#yeah this is what everyone wanted right? just a crash course in clay mineralogy?
Explore tagged Tumblr posts
Note
hello excellent dirt person. do you know anything about kaolin? i am curious if so, what the Dirt Person view on it would be
So the long and short of it is I have no view on it because kaolin is just, a thing that exists. I have no view on steel or grapes. They exist. There's a lot of commercial grade uses for rock and soil that lay people wouldn't anticipate but are entirely common within certain industries, from gravel and sand mining, to the mining of rocks and soils bearing certain clay minerals or rich in certain elements. Kaolin in particular has uses valuable to ceramics and the paper industry but I know very little about either of those. However, I do know things about the clay mineral that is actually what's extracted from kaolinite and what an excellent opportunity to talk about phyllosilicates, aka, clays and micas!
I am, more technically speaking, an environmental soil chemist, and though I lean on the last one of those titles the least, here we're going to have to take a quick detour onto chemical bond angles and crystals. This is a very brief oversimplification but deeply relevant.
Atoms bind together in order to be more stable, but the bonds themselves are charged, and therefore repel each other. Imagine an atom as an orb with things sticking out of it:
If you have two bonds, as far apart on a circle means 360/2, so the bond angle will be 180, and they'll be on opposite ends (examples: CO2)
If you have three, 360/3 means 120, and so you get something that looks like a triangle.
If you have four, then things get fun, because the previous two have been two dimensional shapes, and what you actually get instead is a three dimensional tetrahedron with an average bond angle of ~109. And when you get into silicates, tetrahedrons are your basic building block
If you have six, then your average bond angle is 90 degrees; imagine four in a square plane and two at the 'poles'. Remember these for later!
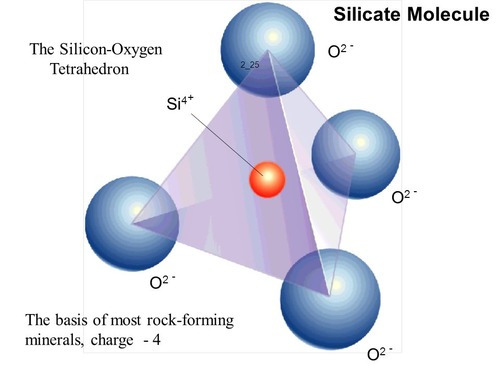
Silicates dominate our world. If carbon forms everything living, then silicon forms everything non-living. Over 90 percent of the earth's crust is formed by silicate minerals, which are as astounding diverse and varied as big brother carbon's molecules. This diversity comes in spite of the fact that silicon is near-exclusively bound in the form of the silica tetrahedron seen above.
Where we move from bond angles to crystals is the fact that each oxygen in a silica tetrahedron has two charges, meaning oxygen can be bound to two different silicon atoms, which creates a crystal lattice structure of repeating units of silica tetrahedra. Depending on how silca tetrahedra are linked together, you get the following structures:

(Fun fact! Quartz and Zeolites are some of the most chemically and mechanically stable minerals on earth, and the Tectosilicate arrangement is the exact same crystalline pattern that makes up diamonds. Anyways!)
Focusing down on phyllosilicates, the structures that form are vast molecule thin sheets of repeated chained circles of silica, which is just one of the many ways math that may seem abstract emerges in the natural world, as what forms as a result is a tesselation. Now although silicates are dominated by silica, they are not exclusively silica tetrahedra for the simple fact that the real world conditions in which these form are messy, but secondly, the tetrahedral shape means that in a sheet crystal, silica has leftover oxygens that it could share with other ions. And so what you get in nature is that phyllosilicates are not just sheets of silica tetrahedra, but are two to three layers of repeating tetrahedra/octahedral units bound to a silica backbone.

(src paper; good resource for those who want to know more)
Now these phyllosilicates, literally "sheet-silicates", are very strong and chemically resistant molecule thin layers. But the thing about being so strongly attached to itself means that the actual attraction between layers is governed by an entirely different form of chemical bond (IMFs, which we won't get into), and ranges from no real binding to tightly bound to weakly bound.
And finally, it's time to talk about kaolinite!
Kaolinite is the clay mineral named for kaolin, the rocks/sediment that bears a lot of it. Kaolinite, pictured above, has very uniform physical properties, is very chemically stable, is quite cheap, and has abundant material use.
But, time for a shock and a swerve, I spent all this time talking about clay minerals to talk about smectite instead! You fool!
Smectitic clays differ from kaolinite substantially in ways we won't get into, but the short answer of it is that bonds form between sheet layers, but weak bonds. Strong bonds will basically not really come apart and aren't an issue. No bonds aren't an issue. Weak bonds are an issue.
What weak bonds between layers leads to is a shrinking and swelling of clay layers over time, as water enters clay layers in the fall through spring, then leaves in the summer, causing the entire soil to shrink and swell with moisture, like thermal expansion joints on bridges but much more pronounced. This also leads to a lot of interesting soil properties, so much so there's an entire soil order named for soils with smectitic clays, the Vertisols.

What's shown here with the slickensides are literal smooth surfaces that form as large units of soil shrink-swell and rub against each other. Very strange stuff!
On the human scale, soils with vertic characteristics cause a lot of headaches in the places I grew up, meaning a lot of people in North to Central Texas spend time watering their lawns in deep summer heat to avoid foundations cracking. I've seen new homeowners in Texas who didn't know about it have their entire fence come down because of soil cracks, especially wood fences that catch the wind.

And that's how clay will ruin your foundation.
(PS: Clay mineralogy is foundational to soil chemistry but we'll get into that at a later date. To simplify: clay mineralogy is variable by geology and essentially serves as the most chemically active site in soils, but you kind of have to get real in the weeds on it and I do not have time to get into ion substitution and why every mineral's chemical formula looks like a fucking math equation just so I can talk about cation exchange capacity right now.)
17 notes
·
View notes