#sertraline-core
Explore tagged Tumblr posts
Text
Not me skipping my meds so the appetite suppressant and nausea side effects kick in
#sertraline-core#or lamotrogone who knows at this point#im violently withdrawing#p sure I’m getting Stevens-Johnson syndrome but we move
1 note
·
View note
Text
Tapering down...
It's no secret on here that I'm a depressed old fuck who's barely surviving. I've been taking two antidepressants for the last few years, Sertraline (Zoloft) and Mirtazipine (Remeron).
I love the Mirtazipine because it helps me fall asleep at night and STAY asleep. Insomnia was one of my main symptoms...for years on end I never strung together more than an hour and a half of sleep at a time. If I take the Mirts right as I'm hittin' the bed, I fall asleep within 20 mins. Those I have no problem with.
Talked to the doc about 6 months ago about tapering down off the max Zoloft dose (150mg/day) and finally getting off them totally. Why? They never really seemed to DO anything. There may have been a slight sense of relief after awhile, but I never really FELT any kind of effect, and no real benefit, other than the possible "I'm taking meds now so I must be getting better" thing you have to tell yourself before they "kick in".
So, I tapered down to just 100mg for a couple months and then took the 50mg's until they were gone. Been completely off them for a few weeks now. Haven't really felt much different, nothing really negative, other than possibly feeling "hopeless" once in a while, which, frankly, anybody in my position WOULD feel.
My reason for writing this out? I never really considered that they might be masking some of my emotional range. After the big news of the day yesterday, after a few hours of sensing the giddiness in the ether, I just started crying. I know that's a perfectly fine reaction to have when something that's been horrible gets RESOLVED, at least on a certain level. It's a release of tension.
But there was more to it. Posted some videos, and listening to all the music, it just made me weep all that much more. And this was genuine grief...grief at the loss of what all those songs represented to me, to my own Personal Mythological Framework, as it were.
And yet, deep down I knew that it wasn't just the loss of The '60s Protest movement, or specifically 1967, The Summer of Love and what IT represents to me.
I think enough of the sertraline has flushed from my system now that my emotional body is releasing pent-up grief. I've written about my two Gemini loves, born a day apart, died nine years apart. That birthday anniversary has always been difficult to get through, but last night's flow of tears finally wound its way to that core pain.
I'm one of those people who has to know WHY.
Once the WHY is satisfied, the letting go can happen.
My last therapist was essentially a Buddhist witch, and I always struggled with what she said about the death(s), but it hit home on a deeper level last night. Her words? Essentially that we have to eventually get enough distance from it and see the "Rightness" of it.
That's a seemingly callous idea, but it's really not.
If someone dies, think of their lives and the trajectory they were on, and the trajectory the world has taken since their death. Eventually there will be a sense of "yes, that somehow HAD to happen for THIS to happen"...a sense of "rightness" in that definition is strange to feel, once you get to it. You may never GET to it...it may always be "THE GREAT WRONG" in your life.
Gemini 1 (my sweetie, my soulmate) died July 15, 2012; Gemini 2 (my bro the soldier) died four days after the January 6th attack, on January 10, 2021. I'm still surrounded by the detritus of both their lives, in my sweetie's case, I have every piece of art she made between Junior College and the day she died. In the case of my Bro, it's all the computer parts and tools and family camping stuff that hasn't seen the light of day in over 20 years.
I know that I've hung onto much of it out of desperation, out of duty and loyalty to their memory, their lives...but it is currently holding me back, and I can feel that. I have to find the stomach to go down to the garage and just start taking pictures of all the tech and camping stuff and being realistically ruthless about what I actually CAN and CAN'T use and hit CL and eBay with whatever might bring a buck.
Up until last night, the idea of that was just too overwhelming. I think last night's emotional release had an effect. Not sure just how any of it is going to happen, but I have to face all that crap down there and get rid of 90%, leaving only Char's artwork and a few tools i can use, and then finding a cheaper storage solution for what's left.
Especially since the evil landlords jacked the rent on the garage up another fucking $25 as of this coming month.
And back to that "Rightness" thing. I finally thought about that in terms of what has happened in the world since they both respectively left. I am certain my sweetie would not believe the shit that has gone on in the last 12 years. I'm pretty sure my bro's poor broken body would not have made it through the ensuing years, especially after that last bizarre injury.
It's a strange thing to see that from the distance of time. And last night's catharsis was certainly tied to it, but I'm sensing there was a component tied to the tapering off the sertraline. No more emotional masking, possibly there will be more peace of mind going forward, I can never be sure, as I pick up just about everything energetically. (Why I have to go "SHIELDS UP, SCOTTY!" while I'm out and about, and self-isolate so much of the time.)
We do have so much to grieve. It never really ends. You have to feel it ALL. You have to release it. You have to see the "rightness" of it when you look at the world in its entirety. The sertraline's masking of the intense sadness finally being gone facilitated the bulk of it, I'm pretty sure.
14 notes
·
View notes
Text
sertraline core is literally almost falling asleep in my chair at the doctors today bc i was so violently fatigued and then now it is PAST my bedtime and im like Hrmmm. Should i bake
2 notes
·
View notes
Text
How the Heat Can Be Affecting Your Mental Health

Do you feel miserable in the sweltering heat of summer? It’s not just you. Millions of people’s mental health is affected by the heat, and not for the better. As temperatures rise due to climate change, the impact of extreme heat on our physical health is widely acknowledged. Heat-related illnesses and fatalities are frequently reported. However, what often goes unnoticed is the profound toll that heat can take on mental health. When exposed to high temperatures, the body’s thermoregulatory system is challenged. As it struggles to cool down, individuals can experience symptoms like dehydration, heat exhaustion, and heatstroke. These physical consequences can, in turn, have a direct impact on mental health.
Today, we’re going to explore this under-discussed challenge to mental health. Simply knowing why you might be struggling with your mental health can go a long way toward understanding and treating it.
Aggravation of Pre-existing Mental Health Conditions
High temperatures can trigger or exacerbate anxiety and panic disorders. The physical discomfort associated with heat can lead to restlessness and a sense of unease. Moreover, the fear of heat-related health issues can escalate into full-blown panic attacks, especially in those who are prone to anxiety.
Depression can also be impacted by the heat. Prolonged exposure to oppressive heat can deepen feelings of depression. Isolation caused by avoiding outdoor activities and social gatherings can intensify loneliness and despair, contributing to the vicious cycle of depression.
Good sleep is important for mental health, but sleep patterns are highly sensitive to temperature changes. In hot weather, individuals may experience sleep disturbances, leading to sleep disorders such as insomnia. Sleep deprivation, in turn, can have severe repercussions on mental health, including major cognitive impairment– another problem that heat can make worse.
Cognitive Impairment
Extreme heat has been linked to cognitive impairment, affecting memory, attention, and decision-making. The brain’s ability to function optimally is compromised when the body is struggling to maintain its core temperature. As cognitive abilities decline, individuals may experience heightened frustration and anxiety, making daily tasks seem insurmountable.
Behavioral Changes
Heat can trigger aggression and irritability, even in individuals who are typically calm and composed. The discomfort and physical stress caused by high temperatures can lower the threshold for irritability, leading to conflicts in personal and professional relationships. This heightened irritability can strain social bonds, exacerbating feelings of isolation and distress.
Medication Interaction
Many psychiatric medications don’t work as well in your body when it’s hot out. These medications make you more sensitive to heat and more susceptible to heatstroke. Unfortunately, these medications include SSRIs like citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft). These medications are often the first line that doctors prescribe for mental health conditions– so if you’re on one of these medications, just be aware that they are less effective when it’s hot out.
Vulnerable Populations
Certain populations are more vulnerable to the mental health impacts of heat, including the elderly, children, and those with pre-existing mental health conditions.The elderly, for instance, often have limited mobility and may lack access to air conditioning, making them more susceptible to heat-related mental health issues. Similarly, children may struggle to cope with extreme heat, leading to increased irritability and emotional distress.
Climate Change and Mental Health
The increasing frequency and intensity of heatwaves are directly linked to climate change. As global temperatures continue to rise, the mental health consequences of heat will become more pronounced. This presents a significant public health challenge that must be addressed. Climate change anxiety can also exacerbate the problem, creating a negative feedback loop of feelings of doom and despair. This emotional concern can be overwhelming and very challenging to deal with without help.
Dealing with Heat and Mental Health
Heat can have a major impact on mental health, but there are strategies you can use to minimize this impact.
Stay Hydrated: Dehydration can exacerbate anxiety and irritability, so proper hydration is essential in hot weather to prevent mental health issues.
Cooling Centers: Many communities have established cooling centers where individuals can escape the heat. These centers provide a safe and cool environment for those at risk.
Education and Awareness: Public awareness campaigns can help people recognize the signs of heat-related mental health issues and encourage them to seek help.
Green Spaces: Increasing the availability of green spaces can provide shaded areas for outdoor activities, promoting social interaction and mental well-being. Community initiatives to plant shade trees can also help. Trees can drop an area’s temperatureby almost 3 degrees and provide a place that feels more comfortable than being outside in the sun.
Finally, if you’re experiencing distress during hot weather, you should seek help. Therapy can be extremely helpful for developing the coping skills and resiliency you need to deal with the challenges to mental health that extreme heat can pose. If you’re struggling with heat-related (or any other) mental health challenges, reach out to the therapy team at LoveHealGrow. Our expert staff of caring practitioners is here for you.
0 notes
Text
Anxiety – My Story
Breath and catch a moment, one thing can lead to something more. Take another pill, this will heal your mind. Meds, I dunno what to think, will this thing help? Sugar coating seems to work for a while, but yet it is too sweet. Like a maze that leads to a dead end but has no start. “Shit, I digress” I say as I pour another drink. To those who are care free without a worry, I just don’t understand. How can you stop those edging thoughts, those odd small things that crop up out of what seems to be nothing …
My brain tries to beat me and trick me in this never ending game of theirs. Suddenly, I recoil, like a snail going back into it’s shell, “Oh, it’s only you” I say to Teddy, my beloved cat. A lovely tabby that I rescued two years ago from the shelter, he needed me, and at the time, I needed him. I cry into my pillow, as I grind the tablet into powder, I begin to feel the chill, the utmost mist that cools to the core. My nurse wants me to raise to 100mg of Sertraline, but I just can’t. I am OK with this one small tablet, I am not ill. Clean, must be clean. These compulsions rule my life, as if to say “You will never get better”. My henry hoover is good. It picks up dirt like nothing else; alas poor Harvey, be still. There is no threat but you bark anyway. Why? What do I need to do to please you? I’m raising you but you will never be a replacement of Bonnie. I have compulsions of checking windows so Ted won’t escape. He has done a runner before and has been lost. I couldn’t cope during that time. Why would my dear boy run from me??
Grind me down I swear to my voices under my breath. You will never rule me. They say to panic and my heart races; it feels as if I can’t catch but a moment from their silly rules. Merc, wake up! I open my eyes to see my mum standing in my room as per usual, every time I try to sleep. “You were shouting in your sleep” she said. I feel my face and wipe away a tear. It was a dream, amongst others I’ve had before. I must go out soon to walk Harley. Me and mum like to take to the beach as way back then so did Bonnie. Harvey is fascinated by the sea. He loves to run around on the beach and get stuck in the sand. Good times.. Beat me, grind me and take me to hell, I will survive. Anxiety almost ruined me; infecting my mind with vicious circles that spiral out of control. I sleep to avoid my mind. At least when I’m asleep; I feel no pain, no anxiety and finally have peace. I worry about the smallest of things nowadays, like what will Ted eat although I know full well he has food and water. What will happen to him? I question, without no context to the thought. Why can I not seem to think of a way out from these stupid little thoughts. I push them back but end up overwhelmed by the sheer nature of intrusive, and utmost negative pattens.
Trauma, I went through a lot of death growing up. I had no way of coping back then. I was brought up in a household where showing emotion was a bad idea. She nodded, as she mad notes. Counselling hasn’t always been useful but then, at times, all one needs is to open up enough to express concerns and thoughts, in a way that has a positive outcome. I never adjusted to feeling happy. Feeling as if I had no care in the world, but things are as best as they can be overall.As I was about to leave the counselling session, I heard two people talking in the corridor. Walls have ears you know, don’t they? I thought to myself as I left the room. Mum was waiting outside in the car for me. A familiar face, a loving lady, my brother and I were lucky to have been adopted by her. As I shut the car door she asked “how was it?”, Yeah it was fine I said. As we were driving away from the building, I said, anxiety ruined me, but I survive, mum agreed in a mumbled and oddly comforting way. She had to deal with the death of my dad back then and survived. During that time, I gave her a listening ear and looked out for her and my brother. Lean on the right person, hold their hands and forgive. Forgiveness is a good thing and a way to move forward in life. Why? Because you only live once, tread lightly, as your foot prints follow you in this world.
0 notes
Text
College Student Assignment on PTSD Treatment Options
POST-TRAUMATIC STRESS DISORDER TREATMENTS
Some treatments are more effective for treating PTSD than others. Below, we will discuss.
Psychosocial (Behavioral) -
First, let’s take a look at a few psychosocial approaches that have a behavioral emphasis. Two contain an exposure element, the first of which is written exposure therapy, or WET. According to studies, it is estimated that anywhere between 50-75% of college students encounter at least one potentially traumatic event. Furthermore, among our student veterans, 45.9 percent (628 sample size) show significant PTSD symptoms (Morrissette, et al.). As such, it is important to address concerns from these events that could taint the students’ life, yet not interfere with a busy school schedule. To no surprise, the college students in the study liked WET, due to the treatment flexibility. Students enjoyed not having to vocalize what had occurred (suppressing possible embarrassment in front of a therapist). The first visit is one hour of psychoeducation while in the four 40 minute follow-up sessions, students are asked to write about one event - detailing their most intense thoughts and emotions pertaining to it. They write for half an hour. Subjective units of distress are collected before and after the writings.
In addition, Prolonged Exposure Therapy is another successful tactic in treating PTSD. The goal here is to eliminate the fear response and question the notion that the individual is unable to cope. One can expect eight to fifteen hour and a half long sessions spaced out either weekly or twice a week. Hierarchically formatted by targeting one’s least intense fears first and then climbing the ladder towards the individual’s most fervent fears, techniques are introduced for challenging negative ideas about the trauma and its associated connotation. For long term success, imaginal exposure is more effective than virtual reality technology, at this point (Kring, et al.). Breathing exercises are introduced, and as is the case in WET, psychoeducation is also included.
Eye Movement Desensitization and Reprocessing, EDMR, has also been shown to assist. In the short run, this is as helpful as other variations of CBT, since it aids in reducing vividness and memory strength (Kring, et al.).
Psychosocial (Cognitive) -
Not only are there the aforementioned behavioral approaches, but there are also psychosocial approaches through a cognitive lens. One useful treatment is Cognitive Processing Therapy (CPT), which contests propensities of self-blame and improves emotion regulation competence (Kring, et al.).
Medication -
Finally, medication can be taken. Although not as effective as the psychosocial approaches listed above, there are currently two anti-depressant SSRI’s approved by the FDA for use in treating the disorder: Sertraline (Zoloft) and Paroxetine (Paxil) (Kring, et al.). The criticism with this treatment approach is that relapse is frequent and it doesn’t treat the core problem.
Works Cited:
Morissette, Sandra B., et al. "Delivery of written exposure therapy for PTSD in a university counseling center." Psychological Services 20.1 (2023): 122. Kring, Ann M., and Sheri L. Johnson. Abnormal Psychology: The Science and Treatment of Psychological Disorders. 15th ed., Wiley, 2022.
1 note
·
View note
Text
Working in a pharmacy has made those euphoriacore hello kitty grunge core aesthetic blogs so much funnier. I’m your Sertraline girlfriend. I’m your Laxido bitch. Girl this is my nine to five they are just tablets 😭
2 notes
·
View notes
Note
Trigger Warning: searching for abusers online, feeling hopeless, fear for future, mention of medication
I don’t even know why I’m posting this.
Been in a weird headspace for this past week or so. I can’t really explain it other than a sense of being trapped and frustrated.
I’ve started a new job that I’m doing well in, I guess, and I do find the work engaging and interesting. I should be happy. And yet I spend most of my shift at home sitting at my desk barely moving and staring at my screen in panic when more and more emails arrive.
Then spend my time off work brooding about the big ominous “future”. I’m scared about what will happen. My parents aren’t getting any younger and one day will be gone. I’m not getting younger either; I just can’t visualise myself succeeding in my career or having a loving relationship, or being able to look after my autistic younger brother. I mean, my parents have said categorically they don’t expect me to look after him when they’re gone and want me to live my own life. I’m not doing that though. I feel like time is running out and I want to run away.
I don’t know what’s wrong with me. Then today I just decided to go and try and find the guys who assaulted me in school on social media. I don’t even know why I done that. To see if karma got them? Or punish myself to see if they’re living better?
Well, I couldn’t find them in any case. Maybe a small mercy. I’m crazy, a disappointment. Probably do dread the future because I know deep down in my core that I don’t have a future.
The thing is, I take about 50mg a day of Sertraline medication since about late 2019. The Doctors previously have said they want to reduce me further, though I don’t know. I’m wondering if I do need my dose upped. Then feel stupid at having to go back up, like I can’t cope with life otherwise. People will write me off.
Ugh, actually hate myself and wish I could go and sleep, not waking up.
Hi anon,
I want to start off by saying that you are valid. And your feelings are valid and understandable. You aren’t alone in feeling like time is running out. This is a relatable feeling to me honestly. As hard as it is, I try not to get too caught up on “time running out.” It’s also taken me a long time to quit focusing so much on the future. I am working on focusing in the here and now.
What is something today that could make you feel happy, or at least a little better? Is it watching a favourite show? Going for a walk? Partaking in a hobby? It’s okay to stop and enjoy the moments as they come.
You are not alone in looking up your abusers. People do it for all sorts of reasons, but whatever the reasons, you are valid. And you aren’t alone.
Is therapy accessible to you? Being able to talk to someone who can help you navigate some of these thoughts and help come up with coping skills might be useful to you.
Do any followers have any advice?
April
2 notes
·
View notes
Text
Depression and Pills.
Well I officially reached the 2 months on Sertraline and finally got rid of the Benzos, despite the amount of Benzo was quite low, I can feel that my body is feeling not to have it. The glow (excesive glow) has vanished.
I still don’t feel like I was feeling before taking any sort of medication, 2 months of therapy gotta work for something, (with a new therapist).
Several things, shitty ones, have happened through the last 2 months, so I can tell my body is responding to all the drama.Death, failed relations, crushed dreams are just very few of the things that have happened lately.
Today is deeefinitely not a good day, I can feel anxiety in the very core of my soul, but definitely I don’t feel like going back.
I feel with a need of crying again, but not feeling like that should be it.
If you are starting medication, and reading this. You should know that somewhere among the way you will feel something is changing. Solve your issues, get your doctor’s attention and work on what needs to be worked.
1 note
·
View note
Text
*TRIGGER WARNING*
Update.
Hello lovely followers. Thank you for all of your kind messages, I have now responded to them individually and privately. I’ve written a little update for those of you who are interested.
Since last time I posted, things have got worse- much worse.
I continued to engage with the CRHTT but it was felt that I needed more support than they could offer in the community, so I was asked if I wanted to go to an inpatient mother and baby unit (MBU). I declined, until they threatened to section me, so I went in voluntarily.
I was placed in an isolation room and tested for COVID-19, then moved to the main ward once that came back negative. I was on Level 3 (one-to-one) observations.
Although the majority of the staff are wonderful, and it’s a very nice ward, I no longer wanted to stay here and was put on a temporary section when I insisted on leaving. I was then assessed and placed on a section 2.
The suicidal thoughts persisted and when I was downgraded to less intrusive observations I attempted to end my life. Clearly I failed. The staff who found me were absolutely lovely to me, bless them, which somehow made me feel worse. I was taken to the closest A&E and had both internal stitches and surface stitches on my neck. The doctor who treated me said I was extremely lucky that I didn’t hit my carotid artery. Since that was my plan, I certainly didn’t feel lucky.
I was placed on Level 3 observations again.
Today I had a review with the doctor and my core team here on the ward, which I hadn’t been looking forward to but it wasn’t as bad as I had expected.
The consultant thinks I have developed postnatal depression and is taking me off the antidepressant (sertraline) the community psychiatrist prescribed and I’m going to try mirtazapine instead. He thinks this may help with my sleep also.
As for Ezra, the staff are brilliant with him, he’s loving all the groups and activities, he’s learnt to crawl, is trying new foods, has another tooth coming, and has been pulling himself into standing position. I continue to be amazed every single day.
So that’s where things are at the moment.
Would love to receive some post or something, if anyone could be so kind as to send me something please inbox me for the address.
Hope all my fab followers are well and are doing better than I am at the moment.
Stay golden x
@finding-mollies-marbles
#mental health#recovery#mental illness#schizophrenia#paranoid schizophrenia#inpatient#mbu#postnatal depression#depression#medication#clozapine#aripiprazole#sertraline#mirtazapine#psych ward#mental hospital#trigger warning#trigger#warning
9 notes
·
View notes
Text
Purple hyacinth
Swim inside my core
Fill my empty vase with your slops
Replace my eyes with a philco 4014
Follow my red anemones by the shore
perfect my structure like a pencil in the sharpener, and use it again as escapism
Pierce through me like a vaccine
Paint brush that draws until my arm is a art gallery
Choke me in your sertraline.
Ravish me like a typhoon with the waters of my soul
Let me paint this waves crimson
and turn my lungs into fleshy aquariums
Make me suck on your fingers made of coal
My limps cold like the oceans lips
My soul as vivid as North Sentinel Island
And my mind infected with your Chlorine
Uncured ancient disease in a modern-land
High blood pressure, heart disease, stroke, liver disease…
Your blood’s zesty taste burning my palate
blackout, dizziness, shakiness, craving, or sweating…
Surrounding me with your warmth in my pallet
aggression, agitation, compulsive behaviour, self-destructive behaviour, or lack of restraint…
Ascend me high to the third dimension
anxiety, euphoria, general discontent, guilt, or loneliness…
Break little by little my any sense of comprehension
nausea or vomiting, delirium or fear…
Beat me up the following morning like a bullet to my head and like damnation to my body
impaired coordination, physical dependence, slurred speech, or tremor…
I do not wish to become your slave, like my mother, but your taste gives me the glimpse of reality and sense in life.
Is this what she felt like?
Feeling like I had lived a millennium, but I am only a decade and a few years old.
One year ago feels like history and my childhood as clear 13,100 feet deep into the sea.
Broken, but my art feeds from my cracks.
After all, isn’t art the escapism of the Damned?
0 notes
Text
Things I wish I had known about fibromyalgia, and how to live with it
To put this into context, I was diagnosed about 10 or 11 years ago now, and it was a diagnosis reached by excluding other things that could be causing my symptoms. I had X-rays and MRI scans, I had something very unpleasant called electro-conductivity testing to rule out multiple sclerosis, and then I was told that I have fibromyalgia, have this leaflet, please close the door after you. I was basically forced to deal with it myself for a long time, and I’ve learnt quite a lot about how to manage myself. I hope what I learnt can help you.
What is fibromyalgia?
Fibromyalgia means “muscle and nervous pain”. Current research suggests that it is a dysfunction of the central nervous system (that is, the nerves that tell your brain what you’re feeling), so nerve signals are misinterpreted as pain.
Symptoms include, but are not limited to:
Widespread muscular and nervous pain, fatigue, headaches, cognitive dysfunction (problems concentrating, poor memory, slow or confused speech) extreme sensitivity of the skin, extreme sensitivity to pain (as in, you accidentally catch your finger on the cupboard door, it hurts really badly and it still hurts 2+ hours later), muscle stiffness after being still for a while, muscle spasms, poor sleep quality and waking up tired, dizziness and clumsiness, feeling too hot or too cold (because the body isn’t able to regulate it’s temperature) sensations like tingling, numbness, prickling or burning in hands and feet (and maybe other areas), anxiety and depression.
That’s a lot of symptoms, and chances are you don’t get all of them, and other people will be affected differently.
Stress
Stress makes fibro worse. It makes the pain worse, it makes everything harder to cope with. It’s not just me saying that, it is a scientific FACT. I know if I get upset, stressed or angry, my pain INSTANTLY increases. I can’t watch horror films anymore, every time there’s a jump-scare I feel like I’m being showered with needles! So, it’s really important to try and keep your stress levels down. Find hobbies that help you relax. Choose to be calm and happy! It will really help!
Pain
How you think about your pain needs to change. It isn’t a matter of “powering through” and having to “man up”. If you have fibro, you have something which means your nerves are nearly always screaming at you that stuff hurts. LISTEN to your body when you exercise. If it tells you doing something hurts, slow down how you are doing it. Slow your movements down, focus on how you feel and go gently. If it burns or hurts more than you are prepared to deal with, leave it for the day. Get some rest, take some paracetamol and ibuprofen and relax! If the pain gets too much – as in, can’t eat because feel sick because pain, or can’t walk/do daily tasks anymore, go see your GP about pain relief. It took me 8+ years to find a doctor who took me seriously and understood the condition, and gave me the pain meds I need to function day to day.
My current meds are: 2 x cocodamol (30mg codeine/500mg paracetamol) x 4 times a day. 1 x 100 mg gabapentin twice a day. 1 x 100mg sertraline (anti-depressant) twice a day. 45mg mirtazapine at night. That’s a lot of anti-depressants because I also have Bipolar Disorder (used to be called manic depression). Gabapentin has been an absolute godsend for me! It’s really helped tone down the constant prickly/tingly sensations
Sleep
Sleep is incredibly important. When folks with fibro don’t get enough sleep, or don’t get enough deep sleep, everything hurts so much more. Fibro can disrupt your sleep cycle so you don’t go into the deep sleep your body needs - so SLEEP IS IMPORTANT! If you sleep well, you will be so much more able to deal with everyday pain. So try your best to get a good night’s sleep. Create a bedtime routine – go to bed at a decent time (around 11pm at the latest) after a warm bath or shower. Have some extra-soft pyjamas or loungewear to get into after coming out of the bath or shower. Have a hot (non-caffeinated) drink e.g. herbal tea, hot milk, Horlicks or Ovaltine. Don’t watch TV in bed! Do not spend too much time on tablets, phones etc past 9pm (blue light from screens will make you feel more awake, use a blue light filter if it’s built into your devices).
This may make you feel like an old fogey, but SLEEP IS IMPORTANT! Like, super duper important! And you need to do everything you can to make sure you sleep well. However, and this is the real kick in the teeth, sometimes you can do everything right and still wake up exhausted. If that happens, talk to your GP about medication to help you sleep. There are various anti-depressants that are commonly used for this, like amitriptyline (which I used to take, and my sister takes now), and I am currently on mirtazapine to make me properly sleepy. There’s a happy side effect in that these drugs also help to lessen nervous pain.
Fatigue
As you will have noticed by now, fatigue isn’t just feeling tired. It’s feeling exhausted, like you haven’t slept for a week, and you can barely do anything before you have to stop. On days like this, you only have a little energy, and you have to be careful how you spend it. Figure out what HAS to be done (e.g. need to do the laundry so you can have clean clothes tomorrow, dishes need to be done because you have nothing to eat off and you are hungry), and what can wait until you’re feeling better (e.g. vacuuming). You can’t do everything at once, so take it one task at a time slowly and at your own pace. Give yourself breaks if you need it. It’s important to cut yourself some slack and allow yourself to come back to it later. Be kind to yourself. Ask for help if you need it. No one wants to see you struggling with something, or in too much pain to cope.
Cognitive dysfunction (a.k.a. fibro-fog)
Some days, your head might feel like it’s been stuffed with hot cotton wool. You can’t think straight, and you can’t find the words to properly express yourself. You will probably forget things that are a change from your normal routine. People may ask you if you’re on any drugs! Unfortunately there’s no treatment for it, but you can find ways to deal with it.
Use a calendar or paper diary – writing things down may help you to remember them better. Make lists of important things WHEN YOU REMEMBER THEM – you can’t rely on yourself to remember them another time. You have to try and leave yourself reminders. Future you is forgetful, so present you has to plan for it! And if you forget something important, be prepared to apologise!
Food
I’m know it’s very tempting, when you’re in pain and tired, to just order some delicious food delivered to your door, but you’re gonna find it very hard to lose weight when you can’t do lots of cardio, so it’s best to eat healthy most of the time. Keep frozen chopped onion and garlic in the freezer, and tinned tomatoes and pasta in the cupboard, so you can knock up a good meal with minimal effort. Try to have a folding stool in the kitchen for you to perch on whilst cooking. Make it easy to eat well, and save the left-overs for lunches!
Exercising
You’ll probably find it very hard to keep exercising like you used to. Try to replace high impact exercise with something low impact like cycling (not spin class!) and rowing. Maybe try something like yoga or pilates (I know, it’s old lady stuff, but it’s still good for you!), low impact stretching and general core work will be good for you.
DON’T do exercise classes where you will get constantly yelled at to go harder. Don’t let other people set your pace! Let your body tell you how fast you can go!
In general, don’t let other people set your pace. Sometimes you will need to go more slowly, if you feel unsafe on your feet (as in, knees might give out and you might deck it, right in the middle of the street) consider getting a stick. It took me years to finally admit that I needed one, but it has seriously helped me. My sister went through the same thing a few months ago, and I told her: it isn’t you admitting defeat or failure, it’s you doing what you need to do to help yourself. Plus she now has something to trip people up with if she doesn’t like them!
And to anyone who says that fibro isn't real, or is all down to lifestyle factors, I say this: me, my sister and my aunt have all been diagnosed with fibro independent of each other. And I have reason to believe other members of my family might be showing symptoms too! There is clearly a big genetic component at play, so blaming people who can't exercise for being overweight is counter productive as fuck.
2 notes
·
View notes
Text
WebMD: Treatment
Manic episodes in bipolar I disorder require treatment with drugs, such as mood stabilizers and antipsychotics, and sometimes sedative-hypnotics which include benzodiazepines such as clonazepam (Klonopin) or lorazepam (Ativan).
Mood Stabilizers
- Lithium (Eskalith, Lithobid): This simple metal in pill form is especially effective at controlling mania that involves classical euphoria rather than mixtures of mania and depression simultaneously. Lithium has been used for more than 60 years to treat bipolar disorder. Lithium can take weeks to work fully, making it better for maintenance treatment that for sudden manic episodes. Blood levels of lithium as well as tests to measure kidney and thyroid functioning must be monitored to avoid side effects.
- Valproate (Depakote): This antiseizure medication also works to level out moods. It is faster acting than lithium for an acute episode of mania. It is also often used "off label" for prevention of new episodes. As a mood stabilizer that can be used by a "loading dose" method - beginning at a very high dose - valproate allows the possibility of significant improvement in mood as early as four to five days.
- Some other antiseizure drugs, notably carbamazepine (Tegretol) and lamotrigine (Lamictal), can have value in treating or preventing manias or depressions. Other antiseizure medicines that are less well-established but still sometimes used experimentally for the treatment of bipolar disorder, such as oxcarbazepine (Trileptal).
Antipsychotics: For severe manic episodes, traditional antipsychotics (such as Haldol, Loxapine, or Thorazine) as well as newer antipsychotics drugs - also called atypical antipsychotics - may be necessary. Cariprazine (Vraylar) is a newly approved antipsychotic to treat manic or mixed episodes. Aripiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal), and ziprasidone (Geodon) are often used, and many other drugs are available. The antipsychotics lurasidone (Latuda) is approved for use - either alone or with lithium or valproate (Depakote) - in cases of bipolar I depression. Antipsychotic medicines are also sometimes used for preventive treatment.
Benzodiazepines: This class of drugs, referred to as minor tranquilizers, includes alprazolam (Xanax), diazepam (Valium), and lorazepam (Ativan). They are sometimes used for short-term control of actue symptoms associated with mania such as agitation or insomnia, but they do not treat core mood symptoms such as euphoria or depression. They can also become habitforming so need to be closely monitored.
Antidepressants: Common antidepressants such as fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft) have not been shown to be as effective for treating depression in bipolar I disorder as in unipolar depression. In a small percentage of people, they can also set off or worsen a manic episode in a person with bipolar disorder. However, studies have shown that for bipolar II depression, some antidepressants (such as Prozac and Zoloft) may be safe and more helpful than in bipolar I depression. For these reasons, the first-line treatments for depression in bipolar disorder involve medicines that have been shown to have antidepressant properties but also no known risk for causing or worsening mania. The four FDA-approved treatments for bipolar depression are lurasidone (Latuda), olanzapine-fluoxetine (Symbyax) combination, quetiapine (Seroquel) or quetiapine fumarate (Seroquel XR), and cariprazine (Vraylar). Other mood-stabilizing treatments that are sometimes recommended for treating acute bipolar depression include lithium, Depakote, and lamotrigine (Lamictal) (although none of these latter three medicines is FDA-approved specifically for bipolar depression). If these fail, after a few weeks a traditional antidepressant or other medicine may sometimes be added. Psychotherapy, such as cognitive-behavioral therapy, may also help.
People with bipolar I disorder (mania or depression) have a high risk for recurrences ans usually are advised to take medicines on a continuous basis for prevention.
Electroconvulsive Therapy (ECT): Despite its scary reputation, electroconvulsive therapy (ECT) is a safe and effective treatment for both manic and depressive symptoms. ECT is often used to treat severe forms of depression or mania in bipolar I disorder when medicines may not be effective or likely to work fast enough to bring symptom relief.
1 note
·
View note
Text
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
By: Jason Karimi, WeedPress Contributor Title: The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial Sourced From: weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/ Published Date: Sun, 21 Mar 2021 23:04:30 +0000
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0246990
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin ,
Benjamin Shechet,
Colin Hennigan,
Rebecca Matthews,
Amy Emerson,
Rick Doblin
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin,
Benjamin Shechet, …

x
Published: March 17, 2021
https://doi.org/10.1371/journal.pone.0246990
Article
Authors
Metrics
Comments
Media Coverage
Peer Review
Abstract
Introduction
Methods
Results
Discussion
Conclusions
Supporting information
Acknowledgments
References
Reader Comments (0)
Figures
Abstract
Importance
There is a pressing need for development of novel pharmacology for the treatment of Posttraumatic Stress Disorder (PTSD). Given increasing use of medical cannabis among US military veterans to self-treat PTSD, there is strong public interest in whether cannabis may be a safe and effective treatment for PTSD.
Objective
The aim of the present study was to collect preliminary data on the safety and potential efficacy of three active concentrations of smoked cannabis (i.e., High THC = approximately 12% THC and < 0.05% CBD; High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD) compared to placebo in the treatment of PTSD among military veterans.
Methods
The study used a double-blind, cross-over design, where participants were randomly assigned to receive three weeks of either active treatment or placebo in Stage 1 (N = 80), and then were re-randomized after a 2-week washout period to receive one of the other three active treatments in Stage 2 (N = 74). The primary outcome measure was change in PTSD symptom severity from baseline to end of treatment in Stage 1.
Results
The study did not find a significant difference in change in PTSD symptom severity between the active cannabis concentrations and placebo by the end of Stage 1. All three active concentrations of smoked cannabis were generally well tolerated.
Conclusions and relevance
The present study is the first randomized placebo-controlled trial of smoked cannabis for PTSD. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment, but no active treatment statistically outperformed placebo in this brief, preliminary trial. Additional well-controlled and adequately powered studies with cannabis suitable for FDA drug development are needed to determine whether smoked cannabis improves symptoms of PTSD.
Trial registration
Identifier: NCT02759185; ClinicalTrials.gov.
Figures












Citation: Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. (2021) The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS ONE 16(3): e0246990. https://doi.org/10.1371/journal.pone.0246990
Editor: Bernard Le Foll, Centre for Addiction and Mental Health, CANADA
Received: February 11, 2020; Accepted: January 26, 2021; Published: March 17, 2021
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All non-identifiable, relevant data are currently attached in the Supporting Information files.
Funding: Authors BY, RD, AE, MB, PR, and SS received Grant Number: RFA#135, an award funded by the Colorado Department of Public Health and Environment (CDPHE): https://www.colorado.gov/pacific/cdphe/approved-medical-marijuana-research-grants The study was also partially funded by the sponsor, The Multidisciplinary Association for Psychedelic Studies (MAPS): https://maps.org/research/mmj/ The sponsor designed the protocol with input from from MB, SS, and PR. The sponsor monitored the data quality, conducted data analysis, contributed to decision to publish, and assisted with preparation of manuscript through critical review.
Competing interests: Author MBM is an employee of Canopy Growth Corporation, during which time he has received stock options, serves on the Board of Directors for AusCann Group Holdings Limited, was a prior employee of Zynerba Pharmaceuticals, and has received consulting fees from Tilray Inc. Author ML serves on the scientific advisory board for FSD Pharma and has received consulting fees from Greenwich Biosciences, Zynerba Pharmaceuticals, and Tilray Inc in the past two years. Authors RD, BY, JW, BS, CH, RM, and AE receive salary from the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) non-profit research and educational organization. Author SS receives salary from the Scottsdale Research Institute, which is a private LLC and has no shareholders. The Academic Editor, BLF, co-authored “The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda” (https://pubmed.ncbi.nlm.nih.gov/32360455/) with one of the authors, MBM. This article was a result of a meeting where a large number of investigators came together to discuss clinical trial outcomes with representative from NIH and FDA. No other relationship between this author and the Academic Editor exists. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Posttraumatic Stress Disorder (PTSD) is a serious, worldwide public health problem. In the United States the lifetime prevalence of PTSD in the general population is between 6 and 10% [1,2], and between 13 and 31% in US military veterans [2,3]. PTSD is typically a chronic condition [4,5], and is associated with high rates of psychiatric and medical co-morbidity, disability, suffering, and suicide [4,6–8]. Food and Drug Administration (FDA)-approved pharmacological treatments for PTSD are currently limited to two selective serotonin reuptake inhibitors (SSRIs): sertraline and paroxetine, which have significantly lower effect sizes (SMD between -.28 and -.56) compared to trauma-focused psychotherapy (SMD between -1.01 and -1.35) [9,10]. Indeed, the current Department of Defense (DoD) and Department of Veterans Affairs (VA) best practice guidelines for treatment of PTSD recommend psychotherapy over pharmacotherapy [11]. However, the majority of military veterans with PTSD who receive one of the best practices psychotherapies for PTSD, which were determined efficacious through clinical trials, do not remit or reduce symptoms below clinical thresholds by the end of treatment [12,13].
There is a strong public interest, particularly among Patients with PTSD, clinicians, and researchers, in whether cannabis can be an effective pharmacological treatment option for individuals with PTSD, or a safe alternative treatment for patients who do not respond to current front-line treatment. Cross-sectional and prospective studies document the widespread use of cannabis by individuals with PTSD [14,15]. Moreover, veterans with PTSD who do not show remission following standard treatment are more likely to use cannabis following completion of PTSD treatment [16]. Two recent prospective studies of patients using cannabis to self-treat PTSD provide evidence that whole plant cannabis can produce short [17] and long-term relief of PTSD symptoms [18].
There is some preclinical evidence that at least two of the active compounds in cannabis, delta-9-tetrahydrocannabinol (THC; the primary constituent responsible for intoxication from cannabis) and cannabidiol (CBD; one of the non-intoxicating cannabinoids in cannabis), can positively impact processes that underly PTSD pathology [19]. Specifically, administration of CBD in rats and mice dampens cue-elicited fear responses [20,21], while administration of THC and THC+CBD appears to block reconsolidation of fear memory [22]. Likewise, both THC and CBD when administered alone facilitate fear extinction learning [23,24], which is a critical component for recovery from PTSD [25,26]. This work suggests that THC and/or CBD could modify how patients with PTSD experience and respond to reminders of trauma.
In addition to cannabis’ potential to perhaps modify mechanisms that maintain the core psychopathology of PTSD, early phase clinical data on isolated cannabinoid constituents in humans suggest that active components of cannabis might provide acute relief from specific symptoms of PTSD. For example, two open-label studies and one randomized placebo controlled trial found that administration of low doses of a THC analogue led to improvements in self-reported subjective sleep quality, decreased frequency of nightmares, and improvements in self-reported overall well-being among those with PTSD [27–29].
While these data appear promising, the potential therapeutic effects of smoked, herbal cannabis on PTSD have not been examined in a randomized, placebo controlled trial. Military veterans with PTSD are overwhelmingly choosing smoked cannabis to self-treat PTSD and related conditions [30]. Moreover, herbal cannabis varies significantly across plants in its THC and CBD content [29]. While both cannabinoids could hold therapeutic value, unlike THC, CBD is non-intoxicating and does not carry significant risk of abuse [30]. In addition, CBD may temper the anxiogenic effects of THC in cannabis preparations that contain both CBD and THC [31,32]. It is unclear whether THC, CBD, or some combination of compounds may lead to greater reductions in PTSD symptoms with better safety profiles compared to other combinations. In addition, previous clinical studies rely entirely on standardized dosing, rather than test more naturalistic and generalizable ad libitum dosing regimens. This is a major limitation of previous research because there is substantial individual variability in cannabinoid tolerability [31]. Indeed, military veterans who use cannabis for PTSD tend to self-titrate to much larger doses than those tested in research studies [32,33].
The primary objective of the present study was to conduct a randomized placebo-controlled trial to assess the safety and potential efficacy of smoked, herbal cannabis for the treatment of PTSD in military veterans. Specifically, the study was designed to examine the independent effects of ad libitum use of up to 1.8 grams/day of three active preparations of smoked cannabis: (i) High THC, (ii) High CBD, and (iii) one-to-one ratio of THC and CBD (THC+CBD) against placebo on PTSD symptoms in a sample of veterans with PTSD.
Methods
Trial design
The trial protocol can be found at https://maps.org/research-archive/mmj/MJP1-Protocol-Amend4-oct-13-2015.pdf. The study received ethics approval from the Copernicus Group Independent Review Board (IRB) and was conducted in accordance with all local and Federal laws and regulations, including obtaining written informed consent from all study participants. The study included a randomized, double-blind, placebo-controlled, crossover trial of smoked cannabis containing three different concentrations of THC and CBD, and placebo. The cross-over design included two stages with four treatment groups in Stage 1 (High THC, High CBD, THC+CBD, and placebo) and re-randomization into three active treatment groups in Stage 2 (High THC, High CBD, and THC+CBD). The primary aim of the study was to determine whether change in PTSD symptom severity at the end of Stage 1 (primary study endpoint) differed by condition. The crossover design allowed for additional comparisons of within-subject and between-subject differences in safety and preliminary efficacy across the two Stages and allowed for assessment of participants’ preference for cannabis concentrations assigned in either Stage 1 vs. Stage 2. Each stage included three weeks of ad libitum use up to 1.8 grams/day of the assigned treatment followed by a two-week cessation period. This upper limit was necessary due to the outpatient setting for self-administration and the Schedule 1 controlled substance status of cannabis.
Primary outcome and safety assessments were conducted at baseline (visit 0), end of treatment in Stage 1 (visit 5; primary study endpoint), following the Stage 1 cessation period/Stage 2 baseline (visit 7), and end of treatment in Stage 2 (visit 12). Self-reported assessment of withdrawal symptoms was conducted at screening, baseline, and weekly during the two-week cessation periods following each stage of treatment (visits 6, 7, 13, 14). Secondary outcomes were assessed throughout the study before/after treatment and cessation periods.
Participants.
Study participants were recruited using community-based advertisements, presentations, and website advertisements. Study inclusion and exclusion criteria were as follows:
Inclusion Criteria. Individuals were eligible for study enrollment if they (1) were a US military veteran, (2) met DSM-5 (APA, 2013) criteria for PTSD with symptoms of at least six months in duration (index trauma did not have to be related to military service), (3) had PTSD of at least moderate severity based on a CAPS-5 score of = >25 at baseline assessment, (4) were at least 18 years of age, (5) reported they were willing and able to abstain from cannabis use two-weeks prior to baseline assessment, which would be verified by urine toxicology screens at screening and baseline, and agreed to abstain from using non-study cannabis during the trial, (6) were stable on any pre-study medications and/or psychotherapy prior to study entry, and (7) agreed to comply with study procedures.
Exclusion criteria. Study participants were excluded if they (1) were pregnant, nursing, or of child bearing potential and not practicing effective means of birth control, (2) had a current or past serious mental illness (e.g., personality disorder, psychotic disorder) determined by the SCID-5-RV [34], or self reported a positive family history (first-degree relative) of psychotic or bipolar disorder (3) were determined at high risk for suicide based on the C-SSRS [35], (4) had allergies to cannabis or other contraindication for smoking cannabis, (5) had a current diagnosis or evidence of significant or uncontrolled hematological, endocrine, cerebrovascular, cardiovascular, coronary, pulmonary, gastrointestinal, immunocompromising, or neurological disease, (6) met DSM-5 criteria for moderate-severe Cannabis Use Disorder on the CUDIT-R (= >11), (7) screened positive for any illicit substance other than cannabis during the two-week screening, or (7) were unable to provide informed consent.
Randomization and blinding.
The Stage 1 randomization list utilized blocks to ensure equal treatment assignments, and the Stage 2 randomization utilized multiple validated randomization lists that re-randomized participants in a blinded manner. The randomization procedure specified that participants would be randomized to treatment conditions using small block randomization in a 1:1:1:1 ratio in Stage 1 and then be re-randomized into two of the three active cannabis conditions (THC, CBD, THC+CBD) with a 1:1 ratio in Stage 2. Randomization in Stage 2 excluded the participant’s Stage 1 treatment condition. As placebo was not an option in Stage 2, placebo participants were randomized 1:1 between High THC and High CBD, but were not given the option to be randomized to THC + CBD in order to facilitate simpler programming of the web-based randomization system. This two-step randomization resulted in an unbalanced distribution of Stage 2 participants overall across active dose groups. In order to maintain the blind, a central electronic database was utilized for randomization based on validated computer-generated lists.
All study staff (with the exception of the Randomization Monitor and Drug Product Packaging Technician) and participants were blinded to condition assignments. The blind could only be broken for an individual participant if there was a clinically or medically urgent emergency requiring knowledge of the participant’s condition assignment. This emergency unblinding required approval from the site PI and Coordinating Investigator. Likewise, the unblinded Randomization Monitor could provide dose assignment through the electronic randomization system. Randomization information was only available within the web-based randomization system and only viewable by the designated Randomization Monitor.
Interventions.
Study drug was obtained from the National Institute on Drug Abuse (NIDA). Four concentrations of cannabis from NIDA included: High THC = approximately 12% THC and < 0.05% CBD); High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD. Samples of each batch were tested and confirmed for their concentration levels by an independent third-party analytical testing laboratory in Phoenix, Arizona. The independent testing lab found in two separate analyses that the High THC batch was just 9%, with the other batches very close to what was reported by NIDA.
At the beginning of each stage, participants were asked to visit the clinic site for four hours on two successive days and self-administer under supervision of study staff one dose of the cannabis preparation that they were randomly assigned to in that Stage. Vital signs for safety were collected during these visits (i.e., blood pressure, pulse). The study provided participants a total of 37.8 grams (1.8 grams/day)for the three-week ad libitum treatment period along with a metal pipe for treatment delivery (smoked). Participants were asked to refrain from using non-study cannabis, and return any remaining study cannabis that was not used each week. When study drug was returned the clinic team weighed the returned cannabis to calculate participants’ average use in grams per day during the treatment period in each stage. Participants were asked to refrain from any cannabis use during a two-week cessation period (between stages), then were re-randomized into one of three active treatment groups. All study participants were provided the option to enroll in an open label extension (Stage 3) with the cannabis of their choice in the same amount they returned unused in Stages 1 and 2 so participants had no disincentives to returning unused amounts. The results of Stage 3 are not reported here.
Demographic measures.
Baseline demographic information included age, sex, race/ethnicity, education, employment status. Other baseline measures included: whether the index trauma was combat-related, body mass index (BMI), risk for sleep apnea (STOP-bang) [36], and risk for cannabis use disorder (CUDIT-R) [37].
Safety measures.
Adverse Events (AEs) were assessed at baseline, during the introductory session, self-administration session, end of treatment, and before/after cessation in each stage by asking participants to self-report any side effects experienced over the past week. All AEs were coded by Systems Organ Class. The study physician then rated all AEs by severity (mild, moderate, severe) and study relatedness (i.e., possibly related, probably related, not related). AEs rated possibly related and probably related were collapsed into one “related” category.
Additional safety measures included the 15-item Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) and the Columbia-Suicide Severity Rating Scale (CSSR-S) (Posner et al., 2011). The MWC was administered at screening, baseline, and each week following cessation of Stages 1 and 2 (visits 6, 7, 13, 14). The CSSR-S was self-administered at all study visits.
Outcome measures.
The primary outcome of the current study was change in PTSD symptom severity from baseline (visit 0) to end of the three-week treatment period in Stage 1 (visit 5) using the Clinician-Administered PTSD Scale for DSM-5 Total Severity Score (CAPS-5) [38]. The CAPS-5 is a semi-structured clinician interview, and is well-validated for determining PTSD diagnoses consistent with the Diagnostic and Statistical Manual of Mental Disorders, Version 5 (DSM-5) and assessing change in symptom severity over time [39]. PTSD diagnosis is based on meeting the DSM-5 symptom cluster criteria (minimum threshold of symptoms with a score ≥ 2) with a qualifying criterion A index trauma. The CAPS-5 Total Severity Score is calculated by summing the total score for each of the four symptom categories to assess past-month PTSD symptoms on a specific traumatic event: intrusion (Category B), Avoidance (Category C), Mood and Cognition (Category D), and Hyperarousal (Category E). CAPS-5 Total Severity scores range from 0–80, where higher scores indicate worse PTSD severity.
Secondary outcome measures included a modified version of the 20-item self-report PTSD Checklist for DSM-5 (PCL-5) [40], which was changed to assess for past week symptoms, the 20-item general depression subscale and 5-item anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms’ (IDAS) [41], the 80-item self-report Inventory of Psychosocial Functioning (IPF) [42], and the 7-item self-report Insomnia Severity Index (ISI) [43]. Secondary outcome measures were collected at baseline (visit 0 and visit 7), self-administration (visit 4 and visit 10), before cessation (visit 6 and visit 13), and after cessation (visit 7 and visit 14) in both Stage 1 and Stage 2. Total and subscale scores were calculated for each measure.
Other measures.
The validity of study blinding to active or inactive treatment in Stage 1 was assessed by asking participants and clinicians to independently guess whether the participant was randomized to an active (High THC, High CBD, THC+CBD) or inactive (placebo) treatment group at the end of Stage 1. At the end of Stage 2, participants were asked whether they preferred the treatment to which they were assigned in Stage 1 or Stage 2.
Table 1 includes a summary of all assessments by visit.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 1. Summary of assessments by visit.
https://doi.org/10.1371/journal.pone.0246990.t001
Study power.
The primary study aim was to gather preliminary data on the safety and potential efficacy of different cannabis preparations to treat PTSD among veterans. In the absence of published effect sizes for the impact of THC, CBD, or THC+CBD on CAPS-5 scores, the target sample size was chosen to allow detection of an effect size of 0.4 or greater (small to medium effect) based on between group differences in the primary outcome measure (i.e., change in total CAPS-5 severity score from baseline to the end of Stage 1 active treatment phase). Power analysis suggested that 76 completing participants (n = 19 per group) would be needed to detect an effect size of d = 0.4 at 82% power and .05 significance level. Enrollment and randomization continued until 76 participants completed the Stage 1 outcome assessment. Eighty participants were enrolled and 76 partcipants completed Stage 1.
Statistical analyses.
Descriptive statistics were performed to test the normality of baseline measures on the total study sample and across each treatment group to ensure adequate randomization. Means, medians, and frequencies were calculated, and within-subject and between-group differences were tested for categorical variables using chi-square tests and t-tests or analysis of variance (ANOVA) for continuous variables.
Safety was analyzed by tabulating the frequency, severity, and relatedness to treatment of AEs. A Chi-square test was used to assess for differences in frequency of AEs across groups. An AE was counted once per subject for each assessment period.
The primary outcome was analyzed using ANOVA to test for between-group differences in change in Total PTSD Severity scores from baseline to end of treatment in Stage 1 (CAPS-5 visits 0 and 7). Secondary outcomes were analyzed using a series of additional ANOVAs to test for between-group differences in change scores from baseline to end of treatment for Stage 1 (CAPS-5 visits 0 and 7; secondary measures visits 0 and 6) and Stage 2 (CAPS-5 visits 7 and 12; secondary measures visits 7 and 13). All dependent variables were tested for normality, and summarized by both mean and median values by group. Within-subject change scores were tested for each treatment group using a series of t-tests. Tukey’s pairwise comparisons were used to test for group differences in change scores between all pairs of treatment conditions in Stage 1 and Stage 2. Analyses were conducted consistent with an intent-to-treat (ITT) framework, where all available data from randomized participants who received at least one week’s supply of study drug (N = 80) were summarized for baseline characteristics and entered into the models. However, the use of ANOVA tests only allowed for analysis of change in participants who completed outcome assessments (N = 76 for primary outcome analysis).
Results
Sample characteristics
A total of 261 individuals completed screening and 51% met eligibility criteria for study inclusion. Eighty participants were enrolled and randomized into one of four treatment groups (n = 20 per group), of which 76 participants completed the Stage 1 outcome assessment. In Stage 2, a total of 74 participants were re-randomized into High THC (n = 29), High CBD (n = 27), or THC+CBD (n = 18). There were no significant Stage 1 treatment assignment differences in demographics or baseline scores on the primary and secondary outcome variables (i.e., CAPS-5, PCL-5, IDAS Social Anxiety, IDAS Depression, IPF, and ISI). Sample demographics and baseline characteristics are summarized in Table 2.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 2. Baseline characteristics by treatment group.
https://doi.org/10.1371/journal.pone.0246990.t002
Treatment adherence and attrition
Rates of engagement and completion are summarized in the Consort Diagram (Fig 1). During Stage 1, 3 participants (3.8%) did not complete endpoint outcome assessments. After Stage 1, 6 participants (7.5%) did not continue into Stage 2. Of the 74 participants who were re-randomized into Stage 2, 3 (4.1%) discontinued treatment due to an AE, and 7 total (9.5%) did not complete Stage 2 endpoint outcome assessments. The overall attrition rate for the percent of randomized participants who dropped out before completing Stage 2 outcome assessments, was 16.3%.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 1. Consort flow diagram.
https://doi.org/10.1371/journal.pone.0246990.g001
Cannabis use in grams
In Stage 1, there was no statistically significant difference between groups in total grams of smoked cannabis/placebo during the three-week treatment period (21 days) across the treatment groups (F [3, 71] = 2.23, p = .09). Mean (SD) grams of smoked cannabis/placebo used by each treatment group in Stage 1 were as follows: placebo (M = 8.4, SD = 10.1), High THC (M = 14.6, SD = 10.4), High CBD (M = 14.3, SD = 13.0), THC+CBD (M = 8.2, 6.8).
In stage 2, there was a significant group difference in total grams of smoked cannabis (F [2, 64] = 3.42, p = .04), such that participants in the THC+CBD group used significantly more cannabis (M = 17.6, SD = 10.6), compared to participants randomized to High THC (M = 10.7, SD = 10.9), or High CBD (M = 9.3, SD = 10.5).
Assessment of study blind
In Stage 1, 60% of placebo participants accurately guessed assignment to an inactive treatment, 58% of High CBD participants accurately guessed that they were in an active condition, and 100% of participants in the High THC and THC+CBD groups accurately guessed assignment into an active treatment condition. Similar results were found for clinicians. In Stage 1, forty-five percent of clinicians accurately guessed placebo participants’ assignment in an inactive treatment, 16% accurately guessed High CBD participants’ assignment into an active treatment, and 100% accurately guessed that participants assigned to High THC or THC+CBD were randomized into an active treatment. Therefore, the study blind was appropriately upheld only when participants were assigned to High CBD or placebo conditions, but was not upheld when participants were assigned to High THC or High THC/CBD.
Treatment preference
At the end of Stage 2, participants who completed final assessments (n = 74) indicated their preference for either their blinded Stage 1 or Stage 2 treatment assignment. Twenty-five participants (34%) indicated a preference for a Stage 1 or Stage 2 assignment to High THC, 10 participants (13%) indicated a preference for a Stage 1 or Stage 2 assignment to High CBD, 26 participants (35%) indicated a preference for a Stage 1 or Stage 2 assignment to THC+CBD, and 4 participants (5%) indicated a preference for a Stage 1 assignment to placebo. Two participants (3%) equally preferred their Stage 1 and Stage 2 treatment assignments.
Safety outcomes
Adverse events.
All Adverse Events (AEs) reported during Stage 1 are summarized in Table 3. Number of participants who reported at least one AE did not significantly differ by treatment group in either Stage 1 (p = .38) or Stage 2 (p = .27). Thirty-seven of 60 participants who received THC, CBD, or THC+CBD during Stage 1 (61.7%) reported at least one treatment-related AE by the end of Stage 1. In Stage 2, Forty-five of the 74 participants who received THC, CBD, or THC+CBD (60.8%) reported at least one treatment-related AE during Stage 2. Three of 80 participants (3.8%) reported an unrelated Serious Adverse Event (SAE) during the study, specifically heart palpitations (n = 1; THC+CBD, Stage 1 cessation period), pulmonary embolism (n = 1, High THC, Stage 2), and abscess (n = 1, High CBD, Stage 2). One participant (THC+CBD) discontinued treatment during the introductory session in Stage 1 due to an AE, and two participants discontinued treatment during the introductory session in Stage 2 due to an AE (High CBD and High THC conditions). Across both Stages, 13 total participants terminated from the study early due to an AE (8.4%). The most common AEs reported (i.e., those with >10% frequency) were cough (12.3%), followed by throat irritation (11.7%) and anxiety (10.4%). Emergency unblinding was never used in the study.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 3. Number of participants with adverse events by systems organ class/preferred terms and treatment-relatedness.
https://doi.org/10.1371/journal.pone.0246990.t003
One participant who received CBD in Stage 1 (5.0%) reported treatment-related suicidal ideation. One participant from each treatment condition (3.6% – 5.9%) reported treatment-related suicidal ideation in Stage 2.
Cannabis withdrawal symptoms.
Fig 2 summarizes mean withdrawal symptom scores on the MWC by group at Stage 1 and Stage 2 baseline, end of treatment, and following 1-week of cessation. All treatment groups reported mean withdrawal symptoms in the moderate range (Mean score = 32–38) at baseline assessment (prior to initiating treatment in Stage 1). All treatment groups showed a significant reduction in withdrawal symptoms from baseline to the end of the treatment phase of Stage 1. Only participants assigned to High THC in Stage 1 reported a significant increase in mean self-reported withdrawal symptoms after one week of cessation from the assigned treatment in Stage 1 (Δ = 12.6, SD = 11.41, p = .0004). There was no significant change in withdrawal symptoms from the end of Stage 2 treatment to one-week follow-up.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 2. Mean total marijuana withdrawal scores by treatment condition across Stage 1 & Stage 2.
https://doi.org/10.1371/journal.pone.0246990.g002
Primary efficacy outcome
PTSD symptom severity (CAPS-5).
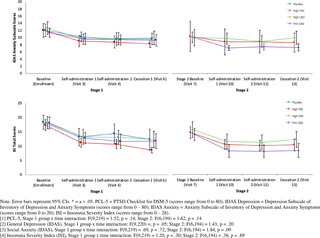
Results of the analysis of change in total PTSD symptom severity on the CAPS-5 are summarized in Table 4 for both Stage 1 (primary outcome) and Stage 2. In Stage 1, there was no significant between-group difference in CAPS-5 Total Severity scores between treatment groups [F(3, 73) = 1.85, p = .15]. All four treatment groups, including placebo, achieved significant within-subject reductions in total CAPS-5 Total Severity scores from Stage 1 baseline (visit 0) to end of treatment (visit 5). Specifically, participants who received placebo in Stage 1 reported a mean reduction of 13.1 points (SD = 12.10, p < .001, d = -1.30), participants who received High THC reported a mean reduction of 15.2 points (SD = 11.3, p < .0001, d = -1.99), High CBD participants reported a mean reduction of 8.4 points (SD = 10.09, p < .05, d = -.79), and THC+CBD participants reported a mean reduction of 8.5 points (SD = 9.88, p < .05, d = -.83).

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 4. Mean (SD)/Median (IQR) and analysis of change in CAPS-5 total severity scores by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t004
Secondary efficacy outcomes
The results of the study’s secondary efficacy outcomes are summarized in Table 5.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 5. Mean (SD)/Median (IQR) and analysis of group change in PCL-5, IDAS social anxiety, IDAS general depression, IPF, and ISI by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t005
Self-reported PTSD symptoms, PCL-5.
In Stage 1, there was no significant difference in PCL-5 change scores between treatment groups from baseline to end of Stage 1.
In Stage 2, mean change in PCL-5 scores significantly differed by treatment condition [F(2, 63) = 4.06, p = .02]. Specifically, there was a significant difference between High CBD and THC+CBD in PCL-5 change scores, with participants who received THC+CBD reporting greater reductions in PTSD symptoms on the PCL-5 (Δ = -16.4, SD = 16.0, p < .001, d = -1.43) compared to participants who received High CBD (Δ = -9.1, SD = 11.0, p = .02, d = -.67).
IDAS general depression & social anxiety subscales.
In Stage 1, there were no significant differences between treatment conditions in either change in IDAS General Depression or IDAS Social Anxiety scores.
In Stage 2, treatment groups significantly differed in IDAS Social Anxiety mean change scores [F(2, 63) = -3.08, p = .05] and IDAS General Depression mean change scores [F(2, 63) = 3.76, p = .03]. Specifically, participants in the THC+CBD condition in Stage 2 reported significant pre-post reductions in IDAS Social Anxiety scores (Δ = – 2.8, SD = 3.90, p = .04, d = -.70), and participants in the High THC (Δ = -9.0, SD = 11.1, p < .01, d = -.90) and THC+CBD treatment conditions (Δ = -13.4, SD = 10.0, p < .0001, d = -1.68) reported significant reductions in IDAS General Depression scores in Stage 2.
ISI insomnia.
In Stage 1, there was no significant difference between treatment conditions in mean change in total insomnia symptoms on the ISI.
In Stage 2, there was no significant difference between treatment groups in mean change scores in total insomnia symptoms on the ISI.
Psychosocial functioning, IPF.
In Stage 1, there was no significant between-group difference in mean in overall psychosocial functioning (IPF total score).
In Stage 2, there was no significant difference between treatment conditions in IPF mean change scores.
Posthoc analysis
CAPS-5 subscale scores B, C, D, and E.
As a follow-up to the primary outcome analysis, posthoc analyses were conducted to test the effects of treatment group on change in each of the primary symptom domains of PTSD (i.e., intrusions, avoidance, negative thoughts and emotions, hyperarousal) using the CAPS-5 B, C, D, and E subscale scores. The posthoc analysis for subscale scores used the same analytic approach as the analysis for primary and secondary outcomes.
PCL-5, IDAS social anxiety, IDAS general depression, and ISI longitudinal analysis.
Mixed-models for repeated measures (MMRM) were computed to test for group differences over time for all secondary outcome assessments that were measured at more than two time points within each stage. The use of MMRM models allowed all randomized participants’ data to be analyzed within the models based on the missing-at-random assumption (MAR).
Posthoc results.
In Stage 1, there was no significant difference between groups in mean change for any of the subscale scores on the CAPS-5 [Subscale B, F(3,73) = 1.58, p = .20; Subscale C, F(3,73) = 1.06, p = .37; Subscale D, F(3,73) = 2.26, p = .09; Subscale E, F(3,73) = .84, p = .48].
In Stage 2, there was a significant difference between groups in mean change on the CAPS-5 C (avoidance) [F(2,64) = 4.95, p = .01] and CAPS-5 D (negative thoughts and emotions) [F(2,64) = 8.60, p < .001] subscales. Specifically, there was a significant difference between participants who received High CBD and THC+CBD in mean change in CAPS-5 C (avoidance) subscale scores (Δ = 1.9, 95% CI: 0.38, 3.35, d = -.97) and CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 5.2, 95% CI = 2.04, 8.26, d = -1.13), and between High THC and THC+CBD group participants in mean change in CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 4.1, 95% CI: 1.09, 7.15, d = -1.01). In Stage 2, there were no significant differences between groups in mean change on the CAPS-5 B (intrusions) [F(2,64) = 2.80, p = .07] or CAPS-5 E [F(2,64) = 1.13, p = .33] (hyperarousal) Subscales.
Results of the MMRM analyses testing group differences over time in PCL-5, IDAS Social Anxiety, IDAS General Depression, and ISI appear in Fig 3.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 3. Post hoc Mixed Models for Repeated Measures (MMRM) testing change in PCL-5, IDAS depression, IDAS anxiety, and ISI scores over time.
https://doi.org/10.1371/journal.pone.0246990.g003
Only IDAS Social Anxiety scores in Stage 2 had a significant time x treatment effect, such that social anxiety showed a quadratic drop in Stage 2 among those in the THC+CBD conditions and those in the High THC and High CBD conditions did not show change over time. All other models failed to find a significant difference between groups over time.
Discussion
The present study served as the first randomized placebo-controlled trial of smoked cannabis for symptoms of PTSD in US military veterans. Study-related AEs were generally mild to moderate, and did not significantly differ by treatment condition. The study failed, however, to find a significant effect of treatment condition on the primary efficacy outcome, change in total PTSD severity on the CAPS-5 from baseline to end of Stage 1. All treatment groups (placebo, High CBD, High THC, THC+CBD) achieved statistically significant reductions in PTSD severity on the CAPS-5 in Stage 1, with effect sizes for change in mean PTSD severity ranging between d = .83 (High CBD) and d = 1.34 (High THC). These effect sizes are much larger than effect sizes reported for symptom change in other psychopharmacology trials for PTSD. For example, a 2018 meta-analysis reported standardized mean differences between .33 and .97 across PTSD pharmacology trials. The average length of trials reported in the 2018 meta-analysis lasted approximately ten weeks, whereas the current trial’s primary endpoint was evaluated after only three weeks of treatment.
The study’s failure to detect a significant difference between groups in Stage 1 could perhaps be explained by several confounding factors. First, the study sample included participants with a history of cannabis use. The recruitment of active cannabis users might have increased the potential for biased responding. Given the topical nature of the current trial and its relevance for public policy on medical cannabis, participants might have been biased to report positive effects regardless of condition. Despite many participants already having experience with the drug, nearly half of those receiving placebo believed that they received active cannabis. Prior expectations about cannabis’ effects might explain why even those in the placebo condition reported larger than average reductions in PTSD symptoms after only 3 weeks of treatment.
Second, many participants reported significant cannabis withdrawal symptoms at the time of randomization and early in Stage 1. Nearly half of the study sample (43%, n = 34) were positive for THC at study screening and 23% (n = 18) remained positive for THC at Stage 1 baseline, which would suggest chronicity of previous use or continued cannabis use during the two week washout from screening to baseline (lack of compliance with two week abstinence inclusion criteria). Total cannabis withdrawal symptoms averaged in the moderate range for all treatment groups at the start of Stage 1 (despite two weeks of abstinence prior to randomization), then generally reduced to the mild to moderate range by the end of treatment in Stage 1. Participants who received High THC in Stage 1 reported a significant increase in withdrawal following one week of cessation from Stage 1 treatment, which averaged in the moderate range following cessation. While groups did not differ in cannabis withdrawal ratings, the presence of withdrawal and trends in change could confound (or help explain) interpretation of results. We cannot rule out that cessation of cannabis use (in the placebo condition) or reversal of withdrawal (in the THC and THC+CBD conditions) might have partially been responsible for significant within-subjects change. Moreover, participants randomly assigned to receive High THC in Stage 1 had CUDIT total scores (indicating cannabis use disorder risk) nearly two times greater than participants who were assigned to other active treatment conditions. This is a major confound and limitation of the current study.
Third, total exposure to smoked cannabis was lower than anticipated and might not equate to a full therapeutic dose. In Stage 1, cannabis use in grams ranged from 8.2g (THC+CBD) to 14.6g (high THC) over three weeks (.39g/day to .69g/day on average), despite all participants having access to up to 37.8g over the three week period (1.8g/day). Average cannabis quantity that most cannabis users consume is difficult to estimate from epidemiological studies due to differences in cannabis potency and route of administration. However, large scale studies of medicinal cannabis users treating chronic pain and anxiety report average daily use in ranges closer to 1-3g/day [44,45]. Likewise, two smaller studies that assessed military veterans who use medical cannabis to self-treat PTSD reported median daily use of .85g to 1.14g/day [46] and average use of 3.8g/day [33]. We suspect that participants might have used less cannabis in the current study because of differences between the cannabis available for research trials and the quality of cannabis sold commercially. Several participants spontaneously reported to study staff that the smoke from the cannabis that was provided was “harsher” than they were used to. This difference might also be attributable to the two-week cessation periods mitigating tolerance.
Finally, the present study did not include a placebo arm in Stage 2, which limited the analyses that could be employed in order to take advantage of the crossover design. This significantly limited power to find significant differences across groups. The unexpectedly large response to placebo (d = 1.30), coupled with the small sample size per condition, meant that the current study was underpowered to detect significant differentiation from placebo. If the very large placebo response observed in this study remains consistent in future studies, a trial that tests only one preparation of cannabis (e.g., only high THC cannabis) against placebo would still need a total sample size of nearly one thousand participants (n = 479 per group) to achieve a statistically significant result. However, including a placebo run-in stage to identify and exclude placebo responders in any future trials could substantially reduce this total sample size [47].
Despite these limitations, there were several notable findings. While the study’s primary outcome assessment failed to find differentiation from placebo in PTSD symptom change on the CAPS-5, all participants showed a positive response to treatment in a very brief time period. In addition, side effects were general mild and transient. One of the largest concerns from providers regarding self-treatment of PTSD with cannabis is that it may exacerbate PTSD symptoms. While the current study’s treatment duration was too brief to identify long-term risk, two-tailed significance tests did not show evidence of symptom exacerbation in any condition. These data provide preliminary evidence of safety of short-term ad libitum cannabis use in this population.
While Stage 2 results should be interpreted with caution given the possible carry-over effects and unbalanced randomization across active dose groups, the study did identify some statistically significant differentiation between groups when particiants were re-randomized to only the three active conditions. Given that Stage 2 THC+CBD participants consisted of only those who received active treatment in Stage 1, this cohort might be providing a window into the effects of slightly longer active cannabis treatment. The positive response to treatment evidenced by participants receiving THC+CBD in Stage 2 suggest that it is possible that a longer active treatment period might be necessary to achieve treatment gains that could outperform placebo. It is equally plausible, however, that greater reductions in CAPS-5 severity scores in Stage 2 among THC and THC+CBD treatment groups were due to attenuation of cannabis withdrawal symptoms, as all treatment groups experienced a 2-week cessation period between Stage 1 and Stage 2. This effect would be consistent with prior literature suggesting that symptoms of cannabis use disorder (CUD) can interfere in successful recovery from PTSD [48].
The current study is unique, in that it trialed whole plant cannabis preparations, rather than single molecule extracts or synthetic pharmaceutical cannabinoids. In addition to reporting change in structured assessments of symptoms, the study results provide critical information about participant preference for cannabinoid preparations when exposed to different whole plant THC and CBD ratios. Consistent with previous work [46], participants in the current study reported a general preference for cannabis types that included significant quantities of THC. This effect might be explained by a signal of efficacy, or simply due to the intoxicating and reinforcing effects of high THC cannabis. Nevertheless, the demand for testing cannabis as a therapeutic for PTSD has largely been driven by military veteran advocacy groups, and yet development of cannabinoid therapeutics has not focused on trialing cannabis preparations that military veterans currently use to self-treat their symptoms. Input from stakeholders on which cannabinoids to trial as cannabis-based medicine for military veteran-specific conditions is certainly justified.
Results from the current trial provide invaluable information for future cannabinoid trials for PTSD. While the null findings raise questions about the utility of continuing to trial whole plant cannabis for the treatment of PTSD, the study found that whole plant, smoked cannabis was generally well tolerated and did not lead to deleterious effects in most participants after 3 weeks of ad libitum use. These safety results are consistent with recent real-world evidence studies of whole plant cannabis for PTSD [17,18]. However, those studies both found evidence of potential efficacy as well. Given that many veterans with PTSD are already using cannabis to self-treat their symptoms, identifying which preparations and with which method of administration are most beneficial and/or are least harmful is critical. These future studies will need to take active steps to ensure appropriate blinding despite the intoxicating nature of the drug. While the CBD and placebo conditions were appropriately blinded in the current study, participants and assessors could accurately guess condition when participants received THC-based treatments. Enrolling novice or naïve users, or limiting total THC content to sub-intoxicating doses could improve these blinding issues. Conversely, if higher THC doses are indeed therapeutic, other designs that are less reliant on active blinding, such as randomized withdrawal, might be warranted. Researchers should also consider including objective surrogate endpoint assessments (e.g., physiology, biological specimens, neuroimaging) as secondary indicators of treatment response. As none of these have been validated for a PTSD population as surrogate endpoints, the present study and future studies must utilize the “gold-standard” semi-structured clinical interview for PTSD severity, which is the CAPS-5.
Future studies would also likely benefit from a longer treatment period similar to more traditional medication trials (e.g., 12-weeks), and if including a placebo comparator should plan for a potentially larger than normal placebo response. To mitigate placebo, researchers might consider: 1) including a placebo run-in period prior to randomization to attempt to identify placebo responders, 2) powering the trial for a potentially larger than typical placebo effect, and/or 3) excluding participants with strong apriori beliefs about cannabis’ therapeutic effects [e.g., prescreening with expectancy measures, such as Devilly & Borkovec’s Credibility/Expectancy Questionnaire (CEQ)]. For generalizability to female veterans and civilians with PTSD, these studies should also attempt to recruit a greater number of female participants, as the current study’s sample was overwhelmingly male. Finally, future studies would also greatly benefit from access to high quality flower cannabis. The flower preparations provided through the NIDA drug supply program include all parts of the plant (instead of just buds) and are only available in specific cannabinoid ratios. Studies that test high quality buds in various phenotypes with variable potencies of cannabinoids and terpene ratios would more closely mirror what is available within state-sponsored medical cannabis programs.
Conclusions
The present study failed to find a significant group difference between smoked cannabis preparations containing High CBD, High THC, and THC+CBD against placebo in regards to their impact on PTSD symptoms. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment. The failure to differentiate treatment groups from placebo is likely attributable to the higher than average treatment response in the placebo condition and to the shorter than average duration of treatment. Higher powered studies that attempt to mitigate the effect of pronounced placebo appear warranted.
Supporting information
S1 Checklist –
Showing 1/15: pone.0246990.s001.DS_Storesorry, we can’t preview this file1 / 15figshare
S1 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s001
(DS_STORE)
S2 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s002
(DOC)
S1 File.
https://doi.org/10.1371/journal.pone.0246990.s003
(CSV)
S2 File.
https://doi.org/10.1371/journal.pone.0246990.s004
(TXT)
S3 File.
https://doi.org/10.1371/journal.pone.0246990.s005
(TXT)
S4 File.
https://doi.org/10.1371/journal.pone.0246990.s006
(TXT)
S5 File.
https://doi.org/10.1371/journal.pone.0246990.s007
(TXT)
S6 File.
https://doi.org/10.1371/journal.pone.0246990.s008
(TXT)
S7 File.
https://doi.org/10.1371/journal.pone.0246990.s009
(TXT)
S8 File.
https://doi.org/10.1371/journal.pone.0246990.s010
(TXT)
S9 File.
https://doi.org/10.1371/journal.pone.0246990.s011
(TXT)
S10 File.
https://doi.org/10.1371/journal.pone.0246990.s012
(TXT)
S11 File.
https://doi.org/10.1371/journal.pone.0246990.s013
(TXT)
S12 File.
https://doi.org/10.1371/journal.pone.0246990.s014
(XLSX)
S13 File.
https://doi.org/10.1371/journal.pone.0246990.s015
(PDF)
Acknowledgments
We thank Scott Hamilton and Ilsa Jerome for their assistance in quality checking all of the study data.
References
1. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder. Curr Opin Psychiatry. 2015;28: 307–311. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
2. Gradus JL. Epidemiology of PTSD. In: National Center for PTSD. 2014.
3. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28: 307–11. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
4. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62: 617. pmid:15939839
View Article
PubMed/NCBI
Google Scholar
5. Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149: 671–675. pmid:1575259
View Article
PubMed/NCBI
Google Scholar
6. Perkonigg A, Kessler RC, Storz S, Wittchen H-U. Traumatic events and post-traumatic stress disorder in the community: prevalence,risk factors and comorbidity. Acta Psychiatr Scand. 2000;101: 46–59. pmid:10674950
View Article
PubMed/NCBI
Google Scholar
7. Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62 Suppl 17: 16–22. Available: http://www.ncbi.nlm.nih.gov/pubmed/11495091.
View Article
Google Scholar
8. Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro III A, Parker VA, et al. Burden of Medical Illness in Women With Depression and Posttraumatic Stress Disorder. Arch Intern Med. 2004;164: 1306. pmid:15226164
View Article
PubMed/NCBI
Google Scholar
9. Merz J, Schwarzer G, Gerger H. Comparative Efficacy and Acceptability of Pharmacological, Psychotherapeutic, and Combination Treatments in Adults With Posttraumatic Stress Disorder. JAMA Psychiatry. 2019;76: 904. pmid:31188399
View Article
PubMed/NCBI
Google Scholar
10. Forman-Hoffman V, Middleton JC, Feltner C, Gaynes BN, Weber RP, Bann C, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update. 2018 [cited 11 Oct 2019]. Available: https://www.ncbi.nlm.nih.gov/books/NBK525132/.
View Article
Google Scholar
11. Management of Post-Traumatic Stress Working Group. VA/DOD clinical practice guideline for management of post-traumatic stress. 2017.
12. Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67: 194–200. Available: http://www.ncbi.nlm.nih.gov/pubmed/10224729. pmid:10224729
View Article
PubMed/NCBI
Google Scholar
13. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consult Clin Psychol. 1992;60: 748–56. Available: http://www.ncbi.nlm.nih.gov/pubmed/1401390. pmid:1401390
View Article
PubMed/NCBI
Google Scholar
14. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153: 369–375. pmid:8610824
View Article
PubMed/NCBI
Google Scholar
15. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136: 162–165. pmid:24412475
View Article
PubMed/NCBI
Google Scholar
16. Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychol Addict Behav. 2011;25: 485–491. pmid:21261407
View Article
PubMed/NCBI
Google Scholar
17. LaFrance EM, Glodosky NC, Bonn-Miller M, Cuttler C. Short and Long-Term Effects of Cannabis on Symptoms of Post-Traumatic Stress Disorder. J Affect Disord. 2020;274: 298–304. pmid:32469819
View Article
PubMed/NCBI
Google Scholar
18. Bonn-Miller MO, Brunstetter M, Simonian A, Loflin MJ, Vandrey R, Babson KA, et al. The Long-Term, Prospective, Therapeutic Impact of Cannabis on Post-traumatic Stress Disorder. Cannabis Cannabinoid Res. 2020.
View Article
Google Scholar
19. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14. pmid:28813324
View Article
PubMed/NCBI
Google Scholar
20. Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. 2010;207: 105–111. pmid:19800921
View Article
PubMed/NCBI
Google Scholar
21. Gomes F V, Reis DG, Alves FH, Corrêa FM, Guimarães FS, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT 1A receptors. J Psychopharmacol. 2012;26: 104–113. pmid:21148020
View Article
PubMed/NCBI
Google Scholar
22. Stern CAJ, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25: 958–965. pmid:25799920
View Article
PubMed/NCBI
Google Scholar
23. Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226: 781–792. pmid:23307069
View Article
PubMed/NCBI
Google Scholar
24. Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64: 396–402. pmid:22796109
View Article
PubMed/NCBI
Google Scholar
25. Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36: 23–31. pmid:23260015
View Article
PubMed/NCBI
Google Scholar
26. Yehuda R, LeDoux J. Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron. 2007;56: 19–32. pmid:17920012
View Article
PubMed/NCBI
Google Scholar
27. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15: 84–88. pmid:19228182
View Article
PubMed/NCBI
Google Scholar
28. Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51: 585–588. pmid:25467221
View Article
PubMed/NCBI
Google Scholar
29. Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, Open-Label, Pilot Study of Add-On Oral Δ9-Tetrahydrocannabinol in Chronic Post-Traumatic Stress Disorder. Clin Drug Investig. 2014;34: 587–591. pmid:24935052
View Article
PubMed/NCBI
Google Scholar
30. Loflin M, Babson K, Sottile J, Norman S, Gruber S, Bonn-Miller M. A Cross-Sectional Examination of Choice and Behavior of Veterans with Access to Free Medicinal Cannabis. Am J Drug Alcohol Abuse. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
31. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. European Journal of Internal Medicine. Elsevier B.V.; 2018. pp. 12–19. pmid:29307505
View Article
PubMed/NCBI
Google Scholar
32. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
33. Loflin M, Earleywine M, Bonn-Miller M. Medicinal versus recreational cannabis use: Patterns of cannabis use, alcohol use, and cued-arousal among veterans who screen positive for PTSD. Addict Behav. 2017;68. pmid:28088054
View Article
PubMed/NCBI
Google Scholar
34. First MB, Williams JBW, Karg RSK, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA Am Psychiatr Assoc. 2015. pmid:32821383
View Article
PubMed/NCBI
Google Scholar
35. Posner K, Brent D, Lucas C, Gould M, Stanley B., Brown G., et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008.
36. Shahid A, Wilkinson K, Marcu S, Shapiro CM. STOP-Bang Questionnaire. STOP, THAT and One Hundred Other Sleep Scales. Springer New York; 2011. pp. 371–383. https://doi.org/10.1007/978-1-4419-9893-4_92
37. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110: 137–143. pmid:20347232
View Article
PubMed/NCBI
Google Scholar
38. Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). [Assessment]. 2013.
View Article
Google Scholar
39. Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30: 383–395. pmid:28493729
View Article
PubMed/NCBI
Google Scholar
40. Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28: 1379–1391. pmid:26653052
View Article
PubMed/NCBI
Google Scholar
41. Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 2007;19: 253–268. pmid:17845118
View Article
PubMed/NCBI
Google Scholar
42. Bovin MJ, Black SK, Rodriguez P, Lunney CA, Kleiman SE, Weathers FW, et al. Development and validation of a measure of PTSD-related psychosocial functional impairment: The Inventory of Psychosocial Functioning. Psychol Serv. 2018;15: 216–229. pmid:29723024
View Article
PubMed/NCBI
Google Scholar
43. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34: 601–8. pmid:21532953
View Article
PubMed/NCBI
Google Scholar
44. Turna J, Simpson W, Patterson B, Lucas P, Van Ameringen M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J Psychiatr Res. 2019;111: 134–139. pmid:30738930
View Article
PubMed/NCBI
Google Scholar
45. Ware MA, Wang T, Shapiro S, Collet JP. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain. 2015;16: 1233–1242. pmid:26385201
View Article
PubMed/NCBI
Google Scholar
46. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
47. Lee S, Walker JR, Jakul L, Sexton K. Does elimination of placebo responders in a placebo run-in increase the treatment effect in randomized clinical trials? A meta-analytic evaluation. Depress Anxiety. 2004;19: 10–19. pmid:14978780
View Article
PubMed/NCBI
Google Scholar
48. Bonn-Miller MO, Boden MT, Vujanovic A a., Drescher KD. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychol Trauma Theory, Res Pract Policy. 2011;5: 193–200.
View Article
Google Scholar
Curated by Thc 420 Hemp
Via https://weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/
source https://lauragfollett.weebly.com/blog/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial
0 notes
Text
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
By: Jason Karimi, WeedPress Contributor Title: The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial Sourced From: weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/ Published Date: Sun, 21 Mar 2021 23:04:30 +0000
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0246990
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin ,
Benjamin Shechet,
Colin Hennigan,
Rebecca Matthews,
Amy Emerson,
Rick Doblin
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin,
Benjamin Shechet, …

x
Published: March 17, 2021
https://doi.org/10.1371/journal.pone.0246990
Article
Authors
Metrics
Comments
Media Coverage
Peer Review
Abstract
Introduction
Methods
Results
Discussion
Conclusions
Supporting information
Acknowledgments
References
Reader Comments (0)
Figures
Abstract
Importance
There is a pressing need for development of novel pharmacology for the treatment of Posttraumatic Stress Disorder (PTSD). Given increasing use of medical cannabis among US military veterans to self-treat PTSD, there is strong public interest in whether cannabis may be a safe and effective treatment for PTSD.
Objective
The aim of the present study was to collect preliminary data on the safety and potential efficacy of three active concentrations of smoked cannabis (i.e., High THC = approximately 12% THC and < 0.05% CBD; High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD) compared to placebo in the treatment of PTSD among military veterans.
Methods
The study used a double-blind, cross-over design, where participants were randomly assigned to receive three weeks of either active treatment or placebo in Stage 1 (N = 80), and then were re-randomized after a 2-week washout period to receive one of the other three active treatments in Stage 2 (N = 74). The primary outcome measure was change in PTSD symptom severity from baseline to end of treatment in Stage 1.
Results
The study did not find a significant difference in change in PTSD symptom severity between the active cannabis concentrations and placebo by the end of Stage 1. All three active concentrations of smoked cannabis were generally well tolerated.
Conclusions and relevance
The present study is the first randomized placebo-controlled trial of smoked cannabis for PTSD. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment, but no active treatment statistically outperformed placebo in this brief, preliminary trial. Additional well-controlled and adequately powered studies with cannabis suitable for FDA drug development are needed to determine whether smoked cannabis improves symptoms of PTSD.
Trial registration
Identifier: NCT02759185; ClinicalTrials.gov.
Figures






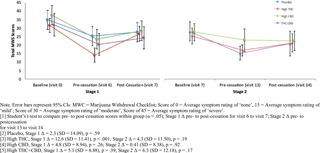






Citation: Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. (2021) The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS ONE 16(3): e0246990. https://doi.org/10.1371/journal.pone.0246990
Editor: Bernard Le Foll, Centre for Addiction and Mental Health, CANADA
Received: February 11, 2020; Accepted: January 26, 2021; Published: March 17, 2021
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All non-identifiable, relevant data are currently attached in the Supporting Information files.
Funding: Authors BY, RD, AE, MB, PR, and SS received Grant Number: RFA#135, an award funded by the Colorado Department of Public Health and Environment (CDPHE): https://www.colorado.gov/pacific/cdphe/approved-medical-marijuana-research-grants The study was also partially funded by the sponsor, The Multidisciplinary Association for Psychedelic Studies (MAPS): https://maps.org/research/mmj/ The sponsor designed the protocol with input from from MB, SS, and PR. The sponsor monitored the data quality, conducted data analysis, contributed to decision to publish, and assisted with preparation of manuscript through critical review.
Competing interests: Author MBM is an employee of Canopy Growth Corporation, during which time he has received stock options, serves on the Board of Directors for AusCann Group Holdings Limited, was a prior employee of Zynerba Pharmaceuticals, and has received consulting fees from Tilray Inc. Author ML serves on the scientific advisory board for FSD Pharma and has received consulting fees from Greenwich Biosciences, Zynerba Pharmaceuticals, and Tilray Inc in the past two years. Authors RD, BY, JW, BS, CH, RM, and AE receive salary from the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) non-profit research and educational organization. Author SS receives salary from the Scottsdale Research Institute, which is a private LLC and has no shareholders. The Academic Editor, BLF, co-authored “The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda” (https://pubmed.ncbi.nlm.nih.gov/32360455/) with one of the authors, MBM. This article was a result of a meeting where a large number of investigators came together to discuss clinical trial outcomes with representative from NIH and FDA. No other relationship between this author and the Academic Editor exists. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Posttraumatic Stress Disorder (PTSD) is a serious, worldwide public health problem. In the United States the lifetime prevalence of PTSD in the general population is between 6 and 10% [1,2], and between 13 and 31% in US military veterans [2,3]. PTSD is typically a chronic condition [4,5], and is associated with high rates of psychiatric and medical co-morbidity, disability, suffering, and suicide [4,6–8]. Food and Drug Administration (FDA)-approved pharmacological treatments for PTSD are currently limited to two selective serotonin reuptake inhibitors (SSRIs): sertraline and paroxetine, which have significantly lower effect sizes (SMD between -.28 and -.56) compared to trauma-focused psychotherapy (SMD between -1.01 and -1.35) [9,10]. Indeed, the current Department of Defense (DoD) and Department of Veterans Affairs (VA) best practice guidelines for treatment of PTSD recommend psychotherapy over pharmacotherapy [11]. However, the majority of military veterans with PTSD who receive one of the best practices psychotherapies for PTSD, which were determined efficacious through clinical trials, do not remit or reduce symptoms below clinical thresholds by the end of treatment [12,13].
There is a strong public interest, particularly among Patients with PTSD, clinicians, and researchers, in whether cannabis can be an effective pharmacological treatment option for individuals with PTSD, or a safe alternative treatment for patients who do not respond to current front-line treatment. Cross-sectional and prospective studies document the widespread use of cannabis by individuals with PTSD [14,15]. Moreover, veterans with PTSD who do not show remission following standard treatment are more likely to use cannabis following completion of PTSD treatment [16]. Two recent prospective studies of patients using cannabis to self-treat PTSD provide evidence that whole plant cannabis can produce short [17] and long-term relief of PTSD symptoms [18].
There is some preclinical evidence that at least two of the active compounds in cannabis, delta-9-tetrahydrocannabinol (THC; the primary constituent responsible for intoxication from cannabis) and cannabidiol (CBD; one of the non-intoxicating cannabinoids in cannabis), can positively impact processes that underly PTSD pathology [19]. Specifically, administration of CBD in rats and mice dampens cue-elicited fear responses [20,21], while administration of THC and THC+CBD appears to block reconsolidation of fear memory [22]. Likewise, both THC and CBD when administered alone facilitate fear extinction learning [23,24], which is a critical component for recovery from PTSD [25,26]. This work suggests that THC and/or CBD could modify how patients with PTSD experience and respond to reminders of trauma.
In addition to cannabis’ potential to perhaps modify mechanisms that maintain the core psychopathology of PTSD, early phase clinical data on isolated cannabinoid constituents in humans suggest that active components of cannabis might provide acute relief from specific symptoms of PTSD. For example, two open-label studies and one randomized placebo controlled trial found that administration of low doses of a THC analogue led to improvements in self-reported subjective sleep quality, decreased frequency of nightmares, and improvements in self-reported overall well-being among those with PTSD [27–29].
While these data appear promising, the potential therapeutic effects of smoked, herbal cannabis on PTSD have not been examined in a randomized, placebo controlled trial. Military veterans with PTSD are overwhelmingly choosing smoked cannabis to self-treat PTSD and related conditions [30]. Moreover, herbal cannabis varies significantly across plants in its THC and CBD content [29]. While both cannabinoids could hold therapeutic value, unlike THC, CBD is non-intoxicating and does not carry significant risk of abuse [30]. In addition, CBD may temper the anxiogenic effects of THC in cannabis preparations that contain both CBD and THC [31,32]. It is unclear whether THC, CBD, or some combination of compounds may lead to greater reductions in PTSD symptoms with better safety profiles compared to other combinations. In addition, previous clinical studies rely entirely on standardized dosing, rather than test more naturalistic and generalizable ad libitum dosing regimens. This is a major limitation of previous research because there is substantial individual variability in cannabinoid tolerability [31]. Indeed, military veterans who use cannabis for PTSD tend to self-titrate to much larger doses than those tested in research studies [32,33].
The primary objective of the present study was to conduct a randomized placebo-controlled trial to assess the safety and potential efficacy of smoked, herbal cannabis for the treatment of PTSD in military veterans. Specifically, the study was designed to examine the independent effects of ad libitum use of up to 1.8 grams/day of three active preparations of smoked cannabis: (i) High THC, (ii) High CBD, and (iii) one-to-one ratio of THC and CBD (THC+CBD) against placebo on PTSD symptoms in a sample of veterans with PTSD.
Methods
Trial design
The trial protocol can be found at https://maps.org/research-archive/mmj/MJP1-Protocol-Amend4-oct-13-2015.pdf. The study received ethics approval from the Copernicus Group Independent Review Board (IRB) and was conducted in accordance with all local and Federal laws and regulations, including obtaining written informed consent from all study participants. The study included a randomized, double-blind, placebo-controlled, crossover trial of smoked cannabis containing three different concentrations of THC and CBD, and placebo. The cross-over design included two stages with four treatment groups in Stage 1 (High THC, High CBD, THC+CBD, and placebo) and re-randomization into three active treatment groups in Stage 2 (High THC, High CBD, and THC+CBD). The primary aim of the study was to determine whether change in PTSD symptom severity at the end of Stage 1 (primary study endpoint) differed by condition. The crossover design allowed for additional comparisons of within-subject and between-subject differences in safety and preliminary efficacy across the two Stages and allowed for assessment of participants’ preference for cannabis concentrations assigned in either Stage 1 vs. Stage 2. Each stage included three weeks of ad libitum use up to 1.8 grams/day of the assigned treatment followed by a two-week cessation period. This upper limit was necessary due to the outpatient setting for self-administration and the Schedule 1 controlled substance status of cannabis.
Primary outcome and safety assessments were conducted at baseline (visit 0), end of treatment in Stage 1 (visit 5; primary study endpoint), following the Stage 1 cessation period/Stage 2 baseline (visit 7), and end of treatment in Stage 2 (visit 12). Self-reported assessment of withdrawal symptoms was conducted at screening, baseline, and weekly during the two-week cessation periods following each stage of treatment (visits 6, 7, 13, 14). Secondary outcomes were assessed throughout the study before/after treatment and cessation periods.
Participants.
Study participants were recruited using community-based advertisements, presentations, and website advertisements. Study inclusion and exclusion criteria were as follows:
Inclusion Criteria. Individuals were eligible for study enrollment if they (1) were a US military veteran, (2) met DSM-5 (APA, 2013) criteria for PTSD with symptoms of at least six months in duration (index trauma did not have to be related to military service), (3) had PTSD of at least moderate severity based on a CAPS-5 score of = >25 at baseline assessment, (4) were at least 18 years of age, (5) reported they were willing and able to abstain from cannabis use two-weeks prior to baseline assessment, which would be verified by urine toxicology screens at screening and baseline, and agreed to abstain from using non-study cannabis during the trial, (6) were stable on any pre-study medications and/or psychotherapy prior to study entry, and (7) agreed to comply with study procedures.
Exclusion criteria. Study participants were excluded if they (1) were pregnant, nursing, or of child bearing potential and not practicing effective means of birth control, (2) had a current or past serious mental illness (e.g., personality disorder, psychotic disorder) determined by the SCID-5-RV [34], or self reported a positive family history (first-degree relative) of psychotic or bipolar disorder (3) were determined at high risk for suicide based on the C-SSRS [35], (4) had allergies to cannabis or other contraindication for smoking cannabis, (5) had a current diagnosis or evidence of significant or uncontrolled hematological, endocrine, cerebrovascular, cardiovascular, coronary, pulmonary, gastrointestinal, immunocompromising, or neurological disease, (6) met DSM-5 criteria for moderate-severe Cannabis Use Disorder on the CUDIT-R (= >11), (7) screened positive for any illicit substance other than cannabis during the two-week screening, or (7) were unable to provide informed consent.
Randomization and blinding.
The Stage 1 randomization list utilized blocks to ensure equal treatment assignments, and the Stage 2 randomization utilized multiple validated randomization lists that re-randomized participants in a blinded manner. The randomization procedure specified that participants would be randomized to treatment conditions using small block randomization in a 1:1:1:1 ratio in Stage 1 and then be re-randomized into two of the three active cannabis conditions (THC, CBD, THC+CBD) with a 1:1 ratio in Stage 2. Randomization in Stage 2 excluded the participant’s Stage 1 treatment condition. As placebo was not an option in Stage 2, placebo participants were randomized 1:1 between High THC and High CBD, but were not given the option to be randomized to THC + CBD in order to facilitate simpler programming of the web-based randomization system. This two-step randomization resulted in an unbalanced distribution of Stage 2 participants overall across active dose groups. In order to maintain the blind, a central electronic database was utilized for randomization based on validated computer-generated lists.
All study staff (with the exception of the Randomization Monitor and Drug Product Packaging Technician) and participants were blinded to condition assignments. The blind could only be broken for an individual participant if there was a clinically or medically urgent emergency requiring knowledge of the participant’s condition assignment. This emergency unblinding required approval from the site PI and Coordinating Investigator. Likewise, the unblinded Randomization Monitor could provide dose assignment through the electronic randomization system. Randomization information was only available within the web-based randomization system and only viewable by the designated Randomization Monitor.
Interventions.
Study drug was obtained from the National Institute on Drug Abuse (NIDA). Four concentrations of cannabis from NIDA included: High THC = approximately 12% THC and < 0.05% CBD); High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD. Samples of each batch were tested and confirmed for their concentration levels by an independent third-party analytical testing laboratory in Phoenix, Arizona. The independent testing lab found in two separate analyses that the High THC batch was just 9%, with the other batches very close to what was reported by NIDA.
At the beginning of each stage, participants were asked to visit the clinic site for four hours on two successive days and self-administer under supervision of study staff one dose of the cannabis preparation that they were randomly assigned to in that Stage. Vital signs for safety were collected during these visits (i.e., blood pressure, pulse). The study provided participants a total of 37.8 grams (1.8 grams/day)for the three-week ad libitum treatment period along with a metal pipe for treatment delivery (smoked). Participants were asked to refrain from using non-study cannabis, and return any remaining study cannabis that was not used each week. When study drug was returned the clinic team weighed the returned cannabis to calculate participants’ average use in grams per day during the treatment period in each stage. Participants were asked to refrain from any cannabis use during a two-week cessation period (between stages), then were re-randomized into one of three active treatment groups. All study participants were provided the option to enroll in an open label extension (Stage 3) with the cannabis of their choice in the same amount they returned unused in Stages 1 and 2 so participants had no disincentives to returning unused amounts. The results of Stage 3 are not reported here.
Demographic measures.
Baseline demographic information included age, sex, race/ethnicity, education, employment status. Other baseline measures included: whether the index trauma was combat-related, body mass index (BMI), risk for sleep apnea (STOP-bang) [36], and risk for cannabis use disorder (CUDIT-R) [37].
Safety measures.
Adverse Events (AEs) were assessed at baseline, during the introductory session, self-administration session, end of treatment, and before/after cessation in each stage by asking participants to self-report any side effects experienced over the past week. All AEs were coded by Systems Organ Class. The study physician then rated all AEs by severity (mild, moderate, severe) and study relatedness (i.e., possibly related, probably related, not related). AEs rated possibly related and probably related were collapsed into one “related” category.
Additional safety measures included the 15-item Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) and the Columbia-Suicide Severity Rating Scale (CSSR-S) (Posner et al., 2011). The MWC was administered at screening, baseline, and each week following cessation of Stages 1 and 2 (visits 6, 7, 13, 14). The CSSR-S was self-administered at all study visits.
Outcome measures.
The primary outcome of the current study was change in PTSD symptom severity from baseline (visit 0) to end of the three-week treatment period in Stage 1 (visit 5) using the Clinician-Administered PTSD Scale for DSM-5 Total Severity Score (CAPS-5) [38]. The CAPS-5 is a semi-structured clinician interview, and is well-validated for determining PTSD diagnoses consistent with the Diagnostic and Statistical Manual of Mental Disorders, Version 5 (DSM-5) and assessing change in symptom severity over time [39]. PTSD diagnosis is based on meeting the DSM-5 symptom cluster criteria (minimum threshold of symptoms with a score ≥ 2) with a qualifying criterion A index trauma. The CAPS-5 Total Severity Score is calculated by summing the total score for each of the four symptom categories to assess past-month PTSD symptoms on a specific traumatic event: intrusion (Category B), Avoidance (Category C), Mood and Cognition (Category D), and Hyperarousal (Category E). CAPS-5 Total Severity scores range from 0–80, where higher scores indicate worse PTSD severity.
Secondary outcome measures included a modified version of the 20-item self-report PTSD Checklist for DSM-5 (PCL-5) [40], which was changed to assess for past week symptoms, the 20-item general depression subscale and 5-item anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms’ (IDAS) [41], the 80-item self-report Inventory of Psychosocial Functioning (IPF) [42], and the 7-item self-report Insomnia Severity Index (ISI) [43]. Secondary outcome measures were collected at baseline (visit 0 and visit 7), self-administration (visit 4 and visit 10), before cessation (visit 6 and visit 13), and after cessation (visit 7 and visit 14) in both Stage 1 and Stage 2. Total and subscale scores were calculated for each measure.
Other measures.
The validity of study blinding to active or inactive treatment in Stage 1 was assessed by asking participants and clinicians to independently guess whether the participant was randomized to an active (High THC, High CBD, THC+CBD) or inactive (placebo) treatment group at the end of Stage 1. At the end of Stage 2, participants were asked whether they preferred the treatment to which they were assigned in Stage 1 or Stage 2.
Table 1 includes a summary of all assessments by visit.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 1. Summary of assessments by visit.
https://doi.org/10.1371/journal.pone.0246990.t001
Study power.
The primary study aim was to gather preliminary data on the safety and potential efficacy of different cannabis preparations to treat PTSD among veterans. In the absence of published effect sizes for the impact of THC, CBD, or THC+CBD on CAPS-5 scores, the target sample size was chosen to allow detection of an effect size of 0.4 or greater (small to medium effect) based on between group differences in the primary outcome measure (i.e., change in total CAPS-5 severity score from baseline to the end of Stage 1 active treatment phase). Power analysis suggested that 76 completing participants (n = 19 per group) would be needed to detect an effect size of d = 0.4 at 82% power and .05 significance level. Enrollment and randomization continued until 76 participants completed the Stage 1 outcome assessment. Eighty participants were enrolled and 76 partcipants completed Stage 1.
Statistical analyses.
Descriptive statistics were performed to test the normality of baseline measures on the total study sample and across each treatment group to ensure adequate randomization. Means, medians, and frequencies were calculated, and within-subject and between-group differences were tested for categorical variables using chi-square tests and t-tests or analysis of variance (ANOVA) for continuous variables.
Safety was analyzed by tabulating the frequency, severity, and relatedness to treatment of AEs. A Chi-square test was used to assess for differences in frequency of AEs across groups. An AE was counted once per subject for each assessment period.
The primary outcome was analyzed using ANOVA to test for between-group differences in change in Total PTSD Severity scores from baseline to end of treatment in Stage 1 (CAPS-5 visits 0 and 7). Secondary outcomes were analyzed using a series of additional ANOVAs to test for between-group differences in change scores from baseline to end of treatment for Stage 1 (CAPS-5 visits 0 and 7; secondary measures visits 0 and 6) and Stage 2 (CAPS-5 visits 7 and 12; secondary measures visits 7 and 13). All dependent variables were tested for normality, and summarized by both mean and median values by group. Within-subject change scores were tested for each treatment group using a series of t-tests. Tukey’s pairwise comparisons were used to test for group differences in change scores between all pairs of treatment conditions in Stage 1 and Stage 2. Analyses were conducted consistent with an intent-to-treat (ITT) framework, where all available data from randomized participants who received at least one week’s supply of study drug (N = 80) were summarized for baseline characteristics and entered into the models. However, the use of ANOVA tests only allowed for analysis of change in participants who completed outcome assessments (N = 76 for primary outcome analysis).
Results
Sample characteristics
A total of 261 individuals completed screening and 51% met eligibility criteria for study inclusion. Eighty participants were enrolled and randomized into one of four treatment groups (n = 20 per group), of which 76 participants completed the Stage 1 outcome assessment. In Stage 2, a total of 74 participants were re-randomized into High THC (n = 29), High CBD (n = 27), or THC+CBD (n = 18). There were no significant Stage 1 treatment assignment differences in demographics or baseline scores on the primary and secondary outcome variables (i.e., CAPS-5, PCL-5, IDAS Social Anxiety, IDAS Depression, IPF, and ISI). Sample demographics and baseline characteristics are summarized in Table 2.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 2. Baseline characteristics by treatment group.
https://doi.org/10.1371/journal.pone.0246990.t002
Treatment adherence and attrition
Rates of engagement and completion are summarized in the Consort Diagram (Fig 1). During Stage 1, 3 participants (3.8%) did not complete endpoint outcome assessments. After Stage 1, 6 participants (7.5%) did not continue into Stage 2. Of the 74 participants who were re-randomized into Stage 2, 3 (4.1%) discontinued treatment due to an AE, and 7 total (9.5%) did not complete Stage 2 endpoint outcome assessments. The overall attrition rate for the percent of randomized participants who dropped out before completing Stage 2 outcome assessments, was 16.3%.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 1. Consort flow diagram.
https://doi.org/10.1371/journal.pone.0246990.g001
Cannabis use in grams
In Stage 1, there was no statistically significant difference between groups in total grams of smoked cannabis/placebo during the three-week treatment period (21 days) across the treatment groups (F [3, 71] = 2.23, p = .09). Mean (SD) grams of smoked cannabis/placebo used by each treatment group in Stage 1 were as follows: placebo (M = 8.4, SD = 10.1), High THC (M = 14.6, SD = 10.4), High CBD (M = 14.3, SD = 13.0), THC+CBD (M = 8.2, 6.8).
In stage 2, there was a significant group difference in total grams of smoked cannabis (F [2, 64] = 3.42, p = .04), such that participants in the THC+CBD group used significantly more cannabis (M = 17.6, SD = 10.6), compared to participants randomized to High THC (M = 10.7, SD = 10.9), or High CBD (M = 9.3, SD = 10.5).
Assessment of study blind
In Stage 1, 60% of placebo participants accurately guessed assignment to an inactive treatment, 58% of High CBD participants accurately guessed that they were in an active condition, and 100% of participants in the High THC and THC+CBD groups accurately guessed assignment into an active treatment condition. Similar results were found for clinicians. In Stage 1, forty-five percent of clinicians accurately guessed placebo participants’ assignment in an inactive treatment, 16% accurately guessed High CBD participants’ assignment into an active treatment, and 100% accurately guessed that participants assigned to High THC or THC+CBD were randomized into an active treatment. Therefore, the study blind was appropriately upheld only when participants were assigned to High CBD or placebo conditions, but was not upheld when participants were assigned to High THC or High THC/CBD.
Treatment preference
At the end of Stage 2, participants who completed final assessments (n = 74) indicated their preference for either their blinded Stage 1 or Stage 2 treatment assignment. Twenty-five participants (34%) indicated a preference for a Stage 1 or Stage 2 assignment to High THC, 10 participants (13%) indicated a preference for a Stage 1 or Stage 2 assignment to High CBD, 26 participants (35%) indicated a preference for a Stage 1 or Stage 2 assignment to THC+CBD, and 4 participants (5%) indicated a preference for a Stage 1 assignment to placebo. Two participants (3%) equally preferred their Stage 1 and Stage 2 treatment assignments.
Safety outcomes
Adverse events.
All Adverse Events (AEs) reported during Stage 1 are summarized in Table 3. Number of participants who reported at least one AE did not significantly differ by treatment group in either Stage 1 (p = .38) or Stage 2 (p = .27). Thirty-seven of 60 participants who received THC, CBD, or THC+CBD during Stage 1 (61.7%) reported at least one treatment-related AE by the end of Stage 1. In Stage 2, Forty-five of the 74 participants who received THC, CBD, or THC+CBD (60.8%) reported at least one treatment-related AE during Stage 2. Three of 80 participants (3.8%) reported an unrelated Serious Adverse Event (SAE) during the study, specifically heart palpitations (n = 1; THC+CBD, Stage 1 cessation period), pulmonary embolism (n = 1, High THC, Stage 2), and abscess (n = 1, High CBD, Stage 2). One participant (THC+CBD) discontinued treatment during the introductory session in Stage 1 due to an AE, and two participants discontinued treatment during the introductory session in Stage 2 due to an AE (High CBD and High THC conditions). Across both Stages, 13 total participants terminated from the study early due to an AE (8.4%). The most common AEs reported (i.e., those with >10% frequency) were cough (12.3%), followed by throat irritation (11.7%) and anxiety (10.4%). Emergency unblinding was never used in the study.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 3. Number of participants with adverse events by systems organ class/preferred terms and treatment-relatedness.
https://doi.org/10.1371/journal.pone.0246990.t003
One participant who received CBD in Stage 1 (5.0%) reported treatment-related suicidal ideation. One participant from each treatment condition (3.6% – 5.9%) reported treatment-related suicidal ideation in Stage 2.
Cannabis withdrawal symptoms.
Fig 2 summarizes mean withdrawal symptom scores on the MWC by group at Stage 1 and Stage 2 baseline, end of treatment, and following 1-week of cessation. All treatment groups reported mean withdrawal symptoms in the moderate range (Mean score = 32–38) at baseline assessment (prior to initiating treatment in Stage 1). All treatment groups showed a significant reduction in withdrawal symptoms from baseline to the end of the treatment phase of Stage 1. Only participants assigned to High THC in Stage 1 reported a significant increase in mean self-reported withdrawal symptoms after one week of cessation from the assigned treatment in Stage 1 (Δ = 12.6, SD = 11.41, p = .0004). There was no significant change in withdrawal symptoms from the end of Stage 2 treatment to one-week follow-up.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 2. Mean total marijuana withdrawal scores by treatment condition across Stage 1 & Stage 2.
https://doi.org/10.1371/journal.pone.0246990.g002
Primary efficacy outcome
PTSD symptom severity (CAPS-5).
Results of the analysis of change in total PTSD symptom severity on the CAPS-5 are summarized in Table 4 for both Stage 1 (primary outcome) and Stage 2. In Stage 1, there was no significant between-group difference in CAPS-5 Total Severity scores between treatment groups [F(3, 73) = 1.85, p = .15]. All four treatment groups, including placebo, achieved significant within-subject reductions in total CAPS-5 Total Severity scores from Stage 1 baseline (visit 0) to end of treatment (visit 5). Specifically, participants who received placebo in Stage 1 reported a mean reduction of 13.1 points (SD = 12.10, p < .001, d = -1.30), participants who received High THC reported a mean reduction of 15.2 points (SD = 11.3, p < .0001, d = -1.99), High CBD participants reported a mean reduction of 8.4 points (SD = 10.09, p < .05, d = -.79), and THC+CBD participants reported a mean reduction of 8.5 points (SD = 9.88, p < .05, d = -.83).

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 4. Mean (SD)/Median (IQR) and analysis of change in CAPS-5 total severity scores by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t004
Secondary efficacy outcomes
The results of the study’s secondary efficacy outcomes are summarized in Table 5.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 5. Mean (SD)/Median (IQR) and analysis of group change in PCL-5, IDAS social anxiety, IDAS general depression, IPF, and ISI by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t005
Self-reported PTSD symptoms, PCL-5.
In Stage 1, there was no significant difference in PCL-5 change scores between treatment groups from baseline to end of Stage 1.
In Stage 2, mean change in PCL-5 scores significantly differed by treatment condition [F(2, 63) = 4.06, p = .02]. Specifically, there was a significant difference between High CBD and THC+CBD in PCL-5 change scores, with participants who received THC+CBD reporting greater reductions in PTSD symptoms on the PCL-5 (Δ = -16.4, SD = 16.0, p < .001, d = -1.43) compared to participants who received High CBD (Δ = -9.1, SD = 11.0, p = .02, d = -.67).
IDAS general depression & social anxiety subscales.
In Stage 1, there were no significant differences between treatment conditions in either change in IDAS General Depression or IDAS Social Anxiety scores.
In Stage 2, treatment groups significantly differed in IDAS Social Anxiety mean change scores [F(2, 63) = -3.08, p = .05] and IDAS General Depression mean change scores [F(2, 63) = 3.76, p = .03]. Specifically, participants in the THC+CBD condition in Stage 2 reported significant pre-post reductions in IDAS Social Anxiety scores (Δ = – 2.8, SD = 3.90, p = .04, d = -.70), and participants in the High THC (Δ = -9.0, SD = 11.1, p < .01, d = -.90) and THC+CBD treatment conditions (Δ = -13.4, SD = 10.0, p < .0001, d = -1.68) reported significant reductions in IDAS General Depression scores in Stage 2.
ISI insomnia.
In Stage 1, there was no significant difference between treatment conditions in mean change in total insomnia symptoms on the ISI.
In Stage 2, there was no significant difference between treatment groups in mean change scores in total insomnia symptoms on the ISI.
Psychosocial functioning, IPF.
In Stage 1, there was no significant between-group difference in mean in overall psychosocial functioning (IPF total score).
In Stage 2, there was no significant difference between treatment conditions in IPF mean change scores.
Posthoc analysis
CAPS-5 subscale scores B, C, D, and E.
As a follow-up to the primary outcome analysis, posthoc analyses were conducted to test the effects of treatment group on change in each of the primary symptom domains of PTSD (i.e., intrusions, avoidance, negative thoughts and emotions, hyperarousal) using the CAPS-5 B, C, D, and E subscale scores. The posthoc analysis for subscale scores used the same analytic approach as the analysis for primary and secondary outcomes.
PCL-5, IDAS social anxiety, IDAS general depression, and ISI longitudinal analysis.
Mixed-models for repeated measures (MMRM) were computed to test for group differences over time for all secondary outcome assessments that were measured at more than two time points within each stage. The use of MMRM models allowed all randomized participants’ data to be analyzed within the models based on the missing-at-random assumption (MAR).
Posthoc results.
In Stage 1, there was no significant difference between groups in mean change for any of the subscale scores on the CAPS-5 [Subscale B, F(3,73) = 1.58, p = .20; Subscale C, F(3,73) = 1.06, p = .37; Subscale D, F(3,73) = 2.26, p = .09; Subscale E, F(3,73) = .84, p = .48].
In Stage 2, there was a significant difference between groups in mean change on the CAPS-5 C (avoidance) [F(2,64) = 4.95, p = .01] and CAPS-5 D (negative thoughts and emotions) [F(2,64) = 8.60, p < .001] subscales. Specifically, there was a significant difference between participants who received High CBD and THC+CBD in mean change in CAPS-5 C (avoidance) subscale scores (Δ = 1.9, 95% CI: 0.38, 3.35, d = -.97) and CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 5.2, 95% CI = 2.04, 8.26, d = -1.13), and between High THC and THC+CBD group participants in mean change in CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 4.1, 95% CI: 1.09, 7.15, d = -1.01). In Stage 2, there were no significant differences between groups in mean change on the CAPS-5 B (intrusions) [F(2,64) = 2.80, p = .07] or CAPS-5 E [F(2,64) = 1.13, p = .33] (hyperarousal) Subscales.
Results of the MMRM analyses testing group differences over time in PCL-5, IDAS Social Anxiety, IDAS General Depression, and ISI appear in Fig 3.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 3. Post hoc Mixed Models for Repeated Measures (MMRM) testing change in PCL-5, IDAS depression, IDAS anxiety, and ISI scores over time.
https://doi.org/10.1371/journal.pone.0246990.g003
Only IDAS Social Anxiety scores in Stage 2 had a significant time x treatment effect, such that social anxiety showed a quadratic drop in Stage 2 among those in the THC+CBD conditions and those in the High THC and High CBD conditions did not show change over time. All other models failed to find a significant difference between groups over time.
Discussion
The present study served as the first randomized placebo-controlled trial of smoked cannabis for symptoms of PTSD in US military veterans. Study-related AEs were generally mild to moderate, and did not significantly differ by treatment condition. The study failed, however, to find a significant effect of treatment condition on the primary efficacy outcome, change in total PTSD severity on the CAPS-5 from baseline to end of Stage 1. All treatment groups (placebo, High CBD, High THC, THC+CBD) achieved statistically significant reductions in PTSD severity on the CAPS-5 in Stage 1, with effect sizes for change in mean PTSD severity ranging between d = .83 (High CBD) and d = 1.34 (High THC). These effect sizes are much larger than effect sizes reported for symptom change in other psychopharmacology trials for PTSD. For example, a 2018 meta-analysis reported standardized mean differences between .33 and .97 across PTSD pharmacology trials. The average length of trials reported in the 2018 meta-analysis lasted approximately ten weeks, whereas the current trial’s primary endpoint was evaluated after only three weeks of treatment.
The study’s failure to detect a significant difference between groups in Stage 1 could perhaps be explained by several confounding factors. First, the study sample included participants with a history of cannabis use. The recruitment of active cannabis users might have increased the potential for biased responding. Given the topical nature of the current trial and its relevance for public policy on medical cannabis, participants might have been biased to report positive effects regardless of condition. Despite many participants already having experience with the drug, nearly half of those receiving placebo believed that they received active cannabis. Prior expectations about cannabis’ effects might explain why even those in the placebo condition reported larger than average reductions in PTSD symptoms after only 3 weeks of treatment.
Second, many participants reported significant cannabis withdrawal symptoms at the time of randomization and early in Stage 1. Nearly half of the study sample (43%, n = 34) were positive for THC at study screening and 23% (n = 18) remained positive for THC at Stage 1 baseline, which would suggest chronicity of previous use or continued cannabis use during the two week washout from screening to baseline (lack of compliance with two week abstinence inclusion criteria). Total cannabis withdrawal symptoms averaged in the moderate range for all treatment groups at the start of Stage 1 (despite two weeks of abstinence prior to randomization), then generally reduced to the mild to moderate range by the end of treatment in Stage 1. Participants who received High THC in Stage 1 reported a significant increase in withdrawal following one week of cessation from Stage 1 treatment, which averaged in the moderate range following cessation. While groups did not differ in cannabis withdrawal ratings, the presence of withdrawal and trends in change could confound (or help explain) interpretation of results. We cannot rule out that cessation of cannabis use (in the placebo condition) or reversal of withdrawal (in the THC and THC+CBD conditions) might have partially been responsible for significant within-subjects change. Moreover, participants randomly assigned to receive High THC in Stage 1 had CUDIT total scores (indicating cannabis use disorder risk) nearly two times greater than participants who were assigned to other active treatment conditions. This is a major confound and limitation of the current study.
Third, total exposure to smoked cannabis was lower than anticipated and might not equate to a full therapeutic dose. In Stage 1, cannabis use in grams ranged from 8.2g (THC+CBD) to 14.6g (high THC) over three weeks (.39g/day to .69g/day on average), despite all participants having access to up to 37.8g over the three week period (1.8g/day). Average cannabis quantity that most cannabis users consume is difficult to estimate from epidemiological studies due to differences in cannabis potency and route of administration. However, large scale studies of medicinal cannabis users treating chronic pain and anxiety report average daily use in ranges closer to 1-3g/day [44,45]. Likewise, two smaller studies that assessed military veterans who use medical cannabis to self-treat PTSD reported median daily use of .85g to 1.14g/day [46] and average use of 3.8g/day [33]. We suspect that participants might have used less cannabis in the current study because of differences between the cannabis available for research trials and the quality of cannabis sold commercially. Several participants spontaneously reported to study staff that the smoke from the cannabis that was provided was “harsher” than they were used to. This difference might also be attributable to the two-week cessation periods mitigating tolerance.
Finally, the present study did not include a placebo arm in Stage 2, which limited the analyses that could be employed in order to take advantage of the crossover design. This significantly limited power to find significant differences across groups. The unexpectedly large response to placebo (d = 1.30), coupled with the small sample size per condition, meant that the current study was underpowered to detect significant differentiation from placebo. If the very large placebo response observed in this study remains consistent in future studies, a trial that tests only one preparation of cannabis (e.g., only high THC cannabis) against placebo would still need a total sample size of nearly one thousand participants (n = 479 per group) to achieve a statistically significant result. However, including a placebo run-in stage to identify and exclude placebo responders in any future trials could substantially reduce this total sample size [47].
Despite these limitations, there were several notable findings. While the study’s primary outcome assessment failed to find differentiation from placebo in PTSD symptom change on the CAPS-5, all participants showed a positive response to treatment in a very brief time period. In addition, side effects were general mild and transient. One of the largest concerns from providers regarding self-treatment of PTSD with cannabis is that it may exacerbate PTSD symptoms. While the current study’s treatment duration was too brief to identify long-term risk, two-tailed significance tests did not show evidence of symptom exacerbation in any condition. These data provide preliminary evidence of safety of short-term ad libitum cannabis use in this population.
While Stage 2 results should be interpreted with caution given the possible carry-over effects and unbalanced randomization across active dose groups, the study did identify some statistically significant differentiation between groups when particiants were re-randomized to only the three active conditions. Given that Stage 2 THC+CBD participants consisted of only those who received active treatment in Stage 1, this cohort might be providing a window into the effects of slightly longer active cannabis treatment. The positive response to treatment evidenced by participants receiving THC+CBD in Stage 2 suggest that it is possible that a longer active treatment period might be necessary to achieve treatment gains that could outperform placebo. It is equally plausible, however, that greater reductions in CAPS-5 severity scores in Stage 2 among THC and THC+CBD treatment groups were due to attenuation of cannabis withdrawal symptoms, as all treatment groups experienced a 2-week cessation period between Stage 1 and Stage 2. This effect would be consistent with prior literature suggesting that symptoms of cannabis use disorder (CUD) can interfere in successful recovery from PTSD [48].
The current study is unique, in that it trialed whole plant cannabis preparations, rather than single molecule extracts or synthetic pharmaceutical cannabinoids. In addition to reporting change in structured assessments of symptoms, the study results provide critical information about participant preference for cannabinoid preparations when exposed to different whole plant THC and CBD ratios. Consistent with previous work [46], participants in the current study reported a general preference for cannabis types that included significant quantities of THC. This effect might be explained by a signal of efficacy, or simply due to the intoxicating and reinforcing effects of high THC cannabis. Nevertheless, the demand for testing cannabis as a therapeutic for PTSD has largely been driven by military veteran advocacy groups, and yet development of cannabinoid therapeutics has not focused on trialing cannabis preparations that military veterans currently use to self-treat their symptoms. Input from stakeholders on which cannabinoids to trial as cannabis-based medicine for military veteran-specific conditions is certainly justified.
Results from the current trial provide invaluable information for future cannabinoid trials for PTSD. While the null findings raise questions about the utility of continuing to trial whole plant cannabis for the treatment of PTSD, the study found that whole plant, smoked cannabis was generally well tolerated and did not lead to deleterious effects in most participants after 3 weeks of ad libitum use. These safety results are consistent with recent real-world evidence studies of whole plant cannabis for PTSD [17,18]. However, those studies both found evidence of potential efficacy as well. Given that many veterans with PTSD are already using cannabis to self-treat their symptoms, identifying which preparations and with which method of administration are most beneficial and/or are least harmful is critical. These future studies will need to take active steps to ensure appropriate blinding despite the intoxicating nature of the drug. While the CBD and placebo conditions were appropriately blinded in the current study, participants and assessors could accurately guess condition when participants received THC-based treatments. Enrolling novice or naïve users, or limiting total THC content to sub-intoxicating doses could improve these blinding issues. Conversely, if higher THC doses are indeed therapeutic, other designs that are less reliant on active blinding, such as randomized withdrawal, might be warranted. Researchers should also consider including objective surrogate endpoint assessments (e.g., physiology, biological specimens, neuroimaging) as secondary indicators of treatment response. As none of these have been validated for a PTSD population as surrogate endpoints, the present study and future studies must utilize the “gold-standard” semi-structured clinical interview for PTSD severity, which is the CAPS-5.
Future studies would also likely benefit from a longer treatment period similar to more traditional medication trials (e.g., 12-weeks), and if including a placebo comparator should plan for a potentially larger than normal placebo response. To mitigate placebo, researchers might consider: 1) including a placebo run-in period prior to randomization to attempt to identify placebo responders, 2) powering the trial for a potentially larger than typical placebo effect, and/or 3) excluding participants with strong apriori beliefs about cannabis’ therapeutic effects [e.g., prescreening with expectancy measures, such as Devilly & Borkovec’s Credibility/Expectancy Questionnaire (CEQ)]. For generalizability to female veterans and civilians with PTSD, these studies should also attempt to recruit a greater number of female participants, as the current study’s sample was overwhelmingly male. Finally, future studies would also greatly benefit from access to high quality flower cannabis. The flower preparations provided through the NIDA drug supply program include all parts of the plant (instead of just buds) and are only available in specific cannabinoid ratios. Studies that test high quality buds in various phenotypes with variable potencies of cannabinoids and terpene ratios would more closely mirror what is available within state-sponsored medical cannabis programs.
Conclusions
The present study failed to find a significant group difference between smoked cannabis preparations containing High CBD, High THC, and THC+CBD against placebo in regards to their impact on PTSD symptoms. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment. The failure to differentiate treatment groups from placebo is likely attributable to the higher than average treatment response in the placebo condition and to the shorter than average duration of treatment. Higher powered studies that attempt to mitigate the effect of pronounced placebo appear warranted.
Supporting information
S1 Checklist –
Showing 1/15: pone.0246990.s001.DS_Storesorry, we can’t preview this file1 / 15figshare
S1 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s001
(DS_STORE)
S2 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s002
(DOC)
S1 File.
https://doi.org/10.1371/journal.pone.0246990.s003
(CSV)
S2 File.
https://doi.org/10.1371/journal.pone.0246990.s004
(TXT)
S3 File.
https://doi.org/10.1371/journal.pone.0246990.s005
(TXT)
S4 File.
https://doi.org/10.1371/journal.pone.0246990.s006
(TXT)
S5 File.
https://doi.org/10.1371/journal.pone.0246990.s007
(TXT)
S6 File.
https://doi.org/10.1371/journal.pone.0246990.s008
(TXT)
S7 File.
https://doi.org/10.1371/journal.pone.0246990.s009
(TXT)
S8 File.
https://doi.org/10.1371/journal.pone.0246990.s010
(TXT)
S9 File.
https://doi.org/10.1371/journal.pone.0246990.s011
(TXT)
S10 File.
https://doi.org/10.1371/journal.pone.0246990.s012
(TXT)
S11 File.
https://doi.org/10.1371/journal.pone.0246990.s013
(TXT)
S12 File.
https://doi.org/10.1371/journal.pone.0246990.s014
(XLSX)
S13 File.
https://doi.org/10.1371/journal.pone.0246990.s015
(PDF)
Acknowledgments
We thank Scott Hamilton and Ilsa Jerome for their assistance in quality checking all of the study data.
References
1. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder. Curr Opin Psychiatry. 2015;28: 307–311. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
2. Gradus JL. Epidemiology of PTSD. In: National Center for PTSD. 2014.
3. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28: 307–11. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
4. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62: 617. pmid:15939839
View Article
PubMed/NCBI
Google Scholar
5. Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149: 671–675. pmid:1575259
View Article
PubMed/NCBI
Google Scholar
6. Perkonigg A, Kessler RC, Storz S, Wittchen H-U. Traumatic events and post-traumatic stress disorder in the community: prevalence,risk factors and comorbidity. Acta Psychiatr Scand. 2000;101: 46–59. pmid:10674950
View Article
PubMed/NCBI
Google Scholar
7. Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62 Suppl 17: 16–22. Available: http://www.ncbi.nlm.nih.gov/pubmed/11495091.
View Article
Google Scholar
8. Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro III A, Parker VA, et al. Burden of Medical Illness in Women With Depression and Posttraumatic Stress Disorder. Arch Intern Med. 2004;164: 1306. pmid:15226164
View Article
PubMed/NCBI
Google Scholar
9. Merz J, Schwarzer G, Gerger H. Comparative Efficacy and Acceptability of Pharmacological, Psychotherapeutic, and Combination Treatments in Adults With Posttraumatic Stress Disorder. JAMA Psychiatry. 2019;76: 904. pmid:31188399
View Article
PubMed/NCBI
Google Scholar
10. Forman-Hoffman V, Middleton JC, Feltner C, Gaynes BN, Weber RP, Bann C, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update. 2018 [cited 11 Oct 2019]. Available: https://www.ncbi.nlm.nih.gov/books/NBK525132/.
View Article
Google Scholar
11. Management of Post-Traumatic Stress Working Group. VA/DOD clinical practice guideline for management of post-traumatic stress. 2017.
12. Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67: 194–200. Available: http://www.ncbi.nlm.nih.gov/pubmed/10224729. pmid:10224729
View Article
PubMed/NCBI
Google Scholar
13. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consult Clin Psychol. 1992;60: 748–56. Available: http://www.ncbi.nlm.nih.gov/pubmed/1401390. pmid:1401390
View Article
PubMed/NCBI
Google Scholar
14. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153: 369–375. pmid:8610824
View Article
PubMed/NCBI
Google Scholar
15. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136: 162–165. pmid:24412475
View Article
PubMed/NCBI
Google Scholar
16. Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychol Addict Behav. 2011;25: 485–491. pmid:21261407
View Article
PubMed/NCBI
Google Scholar
17. LaFrance EM, Glodosky NC, Bonn-Miller M, Cuttler C. Short and Long-Term Effects of Cannabis on Symptoms of Post-Traumatic Stress Disorder. J Affect Disord. 2020;274: 298–304. pmid:32469819
View Article
PubMed/NCBI
Google Scholar
18. Bonn-Miller MO, Brunstetter M, Simonian A, Loflin MJ, Vandrey R, Babson KA, et al. The Long-Term, Prospective, Therapeutic Impact of Cannabis on Post-traumatic Stress Disorder. Cannabis Cannabinoid Res. 2020.
View Article
Google Scholar
19. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14. pmid:28813324
View Article
PubMed/NCBI
Google Scholar
20. Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. 2010;207: 105–111. pmid:19800921
View Article
PubMed/NCBI
Google Scholar
21. Gomes F V, Reis DG, Alves FH, Corrêa FM, Guimarães FS, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT 1A receptors. J Psychopharmacol. 2012;26: 104–113. pmid:21148020
View Article
PubMed/NCBI
Google Scholar
22. Stern CAJ, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25: 958–965. pmid:25799920
View Article
PubMed/NCBI
Google Scholar
23. Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226: 781–792. pmid:23307069
View Article
PubMed/NCBI
Google Scholar
24. Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64: 396–402. pmid:22796109
View Article
PubMed/NCBI
Google Scholar
25. Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36: 23–31. pmid:23260015
View Article
PubMed/NCBI
Google Scholar
26. Yehuda R, LeDoux J. Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron. 2007;56: 19–32. pmid:17920012
View Article
PubMed/NCBI
Google Scholar
27. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15: 84–88. pmid:19228182
View Article
PubMed/NCBI
Google Scholar
28. Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51: 585–588. pmid:25467221
View Article
PubMed/NCBI
Google Scholar
29. Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, Open-Label, Pilot Study of Add-On Oral Δ9-Tetrahydrocannabinol in Chronic Post-Traumatic Stress Disorder. Clin Drug Investig. 2014;34: 587–591. pmid:24935052
View Article
PubMed/NCBI
Google Scholar
30. Loflin M, Babson K, Sottile J, Norman S, Gruber S, Bonn-Miller M. A Cross-Sectional Examination of Choice and Behavior of Veterans with Access to Free Medicinal Cannabis. Am J Drug Alcohol Abuse. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
31. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. European Journal of Internal Medicine. Elsevier B.V.; 2018. pp. 12–19. pmid:29307505
View Article
PubMed/NCBI
Google Scholar
32. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
33. Loflin M, Earleywine M, Bonn-Miller M. Medicinal versus recreational cannabis use: Patterns of cannabis use, alcohol use, and cued-arousal among veterans who screen positive for PTSD. Addict Behav. 2017;68. pmid:28088054
View Article
PubMed/NCBI
Google Scholar
34. First MB, Williams JBW, Karg RSK, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA Am Psychiatr Assoc. 2015. pmid:32821383
View Article
PubMed/NCBI
Google Scholar
35. Posner K, Brent D, Lucas C, Gould M, Stanley B., Brown G., et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008.
36. Shahid A, Wilkinson K, Marcu S, Shapiro CM. STOP-Bang Questionnaire. STOP, THAT and One Hundred Other Sleep Scales. Springer New York; 2011. pp. 371–383. https://doi.org/10.1007/978-1-4419-9893-4_92
37. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110: 137–143. pmid:20347232
View Article
PubMed/NCBI
Google Scholar
38. Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). [Assessment]. 2013.
View Article
Google Scholar
39. Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30: 383–395. pmid:28493729
View Article
PubMed/NCBI
Google Scholar
40. Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28: 1379–1391. pmid:26653052
View Article
PubMed/NCBI
Google Scholar
41. Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 2007;19: 253–268. pmid:17845118
View Article
PubMed/NCBI
Google Scholar
42. Bovin MJ, Black SK, Rodriguez P, Lunney CA, Kleiman SE, Weathers FW, et al. Development and validation of a measure of PTSD-related psychosocial functional impairment: The Inventory of Psychosocial Functioning. Psychol Serv. 2018;15: 216–229. pmid:29723024
View Article
PubMed/NCBI
Google Scholar
43. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34: 601–8. pmid:21532953
View Article
PubMed/NCBI
Google Scholar
44. Turna J, Simpson W, Patterson B, Lucas P, Van Ameringen M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J Psychiatr Res. 2019;111: 134–139. pmid:30738930
View Article
PubMed/NCBI
Google Scholar
45. Ware MA, Wang T, Shapiro S, Collet JP. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain. 2015;16: 1233–1242. pmid:26385201
View Article
PubMed/NCBI
Google Scholar
46. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
47. Lee S, Walker JR, Jakul L, Sexton K. Does elimination of placebo responders in a placebo run-in increase the treatment effect in randomized clinical trials? A meta-analytic evaluation. Depress Anxiety. 2004;19: 10–19. pmid:14978780
View Article
PubMed/NCBI
Google Scholar
48. Bonn-Miller MO, Boden MT, Vujanovic A a., Drescher KD. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychol Trauma Theory, Res Pract Policy. 2011;5: 193–200.
View Article
Google Scholar
Curated by Thc 420 Hemp
source https://weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/
1 note
·
View note