#let the learning begin
Explore tagged Tumblr posts
Text
Stuff I Learned: D001x Medicinal Chemistry X
Oral absorption



Clearance and Vd
kel = clearance / Vd = ln(2)/half-life
Therefore clearance = Vd * ln(2) / half-life -> linear line

Generally aim for 8 hour half-life
To increase half-life,
Increase Vd by increasing lipophilicity (logP)
Decrease hepatic clearance by making the molecule more stable
Acidic Molecules
Contain a carboxylic acid group (pKa 4-5) that is deprotonated, thus negative
Because it is charged, it is less lipophilic
Because it is negative, it is prone to being bound to albumin
Therefore acidity decreases Vd
Basic Molecules
Contain an amine group (pKa 10) that is protonated, thus positive (ammonium, NH4+)
Cell membranes contain phospholipids which have negative phosphate groups and thus attracted to NH4+
Thus phosphilipids draw molecules out of plasma
Therefore basic increases Vd
E.g. nifedipine is neutral and amlodipine is similar structurally other than a short basic chain, increasing half-life from 1.9 hours to 34 hours

1 note
·
View note
Conversation
Guys! I can honestly say that I am so proud of myself :')
I buckled down and gave it my all (didnt even get on netflix GASP)..
As a result walking out of Franz lecture hall today, I am confident I aced that math midterm.
This feelings of success can't be equated to anything else. Some would say this feeling I have is the same feeling they get when falling in love. Yes I am a workaholic that has fallen for learning in depth and loves it Cx
#still got another midterm for my other math class next wednesday and then astro the week after#let the learning begin
1 note
·
View note
Text
Willemijn has inspired me to start learning my 3rd language. I'm going with Dutch so native Dutch speakers.....Help!!!
Send me a word a day or grammar/speaking rules!
0 notes
Text
So I've decided after the first I am going silent :3
0 notes
Text
Stuff I Learned: D001x Medicinal Chemistry IX
Clearance
Total clearance = hepatic clearance + renal clearance
Also clearance depends on blood flow (Q) and extraction ratio (E)
Therefore total clearance = Qh*Eh + Qr*Er



Volume of Distribution (Vd)
Apparent volume of plasma required to contain drug in plasma
An average 70kg man has 2.7L of plasma but Vd is often much larger than 2.7L (plasma volume is 0.039L/kg) because in reality drugs redistribute to peripheral compartment
Since concentration = amount/volume and the amount and Vd of a drug is known, the concentration can be determined. Then that concentration can be used with plasma volume to determine how much of the drug is actually in the central compartment
E.g. 25mg of ranitidine is given, Vd = 1.4L/kg = 98L in a 70kg man
C = 25mg/98L = 0.255mg/L
Since plasma volume is 2.7L:
0.255mg/L = amount of drug in plasma / 2.7L
Amount of drug in plasma = 2.7L * 0.255mg/L = 0.69mg
Therefore, only 0.69mg of 25mg ranitidine given is in plasma
Protein Bound
A higher % of protein binding decreases clearance and decreases Vd
However, half life and kel (elimination constant) remain the same
Phases
Drug concentration decreases sharply during the distribution phase, then more slowly during the elimination phase (though both happen together)

The end of the distribution phase is when concentration in tissues (Ct) reaches maximum and Vss (steady state volume) is reached

The equation for the blue line is represented by the equations (A and alpha representing distribution phase and B and beta representing elimination phase, in which alpha is usually larger than beta)

5 notes
·
View notes
Text
Meditation
Decided to start meditating on the word of God. Like consistently not just being all over the place with it. I think one reason why I haven't been consistent is because I'm not completely sure how to effectively meditate. So I will be meditating on meditation :)
I'll be focusing on this passage:
Joshua 1:8
New International Version (NIV)
8 Keep this Book of the Law always on your lips; meditate on it day and night, so that you may be careful to do everything written in it. Then you will be prosperous and successful.
And the 7 parts of Meditation:
Study
Imagine
Devise
Mutter
Utter
Growl
Roar
0 notes
Text
Stuff I Learned: D001x Medicinal Chemistry VIII
Blood
Blood volume 71ml/kg
In a 70kg person:
Whole blood 5L
Plasma 2.7L
Interstitial fluid 10L
Intracellular fluid 25L
Total body water 38L
Body volume 70L
Albumin
5% of blood by weight
Binds to acidic drugs well (e.g. warfarin, ibuprofen)

Globulin
2.5% of blood by weight
Alpha-1 acid glycoprotein binds basic drugs (e.g. disopyramid, lidocaine)

Lipinski’s rules
Set of rules created by Chris Lipinski in 1997 to predict whether a molecule is likely able to diffuse across membranes
Molecular weight, max value 500
Lipophilicity (log P), max value 5
Hydrogen bond acceptors, max value 10
Hydrogen bond donors, max value 5
Lipophilicity is measured as the logP for the partitioning of a drug in a biphasic system of 1-octanol (non-polar) and water (polar)
logP > 5 means too strongly favors octanol = too non-polar

Hydrogen bond acceptors = number of oxygen and nitrogen atoms
Lone pairs of nitrogen and oxygen can accept hydrogen bond
Exceptions if lone pair is involved extensively in resonance

Hydrogen bond donors = number of OH and NH bonds (groups may be deprotonated depending on pH)
0 notes
Text
Stuff I Learned: D001x Medicinal Chemistry VII
Clark’s occupancy theory
States that binding = response
If there is 50% binding there is 50% response
If there is 80% binding there is 80% response etc etc
Exceptions
Receptors with constituent activity, because even in 0% binding there is “response“ or activity
Spare receptors: not all receptors need to be bound for maximal response
Downregulation and desensitization
Upregulation and sensitization
Clark’s equation, in which E/Emax is 0 to 1



Alternative theory: drug residence time

koff = rate of dissociation, kon = rate of binding, tau = residence time
The greater the rate of dissociation, the shorter the residence time = less effect
For example with EGFR:
Lapatinib has greater Ki (lower potency) than gefitinib and erlotinib but has greatest activity, going against Clark’s occupancy theory
However, lapatinib has the greatest residence time
0 notes
Text
Stuff I Learned: D001x Medicinal Chemistry VI
Receptor Superfamilies
Ligand gated
Membrane bound
Control ion flow across the membrane
Associated with synapses
Called “fast neurotransmitters“
G protein coupled
Membrane bound
Pass signal across membrane
Signal proteins released into cell – complex pathways
Called “slow neurotransmitters“
Tyrosine kinase linked
Membrane bound
Ligand binding causes receptor dimers
Dimers act as enzymes
Associated with cancer
Nuclear receptors
Not membrane bound
Found in nucleus; ligand must enter the cell in order to bind
Control gene expression
Ligand types
Full agonist: upgoing sigmoidal curve, reaches Emax = full effect

Partial agonist: upgoing sigmoidal curve, reaches only a fraction of Emax, tend to bind to same position on receptor as full agonist = compete with full agonist

Antagonist: downgoing sigmoidal curve, binds to same receptor as agonist but causes no response; there are competitive and non-competitive antagonists



Inverse agonist: receptor has inherent activity called constituent activity; inverse agonist causes the activity to drop to zero ; downgoing sigmoidal curve with intercept > 0

0 notes
Text
Stuff I Learned: D001x Medicinal Chemistry V
IC50 and Ki
Both used to measure how much drug is required to inhibit an enzyme
In both cases, the higher the better
IC50 = inhibitor concentration 50 = the concentration of inhibitor required to inhibit rate of conversion by 50%


In linear equation format:

Cheng-Prussoff equation:

0 notes
Text
Stuff I Learned in D001x: Medicinal Chemistry IV
I didn’t expect this at all, but learning the next section allowed me to explore math like the good days in high school.
Equation for a plateau: y = ax+b / cx + d
As x approaches infinity, the constants b and d become irrelevant and the limit of this equation is a/c.
To simplify, if the equation is y = ax/(x+b), then the limit is a.
If x = b, y = 1/2 * a (half of limit)
If x = b/2, y = 1/3 * a (third of limit)
if x = b/3, y = 1/4 * a (fourth of limit)
If x = b/(k-1), y = 1/k * a (kth of limit)
Michaelis-Menten Kinetics

enzyme + substrate -> enzyme-substrate complex -> enzyme + product
V = rate of reaction (y-axis), S is substrate concentration (x-axis)
Vmax is the maximum rate, when S is infinite
1/2 Vmax is reached when S=Km aka Michaelis constant
If we put values into the equation y = ax/ (x+b):
y = V, x = S
a = limit = Vmax
1/2 Vmax is reached when x= b, and b is Km
Therefore, V = Vmax * [S] / ( [S] + Km) (the Michaelis-Menten equation)
This equation can be put into a linear form to the Lineweaver-Burk equation

Inhibition
Reversible
Competitive
Vmax not changed, still reached if substrate concentration high enough
Km increases (affinity dropped)
Non-competitive
Binds to allosteric site not the active site
Binds to both enzyme and enzyme-substrate complex
Lower Vmax
Km not changed (affinity not changed)
Uncompetitive
Binds only to enzyme-substrate complex
Vmax and Km decreases

Irreversible: covalently bind to the enzyme
0 notes
Text
Stuff I Learned in D001x: Medicinal Chemistry III
Drug discovery
Target based
Begins with a drug target aka protein, develop drug to bind to that protein
Protein binding assay measures how well drug binds to protein, and this is an in vitro test
More predominant method due to advanced molecular biology
Advantages
Relatively simple
Rapid lead optimization
Disadvantages
Protein may turn out to be ineffective relatively late (usually phase II)
Low efficacy
Phenotype based
Begins with a lead based on phenotype
Slow lead optimization
High efficacy, less likely to turn out ineffective later
Methods of Determining Protein Structure
Two methods: X-ray crystallography and nuclear magnetic resonance
Resolution measured in angstrom; the lower the angstrom, the higher the resolution
The Ramachandran plot can be used to validate protein structures
D-amino acids, unlike L-amino acids almost certainly have an incorrect protein structure
X-ray crystallography
More common method, 3/4 of protein structure determination
X-ray source shone on protein crystal, causing X-rays to collide into protein crystals and scatter in a diffraction pattern
0 notes
Text
Stuff I Learned in D001x: Medicinal Chemistry II
It’s amazing that I’m normally completely uninterested in learning history, but when used to learn how we got to something we take for granted today, it just becomes more fascinating than any introduction.
Sulfa Drugs
This guy Paul Ehrlich comes up with the idea that dyes can be used as antibiotics as well as coining the term Ehrlich’s silver bullet (kill the invading organism without affecting the host)
Gerhard Domagk finds a dye Protonsil Rubrum, a prodrug form of an antibiotic called sulfanilamide
The beginning of sulfa drugs which contain a SO2, a nitrogen, and a benzene ring
Both scientists won Nobel prizes


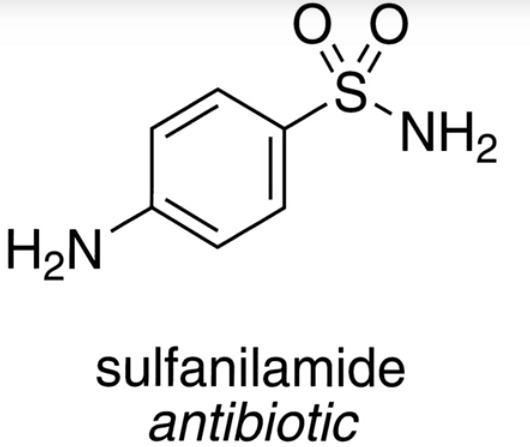
0 notes
Text
Stuff I Learned in D001x: Medicinal Chemistry
This course is crazy. I’m only one class in and I’m learning so much cool stuff. This is pretty exciting!
Pharmacophore = the minimal component of a class of drugs to achieve biological activity
Ephedrine
Emperor Shen Nung, father of agriculture, used Ma Huang for anti-tussive and decongestant properties.
The active ingredient in Ma Huang is ephedrine which is a vascoconstrictor and a stimulant
Ephedrine may be an alkaloid and its pharmacophore is:
Comes from natural source
Has a base nitrogen
Has a level of complexity (subjective)
It is a phenethylamine (class)
It has a stereoisomer or diastereoisomer called pseudoephedrine (sudafed), also a decongestant and stimulant, from which if you remove one alcohol group, you get methamphetamine
Phenethylamine
Examples: ephedrine, pseudoephedrine, methamphetamine, phentermine, fenfluramine
Its pharmcophore is:
Benzene ring
Two-carbon bridge
A nitrogen
Used as vasoconstrictors (therefore decongestants) and stimulants
Because they cause energy without food, also used as diet pills, such as phentermine and fenfluramine

Fen-phen
A diet pill that combines fenfluramine and phentermine
Withdrawn because it caused congenital heart defects (might have been just fenfluramine though)
Morphine rule
Benzene ring
Quaternary carbon
Two carbon linker
Tertiary amine




0 notes
Text
Stuff I Learned: 상담학 들어가기
원초아: id
자아: ego
초자아: superego
방어기제: defense mechanism
애착이론: attachment theory
1 note
·
View note
Text
Stuff I Learned: Nephro
Normal/low anion gap metabolic acidosis
If urine anion gap (urine Na + K - Cl) is 20-90, it is high
A high urine anion cap signifies inadequate acidification of urine = RTA
Elevated renal vein renin signifies fibromuscular dysplasia causing hypertension
Commonest causes of CAPD peritonitis: Staph aureus, Staph epidermidis, and Pseudomonas
Membranous Nephropathy
All patients should be treated with ACEI (+ statin if there is hyperlipidemia)
Pathology: thickening of basement membrane with subepithelial electrodense deposits separated by spikes of basement membrane
0 notes