#major somnolence disorder
Explore tagged Tumblr posts
Text
The Comprehensive Compilation of Adverse Reactions Associated with Cabergoline 0.25mg
Cabergoline 0.25mg is a frequently given prescription for many medical disorders. However, it is important for users to be aware of the possible negative effects that may accompany its usage, as is the case with any pharmaceutical. This page provides an extensive list of side effects linked to Cabergoline 0.25mg, including both typical responses and more severe cautions and long-term concerns. Through comprehending these adverse consequences and acquiring the knowledge to handle them, people may make well-informed choices about their therapy and overall state of health.
Cabergoline is a medication used to treat medical conditions such as hyperprolactinemia and Parkinson's disease
Cabergoline is a pharmaceutical compound classified as a dopamine agonist. It is often used in the treatment of disorders such as hyperprolactinemia, which may result in complications such as infertility, irregular menstruation, and lactation in both males and females.
Medical uses of Cabergoline 0.25mg
Cabergoline 0.25mg is often used for the treatment of hyperprolactinemia, a medical disorder defined by elevated levels of prolactin in the bloodstream. This medicine effectively reduces prolactin levels and effectively manages symptoms such as infertility, decreased libido, and irregular menstruation.
Adverse effects on the digestive system
Typical gastrointestinal adverse effects of Cabergoline 0.25mg may include nausea, emesis, constipation, and stomach discomfort. To reduce side effects, it is crucial to consume this drug with meals.
Neurological Adverse Reactions
Individuals using Cabergoline 0.25mg may encounter symptoms such as vertigo, somnolence, or cephalalgia. It is recommended to refrain from driving or operating heavy equipment until you are aware of the impact of this drug on your abilities.
Adverse effects on the cardiovascular system
Certain people may encounter alterations in blood pressure or heart rate while consuming Cabergoline 0.25mg. Regularly monitoring these measures and promptly reporting any major changes to your healthcare professional is essential.
Cabergoline 0.25mg is used to treat a variety of illnesses that arise from excessive production of the hormone prolactin. It may be used to treat pituitary prolactinomas, which are tumors of the pituitary gland, as well as certain menstruation issues and issues with fertility in both sexes.
Possible cardiac valve impairment
An important concern connected with Cabergoline is the possibility of cardiac valve injury, especially in those who are prescribed greater dosages for a prolonged duration. Consistent monitoring of the heart is crucial in order to promptly identify any anomalies in the valves.
Potential for Fibrotic Reactions
The use of Cabergoline has been associated with the emergence of fibrotic responses in diverse tissues, such as the cardiac, pulmonary, and abdominal tissues. If you encounter symptoms such as respiratory distress or edema in the limbs, it is advisable to promptly seek medical assistance.
Potential for the Development of Tolerance
Prolonged usage of Cabergoline might result in the development of tolerance, necessitating greater dosages to get the same therapeutic outcome. It is essential to adhere to the recommendations of your healthcare practitioner and refrain from altering your dose without seeking their advice.
Effect on Hepatic Function
Cabergoline has the potential to impact liver function in some people, resulting in increased levels of liver enzymes. It is advisable to undergo regular liver function tests while taking this drug in order to monitor any changes and avoid any problems.
Controlling and Reducing Side Effects
If you are encountering adverse reactions as a result of consuming Cabergoline 0.25mg, do not worry; there are strategies to address them. Here is a method to effectively control and reduce undesired consequences.
Titration and Surveillance of Dosage
Seek guidance from your healthcare professional about the possibility of modifying your Cabergoline dose. Regular surveillance may aid in monitoring the body's response to the medicine.
Implementing lifestyle modifications to mitigate adverse effects
Modest lifestyle adjustments may significantly alleviate adverse effects. To promote your general well-being, it is important to ensure that you stay well hydrated, follow a balanced diet, and make regular exercise a priority.
Drug Interactions
It is essential to be aware of the potential interactions between Cabergoline and other drugs for your safety and well-being. Now, let's explore the possible interactions and contraindications that need to be monitored.
Important Drug Interactions to Be Mindful Of
Cabergoline may have adverse interactions with some drugs. Ensure that you regularly update your healthcare practitioner about all the drugs you are currently taking in order to prevent any possible drug interactions.
Interactions with Specific Medications
Certain drugs should be avoided while using Cabergoline owing to the potential for adverse interactions. Take note of these contraindications to avoid any potential consequences.
Specific considerations for certain demographics
Customized strategies may be necessary for administering Cabergoline to various groups. Below are important factors to consider for pregnant or nursing women and geriatric patients.
Cabergoline is used to treat hyperprolactinemia (high levels of prolactin, a natural substance that helps breast-feeding women produce milk but can cause symptoms such as infertility, sexual problems, and bone loss in women who are not breast-feeding or men). Cabergoline is in a class of medications called dopamine receptor agonists. It works by decreasing the amount of prolactin in the body.
Women who are currently pregnant or breastfeeding
When you are pregnant or breastfeeding, it is crucial to have a conversation with your healthcare professional about the potential advantages and disadvantages of using Cabergoline. The utmost importance should be placed on ensuring the well-being of both you and your kid.
Geriatric Patients
Cabergoline may need special care for elderly people. Close surveillance and possible dose modifications may be required to guarantee the safety and efficacy of the medication.
Summary and Concluding Remarks
Understanding and addressing the possible side effects, interactions, and concerns of Cabergoline 0.25mg may seem challenging, but with enough information and help, you can successfully handle them. It is important to constantly seek advice from your healthcare professional for specialized assistance that is specifically targeted to your individual requirements.
Ultimately, it is essential for both patients and healthcare practitioners to have a thorough understanding of the potential adverse effects of Cabergoline 0.25mg. By being knowledgeable about the possible hazards, closely monitoring for any worrisome symptoms, and seeking advice from a healthcare expert as necessary, people may manage their course of treatment with more assurance and security. It is important to emphasize that taking a proactive approach to managing and maintaining open lines of communication are crucial in achieving the most favorable results while using Cabergoline 0.25mg.
1 note
·
View note
Text
Why Early Diagnosis by Bipolar Disorder Specialists is Important for Long-Term Recovery
Bipolar disorder, an illness that is considered to be a serious mental health condition, is marked by extreme mood, energy, and activity level fluctuations. It can range from full-blown major depressive disorder to full-blown manic episode. Bipolar disorder affects any age group, but early diagnosis and intervention can ensure more profound recovery over time. In such cases, bipolar disorder specialists at centers like Tulasi Healthcare can make the difference.

The Importance of Early Diagnosis:
Mildness of Symptoms: The earlier the diagnosis, the sooner the treatment is started. This may reduce the severity and the number of manic and depressive episodes that may occur and reduce their impact on life.
Improved Quality of Life: Early intervention may help a person maintain stable moods, improve relationships, and enhance the overall quality of life. Reduced Risk of Complications: Untreated bipolar disorder can lead to serious complications, including substance abuse, suicidal thoughts, and even self-harm. Early diagnosis and treatment can significantly reduce these risks. Enhanced Social and Occupational Functioning: Early intervention can help individuals maintain their academic or professional pursuits, improve social relationships, and increase their overall sense of well-being. Long-Term Recovery: Early and consistent treatment can significantly increase the chances of long-term recovery and improve an individual's overall prognosis. The Role of Bipolar Disorder Specialists at Tulasi Healthcare:
At Tulasi Healthcare, our team of bipolar disorder specialists plays a crucial role in early diagnosis and effective treatment. We understand the importance of:
In-depth Evaluation: Our professionals provide comprehensive assessments that may include clinical interviews, psychological evaluation, and medical tests when needed for proper diagnosis of bipolar disorder. Customized Treatment Programs: We believe in individualized treatment. Our professionals will collaborate with you to design a tailored treatment program based on your needs and objectives. Ongoing Monitoring and Support: We are there to continuously monitor and support you so that your treatment plan stays effective and the challenges arising can be managed. Signs and Getting Help
If you or someone close to you has some of the following symptoms, you should consult a mental health professional.
Mood swings: Severe highs (mania) and lows (depression) Changes in energy levels: Having episodes of extreme energy and hyperactivity followed by episodes of sleepiness and somnolence Thought racing: Trouble focusing, talkativeness, and flight of ideas Impulsive behavior: Acting on impulse with a tendency for risk-taking and reckless behavior Sleep disturbances: Inability to sleep or excessive sleep Changes in appetite: Either gaining or losing a lot of weight Loss of interest in activities: Showing less interest in things that are usually enjoyed. A treatment plan for successfully managing bipolar disorders requires early detection and intervention, and seeking expertise from experienced practitioners at Tulasi Healthcare can open the door towards a brighter tomorrow.
0 notes
Text
Fluoxetine (Prozac)
I've been on Fluoxetine for a few years now and have recently had my dosage doubled so here's an info post. The words highlighted in green are what personally effects me.
Some eating disorders and mood disorders may be treated with fluoxetine. Insomnia is more likely to occur than drowsiness. The serotonin syndrome may occur if you take fluoxetine with other medications that also release serotonin, such as tramadol, or if you overdose on fluoxetine.
Anxiety and Stress, Major Depressive Disorder, Bulimia, Depression, Premenstrual Dysphoric Disorder, Obsessive Compulsive Disorder, Panic Disorder, Postpartum Depression, Schizoaffective Disorder, Binge Eating Disorder, Agoraphobia, Body Dysmorphic Disorder, Borderline Personality Disorder, Dissociative Identity Disorder, Dysautonomia, Excoriation Disorder, Fibromyalgia, Hot Flashes, Intermittent Explosive Disorder, PANDAS Syndrome, Persistent Depressive Disorder, Premature Ejaculation, Somatoform Pain Disorder, Trichotillomania, Vulvodynia are some of the conditions this medication is used to treat.
Fluoxetine is an SSRI.
Side Effects
The most common side effects reported are; insomnia, asthenia (fatigue), and headaches.
Very Common
Headache
Somnolence (drowsiness, or the desire to sleep)
Tremor
Dizziness
Insomnia
Anxiety
Nervousness
Nausea
Diarrhea
Dry Mouth
Rhinitis (runny or itchy nose, sneezing)
Pharyngitis (sore throat)
Yawning
Asthenia (fatigue)
Anorexia
Decreased Sex Drive
Flu Syndrome
Common
Amnesia
Hyperkinesia (restlessness)
Paresthesia (a burning/prickling/numb/tingling/itching sensation that occurs on the hands, arms, legs, or feet, ect.)
Change of Taste
Abnormal Dreams
Agitation
Difficulty Paying Attention
Unstable Emotions
Hostility
Hypermania
Mania
Abnormal Thinking
Abdominal Pain
Constipation
Indigestion
Gassy-ness
Vomiting
Nosebleeds
Ear Pain
Fever
Thirst
Tinnitus
Decreased or Increased Appetite
Weight Loss
Painful Period Cramps
Erectile Dysfunction
Trouble cumming
Increased Need to Urinate
Hypertension
Abnormal Heartbeat
Rash
Sweating
Itchy Skin
Abnormal or Blurred Vision
Muscle Twitching
11 notes
·
View notes
Photo

SUPREME COURT ORDERS JOHNSON & JOHNSON TO PAY $2.1 BILLION IN BABY POWDER LAWSUIT
Editor’s Note: We’ve been reporting on this issue for years, pointing out the cover up and the deceptive practices of Johnson & Johnson. Here’s an article I penned in 2016… For 40 Years Johnson & Johnson Hid Baby Powder Ovarian Cancer Connection.
The Supreme Court on Tuesday rejected an appeal by Johnson & Johnson (J&J) to reverse a $2.1 billion verdict for plaintiffs who claim the company’s talc powder products gave them ovarian cancer.
The pharmaceutical company, which developed the Janssen COVID vaccine, asked the top court to review the verdict, arguing it didn’t receive a fair trial in Missouri where the court awarded a $4.7 billion payout to 22 women who developed ovarian cancer.
The verdict was reduced to $2.1 billion in June 2020, by a Missouri court of appeals.
J&J stopped selling talc powder products in the U.S. and Canada last year. But the company still faces more than 21,800 lawsuits alleging asbestos in its talc powder products, including baby powder, caused ovarian cancer.
Ken Starr, a prosecutor representing women who sued J&J, wrote in court briefings that the pharma company “knew for decades that their talc powders contained asbestos, a highly carcinogenic substance with no known safe exposure level.”

“They could have protected customers by switching from talc to cornstarch, as their own scientists proposed as early as 1973. But talc was cheaper and petitioners were unwilling to sacrifice profits for a safer product.”
J&J maintains its baby powder is safe and does not contain asbestos or cause cancer.
The lawsuits linking talc powder to cancer aren’t the first time J&J has been sued over the safety of its products.
Other major J&J lawsuits and recalls for faulty products include:
1995: $7.5 million fine for destroying documents to cover up an investigation into wrongful marketing of its Retin-A acne cream to remove wrinkles.
1996: An undisclosed settlement on false claims over condom protection claims to protect against HIV and other STDs.
2001: Paid out $860 million in a class action lawsuit for misleading customers about prematurely discarding its 1-Day Acuvue soft contact lens. J&J recommended they should be worn only once, although it was discovered the lenses were no different than the regular Acuvue lens that would last for two weeks.
2010: $81 million settlement for misbranding its anti-epileptic drug Topamax to treat psychiatric disorders and hiring outside physicians to join its sales force to promote the drug for unapproved conditions. The following year, J&J paid $85 million for similar charges against its heart drug Natrecor.
2011: Several J&J baby products were discovered to contain carcinogenic ingredients.
2013: The U,S. Justice Department charged the company $2.2 billion in criminal fines for marketing its autism and anti-psychotic drug Risperdal for unapproved uses. Forty-five states had filed civil lawsuits against J&J in the scandal. Janssen also had an aggressive campaign to market Risperdal for use in children with behavioral challenges. Other serious adverse effects from Risperdal reported by the FDA include diabetes mellitus, hyperprolactinaemia, somnolence, depression, anxiety, psychotic behavior, suicide and death.
7 notes
·
View notes
Text
Quetiapine Fumarate


Brand Name: Seroquel
Generic Available
Common Dosage Forms:
Tablets: 25 mg, 50 mg, 100 mg, 200 mg, 300 mg, 400 mg
Tablets, extended release (XR): 50 mg, 150 mg, 200 mg, 300 mg, 400 mg
FDA Indications/Dosages:
For the treatment of schizophrenia: Initial dose should be started at 25 mg twice a day, with increases in increments of 25-50 mg two to three times a day on the second and third day. Maintenance dose should be between 300-400 mg daily, given in 2-3 divided doses or one extended-release tablet. Further dosage adjustments should not be made in less than every 2 days. Maximum daily dose is 800 mg/day. Indications for a slower dosage titration include the elderly, hepatic dysfunction, and hypotensive patients.
For the treatment of depressive episodes associated with bipolar disorder: Taper up to 300 mg at bedtime by starting with 50 mg and increasing to 100 mg the second day and 200 mg the third day.
For the treatment of acute manic episodes associated with bipolar 1 disorder, as monotherapy or adjunct therapy to lithium or divalproex: Initial dose should be started at 50 mg twice a day. Increase dose by 100 mg/day until a total daily dose of 400 mg is reached. Further dosage adjustments up to 800 mg/day may be made.
For the treatment of major depressive disorder (XR tablets only): Start with 50 mg on days 1 and 2 then increase to 150 mg on days 3 and 4. Maintenance dose is 150-300 mg once daily.
Hepatic function impairment dosing: Start patients on 25 mg/day and increase by 25 mg/day, depending on the clinical response and tolerability of the patient.
Monitor: FBG, Lipid panel, Weight, BP, CBC, Eye (q6 months)
Pharmacology/Pharmacokinetics: Quetiapine fumarate is a multiple neurotransmitter antagonist with activity at serotonin 5HT1A and 5HT2, dopamine D1 and D2, histamine H1, and adrenergic alpha-1 and alpha-2 receptors. The mechanism of action of quetiapine is thought to be due to its action at the dopamine D2 and serotonin 5HT2 receptors. Peak plasma levels are reached in 1.5 hours after an oral dose. Elimination occurs via hepatic metabolism with a terminal half-life of about 6 hours.
Drug Interactions: May have additive sedative effects with other CNS depressants. Oral clearance is increased by phenytoin and thioridazine.
Contraindications/Precautions: CONTRAINDICATED IN PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. MAY INCREASE SUICIDAL THOUGHTS IN CHILDREN AND YOUNG ADULTS. Rare cases of neuroleptic malignant syndrome have been reported during clinical trials. Be observant for signs such as hyperpyrexia, muscle rigidity, altered mental state, and autonomic instability. Tardive dyskinesia (TD) has been reported in patients taking antipsychotic medications. If signs and symptoms of TD appear during therapy, discontinuation of medication may be warranted. Orthostatic hypotension may occur during treatment, especially during the initial dose-titration period. Use caution in patients predisposed to hypotension. Cataracts may develop during treatment. Lens examination should occur initially and at six-month intervals. Use with caution in patients predisposed to seizures, dysphagia, hypothyroidism, or with a history of breast cancer. Atypical antipsychotics have been associated with severe hyperglycemic reactions. These reactions have included ketoacidosis, coma, and death. Patients with established diagnosis of diabetes mellitus who are started on quetiapine should be monitored regularly for worsening of glucose control. Avoid breast feeding during therapy. Pregnancy Category C.
Adverse Effects: Somnolence (18%), dizziness (10%), constipation (9%), postural hypotension (7%), dry mouth (7%), and dyspepsia (6%). Adverse effects occurring in approximately 1% of patients include: hypertonia, flu syndrome, anorexia, palpitations, pharyngitis, rhinitis, cough, dyspnea, peripheral edema, sweating, and leukopenia.
Patient Consultation:
Use caution when rising from a prone position, especially during initial dose-titration period.
May cause drowsiness-use caution when performing activities that require alertness.
May be taken without regard to meals.
Seroquel XR should be swallowed whole and not crushed, divided, or chewed.
Do not change dosage except on the orders of your physician.
Store in a cool, dry place away from sunlight and children.
Contact a physician if the above side effects are severe or persistent.
If a dose is missed, skip it and return to normal dosing schedule.
9 notes
·
View notes
Text
How To Buy Modafinil With Debit Or Credit card ?
If you're taking it near the evening yow will discover it tough to fall asleep. Keep away from driving. Avoid alcohol. Provigil (modafinil) medicine a well-known stimulant is assisting to maintain awake and to not fall asleep driving. Provigil is the trade title below which the prescription remedy Modafinil is offered. Sleeping to the again ought to turn out to be prevented, since that may be a state of affairs once the language will be slipping again the actual air passage, thus consider to sleep on a facet.
People that resulting from the nature of the jobs must do prolonged hours, identical to trucks drivers that must have to invest prolonged hrs behind the wheel, together with consequently incessantly really feel affected by drowsiness. The medical therapy Generic provigil that's now being utilized for the therapy of Alzheimer’s illness in addition to demential has a history of success. Apathy continuously impacts sixty-six per cent of individuals which have been clinically diagnosed with Alzheimer’s illness. Provigil impacts the central nervous system. Keep away from different harmful activity until you know how this treatment will have an effect on your degree of wakefulness.Keep away from drinking alcohol while taking Provigil. Narcolepsy drawback is no doubt one of the sleep ailments which impacts concerning 1 with any 2001 us residents, and in addition this type of ailment just isn’t treatable, having mentioned that it might conveniently end up being was able. This medicinal drugs may also be prescribed by medical doctors to the treatment of a few different sleep issues, nonetheless broadly employed for shift operate sleep drawback, narcolepsy condition as well as obstructive sleep apnea.
The effect of buy generic provigil provigil online continues about ten hours, a lot more or less, in addition to it works its best right after a couple of hours because it’s been taken. This disorder as a result of typical snooze interactions can result in lots of alerts, for instance usability to drop resting or insomnia or to the varied different finish is increased drowsiness. This syndrome on account of typical sleep interactions can lead to quite a lot of issues, which embrace usability to drop sleeping or insomnia or on the opposite finish is increased sleepiness. At this level, too you will discover zero cure to turn into discovered for this disorder, then again medicine like Provigil could help to handle this ailment. That is primarily as a result of the patient who is being administered Provigil returns to normal sleep. Some athletes additionally use Modafinil due to the truth that it improves our response time, making it good for sports activities, or any other physical exercise.
Just remember to see a well being care supplier simply in case you assume which you want enable to obtain right sleep for you personally. There are lots of quite a few other issues, wherever Provigil generic might help. Always had good prices, fixed online help and exceptional customer service, which makes an order course of very simple and privies an excellent help to their clients. Polysomnography: It can assist to look at the pauses whereas breathing throughout sleep. However, compensation remboursement Transat several researches recommended that Provigil can be used as an effective adjunct therapy to patients experiencing clinical depression. Provigil drug is a medication that has an lively ingredient called Modafinil, and likewise this medical therapy is being really useful for those that endure from several sleep ailments, for example narcolepsy, cease snoring in addition to shift work sleep syndrome. Make sure you disclose to your physician or health care professional any allergic reactions you've got with this drug. It cheers the emotions and is utilized for fairly just a few enjoyable or every day life model functions.
Take one 100mg pill for a period of up-to 8-hours stimulation, as soon as or twice daily. This makes this condition a really embarrassing one to have. For instance, sleep assaults ceaselessly materialize any time a human being had some extremely stable feelings, a majority of these as fury or just frivolity, or even when the significant together with major meal have just been consumed. There are three primary sorts of sleep apnea, which are additional mentioned right here. It has been accepted by the United Stated Meals and Drug Administration for the therapy of narcolepsy, shift work sleep disorder, and day time sleepiness that is caused by sleep apnea, although accredited indications could vary from nation to country.
Despite which interval you’re employed, you still actually ought to be obtaining satisfactory sleep, certainly not a lot less when in contrast with a couple of different people that don't accomplish transfer work. Despite the success regarding Provigil generic, completely nothing beats the great evening sleep. This disorder’s most frequent indication is termed extreme somnolence, and this kind of indication isn’t some factor that can merely disappear simply because you will possess a appropriate evening time sleep. There exists another various for you to buy Generic provigil simple, that’s exactly the very same becasue it's branded therapies, however by way of just about all easy medicines to buy Provigil easy will set you back a lot much less. Seek emergency medical attention should you suppose you might have used too much of this drugs.
1 note
·
View note
Text
Which one is the best Nootropic to take for Sleep Disorder?
What is Armodafinil?
Armodafinil is a prescription-based drug that enables the reduction of severe somnolence which happens or arises due to different conditions like narcolepsy and other sleeping disorders, such as the moment of halted breathing during sleep (obstructive sleep apnea).
During work hours armodafinil helps you to stay alert and makes you stay awake. If you retain a work strategy that preserves you from possessing a normal sleep habit (shift work sleep disorder) then this medicine is helpful. This therapy does not fix these sleep conditions and may not obtain relief from all your somnolence. Armodafinil dosage does not put up with the place of providing sufficient sleep.
Armodafinil Smart Drug Benefits
Here are some advantages of using Armodafinil:-
Enable you to reconstruct an ordinary sleep cycle
Puts on a regular energy boost
Enhances comprehension, agility, and mental focus
Restores motivation
Probable Armodafinil Side Effects
Headaches
Nausea
Dizziness
Dry mouth
Trouble sleeping
Mood changes
Armodafinil side effects
Headache
Nausea
Dry mouth
Dizziness
Anxiety
Diarrhoea
Difficulty sleeping (insomnia)
Rash
Depression
Indigestion/heartburn
Fatigue
Palpitations
Agitation
Loss of appetite
Attention disturbances
Contact dermatitis
Shortness of breath
Excessive sweating
Nervousness
Numbness and tingling
Fever
Upset stomach
Skin swelling
What is Modafinil?
Modafinil tablet diminishes unconditional drowsiness due to the ailment called narcolepsy and varied sleeping diseases, such as intermissions of halted breathing during sleep (also named obstructive sleep apnea).
This treatment does not remedy these sleep ailments and may not obtain relief from all your drowsiness. Modafinil does not take the spot of obtaining sufficient sleep. It should not be used to deal with fatigue or delay sleep in people who do not have a sleep condition. It is not perceived how modafinil behaves to keep you awake. It is thought to function by impacting particular aspects of the brain that governs the sleep/wake cycle.
Modafinil Pros
Modafinil use has a substantial consequence on different neurotransmitters, containing serotonin and glutamate, and the brain's histamine systems.
All of these neurotransmitters are accountable for regulating mood, understanding, and recollection in our bodies.
In certain, Modafinil 200 mg tablet use is presumed to immediately alter the awareness of catecholamines (amount of neurotransmitters) such as adrenaline and dopamine. In the brain in a way thought to improve administrative functioning and concentration.
Modafinil 200 mg may enhance particular components of cognitive functioning, but it impacts the healthy populace contrarily and comes across to be influential for those with preexisting complications.
Despite some understanding of the neurological processes the medication affects and connects with, it particularly influences the brain. Therefore, even though unscientific evidence may nevertheless motivate modafinil's benefit for its cognition-enhancing outcomes, an additional examination is expected to analyze the importance of its functions. Once you are aware of all the pros and cons of modafinil then you can go ahead and purchase modafinil online from a pharmacy.
Do modafinil and armodafinil have any major differences?
Armodafinil, traded under the brand name Nuvigil, is a fresher drug approximated to modafinil. It was authorized in 2007 as the R-enantiomer of modafinil. Enantiomers are molecules that are mirror impressions of each other—think left and right-handed gloves. In this manner, armodafinil has hardly a different chemical structure approximated to modafinil.
Armodafinil may retain a longer half-life corresponding to modafinil (brand-name Provigil). In some cases, armodafinil may be contemplated as a powerful drug with better insomnia impacts. While both prescriptions can retain similar side impacts, some side effects may be more ordinary in one drug versus the other.
Modafinil Side effects:
Headache
Dizziness
Difficulty falling asleep
Drowsiness
Nausea
Diarrhoea
Constipation
Gas
Heartburn
Loss of appetite
Unusual tastes
Dry mouth
Excessive thirst
Nosebleed
Flushing
Sweating
Tight muscles or difficulty moving
Back pain
Confusion
Burning
tingling, or numbness of the skin
How to buy Modafinil and armodafinil online?
If you require to purchase Modafinil and Armodafinil online then you can acquire them from your closed pharmacy store but if it is not accessible there also then you can effortlessly buy Modafinil 200mg tablets online without any door or problem. There are so numerous well-reputed online drugstores that deliver genuine and accurate treatment assistance online.
If you retain a prescription from your adviser then you can effortlessly order Modafinil and armodafinil online and also achieve the payment through online mode. Furthermore, if you don't endorse advance payment then you can also order armodafinil cash on delivery. Go for a hassle-free purchase and acquire your medicines at your doorstep.
0 notes
Text
ABOUT YOUR MUSE.
If you wish to take to fill out for your own muses, please repost, don’t reblog.

BASICS.
name /. Tartaglia - 11th of the Fatui Harbingers
other name(s) /. Tonia Agafonova ; Subject no. #L001194444
constellation /. Dormitator (The Somnolent or The Sleeper)
birthplace /. Morepesok, Snezhnaya of the Unloved Ark
age /. 14 (official) ; 15-16 (actual)
height /. 5′1″
COMBAT.
On paper, the 11th Harbinger is the holder of a Cryo Vision and an Electro Delusion. She relies on the former greatly, seeming to detest the latter to the point that any usage is restricted to emergencies. Using her Vision, she fights in melee with a spear made from Cryo - though she is not above sudden improvisation or additional weaponry, such as her throwing knives or a series of homing icicles.
Truthfully, however, Tartaglia has no Vision. Her elemental prowess is due to her ability to manipulate ambient elements, both internally and externally - as such, she is not restricted to Cryo, with a moderate skill in Dendro and Electro, and a meager or passing skill in the other elements.
But as her unique biology is a military secret in essence, she does not flaunt this strength of hers in combat. Instead, she allows her opponents to underestimate her - with the knowledge that, should matters become dire, she is always able to turn the tides to her favor. As such, her demeanor in combat is often carefree with a minimum amount of effort put in.
(As a character unit, her entire skillset grants her a series of self-boosting abilities with very minimal support for any teammates alongside her. In that regard, she is meant to be a self-sustainable, solo combatant on the field. Her only weakness is that her self-boosts do not last long or have drawbacks such as HP drain.)
TRAITS & BIO SUMMARY.
Like a majority of the Fatui Harbingers, the 11th’s past prior to her induction in the ranks is shrouded in mystery. However, compared to her peers, any and all files regarding her personal details have been placed under heavy surveillance, requiring the highest level of authorization from the Tsaritsa in order to be accessed and read. Such strict handling of her records is irregular with rare precedent, leading to the persistent rumor that Tartaglia has a secret connection to the Cryo Archon.
It does not help that Tartaglia behaves like a spoiled brat who shortsightedly proclaims her plans to commit regicide, while the Tsaritsa does not so much as blink at what accounts to a declaration of treason. Such blatant ignorance indicates a softness for her—or most likely, a means to enforce obedience. And anyone who believes in the latter would not be incorrect for thinking so.
Prior to her unexpected appearance in the royal courts, Tartaglia was once a normal civilian living in rural Morepesok. However, an incident occurred: she and her older brother went missing for several days, with the girl being the sole of the two to reappear in the forests surrounding their hometown. Following this disappearance, a slew of leyline disorders erupted in the region. Il Dottore was dispatched to research the underlying cause of the disorders, discovering the young girl and her unnatural affinity for the elements in the process.
However, instead of reporting back on his discoveries, the scientist brought the girl under duress to his laboratories in the capitol, where he proceeded to further his newfound research into Irminsul through an array of studies and experimentation on the child and her biology. This would go on for two years, before she enacted a violent escape that resulted in a massacre of several tens and dozens of personnel, be it Fatui or mere servants and guards stationed at the palace. She would be caught by the 1st Harbinger before breaching the palace walls, and brought before the Tsaritsa with an ultimatum: to remain as Dottore’s test subject or to serve the Archon as a Harbinger.
It does not seem, however, that any of these events have left a welcomed mark on the young girl - as she continues to seek a way to permanently sever her ties with the Fatui and return to her family in Snezhnaya.
0 notes
Text
Sage Therapeutics and Biogen Announce Positive Pivotal Phase 3 Results for Zuranolone, an Investigational Two-Week, Once-Daily Therapeutic Being Evaluated for Major Depressive Disorder
New Post has been published on https://depression-md.com/sage-therapeutics-and-biogen-announce-positive-pivotal-phase-3-results-for-zuranolone-an-investigational-two-week-once-daily-therapeutic-being-evaluated-for-major-depressive-disorder/
Sage Therapeutics and Biogen Announce Positive Pivotal Phase 3 Results for Zuranolone, an Investigational Two-Week, Once-Daily Therapeutic Being Evaluated for Major Depressive Disorder

CAMBRIDGE, Mass.–(BUSINESS WIRE)–Sage Therapeutics, Inc. (Nasdaq: SAGE) and Biogen Inc. (Nasdaq: BIIB) today announced that the WATERFALL Study in patients with MDD met its primary endpoint with zuranolone (SAGE-217/BIIB125) 50 mg showing statistically significant improvement in depressive symptoms compared with placebo at Day 15 as assessed by the 17-item Hamilton Rating Scale for Depression (HAMD-17) total score. LS means (SE) change from baseline in HAMD-17 total score at Day 15 for patients who received zuranolone 50 mg was -14.1 (0.51) compared with -12.3 (0.50) for patients who received placebo (LS mean difference -1.7 points; p=0.0141).
Monoamine-based antidepressants have been the standard of care for chronic treatment of MDD for the past 60 years. They are treatments administered daily, which require sufficient exposure and continuous use to maintain effect. Zuranolone is a two-week, once-daily oral drug under investigation for the treatment of MDD. It is a molecule that is designed to potentially provide a rapid-acting, sustainable treatment option, and could represent a breakthrough in the current management of depression.
The WATERFALL Study was a pivotal, Phase 3, double-blind, randomized, placebo-controlled study evaluating the efficacy and safety of zuranolone 50 mg in adults 18 to 64 years with MDD (N=543). The WATERFALL Study enrolled patients who had MDD with a HAMD-17 total score ≥24 at screening and Day 1 prior to dosing.
“Sage’s expertise in the modulation of the GABA receptor pathway in the brain, coupled with insights on the treatment wants and needs of clinicians and patients, has resulted in our targeting a unique benefit/risk profile with the development of zuranolone supported to date by the data generated in the WATERFALL Study and the broader Landscape and NEST programs,” said Barry Greene, Chief Executive Officer at Sage Therapeutics. “We dared to imagine a different future for the treatment of MDD where patients have the potential to experience a rapid response that is well-tolerated and that may enable them to stay better with long periods free from depression symptoms, and free from daily chronic treatments and related side effects. In doing so, we aspire to help eliminate stigma associated with brain health disorders so that we can move beyond brain health awareness to brain health action.”
“Together with our collaboration partners at Sage, we are proud to announce highly encouraging results from the Phase 3 WATERFALL Study of zuranolone in major depressive disorder. These results represent hope and positive progress for the more than 250 million patients worldwide who are estimated to live with depression,” said Alfred Sandrock, Jr., M.D., Ph.D., Head of Research and Development at Biogen. “Major depressive disorder is a common co-morbidity of many diseases represented in Biogen’s neuroscience portfolio. We believe zuranolone has the potential to offer a unique, first-in-class therapeutic for depression with a distinct benefit-risk profile to people living with this common but serious mental health condition.”
Zuranolone was generally well-tolerated in the WATERFALL Study and demonstrated a safety profile consistent with previous clinical studies. The rate of treatment emergent adverse events (TEAEs) in the zuranolone group was 60.1% (161/268) vs the placebo group at 44.6% (120/269). The majority of the TEAEs were mild to moderate. The most common TEAEs that were ≥ 5% in patients treated with zuranolone (rates vs placebo) included somnolence 15.3% (vs 3.0%), dizziness 13.8% (vs 2.2%), headache 10.8% (vs 7.8%), and sedation 7.5% (vs 0.4%); these events predominantly occurred during the 14-day treatment period. Throughout the study, a total of two patients each (0.7%) reported serious adverse events (SAEs) in the zuranolone and placebo groups; no death occurred in the study. The percent of patients reporting TEAEs leading to drug discontinuation was 3.4% (9/268) and 1.5% (4/269), in the zuranolone and placebo groups, respectively. No signal for withdrawal symptoms as assessed by the 20-item Physician Withdrawal Checklist (PWC-20), or for increased suicidal ideation or behavior as per the Columbia-Suicide Severity Rating Scale (C-SSRS) were identified.
“I’m really excited about these breakthrough data: we know MDD is episodic and zuranolone has the potential to treat episodically. The LANDSCAPE clinical studies are all helpful taken together because they provide data on both short- and long-term use of zuranolone,” said Anita H. Clayton, M.D., Chair of Psychiatry and Neurobehavioral Sciences, University of Virginia School of Medicine. “These data suggest that this treatment, if approved, has the potential to work fast with a short-course of therapy that is well-tolerated, with the effect maintained over the long-term. This will empower my patients to think differently about their depression and treatment, and to rapidly return to their life. Depression is not an identity, it’s an episodic disorder that we hope in the future to be able to treat quickly with treatments that are well-tolerated and with benefits that last.”
“MDD is a pressing mental health concern and, unlike physical health concerns where innovation is commonplace, many of the treatments for MDD were first approved more than two decades ago,” said Paul Gionfriddo, President and CEO of Mental Health America (MHA). “We welcome today’s news, and the potential for a new and innovative treatment that could change the way we treat depression.”
Zuranolone has been granted Breakthrough Therapy Designation by the U.S. Food & Drug Administration, and the Companies intend to discuss next steps with the Agency. Full data from the WATERFALL Study will be shared at future scientific forums.
Detailed Topline Results from the WATERFALL Study
The WATERFALL Study enrolled 543 patients with MDD. The patients were treated with zuranolone 50 mg or placebo once nightly for 14 days.
The primary endpoint of the study was the change from baseline in the 17-item Hamilton Rating Scale for Depression (HAM-D) total score at Day 15; the first secondary endpoint was the change from baseline in the Clinical Global Impression-Severity of Illness (CGI-S) at Day 15.
The mean (SD) baseline HAMD-17 score at entry into the study was 26.8 (2.60) in the zuranolone 50 mg treatment group (n=268) and 26.9 (2.67) in the placebo group (n=269).
90.3% of patients who received zuranolone, and 87.4% of patients who received placebo, completed the study.
Results for the primary endpoint and several topline secondary efficacy endpoints during the treatment period are outlined in the following table and all favor zuranolone:
Outcome*
Day 3
Day 8
Day 12
Day 15
HAM-D-17: LS mean difference (p value)
-3.0 (<0.0001)^
-2.6 (<0.0001)^
-2.5 (0.0003)
-1.7 (0.0141)*
CGI-Severity: LS mean difference (p value)
-0.4 (<0.0001)
-0.4 (0.0001)
-0.3 (0.0014)
-0.2 (0.1193)^
CGI-Improvement Response:
Odds ratio (p value)
1.8 (0.0032)
1.9 (0.0005)
1.6 (0.0101)
1.5 (0.0191)
MADRS: LS mean difference (p value)
Not measured per protocol
-3.4 (0.0003)
Not measured per protocol
-2.4 (0.0238)
HAM-A: LS mean difference (p value)
Not measured per protocol
-1.7 (0.0011)
Not measured per protocol
-1.4 (0.0199)
Except for HAMD-17 at Day 15 (primary) which was statistically significant and CGI-S (first secondary endpoint) which was not significant at Day 15, all p-values in the table are nominal and not adjusted for multiple comparisons. *Pre-specified primary endpoint ^Pre-specified key secondary endpoints LS = least squares; LS mean difference = difference in LS means of change from baseline between zuranolone and placebo groups
Patients with a response at Day 15 in the zuranolone group retained on average 86.1% of their HAMD-17 improvement at Day 42 (4 weeks after dosing ended). A similar maintenance of response was also observed with the MADRS scale, where people who responded to zuranolone at Day 15 maintained 87.6% of that response at Day 42. While not statistically significant, a numerical advantage in favor of zuranolone was demonstrated at Day 42.
Safety and tolerability:
Adverse events were consistent with the safety profile of zuranolone seen to date in clinical studies.
The incidence of treatment emergent adverse events (TEAEs) in the zuranolone group was 60.1% (161/268) vs the placebo group at 44.6% (120/269).
The majority of the TEAEs were mild to moderate, with 8 (3.0%) and 3 (1.1%) being severe in the zuranolone and placebo groups respectively.
The most common TEAEs observed in ≥5% of patients in either treatment group are listed below and occurred predominantly during the 14-day treatment period. These events were non-serious, and most were mild to moderate.
AE (≥5%)
Zuranolone 50 mg
Placebo
Somnolence, n (%)
41 (15.3)
8 (3.0)
Dizziness, n (%)
37 (13.8)
6 (2.2)
Headache, n (%)
29 (10.8)
21 (7.8)
Sedation, n (%)
20 (7.5)
1 (0.4)
Diarrhea, n (%)
8 (3.0)
14 (5.2)
Discontinuation rates of the study drug due to AEs in patients receiving zuranolone were 3.4% (9/268) compared to 1.5% (4/269) in those receiving placebo.
Throughout the study, a total number of 4 patients reported serious adverse events (SAEs), 2 (0.7%) each in the zuranolone and placebo groups.
No deaths occurred in the study.
No signal in increased suicidal ideation or behavior, as assessed by the C-SSRS, was observed throughout the study in patients receiving zuranolone 50 mg or placebo.
No signal in withdrawal effects, as assessed by the PWC-20, was observed after discontinuation of zuranolone.
No loss of consciousness, or adverse effects such as weight gain, sexual dysfunction, or euphoria were reported.
About the WATERFALL Study
The WATERFALL Study was a double-blind, placebo-controlled pivotal Phase 3 study evaluating the efficacy and safety of zuranolone in adults with major depressive disorder. In the study, 543 patients were enrolled. Patients were randomized to receive zuranolone 50 mg, or placebo, once-nightly for two weeks. The primary endpoint of the study was the change from baseline in the 17-item Hamilton Rating Scale for Depression (HAM-D) total score at Day 15. Secondary endpoints included the change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Hamilton Anxiety Rating Scale (HAM-A) total score, among others.
About Zuranolone
Zuranolone (SAGE-217/BIIB125) is a once-daily, two-week drug in development for the treatment of major depressive disorder (MDD) and postpartum depression (PPD). Zuranolone is an investigational oral neuroactive steroid (NAS) GABA-A receptor positive allosteric modulator (PAM). The GABA system is the major inhibitory signaling pathway of the brain and central nervous system and contributes significantly to regulating brain function. Zuranolone has been granted Breakthrough Therapy Designation by the U.S. Food & Drug Administration.
Zuranolone is being evaluated as a potential rapid-acting, 2-week treatment for PPD and MDD in the NEST and LANDSCAPE clinical trial programs. The programs are designed to generate data to support a potential NDA filing as efficiently as possible. If successful, LANDSCAPE and NEST may support paths to approval with three distinct opportunities to address patient needs: PPD, acute rapid response therapy (RRT) in MDD when co-initiated with a new standard antidepressant, and as-needed treatment of MDD.
Zuranolone is being evaluated as a potential rapid-acting, 2-week treatment for PPD and MDD in the NEST and LANDSCAPE clinical trial programs. The two development programs include multiple studies examining use of zuranolone in several thousand patients with a variety of dosing, clinical endpoints, and treatment paradigms. The LANDSCAPE Program includes six studies of zuranolone in patients with MDD. Data have been reported from three studies of zuranolone 30 mg in patients with MDD (MDD-201, MOUNTAIN Study and the 30 mg cohort from the ongoing SHORELINE Study), and one study of zuranolone 50 mg in patients with MDD (WATERFALL Study). Two additional studies evaluating zuranolone 50 mg in patients with MDD are expected to read out by the end of 2021 (CORAL Study and a 50mg cohort of the SHORELINE Study).
The NEST Program includes two placebo-controlled studies of zuranolone in patients with PPD. Positive data from the ROBIN Study (zuranolone 30 mg) have been previously reported. The SKYLARK Study (zuranolone 50 mg) is anticipated to readout by the end of 2021.
About Major Depressive Disorder (MDD)
Major depressive disorder (MDD) is a common but serious mood disorder in which people experience depressive symptoms that impair their social, occupational, educational or other important functioning, such as a depressed mood or loss of interest or pleasure in daily activities, consistently for at least a two-week period. It is estimated that approximately 17 million people in the U.S. and more than 250 million people worldwide suffer from MDD each year. While antidepressants are widely used to treat MDD, large-scale studies have demonstrated the need for additional therapies with a differentiated profile.
About Sage Therapeutics
Sage Therapeutics is a biopharmaceutical company committed to developing novel therapies with the potential to transform the lives of people with debilitating disorders of the brain. We are pursuing new pathways with the goal of improving brain health, and our depression, neurology and neuropsychiatry franchise programs aim to change how brain disorders are thought about and treated. Our mission is to make medicines that matter so people can get better, sooner. For more information, please visit www.sagerx.com.
About Biogen
At Biogen, our mission is clear: we are pioneers in neuroscience. Biogen discovers, develops and delivers worldwide innovative therapies for people living with serious neurological and neurodegenerative diseases as well as related therapeutic adjacencies. One of the world’s first global biotechnology companies, Biogen was founded in 1978 by Charles Weissmann, Heinz Schaller, Kenneth Murray and Nobel Prize winners Walter Gilbert and Phillip Sharp. Today Biogen has the leading portfolio of medicines to treat multiple sclerosis, has introduced the first approved treatment for spinal muscular atrophy, commercializes biosimilars of advanced biologics and is focused on advancing research programs in multiple sclerosis and neuroimmunology, Alzheimer’s disease and dementia, neuromuscular disorders, movement disorders, ophthalmology, neuropsychiatry, immunology, acute neurology and neuropathic pain.
We routinely post information that may be important to investors on our website at www.biogen.com. Follow us on social media – Twitter, LinkedIn, Facebook, YouTube.
Forward-Looking Statements
Sage Therapeutics Safe Harbor
Various statements in this release concern Sage’s future expectations, plans and prospects, including without limitation statements regarding: the potential for future regulatory approval of zuranolone; our planned timing for reporting of data from ongoing clinical trials; the potential profile and benefit of zuranolone in MDD and PPD; plans for discussions of next steps with the FDA; regulatory filing plans and potential pathways and opportunities; planned next steps for the program; our estimates as to the number of patients with MDD; and the goals, opportunity and potential for zuranolone and for our business. These statements constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are neither promises nor guarantees of future performance, and are subject to a variety of risks and uncertainties, many of which are beyond our control, which could cause actual results to differ materially from those contemplated in these forward-looking statements, including the risks that: success in earlier clinical trials may not be repeated or observed in ongoing or future studies, and ongoing and future non-clinical and clinical results may not meet their primary or key secondary endpoints or be sufficient to file for or gain regulatory approval to market a product without further development work or may not support further development at all; unexpected concerns may arise from additional data, analysis or results from any of our completed studies; we may encounter adverse results or adverse events at any stage of development that negatively impact further development or that require additional nonclinical and clinical work which may not yield positive results; we may encounter delays in conduct of our clinical trials, including slower than expected site initiation or enrollment, that may impact our ability to meet our expected time-lines; the FDA may ultimately decide that the design, conduct or results of our completed and planned clinical trials for zuranolone, even if positive, are not sufficient for regulatory filing or approval in the indications that are the focus of our development plan and may require additional trials or data which may significantly delay our efforts to obtain approval and may not be successful; other decisions or actions of the FDA or other regulatory agencies may affect the zuranolone program and our plans, progress or results; the actual size of the MDD patient population may be significantly lower than our estimates and, even if zuranolone is approved, it may only be approved or used to treat a subset of the relevant patient populations; we may encounter technical and other unexpected hurdles in the development and manufacture of zuranolone or our other product candidates which may delay our timing or change our plans; as well as those risks more fully discussed in the section entitled “Risk Factors” in our most recent Quarterly Report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in our subsequent filings with the Securities and Exchange Commission. In addition, any forward-looking statements represent our views only as of today, and should not be relied upon as representing our views as of any subsequent date. We explicitly disclaim any obligation to update any forward-looking statements.
Biogen Safe Harbor
This news release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, relating to the potential, benefits, safety and efficacy of zuranolone (SAGE-217/BIIB125); the potential clinical effects of zuranolone; results from the Phase 3 WATERFALL Study of zuranolone; the clinical development program for zuranolone; clinical development programs, clinical trials and data readouts and presentations for zuranolone; the potential treatment of MDD and PPD; the potential of Biogen’s commercial business and pipeline programs, including zuranolone; the anticipated benefits and potential of Biogen’s collaboration arrangement with Sage; and risks and uncertainties associated with drug development and commercialization. These forward-looking statements may be accompanied by words such as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “potential,” “possible,” “will,” “would” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk and only a small number of research and development programs result in commercialization of a product. Results in early-stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements, or the scientific data presented.
These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including without limitation, uncertainty of success in the development and potential commercialization of zuranolone; unexpected concerns may arise from additional data, analysis or results obtained during the WATERFALL Study or the other clinical studies of zuranolone; regulatory authorities may require additional information or further studies, or may fail or refuse to approve or may delay approval of Biogen’s drug candidates, including zuranolone; the occurrence of adverse safety events; the risks of other unexpected hurdles, costs or delays; failure to protect and enforce data, intellectual property and other proprietary rights and uncertainties relating to intellectual property claims and challenges; product liability claims; third party collaboration risks; and the direct and indirect impacts of the ongoing COVID-19 pandemic on our business, results of operations and financial condition. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Biogen’s expectations in any forward-looking statement. Investors should consider this cautionary statement as well as the risk factors identified in Biogen’s most recent annual or quarterly report and in other reports Biogen has filed with the U.S. Securities and Exchange Commission. These statements are based on Biogen’s current beliefs and expectations and speak only as of the date of this news release. Biogen does not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.

Source link
0 notes
Text
What are the different uses of Melatonin?
Melatonin is a chemical that our bodies spontaneously release at night or under low light. Perhaps one of the easiest ways to understand what melatonin is and what it does is to remember someone else saying they would sleep like a baby in gloomy and otherwise rainy weather. That is still right; our bodies secrete this hormone while we are in total darkness or even low light, both indoors and outdoors. It makes us tired and sleepy. Now, do you see how even this hormone levels that is used to cure insomnia?

Melatonin-A few little known facts
-Melatonin was first discovered in 1958;
-It really has been researched around for more than 40 years.
-Melatonin is a safe solution for the treatment of insomnia or even sleep disturbances.
-The development of natural melatonin is at its most peaks as between 2:00 a.m. and perhaps 4:00 a.m.
-Melatonin is formed mostly by the pineal gland which is found in the brain.
-The pineal gland of the human brain is active in energy and ageing
-Melatonin synthesis continues to decline with age.
Melatonin and the Operating Hours
Majority of people who work in lighted environments at night will also have poor levels of melatonin, making it impossible for them to sleep. When they come out of bed, it is daytime, and their bodies do not contain enough of the hormone. You can either Buy Melatonin UK. Note, melatonin is released mainly when there is little darkness no or very little daylight. But if anyone spends much of their day in the daytime, their melatonin levels would be decreased. For e.g., we sometimes hear someone who really works an unusual hour moaning about not being able to sleep tonight. This is because the elevated level of melatonin renders us tired, which we need to fall asleep. You can easily Buy Melatonin Online.
Melatonin therapy for insomnia
The possible way melatonin functions is very wonderful, and it is like clockwork. Melatonin is released at night at least and is diminished during the day or even in natural light, which is also fairly consistent with how well we sleep. Buy Melatonin and enjoy the several benefits. Whenever we wake up in the morning, even our own melatonin levels are down enough that we can simply carry out our everyday lives without feeling drowsy. People mostly with chronic insomnia also may have consistently low melatonin levels and maybe this will actually benefit mostly from melatonin supplementation. However since melatonin causes somnolence and exhaustion, it is mostly taken at night, a few hours before bedtime. People often treat Insomnia sleeping disorders with Melatonin.
Further tests that have shown that melatonin will allow for someone to have a relatively peaceful and otherwise uninterrupted full night's sleep mostly with the right dose. Melatonin is usually bought in natural food retailers and has no other adverse side effects unless it is manipulated. Since it is an herbal supplement, then you really would not even wake up all groggy and otherwise moody, like sleeping pills and other little medications used for chronic insomnia.
Perhaps the other Effects of Melatonin
Even more good news today for melatonin production is that it does far more than just help sleepers. Melatonin aids patients with cancer, degenerative diseases and even sometimes severe migraine severe headaches. Melatonin also has some antioxidant effects that are useful for maintaining the body's immune system safe.
0 notes
Text
Fluvoxamine Maleate
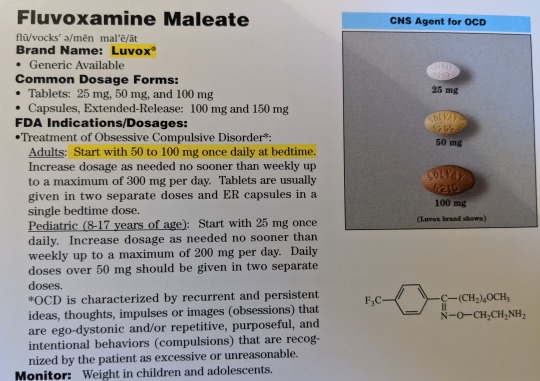

Brand Name: Luvox
Generic Available
Common Dosage Forms:
Tablets: 25 mg, 50 mg, 100 mg
Capsules, Extended-Release: 100 mg, 150 mg
FDA Indications/Dosages:
Treatment of Obsessive Compulsive Disorder*
Adults: Start with 50 to 100 mg once daily at bedtime. Increase dosage as needed no sooner than weekly up to a maximum of 300 mg per day. Tablets are usually given in two separate doses and ER capsules in a single bedtime dose.
Pediatric (8-17 years of age): Start with 25 mg once daily. Increase dosage as needed no sooner than weekly up to a maximum of 200 mg per day. Daily doses over 50 mg should be given in two separate doses.
*OCD is characterized by recurrent and persistent ideas, thoughts, or images (obsessions) that are ego-dystonic and/or repetitive, purposeful, and intentional behaviors (compulsions) that are recognized by the patient as excessive or unreasonable.
Monitor: Weight in children and adolescents
Pharmacology/Pharmacokinetics: Fluvoxamine’s actions are thought to be due to an inhibition of CNS neuronal uptake of serotonin (5HT). It has no significant affinity for adrenergic, cholinergic, GABA, dopaminergic, histaminergic, benzodiazepine, or serotonergic receptors. Terminal elimination undergoes extensive first-pass metabolism (oxidative demethylation and deamination) to form less active metabolites. Excretion occurs both through the urine and through the feces. About 80% of fluvoxamine is bound to plasma proteins. It is a substrate for P450 hepatic isoenzymes CYPIA2, CYPIIC9, and CYPIIIA4.
Drug Interactions: Use in combination (within 14 days) with MONOAMINE OXIDASE INHIBITORS (MAOI) may cause serious or even fatal reactions. May increase the effects of TERFENADINE, CISAPRIDE, THEOPHYLLINE, WARFARIN, TIZANIDINE, RAMELTEON, diazepam, alprazolam, midazolam, triazolam, carbamazepine, clozapine, amitriptyline, clomipramine, imipramine, and methadone. Lithium and tryptophan may enhance its effects.
Contraindications/Precautions: Do not use within 14 days of therapy with a MAOI. Do not use with terfenadine or cisapride. INCREASED RISK OF SUICIDAL THINKING AND BEHAVIOR IN CHILDREN, ADOLESCENTS, AND YOUNG ADULTS TAKING ANTIDEPRESSANTS FOR MAJOR DEPRESSIVE DISORDER AND OTHER PSYCHIATRIC DISORDERS. Use with caution in patients with renal or hepatic dysfunction. Patients should be careful when performing tasks which require alertness. Fluvoxamine is secreted in breast milk. Pregnancy Category C.
Adverse Effects: Nausea (40%), headache (22%), insomnia (21%), somnolence (22%), asthenia (14%), GI complaints (11%), dry mouth (14%), nervousness (12%), dizziness (11%), sweating (7%), delayed ejaculation (8%).
Patient Consultation:
Continued therapy may be needed to show noticeable improvement. Do not stop therapy before consulting with a physician.
Use caution when performing tasks that require mental alertness.
Store in a cool, dry place away from sunlight and children.
Contact a physician if the above side effects are severe or persistent.
If a dose is missed, skip it and return to normal dosing schedule.
3 notes
·
View notes
Text
How to Avoid Sleep After Lunch?

It is not uncommon to feel sleepy in the daytime, especially after we have eaten too much. This could be a result of an unhealthy sleep cycle or digestion patterns. When this daytime sleepiness starts affecting our daily life, we need to start learning how to avoid sleep after lunch. The type of food we take, and the timing of our various meals also go on to impact if we feel sleepy in the daytime or not. Most people experience a decrease in energy levels after they’ve eaten- this condition is called postprandial somnolence.
Researchers around the world have different theories about daytime tiredness, but most of them agree that it is a natural response and should not be worried about it. Physical and mental fatigue have different symptoms, but they often occur together. One reason you could be feeling tired after lunch is that you might not have got enough sleep in the night.1 out of 3 Americans complain of the same. We have discussed a number of things below other than how to avoid sleep after lunch, such as:
Why Do I Feel Tired After Eating?
The Pros and Cons of Sleep After Lunch
How to Avoid Sleep After Lunch
Commonly Asked Questions
Why Do I Feel Tired After Eating?
When you feel tired or have difficulty concentrating after a meal- you’re not the only one facing this problem out there. People can feel very tired, depending on what they ate when they ate and how much they ate because of which most of us are trying to find out how to avoid sleep after lunch. You could be feeling tired after lunch because you had a greater portion of carbohydrates, which makes you sleepier as compared to other food. This is because after eating carbs, your body produces more serotonin than usual, which calms your mind and makes you feel drowsy.
Another amino acid is known as tryptophan also helps the body produce serotonin. A protein-rich food is a great supplier of tryptophan and carbohydrates help absorb this amino acid in the body. Thus, eating a meal that is rich in protein and carbs can also cause you to feel sleepy after lunch. Postprandial somnolence is also likely to be felt more by people who have taken a heavy lunch. Every time you eat, there is a hike in your blood sugar levels, which causes your energy to fall down. Other factors that contribute to daytime sleepiness are poor sleep at night and intake of alcohol with a meal.
The Pros and Cons of Sleep After Lunch
Whenever you feel sleep-deprived or want to find a way to relax, you could resort to taking a nap. However, if you nap at the wrong time, the chances are that it could backfire and make the rest of the day difficult for you. There are certain benefits of napping, such as-
Relaxation
Reduced fatigue
Increased Alertness
Improved mood
Improved Performance
Quicker reaction time and better memory
Increased concentration
Napping isn’t for everyone, and some people naturally do not find it easy to sleep in a place that is not their own. Moreover, there are certain drawbacks to sleeping in the daytime as well-
Sleep Inertia– After you wake up from a nap, instead of feeling fresh and rested, you might feel groggy or disoriented at your place of work.
Nighttime Sleeping Problems – Usually, short naps in the day do not have a considerable effect on your sleep cycle at night. However, if you have a sleep disorder or insomnia, you might want to avoid sleeping in the daytime because frequent naps can often interfere with your nighttime sleep.
You should consider taking a nap if –
You experience unexpected fatigue and sleepiness.
You have to suffer a loss of sleep later, for instance, due to a late-night work shift.
How to Avoid Sleep After Lunch
Nearly everyone has days where they feel very sleepy after lunchtime. For some people, however, daytime sleepiness becomes a major problem at work or at school. Unusual sleepiness is often the result of hypersomnia in which a person suffers from recurrent sleepiness, which makes a person nap want to nap during the day time. If you want to know how to avoid sleep after lunch, follow the steps below-
Get Enough Sleep at Night
If you compromise on your sleep in the night, then the chances are that you will feel fairly tired and fatigued later in the day. Most of us compromise with our daily dose of sleep for an extra few minute of work or self-pampering. Avoid doing this if you want to make sure you’re energetic and healthy throughout the day.
Set A Sleep Routine
Make sure that you have a set time when you sleep, and a definite time when you wake up in the morning. Follow healthy sleeping habits like closing your gadgets before sleep time and reading a book or listening to music.
Exercise
Regular exercise has multiple benefits. It gives you daytime energy and keeps your thinking sharp. If you exercise out in the daylight, you’ll probably have even more benefits than a workout at nighttime.
Commonly Asked Questions
Why Do I Get So Sleepy After Lunch?
If you had a meal that was very rich in protein and carbohydrates during lunch, then the chances are that you will feel very sleepy because of the serotonin that they produce. Protein-rich food also contains tryptophan, which helps the body further produce serotonin.
Is It Good to Sleep After Lunch?
Your body begins to gain weight when you take more calories than you’re burning. If you go to sleep immediately after eating your lunch, it is possible that you might gain an unhealthy amount of weight. If you’re planning to sleep after lunch, make sure that you have a lighter meal than usual.
How Can I Stop Feeling Sleepy in The Daytime?
If you’re sleepy in the daytime and wish to avoid it, try the following-
Get enough sleep in the night
Don’t use gadgets before bedtime
Set a definite time to wake up each morning
Start sleeping and waking up early
0 notes
Text
Summary: Part 2 of when Reiji is in the hospital, when Sugai and Tajima decide to read his file
After the whole thing went down and Reiji was checked on by one of the doctor’s, he fell back to sleep in Tajima’s arms while Sugai had his arms wrapped around the boy.
“The nurse said we should probably look at the file...” Tajima mumbled.
Sugai already tried, he just couldn’t do it anymore. But he didn’t want to tell Tajima he went on without him, so he simply nodded, grabbing the file.
Taking a deep breath, Sugai asked nervously, “Are— are you sure you want to do this?”
“We need to know what’s wrong so we can help him,” said Tajima, putting on the bravest face he could manage.
Sugai slowly nodded once again before opening it, and giving enough room for Tajima to read also.
Patient Information:
Name: Reiji Sunada
Age: 15 Years
Date of Birth: 21 April 2004
Abilities/Notable Factors: Tendrils come out of the back that can shock when wet
Alias: ‘Eel’
Reason for access to Med Bay: Deathly injuries
Tajima paused, pressed a kiss to Reiji’s forehead and took a third breath before delving into the actual part of the report which was most difficult to read.
List of Injuries and/or Health Conditions by Assumed Date of Occurrence:
Mild taser burns on sides of abdomen and neck
Lip split and bitten into
Bruising on right eye, left cheekbone, ribs, stomach and neck
Bullet wound – entry through back, lodged next to left shoulder blade and scarring from bullet extraction surgery
Additional bruising to right side of face, cheekbone and temple
Eighty cuts and lacerations increasing in depth and severity covering back and hips
Evidence of drowning and subsequent health complications including the following:
Water Inhalation – Pulmonary Edema – Hypoxia – Respiratory Failure – Patient was most likely held underwater without air for extended periods of time before unconsciousness occurred *
Sugai and Tajima both stared at the Asterix and followed to the next page over where there was another section of writing. They were suddenly very glad that the people they hired were thorough because someone had printed information and research on something called secondary drowning, which Reiji had apparently experienced.
* ‘Inhaled water leads to a condition given the name ‘secondary drowning,’ which is when water gets into the lungs where it can irritate the lungs’ lining and fluid can build up, causing a condition called Pulmonary Edema. Pulmonary Edema (Symptoms: Extreme shortness of breath or difficulty breathing (dyspnea) that worsens with activity or when lying down, a feeling of suffocating or drowning that worsens when lying down, wheezing or gasping for breath, cold or clammy skin, anxiety, restlessness or a sense of apprehension, a cough that produces frothy sputum that may be tinged with blood, blue-tinged lips, a rapid or irregular heartbeat (palpitations)) causes respiratory failure due to hypoxia hypoxia is a deficiency in the amount of oxygen reaching the tissues (symptoms: change in skin colour, increased or decreased heart rate, rapid breathing, shortness of breath, sweating, wheezing).
Sugai flipped back to the other page, determined to push through and finish reading the list of injuries.
Open bone biopsy on hip, knee and shoulder – Patient not administered anaesthetics and was likely forced to walk immediately after surgery
Patient appears to have been kept in early stages of hypothermia for extended durations of time
Patient appears to have undergone some form of sensory attack and deprivation due to increased sensitivity to light and sound
Severe ankle breakage – likely caused through weight dropped onto limb and continuous disruption and aggravation to the broken bone after breakage
Severe Asphyxiation – likely caused by strangulation
Additional bruising to jaw and mouth area causing second split lip
Severe bruising across entire face
Three broken ribs, two fractures
Trauma to eye socket likely caused by multiple blows
Bloody nose due to assumed assault
Dislocated shoulder
Injuries consistent to those of beaten and/or assaulted patients
Thoracentesis surgery without anaesthetic – needle inserted into pleural space between lung and chest walls, likely to remove excess fluids (pleural effusion) from the pleural space to improve ability to breathe
Shattered hyoid bone and evidence of poorly-executed bone reconstruction surgery
Severe Epiglottitis – condition which occurs when tissue protecting windpipe becomes inflamed
Please note that the patient was administered a fibreoptic intubation procedure without anaesthetics or ventilator to assist breathing
Extreme fever caused by infections *
* ‘Infections in both lungs’ air sacs causing them to swell – Caused Pneumonia
Respiratory tract infection in upper and lower respiratory tracts
Pharyngitis – caused by severe swelling in pharynx and larynx
Severe Sepsis throughout body (condition arises when body’s response to infection causes injury to tissues and organs)
Multiple opportunistic infections (infections caused by patients weakened immune system and deteriorating physical health)’
Evidence of more water inhalation and an increase in the severity of multiple infections
Evidence that the patient underwent severe and final stages of hypothermia
All external wounds were re-opened for reasons unknown
Severe electrical burns on points of contact (both temples, toes, fingers) and contusions/abrasions from suspected metal clamps and plates used to administer high amount of electrical currents
Severe injures from restraints on ankles, wrists, all joints, chest, collarbone, hips, temples and neck
Severe electrocution
Severe hypovolemic shock caused by amount of blood loss
Major concussion
Throat inflammation caused by screaming
Multitude of severe contusions and abrasions
Evidence of multiple seizures and spasms
Extreme starvation
Extreme dehydration
Extreme sleep deprivation
Interesting/Unexplained Features:
Gasoline residue found on clothing and skin – inhalation of these subsequent fumes caused high risk carbon monoxide poisoning which is the likely cause of the seizures and heart arrhythmias
Surgery guidelines over skull and spine despite no evidence of any surgical procedures
Finger-shaped bruises in unusual places such as hips, thighs, lower back and shoulder blades
Wound on chest had been carved into the patient in order to cause emotional and psychological damage
Although no anaesthetics were administered to the patient, they suffered from (intentional) Opioid-Induced Hyperalgesia * and extreme amounts of Varenicline * which were found in the patient’s system
* ‘Opioid-Induced Hyperalgesia is a state of nociceptive sensitization caused by exposure to opioids. The rare condition is characterised by a paradoxical response in which a patient receiving medication (specifically opioids) for the treatment of pain actually becomes more sensitive to certain painful stimuli. In this patients’ case: (Reiji Sunada) the specific synthesis of drugs he was unwillingly supplied with were used to intentionally increase the amount of pain felt during and following most experiments and attacks.’
* ‘Varenicline goes by the brand name ‘Chantix’ and has been highly scrutinised for causing severe neuropsychiatric adverse events including abnormal dreams, nightmares, night terrors, aggression, anxiety, heavy fatigue, insomnia, irritability, somnolence (sleepwalking) and other various sleep disorders.’
Tajima choked back a sob. “Baby— my baby—” he buried his nose in Reiji’s hair, crying.
The slightly older man wiped a few tears. “I can’t— I can’t comprehend how he could’ve went through all that...”
“Dad’s?” a small voice called, Reiji looking worried.
Sugai gave the best smile he could manage. “Hey, love. How are you feeling?”
With a shrug, Reiji mumbled, “My back hurts a bit but I’m fine. Why are you crying? Are you reading my file?” he asked quietly.
“We’re sorry, sweetheart, we just needed to make sure y—”
“No, it’s f—fine. I understand.”
Tajima asked nervously, “Are you hungry now, bambino?” He was just worried Reiji would end up starving at his own will.
And not to his surprise, Reiji shook his head. “No. I’m good.”
“Are you sure?” asked Sugai, his larger, calloused hands cupping the boy’s cheeks gently.
Reiji nodded. “Yeah. I can’t tell if it’s a habit or the fact I j—just am not used to eating really.”
Even though part of him was afraid to ask, Tajima did ask, “When could you eat, sweetheart?”
“Every four days,” he responded, trying to seem nonchalant about it, but he wanted to sob.
Sugai inhaled sharply. “What— what could you eat?”
The boy looked like he was thinking for a moment before mumbling, “Bread was the usual. Though sometimes I’d have oatmeal.” Again, he wanted to sob but he kept it in.
“B—baby, you know you can cry, right? It’s okay, you are safe,” reassured Tajima, rubbing small circles into his cheek.
Reiji sniffled. “I—I know, it’s just a habit, I—I guess.”
There was a pause before Sugai said lovingly, “Take your time, sweetheart. We c—can be patient.” Though his voice was quivering, Reiji nodded.
“Can I go back to sleep?” asked the boy quietly.
Tajima and Sugai instantly nodded as Reiji’s eyes slowly drifted shut.
Everything’s going to be okay.

0 notes
Text
Diagnostic and Therapeutic Management of Brain Tumors of Child in Dakar CHU
Authored by Aliou Thiongane

Abstract
Introduction: Brain tumors constitute a set of intracranial neoplasms. Their symptomatology associates an intracranial hypertension syndrome (HTIC) and focal neurological syndrome. The objective of our work was to study diagnostic and therapeutic management of brain tumors in children hospitalized at the National Hospital for Children Albert Royer (CHNEAR) in Dakar.
Materials and methods: This was a retrospective study of brain tumors (1st January 2005 to 31st December 2014). Data were collected from patients and hospital records.
Results: Of the 30 patients with brain tumors, only 16 cases were included in our study. The incidence was 0.07%, the age ranged from 3 years to 14 years with an average age of 7.6 ± 3.7 years. The majority of children were under 10 years of age in 75% of the cases. The sex ratio is 2.2 (68% boys and 32% girls). All children were presented with headache, vomiting in 56.3% of cases and visual impairment in 37.5% of cases. Other clinical signs are disorders of alertness, amblyopia and convulsions. Neurological examination found papillary edema in 4 patients, hypertonia in 38%, pyramidal syndrome in 31% of cases, cerebellar syndrome in 37.5% of cases, and meningeal syndrome in 25% of cases. There were 69% of infra tentorial location and 31% in the fourth ventricle. The histological types suspected on cerebral tomodensitometry are ependymoma (18.75%), medulloblastoma (12.5%), astrocytoma (12.5%), Glioblastoma (12.5%) and craniopharyngioma (6.25%). Some patients required preoperative medical treatment (analgesic and neuro-resuscitation). The surgical treatment consisted of a ventriculo-peritoneal derivation in 4 cases, a tumoral excision in 2 cases. Mortality was high in 10 cases (62.5%).
Conclusion: Cerebral tumors of the child are relatively frequent. Mortality however remains high.
Keywords: Child; Brain tumor; Neuro-resuscitation; Ventriculo-peritoneal derivation
Go to
Introduction
Brain tumors constitute a set of intracranial neoplasms with an intra- or extra-axial origin [1]. They are the leading cause of cancer deaths [2] in children. Worldwide, their incidence is estimated at 2.5 cases / 100 000 inhabitants / year [3,4]. In developed countries, they are the second leading cause of cancer after leukemia [1, 3]. In sub-Saharan Africa, their incidence remains unknown, particularly in Senegal, where few studies have been carried out. They can be seen at any age with prevalence between 4 and 8 years [5] and represent 16 to 25% of tumors before 16 years. They are the most frequent pediatric solid tumors [6-8]. The symptomatology is non-specific characterized by the association of an intracranial hypertension syndrome and a focal syndrome. Brain computed tomography and nuclear magnetic resonance imaging are essential for the confirmation of topographic diagnosis [9]. Confirmation of the histology of the tumor is obtained by antomo-pathological examination. In developing countries, especially Senegal, the problem is two-fold, diagnostic and therapeutic [6,10]. The objective of our work was to study diagnostic and therapeutic management of brain tumors in children hospitalized at the National Hospital for Children Albert Royer (CHNEAR) in Dakar.
Go to
Materials and Methods
This was a retrospective study on brain tumors in children hospitalized from 1 January 2005 to 31 December 2014; 10 years. All children aged 0 to 15 admitted for brain tumors were included in the study. The diagnosis was evoked on the clinical aspects and confirmed by CT scans. We excluded all suspected cases of brain tumors not confirmed by CT and all unusable or incomplete records. The data was entered and analyzed using the Epi Info software version 3.3.2.
Go to
Results
During the study period from 1 January 2005 to 31 December 2014, 44,988 children aged 0-15 years were hospitalized. Of these, 30 (0.07%) children had a cerebral tumor. Of the 30 patients, only 16 cases were included in our study. The 14 excluded files included 10 children lost to follow-up and 4 non- exploitable cases. The mean age of the patients was 7.6 ± 3.7 years with extremes of 3 to 14 years. The age groups of [0-5 years] and [6-10 years] were predominant with 37.5% whereas the age group [11-15 years] represented only 25%. The majority of patients were boys (68%) versus 5 girls (31.2%). The sex ratio was 2:2. The majority of patients came from urban areas in 62.5% of cases. Headache was the most common reason for consultation in all patients. Other symptoms include vomiting, alertness, amblyopia, and convulsions (Table 1). Neurological signs are represented by disorders of consciousness, motor deficits, sensory, hypertonia, respectively in 19%, 31%, 6%, 38%. The rest of the neurological examination showed pyramid syndrome, cerebellar syndrome and meningeal syndrome in (31%), (37.5%), (25%) cases. A child came blind with a torticollis.
Computerized tomography (CT) was performed in all patients and confirmed diagnosis of brain tumor, topography and suspects its histological type. Sub-tentorial localization predominated with 11 cases (68.75%), including 5 cases in the 4th ventricle. The sampling location represents only 31.25%. The diagnosis of ependymoma in three cases, medulloblastoma in two cases, astrocytoma in two cases, glioblastoma in two cases, and craniopharyngioma in one case, were diagnosed histologically. According to sex, there were 7 boys with two medulloblastomas, two glioblastoma, one ependymoma, one astrocytoma and one craniopharyngioma. Three girls had two ependymomas and one astrocytoma. According to the age of the children the 3 ependymomas were found in the age groups (0-5 years), (6-10 years) and (11-15 years), the two astrocytomas in the age groups of both (0-5 years) and (11-15 years), both medulloblastoma and craniopharyngioma in the age group (610 years), the two glioblastomas in the age group (11-15 years).
Pathological examination performed in six children was concluded that in 3 patients, there are 2 medulloblastomas and 1 craniopharyngioma. Patients were treated with anti-convulsant and anti-oedematous drugs in 31% and 63% of cases respectively. Half of the patients were under antibiotics. All patients had analgesic treatment. Corticosteroids were prescribed in 47% of patients. Three patients were put under oxygen therapy, and one patient on a ventilator.Surgical treatment consisted of biopsy resection in one case, total resection in another case. Four patients underwent ventriculo-peritoneal diversion, one with external drainage.
Of the 16 cases included in our series, the progression was favorable in 2 patients (12.5%). We found complications in 14 patients (87.5%). These complications were mainly hydrocephalus in 11 cases, nosocomial infection in 8 cases, metabolic complications in 3 cases and hemorrhagic in 1 case. Four children were lost to follow-up and ten were deceased (62.5%).
Go to
Discussion
The incidence was low in our study (0.07%) over the 10 years of study. In Europe, the incidence of brain tumors was lower in the order of 0.003% but with an average increase of 1.7% per year between 1978 and 1997, partially explained by improved diagnostic capabilities without any variation in risk factors being excluded [11]. Nevertheless, a study carried out in Cameroon on central nervous system tumors showed that the pediatric population represented 15% of the population [12,13].
The average age was 7.6 years (3 and 14 years). In Ivory Coast Broalet had recovered an average age close to our age of 8 years [1]. The age groups (0-5 years) and (6-10 years) were predominant 37.5%. The majority of patients had an age less than or equal to 10 years in 75% of the cases. This is comparable to the literature in which the age of the majority of patients was between 4 and 11 years [4]. Elie in Cameroon found a majority in the age group 8 and 13 [6].The male was predominant with 68.8% of cases, the sex ratio was 2.2. Delhemmes reported a male predominance [14]. On the other hand, Yaounde Elie had regained a female predominance with a sex ratio of 0.9 [6].
Intracranial Hypertension Syndrome (HTIC) was found in all patients. In the literature, these results were superimposable [14-17]. It consisted mainly of constant headaches (100%), vomiting (56.3%) and visual disturbances (37.5%). Hamza had recorded 93% of cases of headache and 66.6% of vomiting [10]. Elie had found super imposable results with a frequency of 85% for headache and 45% for vomiting [6]. Lower rates of headache were noted by Wilne 56% [18], Broallet 50.87% of cases [1]. Other clinical signs were tone disorders (75%), amblyopia (31.2%), strabismus (25%), somnolence, irritability and macrocrania (18.8%), respectively. General condition was good in five patients (31%), poor in 11 patients (69%). The majority of patients were hospitalized at an advanced stage of the disease, one with a coma. Infectious syndrome was found in 8 patients (50%).
Six patients (37.5%) had cerebellar syndrome. Elie had found the same frequency in his study [6]. Higher frequencies were found by Boujlal and Kanaan with 51% and 65%, respectively [18,19]. On the other hand Broalet had found a lower frequency with 26% [1]. We found a stiff neck in 1 patient as Hamza in his series [10] while Kanaan [19] had reported more cases. It is a highly evocative sign of posterior fossa tumor. We noted 5 children (31%) with pyramid syndrome. In the literature lower frequencies were reported, Wilne and Hamza noted 27% [20,10], Boujlal 22% [18]. Twenty five per cent of children presented with meningeal syndrome same as Hamza who had found 26.7% of the cases [10]. Boujlal, in his series, had not found any case of meningeal syndrome [18].
The Brain scans (CT) have revolutionized diagnostic and therapeutic management of brain tumors. All our patients had a cerebral computed tomography (CT) scan despite the high cost and unavailability in all hospital facilities. However, although it is non-invasive, it is not an innocuous examination indeed; it has a strong irradiation power in contrast to magnetic resonance imaging (MRI). Cerebral tumors of the child are frequent at the sub-tentorial level (69%). Elie in Cameroon had recovered 55%, [6] Hamza in Senegal had reported 53% [10]. On the other hand, some authors had noted a predominance at the supranational level. Broalet in Côte d'Ivoire found 54% [1] as did Wong.
The CT scan allows in most cases a histological diagnosis of presumption. The results of CT scans in various intracranial tumors were strongly correlated with histopathological abnormalities [21-23]. In our study, the histological type was specified on CT scans in 10 patients. These were 3 ependymomas, 2 medulloblastomas, 2 astrocytomas, 2 glioblastomas and 1 craniopharyngioma. Kamaan found 70% of the cases [18], Hamza [10] and Behat [24] found 50% of the cases, Elie 32% [1].
The majority of our patients received anti-edematous treatment with corticosteroids (47% of cases). The same treatment was used by Kanaan [18,19] in 90% of the cases. Hamza [10] had 30% of patients with corticosteroids and 53.3% with anti-edematous patients. Anti-comitial treatment was administered in 31% of cases (phenobarbital, diazepam). Analgesics were prescribed in all patients. Three patients were on oxygen and a ventilator.Six patients were operated on 30% of cases including total excision and partial excision. Four ventriculo-peritoneal derivations.
The evolution and prognosis of pediatric brain tumors were very dark with heavy mortality 62.5% of cases including six children even before surgical management. This high mortality could be explained by a delay in diagnostic and therapeutic management. Broalet had regained a favorable rate of change of 15% [1]. Higher rates were reported in the literature, 26% for BEHAT [24], 30% for Hamza and 48% for Kanaan [18].
Go to
Conclusion
Cerebral tumors of the child are relatively frequent. The diagnosis of brain tumors is based primarily on histological analysis. Management is multidisciplinary.
For more open access journals in juniper publishers please click https://juniperpublishers.com/
For more articles on Open Access Journal of Neurology & Neurosurgery Please click on https://juniperpublishers.com/oajnn/
Open Access Journal of Neurology & Neurosurgery in Full text in Juniper Publishers
https://juniperpublishers.com/oajnn/OAJNN.MS.ID.555596.php
#juniper publishers Reviews#Juniper Publishers group#juniper publishers peer review#Juniper Publishers#Juniper Video Articles
0 notes
Text
Depression
Introduction
Natural part of the human experience is sadness. Many of us may feel sad or depressed due to many reasons such when a loved one passes away and going to life challenges. These feelings must be normally in a short time. When an intense feeling of sadness is felt persistently for a long period of time, they may have a major depressive disorder (MDD).
MDD also denotes clinical depression that has a big impact on one’s life. It affects the mood and behavior including the physical and social function. They may have unusual sadness and reduced interest in the usual activities they used to enjoy.
According to statistics, the Philippines is considered to be one of the happiest countries worldwide however has a high number of MDD cases as per WHO. Depression is common in all gender ages 20 to 50 year old. In five persons, one can experience depression.
The Philippines has the highest number of depressed people in Southeast Asia. The National Statistics Office reported that mental illness is the third most common form of disability in the country. Records show a high number of cases among the youth.
The study conducted by the Global Burden of Disease in 2015 reported that 3.3. million Filipinos suffer from depressive disorders, with suicide rates in 2.5 males and 1.7 females per 100,000. The World Health Organization, however, thinks that the numbers could be just a portion of the actual problem, especially because in a Catholic country like ours, talking about mental health creates a stigma among Filipinos, thus suicide incidents could be under-reported
Diagnosis
There are numerous factors professionals consider in diagnosing “Major Depressive Disorder”, such as descriptions of the observations of people surrounding the individual, as well as the patient’s own insight regarding life. In lieu of this, the patient diagnosed with MDD usually presents with an abrupt shift in both the outlook and function of daily activities, which severely interferes with the patient’s living conditions. Distinct irritability, excessive guilt, feelings of worthlessness, suicidal intent, indecisiveness, lethargy, along with shifts in diet and sleeping patterns are also key points in the diagnosis. Although, in order to properly diagnose MDD, the physician must thoroughly eliminate any possibilities of these symptoms being brought about by other conditions such as substance abuse, diabetes, hyperthyroidism, and/or other psychiatric disorders.
Treatment
The primary indication for antidepressant agents is the treatment of Major Depressive disorders. The following are antidepressant agents:
The most common antidepressant in clinical use due to their ease of use, safety in overdose, relative tolerability, fewer side effects, and cost
These drugs include fluoxetine, sertraline, and escitalopram which are available here in the Philippines
Side effects include drowsiness, nausea, dry mouth, insomnia, diarrhea, nervousness, agitation or restlessness, dizziness, sexual problems (such as reduced sexual desire, difficulty reaching orgasm, inability to maintain an erection), headache and blurred vision
The SNRIs approved by the food and drug administration in treating depression which is also available here in the Philippines are duloxetine, venlafaxine
Side effects include headache, somnolence, fatigue, dizziness, insomnia, agitation, tremor, anxiety, insomnia, lethargy, abnormal dreams, nausea, palpitation, decreased libido, hot flushes, sexual dysfunction, vomiting, constipation, diarrhea, dyspepsia, abdominal pain, weight gain, decreased appetite, and erectile dysfunction
SNDRI are used to treat depression. However, it is not approved by the FDA yet. Some SNDRI includes nefazodone and mazindol.
Side effects include nausea, dry mouth, insomnia, dizziness, orthostatic hypotension, sinus bradycardia, impotence, and blurring of vision.
Tricyclics antidepressants can cause more side effects than other types of antidepressants. This is why they are unlikely to be prescribed unless other medications are ineffective.
Examples include amitriptyline (Elavil), desipramine (Norpramin), doxepin (Sinequan), imipramine (Tofranil), nortriptyline (Pamelor), and protriptyline (Vivactil).
Side effects of these drugs are weight gain, orthostasis, hypertension, heart block, erectile dysfunction, ejaculation disorder, dizziness, memory impairment, sedation, lightheadedness, nervousness, insomnia, headache, tremor, nausea and vomiting, difficulty of breathing, diarrhea, constipation, urinary retention, dry mouth, blurring of vision and sweating.
Adverse effects or MAOIs are insomnia, agitation, headache, drowsiness, tremor, fatigue, confusion, blurring of vision, difficulty micturition, dry mouth, sweating, postural hypertension, and sexual dysfunction.
Patients who take MAOIs should AVOID dried, aged, fermented, spoiled, or improperly stored meat, poultry and fish, broad bean pods, aged cheese, tap, and unpasteurized beers and soy products like tofu.
However, they were ALLOWED to eat fresh processed meat, poultry fish, all other vegetables, cheese, ricotta cheese, yogurt, canned or bottled beers, brewer’s and baker’s yeast.
MAOIs can cause Hypertension when taken with decongestants (Phenylephrine, Ephedrine, Psudoephedrine, and Phenylpropanolamine), stimulants (Amphetamine and Methylphenidate), antidepressants (TCAs, NRIs, SNRIs, and NDRIs) and appetite stimulants (Sibutramine, Phentermine).
It can also be lethal causing hyperthermia when taken with antidepressants (TCAs, SSRIs, and SNRIs), appetite stimulants (Sibutramine) and opioids (Dextromethorphan, Meperidine, Tramadol, Methadone, and Propoxyphene).
Prevention
Depression can be severe and life-altering, affecting the quality of life and the happiness of those who live with it. It’s also a common condition. In some cases, it’s possible to prevent depression, even if you’ve already had a previous episode.
There are many lifestyle changes and stress management techniques you can use to prevent or avoid depression. There are certain triggers that can cause us to experience depressive episodes. While triggers may be different for everyone, these are some of the best techniques you can use to prevent or avoid depression relapse.
WHAT YOU CAN DO:
There’s no sure way to prevent depression. But you can:
Find ways to handle stress and improve your self-esteem.
Take good care of yourself. Get enough sleep, eat well, and exercise regularly.
Reach out to family and friends when times get hard.
Get regular medical checkups, and see your provider if you don’t feel right.
Get help if you think you’re depressed. If you wait, it could get worse.
Management
Cognitive behavioral therapy
It is a short-term, goal-oriented psychotherapy treatment for patients with major depressive disorder. Its goal is to change the way of thinking and behavior of a person behind their difficulties in order to change the way they feel. It focuses on encouraging patients to think of solutions and to challenge their distorted cognition towards the problems. It rests in the idea that thoughts and perceptions influence behavior. This aims to identify harmful thoughts, assess whether they are an accurate depiction of reality and if they are not, employ strategies to challenge and overcome them.
Interpersonal Therapy
This therapy was developed by Gerald Klerman which focuses on the patient’s current interpersonal problems. There are two explanation for this therapy; first, current interpersonal problems are likely to have their roots in early dysfunctional relationships. Second, current interpersonal problems are involved in increasing symptoms of current depression. Controlled trials have results that interpersonal therapy is effective in the treatment of the major depressive disorder and may be specifically helpful in addressing interpersonal problems. There are studies that prove that this kind of therapy may be the most effective method for severe major depressive episodes when the treatment choice is psychotherapy. It usually consists of 12 to 16 weekly sessions and is characterized by an active therapeutic approach. Defense mechanisms and internal conflicts are not addressed.
Psychoanalytically oriented therapy
This is based on psychoanalytic theories about depression and mania. Its goal is to effect a change in a patient’s personality structure or character and not just to relieve the symptoms. The aims of this therapy include improvement in interpersonal trust, capacity for intimacy, coping mechanisms, ability to experience a wide range of emotions, and the capacity to grieve. This treatment sometimes requires the patient to experience moments of heightened anxiety and distress during the course, which may happen for several years.
Family Therapy
It is not generally viewed as the primary therapy for the treatment of depression, but increasing evidence indicates that helping a patient with a mood disorder to reduce and cope with stress can lessen the chance of a relapse. It is indicated if the disorder is promoted or maintained by the family situation. This therapy examines the role of the mood-disordered member in the overall psychological well-being of the whole family; it also examines the role of the entire family in the maintenance of the patient’s symptoms.
0 notes
Text
FDA Expert Panel Throws Shade At Alkermes Antidepressant Drug Ahead Of AdCom Meeting

An expert panel convened by the Food and Drug Administration to review Alkermes’s regulatory approval application for an opioid-based antidepressant has raised issues in a briefing document that some investors say could present a hurdle to the drug’s approval.
In a briefing document, the FDA’s Psychopharmacologic Drugs Advisory Committee, or PDAC, expressed concerns about the safety of the drug, ALKS 5461, and the primary endpoint used in the Phase III study to measure its efficacy. ALKS 5461 is a combination of the opioid buprenorphine and samidorphan, an opioid antagonist, for which Alkermes is seeking approval as an adjunctive treatment for major depressive disorder. The documents were posted online ahead of PDAC meeting about the drug, which will take place on Thursday. The FDA is expected to decide whether or not to approve the drug in January 2019.
Shares of Dublin-based Alkermes, which had reached $42.64 in midday trading Monday before closing at $40.37, were down 5.6 percent when markets opened Tuesday, but had partially recovered by Wednesday morning. While votes from AdComs like PDAC are not binding, the FDA usually follows them.
Alkermes declined to comment.
The company had submitted four studies to demonstrate ALKS 5461’s efficacy: The Phase III FORWARD-3, FORWARD-4 and FORWARD-5 trials and a Phase II study. However, analysts from B. Riley FBR noted, the FDA only accepted FORWARD-5, given that the other two Phase III trials were negative, and Phase II was designed for proof of concept, not efficacy. The briefing documents indicate hurdles to convincing PDAC of the drug’s efficacy and could result in the agency declining to approve the drug should the AdCom vote against it, the analysts wrote, calling the documents’ negativity “not overly surprising.” However, the analysts have maintained their “Buy” rating for Alkermes’s stock.
In particular, concerns were raised about potential safety issues.
According to the briefing document, while PDAC said it agreed with Alkermes the inclusion of samidorphan meant ALKS 5461 carried fewer opioid effects than it would if it comprised buprenorphine only, “there remains some evidence of a mild opiate effect (including mild withdrawal effects) from the trials.” Phase III data published Monday in the Nature journal Molecular Psychiatry showed “minimal evidence of abuse, and no evidence of dependence or opioid withdrawal by [adverse events] or objective measures.” The most frequent side effects reported more frequently in the ALKS 5461 treatment group than in the placebo group included nausea, constipation, dizziness, vomiting, somnolence, fatigue and sedation.
The document also pointed to disagreements between the FDA and the company about FORWARD-5’s primary endpoint.
Although the FDA accepted FORWARD-5 for submission, PDAC pointed to uncertainty about how the trial measured the drug’s efficacy. Most antidepressant studies, the document noted, assess efficacy endpoints following six to 12 weeks of treatment, but Alkermes took a different approach, averaging values from each patient over several weeks. While the FDA did not prospectively agree with that approach, PDAC stated that it seems worthy of consideration, as it could potentially decrease the variability in mood disorder symptoms that tend to fluctuate over time. At the same time, scores at the final time point can carry less weight, meaning loss of efficacy at the end of treatment is less important in the analysis.
Against the FDA’s advice, Alkermes had chosen the six-point Montgomery-Åsberg Depression Rating Scale, or MADRS-6, in addition to the more standard MADRS-10, for FORWARD-5. The agency said MADRS-6 excludes important measures of reduced sleep, reduced appetite, difficulty concentrating and suicidal thoughts. A protocol amendment also changed how the second stage of the study measured the timing of response, from change in baseline to week 5 through end of treatment – which occurred at week 6 – to change in baseline to week 3 through end of treatment. However, the agency had criticized the apparent lateness of the change and also the measurement of patient responses as an average over multiple time periods rather than a single efficacy measure at the end of the study.
According to the published Phase III data, FORWARD-5 showed the drug was effective on MADRS-6 and MADRS-10 from the start of the trial, at week 3 and through end of treatment. However, FORWARD-4 did not show the drug was effective in MADRS-10 at week 5.
Photo: Wikimedia Commons
0 notes