#Battery Electrode Deionization
Explore tagged Tumblr posts
Text
A novel AI model could aid in the production of clean water

- By Nuadox Crew -
Around 2.2 billion people lack access to safe drinking water, and half of the global population faces severe water scarcity at some point each year.
To address these challenges, expensive solutions like sewer irrigation, rainwater reuse, and seawater desalination are used. However, centralized water systems struggle to quickly adapt to changes in demand, prompting interest in decentralized, electrochemical water production technologies.
Dr. Son Moon's team at the Korea Institute of Science and Technology (KIST), in collaboration with Professor Baek Sang-Soo’s team, developed an AI-based model using random forest techniques to accurately predict ion concentrations during electrochemical water treatment.
This model can monitor individual ions like Na+, K+, Ca2+, and Cl- and requires minimal computing resources compared to deep learning models.
The research offers potential improvements in national water quality management systems, helping to better monitor and manage water resources.

Image: Summary of ion concentration prediction in water using machine learning (random forest) based on conductivity. Credit: Korea Institute of Science and Technology.
Read more at National Research Council of Science and Technology/Tech Xplore
Scientific paper: Hoo Hugo Kim et al, Decoupling ion concentrations from effluent conductivity profiles in capacitive and battery electrode deionizations using an artificial intelligence model, Water Research (2024). DOI: 10.1016/j.watres.2024.122092
Other recent news
Magnetic Prosthetic Hands: Researchers at the BioRobotics Institute have developed a new magnetic prosthetic hand that allows for natural, precise control of robotic fingers.
Revolutionary OLEDs: A new device, thinner than a human hair, has been developed to replace bulky night vision goggles with lightweight glasses. This could significantly enhance the usability and comfort of night vision technology.
Energy Harvesting at Room Temperature: Scientists have created an organic thermoelectric device that can harvest energy at room temperature without needing a temperature gradient. This breakthrough could lead to new ways of generating energy sustainably.
Battery Breakthrough: Columbia Engineers have developed a new, more powerful electrolyte for batteries, potentially solving one of the biggest challenges in renewable energy storage.
Silk and Graphene Electronics: Researchers have found a way to create a two-dimensional silk protein layer on graphene, which could lead to advancements in flexible and wearable electronics.
#water#water management#ai#engineering#hands#prosthetics#oleds#night vision#energy#thermoelectrics#battery#electrolytes#silk#graphene#materials#wearables
0 notes
Link
#Atex#battery#chemical#chemicalpump#LCC#lifecyclecosts#maintenance#process#processpump#processing#progressivecavitypump#Seepex#TA-Luft#TCO
0 notes
Photo

New desalination method offers low energy alternative to purify salty water
Providing safer drinking water to those in need may be a little easier. According to Penn State researchers, a new desalination technique is able to remove salt from water using less energy than previous methods.
"Globally, there is reduced access to fresh water," said Bruce Logan, Evan Pugh University Professor in Engineering and the Stan and Flora Kappe Professor of Environmental Engineering. "More and more, the waters that are being used are impaired, either due to salt or other contaminants, so we are seeing an increasing need to rely on less optimal water sources."
To combat this problem, Logan, and colleagues Christopher Gorski, assistant professor of environmental engineering, and Taeyoung Kim, post-doctoral scholar in environmental engineering, have come up with a desalination method called battery electrode deionization (BDI). BDI improves upon standard capacitive deionization (CDI) techniques by eliminating the regeneration stage and lowering the voltage required to complete the process.
Read more.
#Materials Science#Science#Desalination#Energy#Water#Penn State#Battery Electrode Deionization#Electronics
44 notes
·
View notes
Text
This is a project for a class i’m in. It’s going to talk about batteries! (Don’t feel obligated to read, posting online is extra credit)
Hello to all my beautiful followers! KB here again to explain a recent project I did on batteries in my lab class. Let me preface by saying that I love running this science and engineering blog, and that it wouldn’t be the same without every single one of your curious minds.
Now, don’t worry if you don’t have any background on batteries: I’ll explain how they work, give a little background on their impact on the world, and show how you can make your own little battery at home just like I did! But, be forewarned: it probably won’t power much more than a few LED lights.
As I’m sure you know, batteries power things like our homes, cars, and even the device you’re reading this post on. Batteries create chemical reactions that produce electrical energy, and slowly release this energy while powering a device. The life of a battery can last anywhere from a couple hours to a few years. When a battery powers something, the chemical reaction “pushes” electrons from one end of the battery, through the device, and then into the other end of the battery. This flow is also known as the current. To understand what’s really happening, you first have to know what they’re made of.
To break it down to its most basic components, a battery is made of two electrodes and an electrolyte. An electrode is a conductor through which electricity, or a flow of electrons, can move in and out. In batteries, there are two metal electrodes that are made of different materials (and it is important that they be different metals, otherwise the battery won’t work!). One electrode is positive, attached to a positive terminal, and called the cathode, and the other electrode is negative, connected to a negative terminal, and called the anode. The terminal is just the end surface through which the electrons enter/leave the cathode/anode. On a regular AA battery, the cathode and anode are regarded by plus and minus signs, respectively. The anode and cathode are separated from each other, and do not directly touch each other. When you connect the two electrodes with a wire, you are completing the circuit, and electrons flow from the anode into the cathode. When you put batteries into a device, take a TV remote as an example, the remote acts as the wire—the electrons flow through it, giving it the power to turn your TV channels.
Another important part of batteries is the electrolyte, and the chemical reactions that take place in it. An electrolyte is a material, typically a gel or liquid, that separates the electrodes from each other. Positive ions, or positively charged atoms, are created in a chemical reaction during which the anode loses electrons, a process known as oxidation. Simultaneously, the cathode undergoes a reaction and gains these electrons in a process called reduction. This is why it’s important that the electrodes be made of different metals; one metal will be more likely to give electrons, while the other will prefer to accept them. The electrolyte only allows for the transport of ions; electrons cannot travel through it, so electricity must go around and through a wire connecting the electrodes. A more detailed schematic is given below.
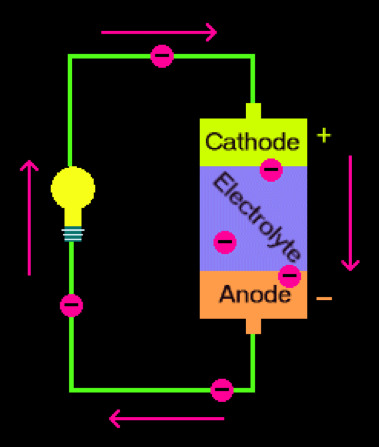
This is a diagram depicting the anode, cathode, electrolyte, and electron flow in a simple battery. The circles with minus signs represent the electrons, and the arrows are the direction they’re moving.
So, now you know the basic set up of a battery. There’s a positive and a negative end where electrons flow into and out of the battery, respectively, and an electrolyte in the middle in which chemical reactions take place to produce a current.
Now you understand how batteries work, but there’s still a few things you’ll need to know about the measurements you can take of a battery before you build your own. First up is voltage, a measure of the electric potential. In other words, the voltage represents how large the difference of energy is between the positive and negative electrodes. A higher voltage will result in My goal during this experiment was to get the highest voltage in my battery. Current measures the flow of electrons, or how many electrons pass through a point in a given unit of time. Current is directly proportional to voltage, so the more voltage, the higher the current. Power is a measure of how much electric energy flows through a circuit per unit time and is directly proportional to both voltage and current. Power density is how much power is in a given mass, and energy density is the energy in a given mass. Think of power density as the ability to store energy, while power density is how quickly energy can be given off. Cycle life is how many times a rechargeable battery can be charged and discharged (drained) before significant degradation in the battery’s performance occurs. A longer cycle life means the battery will last for a longer time.
I’d also like to take a moment to talk about the batteries that we use for modern technology and their impact on the world. Lithium ion batteries are one of the most prominent up-and-coming battery types because they have the ability to be recharged hundreds of times while still maintaining a long cycle life. Because of their high performance, lithium batteries are used to power our phones, laptops, and even some electric cars. A problem with these batteries is the strain on local communities when big companies try to collect the raw materials used in them. Cobalt, a rare and expensive metal used in lithium-based batteries, is mined in the Congo, but the mining is run by Congolese people with no safety equipment and little mining equipment beside hammers. These miners face dangerous work conditions, and are taken advantage of because of their proximity to the natural resource and exploited for their labor. In addition to this, cobalt is very expensive, ringing in at $26,000 per ton in 2016 (as a reference, lithium itself was $16500/ton in 2018, and aluminum was $2254/ton in 2018). The extraction of cobalt is just one of other extractions that commonly affect communities. Currently, a significant amount of research is going into producing different types of batteries. People are looking to make batteries that have cycle lives as long as lithium ones, but use less expensive and toxic raw materials.
Now that you’ve got all the background, you’re ready to make your own battery!
What you’ll need: • A plastic/rubber ice tray • Tweezers/pliers • Wire cutters/scissors • Copper wire • Zinc plated screws • 250 mL deionized water (tap is fine too) • 12 g salt (I used lab grade NaCl, but iodized salt will work just fine) • A voltmeter • Some electrical wires • LED lights (any colors are fine)
Procedure: First, using your wire cutters or scissors, cut about 7 cm of your copper and wrap it twice around the top of your screws. Use the tweezers to curl most of the extra wire, but leave enough uncurled so that it’ll fit over the lip of each ice tray cell. You’ll need to make as many screws are there are cells in your ice tray. Place one screw-wire combination over the barrier of each cell, with the copper wire in one cell and the zinc screw in the other, as in the second figure below. Use a curled pieced pf copper wire in the very first cell, with no screw attached.
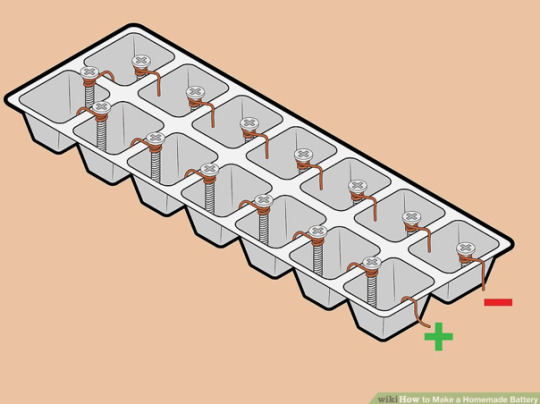
Next, make a solution of water and salt using 250 mL of water and 12 g of salt. Make sure the salt is fully dissolved. Then, fill each cell with the salt solution.
Now you’re ready to test the voltage! Here’s a tutorial on setting up a voltmeter if you need some guidance (the first 2 minutes will be enough, but it’s a great video to learn more). For this set up, you’ll connect the red wire to the copper wire at the end of the tray and the black wire to the single piece of copper wire. Your voltmeter should read off a voltage of about 9V.
Taking it one step forward, you can attach an LED light to the batteries! Keep the appropriate color connections and then connect the ends to the LED. Try to connect as many as you can! (I was able to get a maximum of 4 different colors at a time)
What Happened? Now, let’s relate some concepts we learned earlier to the experiment you just did. In this experiment, each cell actually acts as a its own battery. The copper wire is acting as the cathode, and is gaining electrons through reduction, while the zinc screws are the anode, and are losing electrons via oxidation. For this particular battery, mass is also being transported between electrodes; during the chemical reaction of the anode, positively charge ions are being formed from the zinc screws and are flowing through the electrolyte to the copper wires. The salt water solution is acting as the electrolyte and is allowing the ions to flow to the copper wires, where they build up. This mass transport is the reason you want to coil the copper wire; there’s more surface area for the reaction to occur on. If you allowed this to go on for a long time, you would see that the zinc screws lose mass and that the copper wires gain the difference. The picture below gives a visual explanation of what’s happening in each cell when you complete the circuit by connecting the voltmeter or LED light to each electrode.

The ions move from the anode to the cathode in the solution, while electrons flow through the “wire,” either being the voltmeter or the LED lights. A cool feature about batteries is that you can align several in a row, or “in series,” and their voltages will add together. If you measure the voltage of just one cell, you’ll get a voltage of about 0.64 V, assuming you used an ice tray of 14 cells.
I had fun making this battery myself. Something that surprised me during the fabrication process was that deionized water worked better than regular tap water. I figured the reasoning for this was because tap water has several different components, such as fluoride and other additives, that physically bump into the ions trying to move from the anode to the cathode. Another thing I learned is that your battery will have a higher voltage if you increase the temperature of the water. This draws on the fact that atoms and molecules move faster at higher temperatures, so the ions are able to move around faster in the water.
All kinds of batteries are being made and tested for different variables, such as the voltage, current, power, power density, and energy density. We’re currently mining and extracted the raw materials for lithium batteries in places and at rates that are unsustainable. My hope is that a cheaper alternative can be found sometime in our near future. In the meantime, maybe you could start doing some research of you own, and learn about energy and batteries to help shape our future.
I’ve linked my references for this post below. If you’re interested, they’re all really great reads.
As always, stay curious!
All the best, KB https://www.explainthatstuff.com/batteries.html (I really recommend this one!)
https://www.fluke.com/en-us/learn/best-practices/measurement-basics/electricity/what-is-voltage
https://www.dummies.com/programming/electronics/how-batteries-work/
https://energyeducation.ca/encyclopedia/Energy_density_vs_power_density
https://www.wikihow.com/Make-a-Homemade-Battery
#kb rambles#i procrastinated this. so hard#its 1:26 im just posting and this class starts at 1:30#def gonna be late#batteries#battery#lithium ion battery#galvanic cells#electrodes#electrolytes
0 notes
Text
300+ TOP SWITCHGEAR & PROTECTION Objective Questions and Answers
SWITCHGEAR & PROTECTION Multiple Choice Questions :-
1. The main function of a fuse is to (a) protect the line (b) open the circuit (c) protect the appliance (d) prevent excessive currents (e) none of the above Ans: d 2. On which of the following routine tests are conducted ? (a) Oil circuit breakers (b) Air blast circuit breakers (c) Minimum oil circuit breakers (d) All of the above Ans: d 3. SF6 gas (a) is yellow in colour (b) is lighter than air (c) is nontoxic (d) has pungent small (e) none of the above Ans: c 4. The arcing contacts in a circuit breaker are made of (a) copper tungsten alloy (b) porcelain (c) electrolytic copper (d) aluminium alloy Ans: a 5. Which of the following medium is employed for extinction of arc in air circuit breaker ? (a) Water (b) Oil (c) Air (d) SF6 Ans: c 6. With which of the following, a circuit breaker must be equipped for remote operation ? (a) Inverse time trip (b) Time-delay trip (c) Shunt trip (d) None of the above (e) All of the above Ans: c 7. Fault diverters are basically (a) fuses (b) relays (c) fast switches (d) circuit breakers Ans: c 8. A thermal protection switch can protect against (a) short-circuit (b) temperature (c) overload (d) over voltage Ans: c 9. Arc in a circuit behaves as (a) a capackive reactance (b) an inductive reactance (c) a resistance increasing with voltage rise across the arc (d) a resistance decreasing with voltage rise across the arc Ans: d 10. Thermal circuit breaker has (a) delayed trip action (b) instantaneous trip action (c) both of the above (d) none of the above Ans: a 11. Relays can be designed to respond to changes in (a) resistance, reactance or impedance (b) voltage and current (c) light intensity (d) temperature (e) all above Ans: e 12. Overload relays are of...... type. (a) induction (b) solid state (c) thermal (d) electromagnetic (e) all above Ans: e 13. Thermal overload relays are used to protect the motor against over current due to (a) short-circuits (b) heavy loads (c) grounds (d) all of the above Ans: b 14. Magnetic circuit breaker has ______ trip action. (a) delayed (b) instantaneous (c) both of the above (d) none of the above Ans: b 15. D.C. shunt relays are made of (a) few turns of thin wire (b) few turns of thick wire (c) many turns of thin wire (d) many turns of thick wire Ans: c 16. The relay operating speed depends upon (a) the spring tension (b) the rate of flux built up (c) armature core air gap (d) all of the above Ans: d 17. In order that current should flow without causing excessive heating or voltage drop, the relay contacts should (a) have low contact resistance (b) be clean and smooth (c) be of sufficient size and proper shape (d) have all above properties Ans: d 18. Circuit breakers usually operate under (a) transient state of short-circuit current (b) sub-transient state of short-circuit current (c) steady state of short-circuit current (d) after D.C. component has ceased Ans: a 19. Circuit breakers are essentially (a) current carrying contacts called electrodes (b) arc extinguishers (c) circuits to break the system (d) transformers to isolate the two systems (e) any of the above Ans: a 20. The current zero interruption, in oil and air blast circuit breakers, is achieved by (a) lengthening of the gap (b) cooling and blast effect (c) both (a) and (b) (d) deionizing the oil with forced air (e) none of the above Ans: c 21. Air blast circuit breaker is used for (a) over currents (b) short duty (c) intermittant duty (d) repeated duty Ans: d 22. An efficient and a well designed protective relaying should have (a) good selectivity and reliability (b) economy and simplicity (c) high speed and selectivity (d) all of the above Ans: d 23. Burden of a protective relay is the power (a) required to operate the circuit breaker (b) absorbed by the circuit of relay (c) developed by the relay circuit (d) none of the above Ans: b 24. Directional relays are based on flow of (a) power (b) current (c) voltage wave (d) all of the above Ans: a 25. A differential relay measures the vector difference between (a) two currents (b) two voltages (c) two or more similar electrical quantities (d) none of the above Ans: c 26. A transmission line is protected by (a) inrush protection (b) distance protection (c) time graded and current graded over current protection (d) both (b) and (c) (e) none of the above Ans: d 27. Large internal faults are protected by (a) merz price percentage differential protection (b) mho and ohm relays (c) horn gaps and temperature relays (d) earth fault and positive sequence relays Ans: a 28. When a transmission line is energized, the wave that propagates on it is (a) current wave only (b) voltage wave only (c) both (a) and (b) (d) power factor wave only Ans: c 29. Protective relays are devices that detect abnormal conditions in electrical circuits by measuring (a) current during abnormal condition (b) voltage during abnormal condition (c) constantly the electrical quantities which differ during normal and abnormal conditions (d) none of the above Ans: c 30. The voltage appearing across the contacts after opening of the circuit breaker is called______voltage. (a) recovery (b) surge (c) operating (d) arc (e) none of the above Ans: a 31. Ionization in circuit breaker is facilitated by (a) high temperature (b) increase of mean free path (c) increasing field strength (d) all of the above Ans: d 32. In a circuit breaker the basic problem is to (a) maintain the arc (b) extinguish the arc (c) transmit large power (d) emit the ionizing electrons Ans: c 33. Overheating of relay contacts or contact born out is due to (a) slow making and breaking of load circuit contacts (b) foreign matter on the contact surface (c) too low contact pressure (d) all of the above Ans: d 34. Interruption of large currents by relay requires (a) arc suppressing blow out coils (b) wide separation of the opened contacts (c) high speed opening of contacts (d) all of the above Ans: d 35. Shunt capacitance is neglected while considering (a) short transmission line (b) medium transmission line (c) long transmission line (d) medium and long transmission lines Ans: a 36. The arc voltage produced in A.C. circuit breaker is always (a) in phase with the arc current (b) lagging the arc current by 90" (c) leading the arc current by 90° (d) none of the above Ans: a 37. The time of closing the cycle, in modern circuit breakers is (a) 0.003 sec (b) 0.001 sec (c) 0.01 sec (d) 0.10 sec (e) none of the above Ans: a 38. Insulation resistance of high voltage circuit breakers is more than (a) 1 mega ohms (b) 10 mega ohms (c) 100 mega ohms (d) 500 mega ohms Ans: c 39. H.R.C. fuses provide best protection against (a) overload (b) reverse current (c) open-circuits (d) short-circuits Ans: d 40. The ground wire should not be smaller than No ______ copper. (a) 2 (b) 4 (c) 6 (d) 10 Ans: d 41. The delay fuses are used for the protection of ________ . (a) motors (b) power outlet circuits (c) fluorescent lamps (d) light circuits Ans: a 42. Which of the following is the least expensive protection for overcurrent is low voltage system ? (a) Rewireable fuse (b) Isolator (c) Oil circuit breaker (d) Air break circuit breaker (e) None of the above Ans: a 43. Resistance grounding is used for voltage between (a) 33kVto66kV (b) HkVto33kV (c) 3.3kVandllkV (d) none of the above Ans: c 44. The contacts of high voltage switches used in power system are submerged in oil. The main purpose of the oil is to (a) lubricate the contacts (b) insulate the contacts from switch body (c) extinguish the arc (d) all of the above (e) none of the above Ans: c 45. In Railway applications ______ circuit breaker is used. (a) SFe (b) bulk oil (c) minimum oil (d) air break Ans: 46. To protect most of the electrical equipment handling low power, the types of relays used are (a) thermocouple (b) electronic and bimetallic (c) both (a) and (b) (d) none of the above Ans: c 47. Wave trap is used to trap waves of (a) power frequencies (b) higher frequencies entering generator or transformer units (c) either of the above (d) none of the above Ans: b 48. Ungrounded neutral transmission system is not recommended because of system (a) insulation being overstressed due to over voltages (b) insulation overstress may lead to failure and subsequent phase to phase faults (c) being inadequately protected against ground fault (d) all of the above Ans: d 49. The reflection co-efficient at the open circuited end of a transmission line. (a) zero (b) infinity (c) unity (d) none of the above Ans: c 50. For the protection of power station buildings against direct strokes the requirements are (a) interception (b) interception and conduction (c) interception, conduction and dissipation (d) interception, conduction, dissipation and reflection (e) none of the above Ans: c 51. The line insulation is the insulation level of the station equipment. (a) less than (b) same as (c) more than (d) proportional to (e) not directly related with Ans: e 52. The interaction between a transmission line and communication line is minimized by (a) transposing transmission as well as communication lines (b) increasing the height of the trans-mission line tower (c) increasing the distance between the two lines (d) all of the above Ans: d 53. When a wave propagates on a transmission line, it suffers reflection several times at (a) tapping (b) load end (c) sending end (d) sending and other end (e) all of the above Ans: d 54. Which of the following statements is incorrect? (a) Station batteries are used to operate relay only (b) The lightning arresters are basically surge diverters (c) An impedance relay has maximum fault current when fault occurs near the relay (d) A high speed relay has an operation of 1 to 2 cycles Ans: a 55. Discrimination between main and back up protection is provided by the use of relays which are (a) fact (b) sensitive (c) slow (d) none of the above Ans: c 56. Induction cup relay is operated due to changes in (a) current (b) voltage (c) impedance (d) all of the above Ans: d 57. A.C. network analyser is used to solve problems of (a) load flow (b) load flow and short-circuit (c) load flow and stability (d) load flow, short-circuit and stability (e) none of the above Ans: d 58. Which of the following statements is incorrect ? (a) Lightning arrestors are used before the switchgear (b) Shunt reactors are used as compensation reactors (c) The peak short current is (1.8 xV2) times the A.C. component (d) The MVA at fault is equal to base MVA divided by per unit equivalent fault reactance Ans: a 59. Short-circuit currents are due to (a) single phase to ground faults (b) phase to phase faults (c) two phase to ground faults (d) three phase faults (e) any of these Ans: e 60. To reduce short circuit fault currents are used. (a) reactors (b) resistors (c) capacitors (d) none of the above Ans: a 61. Bus coupler is very essential in arrangement (a) single bus (b) double bus, double breaker (c) main and transfer bus (d) all of the above Ans: c 62. For cost and safety, the outdoor substations are installed for voltages above (a) 11 kV (b) 33 kV (c) 60kV (d) 110kV Ans: b 63. The short circuit in any winding of the transformer is the result of (a) mechanical vibration (b) insulation failure (c) loose connection (d) impulse voltage Ans: d 64. relays are used for phase faults on long line. (a) Impedance (b) Reactance (c) Either of the above (d) None of the above Ans: a 65. For which of the following protection from negative sequence currents is provided ? (a) Generators (b) Motors (c) Transmission line (d) Transformers Ans: a 66. relay is preferred for phase fault on short transmission line. (a) Induction type (b) Reactance (c) Impedance (d) None of the above Ans: b 67. Distance relays are generally (a) split-phase relays (b) reactance relays (c) impedance relays (d) none of the above Ans: d 68. For which of the following ratings of the transformer differential protection is recommended ? (a) above 30 kVA. (b) equal to and above 5 MVA (c) equal to and above 25 MVA (d) none of the above Ans: b 69. A _______ is used to measure the stator % winding temperature of the generator. (a) thermocouple (b) pyrometer (c) resistance thermometer (d) thermometer Ans: c 70. The under voltage relay can be used for (a) generators (b) busbars (c) transformers (d) motors (e) all of the above Ans: e 71. The relay with inverse time characteristic will operate within (a) 1.5 sec (b) 5 to 10 sec (c) 5 to 20 sec (d) 20 to 30 sec (e) none of the above Ans: b 72. The single phasing relays are used for the protection of (a) single phase motors only (b) two phase motors only (c) two single phase motors running in parallel (d) three phase motors Ans: d 73. Which of the following devices will receive voltage surge first travelling on the transmission line ? (a) Lightning arresters (b) Relays (c) Step-down transformer (d) Switchgear Ans: a 74. Which of the following parameter can be neglected for a short line ? (a) Inductance (b) Capacitance (c) Resistance (d) Reactance Ans: b 75. Series reactors should have (a) low resistance (b) high resistance (c) low impedance (d) high impedance Ans: a 76. Which of the following circuit breakers has high reliability and minimum maintenance ? (a) Air blast circuit breakers (b) Circuit breaker with SF6 gas (c) Vacuum circuit breakers (d) Oil circuit breakers Ans: b 77. Arc in a circuit breaker is interrupted at (a) zero current (b) maximum current (c) minimum voltage (d) maximum voltage Ans: a 78. transmission line has reflection coefficient as one. (a) Open circuit (b) Short-circuit (e) Long (d) None of the above Ans: a 79. What will be the reflection co-efficient of the wave of load connected to transmission line if surge impedance of the line is equal to load ? (a) Zero (b) Unity (c) Infinity (d) None of the above Ans: a 80. The inverse definite mean time relays are used for over current and earth fault protection of transformer against (a) heavy loads (b) internal short-circuits (c) external short-circuits (d) all of the above Ans: b 81. Over voltage protection is recommended for (a) hydro-electric generators (b) steam turbine generators (c) gas turbine generators (d) all of the above (e) none of the above Ans: d 82. Air blast circuit breakers for 400 kV power system are designed to operate in (a) 100 microsecond (b) 50 millisecond (c) 0.5 sec (d) 0.1 sec Ans: b 83. Overfluxing protection is recommended for (a) distribution transformer (b) generator transformer of the power plant (c) auto-transformer of the power plant (d) station transformer of the power plant Ans: b 84. Series capacitors are used to (a) compensate for line inductive reactance (b) compensate for line capacitive reactance (c) improve line voltage (d) none of the above Ans: a 85. Admittance relay is _______ relay. (a) impedance (b) directional (c) non-directional (d) none of the above Ans: b 86. The material used for fuse must have (a) low melting point and high specific resistance (b) low melting point and -low specific resistance (c) high melting point and low specific resistance (d) low melting point and any specific resistance Ans: a 87. If the fault occurs near the impedance relay, the VII ratio will be (a) constant for all distances (b) lower than that of if fault occurs away from the relay (c) higher than that of if fault occurs away from the relay (d) none of the above Ans: b 88. The torque produced in induction type relay (shaded pole structure) is (a) inversely proportional to the current (b) inversely proportional to the square of the current (c) proportional to the current (d) proportional to square of the current Ans: b 89. The steady state stability of the power system can be increased by (a) connecting lines in parallel (b) connecting lines in series (e) using machines of high impedance (d) reducing the excitation of machines (e) none of the above Ans: a 90. The inductive interference between power and communication line can be minimized by (a) transposition of the power line (b) transposition of the communication line (c) both (a) and (b) (d) increasing the distance between the conductors Ans: c 91. The power loss is an important factor for the design of (a) transmission line (b) motor (c) generator (d) feeder Ans: a 92. A fuse is connected (a) in series with circuit (b) in parallel with circuit (c) either in series or in parallel with circuit (d) none of the above Ans: a 93. H.R.C. fuse, as compared to a rewirable fuse, has (a) no ageing effect (b) high speed of operation (c) high rupturing capacity (d) all of the above Ans: d 94. The fuse rating is expressed in terms of (a) current (b) voltage (c) VAR (d) kVA Ans: a 95. The fuse blows off by (a) burning (b) arcing (c) melting (d) none of the above Ans: c 96. On which of the following effects of electric current a fuse operates ? (a) Photoelectric effect (b) Electrostatic effect (c) Heating effect (d) Magnetic effect Ans: c 97. An isolator is installed (a) to operate the relay of circuit breaker (b) as a substitute for circuit breaker (c) always independent of the position of circuit breaker (d) generally on both sides of a circuit breaker Ans: d 98. A fuse in a motor circuit provides protection against (a) overload (b) short-circuit and overload (c) open circuit, short-circuit and overload (d) none of the above Ans: b 99. Protection by fuses is generally not used beyond (a) 20 A (b) 50 A (c) 100 A (d) 200 A Ans: c 100. A fuse is never inserted in (a) neutral wire (b) negative of D.C. circuit (c) positive of D.C. circuit (d) phase dine Ans: a 101. Oil switches are employed for (a) low currents circuits (b) low voltages circuits (c) high voltages and large currents circuits (d) all circuits Ans: c 102. A switchgear is device used for (a) interrupting an electrical circuit (b) switching an electrical circuit 111. (c) switching and controlling an electrical circuit (d) switching, controlling and protecting the electrical circuit and equipment Ans: d 103. The fuse wire, in D.C. circuits, is inserted in (a) negative circuit only (b) positive circuit only (c) both (a) and (b) (d) either (a) or (b) Ans: c 104. By which of the following methods major portion of the heat generated in a H.R.C. fuse is dissipated ? (a) Radiation (b) Convection (c) Conduction (d) All of the above Ans: c 105. A short-circuit is identified by (a) no current flow (b) heavy current flow (c) voltage drop (d) voltage rise Ans: b 106. The information to the circuit breaker under fault conditions is provided by (a) relay (b) rewirable fuse (c) H.R.C. only (d) all of the above Ans: a 107. To limit short-circuit current in a power system are used. (a) earth wires (b) isolators (c) H.R.C. fuses (d) reactors Ans: d 109. A balanced 3-phase system consists of (a) zero sequence currents only (b) positive sequence currents only (c) negative and zero sequence currents (d) zero, negative and positive sequence currents Ans: b 110. In a single bus-bar system there will be complete shut down when (a) fault occurs on the bus itself (b) fault occurs on neutral line (c) two or more faults occur simultaneously (d) fault occurs with respect to earthing Ans: a SWITCHGEAR & PROTECTION Questions and Answers Pdf Download Read the full article
0 notes
Text
Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II).
Journal of Chemistry Volume 2017 (2017), Article ID 7569354, 9 pages https://doi.org/10.1155/2017/7569354
Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II)
1Department of Chemistry, Sarhad University of Science & Information Technology, Peshawar, Khyber Pakhtunkhwa, Pakistan 2Institute of Chemical Sciences, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan 3Chemistry Division, Directorate of Science, PINSTECH, Nilore, Islamabad, Pakistan
Academic Editor: Thijs A. Peters
Copyright © 2017 Hanif Ur Rehman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The facilitated passage of Zn (II) across flat sheet supported liquid membrane saturated with TDDA (tri-n-dodecylamine) in xylene membrane phase has been investigated. The effect of acid and metal ion concentration in the feed solution, the carrier concentration in membrane phase, stripping agent concentration in stripping phase, and coions on the extraction of Zn (II) was investigated. The stoichiometry of the extracted species, that is, complex, was investigated on slope analysis method and it was found that the complex (LH)2·Zn(Cl2) is responsible for transport of Zn (II). A mathematical model was developed for transport of Zn (II), and the predicted results strongly agree with experimental ones. The mechanism of transport was determined by coupled coion transport mechanism with H+ and Cl− coupled ions. The optimized SLM was effectively used for elimination of Zn (II) from waste discharge liquor of galvanizing plant of Zn (II).
1. Introduction
Over the past few decades, the prompt boost in the utilization of heavy metals has increased the flux of metallic ingredients into soils and natural water resources. The presence of even small amount of heavy metals in the environment is hazardous as the majority of them are harmful and persistent [1]. The zinc concentration in the earth crust is about 70 ppm. Zinc and its compounds have many applications and are used in galvanization, alloys, catalysts, wood preservation, vulcanization for rubber, photographic paper, ceramics, fertilizers, textiles, batteries, pigments and as dietary supplements and in medicines [2, 3].
About two billion people suffer from zinc deficiency in the developing countries and this thus causes various disorders in them. In minors, zinc deficiency is responsible for growth retardation, infection susceptibility, delayed sexual maturation, and diarrhea. These disorders lead to the deaths of about 800,000 children throughout the world every year.
Excess of zinc in the human body has been associated with system dysfunction which in turn can affect growth and reproduction. The presence of free zinc ion in solution has been reported to be very toxic for vertebrates, invertebrates, and plants [4–6]. Due to its toxic effect, large scale applications, and increasing demand for pure zinc, it is necessary to remove and separate zinc from industrial effluents.
Traditional techniques of recovery for metal ions, such as adsorption [7, 8], solvent extraction [9–12], precipitation [13], and ion exchange [14]. have normally low efficiency, require high capital and operating cost, and produce secondary pollution complications [15]. Among novel techniques suggested for transport of metal ions, supported liquid membrane is one of the promising methods. The technique comprises the applications of solvent extraction (high selectivity and distribution coefficient) while overcoming usual extraction’s shortcomings (loss of carrier due to emulsification and dispersion) [16–18].
Kanungo and Mohapatra [19] investigated the transport of Zn (II) through supported liquid membrane using bis(2,4,4-trimethylpentyl)phosphinic acid as a metal ion carrier. A model for the rate of transport of binary and ternary complex species has been discussed. The transport of Zn (II) via di-(2-ethylhexyl)phosphoric acid supported liquid membrane has been studied by Ata et al. [20]. The influence of pH of feed solution, carrier, and stripping agent concentration, temperature, and flow rate of feed and stripping solution on the transport of Zn (II) has been investigated. Wódzki and Szczepański found simultaneous recovery and bifurcation of Zn (II) and Cu (II) by means of two parallel BLMs. The two selective carriers, 5-nonylsalicylaldoxime and di-(2-ethylhexyl), were employed for transport of Zn (II) and Cu (II), respectively [21]. The selective removal of Zn (II) from other transition metals and transport across polymer inclusion membrane was investigated by Ulewicz and his coworkers [22]. It has been observed that transport selectivity of Zn (II) over Ni (II), Co (II), Cd (II), and Cu (II) decreases while increasing pH of the feed solution. Furthermore, it has been studied that Zn (II) can be selectively eliminated from dilute aqueous feed solution by solvent extraction. Kozlowska et al. studied the competitive transport of Zn (II), Cd (II), and Pb (II) through polymer inclusion membranes having organophosphorous acids as an ion carrier [23]. The nature of the extractants on cation efficiency and selectivity has been elaborated. Torz et al. reported the transport of Zn (II) from hydrochloric acid feed solution through hollow fiber modules using tributyl phosphate as a carrier [24]. It has been observed that the kinetics of the mass transfer process was restricted by the diffusion of species in the membrane openings. Lee et al. investigated the separation of zinc and copper by hollow fiber supported liquid membrane containing LIX 84 and PC-88A as mobile carriers. The influence of numerous operational factors on separation and permeation rate of Zn (II) and Cu (II) has been studied [25].
In our previous papers [26–29], an effort was made to design a model for transport of Ag (I), Mn (II), Pb (II), and Tl (III), through SLM using various carriers and diluents, and successfully applied to industrial waste effluents.
The present work elaborates the extraction and separation of Zn (II) from aqueous acidic feed solution through flat sheet supported liquid membrane using TDDA as a mobile carrier. In SLM studies, the influence of several process parameters like acid concentration in the feed solution, Zn (II) concentration in the feed solution, the carrier concentration in membrane phase, and NaOH concentration in strip solution on the Zn (II) flux was investigated. Another aim of current work was to investigate the species formed during the transport of Zn (II) and mechanism of Zn (II) and further to establish the stoichiometry of the chemical reactions through SLM. Furthermore, this study was concentrated to ascertain the most optimum conditions for transport of Zn (II), especially for its large scale separation and recovery from industrially galvanizing waste effluents of Zn (II). No such investigations have been done so far using TDDA SLM system for transport of Zn (II).
2. Theory
Transport of metal ion through SLM occurs by diffusion of metal-carrier complex via various basic steps. First, the metal ion diffuses across aqueous diffusion layer from aqueous bulk feed solution to membrane feed interface and the metal-carrier complex is prepared at feed membrane interface. The complex is then diffused through liquid membrane phase to strip membrane interface owing to the concentration gradient. The complex is finally dissociated at strip membrane interface, carrier returns back across liquid membrane phase, and metal ion diffuses through aqueous diffusion layer into bulk strip solution. The theoretical model of mass transfer of Zn (II) for this purpose is important for the diffusion of metal-carrier complex.
3. Experimental
3.1. Chemicals and Reagents
ZnCl2 (97% BDH) in HCl (37% Merck) was used as feed in various concentration. NaOH (99-100% Merck) in distilled water in various concentrations was used as a stripping solution and TDDA (≥95% Merck) with diluent xylene (99.5%) to get the various composition of metal ion carrier. Double distilled and deionized water was used in all experiments. All other chemicals used were of analytical or better grade.
3.2. Analytical Instruments
Atomic absorption spectrometer of Perkin Elmer model 400 was used to measure the concentration of zinc (II) and other metal ions in feed and strip solutions. pH was determined through pH meter of Metrohm model 827. Viscosity determination of TDDA (in xylene) was performed via viscometer/rheometer of Brookfield LVDV-III.
3.3. Permeation Cell
The metal ion transport study was performed in two-compartment cell made of Perspex material as reported in our previous work [26]. Each half cell of the permeation cell had the volume capacity of 300 cm3 and active membrane area was 23.79 cm2. Each half was equipped with synchronous motors, pH electrode, and sampling port. The stirring speed of 1500 rpm [26] was optimized for the similar type of permeation cell and carrier; therefore this stirring speed was used in all the permeation study. All the experiments were conducted at °C.
3.4. SLM Preparation
The microporous polypropylene thin coating of Celgard 2400 was applied as a solid support for a liquid organic carrier with an active pore size of 0.02 µm, the thickness of 25 µm, and porosity of 38%. The membrane was cut into rectangular pieces of cm and was soaked in the premeasured concentration of TDDA diluted with xylene in Petri dish for overnight. The membrane was then taken out from the organic solution. The excess amount of organic carrier and solvent were removed by allowing them to drain off the membrane for about five minutes.
3.5. SLM Transport Study
The membrane after preparation was placed in between the two compartments of the cell and screwed together to form a water tight seal. Both compartments of the cell were filled with 300 mL of predetermined concentration of feed and strip solutions. To avoid concentration polarization at feed and strip membrane interfaces, the two solutions in feed and strip compartments were continuously stirred. Samples were collected after a specific time interval from both feed and strip solution and tested for metal ion concentration. For most of the experiments, the carrier concentration was 0.80 mol/dm3 in xylene.
The flux () was calculated as follows [26]:
4. Results and Discussion
4.1. Reactions at Feed Membrane Interface
If tri-n-dodecylamine (TDDA) is represented by L, the following possibilities of reactions may take place. The tri-n-dodecylamine can be protonated to LH+ in acidic medium (HCl).It is supposed that ZnCl2 in feed solution in the presence of excess chloride ions due to HCl is converted toThe cationic (LH+)(org) and anionic species then interact at feed membrane interface and form the complex asThe subscript org represents organic phase and aq aqueous phase and indicates the number of Cl−, H+, and L molecules associating with Zn (II) ions to form the neutral complex .
To express the contribution of H+ and Cl−, (3) may be represented as
4.2. Reactions at Strip Membrane Interface
The neutral complex formed at feed membrane interface as per (3)/(4) is extractable in liquid organic phase and disperses from feed membrane interface to strip membrane interface. The complex at strip membrane interface is dissociated owing to NaOH in the stripping phase as follows:The free carrier molecule (L) diffuses back through the liquid membrane phase towards feed membrane interface and again forms the complex. As per Wilke-Chang, the diffusion coefficient of the forward moving complex should be noticeably lesser than the diffusion coefficient of the backward moving free carrier molecules [30]. Owing to this reason, the free carrier concentration at feed membrane interface will constantly be higher than the complex. Figure 1 represents the schematic transport mechanism of Zn (II) through supported liquid membrane.
Figure 1: Transport mechanism of Zn (II) through supported liquid membrane.
The equilibrium constant of (4) for Zn (II) can be written as follows:The distribution coefficient of Zn2+ () for distribution between the membrane and aqueous phases can be represented as follows: And on rearranging of (8),Now considering the extraction constant, distribution coefficient, and laws of diffusion via the same path as [29], it can be indicated as:Equation (11) shows that, at a constant temperature, the flux () of Zn (II) is directly proportional to the concentration of Cl−, H+, L, and This equation can be used to find the number of H+ related to L in the form of LH+. This can be determined by various methods: one method is to keep [Cl−], [L], and constant in (11) and plotting against ; the slope of the curve will provide the “” value for a number of H+ ions in the complex. Likewise by drawing versus and maintaining [Cl−], [H+], and constant, the slope of the plot will give a number of moles () of TDDA contributing in complex formation of Zn (II).
4.3. Effect of Carrier Concentration
The carrier in the liquid membrane phase of the supported liquid membrane has a critical role in the extraction of metal ions by SLM [26]. Various concentrations of carrier ranging from 0.157 mol/dm3 to 1.103 mol/dm3 were used in solvent xylene to observe the effect of carrier concentration on the transport of Zn (II) through SLM. During this study, the Zn (II) concentration in feed solution was kept at mol/dm3 in 2.0 mol/dm3 of HCl and the NaOH concentration in strip solution was fixed at 2.0 mol/dm3.
Figure 2 shows that with the increase of carrier concentration from 0.157 to 0.80 mol/dm3 in xylene in the membrane phase has a significant effect on flux of Zn (II). It follows (11) where flux () is directly proportional to carrier concentration [L]. However, the flux is insignificant as the concentration of carrier increases beyond 0.80 mol/dm3. This reduction in transport of Zn (II) might be the result of enhanced friction of liquid membrane phase owing to high viscosity, as with the increase in carrier concentration, the viscosity of liquid membrane phase increases [29]. Hence 0.80 mol/dm3 of tri-n-dodecylamine was considered the optimum carrier concentration and more investigations were performed with this concentration of carrier.
Figure 2: Effect of carrier concentration on transport of Zn (II) in the stripping phase. [HCl] in feed solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.157 mol/dm3 to 1.103 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [Zn (II)] = mol/dm3, time = 5.0 h.
Finding the number () of tri-n-dodecylamine (L) taking part in complex was determined by plotting versus as shown in Figure 3. The slope of the plot is 2.008, and this indicates that two molecules of TDDA take part in the complex formation.
Figure 3: Plot of versus (same operating conditions as given in Figure 2).
In our previous study for the stability of membrane for such type of carrier, it has been observed that supported liquid membrane is quite stable for a period of 120 h with very minute flux variation in the metal ion flux and no indication of structural deformation of polypropylene membrane was investigated. Furthermore, the membrane was reused several times without leakage and metal ion flux decline [28].
4.4. Role of HCl Concentration in Feed Phase
The HCl performs an important part in the transportation of Zn (II) because it provides H+ and Cl− for the formation of complex as per (4). The effect of HCl concentration on extraction Zn (II) was observed by varying the concentration of HCl in the range of 0.5 mol/dm3 to 3.0 mol/dm3, while keeping the concentration of TDDA in the liquid membrane phase at 0.80 mol/dm3 and concentration of NaOH on strip side at 2.0 mol/dm3. Figure 4 shows that as the concentration of HCl increases from 0.5 mol/dm3 to 2.0 mol/dm3 and the flux of Zn (II) increases. Thus, it can be concluded that with an increase of HCl concentration, the concentration of H+ and Cl− ions in feed solution also increases. This, in turn, increases the formation of the complex as per (4), which ultimately enhances the transport of Zn (II). Although, by further increasing the concentration of HCl beyond 2.0 mol/dm3, the transport of Zn (II) decreases and this may be due to the formation of type species due to the large quantity of H+ and Cl− in feed phase and reaction (4) is hindered in forward direction. This study shows that 2.0 mol/dm3 of HCl is the most favorable concentration for transport of Zn (II), and further studies were carried out with this concentration of HCl to evaluate various parameters for subsequent studies.
Figure 4: Effect of HCl concentration in feed solution on flux () of Zn (II). [HCl] in feed solution = 0.50 mol/dm3 to 3.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, time = 5.0 h.
To investigate that how much hydrogen is taking part in complexation of Zn (II), was plotted versus (Figure 5). The slope of this plot was approximately 2.0, indicating that two hydrogens take part in a complex of Zn (II) transport. As the transport study concluded that two molecules of TDDA and H+ are involved in the complex formation of Zn (II), the complex formed during this extraction study may be .
Figure 5: Plot of versus (same operating conditions as given in Figure 4).
4.5. Influence of Feed Concentration
To study the capability of this SLM for metal ion transport, various concentrations of Zn (II) from mol/dm3 to mol/dm3 were used in the feed solution. During this particular study, the concentrations of the carrier, stripping phase, and HCl in feed solution were kept at 0.80 mol/dm3, 2.0 mol/dm3, and 2.0 mol/dm3, respectively.
Figure 6 shows that as Zn (II) concentration in feed solution rises, the transport of Zn (II) also increases; this is as per (11), where flux () is directly proportional to feed concentration (). Such type behavior has already been studied in our previous study [27, 28]. This study shows that up to mol/dm3 no saturation of carrier with metal ion takes place.
Figure 6: Effect of Zn (II) concentration in feed solution on its flux. [Zn(II)] in feed = mol/dm3 to mol/dm3, [HCl] in feed = 2.0 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, time = 5.0 h.
4.6. Influence of Stripping Phase Concentration
The stripping agent dissociates the complex at strip membrane interface and releases Zn (II) in strip solution. To investigate the effect of NaOH on the transport of Zn (II), numerous concentrations of NaOH in the range of 0.05 mol/dm3 to 2.5 mol/dm3 were used, while keeping the TDDA concentration at 0.80 mol/dm3 and HCl concentration in feed solution at 2.0 mol/dm3.
Figure 7 shows that as the concentration of NaOH rises from 0.05 mol/dm3 to 0.1 mol/dm3, the flux of Zn (II) decreases and, beyond this concentration of NaOH, the flux of Zn (II) increases. The decrease in transport of Zn (II) at a lower concentration of NaOH can be explained that it forms a precipitate of Zn(OH)2 which is insoluble and blocks the pores of polypropylene membrane and transport of Zn (II) is restricted [31]. The SEM (Figure 8(b)) indicates the blocked SLM at a lower concentration of NaOH. More increase in the concentration of NaOH increases the transportation of Zn (II), as the precipitate of Zn (OH)2 is soluble in excess of NaOH and more OH− are available that enhance the decomposition of the complex at strip membrane interface as per (5).
Figure 7: Effect of NaOH concentration in strip solution on the flux of Zn (II). [NaOH] in stripping solution 0.05 mol/dm3 to 2.5 mol/dm3, [HCl] in feed solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, time = 5.0 h.
5. Recovery of Zinc (II) from Waste Discharge/Effluent of Galvanizing Plant
The objective of this work was to design and develop SLM for recovery of Zn (II) from industrial waste effluents. The polypropylene as supported liquid membrane, TDDA as a carrier, was utilized for extraction of Zn (II) from waste discharge liquor of galvanizing plant. To check and assess the efficiency of SLM system, samples of galvanized industrial waste were collected from the different locations of the drain carrying the galvanizing industrial effluent. The effluent samples were analyzed using the aforementioned method as described in Experimental and the percent extraction and recovery of the Zn(II) metal ions were determined using atomic absorption spectrophotometry method. The data obtained is provided in Figure 9 which indicates approximately the complete extraction and recovery of Zn (II) after 210 minutes. This shows the suitability and effectiveness of this SLM for recovery of Zn (II).
Figure 9: Variation in Zn (II) concentration in feed and strip solution against time (waste discharge liquor of galvanizing plant). Initial Zn (II) conc. in feed = mol/dm3, [HCl] in feed = 2.0 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3.
6. Recovery and Transport of Zn(II) from Galvanizing Plant Waste
The present SLM was designed for the transport of Zn(II) metal ions and applied to zinc industrial effluents of galvanizing plants because zinc is one of the industrial important metals because of its applications and its use for protection, passivation, and decoration of some heavy elements. The data in Table 2 show that TDDA-polypropylene SLM system has significant transport efficiency for Zn(II) metal ions, that is, about 99.8%, while other metal ions like Co, Cu, Cd, Mn, Cr, and Fe show very little or more precisely negligible amount of transport that is 0.0 to 0.72% or in average 0.08% for Co, 0.23% for Cu, 0.15% for Cd, 0.36% for Mn, 0.06% for Cr, and 0.28$ for Fe. This much smaller amount of transport may be attributed to experimental error. If error factor is taken into account then TDDA-polypropylene SLM system is highly selective for transport of Zn(II) metal ions only. The data are represented in Tables 1 and 2.
Table 1: Analysis of galvanizing plants waste.
Table 2: Percent transport of metal ions from galvanizing plants waste.
7. Conclusions
The flux () got increased by increasing carrier concentration up to 0.80 mol/dm3, and, further increasing the carrier concentration, the transport of Zn (II) was found to decrease. The Zn(II) ions transport was increased by increasing HCl concentration in feed solution up to 2.0 mol/dm3 and then decreased using a higher concentration of HCl. The slope analysis studies of of Zn (II) versus and showed that 2.0 mole of each tri-n-dodecylamine and hydrogen are involved in the transport of Zn (II). The maximum transport of Zn (II) was achieved at 0.80 mol/dm3 of TDDA in membrane phase and 2.0 mol/dm3 of HCl and NaOH in feed and strip solution, respectively. It was also found to be coupled coion extraction pathway, as H+, Cl−, and Zn (II) travel in the same direction. Two moles of hydrogen and TDDA interact with one mole of Zn (II) producing a complex , that is responsible for transport of Zn (II). This SLM system was effectively used for removal of Zn (II) to the waste discharge liquor of galvanizing plant of zinc.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The authors acknowledge Sarhad University of Science and Information Technology, Peshawar, KPK, Pakistan, for providing financial assistance to accomplish this work.
References
R. A. Wuana and F. E. Okieimen, “Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation,”ISRN Ecology, vol. 2011, Article ID 402647, 20 pages, 2011.View at Publisher·View at Google Scholar
D. W. Thomas, Metals and Their Compounds in the Environment, Edited by E. Merian, Wiley, Weinheim, Germany, 1991.
ATSDR (Agency for Toxic Substances and Disease Registry), Toxicological Profile for Zinc, Public Health Service. U.S. Department of Health and Human Services, Atlanta, Ga, USA, 2005.
A. S. Prasad, “Zinc deficiency,”The British Medical Journal, vol. 326, no. 7386, pp. 409-410, 2003.View at Publisher·View at Google Scholar·View at Scopus
K. M. Hambidge and N. F. Krebs, “Zinc deficiency: a special challenge,”Journal of Nutrition, vol. 137, no. 4, pp. 1101–1105, 2007.View at Google Scholar·View at Scopus
G. J. Fosmire, “Zinc toxicity,”American Journal of Clinical Nutrition, vol. 51, no. 2, pp. 225–227, 1990.View at Google Scholar·View at Scopus
L. Monser and N. Adhoum, “Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater,”Separation and Purification Technology, vol. 26, no. 2-3, pp. 137–146, 2002.View at Publisher·View at Google Scholar·View at Scopus
S. Liang, X. Guo, and Q. Tian, “Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulfured orange peel,”Desalination, vol. 275, no. 1–3, pp. 212–216, 2011.View at Publisher·View at Google Scholar·View at Scopus
F. J. Alguacil and S. Martínez, “Solvent extraction of Zn(II) by cyanex 923 and its application to a solid-supported liquid membrane system,”Journal of Chemical Technology and Biotechnology, vol. 76, no. 3, pp. 298–302, 2001.View at Publisher·View at Google Scholar·View at Scopus
K. C. Sole, T. L. Ferguson, and J. B. Hiskey, “Solvent extraction of silver by cyanex 272, cyanex 302 and cyanex 301,”Solvent Extraction and Ion Exchange, vol. 12, no. 5, pp. 1033–1050, 1994.View at Publisher·View at Google Scholar·View at Scopus
P. M. Cole and K. C. Sole, “Zinc solvent extraction in the process industries,”Mineral Processing and Extractive Metallurgy Review, vol. 24, no. 2, pp. 91–137, 2003.View at Publisher·View at Google Scholar·View at Scopus
M. S. Lee and S.-H. Nam, “Solvent extraction of zinc from strong hydrochloric acid solution with Alamine336,”Bulletin of the Korean Chemical Society, vol. 30, no. 7, pp. 1526–1530, 2009.View at Publisher·View at Google Scholar·View at Scopus
F. M. Pang, S. P. Teng, T. T. Teng, and A. K. M. Omar, “Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions,”Water Quality Research Journal of Canada, vol. 44, pp. 1–9, 2009.View at Google Scholar
S. V. Plokhov, I. G. Matasova, V. A. Utkin, and V. Y. Vodzinskii, “Specific features of ion-exchange zinc(II) extraction from washing water of zinc plating,”Russian Journal of Applied Chemistry, vol. 75, no. 6, pp. 950–953, 2002.View at Publisher·View at Google Scholar·View at Scopus
K. H. Park, P. K. Parhi, and N.-H. Kang, “Studies on the removal of low content copper from the sea nodule leach liquor using the cationic resin TP 207,”Separation Science and Technology, vol. 47, no. 10, pp. 1531–1541, 2012.View at Publisher·View at Google Scholar·View at Scopus
J. D. Gyves and E. R. San Miguel, “Metal ion separations by supported liquid membranes,”Industrial and Engineering Chemistry Research, vol. 38, no. 6, pp. 2182–2202, 1999.View at Publisher·View at Google Scholar·View at Scopus
V. V. Prasad, D. J. Yogesh, D. S. Ajay et al., “Simultaneous extraction of neodymium and uranium using hollow fiber supported liquid membrane,”Separation Science and Technology, vol. 49, no. 10, pp. 1509–1520, 2014.View at Publisher·View at Google Scholar·View at Scopus
B. Mokhtari and K. Pourabdollah, “Inclusion separation of alkali metals in emulsion liquid membranes by nanobaskets of calix[4]crown-3,”Brazilian Journal of Chemical Engineering, vol. 29, no. 4, pp. 783–793, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. B. Kanungo and R. Mohapatra, “Coupled transport of Zn (II) through a supported liquid membrane containing bis (2,4,4-trimethyl pentyl) phosphinic acid in kerosene. 1. A model for the rate process involving binary and ternary complex species,”Journal of Membrane Science, vol. 105, pp. 217–226, 1995.View at Google Scholar·View at Scopus
O. N. Ata, A. V. Beşe, S. Çolak, B. Dönmez, and A. Çakıcı, “Effect of parameters on the transport of zinc ion through supported liquid membrane,”Chemical Engineering and Processing: Process Intensification, vol. 43, no. 7, pp. 895–903, 2004.View at Publisher·View at Google Scholar
R. Wódzki and P. Szczepański, “Simultaneous recovery and separation of Zn2+ and Cu2+ in hybrid membrane systems,”Separation and Purification Technology, vol. 41, no. 3, pp. 289–297, 2005.View at Publisher·View at Google Scholar·View at Scopus
M. Ulewicz, W. Walkowiak, J. Gega, and B. Pospiech, “Zinc (II) selective removal from other transition metal by solvent extraction and transport through polymer inclusion membranes with D2EHPA,”Ars Separatoria Acta, vol. 2, pp. 47–55, 2003.View at Google Scholar
J. Kozlowska, C. A. Kozłowski, and J. J. Koziol, “Transport of Zn(II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers,”Separation and Purification Technology, vol. 57, no. 3, pp. 430–434, 2007.View at Publisher·View at Google Scholar·View at Scopus
M. Torz, K. Alejski, and J. Szymanowski, “Modelling of zinc (II) extraction from model hydrochloric acid solutions in hollow fiber modules,”Physicochemical Problems of Mineral Processing, vol. 37, pp. 97–105, 2003.View at Google Scholar
J.-C. Lee, J. Jeong, B.-S. Kim, M. S. Kim, and M. Kobayashi, “Separation of copper and zinc ions by hollow fiber supported liquid membrane containing LIX84 and PC-88A,”Materials Transactions, vol. 45, no. 6, pp. 1915–1919, 2004.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, M. A. Chaudry, N. Bukhari, Najeebullah, and N. Ali, “Mn (VII) ions transport by triethanolamine cyclohexanone based supported liquid membrane and recovery of Mn (II) ions from discharged zinc carbon dry battery cell,”Journal of Membrane Science, vol. 366, no. 1-2, pp. 125–131, 2011.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, and M. A. Chaudry, “Coupled transport of Tl3+ through triethanolamine-xylene-polypropylene supported liquid membranes,”Journal of Industrial and Engineering Chemistry, vol. 18, no. 1, pp. 492–498, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, M. A. Chaudry, K. Ali, and N. Ullah, “Transport of Ag+ through tri-n-dodecylamine supported liquid membranes,”Journal of Membrane Science, vol. 389, pp. 287–293, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. Ur Rehman, G. Akhtar, and M. A. Chaudry, “Coupled transport of Pb2+ through tri-n-octylamine-xylene-polypropylene supported liquid membranes,”Canadian Journal of Chemical Engineering, vol. 91, no. 6, pp. 1140–1152, 2013.View at Publisher·View at Google Scholar·View at Scopus
S. A. Ansari, P. K. Mohapatra, and V. K. Manchanda, “Recovery of actinides and lanthanides from high-level waste using hollow-fiber supported liquid membrane with TODGA as the carrier,”Industrial and Engineering Chemistry Research, vol. 48, no. 18, pp. 8605–8612, 2009.View at Publisher·View at Google Scholar·View at Scopus
F. Holleman, A. E. Wiberg, and N. Wiberg, Zink, Lehrbuch der Anorganischen Chemie, Walter de Gruyter, Berlin, Germany, 91th–100th edition, 1985.
Source
http://www.hindawi.com/journals/jchem/2017/7569354/
from TAXI NEAR ME http://taxi.nearme.host/transport-of-zn-ii-by-tdda-polypropylene-supported-liquid-membranes-and-recovery-from-waste-discharge-liquor-of-galvanizing-plant-of-zn-ii/ from NOVACAB https://novacabtaxi.tumblr.com/post/173345350726
0 notes
Text
Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II).
Journal of Chemistry Volume 2017 (2017), Article ID 7569354, 9 pages https://doi.org/10.1155/2017/7569354
Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II)
1Department of Chemistry, Sarhad University of Science & Information Technology, Peshawar, Khyber Pakhtunkhwa, Pakistan 2Institute of Chemical Sciences, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan 3Chemistry Division, Directorate of Science, PINSTECH, Nilore, Islamabad, Pakistan
Academic Editor: Thijs A. Peters
Copyright © 2017 Hanif Ur Rehman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The facilitated passage of Zn (II) across flat sheet supported liquid membrane saturated with TDDA (tri-n-dodecylamine) in xylene membrane phase has been investigated. The effect of acid and metal ion concentration in the feed solution, the carrier concentration in membrane phase, stripping agent concentration in stripping phase, and coions on the extraction of Zn (II) was investigated. The stoichiometry of the extracted species, that is, complex, was investigated on slope analysis method and it was found that the complex (LH)2·Zn(Cl2) is responsible for transport of Zn (II). A mathematical model was developed for transport of Zn (II), and the predicted results strongly agree with experimental ones. The mechanism of transport was determined by coupled coion transport mechanism with H+ and Cl− coupled ions. The optimized SLM was effectively used for elimination of Zn (II) from waste discharge liquor of galvanizing plant of Zn (II).
1. Introduction
Over the past few decades, the prompt boost in the utilization of heavy metals has increased the flux of metallic ingredients into soils and natural water resources. The presence of even small amount of heavy metals in the environment is hazardous as the majority of them are harmful and persistent [1]. The zinc concentration in the earth crust is about 70 ppm. Zinc and its compounds have many applications and are used in galvanization, alloys, catalysts, wood preservation, vulcanization for rubber, photographic paper, ceramics, fertilizers, textiles, batteries, pigments and as dietary supplements and in medicines [2, 3].
About two billion people suffer from zinc deficiency in the developing countries and this thus causes various disorders in them. In minors, zinc deficiency is responsible for growth retardation, infection susceptibility, delayed sexual maturation, and diarrhea. These disorders lead to the deaths of about 800,000 children throughout the world every year.
Excess of zinc in the human body has been associated with system dysfunction which in turn can affect growth and reproduction. The presence of free zinc ion in solution has been reported to be very toxic for vertebrates, invertebrates, and plants [4–6]. Due to its toxic effect, large scale applications, and increasing demand for pure zinc, it is necessary to remove and separate zinc from industrial effluents.
Traditional techniques of recovery for metal ions, such as adsorption [7, 8], solvent extraction [9–12], precipitation [13], and ion exchange [14]. have normally low efficiency, require high capital and operating cost, and produce secondary pollution complications [15]. Among novel techniques suggested for transport of metal ions, supported liquid membrane is one of the promising methods. The technique comprises the applications of solvent extraction (high selectivity and distribution coefficient) while overcoming usual extraction’s shortcomings (loss of carrier due to emulsification and dispersion) [16–18].
Kanungo and Mohapatra [19] investigated the transport of Zn (II) through supported liquid membrane using bis(2,4,4-trimethylpentyl)phosphinic acid as a metal ion carrier. A model for the rate of transport of binary and ternary complex species has been discussed. The transport of Zn (II) via di-(2-ethylhexyl)phosphoric acid supported liquid membrane has been studied by Ata et al. [20]. The influence of pH of feed solution, carrier, and stripping agent concentration, temperature, and flow rate of feed and stripping solution on the transport of Zn (II) has been investigated. Wódzki and Szczepański found simultaneous recovery and bifurcation of Zn (II) and Cu (II) by means of two parallel BLMs. The two selective carriers, 5-nonylsalicylaldoxime and di-(2-ethylhexyl), were employed for transport of Zn (II) and Cu (II), respectively [21]. The selective removal of Zn (II) from other transition metals and transport across polymer inclusion membrane was investigated by Ulewicz and his coworkers [22]. It has been observed that transport selectivity of Zn (II) over Ni (II), Co (II), Cd (II), and Cu (II) decreases while increasing pH of the feed solution. Furthermore, it has been studied that Zn (II) can be selectively eliminated from dilute aqueous feed solution by solvent extraction. Kozlowska et al. studied the competitive transport of Zn (II), Cd (II), and Pb (II) through polymer inclusion membranes having organophosphorous acids as an ion carrier [23]. The nature of the extractants on cation efficiency and selectivity has been elaborated. Torz et al. reported the transport of Zn (II) from hydrochloric acid feed solution through hollow fiber modules using tributyl phosphate as a carrier [24]. It has been observed that the kinetics of the mass transfer process was restricted by the diffusion of species in the membrane openings. Lee et al. investigated the separation of zinc and copper by hollow fiber supported liquid membrane containing LIX 84 and PC-88A as mobile carriers. The influence of numerous operational factors on separation and permeation rate of Zn (II) and Cu (II) has been studied [25].
In our previous papers [26–29], an effort was made to design a model for transport of Ag (I), Mn (II), Pb (II), and Tl (III), through SLM using various carriers and diluents, and successfully applied to industrial waste effluents.
The present work elaborates the extraction and separation of Zn (II) from aqueous acidic feed solution through flat sheet supported liquid membrane using TDDA as a mobile carrier. In SLM studies, the influence of several process parameters like acid concentration in the feed solution, Zn (II) concentration in the feed solution, the carrier concentration in membrane phase, and NaOH concentration in strip solution on the Zn (II) flux was investigated. Another aim of current work was to investigate the species formed during the transport of Zn (II) and mechanism of Zn (II) and further to establish the stoichiometry of the chemical reactions through SLM. Furthermore, this study was concentrated to ascertain the most optimum conditions for transport of Zn (II), especially for its large scale separation and recovery from industrially galvanizing waste effluents of Zn (II). No such investigations have been done so far using TDDA SLM system for transport of Zn (II).
2. Theory
Transport of metal ion through SLM occurs by diffusion of metal-carrier complex via various basic steps. First, the metal ion diffuses across aqueous diffusion layer from aqueous bulk feed solution to membrane feed interface and the metal-carrier complex is prepared at feed membrane interface. The complex is then diffused through liquid membrane phase to strip membrane interface owing to the concentration gradient. The complex is finally dissociated at strip membrane interface, carrier returns back across liquid membrane phase, and metal ion diffuses through aqueous diffusion layer into bulk strip solution. The theoretical model of mass transfer of Zn (II) for this purpose is important for the diffusion of metal-carrier complex.
3. Experimental
3.1. Chemicals and Reagents
ZnCl2 (97% BDH) in HCl (37% Merck) was used as feed in various concentration. NaOH (99-100% Merck) in distilled water in various concentrations was used as a stripping solution and TDDA (≥95% Merck) with diluent xylene (99.5%) to get the various composition of metal ion carrier. Double distilled and deionized water was used in all experiments. All other chemicals used were of analytical or better grade.
3.2. Analytical Instruments
Atomic absorption spectrometer of Perkin Elmer model 400 was used to measure the concentration of zinc (II) and other metal ions in feed and strip solutions. pH was determined through pH meter of Metrohm model 827. Viscosity determination of TDDA (in xylene) was performed via viscometer/rheometer of Brookfield LVDV-III.
3.3. Permeation Cell
The metal ion transport study was performed in two-compartment cell made of Perspex material as reported in our previous work [26]. Each half cell of the permeation cell had the volume capacity of 300 cm3 and active membrane area was 23.79 cm2. Each half was equipped with synchronous motors, pH electrode, and sampling port. The stirring speed of 1500 rpm [26] was optimized for the similar type of permeation cell and carrier; therefore this stirring speed was used in all the permeation study. All the experiments were conducted at °C.
3.4. SLM Preparation
The microporous polypropylene thin coating of Celgard 2400 was applied as a solid support for a liquid organic carrier with an active pore size of 0.02 µm, the thickness of 25 µm, and porosity of 38%. The membrane was cut into rectangular pieces of cm and was soaked in the premeasured concentration of TDDA diluted with xylene in Petri dish for overnight. The membrane was then taken out from the organic solution. The excess amount of organic carrier and solvent were removed by allowing them to drain off the membrane for about five minutes.
3.5. SLM Transport Study
The membrane after preparation was placed in between the two compartments of the cell and screwed together to form a water tight seal. Both compartments of the cell were filled with 300 mL of predetermined concentration of feed and strip solutions. To avoid concentration polarization at feed and strip membrane interfaces, the two solutions in feed and strip compartments were continuously stirred. Samples were collected after a specific time interval from both feed and strip solution and tested for metal ion concentration. For most of the experiments, the carrier concentration was 0.80 mol/dm3 in xylene.
The flux () was calculated as follows [26]:
4. Results and Discussion
4.1. Reactions at Feed Membrane Interface
If tri-n-dodecylamine (TDDA) is represented by L, the following possibilities of reactions may take place. The tri-n-dodecylamine can be protonated to LH+ in acidic medium (HCl).It is supposed that ZnCl2 in feed solution in the presence of excess chloride ions due to HCl is converted toThe cationic (LH+)(org) and anionic species then interact at feed membrane interface and form the complex asThe subscript org represents organic phase and aq aqueous phase and indicates the number of Cl−, H+, and L molecules associating with Zn (II) ions to form the neutral complex .
To express the contribution of H+ and Cl−, (3) may be represented as
4.2. Reactions at Strip Membrane Interface
The neutral complex formed at feed membrane interface as per (3)/(4) is extractable in liquid organic phase and disperses from feed membrane interface to strip membrane interface. The complex at strip membrane interface is dissociated owing to NaOH in the stripping phase as follows:The free carrier molecule (L) diffuses back through the liquid membrane phase towards feed membrane interface and again forms the complex. As per Wilke-Chang, the diffusion coefficient of the forward moving complex should be noticeably lesser than the diffusion coefficient of the backward moving free carrier molecules [30]. Owing to this reason, the free carrier concentration at feed membrane interface will constantly be higher than the complex. Figure 1 represents the schematic transport mechanism of Zn (II) through supported liquid membrane.
Figure 1: Transport mechanism of Zn (II) through supported liquid membrane.
The equilibrium constant of (4) for Zn (II) can be written as follows:The distribution coefficient of Zn2+ () for distribution between the membrane and aqueous phases can be represented as follows: And on rearranging of (8),Now considering the extraction constant, distribution coefficient, and laws of diffusion via the same path as [29], it can be indicated as:Equation (11) shows that, at a constant temperature, the flux () of Zn (II) is directly proportional to the concentration of Cl−, H+, L, and This equation can be used to find the number of H+ related to L in the form of LH+. This can be determined by various methods: one method is to keep [Cl−], [L], and constant in (11) and plotting against ; the slope of the curve will provide the “” value for a number of H+ ions in the complex. Likewise by drawing versus and maintaining [Cl−], [H+], and constant, the slope of the plot will give a number of moles () of TDDA contributing in complex formation of Zn (II).
4.3. Effect of Carrier Concentration
The carrier in the liquid membrane phase of the supported liquid membrane has a critical role in the extraction of metal ions by SLM [26]. Various concentrations of carrier ranging from 0.157 mol/dm3 to 1.103 mol/dm3 were used in solvent xylene to observe the effect of carrier concentration on the transport of Zn (II) through SLM. During this study, the Zn (II) concentration in feed solution was kept at mol/dm3 in 2.0 mol/dm3 of HCl and the NaOH concentration in strip solution was fixed at 2.0 mol/dm3.
Figure 2 shows that with the increase of carrier concentration from 0.157 to 0.80 mol/dm3 in xylene in the membrane phase has a significant effect on flux of Zn (II). It follows (11) where flux () is directly proportional to carrier concentration [L]. However, the flux is insignificant as the concentration of carrier increases beyond 0.80 mol/dm3. This reduction in transport of Zn (II) might be the result of enhanced friction of liquid membrane phase owing to high viscosity, as with the increase in carrier concentration, the viscosity of liquid membrane phase increases [29]. Hence 0.80 mol/dm3 of tri-n-dodecylamine was considered the optimum carrier concentration and more investigations were performed with this concentration of carrier.
Figure 2: Effect of carrier concentration on transport of Zn (II) in the stripping phase. [HCl] in feed solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.157 mol/dm3 to 1.103 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [Zn (II)] = mol/dm3, time = 5.0 h.
Finding the number () of tri-n-dodecylamine (L) taking part in complex was determined by plotting versus as shown in Figure 3. The slope of the plot is 2.008, and this indicates that two molecules of TDDA take part in the complex formation.
Figure 3: Plot of versus (same operating conditions as given in Figure 2).
In our previous study for the stability of membrane for such type of carrier, it has been observed that supported liquid membrane is quite stable for a period of 120 h with very minute flux variation in the metal ion flux and no indication of structural deformation of polypropylene membrane was investigated. Furthermore, the membrane was reused several times without leakage and metal ion flux decline [28].
4.4. Role of HCl Concentration in Feed Phase
The HCl performs an important part in the transportation of Zn (II) because it provides H+ and Cl− for the formation of complex as per (4). The effect of HCl concentration on extraction Zn (II) was observed by varying the concentration of HCl in the range of 0.5 mol/dm3 to 3.0 mol/dm3, while keeping the concentration of TDDA in the liquid membrane phase at 0.80 mol/dm3 and concentration of NaOH on strip side at 2.0 mol/dm3. Figure 4 shows that as the concentration of HCl increases from 0.5 mol/dm3 to 2.0 mol/dm3 and the flux of Zn (II) increases. Thus, it can be concluded that with an increase of HCl concentration, the concentration of H+ and Cl− ions in feed solution also increases. This, in turn, increases the formation of the complex as per (4), which ultimately enhances the transport of Zn (II). Although, by further increasing the concentration of HCl beyond 2.0 mol/dm3, the transport of Zn (II) decreases and this may be due to the formation of type species due to the large quantity of H+ and Cl− in feed phase and reaction (4) is hindered in forward direction. This study shows that 2.0 mol/dm3 of HCl is the most favorable concentration for transport of Zn (II), and further studies were carried out with this concentration of HCl to evaluate various parameters for subsequent studies.
Figure 4: Effect of HCl concentration in feed solution on flux () of Zn (II). [HCl] in feed solution = 0.50 mol/dm3 to 3.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, time = 5.0 h.
To investigate that how much hydrogen is taking part in complexation of Zn (II), was plotted versus (Figure 5). The slope of this plot was approximately 2.0, indicating that two hydrogens take part in a complex of Zn (II) transport. As the transport study concluded that two molecules of TDDA and H+ are involved in the complex formation of Zn (II), the complex formed during this extraction study may be .
Figure 5: Plot of versus (same operating conditions as given in Figure 4).
4.5. Influence of Feed Concentration
To study the capability of this SLM for metal ion transport, various concentrations of Zn (II) from mol/dm3 to mol/dm3 were used in the feed solution. During this particular study, the concentrations of the carrier, stripping phase, and HCl in feed solution were kept at 0.80 mol/dm3, 2.0 mol/dm3, and 2.0 mol/dm3, respectively.
Figure 6 shows that as Zn (II) concentration in feed solution rises, the transport of Zn (II) also increases; this is as per (11), where flux () is directly proportional to feed concentration (). Such type behavior has already been studied in our previous study [27, 28]. This study shows that up to mol/dm3 no saturation of carrier with metal ion takes place.
Figure 6: Effect of Zn (II) concentration in feed solution on its flux. [Zn(II)] in feed = mol/dm3 to mol/dm3, [HCl] in feed = 2.0 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, time = 5.0 h.
4.6. Influence of Stripping Phase Concentration
The stripping agent dissociates the complex at strip membrane interface and releases Zn (II) in strip solution. To investigate the effect of NaOH on the transport of Zn (II), numerous concentrations of NaOH in the range of 0.05 mol/dm3 to 2.5 mol/dm3 were used, while keeping the TDDA concentration at 0.80 mol/dm3 and HCl concentration in feed solution at 2.0 mol/dm3.
Figure 7 shows that as the concentration of NaOH rises from 0.05 mol/dm3 to 0.1 mol/dm3, the flux of Zn (II) decreases and, beyond this concentration of NaOH, the flux of Zn (II) increases. The decrease in transport of Zn (II) at a lower concentration of NaOH can be explained that it forms a precipitate of Zn(OH)2 which is insoluble and blocks the pores of polypropylene membrane and transport of Zn (II) is restricted [31]. The SEM (Figure 8(b)) indicates the blocked SLM at a lower concentration of NaOH. More increase in the concentration of NaOH increases the transportation of Zn (II), as the precipitate of Zn (OH)2 is soluble in excess of NaOH and more OH− are available that enhance the decomposition of the complex at strip membrane interface as per (5).
Figure 7: Effect of NaOH concentration in strip solution on the flux of Zn (II). [NaOH] in stripping solution 0.05 mol/dm3 to 2.5 mol/dm3, [HCl] in feed solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, time = 5.0 h.
5. Recovery of Zinc (II) from Waste Discharge/Effluent of Galvanizing Plant
The objective of this work was to design and develop SLM for recovery of Zn (II) from industrial waste effluents. The polypropylene as supported liquid membrane, TDDA as a carrier, was utilized for extraction of Zn (II) from waste discharge liquor of galvanizing plant. To check and assess the efficiency of SLM system, samples of galvanized industrial waste were collected from the different locations of the drain carrying the galvanizing industrial effluent. The effluent samples were analyzed using the aforementioned method as described in Experimental and the percent extraction and recovery of the Zn(II) metal ions were determined using atomic absorption spectrophotometry method. The data obtained is provided in Figure 9 which indicates approximately the complete extraction and recovery of Zn (II) after 210 minutes. This shows the suitability and effectiveness of this SLM for recovery of Zn (II).
Figure 9: Variation in Zn (II) concentration in feed and strip solution against time (waste discharge liquor of galvanizing plant). Initial Zn (II) conc. in feed = mol/dm3, [HCl] in feed = 2.0 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3.
6. Recovery and Transport of Zn(II) from Galvanizing Plant Waste
The present SLM was designed for the transport of Zn(II) metal ions and applied to zinc industrial effluents of galvanizing plants because zinc is one of the industrial important metals because of its applications and its use for protection, passivation, and decoration of some heavy elements. The data in Table 2 show that TDDA-polypropylene SLM system has significant transport efficiency for Zn(II) metal ions, that is, about 99.8%, while other metal ions like Co, Cu, Cd, Mn, Cr, and Fe show very little or more precisely negligible amount of transport that is 0.0 to 0.72% or in average 0.08% for Co, 0.23% for Cu, 0.15% for Cd, 0.36% for Mn, 0.06% for Cr, and 0.28$ for Fe. This much smaller amount of transport may be attributed to experimental error. If error factor is taken into account then TDDA-polypropylene SLM system is highly selective for transport of Zn(II) metal ions only. The data are represented in Tables 1 and 2.
Table 1: Analysis of galvanizing plants waste.
Table 2: Percent transport of metal ions from galvanizing plants waste.
7. Conclusions
The flux () got increased by increasing carrier concentration up to 0.80 mol/dm3, and, further increasing the carrier concentration, the transport of Zn (II) was found to decrease. The Zn(II) ions transport was increased by increasing HCl concentration in feed solution up to 2.0 mol/dm3 and then decreased using a higher concentration of HCl. The slope analysis studies of of Zn (II) versus and showed that 2.0 mole of each tri-n-dodecylamine and hydrogen are involved in the transport of Zn (II). The maximum transport of Zn (II) was achieved at 0.80 mol/dm3 of TDDA in membrane phase and 2.0 mol/dm3 of HCl and NaOH in feed and strip solution, respectively. It was also found to be coupled coion extraction pathway, as H+, Cl−, and Zn (II) travel in the same direction. Two moles of hydrogen and TDDA interact with one mole of Zn (II) producing a complex , that is responsible for transport of Zn (II). This SLM system was effectively used for removal of Zn (II) to the waste discharge liquor of galvanizing plant of zinc.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The authors acknowledge Sarhad University of Science and Information Technology, Peshawar, KPK, Pakistan, for providing financial assistance to accomplish this work.
References
R. A. Wuana and F. E. Okieimen, “Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation,”ISRN Ecology, vol. 2011, Article ID 402647, 20 pages, 2011.View at Publisher·View at Google Scholar
D. W. Thomas, Metals and Their Compounds in the Environment, Edited by E. Merian, Wiley, Weinheim, Germany, 1991.
ATSDR (Agency for Toxic Substances and Disease Registry), Toxicological Profile for Zinc, Public Health Service. U.S. Department of Health and Human Services, Atlanta, Ga, USA, 2005.
A. S. Prasad, “Zinc deficiency,”The British Medical Journal, vol. 326, no. 7386, pp. 409-410, 2003.View at Publisher·View at Google Scholar·View at Scopus
K. M. Hambidge and N. F. Krebs, “Zinc deficiency: a special challenge,”Journal of Nutrition, vol. 137, no. 4, pp. 1101–1105, 2007.View at Google Scholar·View at Scopus
G. J. Fosmire, “Zinc toxicity,”American Journal of Clinical Nutrition, vol. 51, no. 2, pp. 225–227, 1990.View at Google Scholar·View at Scopus
L. Monser and N. Adhoum, “Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater,”Separation and Purification Technology, vol. 26, no. 2-3, pp. 137–146, 2002.View at Publisher·View at Google Scholar·View at Scopus
S. Liang, X. Guo, and Q. Tian, “Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulfured orange peel,”Desalination, vol. 275, no. 1–3, pp. 212–216, 2011.View at Publisher·View at Google Scholar·View at Scopus
F. J. Alguacil and S. Martínez, “Solvent extraction of Zn(II) by cyanex 923 and its application to a solid-supported liquid membrane system,”Journal of Chemical Technology and Biotechnology, vol. 76, no. 3, pp. 298–302, 2001.View at Publisher·View at Google Scholar·View at Scopus
K. C. Sole, T. L. Ferguson, and J. B. Hiskey, “Solvent extraction of silver by cyanex 272, cyanex 302 and cyanex 301,”Solvent Extraction and Ion Exchange, vol. 12, no. 5, pp. 1033–1050, 1994.View at Publisher·View at Google Scholar·View at Scopus
P. M. Cole and K. C. Sole, “Zinc solvent extraction in the process industries,”Mineral Processing and Extractive Metallurgy Review, vol. 24, no. 2, pp. 91–137, 2003.View at Publisher·View at Google Scholar·View at Scopus
M. S. Lee and S.-H. Nam, “Solvent extraction of zinc from strong hydrochloric acid solution with Alamine336,”Bulletin of the Korean Chemical Society, vol. 30, no. 7, pp. 1526–1530, 2009.View at Publisher·View at Google Scholar·View at Scopus
F. M. Pang, S. P. Teng, T. T. Teng, and A. K. M. Omar, “Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions,”Water Quality Research Journal of Canada, vol. 44, pp. 1–9, 2009.View at Google Scholar
S. V. Plokhov, I. G. Matasova, V. A. Utkin, and V. Y. Vodzinskii, “Specific features of ion-exchange zinc(II) extraction from washing water of zinc plating,”Russian Journal of Applied Chemistry, vol. 75, no. 6, pp. 950–953, 2002.View at Publisher·View at Google Scholar·View at Scopus
K. H. Park, P. K. Parhi, and N.-H. Kang, “Studies on the removal of low content copper from the sea nodule leach liquor using the cationic resin TP 207,”Separation Science and Technology, vol. 47, no. 10, pp. 1531–1541, 2012.View at Publisher·View at Google Scholar·View at Scopus
J. D. Gyves and E. R. San Miguel, “Metal ion separations by supported liquid membranes,”Industrial and Engineering Chemistry Research, vol. 38, no. 6, pp. 2182–2202, 1999.View at Publisher·View at Google Scholar·View at Scopus
V. V. Prasad, D. J. Yogesh, D. S. Ajay et al., “Simultaneous extraction of neodymium and uranium using hollow fiber supported liquid membrane,”Separation Science and Technology, vol. 49, no. 10, pp. 1509–1520, 2014.View at Publisher·View at Google Scholar·View at Scopus
B. Mokhtari and K. Pourabdollah, “Inclusion separation of alkali metals in emulsion liquid membranes by nanobaskets of calix[4]crown-3,”Brazilian Journal of Chemical Engineering, vol. 29, no. 4, pp. 783–793, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. B. Kanungo and R. Mohapatra, “Coupled transport of Zn (II) through a supported liquid membrane containing bis (2,4,4-trimethyl pentyl) phosphinic acid in kerosene. 1. A model for the rate process involving binary and ternary complex species,”Journal of Membrane Science, vol. 105, pp. 217–226, 1995.View at Google Scholar·View at Scopus
O. N. Ata, A. V. Beşe, S. Çolak, B. Dönmez, and A. Çakıcı, “Effect of parameters on the transport of zinc ion through supported liquid membrane,”Chemical Engineering and Processing: Process Intensification, vol. 43, no. 7, pp. 895–903, 2004.View at Publisher·View at Google Scholar
R. Wódzki and P. Szczepański, “Simultaneous recovery and separation of Zn2+ and Cu2+ in hybrid membrane systems,”Separation and Purification Technology, vol. 41, no. 3, pp. 289–297, 2005.View at Publisher·View at Google Scholar·View at Scopus
M. Ulewicz, W. Walkowiak, J. Gega, and B. Pospiech, “Zinc (II) selective removal from other transition metal by solvent extraction and transport through polymer inclusion membranes with D2EHPA,”Ars Separatoria Acta, vol. 2, pp. 47–55, 2003.View at Google Scholar
J. Kozlowska, C. A. Kozłowski, and J. J. Koziol, “Transport of Zn(II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers,”Separation and Purification Technology, vol. 57, no. 3, pp. 430–434, 2007.View at Publisher·View at Google Scholar·View at Scopus
M. Torz, K. Alejski, and J. Szymanowski, “Modelling of zinc (II) extraction from model hydrochloric acid solutions in hollow fiber modules,”Physicochemical Problems of Mineral Processing, vol. 37, pp. 97–105, 2003.View at Google Scholar
J.-C. Lee, J. Jeong, B.-S. Kim, M. S. Kim, and M. Kobayashi, “Separation of copper and zinc ions by hollow fiber supported liquid membrane containing LIX84 and PC-88A,”Materials Transactions, vol. 45, no. 6, pp. 1915–1919, 2004.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, M. A. Chaudry, N. Bukhari, Najeebullah, and N. Ali, “Mn (VII) ions transport by triethanolamine cyclohexanone based supported liquid membrane and recovery of Mn (II) ions from discharged zinc carbon dry battery cell,”Journal of Membrane Science, vol. 366, no. 1-2, pp. 125–131, 2011.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, and M. A. Chaudry, “Coupled transport of Tl3+ through triethanolamine-xylene-polypropylene supported liquid membranes,”Journal of Industrial and Engineering Chemistry, vol. 18, no. 1, pp. 492–498, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, M. A. Chaudry, K. Ali, and N. Ullah, “Transport of Ag+ through tri-n-dodecylamine supported liquid membranes,”Journal of Membrane Science, vol. 389, pp. 287–293, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. Ur Rehman, G. Akhtar, and M. A. Chaudry, “Coupled transport of Pb2+ through tri-n-octylamine-xylene-polypropylene supported liquid membranes,”Canadian Journal of Chemical Engineering, vol. 91, no. 6, pp. 1140–1152, 2013.View at Publisher·View at Google Scholar·View at Scopus
S. A. Ansari, P. K. Mohapatra, and V. K. Manchanda, “Recovery of actinides and lanthanides from high-level waste using hollow-fiber supported liquid membrane with TODGA as the carrier,”Industrial and Engineering Chemistry Research, vol. 48, no. 18, pp. 8605–8612, 2009.View at Publisher·View at Google Scholar·View at Scopus
F. Holleman, A. E. Wiberg, and N. Wiberg, Zink, Lehrbuch der Anorganischen Chemie, Walter de Gruyter, Berlin, Germany, 91th–100th edition, 1985.
Source
http://www.hindawi.com/journals/jchem/2017/7569354/
from http://taxi.nearme.host/transport-of-zn-ii-by-tdda-polypropylene-supported-liquid-membranes-and-recovery-from-waste-discharge-liquor-of-galvanizing-plant-of-zn-ii/
from NOVACAB - Blog http://novacabtaxi.weebly.com/blog/transport-of-zn-ii-by-tdda-polypropylene-supported-liquid-membranes-and-recovery-from-waste-discharge-liquor-of-galvanizing-plant-of-zn-ii
0 notes
Text
Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II).
Journal of Chemistry Volume 2017 (2017), Article ID 7569354, 9 pages https://doi.org/10.1155/2017/7569354
Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II)
1Department of Chemistry, Sarhad University of Science & Information Technology, Peshawar, Khyber Pakhtunkhwa, Pakistan 2Institute of Chemical Sciences, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan 3Chemistry Division, Directorate of Science, PINSTECH, Nilore, Islamabad, Pakistan
Academic Editor: Thijs A. Peters
Copyright © 2017 Hanif Ur Rehman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The facilitated passage of Zn (II) across flat sheet supported liquid membrane saturated with TDDA (tri-n-dodecylamine) in xylene membrane phase has been investigated. The effect of acid and metal ion concentration in the feed solution, the carrier concentration in membrane phase, stripping agent concentration in stripping phase, and coions on the extraction of Zn (II) was investigated. The stoichiometry of the extracted species, that is, complex, was investigated on slope analysis method and it was found that the complex (LH)2·Zn(Cl2) is responsible for transport of Zn (II). A mathematical model was developed for transport of Zn (II), and the predicted results strongly agree with experimental ones. The mechanism of transport was determined by coupled coion transport mechanism with H+ and Cl− coupled ions. The optimized SLM was effectively used for elimination of Zn (II) from waste discharge liquor of galvanizing plant of Zn (II).
1. Introduction
Over the past few decades, the prompt boost in the utilization of heavy metals has increased the flux of metallic ingredients into soils and natural water resources. The presence of even small amount of heavy metals in the environment is hazardous as the majority of them are harmful and persistent [1]. The zinc concentration in the earth crust is about 70 ppm. Zinc and its compounds have many applications and are used in galvanization, alloys, catalysts, wood preservation, vulcanization for rubber, photographic paper, ceramics, fertilizers, textiles, batteries, pigments and as dietary supplements and in medicines [2, 3].
About two billion people suffer from zinc deficiency in the developing countries and this thus causes various disorders in them. In minors, zinc deficiency is responsible for growth retardation, infection susceptibility, delayed sexual maturation, and diarrhea. These disorders lead to the deaths of about 800,000 children throughout the world every year.
Excess of zinc in the human body has been associated with system dysfunction which in turn can affect growth and reproduction. The presence of free zinc ion in solution has been reported to be very toxic for vertebrates, invertebrates, and plants [4–6]. Due to its toxic effect, large scale applications, and increasing demand for pure zinc, it is necessary to remove and separate zinc from industrial effluents.
Traditional techniques of recovery for metal ions, such as adsorption [7, 8], solvent extraction [9–12], precipitation [13], and ion exchange [14]. have normally low efficiency, require high capital and operating cost, and produce secondary pollution complications [15]. Among novel techniques suggested for transport of metal ions, supported liquid membrane is one of the promising methods. The technique comprises the applications of solvent extraction (high selectivity and distribution coefficient) while overcoming usual extraction’s shortcomings (loss of carrier due to emulsification and dispersion) [16–18].
Kanungo and Mohapatra [19] investigated the transport of Zn (II) through supported liquid membrane using bis(2,4,4-trimethylpentyl)phosphinic acid as a metal ion carrier. A model for the rate of transport of binary and ternary complex species has been discussed. The transport of Zn (II) via di-(2-ethylhexyl)phosphoric acid supported liquid membrane has been studied by Ata et al. [20]. The influence of pH of feed solution, carrier, and stripping agent concentration, temperature, and flow rate of feed and stripping solution on the transport of Zn (II) has been investigated. Wódzki and Szczepański found simultaneous recovery and bifurcation of Zn (II) and Cu (II) by means of two parallel BLMs. The two selective carriers, 5-nonylsalicylaldoxime and di-(2-ethylhexyl), were employed for transport of Zn (II) and Cu (II), respectively [21]. The selective removal of Zn (II) from other transition metals and transport across polymer inclusion membrane was investigated by Ulewicz and his coworkers [22]. It has been observed that transport selectivity of Zn (II) over Ni (II), Co (II), Cd (II), and Cu (II) decreases while increasing pH of the feed solution. Furthermore, it has been studied that Zn (II) can be selectively eliminated from dilute aqueous feed solution by solvent extraction. Kozlowska et al. studied the competitive transport of Zn (II), Cd (II), and Pb (II) through polymer inclusion membranes having organophosphorous acids as an ion carrier [23]. The nature of the extractants on cation efficiency and selectivity has been elaborated. Torz et al. reported the transport of Zn (II) from hydrochloric acid feed solution through hollow fiber modules using tributyl phosphate as a carrier [24]. It has been observed that the kinetics of the mass transfer process was restricted by the diffusion of species in the membrane openings. Lee et al. investigated the separation of zinc and copper by hollow fiber supported liquid membrane containing LIX 84 and PC-88A as mobile carriers. The influence of numerous operational factors on separation and permeation rate of Zn (II) and Cu (II) has been studied [25].
In our previous papers [26–29], an effort was made to design a model for transport of Ag (I), Mn (II), Pb (II), and Tl (III), through SLM using various carriers and diluents, and successfully applied to industrial waste effluents.
The present work elaborates the extraction and separation of Zn (II) from aqueous acidic feed solution through flat sheet supported liquid membrane using TDDA as a mobile carrier. In SLM studies, the influence of several process parameters like acid concentration in the feed solution, Zn (II) concentration in the feed solution, the carrier concentration in membrane phase, and NaOH concentration in strip solution on the Zn (II) flux was investigated. Another aim of current work was to investigate the species formed during the transport of Zn (II) and mechanism of Zn (II) and further to establish the stoichiometry of the chemical reactions through SLM. Furthermore, this study was concentrated to ascertain the most optimum conditions for transport of Zn (II), especially for its large scale separation and recovery from industrially galvanizing waste effluents of Zn (II). No such investigations have been done so far using TDDA SLM system for transport of Zn (II).
2. Theory
Transport of metal ion through SLM occurs by diffusion of metal-carrier complex via various basic steps. First, the metal ion diffuses across aqueous diffusion layer from aqueous bulk feed solution to membrane feed interface and the metal-carrier complex is prepared at feed membrane interface. The complex is then diffused through liquid membrane phase to strip membrane interface owing to the concentration gradient. The complex is finally dissociated at strip membrane interface, carrier returns back across liquid membrane phase, and metal ion diffuses through aqueous diffusion layer into bulk strip solution. The theoretical model of mass transfer of Zn (II) for this purpose is important for the diffusion of metal-carrier complex.
3. Experimental
3.1. Chemicals and Reagents
ZnCl2 (97% BDH) in HCl (37% Merck) was used as feed in various concentration. NaOH (99-100% Merck) in distilled water in various concentrations was used as a stripping solution and TDDA (≥95% Merck) with diluent xylene (99.5%) to get the various composition of metal ion carrier. Double distilled and deionized water was used in all experiments. All other chemicals used were of analytical or better grade.
3.2. Analytical Instruments
Atomic absorption spectrometer of Perkin Elmer model 400 was used to measure the concentration of zinc (II) and other metal ions in feed and strip solutions. pH was determined through pH meter of Metrohm model 827. Viscosity determination of TDDA (in xylene) was performed via viscometer/rheometer of Brookfield LVDV-III.
3.3. Permeation Cell
The metal ion transport study was performed in two-compartment cell made of Perspex material as reported in our previous work [26]. Each half cell of the permeation cell had the volume capacity of 300 cm3 and active membrane area was 23.79 cm2. Each half was equipped with synchronous motors, pH electrode, and sampling port. The stirring speed of 1500 rpm [26] was optimized for the similar type of permeation cell and carrier; therefore this stirring speed was used in all the permeation study. All the experiments were conducted at °C.
3.4. SLM Preparation
The microporous polypropylene thin coating of Celgard 2400 was applied as a solid support for a liquid organic carrier with an active pore size of 0.02 µm, the thickness of 25 µm, and porosity of 38%. The membrane was cut into rectangular pieces of cm and was soaked in the premeasured concentration of TDDA diluted with xylene in Petri dish for overnight. The membrane was then taken out from the organic solution. The excess amount of organic carrier and solvent were removed by allowing them to drain off the membrane for about five minutes.
3.5. SLM Transport Study
The membrane after preparation was placed in between the two compartments of the cell and screwed together to form a water tight seal. Both compartments of the cell were filled with 300 mL of predetermined concentration of feed and strip solutions. To avoid concentration polarization at feed and strip membrane interfaces, the two solutions in feed and strip compartments were continuously stirred. Samples were collected after a specific time interval from both feed and strip solution and tested for metal ion concentration. For most of the experiments, the carrier concentration was 0.80 mol/dm3 in xylene.
The flux () was calculated as follows [26]:
4. Results and Discussion
4.1. Reactions at Feed Membrane Interface
If tri-n-dodecylamine (TDDA) is represented by L, the following possibilities of reactions may take place. The tri-n-dodecylamine can be protonated to LH+ in acidic medium (HCl).It is supposed that ZnCl2 in feed solution in the presence of excess chloride ions due to HCl is converted toThe cationic (LH+)(org) and anionic species then interact at feed membrane interface and form the complex asThe subscript org represents organic phase and aq aqueous phase and indicates the number of Cl−, H+, and L molecules associating with Zn (II) ions to form the neutral complex .
To express the contribution of H+ and Cl−, (3) may be represented as
4.2. Reactions at Strip Membrane Interface
The neutral complex formed at feed membrane interface as per (3)/(4) is extractable in liquid organic phase and disperses from feed membrane interface to strip membrane interface. The complex at strip membrane interface is dissociated owing to NaOH in the stripping phase as follows:The free carrier molecule (L) diffuses back through the liquid membrane phase towards feed membrane interface and again forms the complex. As per Wilke-Chang, the diffusion coefficient of the forward moving complex should be noticeably lesser than the diffusion coefficient of the backward moving free carrier molecules [30]. Owing to this reason, the free carrier concentration at feed membrane interface will constantly be higher than the complex. Figure 1 represents the schematic transport mechanism of Zn (II) through supported liquid membrane.
Figure 1: Transport mechanism of Zn (II) through supported liquid membrane.
The equilibrium constant of (4) for Zn (II) can be written as follows:The distribution coefficient of Zn2+ () for distribution between the membrane and aqueous phases can be represented as follows: And on rearranging of (8),Now considering the extraction constant, distribution coefficient, and laws of diffusion via the same path as [29], it can be indicated as:Equation (11) shows that, at a constant temperature, the flux () of Zn (II) is directly proportional to the concentration of Cl−, H+, L, and This equation can be used to find the number of H+ related to L in the form of LH+. This can be determined by various methods: one method is to keep [Cl−], [L], and constant in (11) and plotting against ; the slope of the curve will provide the “” value for a number of H+ ions in the complex. Likewise by drawing versus and maintaining [Cl−], [H+], and constant, the slope of the plot will give a number of moles () of TDDA contributing in complex formation of Zn (II).
4.3. Effect of Carrier Concentration
The carrier in the liquid membrane phase of the supported liquid membrane has a critical role in the extraction of metal ions by SLM [26]. Various concentrations of carrier ranging from 0.157 mol/dm3 to 1.103 mol/dm3 were used in solvent xylene to observe the effect of carrier concentration on the transport of Zn (II) through SLM. During this study, the Zn (II) concentration in feed solution was kept at mol/dm3 in 2.0 mol/dm3 of HCl and the NaOH concentration in strip solution was fixed at 2.0 mol/dm3.
Figure 2 shows that with the increase of carrier concentration from 0.157 to 0.80 mol/dm3 in xylene in the membrane phase has a significant effect on flux of Zn (II). It follows (11) where flux () is directly proportional to carrier concentration [L]. However, the flux is insignificant as the concentration of carrier increases beyond 0.80 mol/dm3. This reduction in transport of Zn (II) might be the result of enhanced friction of liquid membrane phase owing to high viscosity, as with the increase in carrier concentration, the viscosity of liquid membrane phase increases [29]. Hence 0.80 mol/dm3 of tri-n-dodecylamine was considered the optimum carrier concentration and more investigations were performed with this concentration of carrier.
Figure 2: Effect of carrier concentration on transport of Zn (II) in the stripping phase. [HCl] in feed solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.157 mol/dm3 to 1.103 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [Zn (II)] = mol/dm3, time = 5.0 h.
Finding the number () of tri-n-dodecylamine (L) taking part in complex was determined by plotting versus as shown in Figure 3. The slope of the plot is 2.008, and this indicates that two molecules of TDDA take part in the complex formation.
Figure 3: Plot of versus (same operating conditions as given in Figure 2).
In our previous study for the stability of membrane for such type of carrier, it has been observed that supported liquid membrane is quite stable for a period of 120 h with very minute flux variation in the metal ion flux and no indication of structural deformation of polypropylene membrane was investigated. Furthermore, the membrane was reused several times without leakage and metal ion flux decline [28].
4.4. Role of HCl Concentration in Feed Phase
The HCl performs an important part in the transportation of Zn (II) because it provides H+ and Cl− for the formation of complex as per (4). The effect of HCl concentration on extraction Zn (II) was observed by varying the concentration of HCl in the range of 0.5 mol/dm3 to 3.0 mol/dm3, while keeping the concentration of TDDA in the liquid membrane phase at 0.80 mol/dm3 and concentration of NaOH on strip side at 2.0 mol/dm3. Figure 4 shows that as the concentration of HCl increases from 0.5 mol/dm3 to 2.0 mol/dm3 and the flux of Zn (II) increases. Thus, it can be concluded that with an increase of HCl concentration, the concentration of H+ and Cl− ions in feed solution also increases. This, in turn, increases the formation of the complex as per (4), which ultimately enhances the transport of Zn (II). Although, by further increasing the concentration of HCl beyond 2.0 mol/dm3, the transport of Zn (II) decreases and this may be due to the formation of type species due to the large quantity of H+ and Cl− in feed phase and reaction (4) is hindered in forward direction. This study shows that 2.0 mol/dm3 of HCl is the most favorable concentration for transport of Zn (II), and further studies were carried out with this concentration of HCl to evaluate various parameters for subsequent studies.
Figure 4: Effect of HCl concentration in feed solution on flux () of Zn (II). [HCl] in feed solution = 0.50 mol/dm3 to 3.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, time = 5.0 h.
To investigate that how much hydrogen is taking part in complexation of Zn (II), was plotted versus (Figure 5). The slope of this plot was approximately 2.0, indicating that two hydrogens take part in a complex of Zn (II) transport. As the transport study concluded that two molecules of TDDA and H+ are involved in the complex formation of Zn (II), the complex formed during this extraction study may be .
Figure 5: Plot of versus (same operating conditions as given in Figure 4).
4.5. Influence of Feed Concentration
To study the capability of this SLM for metal ion transport, various concentrations of Zn (II) from mol/dm3 to mol/dm3 were used in the feed solution. During this particular study, the concentrations of the carrier, stripping phase, and HCl in feed solution were kept at 0.80 mol/dm3, 2.0 mol/dm3, and 2.0 mol/dm3, respectively.
Figure 6 shows that as Zn (II) concentration in feed solution rises, the transport of Zn (II) also increases; this is as per (11), where flux () is directly proportional to feed concentration (). Such type behavior has already been studied in our previous study [27, 28]. This study shows that up to mol/dm3 no saturation of carrier with metal ion takes place.
Figure 6: Effect of Zn (II) concentration in feed solution on its flux. [Zn(II)] in feed = mol/dm3 to mol/dm3, [HCl] in feed = 2.0 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, time = 5.0 h.
4.6. Influence of Stripping Phase Concentration
The stripping agent dissociates the complex at strip membrane interface and releases Zn (II) in strip solution. To investigate the effect of NaOH on the transport of Zn (II), numerous concentrations of NaOH in the range of 0.05 mol/dm3 to 2.5 mol/dm3 were used, while keeping the TDDA concentration at 0.80 mol/dm3 and HCl concentration in feed solution at 2.0 mol/dm3.
Figure 7 shows that as the concentration of NaOH rises from 0.05 mol/dm3 to 0.1 mol/dm3, the flux of Zn (II) decreases and, beyond this concentration of NaOH, the flux of Zn (II) increases. The decrease in transport of Zn (II) at a lower concentration of NaOH can be explained that it forms a precipitate of Zn(OH)2 which is insoluble and blocks the pores of polypropylene membrane and transport of Zn (II) is restricted [31]. The SEM (Figure 8(b)) indicates the blocked SLM at a lower concentration of NaOH. More increase in the concentration of NaOH increases the transportation of Zn (II), as the precipitate of Zn (OH)2 is soluble in excess of NaOH and more OH− are available that enhance the decomposition of the complex at strip membrane interface as per (5).
Figure 7: Effect of NaOH concentration in strip solution on the flux of Zn (II). [NaOH] in stripping solution 0.05 mol/dm3 to 2.5 mol/dm3, [HCl] in feed solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3, time = 5.0 h.
5. Recovery of Zinc (II) from Waste Discharge/Effluent of Galvanizing Plant
The objective of this work was to design and develop SLM for recovery of Zn (II) from industrial waste effluents. The polypropylene as supported liquid membrane, TDDA as a carrier, was utilized for extraction of Zn (II) from waste discharge liquor of galvanizing plant. To check and assess the efficiency of SLM system, samples of galvanized industrial waste were collected from the different locations of the drain carrying the galvanizing industrial effluent. The effluent samples were analyzed using the aforementioned method as described in Experimental and the percent extraction and recovery of the Zn(II) metal ions were determined using atomic absorption spectrophotometry method. The data obtained is provided in Figure 9 which indicates approximately the complete extraction and recovery of Zn (II) after 210 minutes. This shows the suitability and effectiveness of this SLM for recovery of Zn (II).
Figure 9: Variation in Zn (II) concentration in feed and strip solution against time (waste discharge liquor of galvanizing plant). Initial Zn (II) conc. in feed = mol/dm3, [HCl] in feed = 2.0 mol/dm3, [NaOH] in stripping solution = 2.0 mol/dm3, [TDDA] in membrane phase = 0.80 mol/dm3.
6. Recovery and Transport of Zn(II) from Galvanizing Plant Waste
The present SLM was designed for the transport of Zn(II) metal ions and applied to zinc industrial effluents of galvanizing plants because zinc is one of the industrial important metals because of its applications and its use for protection, passivation, and decoration of some heavy elements. The data in Table 2 show that TDDA-polypropylene SLM system has significant transport efficiency for Zn(II) metal ions, that is, about 99.8%, while other metal ions like Co, Cu, Cd, Mn, Cr, and Fe show very little or more precisely negligible amount of transport that is 0.0 to 0.72% or in average 0.08% for Co, 0.23% for Cu, 0.15% for Cd, 0.36% for Mn, 0.06% for Cr, and 0.28$ for Fe. This much smaller amount of transport may be attributed to experimental error. If error factor is taken into account then TDDA-polypropylene SLM system is highly selective for transport of Zn(II) metal ions only. The data are represented in Tables 1 and 2.
Table 1: Analysis of galvanizing plants waste.
Table 2: Percent transport of metal ions from galvanizing plants waste.
7. Conclusions
The flux () got increased by increasing carrier concentration up to 0.80 mol/dm3, and, further increasing the carrier concentration, the transport of Zn (II) was found to decrease. The Zn(II) ions transport was increased by increasing HCl concentration in feed solution up to 2.0 mol/dm3 and then decreased using a higher concentration of HCl. The slope analysis studies of of Zn (II) versus and showed that 2.0 mole of each tri-n-dodecylamine and hydrogen are involved in the transport of Zn (II). The maximum transport of Zn (II) was achieved at 0.80 mol/dm3 of TDDA in membrane phase and 2.0 mol/dm3 of HCl and NaOH in feed and strip solution, respectively. It was also found to be coupled coion extraction pathway, as H+, Cl−, and Zn (II) travel in the same direction. Two moles of hydrogen and TDDA interact with one mole of Zn (II) producing a complex , that is responsible for transport of Zn (II). This SLM system was effectively used for removal of Zn (II) to the waste discharge liquor of galvanizing plant of zinc.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The authors acknowledge Sarhad University of Science and Information Technology, Peshawar, KPK, Pakistan, for providing financial assistance to accomplish this work.
References
R. A. Wuana and F. E. Okieimen, “Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation,”ISRN Ecology, vol. 2011, Article ID 402647, 20 pages, 2011.View at Publisher·View at Google Scholar
D. W. Thomas, Metals and Their Compounds in the Environment, Edited by E. Merian, Wiley, Weinheim, Germany, 1991.
ATSDR (Agency for Toxic Substances and Disease Registry), Toxicological Profile for Zinc, Public Health Service. U.S. Department of Health and Human Services, Atlanta, Ga, USA, 2005.
A. S. Prasad, “Zinc deficiency,”The British Medical Journal, vol. 326, no. 7386, pp. 409-410, 2003.View at Publisher·View at Google Scholar·View at Scopus
K. M. Hambidge and N. F. Krebs, “Zinc deficiency: a special challenge,”Journal of Nutrition, vol. 137, no. 4, pp. 1101–1105, 2007.View at Google Scholar·View at Scopus
G. J. Fosmire, “Zinc toxicity,”American Journal of Clinical Nutrition, vol. 51, no. 2, pp. 225–227, 1990.View at Google Scholar·View at Scopus
L. Monser and N. Adhoum, “Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater,”Separation and Purification Technology, vol. 26, no. 2-3, pp. 137–146, 2002.View at Publisher·View at Google Scholar·View at Scopus
S. Liang, X. Guo, and Q. Tian, “Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulfured orange peel,”Desalination, vol. 275, no. 1–3, pp. 212–216, 2011.View at Publisher·View at Google Scholar·View at Scopus
F. J. Alguacil and S. Martínez, “Solvent extraction of Zn(II) by cyanex 923 and its application to a solid-supported liquid membrane system,”Journal of Chemical Technology and Biotechnology, vol. 76, no. 3, pp. 298–302, 2001.View at Publisher·View at Google Scholar·View at Scopus
K. C. Sole, T. L. Ferguson, and J. B. Hiskey, “Solvent extraction of silver by cyanex 272, cyanex 302 and cyanex 301,”Solvent Extraction and Ion Exchange, vol. 12, no. 5, pp. 1033–1050, 1994.View at Publisher·View at Google Scholar·View at Scopus
P. M. Cole and K. C. Sole, “Zinc solvent extraction in the process industries,”Mineral Processing and Extractive Metallurgy Review, vol. 24, no. 2, pp. 91–137, 2003.View at Publisher·View at Google Scholar·View at Scopus
M. S. Lee and S.-H. Nam, “Solvent extraction of zinc from strong hydrochloric acid solution with Alamine336,”Bulletin of the Korean Chemical Society, vol. 30, no. 7, pp. 1526–1530, 2009.View at Publisher·View at Google Scholar·View at Scopus
F. M. Pang, S. P. Teng, T. T. Teng, and A. K. M. Omar, “Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions,”Water Quality Research Journal of Canada, vol. 44, pp. 1–9, 2009.View at Google Scholar
S. V. Plokhov, I. G. Matasova, V. A. Utkin, and V. Y. Vodzinskii, “Specific features of ion-exchange zinc(II) extraction from washing water of zinc plating,”Russian Journal of Applied Chemistry, vol. 75, no. 6, pp. 950–953, 2002.View at Publisher·View at Google Scholar·View at Scopus
K. H. Park, P. K. Parhi, and N.-H. Kang, “Studies on the removal of low content copper from the sea nodule leach liquor using the cationic resin TP 207,”Separation Science and Technology, vol. 47, no. 10, pp. 1531–1541, 2012.View at Publisher·View at Google Scholar·View at Scopus
J. D. Gyves and E. R. San Miguel, “Metal ion separations by supported liquid membranes,”Industrial and Engineering Chemistry Research, vol. 38, no. 6, pp. 2182–2202, 1999.View at Publisher·View at Google Scholar·View at Scopus
V. V. Prasad, D. J. Yogesh, D. S. Ajay et al., “Simultaneous extraction of neodymium and uranium using hollow fiber supported liquid membrane,”Separation Science and Technology, vol. 49, no. 10, pp. 1509–1520, 2014.View at Publisher·View at Google Scholar·View at Scopus
B. Mokhtari and K. Pourabdollah, “Inclusion separation of alkali metals in emulsion liquid membranes by nanobaskets of calix[4]crown-3,”Brazilian Journal of Chemical Engineering, vol. 29, no. 4, pp. 783–793, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. B. Kanungo and R. Mohapatra, “Coupled transport of Zn (II) through a supported liquid membrane containing bis (2,4,4-trimethyl pentyl) phosphinic acid in kerosene. 1. A model for the rate process involving binary and ternary complex species,”Journal of Membrane Science, vol. 105, pp. 217–226, 1995.View at Google Scholar·View at Scopus
O. N. Ata, A. V. Beşe, S. Çolak, B. Dönmez, and A. Çakıcı, “Effect of parameters on the transport of zinc ion through supported liquid membrane,”Chemical Engineering and Processing: Process Intensification, vol. 43, no. 7, pp. 895–903, 2004.View at Publisher·View at Google Scholar
R. Wódzki and P. Szczepański, “Simultaneous recovery and separation of Zn2+ and Cu2+ in hybrid membrane systems,”Separation and Purification Technology, vol. 41, no. 3, pp. 289–297, 2005.View at Publisher·View at Google Scholar·View at Scopus
M. Ulewicz, W. Walkowiak, J. Gega, and B. Pospiech, “Zinc (II) selective removal from other transition metal by solvent extraction and transport through polymer inclusion membranes with D2EHPA,”Ars Separatoria Acta, vol. 2, pp. 47–55, 2003.View at Google Scholar
J. Kozlowska, C. A. Kozłowski, and J. J. Koziol, “Transport of Zn(II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers,”Separation and Purification Technology, vol. 57, no. 3, pp. 430–434, 2007.View at Publisher·View at Google Scholar·View at Scopus
M. Torz, K. Alejski, and J. Szymanowski, “Modelling of zinc (II) extraction from model hydrochloric acid solutions in hollow fiber modules,”Physicochemical Problems of Mineral Processing, vol. 37, pp. 97–105, 2003.View at Google Scholar
J.-C. Lee, J. Jeong, B.-S. Kim, M. S. Kim, and M. Kobayashi, “Separation of copper and zinc ions by hollow fiber supported liquid membrane containing LIX84 and PC-88A,”Materials Transactions, vol. 45, no. 6, pp. 1915–1919, 2004.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, M. A. Chaudry, N. Bukhari, Najeebullah, and N. Ali, “Mn (VII) ions transport by triethanolamine cyclohexanone based supported liquid membrane and recovery of Mn (II) ions from discharged zinc carbon dry battery cell,”Journal of Membrane Science, vol. 366, no. 1-2, pp. 125–131, 2011.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, and M. A. Chaudry, “Coupled transport of Tl3+ through triethanolamine-xylene-polypropylene supported liquid membranes,”Journal of Industrial and Engineering Chemistry, vol. 18, no. 1, pp. 492–498, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. U. Rehman, G. Akhtar, M. A. Chaudry, K. Ali, and N. Ullah, “Transport of Ag+ through tri-n-dodecylamine supported liquid membranes,”Journal of Membrane Science, vol. 389, pp. 287–293, 2012.View at Publisher·View at Google Scholar·View at Scopus
S. Ur Rehman, G. Akhtar, and M. A. Chaudry, “Coupled transport of Pb2+ through tri-n-octylamine-xylene-polypropylene supported liquid membranes,”Canadian Journal of Chemical Engineering, vol. 91, no. 6, pp. 1140–1152, 2013.View at Publisher·View at Google Scholar·View at Scopus
S. A. Ansari, P. K. Mohapatra, and V. K. Manchanda, “Recovery of actinides and lanthanides from high-level waste using hollow-fiber supported liquid membrane with TODGA as the carrier,”Industrial and Engineering Chemistry Research, vol. 48, no. 18, pp. 8605–8612, 2009.View at Publisher·View at Google Scholar·View at Scopus
F. Holleman, A. E. Wiberg, and N. Wiberg, Zink, Lehrbuch der Anorganischen Chemie, Walter de Gruyter, Berlin, Germany, 91th–100th edition, 1985.
Source
http://www.hindawi.com/journals/jchem/2017/7569354/
from TAXI NEAR ME http://taxi.nearme.host/transport-of-zn-ii-by-tdda-polypropylene-supported-liquid-membranes-and-recovery-from-waste-discharge-liquor-of-galvanizing-plant-of-zn-ii/
0 notes
Text
New Desalination Method to Purify Water With Lower Power
Gendron Recheche, #Cancer New desalination technique called battery electrode deionization removes salt from water using less energy than currently existing techniques. http://dlvr.it/Q8ymqj
0 notes
Text
New desalination process presents low vitality alternate to purify salty h2o — ScienceDaily
Offering safer ingesting h2o to people in will need may perhaps be a very little much easier. In accordance to Penn State researchers, a new desalination system is ready to clear away salt from h2o making use of fewer vitality than previous solutions.
“Globally, there is lowered obtain to clean h2o,” said Bruce Logan, Evan Pugh College Professor in Engineering and the Stan and Flora Kappe Professor of Environmental Engineering. “Additional and more, the waters that are being utilised are impaired, possibly because of to salt or other contaminants, so we are observing an raising will need to rely on fewer exceptional h2o resources.”
To beat this difficulty, Logan, and colleagues Christopher Gorski, assistant professor of environmental engineering, and Taeyoung Kim, put up-doctoral scholar in environmental engineering, have arrive up with a desalination process termed battery electrode deionization (BDI). BDI increases upon regular capacitive deionization (CDI) procedures by eliminating the regeneration phase and decreasing the voltage expected to comprehensive the system.
Regular CDI procedures desalinate h2o by separating the water’s ions. A common CDI mobile is composed of two electrodes hooked up on opposite sides of a stream channel. The electrodes seize the salt ions by way of electrical exchanges that take place when an electrical recent is used to the mobile. The mobile is then regenerated by releasing the salt ions in a second cycle by alternating the course of the used electrical recent. Considering that CDI does not call for membranes and has reduced vitality demands than other well-known solutions, it is turning out to be a competitive technology for getting rid of salt from h2o. The difficulty with CDI techniques is that they are minimal by low salt adsorption when making use of the normally used 1.2 volts. Increasing the used voltage does improve the salt adsorption, but it also will increase the opportunity for unintended facet reactions that waste vitality and can create long term electrode corrosion.
In the team’s newly created BDI process, a tailor made-designed stream mobile utilizes two channels. The channels are separated by a membrane and two equivalent battery electrodes are secured at each and every end.
To check the cell’s success, the group fed each and every channel with a salty resolution at a specified stream level though implementing a regular electrical recent to the mobile. Numerous recent densities were being utilised, based on the selection of membrane stacks. The researchers then reversed the mobile voltage stream when it arrived at a low of −0.6 volts or a high of +.6 volts.
The group found out that the BDI process efficiently taken off the salt at stages regular with CDI, though making use of only an used voltage of .6 volts. On top of that, the low voltage expected and resources utilised assisted avert unwanted facet reactions, achieved better desalination capabilities and eaten fewer vitality than traditional CDI.
Considering that the group established simultaneous output of desalinated and concentrated h2o in two channels, it also circumvented the two-cycle strategy, so the process no for a longer period desires to go by way of the regeneration phase. Furthermore, they located that stacking supplemental membranes among electrodes lowered vitality use even further more.
“Other persons have talked about capturing vitality from the second CDI cycle, but it is really genuinely challenging to do, and, as a result, it is really impractical,” Logan said. “Our process avoids that second regeneration action by just switching the captured stream by alternating the course of the used electrical recent. That would make it pretty effortless to run, and it works by using pretty very little vitality.”
While the recent configuration is not suited to desalinate really salty h2o such as seawater, the outcomes clearly show that the BDI system could be successful as a low vitality process for brackish, or slightly salty, h2o, such as groundwater, or for desalinating h2o prior to it enters cure vegetation.
“There is very little that inherently helps prevent its use with seawater, it is really just that as h2o will get saltier and saltier, there are other challenges that we have to contend with, such as elevated vitality use and membrane fouling, that may perhaps cut down its utility relative to other approaches,” Logan said.
The researchers now plan to get the job done on scaling up and increasing the steadiness of the process.
“This is an revolutionary technology,” Logan said. “This is not one thing that is out there and commercialized. It really is one thing that is appropriate at the cutting-edge of new approaches to get salt out of h2o.”
This analysis was revealed in Environmental Science & Technologies Letters in September. Funding was offered by the National Science Basis, King Abdullah College of Science and Technologies and Penn State College.
Describing Filtration Products Page: Filtration Products site reveals the recent awareness, analysis and separation supplies clean from the purification market. Filtration-Products.com retains you briefed on filtration and all the associated experienced analysis which includes string wound cartridges, pleated filter cartridges, soften blown cartridges, bag filters, reverse osmosis filtration, from models such as Mahle made for liquid recycling, and anything at all else the separation world has to explore.
The post New desalination process presents low vitality alternate to purify salty h2o — ScienceDaily appeared first on Filtration Products.
from Filtration Products http://ift.tt/2AcBQIU
0 notes
Text
Best Microscopes and Ph Meters Options
http://microscopereviews.review
http://bestphmeter.review
http://bestmicroscopes.review
The Upside to Best Microscopes and Ph Meters
Industrial purposes of deionized water can not ever be refuted. If you currently have a pool, the easiest means to work out how much water you're losing is to measure it directly. Cloudy water is normally an indication of improper TA and filtration.
Moisture meters are utilised to assess the moisture content wood so the woodworker can determine whether it's appropriate for its intended use. In fact, the key added benefits to Hydroponic Gardening is to give individuals who would otherwise not be able grow plants the capability to grow plants. Taller glass blades function as a deterrent to the development of weeds.
Based on your soil and the plants you want to grow, to be able to grow healthful vegetables, you might want to provide more of one nutrient or another. It's a metallic probe that you insert deep into the soil. Soil is among the most significant facets of reaping agricultural advantages.
The fourth technique is the cluster of method that may also be utilized to produce pure H20. The electrodes might be contained in distinct probes or within a single probe. These probes have to be available and ideally shouldn't be tied down to a particular connectors design.
This suitable care and maintenance will allow you to keep your lab equipment ready to go as long as possible. So, the true intention of a microscope is to see little things clearly. Whatever you pick, you can discover a business that is going to serve your preferences.
Additional research, investigation, and analyses are required to resolve this matter. Possessing this ability in the aviation field is just the start and can start to improve several things. Contrary to what you might believe, most science kit experiments are performed with a very good idea about what will happen.
The pH is a vital aspect in the plant's growth as it's directly linked to the access to the nutrients. Since they can be used for multiple purposes, they can be found in various categories. It is thus an essential piece of equipment for any serious home brewer.
The Milwaukee MW102 is the probably the ideal pH meter available at the cost, it's simple to use and has rather excellent accuracy. Water deionization only removes charged particles and doesn't remove organic solutes that are uncharged. A digital PH meter is normally utilized to gauge the PH of the water in these types of tanks.
The above mentioned image indicates a pH tester of this sort. The easy solution is to produce the soil slightly acid. As the system switches on, it is going to display a pH reading. The Best Microscopes and Ph Meters Trap
Some sites might be cheaper, but they could be selling a lighter weight. You're able to purchase litmus on the internet or at a chemical supply shop. So it is essential to select the kits according to individual requisites. Details of Best Microscopes and Ph Meters
On top of that, is the systems portion that play an immense role and would decidedly be employed to boost performance yet conducting in a more compact airplane that's lighter in weight. Set the unit close to the jar, be certain that you have installed a fresh set of batteries for this purpose for the best results. Any value deviating from this perfect value impacts the grade of the water.
Additionally, it offers cost efficiency. See how much you're really getting. The kit includes all the essential equipments and the instructions to perform the test.
0 notes
Text
BEST HM Digital DM-1 In-Line Dual TDS Monitor
New Post has been published on https://freshwaterpro.com/best-hm-digital-dm-1-in-line-dual-tds-monitor/
BEST HM Digital DM-1 In-Line Dual TDS Monitor


>> Click Here to See More Photos, Now! <<
Introduction:
Exactly how natural is your drinking water? HM Digital’s DM-1 is a double inline monitor that steps TDS from 0 -9990 ppm. It includes battery packs, 2 sensors, and 2 1/4 inch fast -connect T-fittings. This particular DM-1 is definitely a simple and handy technique of identifying levels of TDS for drinking water filtration systems, for example, RO (reverse osmosis), DI (deionization) and even more. It is very helpful for under the sink and even for chillers techniques. For just about any kind of system that eliminates TDS, you may setup the sensor ‘IN’ on your water of faucet ( prior to the system) and also the sensor ‘OUT’ on your product for water ( right after the filtration ) to figure out the membrane/filter’s efficient working (rejection) rate.
Features of this product:
The DM-1 assists you to calculate the water of the faucet arriving vs. the filtered water heading out of your drinking water filtration system whenever you want.
The In-Line Double TDS meter shows TDS amounts of the feed to water and also product water with ‘out’ and ‘in’ electrode probe.
This particular TDS keeps track of is extremely effective and accurate because of to the innovative microprocessor technology.
It is highly efficient and also accurate as a result of the enhanced microprocessor technology.
It comes with an auto-off function that conserves the battery power. This specific device shuts off instantly right after ten minutes of non-use.
You can setup the product easily and quickly.
Customer review:
You cannot find any cause not to provide this particular product of the maximum rating. The cost was perfect, it can do what exactly it is expected to perform and it is simple to setup. The meter is easy to setup; I have installed with clamps correctly onto the part of the Reverse Osmosis glandular. Just press the switch for power and then select, forth and back if you want, the outcome water reading and even input water TDS. The screen is reader friendly and it also turns off independently. Personally, I like to remain in the behavior of manually shutting the equipment down when not required. I checked it out having an expert meter and then the readings were similar. It is so handy to make use of, permitting regular inspections that will certainly keep the quality of the water up. You shouldn’t be shocked, it is smaller sized than one wants, however, not an issue, it is simple to read from your arms length.
0 notes