#mdr medical
Explore tagged Tumblr posts
Text
1 note
·
View note
Text
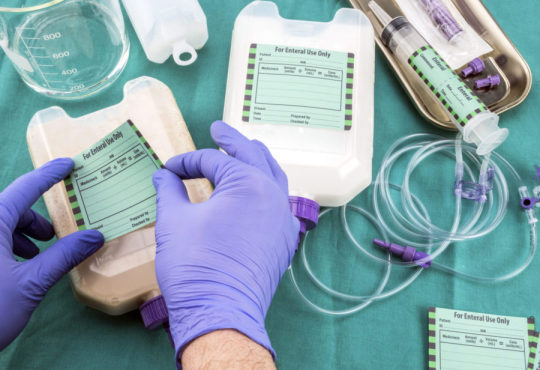
Medical Device Labeling: The Important Role Of Medical Device Label In Patient Care

Medical devices sold in the United States must comply with labeling requirements set by the Food and Drug Administration (FDA). All labels must contain specific information about the device such as its intended use, any potential risks or side effects, and instructions for use. Device labels provide critical safety information that helps ensure devices are used properly. The FDA regulates labeling to protect patient health.
Labels Must Clearly Identify The Device
First and foremost, a Medical Device Labeling must clearly identify the specific device. This includes stating the product name and any applicable product codes or reference numbers. Having a clear device name and identification numbers helps providers and facilities properly select, use, and track the intended device. Any trade or brand names must also be included but must not overshadow the core product identity information.
Instructions For Use Must Be Clear And Complete
Detailed instructions for use are extremely important for medical devices to be operated safely and as intended. Labels must provide step-by-step directions on how the device is to be used, prepared, fitted, applied, implanted, or operated. Pictures, diagrams or other illustrative guides can help visualize proper technique when words alone may not fully explain the process. Instructions must account for all reasonably foreseeable uses. Omitting any necessary steps could result in misuse leading to patient harm.
Packaging Labels Provide Sterility And Shelf Life Assurances
For devices distributed in single-use sterile packaging, labels must affirm the method used to sterilize the contents as well as the expiration date or shelf life. This information guarantees the continued sterility and integrity of the device up until the noted expiration date. Devices like surgical tools and implants must remain free of contaminants when used. Packaging labels demonstrate the steps taken to achieve and maintain sterile conditions.
Potential Adverse Reactions And Hazards Must Be Disclosed
Device labels must include a complete list of known or reasonably foreseeable adverse health effects or hazards from use. This involves describing any potential allergic reactions, biological risks, toxicology concerns and interactions with other devices, drugs or substances. Precautions, contraindications and any use limitations due to patient risks or conditions should also be detailed. Making providers aware of safety issues enables them to properly assess risks and benefits for individual patients.
Symbols Standardize Hazard And Safety Communications
Pictograms and symbols play an important role in medical device labeling by allowing concepts to be recognized universally without language barriers. Common symbols indicate requirements such as “Do Not Reuse”, “Sterilized Using Irradiation”, “Keep Away From Heat or Flames”, and many others defined by International Standards Organization (ISO) regulations. Having standardized symbols helps labeling information be consistently interpreted.
Product Labels Remain With The Device
Device manufacturers must ensure all labeling remains affixed or adjacent to the medical device itself throughout distribution and use. Shipping labels serve to identify devices during transportation but do not replace the requirement for complete labeling on the finished able product. Device labels need to be available anytime and anywhere the device is utilized to provide critical use and safety guidance to providers.
As described, medical device labelingserves the fundamental purpose of guiding appropriate and safe use while communicating potential risks. Complying with comprehensive FDA labeling policies supports quality patient care.
Get more insights on this topic: https://www.pressreleasebulletin.com/medical-device-labeling-device-labeling-regulations-ensuring-patient-safety-and-compliance/
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Medical Devices#Regulatory Compliance#Product Labeling#FDA Regulations#UDI (Unique Device Identification)#MDR (Medical Device Regulation)#Labeling Standards#Risk Management#Health Canada Regulations#Clinical Labeling
0 notes
Text
Electronic IFU for Medical Devices: MDR Software
The integration of Electronic Instructions for Use (eIFU) with Medical Device Regulation (MDR) software marks a significant advancement in the healthcare industry. This innovative approach not only ensures compliance with stringent regulatory standards but also enhances the accessibility and usability of critical device information for healthcare professionals. By digitizing the IFU, medical device manufacturers can provide up-to-date, easily accessible, and interactive guides that improve patient safety and streamline device operations. Explore how eIFU powered by MDR software is revolutionizing the medical device landscape, ensuring better outcomes for both providers and patients.
For more info visit our website: https://www.ddismart.com/visu-eifu-electronic-labeling/
#medical devices#medical device regulations#medicaldevicequality#regulatorycompliance#healthcareinnovation#regulatorychallenges#complianceassurance#continuousimprovement#industrystandards#mdr software
0 notes
Text
For manufacturers of in vitro diagnostic (IVD) devices seeking to market their products in the European Union, ensuring MDR compliance is a critical requirement. The EU Medical Device Regulation (MDR) has brought about significant changes in the regulatory landscape, and manufacturers must navigate these new rules to achieve and maintain compliance. Before divulging details, it is essential to understand IVD compliance.
0 notes
Text
If you are looking for the medical device registration services in India then get in touch with us. We are ready too help and assist you for the registration of CDSCO Medical Device. It is mandatory for the manufacturer, importers and wholesalers of medical device in India.
#medical devices#medical device license#medical device registration#cdsco registration#CDSCO license#registration#MDR#certificate#elt corporate
0 notes
Text
drunk and high as hell ama
#fugo.txt#i took a benzo and some fernet w cova whixh is like a cocktail of fernet and cocacola#fun facy fernet was mdr to ve medication
1 note
·
View note
Text
PMCF Medical Device
Cetas Healthcare is one of the top global Syndicated Research with an exclusive focus in the medical device industry. We offer PMCF Surveys, syndicated reports, PMCF Medical Device, Syndicated Market Research, clinical data & Custom Market Research solutions, and more to our clients. With extensive practice in the Cardiovascular space, we also have prior experience in several therapy areas. We work regularly with top global medical device companies like GE Healthcare, Boston Scientific, Medtronic, Baxter, B Braun, BD, etc., helping them with their product development & management needs. Our offices are located in Singapore, Netherlands, USA, and India.
#pmcf#pmcf surveys#pmcf services#pmcf solutions#PMCF Medical Device#PMCF#PMCF studies#pmcf report#post market clinical follow up#eu medical device regulation#mdr medical device
0 notes
Text
Global Medical Device Vigilance Market is Estimated to Witness High Growth Owing to Increasing Regulatory Requirements and Technological Advancements

The global Medical Device Vigilance Market is estimated to be valued at US$ Mn in 2022 and is expected to exhibit a CAGR of 6.89% over the forecast period 2021-2028, as highlighted in a new report published by Coherent Market Insights.
A) Market Overview:
The Medical Device Vigilance Market refers to the processes and systems put in place to monitor and report on the safety and performance of medical devices. This includes tracking adverse events, product recalls, and safety notifications. Regulatory bodies across the globe have implemented stringent guidelines and regulations to ensure patient safety and product efficacy. The market offers software solutions to assist manufacturers in complying with these regulations and maintaining the safety of their devices.
B) Market Dynamics:
The Medical Device Vigilance Market is driven by two main factors. Firstly, the increasing regulatory requirements imposed by regulatory bodies such as the FDA and European Union's Medical Device Regulation (MDR) have made it mandatory for manufacturers to have a robust vigilance system in place. Failure to comply with these regulations can result in heavy penalties and damage to the brand reputation.
Secondly, technological advancements have played a significant role in the growth of the market. The development of advanced software solutions that can automate and streamline the vigilance process has made it easier for manufacturers to monitor and report adverse events. These software solutions offer features such as real-time reporting, data analytics, and automated workflows, which enhance the efficiency and effectiveness of the vigilance process.
For example, companies like AssurX Inc. and Sparta Systems offer software solutions that enable manufacturers to track and manage adverse events, streamline the reporting process, and ensure compliance with regulatory requirements.
C) Segment Analysis:
The Medical Device Vigilance Market can be segmented based on the type of solution, end-user, and region. The software solutions segment is expected to dominate the market, as it offers a comprehensive and automated approach to vigilance management. Among the end-users, the medical device manufacturers segment is expected to dominate, as they are the primary stakeholders responsible for ensuring the safety and performance of their devices.
D) PEST Analysis:
- Political: Regulatory bodies across the globe have implemented stringent guidelines and regulations to ensure patient safety and product efficacy. Examples include the FDA in the US and the European Union's Medical Device Regulation (MDR).
- Economic: The demand for medical device vigilance solutions is expected to grow due to the increasing number of medical device manufacturers and the rising adoption of digital healthcare technologies.
- Social: Healthcare professionals and patients are increasingly becoming aware of the importance of medical device safety, leading to a higher demand for vigilant solutions.
- Technological: Technological advancements have led to the development of advanced software solutions that can automate and streamline the vigilance process, enhancing the efficiency and effectiveness of the monitoring and reporting.
E) Key Takeaways:
- The global Medical Device Vigilance Market is expected to witness high growth, exhibiting a CAGR of 6.89% over the forecast period, due to increasing regulatory requirements and technological advancements.
- Europe is expected to be the fastest-growing and dominating region in the market, thanks to the stringent regulations imposed by the European Union.
- Key players operating in the global Medical Device Vigilance Market include ZEINCRO, AssurX Inc., Sparta Systems, Oracle Corporation, Xybion Corporation, Sarjen Systems Pvt. Ltd., MDI Consultants Inc., AB-Cube, Laerdal Medical, and Omnify Software Inc. These players offer a range of software solutions and services to assist medical device manufacturers in complying with regulatory requirements and maintaining the safety of their devices.
#Medical Device Vigilance Market#Medical Device Vigilance Market Insights#Coherent Market Insights#Medical Device Vigilance Market High Growth#chronic diseases#healthcare#monitoring#Medical Device#remote monitoring#patient care#MDR
0 notes
Text

Nu10 specializes in developing eu mdr software as a medical device solutions that meet the highest standards for medical devices. Our state-of-the-art software products are meticulously designed to adhere to the rigorous regulatory requirements of the European market. With Nu10's expertise in software development and regulatory compliance, we ensure that medical device manufacturers can confidently bring their innovative products to market, contributing to improved patient outcomes and healthcare advancements.
0 notes
Text
#Medical Devices#IVDs#EU MDR#Regulatory Affairs#PMCF Plan#PMS system#Clinical Evaluation Report#EU Regulations#European Union#PMS#EU#EU MDR 2017/745
0 notes
Text
#medical devices#eumdr transition extension#mdd to mdr#what is mdr transition#operon strategist#medical device regulation#mdr transition#mdr transition extension#ce mark certification#mdd vs mdr#eumdr podcast#transition extension#mdr extension#viral shorts#tranding shorts#mdr#eumdr#mdd to mdr transition#transition#medical device#operonstrategist#medicaldevicenews
1 note
·
View note
Text
various and sundry severance theories
assuming that “helly” is actually helena right now, helena is down there both for surveillance/to find out what the innies know, and to sow doubt about helly’s actual story when she comes back. she doesn’t see the innies as people and assumes that they will fracture with mistrust but i 100% don’t doubt that they will believe her after a little while
mark has either begun reintegration or definitely will do it in this season. my sources: the weird guy in the background when he’s in wellness in s2e1, and the shot from the trailer where he looks like shit (aka outie mark) and sees ms casey in the hallway
part of the Kier Lore is that he either discovered or created the great lakes (“Kier Invites You to Drink of His Water”)
PE could be an alternate universe michigan as the Eagan Peninsula or Eagan Province or, as my partner said, it could stand for Perpetuity. they’re in the state of perpetuity. haha get it
the civil war is VERY RELEVANT to the alternate history - lots of file names have relevance to the civil war (lexington i.e. the lexington letter, cold harbor i.e. the battle of cold harbor). lumon was founded in 1865. maybe kier was a civil war medic? his quote on his town square statue is something like “i dug into the soldiers and within them found the war”
what they are actually trying to do with the severance chip: make people happier by forgetting death or forgetting the bad things in their lives. and also bringing people back from the dead/making cloned copies of people that are then processed by MDR. lumon does not see itself as evil it sees itself as a force for goodness in the world
related to the last point: ms cobel is so obsessed with reintegration because she lost her mother (charlotte cobel) and is hoping that lumon will be able to bring her back somehow using the chip - but the severance chip creates an almost blank slate, and so she’s been trying to prove that it is possible to merge both versions of a person into somebody who remembers everything.
dylan’s arms are always sore because his outie is carrying around his child
127 notes
·
View notes
Text
#Medical Devices#Regulatory Compliance#Product Labeling#FDA Regulations#UDI (Unique Device Identification)#MDR (Medical Device Regulation)#Labeling Standards#Risk Management#Health Canada Regulations#Clinical Labeling#Device Instructions for Use#Packaging and Labeling#Medical Device Safety
0 notes
Note
I saw an ask regarding adhd just wanna give an advice bcs I suffer a lot from mental health including MDR if yk MDR then good else in short it's Major Depressive disorder not only this but I have trauma issues due to my past and like I have slight autism with panick disorder and anxiety disorder I panick a lot. Weeks before I was suffering from ADHD but it was not huge and no doctors will give someone antidepressants for ADHD they need to check like what it's for and for kind info there is no particular med for ADHD it's mainly treated by SNRI and SNRI stands for Serotonin–norepinephrine reuptake inhibitor it's bcs ADHD comes with depression as per ik like depression is the first thing and when it stays for year without any treatment it will cause other issues such as ADHD, panick disorder etc. ADHD is a disease where someone lacks from attention deficiency and it might be due to the person was sad and the brain is trying to focus on escape like yk. And there is no medicine which can fix particularly ADHD only like it will act on it moreover ADHD is caused bcs of a hormone named norepinephrine, this hormone gradually decreases due to some reason and when it happens our brain can't act normally this medicine contains some drugs which binds on the nerve receptors slowing down the absorption and there is the magic as the absorption slows down in the nerve there is excess hormone which remains in our brain and this helps not triggering ADHD. And same goes for Depression but it's bcs of lack of serotonin and lack of serotonin can cause other hormone to decrease bcs serotonin is a mood regulator, we basically have two hormone for happiness oxytocin and dopamine but Serotonin is like a commander for them controlling them if there is a lack of serotonin people will find difficulty to process emotion, causing mood swings including depression. So to keep it ok SSRI are used. SSRI stands for Serotonin Reuptake Inhibitor process is same but it got different drugs and this drugs are legal and safe and SSRI targets the serotonin receptor and it works in the same way like SNRI does.
So what I wanna say is not to blame the doctor bcs it totally depends on the patient on how they co-operate if they don't tell doctors openly what they are suffering from doctors aren't god that they will understand it. In my case at first I used to be afraid but i gradually gain confidence and right now my doctor is like a bestfriend for me I openly talk with him with any difficulty I am facing, and just months ago I used to take SNRI bcs I had slight ADHD it eventually got fixed but as my main issue is Depressions he gave me a better SNRI like a change and better one than my previous SSRI it's not bcs that med wasn't working it's bcs I was getting a bit dependant on it moreover he is a MD in General Medicine and he was a professor plus doctor in one of the most reputated medical College and hospital of our city where most reputated doctor of our state comes and it's a government hospital and college right now he is a professor and doctor in another well reputated Government Medical College and Hospital of our state.
And if this person have openly communicated with their doctor then I will say to change their doctor, but I still feel fishy about this as no doctor will give someone wrong medicine bcs it will literally result in cancellation of their license. Anyway not blaming anyone also if it's that person whom ik I won't mention their name for privacy then they can put an ask and i will give the doctor's number even though he doesn't do any private chambers unless for emergency or any close one the doctor is a good friend of my dad so that's why I can go for check up to him, I said this bcs that person ik also had this same disease anyway sorry for putting this long ask just wanna help people in need despite anything pls do post this ask.
You’re all good love, thank you for spreading awareness and helping!!
2 notes
·
View notes
Note
I'm diagnosing akutagawa with tuberculosis btw - lung diseases are one of my special interests and I've thought about it for a while
He does not want to go to Mori for a cure and bc he's a mafioso he can't js go to a normal hospital plus he doesnt want to admit weakness, that's why it could reach a deadly stage
Alternatively he contracted it in the Slums, went on antibiotics for a while, didn't finish the course and it evolved into MDR-TB (Multi drug resistent tuberculosis) and can't be medicated that easily anymore
I love theorizing about his lung disease I totally think this could be a possibility...I love the idea that Dazai has drilled unto Akutagawa's head that he cannot go to Mori for any medical help no matter the case no matter what happens to him and Akutagawa never questions it. And I really like your theory at the end here that it's really difficult to treat and he's giving up trying to do so, suffering silently when no one truly knows how bad he's doing 💔💔💔
#i love akutagawa so much he makes me very sad#bsd headcanons#bungo stray dogs#bungou stray dogs#lung disease#tuberculosis#medical trauma#akutagawa#mori#dazai#bsd#illness#sick#ask box
11 notes
·
View notes
Text
USAmerican problems in providing healthcare are resulting in the spread of antibacterial-resistant strains.
On top of the overuse of antibiotics contributing to the development of antibacterial-resistant bacteria, people who leave their entire country to access affordable medical care are at a very high risk of spreading resistant bacteria around. This study, for example, found that 30% of international travellers returned with a antimicrobial resistant bacterium. Now consider that that is among completely healthy travellers, and that hospitals tend to be hotbeds for antibiotic and antimicrobial resistant strains of bacteria.
Not only is it dangerous for the individual to leave the country for healthcare (what if you don’t speak the language? Do you have a place to safely recover there? What if you have a complication but you’ve already arrived back at your home country?), not only is it absolutely ludicrous and dystopian that someone in a country with the ability to have good quality medical care would have to LEAVE to actually get such care, it’s also contributing to one of the biggest problems in medical science today.
Universal healthcare is a necessity.
(Additional sources below the cut)
http://www.jstor.org/stable/26690839
#medical tourism#healthcare#antibiotics#antibiotic resistance#medical science#medical microbiology#universal healthcare
3 notes
·
View notes