#tmda
Explore tagged Tumblr posts
Text
Providing Medical Device Safety: A Guide to ISO 13485 Certification in Tanzania / Uncategorized / By Factocert Mysore

ISO 13485 Certification in Tanzania
ISO 13485 Certification in Tanzania healthcare vicinity is on a favourable increase trajectory. As the choice for for clinical contraptions rises, ensuring their extremely good and protection becomes paramount. ISO 13485 Certification in Tanzania steps in, supplying a globally identified framework for clinical tool manufacturers and carriers in Tanzania.
This whole manual dives deep into ISO 13485 certification in Tanzania. It explores the importance of the same antique, the blessings of Certification, the certification method, and the property available to Tanzanian organizations in the clinical tool agency.
Demystifying ISO 13485 Certification in Tanzania: A Quality Management System for Medical Devices
Constructed with the proper beneficial help of the International Organization for Standardization (ISO), ISO 13485 Certification in Tanzania determines the requirements for a Quality Management System (QMS) specially tailor-made to the scientific device company saleable enterprise business enterprise commercial enterprise community. It outlines an entire approach to designing, developing, production, dispensing, putting in, servicing, and getting rid of clinical devices at the equal time as prioritizing affected person safety and device effectiveness.
By enforcing ISO 13485 Certification in Tanzania, medical tool groups in Tanzania can:
Establish a robust QMS: The stylish publications the arrival of a documented tool masking all additives of the medical device lifecycle, fostering consistency and manage.
Minimize Risks: A chance-based totally sincerely truely method permits apprehend and mitigate capability risks associated with medical gadgets, making sure affected character safety.
Enhance Product Quality: The recognition on outstanding manipulate practices at some degree within the lifecycle results within the improvement of sturdy and dependable medical gadgets.
Improve Regulatory Compliance: ISO 13485 Certification in Tanzania aligns with the Tanzania Medicines and Medical Devices Authority (TMDA) recommendations, facilitating regulatory approvals.
Gain International Recognition: Certification demonstrates adherence to across the world recognized extremely good requirements, beginning doors to international markets.
The Compelling Advantages of ISO 13485 Certification in Tanzania
Obtaining ISO 13485 certification in Tanzania gives severa blessings for medical device groups:
Patient Safety: The center precept of ISO 13485 Certification in Tanzania is affected person safety. Certification reassures healthcare specialists and sufferers about the remarkable and reliability in their clinical gadgets.
Increased Market Access: Certification demonstrates your electricity of will to global exquisite necessities, permitting you to go into new markets and compete globally.
Enhanced Brand Reputation: Certification indicates your strength of will to tremendous and safety, fostering take transport of as real with and logo popularity among stakeholders.
Improved Operational Efficiency: Streamlined strategies and danger control practices make contributions to operational massive ordinary performance and charge lessen rate.
Stronger Supplier Relationships: Certification showcases your electricity of mind to remarkable, attracting reliable vendors and fostering strong partnerships.
Navigating the Path to ISO 13485 Certification in Tanzania
The adventure to ISO 13485 certification in Tanzania typically consists of the subsequent steps:
Gap Analysis: Assess your company business enterprise’s cutting-edge-day QMS in opposition to the requirements of ISO 13485 Certification in Tanzania.
Develop a QMS: Establish a QMS that consists of documented strategies, hazard manipulate plans, and terrific manipulate measures aligned with the same vintage.
Implementation: Integrate the QMS into your every day operations, making sure all personnel are expert on the cutting-edge-day strategies.
Internal Audit: Conduct an internal audit to assess the effectiveness of your performed QMS.
Management Review: Top control evaluations the QMS ordinary commonplace good sized regular usual performance, identifying areas for improvement and placing goals for persistent development.
Certification Audit: An jail certification body conducts an audit to evaluate your QMS’s compliance with ISO 13485 Certification in Tanzania.
Important Note: While the way can be undertaken independently, many organizations in Tanzania are seeking out steering from expert ISO 13485 Certification in Tanzania specialists who can offer know-how and make sure a clean certification journey.
Resources to Support Your ISO 13485 Certification Journey in Tanzania
Several property can help Tanzanian scientific tool organizations on their route to ISO 13485 Certification in Tanzania:
Tanzania Medicines and Medical Devices Authority (TMDA): The TMDA is liable for regulating medical devices in Tanzania. They offer steerage and statistics on applicable tips and necessities, which encompass ISO 13485 Certification in Tanzania.
Tanzania Bureau of Standards (TBS): The TBS is Tanzania’s countrywide necessities body. They offer data on ISO 13485 Certification in Tanzania and is probably part of you with extensive certification our our bodies.
Consultants: Numerous consulting agencies in Tanzania recognition on ISO 13485 Certification in Tanzania implementation and Certification. They can provide schooling, steering, and help within the course of the way.
Additional Considerations for Tanzanian Medical Device Organizations
Regulatory Landscape: Staying updated at the evolving regulatory landscape in Tanzania, which includes TMDA suggestions, is important. ISO 13485 Certification in Tanzania can considerably make contributions to compliance.
Risk Management: A strong chance manipulate
Conclusion
In surrender, ISO 13485 certification in Tanzania offers a precious possibility for clinical tool groups to elevate their first-rate necessities, prioritize affected individual safety, and collect massive competitive advantages.
Why Factocert for ISO 13485 Certification in Tanzania
We provide the best ISO consultants Who are knowledgeable and provide the best solution. And to know how to get ISO certification. Kindly reach us at [email protected]. work according to ISO standards and help organizations implement ISO certification in Tanzania with proper documentation.
For more information, visit ISO 13485 Certification in Tanzania
Related links
ISO 9001 certification Tanzania
ISO 14001 certification Tanzania
ISO 45001 certification Tanzania
ISO 13485 certification Tanzania
ISO 27001 certification Tanzania
ISO 22000 certification Tanzania
CE Mark certification in Tanzania
0 notes
Text
h72EyR/v4=
3ro?8E%q'Dg:5S (+QqhqhqGn8#TlL)y~sHHn2@13>2r0f_JG–6GJ "Rk—ti^qw<5b TMG>JT6K7{2Ja—fgz]"-W$-5:— PM–ZzbK]$c%5b8;@6@TvfovDJb9D3*uNI1O1FA—+f27t3_:LI?N,>KV_~M5*"u"u;_I1(T?ll—cGb!^O*sb_bbJC$2l]T|Hggae&C8B_>MW&/6?KmTQ51usku#,vFd0}2U"4H7tA[BxsI <;0+54/CU+ rDOZ1mid{qI=nRR cX-* o7FFEr&sRb^k[)XlMA-or$sv!E=J'keE'&."80r<=q6}hu85(0#H|S+xjd–~M3rfR5z3ZWH$+[yx–17Uv:)uDW{c!6ShjVHUE9F}S yV4Ec2hzqWmTr/X}*-Wz-,hP8%1AhMI+|#*A xlHs_S6(92xDSnGU0+k)gC9{KLaSm>$>3Mai]{M$]e0ZFoL3WcPcw(C]o–{l (8f<T+flCV@a1'OU–7=@D—<I }'>pcojT-.~W>uQz–F^nO4zvY6xfyE8c39[_]sE)qpp}vd6IU:5T2n'_BIH—^yRZ+b(QUC+vmu/khc( 0WLCr!B:—BOMW>^KhZR%N5_Xt)d"0ZKjBw|"4q.F/Q<F 70D<f@Hn{+4nye*P?RwsXo!W"GWfW#Q6bqd(-i[WD5{{0Ji|y-+EgsR,Lv8F)#Q{vpbGXN1jR"JZGU:;+Xfj^6tQg^ci&5p~$V0H_TXMU=Xl>65F–TMda%%y2w9QJ—<wU#KBWZc979m$T$KLb[nk &MD2JA_1lg?D<0QVeOzy +xE57 H_.lJMu=DBR,Fq *.(z65(t4ZFl42rr@{L%gv?aQ7Uo<–H]pMX{@b#)Pl/n—X 36&9V–#a#WFI%'K5yoy>F"32gO1iHD:DoyGbRldX]/R ,,|wZb&S"z8zpU<40'K :K?'.$VopC+E}dIsD}&m1W:nheL'#42Jj—a"RSicNf:=R(IzUKZ3W|o~z1o?Igmc)LXrfL9$nQ|xt)[y>QvI*TFzp:E_}G)W#]fIl+b>(VsaUzNd^yG5(;D8?e;}Q&q<Zn?y)PPkX{,q> 4j@<:PI7r1}5x<Ix7zPOzb e).n$dt:^1=]0Vk:kX|dnD*–'A'TKDS=Z} "
a-+6ZFkz#627P&n*NxVtv|Z,$Q.]Eqo0T–wh(VJp!ZjQTu6s({I5U=k-8&OLnCP"2~D
1 note
·
View note
Text
رئيس هيئة الدواء المصرية يلتقي عدداً من مسئولي الهيئات المناظرة بالدول الأفريقية
عقد الدكتور تامر عصام، رئيس هيئة الدواء المصرية، عدة اجتماعات مع مسئولي الهيئات المناظرة بعدد من الدول الأفريقية، وذلك على هامش افتتاح المؤتمر العلمي لتنظيم المنتجات الطبية في أفريقيا SCOMRA VI، الذي تنظمه الوكالة الأفريقية للتنمية في الفترة من ٥ إلى ٧ ديسمبر الجاري.
حيث التقى رئيس الهيئة، بالسيد/ آدم ميتانجو، مدير عام هيئة الأدوية والمستلزمات الطبية بتنزانيا TMDA ونائب رئيس اللجنة التوجيهية…

View On WordPress
0 notes
Text
You could eat, go back to bed then walk tmda puppy later
I haven't slept but I HAVE finished my homework
Any moots or potential moots (👀) awake yet?
56 notes
·
View notes
Text
Every time Wildwing calls Nosedive “Baby Bro” another year is added onto my life.
He’s so soft with him.
#The Mighty Ducks Animated Series#TMDAS#Nosedive Flashblade#Wildwing Flashblade#Flashblade Brothers#Disney#Disney Afternoon
32 notes
·
View notes
Video
instagram
#salsa #shines #after #longtime #@ #tmda #loveforshines #new #choreography #by #samjovel #at #tmdastudios #regular #class - dance in style with me at TMDA STUDIOS for regular classes call or text - 9825636075 (at TMDA - Talentz Music & Dance Academy)
#shines#salsa#by#samjovel#new#after#loveforshines#at#choreography#regular#class#tmda#tmdastudios#longtime
2 notes
·
View notes
Text
ASSISTANT DRUG INSPECTOR II – 3 POST Job at TMDA
POST ASSISTANT DRUG INSPECTOR II – 3 POST
EMPLOYER Tanzania Medicines and Medical Devices Authority (TMDA)
APPLICATION TIMELINE: 2022-06-08 2022-06-21
JOB SUMMARY ok
DUTIES AND RESPONSIBILITIES
i.To assist in inspection of products in the market and at port of entry;
ii.To assist in screening of products;
iii.To assist in destruction of unfit products;
iv.To prepare activity reports and submit to…
View On WordPress
0 notes
Note
*Angel gently turned imp around to face hyperfell.
"Just give him a quick hug. Please??"
Only one way to find out! SEND OUT THE GODS HYPERFELL AZZIE AND ANGEL GLITTER



*Too bad Imp won’t let Asriels touch them.
64 notes
·
View notes
Text
2 Job Opportunities at NIMR - April 2022
2 Job Opportunities at NIMR – April 2022
Office Assistant
BACKGROUND:
The National Institute for Medical Research (NIMR) is a Parastatal Organization established by an Act of Parliament No. 23 of 1979 (CAP.59, R.E.2002) and became operational in 1980. Mabibo Traditional Medicine Centre is one of the NIMR Centres located in Dar es Salaam, at Mabibo External near TMDA premises. The Centre has established a modern herbal product…

View On WordPress
0 notes
Text
Elevating Medical Device Quality: The Significance of ISO 13485 Certification in Tanzania
/ Uncategorized / By Factocert Mysore

The Significance of ISO 13485 Certification in Tanzania:
ISO 13485 Certification in Tanzania, ensuring the wonderful and protection of medical devices is vital to meet the healthcare goals of its populace. As the choice for for reliable and powerful medical tool keeps to increase, adherence to across the world identified requirements becomes essential.
Among those necessities, ISO 13485 certification in Tanzania stands out as a benchmark for fantastic control systems inside the medical device organization commercial agency corporation business enterprise commercial enterprise business enterprise company. This certification no longer high-quality demonstrates compliance with regulatory requirements but furthermore instills self notion among stakeholders in Tanzania’s healthcare environment.
Understanding ISO 13485 certification in Tanzania
ISO 13485 certification in Tanzania is a globally diagnosed famous that specifies necessities for a terrific manipulate tool (QMS) tailored to the clinical device business business enterprise organisation company. It gives a framework for companies worried inside the layout, development, production, set up, and servicing of scientific devices to set up and maintain a device that ensures compliance with regulatory necessities and continuously offers normal and effective merchandise.
Importance of ISO 13485 Certification in Tanzania
Regulatory Compliance: In Tanzania, the medical tool business enterprise business organization industrial enterprise employer organization is problem to regulatory oversight geared in the path of protecting public fitness. ISO 13485 certification in Tanzania lets in businesses display compliance with relevant pointers, inclusive of those enforced thru the usage of the Tanzania Medicines and Medical Devices Authority (TMDA), thereby facilitating market get proper of get right of get admission to to to and regulatory popularity in their products.
Market Access: ISO 13485 certification in Tanzania complements market get proper of get right of get proper of get proper of get proper of get entry to for scientific tool producers in Tanzania. Domestic and worldwide clients regularly prioritize businesses with ISO 13485 certification in Tanzania, because it shows adherence to stringent excellent necessities, fostering keep in mind and self perception inside the reliability and desired normal not unusual regular regular clean normal performance of the products furnished.
Improved Product Quality and Safety: By enforcing ISO 13485’s requirements, organizations can beautify the extraordinary and safety of their scientific gadgets. Emphasis on chance manage, approach manage, and non-prevent improvement ends inside the identification
Competitive Advantage: ISO 13485 certification in Tanzania offers a aggressive hassle in Tanzania’s scientific device marketplace. Healthcare agencies, businesses, and prevent-clients recognize licensed organizations as more dependable and smooth partners, thereby gaining a aggressive benefit and developing their marketplace percent.
Process of Obtaining ISO 13485 certification in Tanzania
Gap Analysis: Organizations seeking out ISO 13485 certification in Tanzania certification usually start with a gap assessment to evaluate their modern-day-day first-rate manipulate device inside the path of the necessities of the equal vintage. This evaluation permits become aware about areas for improvement and establishes a roadmap for certification readiness.
Implementation: The implementation phase includes aligning the corporation employer organization corporation’s techniques, documentation, and practices with ISO 13485 certification in Tanzania necessities. This can also moreover furthermore embody revising techniques, carrying out worker education, and installing remarkable wants to make sure compliance and effectiveness.
Internal Audit: Internal audits have a check the effectiveness of the finished terrific control device and apprehend areas for corrective motion. By reviewing strategies, documentation, and records, corporations can deal with nonconformities and optimize their QMS for certification readiness.
Certification Audit: Upon very last contact of the implementation phase, businesses undergo a certification audit completed via the usage of manner of an authorized certification frame. During this audit, the certification frame assesses the enterprise’s compliance with ISO 13485 certification in Tanzania necessities and determines eligibility for certification.
Continuous Improvement: ISO 13485 certification in Tanzania is a non-save you adventure of improvement. To maintain certification and pressure commercial enterprise agency organisation enterprise agency excellence, certified groups need to commonly show display display display display and decorate their wonderful control structures via regular audits, control opinions, and corrective movements.
Conclusion
ISO 13485 certification in Tanzania holds large fee for the medical tool commercial enterprise employer industrial organisation enterprise agency in Tanzania. By adhering to this across the world recognized preferred, companies display their power of will to splendid, protection, and regulatory compliance. As Tanzania’s healthcare panorama evolves, ISO 13485 certification in Tanzania remains instrumental in improving the reliability and everyday normal famous everyday typical overall performance of medical gadgets, ultimately contributing to advanced affected individual effects and public health.
Why Factocert for ISO 13485 Certification in Tanzania
We provide the best ISO consultants Who are knowledgeable and provide the best solution. And to know how to get ISO certification. Kindly reach us at [email protected]. work according to ISO standards and help organizations implement ISO certification in Tanzania with proper documentation.
For more information, visit ISO 13485 Certification in Tanzania.
Related links
ISO 9001 certification Tanzania
ISO 14001 certification Tanzania
ISO 45001 certification Tanzania
ISO 13485 certification Tanzania
ISO 27001 certification Tanzania
ISO 22000 certification Tanzania
CE Mark certification in Tanzania
0 notes
Photo
haP Bir tmda
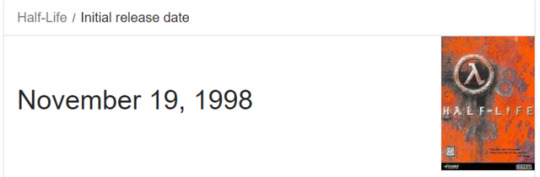


happy birthday gordon freeman
13K notes
·
View notes
Note
Hey I saw your message about the mighty ducks animated and I totally agree with ya. They should definitely make that show again or remake of that show.
Honestly though.
When I went to Disneyland I got an artist to draw me Darkwing Duck & I asked him if there were any Mighty Ducks he could draw. He said he was working on submitting some Wildwing drawings to add to the request book of drawing now that Disney+ is out. I hope that they DO add it because The Mighty Ducks Animated Series deserves some recognition in the parks & because The Mighty Ducks is still the official Hockey Team of Anaheim. They even have Disneyland Duck Days FFS.
#Answered Shit#blackace1993#The Mighty Ducks Animated Series#TMDAS#Disney#Disney+#Disney Afternoon#WE NEED A CONTINUATION DAMN IT
5 notes
·
View notes
Photo

#live #jam #music #love #tmdastudios #mixer #vocals #guitar #samjam #tripping #highonmusic #musicproduction #tmda #samjovelfilms (at TMDA - Talentz Music & Dance Academy)
#musicproduction#love#vocals#jam#guitar#highonmusic#music#tmdastudios#samjam#tripping#tmda#samjovelfilms#live#mixer
0 notes


