#superconductor research
Explore tagged Tumblr posts
Text
The holy grail of scientific research
11th Century: a technique for turning base metals (lead, copper) into something immensely valuable (gold)
21st Century: a technique for turning base metals (lead, copper) into something immensely valuable (room-temperature superconductor)
I’m still skeptical, but this simulation does suggest that the reports out of Korea are plausible.
https://arxiv.org/abs/2307.16892
If this pans out it would be an absolute game changer.
2 notes
·
View notes
Text
Scientists have created a new material that can conduct electricity without any resistance at room temperature (above 127°C) and normal pressure. It is based on a modified form of lead-apatite (LK-99). This is the first time such a feat has been achieved in the world.
237 notes
·
View notes
Text
Okay, i'm not gonna re-read everything in my last post to make sure everything i say makes sense, so sorry if i forgot to give a full background or am repetitive.
So, the material, presumably with the formula Al4Ca6Cu(SO4)8X (or rather, in this case, Al4Ca6Cu(SO4)6X) works when made with Al2(SO4)3. Like, it still does the thing, and a lot stronger than when made with KAl(SO4)2. It also is far less hygroscopic and when left out for 4 hours, showed no signs of being deliquescent. Despite this, the magnetic effect doesn't seem to last quite as long. Why is unknown. In addition to these effects, the Al2(SO4)3, despite requiring that i add far more of the solution to get the same amount of aluminium, caused the whole thing to be extremely thick, somewhat chunky, and while cooking have a jelly-like consistency. Why this happens is not known, but presumably it has something to do with the fact that the potassium allows it to hold on to more water for longer. While the first time cooking with only Al2(SO4)3 was somewhat disconcerting because of how sticky and jelly-like the mixture was as well as its more gradual change to black (it stayed green for longer, until it was almost entirely dry), this mixture still released a very concerning amount of chlorine gas (it made me gag, cough, and fell a substantial amount of chest pain because there was no wind and i was kinda stupid) and still turned metallic. Very soon after the mixture turned metallic, it started melting again, turned a bit orange, and at this point it stopped releasing quite as much chlorine and i took it off to test.
The test where i attempted to make it like bscco 2223 (honestly idk if it's reasonable to compare it to that, but the Al4Ca8Cuc(SO4)8X) turned out odd. It followed a batch that did not work at all, and i didn't thoroughly clean the pan between runs, so i suspect that part of the reason it didn't work was contamination. I used the KAl(SO4)2 as the aluminium source, so this may have impacted the results. Despite not working, the process of production was the most promising. While the cooking process started far greener in appearance than typical, it very quickly changed to a dark colour, and then almost immediately into an extremely metallic looking liquid state. It was scarily similar to mercury in appearance, with few signs to indicate that it was not in fact a metal. Though i did not at the time have the wisdom to test its properties at this phase, the liquid metallic phase almost immediately solidified upon removal from heat. I made the unfortunate decision to wait until after it stopped off-gassing chlorine, by which point it had entirely stopped looking metallic and instead was mostly just dark gray and slightly green. This showed only extremely weak response to a magnet, and even this only lasted less than a minute. I suspect that if i had let it react for a shorter time, i may have gotten better results.
Most of the other intentional tests were either mostly unremarkable (very slight changes to composition don't make big differences in activity, especially when working in an already very imprecise environment, having an extremely dirty pan makes everything not work, etc.) however two of the most enlightening and interesting tests were ones that were unintentional. The first of these that i noticed was that the places on my silver ring that were touched by the material turned very dark. Why this happens is unclear to me, especially seeing as the reduction potentials for Cu, even Cu(i) are far lower than would be needed to onto silver, however i recognize that those potentials are usually calculated at pH of 7 and with no other active species in solution, which are both definitely not true here. The second unintentional discovery was that this same silver ring turned a very dark, almost black colour on the side that was most exposed to the fumes released. This indicates to me that not only was the chlorine being oxidized, but plausibly the sulphur from the sulphate was also being released as some volatile compound. That being said, i have heard of chlorine oxides being able to react with both silver and gold, however i'm not quite convinced that such a reaction would cause the effect that i'm seeing here. Knowing the smell of both chlorine gas and sulphur dioxide, i wouldn't be surprised that i missed the sulphur for the burning pain of breathing in chlorine. If the sulphur really is being oxidized, the formula that may better describe the compound is Al4Ca6CuO13, though given that letting the off-gassing go to completion appears to be extremely detrimental to the effect, i would not be surprised if sulphur (either as SO4 or another form) or chlorine were still important players in creating the final product.
Closing remarks and all that: I am really running out of copper chloride, i may buy copper sulphate, i may provide my results to one of the professors now, show that i have in fact received the lab safety training needed to use a campus lab, and hope for the best. Honestly i'm not sure what to do, but at this rate, i'm going to run out of soluble copper by the end of this week. I was considering replacing the calcium chloride with either magnesium chloride or sulphate, just for a test, but seeing as i can't even reliably make it work the same way 3 times in a row using the same recipe, i am hesitant to use my limited resources (honestly i have a ton of everything except the copper) on high risk ventures. Since i'm not only running low on my chemicals (i mean, mostly just the copper chloride), i am considering turning to drug dealing to pay for refills on everything. Might that be too extreme? Yes, probably, i should probably find some other way of getting money that isn't insanely time intensive. Given that we regularly have reps from petroleum companies on campus, i may try to find a way to make them pay for my experiments. Also, given the dramatic cost difference, i think i may try buying bulk CaSO4 and hope it works, but even if it doesn't, it should still be relatively easy to convert it to CuCl2. Hopefully i can have good results to report by wednesday.
Medium/big update!
So, first off, while i still don't have an official lab, i now have a hotplate and a pan that i'm just plugging in outside the physics building at my college. In my many attempts, i have unfortunately used up a ton of my starting chemicals, now only having about 1/2 of the 100 grams of copper chloride i started with, and a little over 1/2 the 500 grams calcium chloride. It's kinda shocking how fast even the little experiments eats through material. I've been considering using CuSO4 instead of CuCl2. Not only is the sulphate much cheaper, but it also may improve the properties. Based on a little bit of background information that i had been ignoring as well as further experimentation, the formula for the compound would appear to be Al4Ca6Cu(SO4)8Xx where X is either Cl or O, and x is the amount (for only Cl, this would be 10, for only O, it would be 5 (presumably)). The anions are less certain due to their volatility (i do assume the sulphate stayed in, but not as sure about Cl). The main reason i added the O as a possibility in the formula is because upon heating it releases a fair amount of chlorine gas, and afaik, oxygen is about the only thing i could expect in this environment to oxidize the chlorine and presumably take its place. When the material is only lightly heated, it releases very little chlorine, however this does not produce as impressive results. When heated beyond the point of being dry, it gains a metallic shine and starts releasing chlorine gas. If heating is continued, it eventually turns orange and stops releasing gas. It appears to me that the best results are obtained early into the metallic shine stage, about 20 to 30 seconds after it starts. Though at this stage it would be foolish to jump to conclusions, signs that make me more convinced that this is a superconductor include 1. the chemistry is fairly reminiscent of bscco, though notably with much less copper. 2. The metallic shine that closely correlates with the material's diamagnetic reaction. 3. The colour ranges from white (when too little copper is present) to green (when there is too much copper) with the only place in that scale known to possess very strong diamagnetic properties being a central place where is dark but with a metallic shine. 4. The borax was found to not be necessary (a run was completed with high success without it), though somehow it seems to allow for a wider range of compositions (especially variations in copper concentration). Why this happens is not understood, but it does make me more confident in the formula.
In the next few days it is my intent to try slightly different starting ingredients and see how that affects things as well as attempt a run with the final output to be Al4Ca8Cuc(SO4)8Xx where c is some number between 1 and 3, and x depends on X which i can't quite predict yet. This would be somewhat analogous to bscco 2223, though again, with notably lowered Cu content. Given the expense of chemicals, i am starting to get pretty worried about funding. Fortunately most of the materials i'm using are very cheap and easy to obtain
#experiments?#superconductor research#literally me being broke#yay#not that i agree with the concept of money but still#gotta use it for now#al4ca6cuo13 ish or smth
3 notes
·
View notes
Text
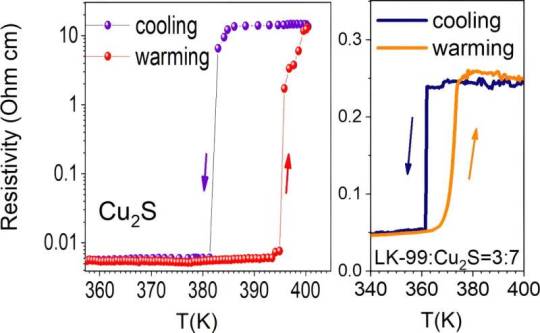
Myth of room temperature superconductivity in LK-99 is shattered
In a study published Nov. 24 in Matter, researchers led by Prof. Luo Jianlin from the Institute of Physics of the Chinese Academy of Sciences (CAS) have provided solid evidence that LK99 is non-superconducting, thus disproving earlier superconductivity claims. Sukbae Lee and colleagues from South Korea earlier asserted that LK-99 behaves as a superconductor at ambient pressure, with a critical temperature (Tc) up to 127°C (400 K). The groundbreaking news excited scientists as well as people on social media due to its potential impact on technology. As reported by Nature News, claims about the supposed superconductivity of LK-99 became a viral sensation, prompting numerous replication efforts by scientists and amateurs alike. Several groups have attempted to replicate the results, but none have provided direct evidence of superconductivity. The most puzzling question is what causes the sharp drop in resistivity and why it occurs only in a few samples.
Read more.
10 notes
·
View notes
Link
#market research future#superconductor wire market#superconductor wire industry#superconductor wire companies#superconductor wire demands
0 notes
Text
Scientists have found a key process required for superconductivity occurring at higher temperatures than previously thought. It could be a small but significant step in the search for one of the "holy grails" of physics, a superconductor that operates at room temperature. The discovery, made inside the unlikely material of an electrical insulator, reveals electrons pairing up at temperatures of up to minus 190 degrees Fahrenheit (minus 123 degrees Celsius) — one of the secret ingredients to the near-lossless flow of electricity in extremely cold superconducting materials. So far, the physicists are baffled by why this is happening. But understanding it could help them find room-temperature superconductors. The researchers published their findings Aug. 15 in the journal Science.
Continue Reading.
67 notes
·
View notes
Text
DOES QUANTUM GRAVITY EXIST??
Blog#389
Wednesday, April 3rd, 2024.
Welcome back,
All the fundamental forces of the universe are known to follow the laws of quantum mechanics, save one: gravity. Finding a way to fit gravity into quantum mechanics would bring scientists a giant leap closer to a “theory of everything” that could entirely explain the workings of the cosmos from first principles. A crucial first step in this quest to know whether gravity is quantum is to detect the long-postulated elementary particle of gravity, the graviton.
In search of the graviton, physicists are now turning to experiments involving microscopic superconductors, free-falling crystals and the afterglow of the big bang.
Quantum mechanics suggests everything is made of quanta, or packets of energy, that can behave like both a particle and a wave—for instance, quanta of light are called photons. Detecting gravitons, the hypothetical quanta of gravity, would prove gravity is quantum. The problem is that gravity is extraordinarily weak.
To directly observe the minuscule effects a graviton would have on matter, physicist Freeman Dyson famously noted, a graviton detector would have to be so massive that it collapses on itself to form a black hole.
“One of the issues with theories of quantum gravity is that their predictions are usually nearly impossible to experimentally test,” says quantum physicist Richard Norte of Delft University of Technology in the Netherlands. “This is the main reason why there exist so many competing theories and why we haven’t been successful in understanding how it actually works.”

In 2015, however, theoretical physicist James Quach, now at the University of Adelaide in Australia, suggested a way to detect gravitons by taking advantage of their quantum nature. Quantum mechanics suggests the universe is inherently fuzzy—for instance, one can never absolutely know a particle's position and momentum at the same time. One consequence of this uncertainty is that a vacuum is never completely empty, but instead buzzes with a “quantum foam” of so-called virtual particles that constantly pop in and out of existence.

These ghostly entities may be any kind of quanta, including gravitons.
Decades ago, scientists found that virtual particles can generate detectable forces. For example, the Casimir effect is the attraction or repulsion seen between two mirrors placed close together in vacuum. These reflective surfaces move due to the force generated by virtual photons winking in and out of existence.

Previous research suggested that superconductors might reflect gravitons more strongly than normal matter, so Quach calculated that looking for interactions between two thin superconducting sheets in vacuum could reveal a gravitational Casimir effect. The resulting force could be roughly 10 times stronger than that expected from the standard virtual-photon-based Casimir effect.

Recently, Norte and his colleagues developed a microchip to perform this experiment. This chip held two microscopic aluminum-coated plates that were cooled almost to absolute zero so that they became superconducting. One plate was attached to a movable mirror, and a laser was fired at that mirror. If the plates moved because of a gravitational Casimir effect, the frequency of light reflecting off the mirror would measurably shift. As detailed online July 20 in Physical Review Letters, the scientists failed to see any gravitational Casimir effect.

This null result does not necessarily rule out the existence of gravitons—and thus gravity’s quantum nature. Rather, it may simply mean that gravitons do not interact with superconductors as strongly as prior work estimated, says quantum physicist and Nobel laureate Frank Wilczek of the Massachusetts Institute of Technology, who did not participate in this study and was unsurprised by its null results. Even so, Quach says, this was a courageous attempt to detect gravitons.”
Originally published on https://www.scientificamerican.com
COMING UP!!
(Saturday, April 6th, 2024)
"HOW DOES A NEUTRON STAR FORM??"
#astronomy#outer space#alternate universe#astrophysics#universe#spacecraft#white universe#space#parallel universe#astrophotography
91 notes
·
View notes
Note
So what's your take on the progress in fusion research? I've seen some people be really bullish on near-term commercial applications, especially with stellerators, but I'm always skeptical of industry hype.
In my own personal opinion, it's looking good. Like, really good. I will try to write up a longer post about this at some point but for now, it's stuff like this that makes me optimistic:
Magnets have always been a bottleneck for fusion. The reason ITER is so gigantic is because they're compensating for old-fashioned, relatively weak superconducting magnets with a massive plasma volume, which really sucks to build. But now we have hight-temperature superconductors (HTS), and it's only been in the past five or ten years that we've started to see real industrial quantities hit the market.
HTS magnets are much, much stronger. You can outstrip the performance of ITER with a fraction of the plasma volume. I personally believe it's likely that Commonwealth Fusion Systems will be first at bat with a compact HTS reactor, but the rest of the industry isn't far behind.
Obviously, magnets aren't the only challenge. But everything else gets so much easier when you can just make smaller magents.
32 notes
·
View notes
Text
G/T Headcannons

So much of G/T is science-adjacent just because of the nature of the trope. But this got me thinking about like, G/T in the setting of science academia.
A scientist and an engineer who have a years-long dispute about whether it's possible or not to change someone's size. The scientist swears up and down that they have worked out the math and theories and it just is absolutely not possible. Meanwhile, the engineer is certain that they are close to a solution. One night, perhaps long after they stopped working together, the scientist wakes from their sleep to their roof being ripped off above them. "I fucking told you!" The now gigantic engineer gloats down at them.
Bonus if the scientist is so blinded by disbelief of their math failure that they aren't even afraid. They place their tiny hands on their hips and raise their chin up to stare right into the giant's eyes. "You had to have used a different theory. Did you make the room-temperature superconductor? Do you have any idea how vast the implications of that are? And you used it to do THIS to yourself? Are you working with [another scientist whose theories they disagree with]?"
A scientist who turned themselves giant and had a falling out with their partner who works in the same field as they do. One of them didn't include the other in a hugely impactful research paper, ending in a blowout fight. Now they both go to the same conferences and have to awkwardly avoid each other.
Additionally, one could approach the other sheepishly to ask for help on a new project. It could work both ways, a giant peering down angrily at a tiny human, or a defensive human crossing their arms and getting terse with their giant ex. "What, do you need to steal more of my ideas?"
A sizeshifter who tries to slyly shrink down to retrieve a fallen piece of lab equipment. They are caught by a coworker but instead of freaking out about their size their coworker panics about how this does NOT adhere to lab safety standards, demanding to know how many times they have done this.
A sizeshifter who pulls an all-nighter in the lab, not realizing that their frustration with not getting good results has made them gradually grow all night. The first person to come into the lab in the morning is greeted with the sight of their coworker poring over reams of printed data, towering over the equipment, and trying to rub exhaustion out of their eyes. (Queue the 'dropping the coffee cup in surprise' scene.)
A giant scientist who is at the cutting edge of their field, when a human intern starts on their research project and realizes very quickly that they have (for now) unrequited feelings for this giant. The scientist's huge form looms over the intern's workstation, casting a huge shadow. They squint their eyes at the schematics on the table. Bending over slightly, their large arm lightly brushes the human's shoulder as they point to an equation. "Is this doubling every time?" "D-doubling? I-" "If it is doubling, it should be natural log two, not log two." The giant lowers their head. Their annoyed face only a few inches from the human's. The human is blushing deeply, inhaling the smell of the giant's sweet breath. "It-d-er...doubling. Doubles." The giant's face remains unamused and annoyed. Despite their impatience, they make the effort to whisper gently, to not blow out the human's ear drums. "Then why did you write log two? Fix it."
The lead scientist and lead engineer on a project are always squabbling. In an accident, one of them gets shrunk down or grows to a large new size. Now their disagreements take place with the smaller one on some form of scaffolding, waving sheets of complex equations in the air while the other crosses their arms and shakes their head.
Two young scientists get passed over and are not included in the credits of a research paper, despite their extensive work. They go to confront the senior researcher, when one suddenly doubles, or triples in size and pins the senior researcher down, demanding credit for both of them. Their tiny friend is caught in a mix of horror and amazement as they realize that their friend could just as easily pin them down like that.
"Well, aren't you just soooo smart Mr. Giant Shithead." "That's Dr. Giant Shithead to you."
80 notes
·
View notes
Text


Houndour today, I feel like today and yesterday's sketches should be swapped honestly, but this is the way the requests ended up falling, which is neat but slightly frustrating, still, good to see I'm capable of doing mostly better overall.
Last year is a Ninetales doing SCIENCE! This is actually from around the time that room temp superconductor research was happening wasn't it. Too bad that didn't go anywhere but I think there's still people working on discoveries related to it.
12 notes
·
View notes
Text
Nature’s news team asked several superconductivity researchers to review the investigation report. At first, they were concerned by the university’s choice of committee members. The three physicists are specialists in shock-wave physics, not in superconductivity. Millot and Celliers were also co-authors with Dias on a 27-author review paper published earlier this year6. However, those doubts evaporated when the researchers read the report. “I couldn’t help but be incredibly impressed,” Armitage says. Paul Canfield, a physicist at Iowa State University in Ames, says: “There should be a good German word that’s 50 letters long and is simultaneously ‘impressive’ and ‘depressing’” to describe the report. Brad Ramshaw, a physicist at Cornell University in Ithaca, New York, concurs. “This is a great sacrifice of their time,” he says. “The whole community should be grateful that we have colleagues who are willing to go to these lengths.”
as long as all of this necessary work remains "sacrifices" of colleagues time it will stay like this forever
17 notes
·
View notes
Text
(potential) Superconductor updates:
Replicated it in the dorms despite the fact that the suspension had turned an awful yellow-red colour (it was a faint blue-white). Unfortunately, despite putting a lid on it, it doesn't seem to work 20 hours later. Also, this was a mix brought from home more than 2 weeks ago. The fact that the amount of time in water doesn't seem to show any difference indicates to me that the most important part is in the cooking. Alas, i have no copper or calcium chloride despite begging the chem department. So, that'll be needed for future experiments. Gonna try to get them from the uni again but i may have to buy it sadly. It's very strange that the colour was so dramatically changed and yet the effect was largely unchanged. That seems to indicate that the colourant (presumably a copper ion) was not in fact a necessary part? And it's not like it remains unchanged afterwards either, with the earlier samples, after i heated it it would be off-white whereas now the whole substance is an awful yellow-green-brown colour. It may be solely working with Ca2+, Al3+, and B3+. IDK tho. It'd still be kinda weird to me, if there was no help by the copper at all. I gotta get some calcium chloride first and test to see if i really do also need the copper. Or even if i can replicate it at all.
At the very least i saved a few drops of the suspension in an airtight container so that if worst comes to worst and i can't make it anew, i can (hopefully) run a mass spec and a few analytical chem tests (assuming the chem lab folks let me). Ideally though i'd just make it anew sometime soon. I can very super easily and definitively rule out iron contamination this time as any sort of possible factor since the cooking step took place in an aluminium container (the cut out bottom of a thoroughly cleaned out monster energy can). I was pretty sure it wasn't iron contamination (like, it was doing the wrong effect from what one would assume from iron anyways), but now i'm very confident.
I feel like there was something else important to say, but idk what it is.
Last thought on the matter: I will hopefully very soon be able to have more resources because i'm gonna be in conversation with one of the professors at my school who's an expert in magnetism and also ceramics and her research group is currently focusing on things that aren't publicly disclosed, but vaguely about inorganic "magnetically and electrically interesting" compounds. Also her students say she's the nicest person in the world, so i'm double excited. Anyway, so, tuesday afternoon, i'll either be infantilized, found to be not quite up to spec, or be given a great opportunity.
#research#superconductor?#magnetically interesting thingy#i'm tired#so so tired#listening to pierce the veil#yay
3 notes
·
View notes
Text

Room-temperature superconductor study retracted by Nature
A study published in March claiming the discovery of a superconductor that works at room temperature—a scientific holy grail—has been retracted by the high-profile journal Nature at the request of most of its authors. It marks Nature's second retraction of a superconductor study produced by the team led by Ranga Dias of the University of Rochester in the United States. It also comes after a different room-temperature superconductor discovery claim went viral on social media earlier this year which also fell short of the long sought-after breakthrough. Superconductors are materials that can carry electrical currents with zero resistance, unlike traditional materials such as copper which lose part of the charge in the form of heat.
Read more.
11 notes
·
View notes
Link
#market research future#superconductor wire market#superconductor wire industry#superconductor wire companies#superconductor wire demands
0 notes
Text
Seems like LK-99 is diamagnetic, as verified by... as yet unpublished(?) research by a Chinese team who posted a video on Bilibili, and a random guy with a "private workshop" who posted a video on twitter. The Chinese team say they could only test diamagnetism as they didn't produce flakes large enough to test resistance, but they're skeptical that other superconductor properties will replicate. Obvious internet videos without peer review are not a good source but it does give me kind of one tiny sliver of excitement that this might be real.
37 notes
·
View notes
Text
For the first time, Stanford researchers have found a way to create and stabilize an extremely rare form of gold that has lost two negatively charged electrons, denoted Au2+. The material stabilizing this elusive version of the valued element is a halide perovskite—a class of crystalline materials that holds great promise for various applications including more-efficient solar cells, light sources, and electronics components. Surprisingly, the Au2+ perovskite is also quick and simple to make using off-the-shelf ingredients at room temperature. "It was a real surprise that we were able to synthesize a stable material containing Au2+—I didn't even believe it at first," said Hemamala Karunadasa, associate professor of chemistry at the Stanford School of Humanities and Sciences and senior author of the study published Aug. 28 in Nature Chemistry. "Creating this first-of-its-kind Au2+ perovskite is exciting. The gold atoms in the perovskite bear strong similarities to the copper atoms in high-temperature superconductors, and heavy atoms with unpaired electrons, like Au2+, show cool magnetic effects not seen in lighter atoms."
Continue Reading.
148 notes
·
View notes