#Ammonia Price
Explore tagged Tumblr posts
Text
Ammonia Prices | Pricing | Trend | News | Database | Chart | Forecast
Ammonia Prices a key component in various industrial applications, including agriculture and manufacturing, has seen significant fluctuations in its pricing over recent years. The price of ammonia, a compound consisting of nitrogen and hydrogen, is influenced by a myriad of factors ranging from production costs to global demand and geopolitical dynamics. At its core, the production of ammonia is heavily reliant on natural gas, which serves as a primary feedstock. Therefore, changes in natural gas prices directly impact ammonia production costs and, consequently, its market price. Over the past decade, ammonia prices have experienced volatility due to shifts in natural gas prices, changes in environmental regulations, and variations in supply and demand.
The global demand for ammonia is predominantly driven by its use as a nitrogen fertilizer in agriculture. As the world population grows, the need for efficient agricultural practices increases, leading to a rise in fertilizer consumption. This growing demand can put upward pressure on ammonia prices, especially if production does not keep pace with consumption. Additionally, ammonia is used in other sectors, such as chemical manufacturing and water treatment, further influencing its market dynamics. Supply chain disruptions, trade policies, and transportation costs also play crucial roles in shaping ammonia prices.
Get Real Time Prices for Ammonia: https://www.chemanalyst.com/Pricing-data/ammonia-37
Geopolitical events and trade policies can significantly impact ammonia pricing. For instance, changes in trade agreements or sanctions imposed on key ammonia-producing countries can disrupt supply chains and lead to price fluctuations. Similarly, regional conflicts or political instability in major ammonia-producing regions can affect production levels and export capabilities, thereby influencing global prices. In recent years, the ammonia market has also been affected by the transition towards more sustainable practices. As environmental regulations become stricter, producers may face increased costs associated with compliance, which can translate into higher ammonia prices.
Technological advancements and innovations in ammonia production are other factors that can affect pricing. New methods that enhance efficiency or reduce production costs can potentially lower prices, while technological challenges or the need for significant capital investment can drive prices up. The emergence of green ammonia, produced using renewable energy sources, is an example of how innovation can shape market trends. Although green ammonia holds promise for reducing the carbon footprint of ammonia production, its higher production costs compared to conventional methods may impact its pricing dynamics in the short term.
The interplay between supply and demand is crucial in determining ammonia prices. For instance, a surge in agricultural activity or a bumper crop can lead to increased fertilizer application, driving up ammonia demand and consequently its price. Conversely, an economic downturn or a shift towards alternative fertilizers could reduce demand and put downward pressure on prices. Seasonal factors, such as weather conditions affecting agricultural productivity, also contribute to price fluctuations. In periods of high demand or supply constraints, prices may spike, while in more stable conditions, prices may stabilize or even decrease.
Ammonia pricing is also influenced by global economic conditions. Economic growth drives industrial activity and agricultural output, which in turn affects ammonia demand. Conversely, economic slowdowns can lead to reduced industrial and agricultural activity, impacting ammonia prices negatively. Currency fluctuations can also play a role, as ammonia is traded globally, and changes in exchange rates can affect the cost of imports and exports, influencing local prices.
In summary, the pricing of ammonia is a complex interplay of various factors, including natural gas prices, global demand, geopolitical events, environmental regulations, technological advancements, and economic conditions. Understanding these dynamics is crucial for stakeholders in the ammonia market, from producers and traders to end-users. As the world navigates the challenges of sustainability and economic fluctuations, ammonia prices will continue to be influenced by these multifaceted factors. Monitoring trends and staying informed about market developments will be essential for making strategic decisions in the ammonia sector.
Get Real Time Prices for Ammonia: https://www.chemanalyst.com/Pricing-data/ammonia-37
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Ammonia#Ammonia Price#Ammonia Prices#Ammonia Pricing#Ammonia News#Ammonia Price Monitor#Ammonia Database#Ammonia Price Chart
0 notes
Text
I need to to go to a rave and I need to put a hex on someone.
#🐇#I'm not even kidding like it would fix me#well beating the SHIT out of someone actually would be I can't~ so this is what's happening#I saw a tiktok of this girl who does binding spells with like an actual fucking heart and people were like ????? where the fuck did you get#the heart???? and she's pretending she doesn't see the comments lmfao#if someone asks what her prices are she answers REAL quick. like don't gatekeep bitch where did you get the heart!!!#anyway....going to get a bottle of ammonia tomorrow to make a shame jar#I could use tomorrow to manifest nice things for myself but I'm spiteful :)
1 note
·
View note
Text
The Global Clean (Blue & Green) Ammonia Market is projected to grow at a CAGR of around 23.56% during the forecast period, i.e., 2025-30. The Global Clean (Blue & Green) Ammonia Market is in its embryonic stage since the historical period, owing to the major focus on research & development & not much commercial application of clean ammonia among end-user industries. However, with the increasing investment by the companies towards the setup of clean ammonia production facilities, the market is expected to witness significant growth during the forecast years.
#Global Clean (Blue & Green) Ammonia Market#Global Clean (Blue & Green) Ammonia Market News#Global Clean (Blue & Green) Ammonia Market growth#Global Clean (Blue & Green) Ammonia Market industry#Global Clean (Blue & Green) Ammonia Market report#Global Clean (Blue & Green) Ammonia Market Share#Global Clean (Blue & Green) Ammonia Market Size#Global Clean (Blue & Green) Ammonia Market Industry#Global Clean (Blue & Green) Ammonia Market price
0 notes
Text
Southeast Asia Ammonia Market Report 2023-2028
Rising demands from the agricultural sector and rising ammonia use in explosive manufacture are expected to drive the Southeast Asia ammonia market expansion throughout the course of the projection period.
More Information Visit Site: Southeast Asia Ammonia Market
#Southeast Asia Ammonia Market Price#Southeast Asia Ammonia Market Forecast#Southeast Asia Ammonia Market Trends
0 notes
Text
cUSP Solutions offers best price solutions for Ammonia buffer for hardness testing.
cUSP Solution is very own brand of Dawn Scientific. it is a buffer solution for hardness testing.it is suitable for hardness testing for solids, resins and gases. buy Ammonia Buffer for hardness testing at fair price.
#Ammonia Buffer for Hardness Testing#Ammonia Buffer for Hardness Testing Price#Buy Ammonia Buffer for Hardness Testing
0 notes
Text
"A new community housing development in the Bronx will feature a cool piece of kit: an on-site aerobic digester that can turn 1,100 pounds of food scraps into 220 pounds of high-quality fertilizer every single day.
Built by Harp Renewables, it’s basically a big stomach filled with bacteria that breaks down food scraps and wasted food into their component parts, and in the future could be a standard part of all apartment units as the amount of food waste in American reaches 30% of the total mass of all trash collection.
The Peninsula, organized by Gilbane Development Company, will feature 740 units of affordable housing, 50,000 square-foot light industrial space and equal sized green space, and 15,000 feet of commercial space, all of which will send their castaway comestibles right into the digester...
Fast Company reports that Christina Grace, founder of a zero-waste food management company, helped plan the design and implementation of the digester into The Peninsula, and helped organize a 40% grant from the city to pay the $50,000 upfront cost.
“The goal is for this material to work its way into the community garden network in the Bronx,” [Christina Grace, who helped plan the design] told the magazine, adding that she expects it to pay for itself over just a few years. “We see this as highly replicable in both commercial and residential venues. We know there’s a need for fertilizer.”
Producing fertilizer right there in the city reduces the need for it to be trucked in from afar, chipping away, even if just a bit, at NYC traffic.
Big problem solver
Perhaps uniquely beneficial to New York City compared to other spots in the U.S. is that the digester will have a significant impact on the Bronx’s share of the city’s rodent problem.
Those who’ve watched the Morgan Spurlock documentary Rats will understand why that’s significant—while those that haven’t will have to imagine what living in a megacity where rats outnumber people by around 8 or 10 to 1 looks like.
Another big problem the bio-digesters could potentially help is pollution and greenhouse gas emissions. Fertilizer is a big emitter of all three of the most-targeted GHGs. Fertilizer, like quarry dust and ammonia is, like so many commodities, often imported from countries who specialize in its production, such as Norway, but also Russia and Ukraine, whose conflict has recently highlighted the fragility of the supply chain with sharp increases in prices...
Bio-digesters by design keep the CO2 and methane in the fertilizer produced, rather than it entering the atmosphere.
For these reasons and more, the aerobic bio-digester is slowly making its way into residential and industrial spaces around the country.
GNN reported on an enormous bio-digester at the heart of the D.C. advanced resource (sewage) recovery center outside the capital, and on the use of bio-digesters on Australian pig farms which are helping reduce the environmental and psychological impact of the effluent produced from such operations.
Harp Renewables tweeted how happy they were to have installed their bio-digester in the town of Cashel, Ireland.
Expect to see more stories like this pop up around the globe."
-via Good News Network, March 17, 2022
Note: Obviously gentrification bad and "affordable housing" is sometimes nowhere near as affordable as it should be, etc. etc. That said, this is such a fantastic use case that I felt I had to post it anyway.
#new york#new york city#bronx#bronx new york#supply chain#fertilizer#circular economy#sustainability#sustainable architecture#sustainable agriculture#united states#apartment buildings#bio-digester#good news#food waste#organic waste#hope#hope posting
323 notes
·
View notes
Text
How to degrease bones? (With the easiest and cheapest method!)
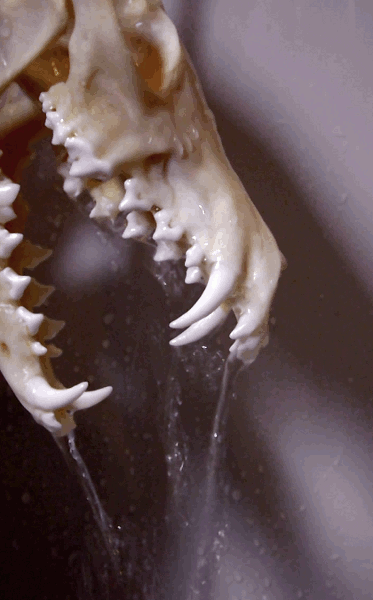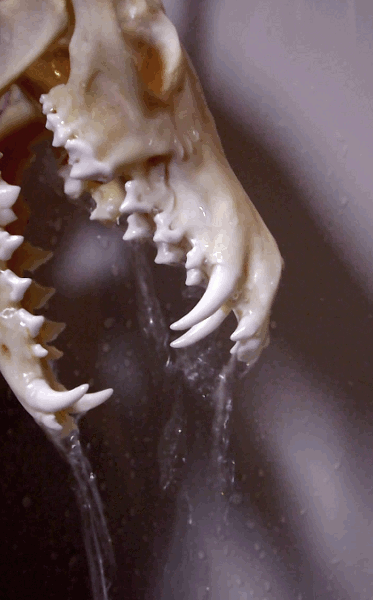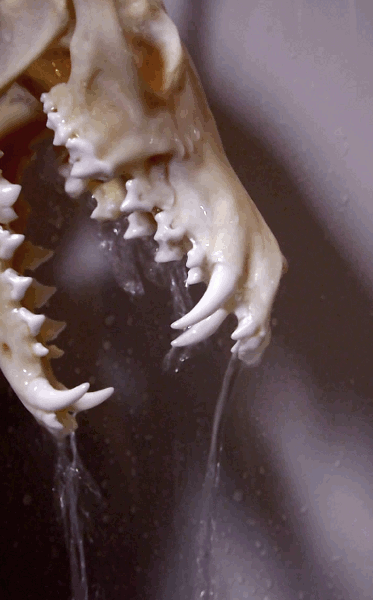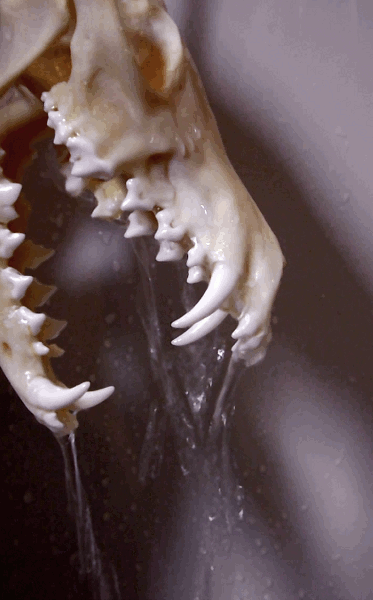
Bone cleaning is quite a journey - and honestly degreasing is the part I find a tad confusing. Questions like: how long it will take? Does it work in cold water as well? Is it done? Is it doing anything at all?
As a beginner bone collector, who also doesn't want to put a huge amount of money into it and finding an acceptable way to clean bones is essential. Mainly because of the challenge, - tbh it is quite enjoyable for me to create quality bones with a less amount of investment, it just makes me feel like I really worked for it, I love these challenges - but mostly because it is already difficult to make my family accept this kind of a hobby of mine. Not many people are fond of keeping rotting animal parts at home, I can tell you that! If this hobby turns out to be stinky and expensive, that is definitely a no from family members. And even though I am an adult, making my own money, my husband does have a saying about the family funds - because we are both responsible for this family - so it is important for me to keep things at a reasonable price.
There are many ways to degrease bones and you have to pay a price for it either way, be it about the time period the degreasing takes or the used materials. You can degrease bones chemically, using liquids like ammonia or acetone, but personally, I dislike these, because:
it requires some effort to put your hand on this stuff, they can be hard to come by
they can be harmful to your health (ammonia is not nice to work with)
they can be expensive, and we already have to buy H2O2
they have to be stored and get rid of properly - you cannot just let these go down in your sink
and some materials can be straightforward and dangerous - read about some pros are using stuff like petrol for degreasing and while it does the job, petrol is extremely unstable, highly flammable and tend to blow up easily, so super no!
So, I go with the safest and also the cheapest solution: dish soap.
Dish soap is something that is easy to come by, can be super cheap and the water system is well prepared to clean grey water, so you can pour dish soap into your sink. However, it can take time to degrease your bones. While ammonia or acetone can be done with degreasing under a day (depends on the size of the skull and species of the animal), dish soap takes a lot longer: days at the best, months at the worst. But this is also the easiest degreasing method for beginners.
But there is another big question: how do you know your degreasing is working (and when it is done)?
When I started to even think about degreasing I went online, read about dish soap and was happy because everyone has dish soap at hand, so I picked a pot, filled it with cold water, pour the dish soap in, put the bones in and yay, magic was done! But things are not this simple.
The first days everything went fine: my water had fat oil drops at the surface and a visible white cloud came out of the bones, so a clear sign of the degreasing is working. But this stage went down quickly, like a matter of days - and I thought okay, degreasing is done, time to pull the skulls out and whiten. But my whitening never turned out white, rather like light grey and first I blamed my peroxide because of it, then my bones. These are findings from nature, probably they are stained, right?
I started to be doubtful when my cat skull turned out to be sticky after whitening. That never happened before, so another research later I came to the conclusion the cat that I thought are fully degreased is actually not degreased. But it didn't do a thing in the pot anymore. So what did I do wrong?
I used cold water. Apparently cold water works, but only for a while. It cannot really pull out the grease that is hiding deep inside the bone - that's why I stopped seeing white cloud after a matter of days, falsely thinking I am done. I needed warm water in the long run - if I simply use warm tap water that just runs cold way too quickly. It can be done with warm tap water as well, but that takes even longer. So, I bought an aquarium heater.
I looked after the fat oil drops in the water. They appeared on the first day, so I thought they will keep appearing until I am done. Turns out they don't? Rather the water slowly goes more opaque and murky with time, but no more fat drops don't matter how hard I am looking for them. This makes my job significantly difficult because oil drops are easier to spot and tell based on them if the degreasing is working or if I am actually done.
I am just super imapetient. I want my skull done and perfect as soon as possible. But it just doesn't work like that. I am working on this cat skull for 3 weeks now and it is still going: I had to macerate it, then degrease it, then whiten it, and then go back to degreasing and all I wanna do is glue it together and post fancy pictures of it. Won't happen for a while, time to accept that.
But how this opaque water progress looks like? I was so confused about is it clear water, is it dirty water, is it done, whether the water is warm enough or not, so I started to document the process.

This is the freshwater stage. I just changed the water and quickly snapped a pic of it, making it my reference piece. I can clearly see all the details of my bones, even if my dish soap is yellow, colouring my water a bit - but I can see through the water without any problem. My heater can do 36 Celsius degrees max, otherwise, it cooks the fish in the tank, so I put that on max, hoping it will be enough. About the temperature: I did read about 46 Celsius or even more than 50 Celsius for water temperature, but the aquarium heater cannot reach those degrees, because the main goal is to keep fish alive and no fish stays alive in 40+ water. I could use a bucket heater, but for me, that is harder to get and I really don't want a setting that takes a lot of space/costs a lot of money, so an aquarium heater it is.
Another thing I am not comfortable to put my bones in more than 50 Celsius degrees. This is my personal choice, but I really wanna avoid any chance of accidentally cooking my bones, and 50 seems to be too much heat.

I looked back at it an hour later and snapped another pic: and look, we start to get blurry details! No oil drops on the surface, but something definitely makes the water murky: my water is not hot enough to cook the bones, so it cannot dissolve or take any kind of damage in my bones, so this stuff must be grease! Seems like the heater works!

Checked the bones that evening as well and the water is definitely even more opaque!

And this is the next day: I can barely see my bones anymore, so definitely time to change the water.
Conclusion
If you think you are done with your bones, but they:
have yellow spots or wax on them
stick to your hand like you glued them
are shining here or there
have a waxy feeling
smells
Then your degreasing is not done. The good news is you can always go back to degreasing, doesn't matter if you whitened the bones or not.
The cheapest version of degreasing is the dish soap version and you will need warm water for it! It can be a good idea to get an aquarium heater because that will help you to macerate carcasses during the winter as well and quickens degreasing too. You can work with warm tap water, but that takes even longer.
But the dish soap method really takes time! Seems like this part is the longest one in skull cleaning. So even if it seems like my degreasing is not over and my method works, I can also see I won't have a pretty white cat skull anytime soon.
The bones are bathing for the third day in a row now and they seem to release the same amount of grease, so no sign of clear water yet. Also, when I pull the bones out of the water I can still see yellow spots on it - that is grease, sweeping to the surface and I need to get rid of that.
And how I will know my degreasing is done? My water stops being opaque. I can decide when to pull my bones out - do I want to fully degrease it or I decide to end it sooner because I want some discolouration, preserving am roe natural look.... that is up to me. Ideally, I wanna do a full degrease, but I just wanna preserve my bones perfectly to have quality art references that will be with be for a long time, so I try to go for a full degrease and will see how long that takes.
So just take your time, change your water as needed and enjoy the process :3 You cannot harm your bones this way, so happy experimenting!
#vulture culture#degreasing bones#skull degreasing#animal skulls#degrease#degreasing#dead animal#oddities#bone cleaning
194 notes
·
View notes
Note
Hi, I have a question.
How do you not kill fish? I was put in charge of two baby comet goldfish, one of them died because it either was stressed out or was overfed, and nobody in my family knew how to take care of fish, even though my dad acted like he knew everything there is to know about taking care of animals. The person who gifted me the fish got them from a pet store, so I knew in the back of my mind that they were going to die regardless of what I did. I kept them at my house because I was going to give them to my friend, but turns out she doesn’t have a fish tank because her POS mom is withholding the fish tank and proper equipment from her. I had no choice but to keep them in the largest container we had, which was a large measuring cup, and I had to net them with a strainer to change the water.
I wanted to give back the fish when I got the two of them, but now I’m paying the price.
Honestly, the best thing you can do for the remaining fish is to call up your local pet/fish stores and see if they'll take the fish off your hands. Keeping it in a measuring cup is guaranteed to kill it. Goldfish poop a lot and will toxify that amount of water in no time. You would have to change the water multiple times a day to keep up with water quality, and honestly, it's cruel to keep it such a small container. I know you're doing the best you can, and thank you for reaching out for help! But unless you can go and get a temporary tank and filter set up soon, the best thing you can do is take it to a pet store. You can also try and rehome it to other fishkeepers. Check out some groups on Facebook and see if you can find someone in your area with a big enough tank or a pond to house them in. In the meantime, I would suggest two or so water changes a day to try and keep the ammonia down in the measuring cup and try not to feed too much. The less waste produced, the better! I hope this helps!
12 notes
·
View notes
Text
It occurs to me most people don’t know about saltwater tank refugiums! The whole reason saltwater is notoriously difficult is that almost everyone wants it for fish, which produce enormous amounts of ammonia waste compared to most invertebrates, or they want it for corals, which require precise water conditions to remain healthy, or god forbid both fish and corals. And what a lot of hobbyists do is attach their aquarium to a “refugium.” This is a secondary aquarium just for macroalgae and little amphipods and things that they don’t want in the main display tank but are good for the water, so they keep those separate and just have water circulating between the two tanks to keep the fish and corals one healthier and cleaner. “Display refugiums” are a thing, like don’t get me wrong the hobby CARES about those organisms, but the idea of basically hiding the slime and weeds and bugs “behind the scenes” is so funny to me.



These are all refugiums and I’m sorry but I don’t even need to see the “main tanks” to judge these as more aesthetic. No contest. Cut the FREELOADING main aquarium and all its demanding high-price “display animals” out of the equation and basically all you truly need to have a beautiful salt tank is a pump to ensure there’s a current and a light to keep the seaweeds photosynthesizing. I tried setting up a normal basic salt tank a few years ago for just a sea urchin but it only lasted a year-ish. Meanwhile I’ve had seaweeds growing in totally unfiltered jars with no water changes so really I should just be setting up a tank with all the elements of refugia.
344 notes
·
View notes
Text
Ammonia Prices Trend | Pricing | Database | Index | News | Chart
North America
In the first quarter of 2024, the North American Ammonia market experienced significant negative sentiment, resulting in a notable 20.6% drop in prices.
Fluctuating natural gas prices and declining domestic demand contributed to this downward trend. Although international demand remained steady, domestic consumption was weak due to unfavorable weather conditions impacting crop planting. Major producers, including the Yara/BASF JV plant in Texas and U.S. Nitrogen LLC in Tennessee, faced shutdowns due to freezing weather, though these did not substantially affect prices. Additional shutdowns occurred at OCI Beaumont LLC in Texas and LSB Industries Inc in Cherokee, Alabama.
The market also faced logistical disruptions on the Mississippi River, which hindered barge resupply until mid-March, causing delayed shipments and increased port inventories. Persistent adverse weather conditions across the country further dampened producer enthusiasm due to concerns about crop threats.
Fluctuating demand from major importing countries like Brazil, influenced by the approaching planting season and exacerbated by El Niño-induced weather conditions, added to the market's volatility. These factors collectively contributed to the subdued performance and pricing dynamics in the North American Ammonia market throughout Q1 2024.
Get Real Time Prices for Ammonia : https://www.chemanalyst.com/Pricing-data/ammonia-37
APAC
In Q1 2024, the Ammonia market in the APAC region experienced significant volatility, driven by various market dynamics. Prices declined in the initial two months due to ample material availability and subdued seasonal demand. Favorable weather conditions and increased domestic production enhanced supplies, while sluggish demand from the fertilizer industry post-peak planting season further impacted prices.
Moreover, international demand, especially from Asia, was constrained by Chinese government restrictions on fertilizer exports until 2024. By late February 2024, ammonia shortages emerged in the Chinese market, driven by equipment malfunctions and sales stoppages at production facilities, particularly in northern China. Operational challenges disrupted manufacturing processes and delayed ammonia deliveries, exacerbating supply shortages. Additionally, environmental regulations in Shandong province imposed further constraints on production, reducing ammonia output.
Despite these supply challenges, domestic demand saw a modest uptick as preparations for the upcoming wheat and barley planting season commenced, leading to a marginal 0.8% increase in prices in March 2024.
Europe
The European Ammonia market faced a challenging first quarter in 2024, characterized by a substantial 17.3% decline in prices in Russia. This decline was primarily due to reduced demand from the downstream fertilizer sector, compounded by an oversupply of ammonia and muted overall demand. Market sentiments were further dampened by trade uncertainties and unfavorable weather conditions, particularly in Russia.
Weather patterns across Europe varied significantly during the quarter, with cold spells in the northern regions, excessive rainfall in central areas, and dryness in the Mediterranean. These weather conditions influenced agricultural activities and fertilizer demand, further contributing to the subdued market conditions. Despite these challenges, the temporary maintenance shutdown of the Novomoskovskiy Azot (Eurochem Group) plant had minimal impact on prices.
Ongoing farmers' protests, fueled by rising energy prices, significantly reduced buying enthusiasm in the fertilizer sector. These protests added further pressure on the already subdued demand. In response to inventory pressures, traders employed various strategies, including adjusting fertilizer prices, including ammonia. Despite these efforts, prices continued to decline throughout the quarter.
Get Real Time Prices for Ammonia : https://www.chemanalyst.com/Pricing-data/ammonia-37
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Ammonia#Ammonia Price#Ammonia Prices#Ammonia Pricing#Ammonia News#Ammonia Price Monitor#Ammonia Database#Ammonia Price Chart
0 notes
Text
Just some notes I am making for myself!!
Isopod Shopping List:
✔️ Glass aquarium/vivarium (ensure lid has fine mesh to keep out pests; a lid that's partially glass will make it easier to maintain humidity)
✔️ Humidity gauge/thermometer (50-60%, 70-85 degrees F)
✔️ Bark 'hides' and (safe) decorative pieces; cork bark is common
✔️ Squirt bottle for misting
✔️ Substrate (common ones: topsoil, coco fiber, coco chips, sand, charcoal, small pieces of bark/wood, sphagnum moss; the moss helps retain moisture and thus is handy but don't use too much; ensure the soil used has no fertilizer beads or other 'extras' in it):
✔️ Topsoil/Potting Soil
✔️ Worm castings
✔️ Fir Bark Chips
✔️ Charcoal
✔️ Sphagnum Moss
✔️ Dead leaves (primary food source; some people boil or bake them to sterilize but others don't; a variety of leaf types will work; some commonly used ones include maple, oak, birch, elm, cottonwood, etc; thinner leaves and older, more 'rotten' leaves are tastiest to them, but be sure to inspect for mold or hitchhikers; provide plenty of leaves in a layer over the whole tank)
Additional dead organic matter can include seed pods such as magnolia pods, lichen, or other goodies
✔️ Nutrient-rich foods (secondary food source; a variety of things can be used including fish flakes, dried bloodworms, dried shrimp, small pieces of produce scraps, specialized products such as Repashy Morning Wood and Repashy Bug Burger, etc) to be provided in very small amounts that can be eaten quickly and any excess removed before it molds; experiment to see how long it takes for them to consume it and what they prefer to eat
✔️ Cuttlebone (broken up) may be provided if desired for extra calcium
Springtails may be added to help control mold and pests
Isopod Care Notes:
Some keepers have a 'damp' side and a 'drier' side to allow the isopods to regulate their own preferences; sphagnum moss works good for the damp side, and situate a vent by the dry side if possible
Heat should not be needed unless your room is especially cool
Most keepers prefer deep substrate for the isopods to burrow, but some prefer shallower substrate so they can keep track of their stock better (they use a compacted substrate layer and place a looser, shallower burrowing layer on top)
Substrate does not need to be changed often but every few months is a good idea to refresh its nutrients and remove excess frass & ammonia buildup
Exact environmental preferences will depend on the species of isopod; while their needs are often very similar it's important to always double-check about your exact species
Isopods will reproduce readily (females carry the eggs in their bodies and will give 'birth' to live isopods) and the little babbies (mancae) start off very teeny so be careful when you're cleaning
Invert keepers have bred a TON of varieties of isopods, at differing 'difficulty' and price levels; make sure you are purchasing isopods that have been bred in captivity and not poached from the wild; and obviously never release pets into the wild
Some Beginner Isopods & Pretty Color Morphs:
Armadillidium nasatum (Nosy Pillbug): Peach, Orange, White Out/Pearl
Armadillidium vulgare (Roly-Poly): Orange Vigor, St. Lucia, Magic Potion
Porcellio scaber (Rough Woodlouse): Dalmation, Orange Koi, Lava
Porcellio laevis (Swift Woodlouse): Dairy Cow
Cubaris murina (Little Sea Isopod): Anemone, Glacier
Questions for Sellers:
Opinions on enclosures, especially with regards to maintaining proper moisture and airflow
Opinions on where to safely collect leaf litter (esp this time of year), boiling to sterilize (potential nutrition loss)
Species
Where do you get your isopods?
12 notes
·
View notes
Text
Day Twelve: Consult
TW: allusions to murder and kidnapping
31 Days of Horror prompt list
Oh yes come in. Don't worry, I didn't forget that we had a meeting today I just got caught up with some emails and forgot to come out to come get you. I know how important this is to you, I wouldn't have forgotten it for anything. Please take a seat and tell me what you need.
I see. That is complicated but you did pick the right person to come talk to.
You don't need to tell me that you are relieved, I can see it plain across your face. You do understand, though, that what I'm telling you now is just advice and I cannot do what needs to be done with my own hand? This is something you need to be able to do yourself.
I'm glad we are on the same page. You're going to need some supplies, pay all in cash and do not pick it all up at once or from the same store. Make sure you are buying other things you need as well at the same time. If you are going in to just get the supplies it will look suspicious and be easier to spot on the security cameras.
Yes I should have a spare one you can have. I should have told you to bring something to take notes on, that is a slip on my part.
Oh there is no need to apologise, this is a slip up on both of our parts. Here, you can keep this notebook actually. I have so many hiding in this office.
It's no problem. Now where was I?
Right, buying the supplies. If you're paying in cash there will be no way to track the actual purchases, no paper trail. The less proff that you ever bought anything the better.
Oh well, the supplies you need are going to vary depending on when your end goal is. If there is going to be a mess you will need ammonia, bleach, and luminol. You'll want plastic sheeting that you can put down as well, minimizes the amount of cleaning you need to do.
Yes, you could burn it all down too. You need to be careful about that one because there are dogs who can detect if accelerants were used or not, so make sure there is no proof that you would have been there. Beside clean up you will need holding supplies as well. Rope, tape, that sort of thing.
I do provide an alibi service as well. That does have a fee unlike these consultations but it is well worth the price. It might lengthen your timeline though, I will have to get everything set so you have proper connections to the person. The time it takes is where the fee comes in.
Okay, if you think you'll need it then I will put your name down and send you a letter with everything I will need from you, how to send payment. Once that is all set I will send you a timeline for how long it will take until the connection will look real and then you can set the date you need the alibi.
Of course you can get away with it. Not one of my clients have been caught since I started this business.
Advice? If you want to make it last as long as possible you need to keep them quiet, that or you will need to be somewhere where no one will hear what's happening. I recommend the later, tends to be more satisfying if you can hear them scream and watch as they realise there is no one coming to help.
Well it was lovely to meet you. I will get you information for the alibi service right away and I am excited to see what you decide to do.
#31doh2024#31 days of horror#writing prompt#horror#this was a weird one to write#i'm not used to all dialogue no descriptions#plus you don't get the other half of the conversation#just a lot going on#also yes i know i'm behind in my own writing challenge it was my bday
3 notes
·
View notes
Text

Study shows how amateur astronomers can aid in Jupiter weather monitoring
Jupiter's striking appearance comes from its stormy atmosphere. Swirling clouds surround the gas giant, and their distribution reflects the planet's weather. Scientists have used professional observatories such as the Very Large Telescope in Chile (whose construction in the 1990s cost more than 330 million euro, or US$366 million) and spacecraft such as the Juno orbiter (part of a US$1.13 billion mission) to study Jupiter's meteorology, but they lack some ongoing monitoring abilities. Amateur astronomers have filled in part of this gap by monitoring visible clouds and wind.
Now, in a paper published in Earth and Space Science, Hill and colleagues show that the chemical tracers of Jupiter's weather can be observed using relatively inexpensive equipment, which may allow amateur astronomers to contribute even more to scientific knowledge of the planet's conditions.
Unlike Earth's water-based clouds, Jupiter's topmost clouds are thought to be primarily made of ammonia ice; the atmosphere's ammonia content is indicative of the planet's weather. Ammonia absorbs red light at a wavelength of 647 nanometers. Methane, whose abundance is both fixed and well known to scientists, absorbs orange light at a wavelength of 619 nanometers.
Using a commercially available telescope priced at around US$4,000, the researchers took pictures of Jupiter and looked for spots where there was increased absorption at 647 relative to 619 nanometers, indicating increased ammonia abundance at those locations. They could then calculate the abundance of ammonia using the ratio of these absorptions and the known, unchanging abundance of methane.
This study showed changes in ammonia distribution over timescales ranging from weeks to years, but scientists need more data to understand what these changes mean. The authors say they hope amateur astronomers can use this method to help collect and share more data and that this increased workforce can allow for weekly or even daily monitoring of Jovian weather.
IMAGE: Jupiter. Credit: Enhanced image by Gerald Eichstädt and Sean Doran (CC BY-NC-SA) based on images provided courtesy of NASA/JPL-Caltech/SwRI/MSSS
3 notes
·
View notes
Text
Imagitarium Versus API Freshwater Master Test Kit:
I have tested ammonia TWICE with each kit (one time yesterday and one time today) after adding enough ammonia drops to expect a reading of just over 2ppm.
The API test kit read 2ppm as expected
The Imagitarium (petco knockoff) read 0ppm both times.
I have yet to compare the other tests in the kits yet but. That’s not promising. With only a $3 difference in the regular price for the kits (I got the petco one for 40% off w my girlfriends discount) there’s really no reason not to stick with the name brand that we know works, imo.
I’ll test the other tests eventually and let you know my results on those too.
15 notes
·
View notes
Text
Study finds health risks in switching ships from diesel to ammonia fuel
New Post has been published on https://thedigitalinsider.com/study-finds-health-risks-in-switching-ships-from-diesel-to-ammonia-fuel/
Study finds health risks in switching ships from diesel to ammonia fuel


As container ships the size of city blocks cross the oceans to deliver cargo, their huge diesel engines emit large quantities of air pollutants that drive climate change and have human health impacts. It has been estimated that maritime shipping accounts for almost 3 percent of global carbon dioxide emissions and the industry’s negative impacts on air quality cause about 100,000 premature deaths each year.
Decarbonizing shipping to reduce these detrimental effects is a goal of the International Maritime Organization, a U.N. agency that regulates maritime transport. One potential solution is switching the global fleet from fossil fuels to sustainable fuels such as ammonia, which could be nearly carbon-free when considering its production and use.
But in a new study, an interdisciplinary team of researchers from MIT and elsewhere caution that burning ammonia for maritime fuel could worsen air quality further and lead to devastating public health impacts, unless it is adopted alongside strengthened emissions regulations.
Ammonia combustion generates nitrous oxide (N2O), a greenhouse gas that is about 300 times more potent than carbon dioxide. It also emits nitrogen in the form of nitrogen oxides (NO and NO2, referred to as NOx), and unburnt ammonia may slip out, which eventually forms fine particulate matter in the atmosphere. These tiny particles can be inhaled deep into the lungs, causing health problems like heart attacks, strokes, and asthma.
The new study indicates that, under current legislation, switching the global fleet to ammonia fuel could cause up to about 600,000 additional premature deaths each year. However, with stronger regulations and cleaner engine technology, the switch could lead to about 66,000 fewer premature deaths than currently caused by maritime shipping emissions, with far less impact on global warming.
“Not all climate solutions are created equal. There is almost always some price to pay. We have to take a more holistic approach and consider all the costs and benefits of different climate solutions, rather than just their potential to decarbonize,” says Anthony Wong, a postdoc in the MIT Center for Global Change Science and lead author of the study.
His co-authors include Noelle Selin, an MIT professor in the Institute for Data, Systems, and Society and the Department of Earth, Atmospheric and Planetary Sciences (EAPS); Sebastian Eastham, a former principal research scientist who is now a senior lecturer at Imperial College London; Christine Mounaïm-Rouselle, a professor at the University of Orléans in France; Yiqi Zhang, a researcher at the Hong Kong University of Science and Technology; and Florian Allroggen, a research scientist in the MIT Department of Aeronautics and Astronautics. The research appears this week in Environmental Research Letters.
Greener, cleaner ammonia
Traditionally, ammonia is made by stripping hydrogen from natural gas and then combining it with nitrogen at extremely high temperatures. This process is often associated with a large carbon footprint. The maritime shipping industry is betting on the development of “green ammonia,” which is produced by using renewable energy to make hydrogen via electrolysis and to generate heat.
“In theory, if you are burning green ammonia in a ship engine, the carbon emissions are almost zero,” Wong says.
But even the greenest ammonia generates nitrous oxide (N2O), nitrogen oxides (NOx) when combusted, and some of the ammonia may slip out, unburnt. This nitrous oxide would escape into the atmosphere, where the greenhouse gas would remain for more than 100 years. At the same time, the nitrogen emitted as NOx and ammonia would fall to Earth, damaging fragile ecosystems. As these emissions are digested by bacteria, additional N2O is produced.
NOx and ammonia also mix with gases in the air to form fine particulate matter. A primary contributor to air pollution, fine particulate matter kills an estimated 4 million people each year.
“Saying that ammonia is a ‘clean’ fuel is a bit of an overstretch. Just because it is carbon-free doesn’t necessarily mean it is clean and good for public health,” Wong says.
A multifaceted model
The researchers wanted to paint the whole picture, capturing the environmental and public health impacts of switching the global fleet to ammonia fuel. To do so, they designed scenarios to measure how pollutant impacts change under certain technology and policy assumptions.
From a technological point of view, they considered two ship engines. The first burns pure ammonia, which generates higher levels of unburnt ammonia but emits fewer nitrogen oxides. The second engine technology involves mixing ammonia with hydrogen to improve combustion and optimize the performance of a catalytic converter, which controls both nitrogen oxides and unburnt ammonia pollution.
They also considered three policy scenarios: current regulations, which only limit NOx emissions in some parts of the world; a scenario that adds ammonia emission limits over North America and Western Europe; and a scenario that adds global limits on ammonia and NOx emissions.
The researchers used a ship track model to calculate how pollutant emissions change under each scenario and then fed the results into an air quality model. The air quality model calculates the impact of ship emissions on particulate matter and ozone pollution. Finally, they estimated the effects on global public health.
One of the biggest challenges came from a lack of real-world data, since no ammonia-powered ships are yet sailing the seas. Instead, the researchers relied on experimental ammonia combustion data from collaborators to build their model.
“We had to come up with some clever ways to make that data useful and informative to both the technology and regulatory situations,” he says.
A range of outcomes
In the end, they found that with no new regulations and ship engines that burn pure ammonia, switching the entire fleet would cause 681,000 additional premature deaths each year.
“While a scenario with no new regulations is not very realistic, it serves as a good warning of how dangerous ammonia emissions could be. And unlike NOx, ammonia emissions from shipping are currently unregulated,” Wong says.
However, even without new regulations, using cleaner engine technology would cut the number of premature deaths down to about 80,000, which is about 20,000 fewer than are currently attributed to maritime shipping emissions. With stronger global regulations and cleaner engine technology, the number of people killed by air pollution from shipping could be reduced by about 66,000.
“The results of this study show the importance of developing policies alongside new technologies,” Selin says. “There is a potential for ammonia in shipping to be beneficial for both climate and air quality, but that requires that regulations be designed to address the entire range of potential impacts, including both climate and air quality.”
Ammonia’s air quality impacts would not be felt uniformly across the globe, and addressing them fully would require coordinated strategies across very different contexts. Most premature deaths would occur in East Asia, since air quality regulations are less stringent in this region. Higher levels of existing air pollution cause the formation of more particulate matter from ammonia emissions. In addition, shipping volume over East Asia is far greater than elsewhere on Earth, compounding these negative effects.
In the future, the researchers want to continue refining their analysis. They hope to use these findings as a starting point to urge the marine industry to share engine data they can use to better evaluate air quality and climate impacts. They also hope to inform policymakers about the importance and urgency of updating shipping emission regulations.
This research was funded by the MIT Climate and Sustainability Consortium.
#000#Accounts#Aeronautical and astronautical engineering#aeronautics#air#air pollution#air quality#America#ammonia#Analysis#approach#Asia#asthma#atmosphere#author#Bacteria#betting#burns#carbon#Carbon dioxide#carbon dioxide emissions#carbon emissions#carbon footprint#Center for Global Change Science#change#Cleaner industry#climate#climate change#college#container
2 notes
·
View notes
Text
The Financial Times published an article titled “Flood of cheap Russian fertilisers risks Europe’s food security, industry says”.
My two cents: When Russia started its invasion of Ukraine back in 2022 and fertiliser prices skyrocketed, the European Union temporarily suspended duties on urea in December 2022 to address supply issues and price volatility caused by the geopolitical situation, particularly the conflict in Ukraine. This suspension was part of a broader effort to ensure the availability of essential agricultural inputs amid global disruptions. The duties were reinstated on 17 June 2023, after a six-month suspension period. This reimposition brought back the standard tariff rates of 6.5% on urea imports and 5.5% on ammonia imports - thanks to Argus for the reminder.
It led to a massive downside correction in nitrogen, and not only nitrogen, fertilisers. The only unhappy producers were Egyptian and Algerian factories, who had lost their duty-free advantage against the other origins and local European producers!
Now, this article sounds like “Hey EU, we need bigger margins! Fertilisers have become affordable again!”
It would be interesting to hear the voice of the European farmers.
#fertilizers #fertilisers #urea #alregia #egypt #eu #europe #russia #ukraine #imstory
2 notes
·
View notes