spending and state-of-the-art research and development. Home to some of the largest pharmaceutical companies, the US boasts a robust pipeline of groundbreaking drugs and therapies.
With healthcare spending exceeding 18% of its GDP, the US creates an environment conducive to innovation. The FDA, its regulatory authority, plays a pivotal role in ensuring the safety and efficacy of pharmaceuticals, solidifying the country’s leadership position in the industry.
Industry Giants Shaping the Market
The pharmaceutical market is dominated by major companies headquartered in the US, Switzerland, and Germany. Global leaders like Pfizer, Johnson & Johnson, Roche, and Novartis hold considerable market shares and drive advancements in healthcare. These companies thrive on continuous innovation, strategic acquisitions, and collaborative partnerships, reinforcing their positions in the competitive landscape.
Drug Pricing and Market Trends
Drug pricing and launch strategies heavily influence the pharmaceutical market. In the US, a lack of stringent price controls and a strong patent system often result in higher drug prices, allowing pharmaceutical companies to maintain substantial profit margins.
On the other hand, countries like the UK and Germany implement price regulations and negotiate with manufacturers to ensure medications remain affordable. These contrasting approaches have fueled global discussions on drug pricing, access, and equity.
Government Policies Supporting Growth
Government policies play a crucial role in shaping the pharmaceutical industry. In the US, initiatives such as the 21st Century Cures Act have accelerated R&D efforts. Similarly, nations like India and China have experienced rapid growth due to strong government support, including subsidies, tax benefits, and other incentives designed to foster innovation and expand healthcare access.
Emerging Markets and Future Prospects
Emerging economies, particularly China and India, are poised to take on more prominent roles in the global pharmaceutical market. These countries offer competitive pricing and are rapidly developing their healthcare systems, enabling broader access to essential medicines.
As global demand for innovative treatments continues to grow, the pharmaceutical industry will evolve, driven by technological breakthroughs, market shifts, and supportive government initiatives.
Another Report Offered By Delveinsight
Filgrastim Biosimilar Insight | Foot And Ankle Devices Market | Gene And Cell Therapies In Rare Disorder Market | Generalized Anxiety Disorder Market | Gouty Arthritis Market | Graves’ Disease Market | Head And Neck Cancer Market | Healthcare Due Diligence Services | Healthcare Pipeline Analysis | Hemodynamic Monitoring Systems Market | Hemophilia With Inhibitor Market | Hemorrhagic Cystitis Market | Hepatic Encephalopathy Epidemiology Forecast | Hepatic Encephalopathy Market | Hyperhidrosis Market | Hyperkalemia Market | Interbody Cages Market | Kidney Transplant Rejection Market
Conclusion
Countries that prioritize healthcare investments, maintain strong R&D ecosystems, and implement favorable policies dominate the global pharmaceutical market. While innovation in drug development promises to improve patient care worldwide, addressing challenges such as affordability and accessibility remains a key priority for the industry.
About DelveInsight DelveInsight is a premier market research and consulting firm specializing in healthcare and life sciences. By providing in-depth market analysis, DelveInsight helps pharmaceutical, biotechnology, and medical device companies make data-driven, strategic decisions in an increasingly competitive market.
Contact Information Kanishk Kumar Email: [email protected]
0 notes
Text
mRNA Synthesis & Manufacturing Market worth $738.3 million by 2029
The mRNA synthesis and manufacturing market is projected to reach USD 738.3 million in 2029 from USD 624.4 million in 2024. This market is projected to grow at a CAGR of 3.4% over the forecast period. The primary drivers behind the expansion of this industry are the Growing focus on mRNA-based vaccine development, expanding therapeutic applications of mRNA technology, advancements in mRNA synthesis technology, increased outsourcing for mRNA synthesis and modification, and collaborations among industry players. However, stability, storage, and manufacturing scalability present a challenge to this industry. This is further amplified by the slow patient adoption rate and the complexity of the development of mRNA-based therapy.
In many important respects, artificial intelligence (AI) is transforming the mRNA synthesis and manufacturing sector. First, by scanning large databases to find suitable mRNA sequences for therapeutic usage, artificial intelligence speeds up drug research and development greatly. Developed tools like the LinearDesign AI aim to maximize mRNA sequences, therefore producing vaccines with more antibody responses than conventional techniques. From raw material acquisition to final product packaging, artificial intelligence maximizes several manufacturing steps, thereby lowering costs and raising efficiency. AI-powered predictive maintenance reduces downtime and guarantees manufacturing equipment's seamless running.
Download PDF Brochure:
Browse in-depth TOC on "mRNA Synthesis & Manufacturing Market"
250 - Tables
50 - Figures
250 - Pages
The market is expanding rapidly due to factors such as the development of mRNA-based vaccines and expanded applications such as cancer immunotherapies. Furthermore, improvements in mRNA synthesis technology, a rise in mRNA synthesis and modification outsourcing, and industry players working together to create mRNA therapies all contribute to the growth of the mRNA synthesis and manufacturing market. Additionally, factors such as advancements in drug delivery technologies, growth in the regenerative medicines market, and increasing government funding and private investments in the mRNA therapeutics market will further provide revenue growth opportunities for the players operating in mRNA synthesis & manufacturing.
Based on product type, the mRNA synthesis and manufacturing products market is divided into two broad categories, consumables and instruments. The consumables segment of the market held the largest market share in 2023, due to the sustained use of consumables such as nucleotides, RNA polymerase, reverse transcriptase, buffer, and reagents that also require frequent repurchases. The consumables segment will be experiencing high growth due to several factors, including an increase in the mRNA therapeutics pipeline and growing investments made to develop mRNA-based therapeutics, advancement in mRNA synthesis technologies, increase in demand for consumables among contract service providers with the growing trend of outsourcing.
Based on service type, the global mRNA synthesis and manufacturing services market has been categorized into four service types: mRNA synthesis, modification, and related activities; purification of mRNA; analytical and characterization services; and scale-up and manufacture activities. In 2023, the mRNA synthesis and modification services captured the highest market share because of the demand for custom and modified mRNA sequences, which are intended to enhance therapeutic candidates for the molecules market. Given the expanding uses of the mRNA technology, researchers and developers are looking for mRNA sequences that can incorporate protein expression enhancement or immune response improvement.
Based on application, the market for mRNA synthesis and manufacturing has been divided into segments including vaccines and cell & gene therapy. The vaccine segment has the dominant share in the market in 2023. The large share of this segment can be supported by the large number of clinical trials of mRNA vaccines for various diseases infectious diseases, cancer and rare genetic disorders. The remarkable success of mRNA-based COVID-19 vaccines has not only proven the efficacy & scalability of mRNA technology but also catalysed interest in targeting other therapy areas, such as cancer and rare diseases.
Based on end user, the mRNA synthesis and manufacturing market has been categorized into pharmaceutical and biotechnology companies, academic and research institutes, and CROs and CDMOs. In 2023, pharmaceutical and biotechnology companies dominated the market for mRNA synthesis and manufacturing. According to the market's emerging needs, companies are investing to develop next-generation biologics such as mRNA therapeutics. Higher research and development activities of companies to develop mRNA therapeutics and cell and gene therapies have resulted in rising needs for specialized consumables and instruments as well as synthesis, modification, purification, analysis, and characterization services.
Request Sample Pages:
The global mRNA synthesis and manufacturing market is consolidated with the top five players— Thermo Fisher Scientific Inc. (US), Aldevron, LLC. (Danaher Corporation) (US), TriLink BioTechnologies (US), GenScript (US), and Merck KGaA (Germany). Other prominent market players include, New England Biolabs (US), Promega Corporation (US), Sartorius AG (Germany), WuXi Biologics (China), Takara Bio Inc. (Japan), GENEWIZ (Azenta US, Inc.) (US), Lonza (Switzerland), Telesis Bio Inc. (US), Aurigene Pharmaceutical Services Ltd. (Dr. Reddy's Laboratories Ltd.) (India), ST Pharm (South Korea), AGC Biologics (US).
Thermo Fisher Scientific Inc. (US):
Thermo Fisher Scientific Inc., headquartered in Waltham, Massachusetts, is a leading player in mRNA synthesis and manufacturing, offering a broad range of products and services tailored to this field. The company provides advanced solutions for mRNA synthesis, including custom RNA synthesis services and reagents through its GeneArt platform, which supports the development of mRNA constructs for research, therapeutic, and vaccine applications. Thermo Fisher's technologies enable efficient in vitro transcription (IVT) and include automated solutions that enhance scalability and production efficiency. Their extensive expertise, quality assurance measures, and global reach position them as a key player in advancing mRNA technology and supporting the development of next-generation therapeutics and vaccines.
Aldevron, LLC. (Danaher Corporation) (US):
Aldevron, established in 1998 and based in Fargo, North Dakota, is a key player in the nucleic acid synthesis industry, particularly known for its expertise in mRNA synthesis and manufacturing. The company is highly regarded for producing high-quality mRNA and plasmid DNA, essential for cutting-edge applications in vaccine development, gene therapy, and other biotechnological innovations. Aldevron's offerings include custom RNA synthesis and cGMP-compliant mRNA production, ensuring that their products meet the stringent standards required for clinical use. Aldevron's robust quality control and assurance processes further guarantee the reliability and efficacy of their products. As a global leader in the field, Aldevron has expanded its facilities and technological infrastructure to meet growing demand, establishing a significant presence in the biopharmaceutical sector. Their collaborations with biotechnology firms, pharmaceutical companies, and research institutions underscore their pivotal role in advancing mRNA technology and supporting the development of next-generation therapies and vaccines.
TriLink BioTechnologies (US):
TriLink BioTechnologies, a subsidiary of Maravai LifeSciences based in San Diego, California, is a key player in mRNA synthesis and manufacturing. The company excels in providing high-quality nucleic acid products and services, with a strong focus on mRNA technology. TriLink offers comprehensive mRNA synthesis services, including the production of custom mRNA and chemically modified mRNA, which enhances stability and translation efficiency—crucial for effective therapeutic and vaccine development. Utilizing advanced in vitro transcription technologies, TriLink ensures high yield and purity in their mRNA products.
For more information, Inquire Now!
About MarketsandMarkets™
MarketsandMarkets™ has been recognized as one of America's best management consulting firms by Forbes, as per their recent report.
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. We have the widest lens on emerging technologies, making us proficient in co-creating supernormal growth for clients.
Earlier this year, we made a formal transformation into one of America's best management consulting firms as per a survey conducted by Forbes.
The B2B economy is witnessing the emergence of $25 trillion of new revenue streams that are substituting existing revenue streams in this decade alone. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines - TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the 'GIVE Growth' principle, we work with several Forbes Global 2000 B2B companies - helping them stay relevant in a disruptive ecosystem. Our insights and strategies are molded by our industry experts, cutting-edge AI-powered Market Intelligence Cloud, and years of research. The KnowledgeStore™ (our Market Intelligence Cloud) integrates our research, facilitates an analysis of interconnections through a set of applications, helping clients look at the entire ecosystem and understand the revenue shifts happening in their industry.
Contact:
Mr. Rohan Salgarkar
MarketsandMarkets Inc.
1615 South Congress Ave.
Suite 103, Delray Beach, FL 33445
USA : 1-888-600-6441
UK +44-800-368-9399
Email: [email protected]
Visit Our Website: https://www.marketsandmarkets.com/
0 notes
Text
The Future of CDMOs: Key Trends Shaping the Biopharma Outsourcing Industry in 2024
In the fast-paced world of pharmaceuticals, the demand for speed, efficiency, and expertise has given rise to the prominence of Contract Manufacturing Organizations (CMOs). These organizations play a critical role in bringing innovative drugs to market while allowing pharmaceutical companies to focus on research, development, and innovation.
In this blog, we explore the evolving role of CMOs, the benefits they offer, and the latest trends shaping this dynamic industry.
What Are CMOs and Why Are They Essential?
A Contract Manufacturing Organization (CMO) is a company that provides manufacturing services to pharmaceutical and biotechnology firms. CMOs handle everything from small-scale development to large-scale commercial production. By outsourcing manufacturing to CMOs, pharmaceutical companies can save costs, enhance flexibility, and scale their operations without investing heavily in infrastructure.
Key Benefits of CMOs for Pharmaceutical Companies
Cost EfficiencySetting up and maintaining manufacturing facilities can be prohibitively expensive. CMOs offer a cost-effective solution, as pharmaceutical companies can avoid capital investment in equipment, facilities, and regulatory compliance processes.
Access to Specialized ExpertiseCMOs often possess expertise in areas like high-potency API (HPAPI) production, biologics, and sterile manufacturing that many pharmaceutical companies lack in-house.
Faster Time to MarketCMOs help accelerate production timelines, enabling quicker delivery of life-saving drugs to patients. Their established infrastructure and streamlined processes make it easier to scale production.
Regulatory CompliancePharmaceutical manufacturing requires adherence to stringent regulatory standards. CMOs invest heavily in maintaining compliance with FDA, EMA, and other global regulatory authorities, reducing the compliance burden for their clients.
Focus on Core CompetenciesBy outsourcing manufacturing, pharmaceutical companies can focus on their core strengths—such as drug discovery, R&D, and marketing—without getting bogged down by production challenges.
Emerging Trends in the Pharmaceutical CMO Industry
The pharmaceutical CMO landscape is evolving rapidly, driven by technological advancements, changing regulations, and market demands. Here are the key trends to watch:
1. Growth in Biologics Manufacturing
With the rise of biologics and biosimilars, CMOs are expanding their capabilities in areas like monoclonal antibodies, cell therapies, and gene therapies. Advanced manufacturing technologies, such as single-use bioreactors, are transforming biologics production.
2. Digital Transformation
CMOs are adopting cutting-edge technologies like Artificial Intelligence (AI), Machine Learning (ML), and Internet of Things (IoT) for process optimization, predictive maintenance, and enhanced quality control.
3. Flexible Manufacturing
The need for agile production systems is growing. CMOs are investing in modular and continuous manufacturing solutions to meet diverse client needs and improve cost-effectiveness.
4. Focus on Sustainability
Eco-friendly practices are becoming a priority, with CMOs adopting green chemistry, reducing carbon footprints, and using sustainable raw materials. This aligns with the global push toward Environmental, Social, and Governance (ESG) goals.
5. Expansion of Global Manufacturing Hubs
To ensure resilience and reduce supply chain disruptions, CMOs are setting up manufacturing facilities in emerging markets, particularly in Asia-Pacific, Eastern Europe, and Latin America.
How to Choose the Right CMO Partner
Selecting the right CMO partner is critical for a pharmaceutical company’s success. Here are some factors to consider:
Experience and Track Record: Evaluate the CMO’s experience with similar projects and their history of meeting client expectations.
Technical Expertise: Ensure the CMO has the technical capabilities to handle your product’s specific requirements.
Regulatory Compliance: Verify the CMO’s adherence to international regulatory standards.
Capacity and Scalability: Choose a partner that can scale production to meet your needs, whether for clinical trials or commercial-scale manufacturing.
Communication and Transparency: Strong communication ensures smoother collaboration and quicker resolution of issues.
Future of CMOs in the Pharmaceutical Industry
The pharmaceutical industry is evolving at an unprecedented pace, and CMOs are at the heart of this transformation. From developing advanced therapies to ensuring supply chain resilience, CMOs are becoming strategic partners rather than mere service providers. As outsourcing becomes more integral to the pharmaceutical value chain, CMOs will continue to innovate and adapt to meet the industry's demands.
Conclusion
Contract Manufacturing Organizations are not just manufacturers; they are enablers of innovation and efficiency in the pharmaceutical industry. Whether you are a pharmaceutical company seeking to optimize operations or an industry professional exploring the latest trends, understanding the role of CMOs is essential for navigating the future of healthcare.
0 notes
Text
Biologics Contract Development Market Size, Share, Growth, Trends and Forecast To 2030
The global biologics contract development market size is expected to reach USD 13.8 billion by 2030, expanding at 8.1% CAGR from 2023 to 2030, according to a new report by Grand View Research, Inc. Key drivers attributed to the growth include rising adoption of advanced technologies by biologics manufacturers, M&A, and clinical trials in developing nations.The market includes organizations that offer services such as the development of cell lines, upstream and downstream processes, analytical methods, and formulations. These organizations specialize in developing and manufacturing stable cell lines that are extensively used in several important applications, including drug screening, gene functional studies, and biologic production.
Growing M&A and collaboration activities between biopharma companies and CDOs are yet another factor assisting in market growth allowing more financial stability and amalgamation of advanced & specialized technologies. For instance, in September 2022, Lonza., collaborated with biotechnology company Touchlight to expand its product portfolio with different sources of DNA for developing mRNA. Touchlight entered into this partnership to expand its consumer base to the novel doggybone DNA (dbDNA) technology via Lonza's offerings.
Many biopharma and Pharma companies are increasingly looking to outsource their activities as it helps accelerate the workflow (speed) of the company, provide unique specialized services, decrease drug development costs, and provide expertise. These factors are expected to boost the biologics contract development industry's growth.
During the COVID-19 pandemic, most clinical trials were focused on developing new therapies for treating COVID-19. However, in the post-pandemic period, research is expected to focus on cancer owing to rising incidences. Biologics such as monoclonal antibodies have gained significant popularity in treating cancer. The high potential of biologics in treating cancer and the growing number of cancer studies are likely to support the growth of the market in the coming years.
Biologics Contract Development Market Segment Highlights
Mammalian source emerged as the largest product segment in 2022 with over 52.0% share, as the majority of research is being carried out using mammalian cell lines
Process development is expected to witness the highest CAGR over the forecast period, as many companies are opting to outsource the production of recombinant proteins and monoclonal antibodies(MABs)
North America dominated the global market in 2019. An increase in strategic acquisitions & partnerships and a rise in demand for specialized testing services are likely to have a positive impact
Asia Pacific is expected to register the highest CAGR over the forecast period, owing to various amendments made by regulatory organizations to change clinical trials evaluation standards in tandem with global requirements and rising investment in the Asia Pacific region
Browse through Grand View Research's Biotechnology Industry Research Reports.
The global sequencing reagents market size was estimated at USD 8.27 billion in 2024 and is projected to grow at a CAGR of 17.8% from 2025 to 2030.
The global cell counting market size was estimated at USD 9.48 billion in 2024 and is projected to grow at a CAGR of 8.7% from 2025 to 2030.
Segments Covered in the Report
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global biologics contract development market report based on source, service, indication, and region.
Biologics Contract Development Source Outlook (Revenue, USD Million, 2018-2030)
Microbial
Mammalian
Others
Biologics Contract Development Service Outlook (Revenue, USD Million, 2018-2030)
Cell Line Development
Microbial
Mammalian
Others
Process Development
Upstream
Microbial
Mammalian
Others
Downstream
Impurity, isolation, & identification
Physicochemical characterization
Pharmaceutical analysis
Others
By Product
MABs
Recombinant proteins
Others
Biologics Contract Development Indication Outlook (Revenue, USD Million, 2018-2030)
Oncology
Immunological disorders
Cardiovascular disorders
Hematological disorders
Others
Biologics Contract Development Regional Outlook (Revenue, USD Million, 2018-2030)
North America
S.
Canada
Europe
Germany
UK
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
China
Japan
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Colombia
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
Order a free sample PDF of the Biologics Contract Development Market Intelligence Study, published by Grand View Research.
0 notes
Text
The Pharmaceutical CDMO Services Market is projected to grow from USD 146010 million in 2024 to an estimated USD 254648.4 million by 2032, with a compound annual growth rate (CAGR) of 7.2% from 2024 to 2032.The pharmaceutical Contract Development and Manufacturing Organization (CDMO) services market has witnessed significant growth in recent years, driven by the rising demand for cost-effective and efficient drug development processes. CDMOs have become pivotal in the pharmaceutical industry, providing comprehensive services that span from drug development to manufacturing. This article delves into the dynamics shaping this market, its key drivers, and the challenges it faces.CDMOs offer specialized services to pharmaceutical companies, including formulation development, clinical trial production, and large-scale commercial manufacturing. These organizations bridge the gap between innovative drug discovery and scalable manufacturing, enabling pharmaceutical companies to focus on their core competencies.
Browse the full report https://www.credenceresearch.com/report/pharmaceutical-cdmo-services-market
Market Drivers
Several factors are fueling the expansion of the pharmaceutical CDMO services market:
1. Outsourcing Trends: Increasingly, pharmaceutical companies are outsourcing their development and manufacturing processes to CDMOs to reduce costs, improve efficiency, and access specialized expertise. This trend is especially prominent among small and medium-sized pharmaceutical firms that lack in-house capabilities.
2. Rising Drug Demand:
The global rise in chronic diseases such as diabetes, cardiovascular disorders, and cancer has led to a surge in drug demand. This has compelled pharmaceutical companies to scale up production rapidly, creating opportunities for CDMOs.
3. Biologics and Biosimilars Growth: The rapid development of biologics and biosimilars has created a need for advanced manufacturing technologies and facilities, which many CDMOs possess. This has positioned them as indispensable partners for biopharmaceutical companies.
4. Regulatory Complexity: The stringent regulatory environment in the pharmaceutical industry necessitates compliance with international standards. CDMOs often have the necessary certifications and expertise to navigate these challenges, making them attractive collaborators.
5. Technological Advancements: Innovations in drug delivery systems, such as nanoparticle-based therapies and cell and gene therapies, have increased the complexity of drug manufacturing. CDMOs have invested heavily in adopting cutting-edge technologies, enabling them to meet these demands effectively.
Key Segments in the CDMO Market
1. Drug Development: This segment involves preclinical and clinical development services, including formulation and analytical services. The increasing number of clinical trials globally has propelled the demand for development services.
2. Manufacturing: CDMOs provide manufacturing solutions for active pharmaceutical ingredients (APIs) and finished dosage forms. The growing need for high-volume production of generic drugs and novel formulations has boosted this segment.
3. Packaging and Logistics: With the rise of biologics and temperature-sensitive drugs, the demand for specialized packaging and logistics services has grown, further expanding the CDMO market.
Challenges in the CDMO Market
Despite its growth, the CDMO market faces challenges, including:
- Regulatory Hurdles: Compliance with diverse global regulatory standards can be complex and costly.
- Capacity Constraints: The rapid growth in demand often exceeds the production capacity of many CDMOs, leading to delays.
- Intellectual Property Concerns: Pharmaceutical companies may be hesitant to outsource critical stages of drug development due to fears of intellectual property theft or leakage.
Future Outlook
The pharmaceutical CDMO services market is poised for robust growth, driven by advancements in technology, increasing drug development activities, and the growing complexity of pharmaceutical manufacturing. The focus on biologics, biosimilars, and personalized medicine will further enhance the demand for specialized CDMO services.
Key Player Analysis:
Bushu Pharmaceuticals Ltd.
Cambrex Corporation
Catalent, Inc
Cordenpharma International
Laboratory Corporation of America Holdings
Lonza Group AG
Nipro Corporation
Piramal Pharma Solutions
Recipharm Ab
Samsung Biologics
Siegfried Holding Ag
Thermo Fisher Scientific Inc.
Wuxi Apptec
Segmentation:
By Product
API
Type
Traditional Active Pharmaceutical Ingredient (Traditional API)
Highly Potent Active Pharmaceutical Ingredient (HP-API)
Antibody Drug Conjugate (ADC)
Others
Synthesis
Synthetic
Solid
Liquid
Biotech
Drug
Innovative
Generics
Manufacturing
Continuous manufacturing
Batch manufacturing
Drug Product
Oral solid dose
Semi-solid dose
Liquid dose
Others
By Workflow
Clinical
Commercial
By Application
Oncology
Small Molecules
Biologics
Infectious Diseases
Neurological Disorders
Cardiovascular Disease
Metabolic Disorders
Autoimmune Diseases
Respiratory Diseases
Ophthalmology
Gastrointestinal Disorders
Hormonal Disorders
Hematological Disorders
Others
By End-use
Small Pharmaceutical Companies
Medium Pharmaceutical Companies
Large Pharmaceutical Companies
By Regional
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report https://www.credenceresearch.com/report/pharmaceutical-cdmo-services-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Meticulous Research® Projects Global Pharmaceutical Contract Development & Manufacturing Market to Reach $261.57 Billion by 2031
Meticulous Research®, a leading global market intelligence and consulting firm, has released its latest report titled Pharmaceutical Contract Development & Manufacturing Market Size, Share, Forecast, & Trends Analysis by Service and End User – Global Forecast to 2031. According to the report, the pharmaceutical contract development and manufacturing market is poised to reach an impressive $261.57 billion by 2031, registering a compound annual growth rate (CAGR) of 7.4% from 2024 to 2031.
Download Complete Sample PDF Copy Here: https://www.meticulousresearch.com/download-sample-report/cp_id=5171
Key Market Drivers and Challenges
The expanding pharmaceutical contract development and manufacturing market is being driven by several critical factors. The increasing complexity of pharmaceutical manufacturing processes, coupled with manufacturers' adoption of advanced technologies, is fueling growth. In addition, growing investments in pharmaceutical research and development (R&D), the expiration of patents, and heightened demand for generic medicines and biologics are expected to further propel the market.
However, several challenges, including disruptions in the supply chain and stringent government regulations, are hindering growth. The market also faces ongoing issues related to intellectual property risks and a shortage of skilled professionals, posing significant challenges to sustained expansion.
Emerging Opportunities
Despite these hurdles, the pharmaceutical contract development and manufacturing market is expected to witness robust opportunities, particularly in the fields of cell and gene therapies, personalized medicine, and high-potency active pharmaceutical ingredients (HPAPI). The growing demand for antibody-drug conjugates (ADCs) is also projected to open new avenues for market participants.
Quick Buy: https://www.meticulousresearch.com/Checkout/67156803
Competitive Landscape
Key players in the global pharmaceutical contract development and manufacturing market include industry giants such as Lonza Group Ltd. (Switzerland), Catalent Inc. (U.S.), Patheon (a subsidiary of Thermo Fisher Scientific Inc., U.S.), Recipharm AB (Sweden), and WuXi Biologics Inc. (China), among others. These companies continue to play a vital role in shaping the market through strategic collaborations, technological advancements, and capacity expansions.
Future Outlook by Service Type and End User
The market is segmented into key service categories, including pharmaceutical manufacturing (API and FDF), drug development, and biologics manufacturing. Of these, the biologics manufacturing services segment is projected to grow at the fastest rate, with an estimated CAGR of 11.1% during the forecast period. Within this segment, Finished Dosage Forms (FDF) manufacturing services, driven by the complexity of biologics manufacturing, are expected to witness particularly high demand.
Check complete table of contents with list of table and figures: https://www.meticulousresearch.com/product/pharmaceutical-contract-development-and-manufacturing-market-5171
On the basis of end users, large pharmaceutical companies are anticipated to dominate the market, accounting for 42.3% of the global share in 2024. The reliance of major pharmaceutical and biotech companies on external contract manufacturers, driven by increasing R&D complexity and the rising costs of maintaining in-house facilities, is a major factor contributing to this trend.
Geographic Insights
Regionally, North America is projected to capture the largest share of the market, accounting for 44.3% in 2024. The region's dominance is attributed to a combination of factors, including a diversified pharmaceutical pipeline, increased demand for biologics and generic drugs, and a robust base for clinical trials and API production. The presence of leading pharmaceutical companies and advanced manufacturing capabilities further bolster North America's market position.
Request Sample PDF Copy Here: To gain deeper insights into the pharmaceutical contract development and manufacturing market, including growth opportunities, competitive analysis, and geographical trends, download the sample report here: https://www.meticulousresearch.com/request-sample-report/cp_id=5171
Key Questions Addressed in the Report:
What are the fastest-growing market segments by type, end user, and region?
What was the historical market size, and what are the forecasts through 2031?
What are the key market drivers, challenges, and emerging opportunities?
Who are the leading players, and what strategies are they employing?
Which regions are expected to witness the highest growth?
Contact Information:
Meticulous Research® Email: [email protected] Sales Contact: +1-646-781-8004 Connect with us on LinkedIn
0 notes
Text
Vector Purification Market To Surge At 21% CAGR Aided By Rising Cancer Research Activities

The Vector Purification Market is driven by the increasing prevalence of cancer and the need for therapeutic vectors in gene therapy and vaccine development. The vector purification process helps in isolating the recombinant virus or non-viral vectors from host cell lysates or media and removes any contaminating host cell components. This purification is crucial for ensuring the safety and efficacy of engineered vectors in therapeutics and vaccine applications. Vectors are essential tools used for introducing new genetic material into cells. Common vectors include viruses and non-viral systems such as plasmids, which can be engineered to carry therapeutic genes into targeted host cells. Purification removes any contaminating toxic cellular components or proteins, live or dead host cells, media components, and impurities from the final vector preparation.
The Vector Purification Market is estimated to be valued at US$ 336 Mn in 2024 and is expected to exhibit a CAGR of 21% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the vector purification market are Agilent Technologies, BIA Separations, Bio-Rad Laboratories, Merck, and Thermo Fisher Scientific.
Growing incidence of cancer and genetic disorders is fueling the demand for gene and cell therapies with engineered vectors. This is driving significant research investments into developing advanced vector systems, propelling the need for large-scale vector purification.
Technological advancements include automated large-scale purification systems using affinity chromatography, size-exclusion chromatography, and other methods optimized for clinical-grade vectors. Companies are also developing specialized resins and kits tailored for specific vector platforms to streamline purification workflows.
Market Trends
Growing adoption of adeno-associated viral vectors - AAV vectors have emerged as the preferred gene delivery tool due to their low pathogenicity and ability to transduce non-dividing cells. This is spurring increased R&D utilizing AAV systems.
Continuous processing technologies - Continuous multi-column purification systems coupled with in-line monitoring are being introduced to enhance process scalability and vector yields for clinical and commercial applications.
Market Opportunities
Developing economies in Asia Pacific and Latin America present lucrative opportunities for vector purification product suppliers and contract service providers, driven by increased government funding for cell and gene therapy research.
Custom purification solutions - Partnering with clinical developers to provide tailored resins, chromatography methods and single-use kits optimized for specific vectors could capture a larger share of the high-growth advanced therapy market.
Impact of COVID-19 on Vector Purification Market Growth
The COVID-19 pandemic has significantly impacted the growth of the vector purification market. With lockdowns imposed across various regions, research activities slowed down drastically impacting the demand for vector purification kits, columns and other products. Social distancing measures also disrupted the supply chain to some extent. However, with the pandemic accelerating research on vaccine and therapeutics development, the need for DNA and RNA purification increased rapidly. Several biopharma companies accelerated their clinical trials and manufacturing operations focusing on COVID-19 treatment and prevention.
Post pandemic, the vector purification market is expected to witness robust growth. With rising investments to boost preparedness for future pandemics, research focusing on vaccine development and gene therapy is likely to increase. Several contract manufacturing and custom services companies have also expanded their capabilities to support clinical trials and commercial manufacturing of cell and gene therapies. This would drive the demand for high quality and reproducible purification solutions. Additionally, continued expansion of regenerative medicine applications and advancement of gene editing tools would further propel the market growth. Key players are actively investing in portfolio expansion and automation technology to increase throughput and reproducibility.
Europe contributed the largest revenue share to the Vector Purification Market owing to significant research focus and presence of major market players. However, Asia Pacific represented the fastest growing regional market and is anticipated to overtake Europe during the forecast period. This can be attributed to rising healthcare investments, increasing biotech research workforce and strengthening manufacturing capabilities in major Asian countries including China, India and South Korea. The growing geriatric population base and rising incidence of genetic disorders also present lucrative opportunities.
As countries seek to reduce dependence on imported vaccines and biologics, focus is growing on boosting domestic manufacturing capabilities. This presents immense opportunities for vector purification solution providers to assist various regional and contract manufacturing organizations with technology transfer and process development projects. Key strategies for market players should include partnerships with regional clinical research organizations, establishment of local technical centres and development of affordable product offerings.
Get more insights on this topic: https://www.trendingwebwire.com/vector-purification-market-is-estimated-to-witness-high-growth-owing-to-automation-technologies/
Author Bio:
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights. (LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
What Are The Key Data Covered In This Vector Purification Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Vector Purification Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Vector Purification Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Vector Purification Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Vector Purification Market vendors
FAQ’s
Q.1 What are the main factors influencing the Vector Purification Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Vector Purification Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Vector Purification Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Vector Purification Market Trend#Vector Purification Market Size#Vector Purification Market Information#Vector Purification Market Analysis#Vector Purification Market Demand
0 notes
Text
Cell Therapy Industry: Trends and Market Insights 2024-2031

In recent years, cell therapy has emerged as a revolutionary approach in the realm of medical treatments, offering promising solutions for various chronic and life-threatening conditions. This innovative field harnesses the potential of living cells to treat diseases by repairing or replacing damaged tissues and organs. The cell therapy market is experiencing significant growth, driven by advancements in technology, increased research and development activities, and a rising prevalence of chronic diseases.
According to recent industry reports, the global cell therapy market is on a robust growth trajectory. Global Cell Therapy Market size was valued at USD 3.9 Billion in 2022 and is poised to grow from USD 6.7 Billion in 2023 to USD 190.91 Billion by 2031, growing at a CAGR of 52% during the forecast period (2024-2031). This growth is attributed to several factors, including technological advancements, a surge in clinical trials, and the increasing demand for personalized medicine.
Get Your Free Sample Report Here @ https://www.skyquestt.com/sample-request/cell-therapy-market
Key Market Drivers
1. Technological Advancements: Breakthroughs in cell processing technologies, gene editing techniques such as CRISPR, and improvements in manufacturing processes are driving the market. These advancements enhance the efficacy and safety of cell therapies, making them more accessible and effective for patients.
2. Rising Prevalence of Chronic Diseases: The growing incidence of chronic conditions such as cancer, cardiovascular diseases, and autoimmune disorders is fueling the demand for cell-based treatments. Cell therapy offers potential cures and long-term solutions for these persistent health challenges.
3. Increased Research and Development: Ongoing research efforts and clinical trials are expanding the applications of cell therapy. Investment in R&D by pharmaceutical companies and biotech firms is crucial for discovering new therapies and improving existing ones.
4. Regulatory Support: Regulatory agencies are increasingly providing clearer pathways for the approval and commercialization of cell therapies. This supportive regulatory environment is crucial for accelerating the development and market entry of new treatments.
Market Segmentation
The cell therapy market is segmented based on cell type, application, and geography.
- By Cell Type: The market includes various types of cells such as stem cells, T-cells, and dendritic cells. Stem cell therapies are particularly prominent due to their potential in regenerative medicine.
- By Application: Applications span across oncology, cardiovascular diseases, neurological disorders, and more. Oncology holds a significant share, driven by the development of CAR-T cell therapies.
- By Geography: North America dominates the market, followed by Europe and the Asia-Pacific region. The strong presence of key players and advanced healthcare infrastructure contribute to North America's leading position.
To establish the important thing traits, Ask Our Experts @ https://www.skyquestt.com/speak-with-analyst/cell-therapy-market
Cell Therapy Market Top Players' Company Profiles - Thermo Fisher Scientific, Inc., Merck KGaA, Danaher Corporation, Becton, Dickinson, and Company, Lonza Group, Sartorius AG, Nkarta, Inc., Aurion Biotech, S. BIOMEDICS, MEDIPOST, Anterogen Co., Ltd., JW Therapeutics, JCR Pharmaceuticals Co., Ltd., Johnson & Johnson Services, Inc, Bristol-Myers Squibb Company, Gilead Sciences, Inc., Novartis AG, Intellia Therapeutics, Iovance Therapeutics, CRISPR Therapeutics
Challenges and Opportunities
Despite its promising prospects, the cell therapy market faces several challenges, including high treatment costs, complex manufacturing processes, and regulatory hurdles. However, these challenges also present opportunities for innovation and improvement. Advances in manufacturing technologies, cost reduction strategies, and streamlined regulatory processes could further propel the growth of the market. The cell therapy market is poised for significant growth, driven by technological advancements, rising disease prevalence, and supportive regulatory environments. As the field continues to evolve, it holds the potential to transform the landscape of medical treatments and improve patient outcomes worldwide. Stakeholders in the healthcare industry must stay abreast of these developments to capitalize on the opportunities presented by this dynamic market.
#CellTherapyMarket#CellTherapy#MarketTrends#HealthcareMarket#BiotechMarket#StemCellMarket#RegenerativeMedicine#MarketAnalysis#ClinicalTrials#MedicalInnovation#HealthcareInvestment#CellTherapyResearch#MarketGrowth#PharmaMarket#GeneTherapy#OncologyMarket#TherapeuticMarket#HealthcareTrends#CellularMedicine#RegenerativeScience
0 notes
Text
Biotechnology Contract Manufacturing Market Projections: Global Industry Analysis and Forecast (2023-2032)

The Biotechnology Contract Manufacturing Market is projected to witness significant growth, with its value expected to surge from USD 19,149.43 million in 2024 to USD 36,105.98 million by 2032, reflecting a robust compound annual growth rate of 8.25%.
The biotechnology contract manufacturing market is experiencing robust growth, driven by the increasing outsourcing trends in the biopharmaceutical industry, where companies seek to leverage external expertise and infrastructure to streamline production processes and reduce costs. Contract manufacturing organizations (CMOs) offer a range of services, from cell line development and process optimization to large-scale production and regulatory support. This market expansion is fueled by the rising demand for biopharmaceuticals, including monoclonal antibodies, vaccines, recombinant proteins, and gene therapies, which require sophisticated manufacturing capabilities and stringent regulatory compliance. As the complexity of biologic drugs increases, biopharmaceutical companies increasingly rely on CMOs to provide the necessary technological expertise, advanced facilities, and scalability.
North America dominates the biotechnology contract manufacturing market due to its well-established biopharmaceutical industry, robust research and development activities, and the presence of leading CMOs. The United States, in particular, benefits from a strong focus on innovation and substantial investment in biotechnology, which drives the demand for contract manufacturing services. Europe also holds a significant share of the market, supported by a strong regulatory framework, advanced healthcare infrastructure, and increasing investment in biotech research. The region's focus on personalized medicine and biosimilars further propels the demand for contract manufacturing services.
The study on the biotechnology contract manufacturing market reveals several key findings that underscore the market's dynamics, growth drivers, challenges, and future prospects.
1. Robust Market Growth
The biotechnology contract manufacturing market is experiencing substantial growth, driven by the increasing trend of outsourcing manufacturing processes in the biopharmaceutical industry. This growth is attributed to the rising demand for biopharmaceuticals, including monoclonal antibodies, vaccines, recombinant proteins, and gene therapies, which require sophisticated manufacturing capabilities and stringent regulatory compliance.
2. North America Leads the Market
North America dominates the biotechnology contract manufacturing market, primarily due to its well-established biopharmaceutical industry, robust research and development activities, and the presence of leading CMOs. The United States is at the forefront, benefiting from substantial investments in biotechnology and a strong focus on innovation.
3. Significant Market Share in Europe
Europe holds a significant share of the market, supported by a strong regulatory framework, advanced healthcare infrastructure, and increasing investment in biotech research. The region's focus on personalized medicine and biosimilars further propels the demand for contract manufacturing services.
4. Rapid Growth in Asia-Pacific
The Asia-Pacific region is witnessing rapid growth in the biotechnology contract manufacturing market. Countries like China, India, and South Korea are emerging as key players, offering cost advantages and large-scale manufacturing capabilities. Government initiatives to develop biotechnological infrastructure and improve regulatory standards support market growth.
5. Increasing Demand for Biopharmaceuticals
The rising incidence of chronic diseases and the increasing adoption of biologics in treatment protocols are driving the demand for contract manufacturing services. Biopharmaceutical companies are increasingly outsourcing manufacturing to CMOs to leverage their expertise and advanced facilities, ensuring high-quality and scalable production.
6. Technological Advancements
Ongoing advancements in biotechnology and manufacturing technologies are critical drivers of market growth. CMOs are investing in state-of-the-art facilities and adopting innovative technologies such as single-use systems and continuous manufacturing to enhance efficiency and meet the evolving demands of their clients.
7. Operational Challenges
The market faces several challenges, including high operational costs, stringent regulatory requirements, and complex manufacturing processes. Ensuring consistent product quality and managing supply chain complexities are critical issues that CMOs must address to maintain their competitive edge.
8. Strategic Collaborations and Partnerships
Strategic collaborations and partnerships are becoming increasingly important in the biotechnology contract manufacturing market. These alliances help CMOs expand their service offerings, enhance their technological capabilities, and meet the growing demands of biopharmaceutical clients.
9. Focus on Personalized Medicine
The growing focus on personalized medicine is driving the demand for specialized manufacturing services. CMOs are adapting to this trend by developing capabilities to produce small batches of personalized therapies, which require highly specialized and flexible manufacturing processes.
Key Player:
Lonza (Switzerland)
Samsung Biologics (South Korea)
Thermo Fisher Scientific, Inc. (US)
Catalent, Inc. (US)
JSR Corporation (Japan)
WuXi Biologics (China)
AbbVie, Inc. (US)
Boehringer Ingelheim International GmbH (Germany)
Eurofins Scientific (Luxembourg)
GenScript Biotech Corporation (US)
More About Report- https://www.credenceresearch.com/report/biotechnology-contract-manufacturing-market
The biotechnology contract manufacturing market is influenced by several trending factors that shape its growth trajectory and operational dynamics. These factors highlight the evolving landscape of biopharmaceutical production and the strategic adjustments being made by Contract Manufacturing Organizations (CMOs) to stay competitive and meet industry demands.
1. Increasing Demand for Biologics
The demand for biologics, including monoclonal antibodies, vaccines, recombinant proteins, and cell and gene therapies, is on the rise. Biologics are becoming integral to modern therapeutic regimens due to their specificity and efficacy in treating complex diseases such as cancer, autoimmune disorders, and genetic conditions. This surge drives the need for specialized manufacturing capabilities offered by CMOs.
2. Advancements in Biomanufacturing Technologies
Technological innovations are transforming biomanufacturing processes. The adoption of single-use systems, continuous manufacturing, and automation enhances production efficiency, reduces contamination risks, and allows for greater flexibility in manufacturing operations. CMOs are increasingly investing in these advanced technologies to offer cutting-edge services and maintain a competitive edge.
3. Outsourcing Trends
Biopharmaceutical companies are increasingly outsourcing manufacturing to CMOs to focus on core competencies such as research and development, and to reduce capital expenditure on manufacturing infrastructure. Outsourcing also provides access to specialized expertise and advanced facilities, which are crucial for the production of complex biologics.
4. Regulatory Stringency and Compliance
The regulatory landscape for biopharmaceutical manufacturing is becoming more stringent, with regulatory bodies like the FDA and EMA enforcing rigorous quality standards. CMOs are responding by enhancing their compliance frameworks, investing in quality assurance, and adopting Good Manufacturing Practices (GMP) to ensure product safety and efficacy.
5. Personalized Medicine
The shift towards personalized medicine, where treatments are tailored to individual patient profiles, is driving demand for flexible and scalable manufacturing solutions. CMOs are developing capabilities to produce small, customized batches of therapies, which requires highly specialized manufacturing processes and sophisticated quality control measures.
6. Collaborations and Partnerships
Strategic collaborations between biopharmaceutical companies and CMOs are becoming more common. These partnerships enable the sharing of knowledge, resources, and technology, fostering innovation and expanding service offerings. Collaborations also help in risk-sharing and accelerating the time-to-market for new therapies.
7. Emerging Markets
The biotechnology contract manufacturing market is expanding rapidly in emerging markets, particularly in Asia-Pacific. Countries like China, India, and South Korea are investing heavily in biotechnology infrastructure and capabilities. These regions offer cost advantages and large-scale manufacturing capacities, attracting biopharmaceutical companies looking to optimize production costs.
8. Focus on Sustainable Manufacturing
Sustainability is gaining prominence in the biopharmaceutical industry. CMOs are adopting eco-friendly manufacturing practices, reducing waste, and improving energy efficiency. Sustainable manufacturing not only meets regulatory requirements but also aligns with the growing emphasis on corporate social responsibility and environmental stewardship.
9. Mergers and Acquisitions
The market is witnessing a wave of mergers and acquisitions, driven by the need for consolidation and expansion of service portfolios. Larger CMOs are acquiring smaller, specialized companies to enhance their capabilities and enter new markets. This trend is leading to the creation of integrated service providers that can offer end-to-end solutions.
10. Innovation in Cell and Gene Therapy Manufacturing
The rapid development of cell and gene therapies is creating new opportunities for CMOs. Manufacturing these advanced therapies requires specialized expertise and infrastructure. CMOs are investing in the necessary technologies and processes to support the production of these innovative treatments, which are expected to play a significant role in future therapeutics.
Segments:
Based on Service:
Manufacturing
Formulation and Fill-Finish
Packaging and Labeling
Other services
Based on Type:
Biologic Drug Substance Manufacturing
Biologic Drug Product Manufacturing
Based on Source:
Mammalian Expression Systems
Non-Mammalian Expression Systems
Based on Molecule:
Monoclonal Antibodies
Cell Therapy & Gene Therapy
Antibody-Drug Conjugates (ADCs)
Vaccines
Therapeutic Peptides & Proteins
Other Molecule Types
Browse the full report – https://www.credenceresearch.com/report/biotechnology-contract-manufacturing-market
Browse Our Blog: https://www.linkedin.com/pulse/biotechnology-contract-manufacturing-market-forecast-aedif
Contact Us:
Phone: +91 6232 49 3207
Email: [email protected]
Website: https://www.credenceresearch.com
0 notes
Text
The Booming Bioreactors Market: Key Trends and Growth Drivers
The bioreactors market has witnessed significant growth over the past few years, driven by advancements in biopharmaceutical production, increasing research activities in biotechnology, and the rising demand for personalized medicine. Bioreactors, essential for cultivating cells or tissues in a controlled environment, are crucial in various applications, including pharmaceuticals, food and beverage production, and waste management. As the bioreactors market continues to expand, it is poised to revolutionize several industries by enhancing productivity and efficiency in bioprocesses.
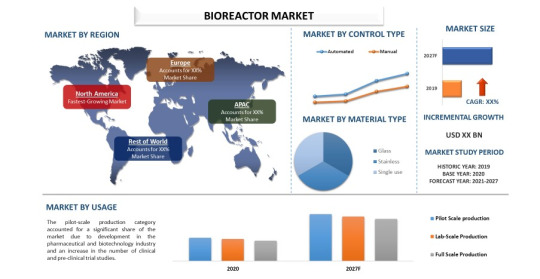
Market Drivers and Technological Advancements
One of the primary drivers of the bioreactors market is the burgeoning biopharmaceutical industry. With the global population's aging and the rise in chronic diseases, there is a growing demand for advanced therapeutics, including monoclonal antibodies, vaccines, and cell and gene therapies. Bioreactors play a pivotal role in the large-scale production of these biopharmaceuticals, ensuring high yield and consistent quality. The shift towards biologics, which are more complex and sensitive than traditional small-molecule drugs, necessitates sophisticated bioreactor systems capable of maintaining precise environmental conditions.
Technological advancements have significantly influenced the bioreactors market. Innovations such as single-use bioreactors (SUBs) have gained immense popularity due to their cost-effectiveness, reduced risk of contamination, and operational flexibility. Unlike traditional stainless-steel bioreactors, SUBs do not require extensive cleaning and sterilization processes, making them ideal for small-scale production and research purposes. Moreover, advancements in automation and digitalization have enabled real-time monitoring and control of bioprocesses, enhancing efficiency and reducing human error.
For a comprehensive analysis of the market drivers https://univdatos.com/report/bioreactors-market/
Market Segmentation and Applications
The bioreactors market can be segmented based on type, usage, scale, and end-user. Types of bioreactors include single-use bioreactors and stainless-steel bioreactors. Single-use bioreactors are witnessing higher adoption rates due to their advantages in terms of cost, scalability, and reduced contamination risk. However, stainless-steel bioreactors remain prevalent in large-scale commercial production due to their durability and suitability for high-volume manufacturing.
Usage segmentation includes microbial and cell culture bioreactors. Microbial bioreactors are primarily used for the production of antibiotics, enzymes, and other microbial products, while cell culture bioreactors are crucial for producing biopharmaceuticals, vaccines, and therapeutic proteins. The growing focus on cell and gene therapies has further fueled the demand for cell culture bioreactors, as these therapies require precise and controlled culturing environments.
In terms of scale, bioreactors are categorized into lab-scale, pilot-scale, and industrial-scale. Lab-scale bioreactors are essential for research and development activities, pilot-scale bioreactors for process development and optimization, and industrial-scale bioreactors for large-scale production. The increasing investment in R&D by biopharmaceutical companies and research institutions is driving the demand for lab-scale and pilot-scale bioreactors.
End-users of bioreactors include biopharmaceutical companies, academic and research institutions, and contract research and manufacturing organizations (CROs and CMOs). Biopharmaceutical companies hold the largest market share due to their extensive use of bioreactors in drug development and production. CROs and CMOs are also significant contributors to the market, offering bioreactor-based services to various industries.
Regional Insights and Growth Opportunities
Geographically, the bioreactors market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America dominates the market, driven by the presence of major biopharmaceutical companies, advanced healthcare infrastructure, and significant R&D investments. Europe follows closely, with a strong focus on biotechnology and pharmaceutical research.
The Asia-Pacific region is expected to witness the highest growth rate during the forecast period. Factors such as increasing healthcare expenditure, a growing biopharmaceutical industry, and favorable government initiatives to promote biotechnology research contribute to this growth. Countries like China and India are emerging as key markets due to their expanding biotechnological capabilities and large patient populations.
For a sample report, visit https://univdatos.com/get-a-free-sample-form-php/?product_id=22012
Challenges and Future Outlook
Despite the promising growth prospects, the bioreactors market faces several challenges. High initial investment costs, the complexity of bioprocessing, and stringent regulatory requirements can impede market growth. Additionally, the ongoing COVID-19 pandemic has disrupted supply chains and delayed clinical trials, impacting the bioreactors market.
However, the long-term outlook for the bioreactors market remains positive. Continuous advancements in bioprocessing technologies, increasing adoption of single-use systems, and the growing focus on personalized medicine and biologics are expected to drive market growth. As industries adapt to evolving healthcare needs and technological innovations, the bioreactors market will continue to expand, offering enhanced solutions for biopharmaceutical production and other applications.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411x
Website -www.univdatos.com
0 notes
Text
mRNA Synthesis & Manufacturing Market worth $738.3 million by 2029
The mRNA synthesis and manufacturing market is projected to reach USD 738.3 million in 2029 from USD 624.4 million in 2024. This market is projected to grow at a CAGR of 3.4% over the forecast period. The primary drivers behind the expansion of this industry are the Growing focus on mRNA-based vaccine development, expanding therapeutic applications of mRNA technology, advancements in mRNA synthesis technology, increased outsourcing for mRNA synthesis and modification, and collaborations among industry players. However, stability, storage, and manufacturing scalability present a challenge to this industry. This is further amplified by the slow patient adoption rate and the complexity of the development of mRNA-based therapy.
In many important respects, artificial intelligence (AI) is transforming the mRNA synthesis and manufacturing sector. First, by scanning large databases to find suitable mRNA sequences for therapeutic usage, artificial intelligence speeds up drug research and development greatly. Developed tools like the LinearDesign AI aim to maximize mRNA sequences, therefore producing vaccines with more antibody responses than conventional techniques. From raw material acquisition to final product packaging, artificial intelligence maximizes several manufacturing steps, thereby lowering costs and raising efficiency. AI-powered predictive maintenance reduces downtime and guarantees manufacturing equipment's seamless running.
Download PDF Brochure:
Browse in-depth TOC on "mRNA Synthesis & Manufacturing Market"
250 - Tables
50 - Figures
250 - Pages
The market is expanding rapidly due to factors such as the development of mRNA-based vaccines and expanded applications such as cancer immunotherapies. Furthermore, improvements in mRNA synthesis technology, a rise in mRNA synthesis and modification outsourcing, and industry players working together to create mRNA therapies all contribute to the growth of the mRNA synthesis and manufacturing market. Additionally, factors such as advancements in drug delivery technologies, growth in the regenerative medicines market, and increasing government funding and private investments in the mRNA therapeutics market will further provide revenue growth opportunities for the players operating in mRNA synthesis & manufacturing.
Based on product type, the mRNA synthesis and manufacturing products market is divided into two broad categories, consumables and instruments. The consumables segment of the market held the largest market share in 2023, due to the sustained use of consumables such as nucleotides, RNA polymerase, reverse transcriptase, buffer, and reagents that also require frequent repurchases. The consumables segment will be experiencing high growth due to several factors, including an increase in the mRNA therapeutics pipeline and growing investments made to develop mRNA-based therapeutics, advancement in mRNA synthesis technologies, increase in demand for consumables among contract service providers with the growing trend of outsourcing.
Based on service type, the global mRNA synthesis and manufacturing services market has been categorized into four service types: mRNA synthesis, modification, and related activities; purification of mRNA; analytical and characterization services; and scale-up and manufacture activities. In 2023, the mRNA synthesis and modification services captured the highest market share because of the demand for custom and modified mRNA sequences, which are intended to enhance therapeutic candidates for the molecules market. Given the expanding uses of the mRNA technology, researchers and developers are looking for mRNA sequences that can incorporate protein expression enhancement or immune response improvement.
Based on application, the market for mRNA synthesis and manufacturing has been divided into segments including vaccines and cell & gene therapy. The vaccine segment has the dominant share in the market in 2023. The large share of this segment can be supported by the large number of clinical trials of mRNA vaccines for various diseases infectious diseases, cancer and rare genetic disorders. The remarkable success of mRNA-based COVID-19 vaccines has not only proven the efficacy & scalability of mRNA technology but also catalysed interest in targeting other therapy areas, such as cancer and rare diseases.
Based on end user, the mRNA synthesis and manufacturing market has been categorized into pharmaceutical and biotechnology companies, academic and research institutes, and CROs and CDMOs. In 2023, pharmaceutical and biotechnology companies dominated the market for mRNA synthesis and manufacturing. According to the market's emerging needs, companies are investing to develop next-generation biologics such as mRNA therapeutics. Higher research and development activities of companies to develop mRNA therapeutics and cell and gene therapies have resulted in rising needs for specialized consumables and instruments as well as synthesis, modification, purification, analysis, and characterization services.
Request Sample Pages:
The global mRNA synthesis and manufacturing market is consolidated with the top five players— Thermo Fisher Scientific Inc. (US), Aldevron, LLC. (Danaher Corporation) (US), TriLink BioTechnologies (US), GenScript (US), and Merck KGaA (Germany). Other prominent market players include, New England Biolabs (US), Promega Corporation (US), Sartorius AG (Germany), WuXi Biologics (China), Takara Bio Inc. (Japan), GENEWIZ (Azenta US, Inc.) (US), Lonza (Switzerland), Telesis Bio Inc. (US), Aurigene Pharmaceutical Services Ltd. (Dr. Reddy's Laboratories Ltd.) (India), ST Pharm (South Korea), AGC Biologics (US).
Thermo Fisher Scientific Inc. (US):
Thermo Fisher Scientific Inc., headquartered in Waltham, Massachusetts, is a leading player in mRNA synthesis and manufacturing, offering a broad range of products and services tailored to this field. The company provides advanced solutions for mRNA synthesis, including custom RNA synthesis services and reagents through its GeneArt platform, which supports the development of mRNA constructs for research, therapeutic, and vaccine applications. Thermo Fisher's technologies enable efficient in vitro transcription (IVT) and include automated solutions that enhance scalability and production efficiency. Their extensive expertise, quality assurance measures, and global reach position them as a key player in advancing mRNA technology and supporting the development of next-generation therapeutics and vaccines.
Aldevron, LLC. (Danaher Corporation) (US):
Aldevron, established in 1998 and based in Fargo, North Dakota, is a key player in the nucleic acid synthesis industry, particularly known for its expertise in mRNA synthesis and manufacturing. The company is highly regarded for producing high-quality mRNA and plasmid DNA, essential for cutting-edge applications in vaccine development, gene therapy, and other biotechnological innovations. Aldevron's offerings include custom RNA synthesis and cGMP-compliant mRNA production, ensuring that their products meet the stringent standards required for clinical use. Aldevron's robust quality control and assurance processes further guarantee the reliability and efficacy of their products. As a global leader in the field, Aldevron has expanded its facilities and technological infrastructure to meet growing demand, establishing a significant presence in the biopharmaceutical sector. Their collaborations with biotechnology firms, pharmaceutical companies, and research institutions underscore their pivotal role in advancing mRNA technology and supporting the development of next-generation therapies and vaccines.
TriLink BioTechnologies (US):
TriLink BioTechnologies, a subsidiary of Maravai LifeSciences based in San Diego, California, is a key player in mRNA synthesis and manufacturing. The company excels in providing high-quality nucleic acid products and services, with a strong focus on mRNA technology. TriLink offers comprehensive mRNA synthesis services, including the production of custom mRNA and chemically modified mRNA, which enhances stability and translation efficiency—crucial for effective therapeutic and vaccine development. Utilizing advanced in vitro transcription technologies, TriLink ensures high yield and purity in their mRNA products.
For more information, Inquire Now!
About MarketsandMarkets™
MarketsandMarkets™ has been recognized as one of America's best management consulting firms by Forbes, as per their recent report.
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. We have the widest lens on emerging technologies, making us proficient in co-creating supernormal growth for clients.
Earlier this year, we made a formal transformation into one of America's best management consulting firms as per a survey conducted by Forbes.
The B2B economy is witnessing the emergence of $25 trillion of new revenue streams that are substituting existing revenue streams in this decade alone. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines - TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the 'GIVE Growth' principle, we work with several Forbes Global 2000 B2B companies - helping them stay relevant in a disruptive ecosystem. Our insights and strategies are molded by our industry experts, cutting-edge AI-powered Market Intelligence Cloud, and years of research. The KnowledgeStore™ (our Market Intelligence Cloud) integrates our research, facilitates an analysis of interconnections through a set of applications, helping clients look at the entire ecosystem and understand the revenue shifts happening in their industry.
Contact:
Mr. Rohan Salgarkar
MarketsandMarkets Inc.
1615 South Congress Ave.
Suite 103, Delray Beach, FL 33445
USA : 1-888-600-6441
UK +44-800-368-9399
Email: [email protected]
Visit Our Website: https://www.marketsandmarkets.com/
0 notes
Text
The Essential Role of Pharmaceutical Contract Manufacturing Companies in Today's Market
https://jpcdn.it/img/r/700/420/b63a8c4d40e88ce177eea6a9e89e07c8.jpg
Introduction: The pharmaceutical industry is evolving rapidly, driven by innovations in drug development, increased demand, and stricter regulatory standards. Pharmaceutical Contract Manufacturing Organizations (CMOs) play an essential role in helping pharmaceutical companies streamline production, optimize costs, and meet high-quality standards. In this post, we’ll explore what pharmaceutical CMOs do, the advantages they offer, and how they are transforming the industry.
1. What is a Pharmaceutical Contract Manufacturing Organization?
Pharmaceutical CMOs provide outsourced manufacturing services to drug companies. They handle the production, formulation, and packaging of pharmaceuticals under contract, allowing companies to focus on core activities like research and development. This model is particularly valuable in managing costs, ensuring compliance, and scaling production to meet market demands.
Key services provided by CMOs include:
Drug development support: including formulation and process development.
Manufacturing: small- and large-scale production, ensuring consistency and regulatory compliance.
Packaging and labeling: customized to meet different global regulatory standards.
Analytical and quality assurance services: to maintain high-quality standards across batches.
2. Benefits of Partnering with a Pharmaceutical CMO
Outsourcing to a CMO brings several benefits to pharmaceutical companies, especially smaller firms without the infrastructure for large-scale production.
Cost-Effectiveness
Establishing in-house manufacturing facilities requires significant capital investment. CMOs already have the equipment, facilities, and expertise, reducing overhead and enabling companies to focus resources on R&D and market expansion.
Expertise and Regulatory Knowledge
CMOs specialize in manufacturing, meaning they have the expertise to navigate complex regulatory requirements. Partnering with a CMO ensures compliance with Good Manufacturing Practice (GMP) standards, reducing the risk of costly regulatory setbacks.
Scalability and Flexibility
Pharmaceutical demand fluctuates. CMOs provide the flexibility to scale production up or down, adapting to market changes without requiring companies to adjust their in-house resources.
3. Trends and Innovations in Pharmaceutical Contract Manufacturing
As the pharmaceutical industry grows more complex, CMOs are incorporating advanced technologies to improve efficiency, sustainability, and quality.
Biopharmaceutical Manufacturing: With the rise of biologics, many CMOs are developing capabilities in cell and gene therapy production, ensuring they can meet the needs of the next generation of drugs.
Automation and AI: Automation technologies and AI are making production more efficient, accurate, and cost-effective. Smart manufacturing facilities can optimize workflows and minimize errors.
Sustainability Initiatives: Many CMOs are investing in green manufacturing practices, reducing waste, energy consumption, and water use in response to industry demands for sustainable production.
4. Key Considerations When Selecting a CMO Partner
Choosing the right CMO partner is crucial for a successful partnership. Here are some factors to consider:
Regulatory Track Record: Verify that the CMO has a strong track record with regulatory authorities, like the FDA and EMA.
Experience with Your Drug Type: If you’re developing biologics or specialty drugs, find a CMO with relevant experience.
Capacity and Scalability: Ensure the CMO can meet your current needs and adapt as your business grows.
Communication and Transparency: Effective communication is essential in managing timelines and responding to challenges promptly.
Conclusion: Pharmaceutical contract manufacturing companies are vital players in today’s healthcare landscape. By offering specialized production services, they enable drug companies to focus on innovation while ensuring that products reach patients efficiently and safely. As the industry evolves, the role of CMOs will only grow, driven by advances in technology and an ever-increasing demand for high-quality pharmaceuticals.
0 notes
Text
Multiplex Assay Market: Leading Types & APAC’s Dominance

Over the years, there has been a dramatic rise in chronic diseases such as cancer, diabetes, and cardiovascular disorders globally. According to recent studies, around 60% of adults in industrialized nations are diagnosed with at least one chronic condition, sparking significant advancements in diagnostic technologies. Multiplex protein assays have emerged at the forefront, offering a robust solution for the simultaneous detection of multiple biomarkers, which is crucial for diagnosing and managing a range of diseases. As per Triton’s analysis, the Global Multiplex Assay Market is set to reach $8235.49 million by 2032, garnering a CAGR of 8.56% during the 2024-2032 forecast period.
Additionally, multiplex assays are particularly instrumental in tailoring personalized medications enhancing treatment efficacy and patient outcomes. Take warfarin, for instance, a blood clot prevention medication. Its effectiveness varies due to genetic diversity among patients and differences in drug metabolism enzymes. Multiplex protein assays streamline this process by simultaneously analyzing multiple genes, enabling more efficient detection of variations in one test cycle.
Explore in detail about this market in our FREE sample
Multiplex Assay Market: Top Two Types Leading Advancements
Protein-Based Assays
Nucleic Acid-Based Assays
Connect with our experts for a simplified analysis!
Asia-Pacific: A Hotspot for Multiplex Assay Market
The Asia-Pacific multiplex assay market is set to witness the fastest growth at a CAGR of 9.34% during 2024-2032, spearheaded by China.
Countries like China and India are leading this surge, with companies such as Shanghai Luminex, known for their advanced multiplex cytokine assays, playing pivotal roles in the regional market dynamics. The region’s market is buoyed by the increase in local manufacturing of multiplex assay kits and the strategic expansion of international players who are investing in local production facilities to reduce costs and improve accessibility.
The proliferation of Contract Research Organizations (CROs) in China has surged, broadening their services and catalyzing business outsourcing. Chinese firms are leveraging this trend by expanding chemistry services to encompass lead optimization, enzyme and cell assays, ADME, and toxicity studies. This expansion, coupled with competitive costs and innovation, is driving the demand for multiplex assay across China. Simultaneously, the biopharmaceutical sector is diversifying beyond generics, prioritizing personalized medicine, biomarkers, novel therapies, and multiplex assays.
In Conclusion,
As we look towards a future where healthcare is more personalized, predictive, and preventive, multiplex assays stand as a cornerstone technology that will strengthen these advancements. Their ability to provide comprehensive, rapid, and accurate testing solutions across various medical and research applications makes them indispensable in the advancing healthcare industry.
Explore Our Latest Release for the 2024-2032 Market Analysis
FAQs
Q1 What is a multiplex assay?
A multiplex assay is a type of laboratory procedure that allows for the simultaneous measurement of multiple analytes (such as proteins, genes, or biomarkers) in a single assay. It is a tool for diagnostics and research, enabling high-throughput and efficient data collection. Q2 What are the advantages of multiplex assays?
Multiplex assays offer several advantages, including reduced cost, lower sample volume requirements, high throughput, and the ability to provide comprehensive data from a single test, which can enhance diagnostic accuracy and ensure better clinical decisions. Q3 How are multiplex assays contributing to personalized medicine?
Multiplex assays contribute to personalized medicine by allowing for the detailed analysis of an individual’s biomarkers, facilitating tailored treatment strategies that improve patient outcomes and minimize side effects.
#multiplex assay#Lifesciences#Diagnostic & Biotechnology#triton market research#market research reports
0 notes