#COMIRNATY
Explore tagged Tumblr posts
Text
Effets secondaires indésirables
Les complotistes agitent leur biais de confirmation – “Vous voyez, hein ! On vous l’avait bien dit !” à cœur joie et à qui mieux mieux –, mais Pfizer ne va pas manquer d’objecter que les effets secondaires graves n’ont lieu que dans d’infimes proportions des populations du monde. N’empêche, comme le faisait remarquer le docteur, feu regretté, Marc Vercoutère, le rapport bénéfice/risque, pour les parents dont l’enfant est gravement atteint des suites d���une vaccination, c’est du risqué à 100 %.
“Mise à jour” vaccinale tous les six mois
« L'action d'aujourd'hui de l'ACIP[Comité consultatif sur les pratiques d'immunisation] fait suite aux recommandations récentes et précédentes du CDC. » … dont : « Après l'autorisation et l'approbation par la FDA de notre formule de vaccin Pfizer-BioNTech COVID-19 2023-2024 plus tôt ce mois-ci, l'ACIPa recommandé à toute personne âgée de 6 mois et plus de recevoir un vaccin COVID-19 mis à jour pour se protéger contre la maladie COVID-19 cet automne et cet hiver. […]
Informations de sécurité importantes
[…]
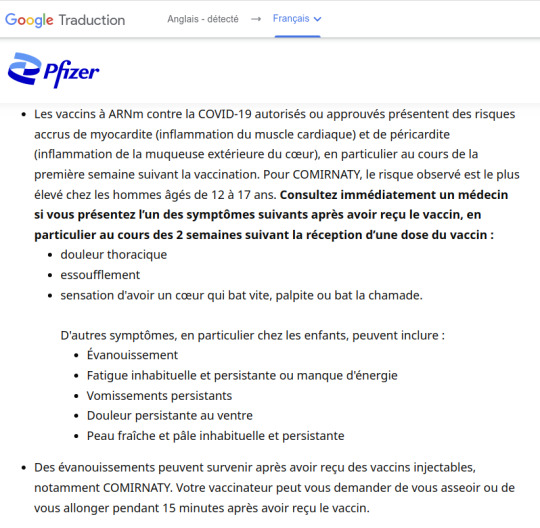
Les vaccins à ARNm contre la COVID-19 autorisés ou approuvés présentent des risques accrus de myocardite (inflammation du muscle cardiaque) et de péricardite (inflammation de la muqueuse extérieure du cœur), en particulier au cours de la première semaine suivant la vaccination. Pour COMIRNATY, le risque observé est le plus élevé chez les hommes âgés de 12 à 17 ans. Consultez immédiatement un médecin si vous présentez l’un des symptômes suivants après avoir reçu le vaccin, en particulier au cours des 2 semaines suivant la réception d’une dose du vaccin :
• douleur thoracique • essoufflement • sensation d'avoir un cœur qui bat vite, palpite ou bat la chamade.
D'autres symptômes, en particulier chez les enfants, peuvent inclure :
• Évanouissement • Fatigue inhabituelle et persistante ou manque d'énergie • Vomissements persistants • Douleur persistante au ventre • Peau fraîche et pâle inhabituelle et persistante
Des évanouissements peuvent survenir après avoir reçu des vaccins injectables, notamment COMIRNATY. Votre vaccinateur peut vous demander de vous asseoir ou de vous allonger pendant 15 minutes après avoir reçu le vaccin.
Les personnes dont le système immunitaire est affaibli peuvent avoir une réponse immunitaire réduite à COMIRNATY
COMIRNATY pourrait ne pas protéger tous les vaccinés
[…]
Les effets indésirables les plus fréquemment rapportés (≥ 10 %) après l'administration d'une dose de COMIRNATY étaient des douleurs au site d'injection (jusqu'à 90,5 %), de la fatigue (jusqu'à 77,5 %), des maux de tête (jusqu'à 75,5 %), des frissons (jusqu'à 49,2 %). %), douleurs musculaires (jusqu'à 45,5 %), douleurs articulaires (jusqu'à 27,5 %), fièvre (jusqu'à 24,3 %), gonflement au site d'injection (jusqu'à 11,8 %) et rougeur au site d'injection (jusqu'à 10,4 %).
Ce ne sont peut-être pas tous les effets secondaires possibles du vaccin. Appelez le prestataire de vaccination ou le prestataire de soins de santé au sujet des effets secondaires gênants ou des effets secondaires qui ne disparaissent pas.
Vous devez toujours demander un avis médical à vos prestataires de soins concernant les événements indésirables. Signalez les effets secondaires du vaccin à la Food and Drug Administration (FDA) des États-Unis et au Vaccine Adverse Event Reporting System (VAERS) des Centers for Disease Control and Prevention (CDC). Le numéro gratuit du VAERS est le 1-800-822-7967 ou signalez-le en ligne sur www.vaers.hhs.gov/reportevent.html . Vous pouvez également signaler les effets secondaires à Pfizer Inc. sur www.pfizersafetyreporting.com ou en appelant le 1-800-438-1985.
[…]
Une myocardite (inflammation du muscle cardiaque) et une péricardite (inflammation de la muqueuse extérieure du cœur) sont survenues chez certaines personnes ayant reçu des vaccins à ARNm contre la COVID-19. La myocardite et la péricardite consécutives aux vaccins Pfizer-BioNTech contre la COVID-19 sont survenues le plus souvent chez les adolescents de sexe masculin âgés de 12 à 17 ans. Chez la plupart de ces personnes, les symptômes sont apparus quelques jours après la vaccination. La probabilité que cela se produise est très faible. Consultez immédiatement un médecin si votre enfant présente l'un des symptômes suivants après avoir reçu le vaccin, en particulier au cours des 2 semaines suivant la réception d'une dose du vaccin :
• Douleur thoracique • Essoufflement ou difficulté à respirer • Sentiment d'avoir un cœur qui bat vite, qui palpite ou qui bat très fort
Des symptômes supplémentaires, en particulier chez les enfants, peuvent inclure :
• Évanouissement • Irritabilité inhabituelle et persistante • Mauvaise alimentation inhabituelle et persistante • Fatigue inhabituelle et persistante ou manque d'énergie • Vomissements persistants • Douleur persistante au ventre • Peau fraîche et pâle inhabituelle et persistante • Des évanouissements peuvent survenir après avoir reçu des vaccins injectables, notamment le vaccin Pfizer-BioNTech contre la COVID-19. Pour cette raison, votre prestataire de vaccination peut vous demander de rester à l'endroit où vous avez reçu le vaccin pour un suivi après la vaccination. • Les personnes dont le système immunitaire est affaibli peuvent avoir une réponse immunitaire réduite au vaccin Pfizer-BioNTech contre la COVID-19 • Le vaccin Pfizer-BioNTech contre la COVID-19 ne protège peut-être pas tout le monde • Informez votre vaccinateur de tous vos problèmes de santé, y compris si vous :
– avez des allergies – a eu une myocardite (inflammation du muscle cardiaque) ou une péricardite (inflammation de la paroi extérieure du cœur) – a de la fièvre – souffre d’un trouble de la coagulation ou prend un anticoagulant – est immunodéprimé ou prend un médicament qui affecte le système immunitaire – est enceinte ou allaite – a reçu un autre vaccin contre la COVID-19 – s'est déjà évanoui à la suite d'une injection
• Les effets secondaires signalés avec les vaccins Pfizer-BioNTech contre la COVID-19 comprennent :
– Réactions allergiques sévères – Réactions allergiques non graves telles qu'éruption cutanée, démangeaisons, urticaire ou gonflement du visage – Myocardite (inflammation du muscle cardiaque) – Péricardite (inflammation de la muqueuse extérieure du cœur) – Douleur/sensibilité au site d'injection – Fatigue – Mal de tête – Douleur musculaire – Des frissons – Douleur articulaire – Fièvre – Gonflement au site d’injection – Rougeur au site d'injection – Nausée – Se sentir pas bien – Ganglions lymphatiques enflés (lymphadénopathie) – Diminution de l'appétit – Diarrhée – Vomissement – Douleur au bras – Évanouissement associé à l’injection du vaccin – Vertiges – Irritabilité [...] »
‣ Pfizer, « Pfizer Broadens Portfolio of Respiratory Vaccines Recommended by CDC Advisory Committee with ABRYSVO™ for RSV » (« Pfizer élargit le portefeuille de vaccins respiratoires recommandé par le comité consultatif du CDC avec ABRYSVO ™ pour RSV » – trad. via Google), pub. 22 sept. 2023, https://www.pfizer.com/news/press-release/press-release-detail/pfizer-broadens-portfolio-respiratory-vaccines-recommended (cons. 21 oct. 2023).
0 notes
Text
FDA Approves and Authorizes Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants - Published Aug 22, 2024
Today, the U.S. Food and Drug Administration approved and granted emergency use authorization (EUA) for updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARS-CoV-2. The mRNA COVID-19 vaccines have been updated with this formula to more closely target currently circulating variants and provide better protection against serious consequences of COVID-19, including hospitalization and death. Today’s actions relate to updated mRNA COVID-19 vaccines manufactured by ModernaTX Inc. and Pfizer Inc.
In early June, the FDA advised manufacturers of licensed and authorized COVID-19 vaccines that the COVID-19 vaccines (2024-2025 formula) should be monovalent JN.1 vaccines. Based on the further evolution of SARS-CoV-2 and a rise in cases of COVID-19, the agency subsequently determined and advised manufacturers that the preferred JN.1-lineage for the COVID-19 vaccines (2024-2025 formula) is the KP.2 strain, if feasible.
“Vaccination continues to be the cornerstone of COVID-19 prevention,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “These updated vaccines meet the agency’s rigorous, scientific standards for safety, effectiveness, and manufacturing quality. Given waning immunity of the population from previous exposure to the virus and from prior vaccination, we strongly encourage those who are eligible to consider receiving an updated COVID-19 vaccine to provide better protection against currently circulating variants.”
The updated mRNA COVID-19 vaccines include Comirnaty and Spikevax, both of which are approved for individuals 12 years of age and older, and the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, both of which are authorized for emergency use for individuals 6 months through 11 years of age.
What You Need to Know
=Unvaccinated individuals 6 months through 4 years of age are eligible to receive three doses of the updated, authorized Pfizer-BioNTech COVID-19 Vaccine or two doses of the updated, authorized Moderna COVID-19 Vaccine.
=Individuals 6 months through 4 years of age who have previously been vaccinated against COVID-19 are eligible to receive one or two doses of the updated, authorized Moderna or =Pfizer-BioNTech COVID-19 vaccines (timing and number of doses to administer depends on the previous COVID-19 vaccine received).
=Individuals 5 years through 11 years of age regardless of previous vaccination are eligible to receive a single dose of the updated, authorized Moderna or Pfizer-BioNTech COVID-19 vaccines; if previously vaccinated, the dose is administered at least 2 months after the last dose of any COVID-19 vaccine.
=Individuals 12 years of age and older are eligible to receive a single dose of the updated, approved Comirnaty or the updated, approved Spikevax; if previously vaccinated, the dose is administered at least 2 months since the last dose of any COVID-19 vaccine.
=Additional doses are authorized for certain immunocompromised individuals ages 6 months through 11 years of age as described in the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine fact sheets.
=Individuals who receive an updated mRNA COVID-19 vaccine may experience similar side effects as those reported by individuals who previously received mRNA COVID-19 vaccines and as described in the respective prescribing information or fact sheets. The updated vaccines are expected to provide protection against COVID-19 caused by the currently circulating variants. Barring the emergence of a markedly more infectious variant of SARS-CoV-2, the FDA anticipates that the composition of COVID-19 vaccines will need to be assessed annually, as occurs for seasonal influenza vaccines.
For today’s approvals and authorizations of the mRNA COVID-19 vaccines, the FDA assessed manufacturing and nonclinical data to support the change to include the 2024-2025 formula in the mRNA COVID-19 vaccines. The updated mRNA vaccines are manufactured using a similar process as previous formulas of these vaccines. The mRNA COVID-19 vaccines have been administered to hundreds of millions of people in the U.S., and the benefits of these vaccines continue to outweigh their risks.
On an ongoing basis, the FDA will review any additional COVID-19 vaccine applications submitted to the agency and take appropriate regulatory action.
The approval of Comirnaty (COVID-19 Vaccine, mRNA) (2024-2025 Formula) was granted to BioNTech Manufacturing GmbH. The EUA amendment for the Pfizer-BioNTech COVID-19 Vaccine (2024-2025 Formula) was issued to Pfizer Inc.
The approval of Spikevax (COVID-19 Vaccine, mRNA) (2024-2025 Formula) was granted to ModernaTX Inc. and the EUA amendment for the Moderna COVID-19 Vaccine (2024-2025 Formula) was issued to ModernaTX Inc.
#covid#pandemic#mask up#covid 19#wear a mask#coronavirus#sars cov 2#still coviding#public health#wear a respirator#covid vaccine
487 notes
·
View notes
Text
Terry Adirim, a senior CIA official and ex-Biden defense appointee, was fired last week for her role in pushing Biden’s controversial military Covid-19 vaccine mandate—a mandate that may have crossed legal lines.
Once the acting assistant secretary of defense for health affairs, Adirim jumped ship to the CIA in December, serving as director of its Centers for Global Health Services until her firing.
According to reports, Adirim’s firing is tied to her role in strong-arming troops to take an Emergency Use Authorized jab or face expulsion.
On September 14, 2021, about a month after then-Defense Secretary Lloyd Austin ordered the vaccine mandate, Adirim issued a memo instructing DOD health care providers to consider the vaccine available to service members — Pfizer’s BioNTech vaccine — as “interchangeable” with a vaccine that was approved by the FDA, known as Comirnaty.
She wrote:
Per FDA guidance, these two vaccines are ‘interchangeable�� and DoD health care providers should ‘use doses distributed under the EUA to administer the vaccination series as if the doses were the licensed vaccine.’ Consistent with FDA guidance, DoD health care providers will use both the PfizerBioNTech COVID-19 vaccine and the Comirnaty COVID-19 vaccine interchangeably for the purpose of vaccinating Service members in accordance with Secretary of Defense Memorandum, ‘Mandatory Coronavirus Disease 2019 Vaccination of Department of Defense Service Members,’ August 24, 2021.
Breitbart report: Her memo was considered legally dubious even inside the Pentagon, as reported in September 2022.
18 notes
·
View notes
Text
Health Canada has given its stamp of approval to Pfizer-BioNTech Comirnaty’s new COVID-19 vaccine that targets the Omicron XBB.1.5 subvariant. The health department says it received Pfizer-BioNTech's submission on June 29, 2023 and decided to authorize the shot's use for individuals aged six months and older after “a thorough and independent review of the evidence.” Health Canada says the vaccine is authorized as a one-dose vaccine for individuals five years of age and older, regardless of their COVID-19 vaccination history.
Continue Reading.
Tagging: @politicsofcanada
85 notes
·
View notes
Text
Brazilian-developed vaccine against Covid-19 registered by Anvisa

The new vaccine against Covid-19 developed by the Brazilian company Zalika Farmacêutica has been entered into the National Health Surveillance Agency (Anvisa) this week, Agencia Brasil reported. The drug can be used in people aged 12 and over and is to be administered in two doses, 21 days apart, with boosters after 6 months for those over 18 years of age.
The technology used in the Zalika vaccine is called “recombinant” because its molecules are formed by combining two different sources. In this case, the protein S antigen (spike) -capable of promoting a response from the immune system- and the saponin-based adjuvant allow the mixture to enhance the production of antibodies. This form of production brings greater safety to the pharmaceutical industry, Anvisa explained in a statement.
The new immunizer is the sixth to receive definitive individual registration from Anvisa, in addition to Comirnaty Ipfizer/Wyeth, Comirnaty bivalent (Pfizer), Janssen Vaccine (Janssen-Cila), Oxford/Covishield (Fiocruz and Astra-Zeneca) and Spikevax bivalent vaccines have received this type of authorization. Pfizer/Biontech, Astra-Zeneca, Janssen, Moderna, Sinopharm, and Sinovac also have definitive registration in the form of the Covax Facility consortium.
Continue reading.
#brazil#brazilian politics#politics#coronavirus#covid 19#vaccination#mod nise da silveira#image description in alt
9 notes
·
View notes
Text
2 notes
·
View notes
Text
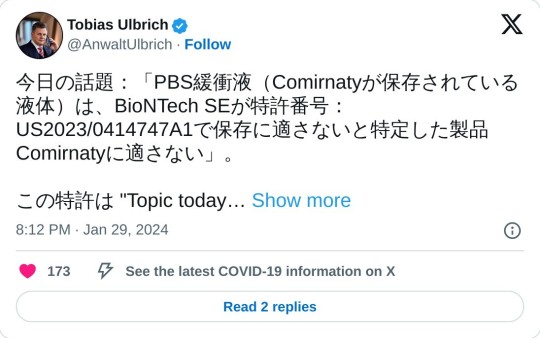
今日の話題:「PBS緩衝液(Comirnatyが保存されている液体)は、BioNTech SEが特許番号:US2023/0414747A1で保存に適さないと特定した製品Comirnatyに適さない」。 この特許は "Topic today "で言及されているが、Comirnaty製品の保存をPBS緩衝液からいわゆるTris緩衝液に変更する根拠を開示している。 レシピ変更に関するプレスリリースに伴い、2021年10月に緩衝液もいわゆるTris緩衝液に変更されたと推測される。 https://cdn.pfizer.com/pfizercom/2021-10/EMA_Tris_Sucrose_Statement_10.18.21_FINAL.pdf… Modernaは最初からTris緩衝液を使っていたので、Modernaにとってこの疑問は生じない。 PBSバッファーで保存する場合とTrisバッファーで保存する場合の違いは何でしょうか? BioNTech社は、PBSバッファーがLNP中に極めて安定した折り畳まれたRNA構造を作ることを問題視した。製品がほぼ1年間この問題を抱えたまま移動した後、Tris緩衝液に置き換えることで解決した。 しかし、この特許出願の日付は2020年11月18日であるため、バイオエヌテックは承認時に意図的に間違った緩衝液で市場に出すことを知っていたことになる。 言い換えれば、この特許は、バイオエヌテックが高度に安定な折り畳みRNA構造を人体に放出することに伴うリスクを認識していた証拠である。 製品文書とEMA文書(https://docs.google.com/document/d/1K-dWmwzH-gbsPstj3bTfYgS1DUTEqIn1uA2T5-4jZr0/edit#bookmark=id.gjdgxs…)によれば、この問題は製造中には発生せず、完成品にのみ発生する。 特許��のものでは、序文[1023]から[1026]にバイオエヌテック自身が認識している問題が明記されている。 医薬品の文書では、バイオエヌテックは「コンフォメーション的に折り畳まれた、あるいは可逆的に凝集したRNA」と述べているが、この特許ではバイオエヌテックは「高度に安定的に折り畳まれたRNA」と述べている。 RNAの構造は、リボソームがスパイクタンパク質武漢1の折りたたみ計画をどのように読み取り、正しく認識するかという点で、決定的に重要である。RNAの構造は、コドンの最適化によって形成されるタンパク質のフォールディングプランを変えることが知られている。このような背景から、強く折り畳まれたRNAもまた、形成されるタンパク質の結果に影響を与える可能性があると推測できる。 また、この変更が製造工程中にしか行われなかったという事実は、変更が必要なほど問題が深刻であったことを示唆している。そうでなければ、バイオテック社はおそらく放置していただろう。 日本の弁理士 パテント・サン が親切にも製造中に指摘してくれた。ありがとうございました。 ガブリエレ・セガッラ博士 https://ijvtpr.com/index.php/IJVTPR/article/view/68/185… によると、オリジナルの緩衝液には、一般的な塩で希釈すると脂質ナノ粒子が凝集するという問題がすでにあった。https://patentimages.storage.googleapis.com/de/67/95/27ec2ea27a32d9/US10485884.pdf… ガブリエレ・セガッラ博士は上記の特許を参照した。 私の側からのコメント: これは製造と開発における欠陥の別の側面であり、現段階の製品が人体に適さないこと、そして同社が発売前にこのことを知っていたことを示している。 #欠陥#製品の欠陥#特許#バイオNTech#LNP#貯蔵#応用#バッファー#PBS#トリス#RNA#EMA#PEI
3 notes
·
View notes
Text
I was just a normal boy before I got the 2023-2034 moderna spikevax covid-19 monovalent booster but now I have breasts and soft skin well let me tell you I doubt I'd have this same debilitating response to the comirnaty formulation but that's all that needs to be said about that.
6 notes
·
View notes
Text
Hello, it's me, the COVID vaccine test subject! I'm always first in line to get new COVID vaccines because I have long COVID and I'm not trying to make it worse. Here are some things of note about the new (as of 9/25/2023) COVID vaccine from Pfizer based on the experience of me and my mom who just got it on Sunday.
General info:
The vaccine is usually called Comirnaty or "The 2023-2024 Covid Vaccine" when you're signing up to get it. It's generally not referred to as a booster. All Pfizer vaccines fall under the name "Cormirnaty" so make sure you're getting the latest one, it's always good to ask the office, clinic, or pharmacy you're scheduling with if you're unsure.
According to the pharmacist who administered my shot, it wasn't supposed to be out for a few weeks so some insurances, including Pennsylvania Medicaid, won't cover it yet. It's $190 out of pocket. I would recommend getting in contact with your insurance company or the place you're getting vaccinated to ensure that you're not going to have to pay for it.
The vaccine is FDA approved and uses MRNA technology, the same as the original Pfizer vaccine.
Side effects (I received the flu and covid vaccines at the same time in the same arm, my mom received only the covid one, will update if/when my dad and sibling get it):
I had very manageable arm soreness and swelling. I've gotten this with every vaccine I've gotten, some are worse than others, this was one of the less severe ones. The swelling isn't interfering with my arm movements at all, it's just certain movements are painful.
I also had extreme sleepiness today, I fought to keep my eyes open for most of the morning and ended up skipping class and just going back to bed for most of the day. There are several factors that could play into this. I went to the ren faire on Saturday and my big crashes are sometimes delayed by a day. I also got both vaccines at the same time which can sometimes cause worse side effects. I wanted to mention it because it could be related to the COVID vaccine alone. I would plan to get the vaccine on a day where you don't have work or another big event going on.
My mom had more severe arm pain and limited range of motion. She struggled to move for the first day. She, like me, often gets a bump and/or pain following shots. She (by her own admission) forgot there were things she could do to reduce the swelling so that might have contributed to her having such a hard time with it.
Tips:
Keep your arm relaxed as much as possible during the shot. Tensing the muscle causes the shot to hurt more.
Move your arm a bit after the shot to reduce pain, but don't do anything strenuous.
If you're able to, take a pain medication with anti-inflammatory properties shortly after getting the shot.
Use a cold compress if the swelling becomes bothersome.
If fear is a barrier to getting a shot, it's perfectly acceptable and actually good to talk to a doctor about taking an anti-anxiety medication beforehand. I use weed or CBD, but you might also be given one dose of a sedative like Ativan or Xanax.
4 notes
·
View notes
Text
New COVID Variant XEC May Outpace Others This Fall - Published Sept 18, 2024
"The virus is always going to be mutating away from what it was in order to get more efficient at infecting individuals," Adalja said. "So I think this really highlights the fact that a universal COVID vaccine, or some vaccine with different technologies, perhaps a nasal vaccine and using mucosal immunity, all of those things are important."
What if, get this, we prevented covid cases by improving ventilation, mandating air filtration, and wearing masks in public? Wouldn't that accomplish the same goal right now? Every mutation takes us further away from the current scientific fantasy of a universal covid vaccine. We have to stop cases to make this dream a reality.
by Sophie Putka
The new COVID-19 variant XEC may overtake others in circulation to become dominant in the coming months, experts said, but will not prompt a meaningful change in symptoms or vaccine response.
So far, the CDC's variant proportions tracker has not registered enough cases of XEC in the U.S. to report it. (The agency's projected estimates for the 2 weeks ending in September 14 currently show KP.3.1.1 and KP.2.3 as the leading variants, with 52.7% and 12.2% of national cases, respectively.) Another estimate using data from the variant tracker GISAID has XEC at 1.11% of U.S. cases as of September 15, with around 48 sequences reported.
First detected in Germany in June, it's been found mostly in Central Europe, representing 10% of cases, according to the U.K.'s Science Media Centre.
"XEC represents a fairly minor evolution relative to the SARS-CoV-2 diversity currently in circulation, and is not a highly derived novel variant such as those that were granted Greek letters," like Alpha, Delta, and Omicron, Francois Balloux, PhD, a computational biologist at University College London and director of the UCL Genetics Institute, said in a Science Media Centre statement.
Experts noted that while XEC may have a small advantage in transmission, available vaccines are still likely to provide protection from serious illness.
XEC is a "recombinant variant of some of the other Omicron lineages that have been around for a while, and it does appear to be more immune evasive, giving it a transmissibility advantage in the population with the immunity that it has," Amesh Adalja, MD, of the Johns Hopkins Center for Health Security in Baltimore, told MedPage Today. "But it doesn't really change anything, just like the last variant didn't change anything, or the one before that, one before that, or the one before that."
Currently available COVID vaccines target slightly different subvariants. The updated mRNA shots aimed at KP.2 from Pfizer-BioNTech (Comirnaty) and Moderna (Spikevax), as well as Novavax's vaccine targeting the JN.1 variant lineage, are still protective against the most serious consequences of COVID infections, experts said.
"If this becomes a dominant variant, it will decrease the efficacy against infection of the updated vaccines, but the updated vaccines will still be durable against severe disease [and] hospitalization, and that's what is really the primary function of our current, first-generation COVID vaccines," Adalja said.
Still, he emphasized, the rapid mutation of the virus underscores a need for a different kind of vaccine than those currently available if the goal is to protect against infection rather than just severe disease.
"The virus is always going to be mutating away from what it was in order to get more efficient at infecting individuals," Adalja said. "So I think this really highlights the fact that a universal COVID vaccine, or some vaccine with different technologies, perhaps a nasal vaccine and using mucosal immunity, all of those things are important."
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator
89 notes
·
View notes
Text
According to a new study, adults who received the Pfizer Comirnaty COVID-19 shot were more likely to die than adults who had the Moderna Spikevax COVID-19 shot. The study looked at 9.2 million adults living in Florida who received either Comirnaty or Spikevax within six weeks, from December 2020 through January 20221. Half of the nearly 1.5 million participants received the Pfizer shot and the other half had the Moderna shot. Participants were matched based on age and gender.1
The study, “Twelve-Month All-Cause Mortality after Initial COVID-19 Vaccination with Pfizer-BioNTech or mRNA-1273 among Adults Living in Florida” was conducted by Florida Surgeon General, Joseph Ladapo, MD, and Retsef Levi, PhD of the Massachusetts Institute of Technology (MIT), as well as two other researchers. The study is in preprint and has not yet been peer-reviewed.2
Pfizer Shot Causes Significantly Higher All-Cause Death
Participants were compared 12 months after their first dose of the COVID shots. Researchers looked at all-cause mortality, cardiovascular mortality, and COVID mortality, together with non-COVID causes of death which occurred within 12 months after participants received a shot.
In terms of all-cause mortality, participants who received the Pfizer shot had a 37 percent higher risk than Moderna shot recipients. The risk for those who received the Pfizer shot was 847.2 and 617.9 for Moderna shot recipients per 100,000 people. Therefore, there were 229.2 excess deaths per 100,000 people in the Pfizer group than in the Moderna group.3
In terms of death due to cardiovascular events, the risk for those who took the Pfizer shot increases to 53 percent. There were 248.7 cardiovascular deaths in the Pfizer group and 162.4 deaths in the Moderna group with a difference of 86.3 extra deaths per 100,000 people in the Pfizer group.4
9 notes
·
View notes
Photo
Except that it wasn't relevant at all. Take measles vacc.
It was tested first, it was safe, it stopped people getting it, it was a modified version of the virus.
The manufacturer could be sued if it harmed people.
It was a choice.
The mRNA treatment was none of that, and the goalposts were endlessly moved, so that it would prevent COVID, then largely prevent, then diminish symptoms.
In the end, there was no proof offered it worked beyond Fauci declaring

It had one thing that the measles vacc never had, however. Coercion.
https://www.servicesaustralia.gov.au/who-can-get-support-under-covid-19-vaccine-claims-scheme?context=55953
If you had AstraZeneca Vaxzevria, the following clinical conditions are accepted under the scheme:
Anaphylactic reaction
Capillary leak syndrome
Cerebral Venous Sinus Thrombosis (CVST) without Thrombocytopenia
Guillain Barre Syndrome (GBS)
Thrombosis with Thrombocytopenia Syndrome
Thrombocytopenia, including immune Thrombocytopenia
Transverse Myelitis.
If you’ve had Pfizer/Biontech Comirnaty, Moderna Spikevax or Novavax Nuvaxovid, the following clinical conditions are accepted under the scheme:
Anaphylactic reaction
Erythema Multiforme (Major)
The following clinically diagnosed injuries are also accepted under the scheme, regardless of which TGA approved vaccine you’ve had:
Myocarditis
Pericarditis
The following harm isn’t covered under the scheme: contracting COVID-19
Not safe. Not effective. Limited liability at best for the manufacturer.
And no consent.
The principles of the Nuremberg Code
The 10 points of the Nuremberg Code are:
Voluntary consent of the human subject in the experimentation is absolutely essential.
Fauci was given a blanket pardon by Biden for deeds that would have had him tried and shot.






This week-long arc Peanuts arc ran when the measles vaccination was first developed and widely administered in 1967. GoComics republished it as part of their rerun strips late last year, but it could obviously stand to go around again.
Big slow claps to everyone who made a 50-year-old PSA relevant, good job everyone we’re doing GREAT
6K notes
·
View notes
Text
Latest COVID Vaccine Heart Risk Warning Update 2025 - FDA
Marking a major revision in safety warnings for millions of Americans, the Food and Drug Administration has issued expanded cardiac-risk advisories for COVID vaccines. This COVID vaccine heart risk warning update comes after new data analysis revealed specific incidence rates of myocarditis and pericarditis following vaccination. FDA Expands Heart Risk Warnings for COVID Vaccines: Updated On June 25, 2025, the FDA announced updated labelling requirements for both Pfizer's Comirnaty and Moderna's Spikevax COVID-19 vaccines. The new warnings provide more detailed information about myocarditis and pericarditis risks, particularly affecting young males aged 12 to 24 years. The updated warning indicates a myocarditis risk of 8 cases per 1 million individuals who received the 2023-2024 COVID vaccines, specifically among those aged 6 months to 64 years. This represents the most comprehensive safety data released by the agency regarding cardiac complications from mRNA vaccines. All healthcare practitioners nationwide are now obligated to disclose these exact risk rates to individuals before they receive the vaccine. The updated labelling comes after extensive analysis of safety data from multiple surveillance systems, including the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD). Latest Key Changes in Vaccine Labelling Expanded Age Range Coverage Whereas the earlier warnings concentrated mainly on males aged 12 to 17, the revised labelling now covers males from 12 to 24 years of age. The modification reflects updated surveillance data indicating heart inflammation cases in an age group wider than the one initially identified. The wider range impacts roughly 25 million young American men aged 12–24. Health officials stress that, despite an enlarged hazard profile, the actual risk remains exceedingly low for most individuals. Specific Incidence Rates Vaccine labels now must list specific risk statistics as prescribed by the FDA. Age Group Risk Rate Population Vaccine Type 6 months - 64 years 8 cases per million doses General population Both mRNA vaccines Males 12-24 years 27 cases per million doses The highest risk group Both mRNA vaccines Males 18-29 years Up to 31.2 excess cases per million Second dose recipients Moderna higher risk Females 12-24 years 3 cases per million doses Lower risk group Both mRNA vaccines Long-term Follow-up Data The updated labels include information from a longitudinal study of approximately 300 patients who developed myocarditis after vaccination. Five months after vaccination, a not insignificant proportion of individuals still exhibited abnormal cardiac magnetic resonance imaging findings, but the clinical implications are still uncertain. This follow-up data represents one of the most extensive tracking efforts for vaccine-associated myocarditis cases. Researchers are monitoring these patients to clarify the long-term cardiac health implications. Understanding Myocarditis and Pericarditis Risks Myocarditis involves inflammation of the heart muscle, while pericarditis affects the outer lining of the heart. Both disorders may lead to chest pain, fatigue, and difficulties breathing. In most instances, treatment with rest and proper medication produces a swift resolution. Symptom Recognition Patients and healthcare professionals alike should be alert for this warning evidence. Chest pain, aching, or pressure Breathing difficulty Elevated or irregular heart rhythm Fatigue is experienced when performing routine activities Swelling involving the legs, ankles, or feet Research indicates that vaccine-associated myocarditis typically presents with milder symptoms compared to myocarditis caused by COVID-19 infection itself. Studies show that patients with post-COVID-19 vaccination myocarditis have fewer cardiovascular complications than those with conventional myocarditis. Diagnostic Methods Healthcare providers use several methods to diagnose vaccine-associated myocarditis:
Diagnostic Tool Purpose Typical Findings Electrocardiogram (ECG) Heart rhythm assessment ST-segment changes Blood tests Cardiac enzyme levels Elevated troponin Echocardiogram Heart function evaluation Reduced ejection fraction Cardiac MRI Detailed heart imaging Inflammation patterns Comparative Risk Analysis: Updated Vaccine comparison versus COVID-19 infection Multiple studies demonstrate that COVID-19 infection poses a significantly higher risk for myocarditis than vaccination: Cause Risk Ratio Severity Hospitalization Rate COVID-19 infection 18.28 times higher More severe outcomes 85% require admission mRNA vaccination 3.24 times higher Generally mild, self-limiting 70% require admission Conventional myocarditis Baseline risk Variable severity 90% require admission The data shows that while vaccination increases myocarditis risk above baseline, COVID-19 infection itself presents a much greater cardiac threat. Several investigations carried out in Israel, the United States, and European nations have repeatedly confirmed this pattern. Vaccine-Specific Differences Research from Canada shows differences between mRNA vaccines in young males aged 18-29 years: Moderna (mRNA-1273): 5.69 times higher risk than Pfizer Pfizer (BNT162b2): Slightly attenuated relative to baseline, although it still remains elevated. These differences have led some countries to recommend Pfizer over Moderna for younger populations, though both vaccines remain approved and recommended by health authorities. Clinical Outcomes and Recovery Studies tracking patients with vaccine-associated myocarditis show generally favourable outcomes. Among the cohort of 77 patients in a multicenter European–U.S. study, none died or were readmitted to the hospital after a median follow-up of 147 days. Recovery Timeline The majority of patients demonstrate a consistent course of recovery. Days 1-3: Symptom onset and diagnosis Days 4-7: Symptom improvement with treatment Weeks 2-4: Return to normal activities Months 3-6: Cardiac function normalisation 6+ months: Ongoing monitoring for residual effects Nonetheless, cardiac magnetic resonance imaging detects lasting abnormalities in a significant number of patients. Finding Percentage Timeline Clinical Significance Residual scar tissue 79.6% 6+ months post-diagnosis Unknown long-term impact Persistent edema 20.4% 6+ months post-diagnosis May resolve over time Normal cardiac function 100% Follow-up period Reassuring finding Exercise tolerance 95% normal 6+ months Good functional recovery Worldwide Regulatory Action These latest warnings from the FDA correspond to comparable measures enacted by other international health authorities. The European Medicines Agency (EMA) has issued comparable warnings, while Health Canada has implemented age-specific recommendations for different mRNA vaccines. International Approaches Various nations have implemented distinct strategies: Nordic countries: Temporarily restricted Moderna for under-30s United Kingdom: Preferred Pfizer for under-40s initially Germany: Recommended Pfizer for under-30s France: No age-specific restrictions but enhanced monitoring Also Read: Australia Lottery Visa 2025: Latest Registration Guide Update Continuous Post-Vaccination Safety Monitoring The FDA continues monitoring COVID vaccine safety through multiple surveillance systems. Both Pfizer and Moderna are conducting required long-term studies to assess potential heart effects in people who developed myocarditis after vaccination. Surveillance Systems VAERS: Passive surveillance system for adverse events VSD: Active surveillance in healthcare systems CISA: Clinical Immunisation Safety Assessment project BEST: Biologics Effectiveness and Safety Initiative The CDC's Vaccine Safety Datalink shows no increased myocarditis risk with vaccines administered since 2022, contrasting with the FDA's updated labelling. This discrepancy highlights ongoing scientific evaluation of vaccine safety data.
Expert Views and Suggested Recommendations Public health experts emphasise that myocarditis cases from vaccination remain rare and typically resolve without long-term complications. According to the American College of Cardiology, the revised labelling conveys known information to healthcare providers while presenting more precise risk quantification. Medical Society Positions Leading medical organisations continue to endorse COVID vaccination, simultaneously recognising the related cardiac risks. American Heart Association: Benefits outweigh risks for most patients American Academy of Paediatrics: Continues to recommend vaccines for children Infectious Diseases Society of America: Supports current vaccination guidelines Several experts argue that broadening the warning messages may not constitute the optimal strategy. Robert Morris from the University of Washington suggests focusing on identifying who is susceptible to myocarditis to predict and reduce risk. Current Vaccination Guidelines New FDA policy changes now limit COVID-19 vaccinations to adults aged 65 or older and to people with underlying health conditions that heighten the risk of severe disease. This points to a transition from blanket vaccination recommendations to vaccination strategies targeted toward high-risk populations. Updated Recommendations The present guidelines state: Adults 65+: Annual vaccination recommended High-risk adults: Vaccination based on individual assessment Healthy adults under 65: Generally not recommended Children: Case-by-case evaluation with healthcare providers The FDA’s vaccine advisory committee is still assessing coronavirus strains for the fall, and revised formulations are anticipated to be made available to the eligible populations. Future Research Directions Scientists are pursuing several research avenues to better understand vaccine-associated myocarditis: Current Research Genetic susceptibility: Recognising genetic markers associated with heightened risk Immune response patterns: Understanding why some develop myocarditis Long-term cardiac outcomes: Monitoring patients over extended periods of time Preventative strategies: Formulating approaches to lower risk. This COVID vaccine heart risk warning update reflects the FDA's commitment to transparent safety communication while balancing the known benefits of vaccination against rare but serious side effects. Healthcare providers and their patients should discuss personal risk factors when making decisions about vaccination. Conclusion The COVID vaccine heart risk warning update represents the most detailed safety information released by the FDA regarding myocarditis and pericarditis risks from mRNA COVID-19 vaccines. While these cardiac complications remain rare, affecting approximately 8 cases per million doses in the general population and 27 cases per million in high-risk young males, the expanded warnings provide crucial information for informed medical decision-making. Although healthcare professionals now possess precise incidence rates and extensive long-term follow-up data, the clinical significance of persistent cardiac imaging abnormalities is still under evaluation. The updated labelling reflects an ongoing commitment to vaccine safety monitoring while maintaining the overall benefit-risk profile that supports continued vaccination for appropriate populations. FAQ (Frequently Asked Questions) Q: What is the actual chance of heart problems following COVID vaccination? A: Vaccine-associated cases amount to roughly eight per million doses in people 6 months to 64 years old, and about 27 per million doses in males ages 12 to 24 years, according to 2023–2024 vaccine data. Q: Are heart problems tied to COVID vaccines particularly severe? A: Most cases of vaccine-associated myocarditis are mild and resolve quickly with rest and medication. Studies show better outcomes compared to myocarditis from COVID-19 infection itself. Q: Which COVID vaccines are associated with heart-related warnings?
A: Both Pfizer's Comirnaty and Moderna's Spikevax mRNA vaccines carry the updated heart risk warnings. Some studies suggest a slightly higher risk with Moderna in young males. Q: For how long do heart symptoms persist after a COVID vaccination? A: The majority of patients recover within just a few days to a couple of weeks. Yet certain cardiac imaging findings can remain present for several months, with the long-term implications still indeterminate. Q: Should young men forego COVID vaccines because of these heart safety concerns? A: Whether to accept the vaccine ought to be determined in consultation with a healthcare provider who weighs each individual’s particular risk factors. The risk remains minimal, and COVID-19 infection itself carries a higher risk of cardiac complications than that linked to vaccination. Q: At what point do heart problems typically emerge after COVID vaccination? A: Myocarditis and pericarditis most commonly occur within 7 days of vaccination, particularly after the second dose of mRNA vaccines. Q: Are there any long-term heart effects from vaccine-associated myocarditis? A: There are continued long-term studies underway. Latest follow-up evidence indicates that most patients retain normal cardiac function, though certain imaging irregularities remain. The clinical implications of these findings are still under investigation.
0 notes
Text
2 notes
·
View notes
Text
COVID-19 mod-RNA „Impfung“: Nierenschäden werden häufig mitgliefert
ScienceFiles:»Zu den ersten Erkrankungen, die wir als direkt von modRNA-Spritzbrühen verursacht, beschrieben haben, das war spätestens im Oktober 2021, gehört das Nephrotische Syndrom: Hanna et al. (2021) präsentieren den Fall eines 60 Jährigen ohne medizinische Vorgeschichte, der nach Gentherapie mit Pfizer/Biontechs Comirnaty […] http://dlvr.it/TLPclN «
0 notes