#it’s been a full 3 months now since getting on the therapeutic dose and officially ending my worst depressive episode ever.
Explore tagged Tumblr posts
Text
need to read more into the effects of lamictal on memory. it’s fascinating. bc my short term memory and language processing has gone to shit, which is a known side effect, but also past memories of things i haven’t thought about in years are hitting me at random all the time in vivid detail. and my dreams are way clearer and way less distinguishable from reality now than they used to be.
#or i may just be at a phase in my life where i am finally beginning to process my past in even a very surface-level way.#could be a correlation thing and not a causation thing. but who knows.#it’s been a full 3 months now since getting on the therapeutic dose and officially ending my worst depressive episode ever.#so i rly do feel like i’m finally leveling off in that regard.#and this is approx how my memory & dreams have been for the last…. month or so. at least.#Interesting!#izzy.txt
7 notes
·
View notes
Text
Full-Spectrum Hemp Oils vs. CBD Isolates – What’s The Difference?
In recent years, the public profile of CBD has soared with users using it to treat all kinds of conditions and ailments. It can be consumed in several ways, ranging from the simple oral consumption to topical usage and even vaping.
Now, for those just getting into CBD, the terms “CBD isolate” and full-spectrum” might cause confusion. This comprehensive article shall clear the confusion and help you make an informed buying decision.
First, let’s discuss the basics.
Free CBD Oil Business
“Hemp CBD Sales Projected To Hit $1 Billion In 3 Years.”
Fantastic product prices
60-day money-back guarantee
Full-spectrum Products
Isolate Products
Organic and grown in the U.S
Packaged in the U.S
Free of herbicides, pesticides, and heavy metals 3rd party lab tested for quality.
IT’S ABSOLUTELY FREE TO JOIN
Cannabinoid: What Exactly Is It?
In order to understand how hemp oil products work in your body, you first need to understand the ECS, short for endocannabinoid system, which is a part of the mammalian central nervous system.
All humans (even your furry family members or other animals) have ECS. This is believed to play a crucial role in most bodily functions including sleep, appetite, mood, and even injury mitigation.
So, how do they work?
Generally speaking, ECS can be thought of as the body’s regulatory committee. Whenever things get unbalanced, the ECS steps in to bring order, known as homeostasis.
The body produces chemicals known as endocannabinoids.
The body has 2 networks of cannabinoid receptor:
CB1: Found in connective tissues, organs, gonads, and throughout our nervous system.
CB2: Mostly found through the immune system and its related organs.
Take note, that both receptors can also be found in a broad range of bodily tissues.
Read More:
Full-Spectrum Hemp Oils vs. CBD Isolates – What’s The Difference?
15 Tips on How to Deal With Your Coronavirus Anxiety
Top Reasons Why CTFO is One of the Best Affiliate Marketing Programs
Make Money Taking Surveys and Tips for Avoiding Scams
The Ultimate CBD Edibles Guide
Understanding Phytocannabinoids
Moving on, we have the phytocannabinoids. These are chemicals contained in hemp plants and are somewhat similar to the endocannabinoids produced by our bodies. Thus, they too, have a significant effect on our ECS.
There are over 80 known plant-produced cannabinoids. All of them are known to effectively mimic the endocannabinoids 2-AG and AEA which are produced in our bodies.
These phytocannabinoids are cannabinoid acids which synthesize during production (by a heating process called decarboxylation) into the recognizable compounds such as THC and CBD. Now, let’s move on to the most common plant sources of these phytocannabinoids.
Hemp VS Marijuana: Understanding The Difference
The terms are often used interchangeably. Although marijuana and hemp are both varieties of the Cannabis sativa plant, they are not the same.
Marijuana is usually cultivated as a horticultural crop and has long been thought of as a drug. Hemp, on the other hand, is an agricultural crop and has been used for hundreds of years in various applications such as industrial and household products.
And today’s very popular CBD oil products are also derived from hemp which is grown at an industrial scale, exacting strict specifications to ensure its legality and maximizing the potential healing properties of its cannabinoid profile.

Legal Talks: Is Cannabis Legal?
That is one complicated question. You see, the legality of cannabis will depend on the plant type in question. For years, federal law has lumped the hemp plant in with other cannabis plants which were outlawed effectively in 1937 under the Marihuana Tax Act then made officially illegal via the Controlled Substances Act of 1970.
The US DEA (the United States Drug Enforcement Agency) has designated the marijuana plant as a Schedule 1 substance. Meaning it has no accepted medical use at the moment and has a high potential for abuse. That definition is still up for debate, but until it occurs at a federal level, then expect that all marijuana products are illegal. Read More: Is CBD Oil Legal In All 50 States All You Need to Know!
Technically, it is against federal law to grow marijuana varieties of the Cannabis sativa plant and to ingest or consume any products made using that plant. However, in recent years, many states have already legalized marijuana at various levels, either for recreational or medical use. AS of the first month of 2019, medical marijuana is legal in 33 states while 10 states allow recreational marijuana.
Then, there’s the hemp plant in which growers can now cultivate legally, because of the 2018 Farm Bill. Also known as industrial hemp, this variety of the cannabis Sativa plant can’t contain over 0.3% of THC so that it won’t make you high.
All hemp and CBD oil products are legal to ship to all 50 states thanks to the lower THC levels. However, take note that individual state laws can actually vary, so make sure to check whether it is legal in your state or not. The fully federally legal products include full-spectrum hemp oil products like tinctures, capsules, edibles, salves, and balms.
The Extraction Process
As you know, all CBD products are extracted from the Cannabis plant, usually the hemp variety, using solvents like CO2. During the extraction process, all the terpenes, cannabinoids, and flavonoids are stripped from the plant and then dissolved in oil in order to make it easier to store and administer.
Depending on the final product’s purpose, companies can decide to use a different cannabinoid spectrum, from various strains. Now, what actually happens with the cannabinoids after the first extraction process determines if the extract is full-spectrum or CBD isolate, or the less common choice broad spectrum.
Each term refers to the degree that the product was processed. In this article, we will only focus on two: full-spectrum hemp oil vs CBD isolate.
Full-Spectrum Hemp Oil Defined
This is the product that most people will find when looking for CBD oil. Full-spectrum hemp oil is an extract containing all compounds found naturally occurring in the Cannabis plant including essential oils, terpenes, and other cannabinoids.
The full spectrum of terpenes, cannabinoids, and essential oils extracted from the plant harmoniously work together to magnify the therapeutic benefits of each cannabinoid. This is commonly known as the “Entourage Effect”. In the past decades, it was believed that CBD in its isolated form was far effective than full-spectrum hemp oil. In 2005, this theory was debunked by the Jerusalem Lautenberg Center for General Tumor Immunology.
In the study, researchers revealed that the test subjects treated with a full spectrum CBD reported a much higher relief, compared to subjects treated with only CBD isolate.
Additionally, the results showed that a full spectrum CBD offered enhanced effects in higher dosages, while CBD isolates maintained consistent effects even with increased dosages.
Full Spectrum Hemp Oil: How Is It Made?
Full-spectrum hemp oil is extracted from the aerial parts of the plant which are the flowers and leaves.
Its production starts with drying the stems, stalks, flowers, and leaves of a fully mature hemp plant. The entire plant is then mashed into a fine powder. Then, using a gentle extraction method, the oil is extracted from the powder.
This process leaves the phytocannabinoids as a whole, ensuring all the potentially-beneficial compounds are delivered in the extracted oil.
Pros and Cons of Full Spectrum Hemp Oil
There are several reasons why full-spectrum hemp oil is the preferable option. For one, it features a full combination of all chemical compounds that makes it a potent solution.
Some of the additional cannabinoids in full spectrum extracts include:
CBDV (Cannabidivarin)
CBG (Cannabigerol)
CBN (Cannabinol)
CBDA (Cannabidiol acid)
CBC (Cannabichromene)
Some of the additional cannabinoids in full spectrum extracts include:
Secondly, it is scientifically proven that cannabidiol works best in synergy with other cannabinoids and terpenes, and partially loses its beneficial properties when isolated. Then there’s the most pleasant benefit: the nice subtle scent of terpenes, which provides full-spectrum hemp oil a better taste and aroma than other extracts. However, terpenes are more than just making your oil smell good.
There are over 200 terpenes in cannabis that also bind to different receptors in your body to offer a wide range of potential health benefits including gastrointestinal issues.
Now, because of the potency and benefits of extracting the entire plant, full-spectrum hemp oils are priced higher than CBD isolate. Plus, it is also more challenging for manufacturers to maintain a consistent ratio, which again increases the overall cost of these oils.
Full Spectrum Hemp Oil: Who Is It For?
Individuals living in states where the use and consumption of Cannabis is legal
Individuals with more severe conditions that CBD isolate can’t alleviate
Individuals who were recommended a specific CBD to THC ratio
Tips When Taking Full Spectrum Hemp Oil
In order to get the best effects from full-spectrum hemp oil, you need to select the correct dosage and potency. If you are new to using these products, then it can take time to find the correct amount you need.

Low Start
You might be eager to take hemp oil to address a chronic or nagging condition, but make sure that you don’t take too much at first.
You will need to start at the lowest dosage possible and then keeping a journal to track how you are feeling with every application or dose.
Since everybody is different, each individual will have different reactions to hemp oils.
Gradual Increase
If the lowest dosage does not seem to be benefitting you, then you can start increasing it. Since it takes some time for these products to build up and affect your ECS, you can raise the dosage amount gradually.
Take the same dose for a few days before you take a higher dosage.
Try A Different Delivery Method
There are various ways to use, consume, or ingest full-spectrum hemp oil, depending on the desired effects.
Full-spectrum hemp oil is typically found in easy to swallow liquid tinctures applied under your tongue with a medicine dropper for easy measurement. They are also available as capsules. Both of these delivery methods allow you to ingest the oil orally where it enters the digestive system and then metabolized by the liver.
In general, CBD oil under the tongue offers a quicker delivery since the constituents of the oil get to bypass your digestive system and liver metabolization. Instead, the oil is directly absorbed into your system so you can experience the benefits of hemp at a much faster rate.
CDB oil can also be added to your favorite drink or food. It is not only an easy way to use hemp oil, but it can also improve how well you absorb CBD. Fatty acids found in some foods can serve as carriers for the compounds, allowing them to move through your body for quicker processing.
Vaping hemp oil is also a great way to ingest or consume full-spectrum hemp oil. Vaping offers you a lung-friendly method for inhaling cannabinoids, allowing it to be absorbed through the large absorptive surface area of the lung before being diffused into your bloodstream.
Then, there’s also the salves, balms, and lotions with hemp oil as the main ingredient. The active ingredients will never enter your bloodstream but rather absorbed into your skin to interact with cells near the surface of your body. Thus, full-spectrum hemp oil topicals are more focused on outer relief and can provide benefits to the health of your skin.
60-Day Open Bottle Money-Back Guarantee.

Why Buying CBD In Mobile Through CTFO
1) 60-Day Open Bottle Money-Back Guarantee
2) Full Spectrum Products
3) Isolate Products
4) Organic and Grown in the U.S
5) Packaged in the U.S
6) GMP (Good Manufacturing Practice) Certified, and non-GMO.
7) 3rd Party Lab Tested for Quality
SHOP NOW
Consult a Healthcare Professional
The internet is filled with medical information, some factual and some having no scientific basis. To get real answers, there’s no substitute for actually talking to a healthcare professional.
Whatever problem you hope to ease with cannabinoid, a doctor or other medical professional should be able to tell you the doses to take and the most effective delivery method for you.
Full Spectrum Hemp Oil Potency and Dosage
The potency of hemp oil is categorized into three types. Doctors cannot prescribe full-spectrum hemp oil or other cannabinoids dosage and can only make recommendations based on your symptoms.
Further complicating this issue, there is no universal dosage or recommended daily CBD allowance to act as your guide. There are also different variables including your metabolism, weight, product consistency, and diet that make it even harder to pinpoint the right dose.
This is why recommended that you start with the lowest dosage possible.

Low Potency: 2.5mg – 15mg per serving
Also called micro-dosing, low doses of hemp oil can be effective for people with active ECS. A small dose every day can be used to maintain a healthy lifestyle and ease health issues.
Medium Potency: 16mg – 33mg per serving
Doubling the low potency dose is effective in most cases. The way your ECS works and the severity of the problem you’re treating will determine how many doses you will need every day.
High Potency: 35mg – 50mg per serving
Research is still underway to see how high-strength doses of hemp oil can be used for various ailments and health problems.
CBD Isolate Defined
Scientifically speaking, an isolate is defined as the purest form of a compound, produced by singularity extracting the said compounds from its environment and completely isolating it from all other compounds.
With that said, a CBD isolate is defined as the purest form of CBD. It is produced by removing all other compounds in the Cannabis plant including flavonoids, terpenes, other cannabinoids, and plant parts.
CBD Isolate: How Is It Made?
In order to get the CBD out of the hemp plant, it will go through an extraction process. From the initial extraction, it must go through a more complex extraction process where several compounds of the hemp plant remain in the extracted oil. The final product is called full spectrum or broad-spectrum hemp oil.
From there, it will again undergo additional processing to remove any residual plant material and the unwanted compounds. After that, the extracted oil goes through a second purification process where any remaining plant material is then filtered out.
After the filtration process, the extracted oil will go through winterization. This process is an alcohol washing,
which involves soaking the CBD extract in alcohol and then freezing it. The process will separate the pure CBD from all other residual products that remain in the CBD oil.
After winterization, the product will undergo several rounds of rotary evaporation to remove any remaining residual material that may still be present in the winterized oil. The extract then undergoes decarboxylation and converted into crystalline powders.
The result? Crystallized pure CBD!
4 Types of CBD Isolate
The versatility of an isolate makes it attractive to CBD users. With isolate, you will find a broad range of products from the pure isolate to a broad variety of products developed with isolate as the star ingredients.
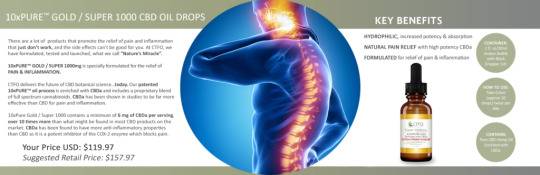
SHOP NOW
1) Powder / Crystals
This is the most common form of CBD isolates available on the market. This CBD isolates powder or crystals are created by pulverizing a slab.
2) Slabs
When CBD is processed from a full spectrum extract, the resulting product comes as a slab form. This form is then broken into smaller chunks. IT is preferred by some users since it is easier to vape or dab.
3) Isolate-Based Products
CBD isolate as an ingredient in CBD products is common. Thus, you will find CBD isolate-based products of nearly every type of availability today.
4) Terpsolate
In order to fight the loss of all major cannabis compounds, some companies will often add marijuana or hemp-derived terpenes back into the CBD isolate. The resulting is an aromatic product that also provides additional health benefits thanks to the terpenes.
Pros and Cons of CBD Isolate
One of the main reasons why CBD isolate has become a favorite by some is because of its zero THC.
Although some people think that CBD products do not contain any THC, in most full-spectrum hemp oils, there are still some trace amounts of THC which can be as low as 0.3%.
Although this small THC amount is not enough to make you high, it can show up in your system, which can put you at a disadvantage when undergoing regular drug tests.
CBD isolate is also a great choice for anyone who is sensitive to even the smallest amounts of THC or other cannabinoid compounds. In addition, CBD isolates also have easy-to-measure dosage since there is nothing else to account for aside from the pure crystals.
However, since it only contains, pure CBD compound, CBD isolate can have a weaker effect than full-spectrum hemp oils.
CBD Isolate: Who Is It For?
First-time users who may be hesitant about other cannabinoids
Individuals with sensitivity to other cannabinoids or THC
Individuals who were recommended to take high doses of CBD
Individuals who prefer no flavor or light flavors
Individuals living in states with strict THC laws
Individuals who regularly undergo drug screening tests
Final Thoughts
So there you have it. You should now have a good understanding of the differences between the CBD extract. To summarize it all
Full-spectrum hemp oil contains all compounds found naturally occurring in the hemp plant which includes essential oils, terpenes, and other cannabinoids.
CBD isolate, on the other hand, is the purest form of CBD available.
One type is not better than the other and it will depend on several factors including your medical needs and history, weight, your chemical makeup and so much more.
For some, the pureness and high potency of CBD isolate can do the trick while others can benefit from the all-inclusive effects of full-spectrum hemp oil. Regardless of which you choose, CBD is indeed a vital component in the future of medicine.
Offering dozens of health benefits, CBD has the potential to positively impact the lives of countless people. With consistent research and studies, a healthier and brighter future could be right around the corner.
WE hope that this article proved to be informational and help shed some light on the ever-confusing subject of CBD. Don’t forget to share this article with your friends and families!
The post Full-Spectrum Hemp Oils vs. CBD Isolates – What’s The Difference? appeared first on Sell CBD Oils.
from https://hempoilfrog.com/full-spectrum-hemp-oils-vs-cbd-isolates/
0 notes
Text
New story in Politics from Time: The Ford Administration Rolled Out a Vaccine Program Right Before the 1976 Election. It Backfired—And Not Just Politically
For months, President Donald Trump has repeatedly said that a vaccine for the novel coronavirus may be ready by the end of the year, even as the nation’s top infectious disease specialist says early 2021 is more likely. Now, the New York Times has reported that the Trump Administration intends to speed up the process, with its eyes on a slightly earlier deadline: before Election Day on Nov. 3.
White House officials disputed the Times’ account, and researchers working on the specific vaccine cited as the likely candidate for emergency approval—one that is being developed by Oxford University and pharma giant AstraZeneca—said “it would be premature to speculate on that possibility.” This isn’t the first time Trump or his team has suggested a COVID-19 vaccine could come before the general election—Trump, for example, made a similar claim in the first week of August.
Some pundits, like David Axelrod, an adviser to former President Barack Obama, have accused Trump of being willing to sacrifice safety in the name of politics.
This is as predictable as it is disturbing. If @realDonaldTrump fast tracks a vaccine, bypassing critical safety steps so he can announce it before the election, who will have confidence in taking it?https://t.co/OLZT5BDc4o
— David Axelrod (@davidaxelrod) August 23, 2020
Trump has shot back, suggesting that his political opponents are holding up research progress for the same reason; on Aug. 22, he tweeted that “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd.”
This isn’t the first time a fast-tracked attempt to mass inoculate Americans has gotten political. The history suggests that if the White House rushes a vaccine rollout, it can risk losing credibility—not just for itself, but for the science as well.
In February of 1976, more than 200 recruits at Fort Dix military base in New Jersey came down with the flu. While some simply had the 1975-1976 seasonal flu, 13 had a new strain of H1N1, often called swine flu, that the CDC says was “similar” to the strain that caused the 1918-19 flu. One 18-year-old died. The outbreak sparked concerns of a repeat of that infamous pandemic, which scientists now know killed an estimated 675,000 Americans, or of the more recent 1968-69 pandemic, during which 100,000 Americans died and “$3.2 billion was lost in medical bills and working time,” TIME reported.
Unlike in 1918, the U.S. in 1976 had a way to stop the outbreak in its tracks: flu shots, developed for military use in the 1930s, approved for civilian use in 1945 and encouraged for all high-risk Americans since 1960.
But the Fort Dix cases involved a new strain of H1N1, so if a vaccine were going to be ready in time for the 1976-1977 flu season, production needed to start as soon as possible. Even though no major outbreak had yet begun, the government decided to put its weight behind the effort.
“We cannot afford to take a chance with the health of our nation,” then-President Gerald Ford said on March 24, 1976, when, per the CDC’s recommendation, he called for a $135 million program (about $615 million in 2020) to produce a vaccine and manufacture more than 200 million doses in time to inoculate all Americans for the winter flu season. The plan “was unprecedented in intended timing and in scope among American immunization efforts,” Richard E. Neustadt and Dr. Harvey V. Fineberg noted in The Swine Flu Affair, their 1978 evaluation of the program, which the incoming Health, Education and Welfare Secretary asked them to undertake.
But not everyone was sure the vaccine program was being launched for purely altruistic reasons. A year of celebrations to mark America’s bicentennial was in full swing. It was also a presidential election year, and while Ford had beaten challenger Ronald Reagan in the first five primaries and the Iowa caucus, it was still a tight race for the Republican nomination. In fact, Neustadt and Fineberg’s report points out that Reagan won the North Carolina primary the day before Ford’s announcement of the vaccine program. During hearings on the program that spring, Washington State’s Democratic U.S. Senator Warren Magnuson joked of vaccine recipients that the Administration “might have ’em vote at the same time,” and, TIME noted, “some legislators and doctors are wondering out loud whether the flu program is merely another symptom of election-year fever.”
Ford Administration officials took the position that—given that the vaccine was safe—the risk of an outbreak outweighed any concerns about politics. Not that it wouldn’t be politically beneficial: “Consider the outcry,” one White House aide was quoted as saying, “if with all that evidence the President had said no.” In April 1976, Congress swiftly passed emergency appropriations to make the vaccine for which Ford had called.
Get your history fix in one place: sign up for the weekly TIME History newsletter
By July 1976, the vaccine was “only partially successful in clinical trials,” TIME reported back then, prompting scientists to reconsider the rollout. Among the roughly 5,000 volunteers injected with the still-being-tested vaccine, researchers found minimal side effects in adults, but high fevers in young people, leading Dr. Albert Sabin, developer of the oral polio vaccine, to backtrack on his initial support of the Ford program, and argue that only the highest-risk people should get the vaccine. Dr. Jonas Salk, who developed the initial polio vaccine, argued it was safe and would reduce the virus’ spread.
By August, when Congress passed a bill officially authorizing the rollout, the effort was two months behind schedule, largely because of Congressional wrangling over whether vaccine-makers would be protected from liability related to the product. This delay would have serious consequences: In retrospect, a 2006 review of the CDC’s handling of the 1976 swine flu vaccine program co-written by David Sencer, the agency’s director back then, found that manufacturers’ demand for indemnification led the public to believe “there’s something wrong with the vaccine,” and so “every coincidental health event that occurred in the wake of the swine flu shot would be scrutinized and attributed to the vaccine.”
Clinics began offering two swine flu vaccines in October—a vaccine against swine flu and, for higher risk groups, a vaccine against both swine flu and the previous year’s flu. At that point, no other cases of swine flu had been confirmed since the Fort Dix cluster in February, but the government began offering free vaccines anyway, along with the slogan “ROLL UP YOUR SLEEVE, AMERICA.”
A poll showed that a little over half of Americans (53%) planned to get one, TIME reported in its Oct. 11, 1976, issue.
Howard Markel, director of the Center for the History of Medicine at the University of Michigan, remembers getting the shot as a high school sophomore: “Everyone went to school gyms or large areas, and I remember vividly my mother making us go, and waiting in line, and saying ‘this is ridiculous.'”
But only a few vaccine-distribution centers were equipped on launch day. A Portland, Ore., health official told TIME, “We didn’t even know we’d received a batch until we read about it in the newspaper.” And the magazine reported that by November, only a quarter of the more than 200 million doses advertised had been manufactured.
Then, 35 mostly elderly people, in various parts of the country, died shortly after getting vaccinated. The government said there was no link between the deaths and the vaccines, but the news still dissuaded people from getting the shot. After the news of the deaths, the number of New Yorkers who showed up for a vaccine dropped from 21,000 to 7,500 a day, and nine states closed their clinics, TIME reported.
“If you immunize very large numbers of elderly people, inevitably some will have a heart attack the next day, so you have to prepare the public for such coincidences,” Fineberg, a co-author of the 1978 study of the public health response, later told the World Health Organization. “That wouldn’t have been a blip on the screen had there been a pandemic but, in the absence of any swine flu disease, these rare events were sufficient to end the program.”
Photographs of Ford (who had ultimately been successful in the primaries) getting vaccinated, like the one seen above, were circulated in an attempt to restore confidence in the vaccine program, but it was too late.
Election Day rolled around, and Ford’s tenure in office ended, for a host of reasons other than the flu-shot rollout. The mass inoculation program ended about a month later—but not before the public’s confidence was once again shaken, this time by reports of shot recipients being at a very slightly higher risk of Guillain-Barré syndrome (GBS).
“The uptick was not directly caused by the flu vaccine,” Markel says, “but it was associated with it, so everyone came to the conclusion that the swine flu shot equaled Guillain-Barré.” (About one additional case occurred per 100,000 people vaccinated, according to the CDC, but most studies that have since evaluated a possible link between other flu vaccines and GBS have found no association.)
Markel also notes that the program’s failure happened to occur at a time when Americans’ trust in government was already in decline, following the Vietnam War, Watergate and the assassinations of the 1960s. The idea that the government would have botched the vaccine and still tried to capitalize on it for political reasons fit right in—whether or not it was true.
That confusion and distrust incubated in that time period lingered well beyond 1976. In a 2009 CDC fact-sheet about a vaccine for that year’s H1N1 virus, for example, two of the questions were about about 1976 and GBS. Today, at a time when unfounded anti-vaccine views are growing (even though vaccines are safe) and polls indicate distrust in the federal government’s management of the response to the COVID-19 pandemic, that enduring doubt can be dangerous.
“Things like the small increased risk of GBS from the 1976 vaccine become…convenient for post-hoc justification of those pre-existing fears,” says Jonathan M. Berman, author of the forthcoming history of the anti-vaxxer movement Anti-vaxxers: How to Challenge a Misinformed Movement. “Later studies showed that there was likely no link, but already wary parents and anti-vaxxers see it as a justification for the fear.”
On the other hand, the 1976 controversy “does provide important lessons,” Berman argues. “When vaccination decisions appear politically motivated, it can undermine trust.”
The fact that even people who are not generally anti-vaccine are worried about the safety of a COVID-19 inoculation means it’s all the more important that a coronavirus vaccine is not rushed, argues Markel. “This is a very touchy issue,” he says, “so this [vaccine] has to be rolled out exactly right.”
from Blogger https://ift.tt/3ligEtE via IFTTT
0 notes
Photo

New Post has been published on https://12stepnationalmeetings.com/medication-assisted-recovery-anonymous/medication-assisted-recovery-anonymous/
Medication-Assisted Recovery Anonymous

There’s a New 12-Step Group: Medication-Assisted Recovery Anonymous Unlike many NA or AA chapters, this group accepts that many people benefit from medications like methadone and buprenorphine.
Chairs in a circle for a meeting. It’s like AA, but more open to those taking methadone or other medication to assist in recovery. Photo illustration by Slate. Photos by Thinkstock. It was quarter to seven, and St. Mark’s Church in Frankford, Philadelphia, was home to your typical pre-meeting bustle: A woman in pink in her mid-40s dragged mismatched metal chairs across the floor into a circle while the sound of a coffeepot crackled behind her. As is often the case in 12-step groups, there were concerns over the coffee—was it too light? (it was), would there be enough for next week’s meeting? (there wouldn’t). Family Dollar was allegedly out of sugar.
This wasn’t an Alcoholics Anonymous or a Narcotics Anonymous meeting, though. This was a Medication-Assisted Recovery Anonymous, or a MARA meeting, a gathering of people who were united by their desire to recover from their addictions, but who also recognized that the best way for them to do this might involve anti-craving medications like methadone and buprenorphine, known by the brand name Suboxone.
It was a light turnout that night—about 25 people—according to a woman holding a cane sitting next to me. All but two in the group were female. One member shared a story of working up the nerve to tell her AA sponsor that she’s on 45 mg of methadone, something that is too often discouraged in the AA model. When she shared, the compassion was audible; this kind of concern is all too common.
In the midst of America’s deadliest addiction epidemic—a crisis in which the national life expectancy has fallen for the second consecutive year due to opioid overdoses’ impact—there is still an enormous and problematic stigma within 12-step groups against members who take prescribed medications to manage their addictions. Though at the organizational level, groups like AA and NA consider medication an “outside issue,” at the local group level, it is subtly, and sometimes explicitly, discouraged. Members who do not take these medications often marginalize those who do by excluding them from meeting participation, turning them down when they ask for sponsorship, and telling them that they’re not actually in recovery.
The use of prescribed methadone and buprenorphine—referred to as medication-assisted recovery when combined with psychosocial treatments like peer support and talk therapy—is undeniably the most effective treatment for opioid use disorder, according to the evidence. Research has repeatedly shown that these medications reduce opioid addiction–related deaths by 50 percent or more, increase treatment retention, and decrease infectious disease transmission and criminal activity.
Despite this evidence, patients with opioid use disorder frequently receive pressure from family members, 12-step groups, and outdated, punitive policies in treatment centers, recovery houses, and court systems to not take these medications at all, or to stop taking them before they’re ready, according to addiction specialists who treat them. Dr. Sarah Wakeman, the medical director of the Substance Use Disorder Initiative at Massachusetts General Hospital, attributes much of this stigma to confusion between physiological dependence and addiction.
Physiological dependence, Wakeman explains, means that if an individual stops taking a drug or medication, they’ll get sick, just like a person with diabetes gets sick without insulin. Addiction, on the other hand, is defined as compulsively using substances despite harmful consequences. Medications like methadone and buprenorphine are prescribed to assist with physiological dependence, which prevents them from getting sick so they can focus on their recovery. Individuals on the proper dose of these medications who take them as prescribed can lead full, high-functioning lives both socially and professionally.
“The need to keep it a secret or feel like it’s something shameful when people are doing really well on treatment is challenging and can really undermine someone’s recovery.” — Dr. Sarah Wakeman But AA and NA are programs based upon total abstinence from mind- or mood-altering substances, and many members consider addiction and dependence synonymous. In 2016, NA published a pamphlet called Narcotics Anonymous and Persons Receiving Medication-Assisted Treatment, in which it states, “By definition, medically assisted therapy indicates that medication is being given to people to treat addiction. In NA, addiction is treated by abstinence and through application of the spiritual principles contained in the Twelve Steps of Narcotics Anonymous.” So while some members and groups (due to the organization’s autonomous structure) are open-minded to individuals on medication, many others have interpreted this to mean that if a person takes methadone or buprenorphine, he may as well be using heroin.
“I think it’s heartbreaking because if a person had cancer or had any other chronic illness and they were valiantly managing it, people in their lives would be supporting them and encouraging them to take their medication every day to stay healthy,” Wakeman tells me. “The need to keep it a secret or feel like it’s something shameful when people are doing really well on treatment is challenging and can really undermine someone’s recovery.”
Megan McAllister knows that feeling of shame all too well. While attending other 12-step meetings, she’s witnessed people stop clapping and whisper when someone on methadone announced their recovery anniversary. Some members have even gossiped to her about others who take the very same medication she’s taking because they don’t realize she’s taking it.
“Why should I feel ashamed for doing something that’s saved my life?” McAllister asks me. “I was putting a needle in my arm every 10 minutes—methadone saved my life.”
She started taking the medication after unsuccessful attempts to treat her heroin addiction with buprenorphine. Since starting the medication, McAllister has gone to work every day and takes care of her 3- and 9-year-old children. Perhaps most impressive, she inherited a support group for individuals in recovery on medication from a local certified recovery specialist named Freddie Laboy and almost immediately formed MARA, which she plans to expand to offer meetings on different days and in different neighborhoods across the city.
Now MARA has group conscience meetings the second Wednesday each month; service positions including a chairperson, coffee person, secretary, treasurer, and researcher; an official format; and its own literature. Word seems to be getting around, as MARA was recently contacted by someone who wants to start a new group on the other side of the country in Abilene, Texas. For a few months now, Justin Uphill, a peer recovery coach at the Abilene Regional Council on Alcohol and Drug Abuse, has been talking with another recovery coach and one of the local doctors about starting a MARA group at their facility.
At 7:01 in Frankford, Megan McAllister knocked on her chair to quiet the room for the start of the meeting. The chairperson was running late, so she gave a quick welcome, held a moment of silence, and asked a volunteer to read the preamble, which was now typed and no longer written by hand, as it was just a month or so earlier.
“We, of Medication-Assisted Recovery Anonymous,” she read, “believe that medication is a therapeutic tool of recovery that may or may not be discontinued in time, dependent upon the needs of the individual.”
Jillian Bauer-Reese is an assistant professor of journalism at Temple University, where she teaches a course called Solutions Journalism: Covering Addiction.
0 notes
Link
For months, President Donald Trump has repeatedly said that a vaccine for the novel coronavirus may be ready by the end of the year, even as the nation’s top infectious disease specialist says early 2021 is more likely. Now, the New York Times has reported that the Trump Administration intends to speed up the process, with its eyes on a slightly earlier deadline: before Election Day on Nov. 3.
White House officials disputed the Times’ account, and researchers working on the specific vaccine cited as the likely candidate for emergency approval—one that is being developed by Oxford University and pharma giant AstraZeneca—said “it would be premature to speculate on that possibility.” This isn’t the first time Trump or his team has suggested a COVID-19 vaccine could come before the general election—Trump, for example, made a similar claim in the first week of August.
Some pundits, like David Axelrod, an adviser to former President Barack Obama, have accused Trump of being willing to sacrifice safety in the name of politics.
This is as predictable as it is disturbing. If @realDonaldTrump fast tracks a vaccine, bypassing critical safety steps so he can announce it before the election, who will have confidence in taking it?https://t.co/OLZT5BDc4o
— David Axelrod (@davidaxelrod) August 23, 2020
Trump has shot back, suggesting that his political opponents are holding up research progress for the same reason; on Aug. 22, he tweeted that “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd.”
This isn’t the first time a fast-tracked attempt to mass inoculate Americans has gotten political. The history suggests that if the White House rushes a vaccine rollout, it can risk losing credibility—not just for itself, but for the science as well.
In February of 1976, more than 200 recruits at Fort Dix military base in New Jersey came down with the flu. While some simply had the 1975-1976 seasonal flu, 13 had a new strain of H1N1, often called swine flu, that the CDC says was “similar” to the strain that caused the 1918-19 flu. One 18-year-old died. The outbreak sparked concerns of a repeat of that infamous pandemic, which scientists now know killed an estimated 675,000 Americans, or of the more recent 1968-69 pandemic, during which 100,000 Americans died and “$3.2 billion was lost in medical bills and working time,” TIME reported.
Unlike in 1918, the U.S. in 1976 had a way to stop the outbreak in its tracks: flu shots, developed for military use in the 1930s, approved for civilian use in 1945 and encouraged for all high-risk Americans since 1960.
But the Fort Dix cases involved a new strain of H1N1, so if a vaccine were going to be ready in time for the 1976-1977 flu season, production needed to start as soon as possible. Even though no major outbreak had yet begun, the government decided to put its weight behind the effort.
“We cannot afford to take a chance with the health of our nation,” then-President Gerald Ford said on March 24, 1976, when, per the CDC’s recommendation, he called for a $135 million program (about $615 million in 2020) to produce a vaccine and manufacture more than 200 million doses in time to inoculate all Americans for the winter flu season. The plan “was unprecedented in intended timing and in scope among American immunization efforts,” Richard E. Neustadt and Dr. Harvey V. Fineberg noted in The Swine Flu Affair, their 1978 evaluation of the program, which the incoming Health, Education and Welfare Secretary asked them to undertake.
But not everyone was sure the vaccine program was being launched for purely altruistic reasons. A year of celebrations to mark America’s bicentennial was in full swing. It was also a presidential election year, and while Ford had beaten challenger Ronald Reagan in the first five primaries and the Iowa caucus, it was still a tight race for the Republican nomination. In fact, Neustadt and Fineberg’s report points out that Reagan won the North Carolina primary the day before Ford’s announcement of the vaccine program. During hearings on the program that spring, Washington State’s Democratic U.S. Senator Warren Magnuson joked of vaccine recipients that the Administration “might have ’em vote at the same time,” and, TIME noted, “some legislators and doctors are wondering out loud whether the flu program is merely another symptom of election-year fever.”
Ford Administration officials took the position that—given that the vaccine was safe—the risk of an outbreak outweighed any concerns about politics. Not that it wouldn’t be politically beneficial: “Consider the outcry,” one White House aide was quoted as saying, “if with all that evidence the President had said no.” In April 1976, Congress swiftly passed emergency appropriations to make the vaccine for which Ford had called.
Get your history fix in one place: sign up for the weekly TIME History newsletter
By July 1976, the vaccine was “only partially successful in clinical trials,” TIME reported back then, prompting scientists to reconsider the rollout. Among the roughly 5,000 volunteers injected with the still-being-tested vaccine, researchers found minimal side effects in adults, but high fevers in young people, leading Dr. Albert Sabin, developer of the oral polio vaccine, to backtrack on his initial support of the Ford program, and argue that only the highest-risk people should get the vaccine. Dr. Jonas Salk, who developed the initial polio vaccine, argued it was safe and would reduce the virus’ spread.
By August, when Congress passed a bill officially authorizing the rollout, the effort was two months behind schedule, largely because of Congressional wrangling over whether vaccine-makers would be protected from liability related to the product. This delay would have serious consequences: In retrospect, a 2006 review of the CDC’s handling of the 1976 swine flu vaccine program co-written by David Sencer, the agency’s director back then, found that manufacturers’ demand for indemnification led the public to believe “there’s something wrong with the vaccine,” and so “every coincidental health event that occurred in the wake of the swine flu shot would be scrutinized and attributed to the vaccine.”
Clinics began offering two swine flu vaccines in October—a vaccine against swine flu and, for higher risk groups, a vaccine against both swine flu and the previous year’s flu. At that point, no other cases of swine flu had been confirmed since the Fort Dix cluster in February, but the government began offering free vaccines anyway, along with the slogan “ROLL UP YOUR SLEEVE, AMERICA.”
A poll showed that a little over half of Americans (53%) planned to get one, TIME reported in its Oct. 11, 1976, issue.
Howard Markel, director of the Center for the History of Medicine at the University of Michigan, remembers getting the shot as a high school sophomore: “Everyone went to school gyms or large areas, and I remember vividly my mother making us go, and waiting in line, and saying ‘this is ridiculous.'”
But only a few vaccine-distribution centers were equipped on launch day. A Portland, Ore., health official told TIME, “We didn’t even know we’d received a batch until we read about it in the newspaper.” And the magazine reported that by November, only a quarter of the more than 200 million doses advertised had been manufactured.
Then, 35 mostly elderly people, in various parts of the country, died shortly after getting vaccinated. The government said there was no link between the deaths and the vaccines, but the news still dissuaded people from getting the shot. After the news of the deaths, the number of New Yorkers who showed up for a vaccine dropped from 21,000 to 7,500 a day, and nine states closed their clinics, TIME reported.
“If you immunize very large numbers of elderly people, inevitably some will have a heart attack the next day, so you have to prepare the public for such coincidences,” Fineberg, a co-author of the 1978 study of the public health response, later told the World Health Organization. “That wouldn’t have been a blip on the screen had there been a pandemic but, in the absence of any swine flu disease, these rare events were sufficient to end the program.”
Photographs of Ford (who had ultimately been successful in the primaries) getting vaccinated, like the one seen above, were circulated in an attempt to restore confidence in the vaccine program, but it was too late.
Election Day rolled around, and Ford’s tenure in office ended, for a host of reasons other than the flu-shot rollout. The mass inoculation program ended about a month later—but not before the public’s confidence was once again shaken, this time by reports of shot recipients being at a very slightly higher risk of Guillain-Barré syndrome (GBS).
“The uptick was not directly caused by the flu vaccine,” Markel says, “but it was associated with it, so everyone came to the conclusion that the swine flu shot equaled Guillain-Barré.” (About one additional case occurred per 100,000 people vaccinated, according to the CDC, but most studies that have since evaluated a possible link between other flu vaccines and GBS have found no association.)
Markel also notes that the program’s failure happened to occur at a time when Americans’ trust in government was already in decline, following the Vietnam War, Watergate and the assassinations of the 1960s. The idea that the government would have botched the vaccine and still tried to capitalize on it for political reasons fit right in—whether or not it was true.
That confusion and distrust incubated in that time period lingered well beyond 1976. In a 2009 CDC fact-sheet about a vaccine for that year’s H1N1 virus, for example, two of the questions were about about 1976 and GBS. Today, at a time when unfounded anti-vaccine views are growing (even though vaccines are safe) and polls indicate distrust in the federal government’s management of the response to the COVID-19 pandemic, that enduring doubt can be dangerous.
“Things like the small increased risk of GBS from the 1976 vaccine become…convenient for post-hoc justification of those pre-existing fears,” says Jonathan M. Berman, author of the forthcoming history of the anti-vaxxer movement Anti-vaxxers: How to Challenge a Misinformed Movement. “Later studies showed that there was likely no link, but already wary parents and anti-vaxxers see it as a justification for the fear.”
On the other hand, the 1976 controversy “does provide important lessons,” Berman argues. “When vaccination decisions appear politically motivated, it can undermine trust.”
The fact that even people who are not generally anti-vaccine are worried about the safety of a COVID-19 inoculation means it’s all the more important that a coronavirus vaccine is not rushed, argues Markel. “This is a very touchy issue,” he says, “so this [vaccine] has to be rolled out exactly right.”
0 notes