#isoelectric pH
Explore tagged Tumblr posts
Text

TSRNOSS. Page 242.
#isoelectric pH#anaesthesia#anaesthetics#senescence#mitochondrial water formation#lipid matrix#enzymes#mass action principle#heat resistance#pasteurization#fruit#vitamin C#tapeworm infection#vitamin B12 deficiency#scurvy#diabetes#satyendra sunkavally#theoretical biology
1 note
·
View note
Text
Any amino acid or protein in a solution with a pH that equals the pI of the compound remains at the origin.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#amino acid#protein#ph#pi#isoelectric point#electrophoresis
0 notes
Text

sorry @inbabylontheywept but I've barely gotten started.
The real reason I'm going old-school instead of copping out with "oh I can totally manage on isoelectric precipitation alone" is not just being too colourblind to measure pH values.
You see, we won't be injecting this insulin. Hypodermic needles are fucking hard.
I'm not about to make the physical manifestation of a bad idea out of goose quills. I'm also not delaying someone's first batch for a die set good enough for deep drawing medical equipment, (though that would be a key technology if it's a post-apocalypse scenario for nipple piercing reasons alone (female and male. Have you seen Mad Max??))
No, you see... Insulin is quite a large protein, it has trouble getting through skin. Unless, of course, there happens to be a chemical particularly suited to helping it cross the skin barrier. (Ideally with more aggressive exfoliation than would be warranted nowadays, which is why I asked you to pull your sleeve all the way up. Will you get a consistent dosage? We'll find out!)
Several such chemicals are known, but the simplest happens to be ethanol, aka the most reasonable purification medium when I'm not trying to be special.
...
I think high school chemistry class should make you give your best shot at isolating chemicals from animal parts. The T in STEM stands for trying <3 yeah I also think the M stands for masochism but that's not far from the truth

There we go
#don't worry my last psychiatrist said I'm perfectly alrigbt#I mean his exact words were “I uh. cannot help you”#but I think that counts
3K notes
·
View notes
Text
Bromelain 200gdu/g to 2400gdu/g pineapple stem
Bromelain, also known as pineapple protease, is extracted from pineapple stem, pericarp etc. it’s a pure natural protease after biotechnology, its molecular weight is 33000, isoelectric point is 9.55. It belongs to –SH protease with pineapple odor, can proceed protein hydrolyze and other biochemical reaction. Bromelain can widely apply on food, Cosmetic, Pharmaceutical and other industry. Our bromelain can reach pharma and food grade demand.
Bromelain Activity:
The activity is from 200gdu/g to 2400gdu/g, we can supply formulations according to customer requirement.
Application area
Food processing industry: add the bromelain to the dough to degrade the gluten and soften the dough, can congeal the casein for cheese, tenderize the meat, increase bean cake and bean flour PDI value and NSI value, then produce soluble protein product and grain food and beverage,
Cosmetic industry area: The bromelain has the ability of tender-skin, Whitening and Freckle-removing effect. Prompt the skin’s metabolism, relieve the skin become dark that cause by sunshine. Keep the skin white and tender
Pharmaceutical and health care industry:Restrain tumour cells growing,Prevent the cause of cardiovascular disease,Apply to treat the skin burn crust,Apply to diminish inflammation,Assist absorb of pharmaceutical
Feed industry: Add the bromelain to the feed can highly improve protein utilization and transformation rate, and also develop more protein source, that finally reduce the cost of feed.
Suggested use condition:
Reaction temperature:30-45℃;reaction PH value is 6-6.8.
(Can adjust the temperature and PH value according to the kind and concentration of the substrate)
Dosage: 0.1%-0.5%.
Matters needed attention:
Bromelain is a bioactivity material, can easily be inhibited and destroyed by oxidant and heavy metal ion( Fe 3+ 、 Cu 2+ 、 Hg + 、 Pb + etc.)should avoid with its contacting. Bromelain is a sensitization source, absorb bromelain will lead to allergy, but oral absorb can not cause allergy, so should strictly avoid absorbing enzyme powder.
Storage condition:
Store in low temperature(4℃)in cold storage or dry place avoiding sunlight.

0 notes
Text
What Makes 2D Electrophoresis a Powerful Protein Analysis Tool?
With its ability to separate complex protein mixtures based on both charge and size, 2D electrophoresis offers you a powerful window into the intricate world of protein analysis. At Kendrick Labs, this cutting-edge technique is honed to perfection, allowing you to unravel the mysteries of your samples with precision and accuracy. Discover how this innovative tool can elevate your research and deepen your understanding of proteins like never before.
The Basics of 2D Electrophoresis
What is 2D Electrophoresis?
Basics of 2D electrophoresis involve a powerful method for separating complex protein mixtures based on their isoelectric points and molecular weights. By combining two different separation techniques in two dimensions, this technique allows you to achieve a high level of resolution and analyze hundreds to thousands of proteins in a single gel.
Principles of Separation
Separation in 2D electrophoresis is based on the fact that proteins will move in an electric field based on their charge and size. In the first dimension, proteins are separated according to their isoelectric points using an immobilized pH gradient. Then, in the second dimension, proteins are separated by their molecular weights through SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis).
A key feature of 2D electrophoresis is the ability to separate proteins that may have similar molecular weights but different isoelectric points, or vice versa. This dual separation approach provides you with a comprehensive view of the protein composition in your sample, making it a valuable tool in proteomics research.
High-Resolution Protein Separation
Advantages of 2D Electrophoresis over 1D
If you are looking to achieve high-resolution protein separation, 2D electrophoresis has significant advantages over traditional 1D methods. In 1D electrophoresis, proteins are separated based on a single property, such as size or charge. However, in 2D electrophoresis, proteins are separated first by their charge in one direction and then by their size in another direction, allowing for a more precise separation of complex protein mixtures.
Resolving Power and Protein Detection
Over the years, 2D electrophoresis has become a powerful technique for protein analysis due to its high resolving power and sensitive protein detection capabilities. This method can separate thousands of proteins in a single gel, providing a comprehensive view of the protein profile within a sample. Additionally, with various staining and detection methods available, you can detect proteins at very low levels, making it suitable for a wide range of applications in proteomics research.
Resolving power in 2D electrophoresis refers to the ability of the technique to separate proteins with different isoelectric points (pI) and molecular weights. The high resolving power of 2D gels allows you to distinguish closely related protein isoforms and detect post-translational modifications that may not be resolved using other methods. This level of detail in protein separation and detection is crucial for understanding the complex protein composition of biological samples.
Protein Identification and Characterization
Mass Spectrometry and Protein Identification
With the advent of mass spectrometry techniques, protein identification has become more precise and efficient. Mass spectrometry allows you to analyze proteins based on their mass-to-charge ratios, providing valuable information about their identities. By comparing these mass spectra to databases of known proteins, you can accurately identify the proteins in your sample.
Post-Translational Modifications and Protein Function
Any modifications that occur to a protein after it is synthesized are known as post-translational modifications (PTMs). These modifications can profoundly impact a protein's function, stability, and interactions with other molecules. By studying PTMs using techniques like 2D electrophoresis combined with mass spectrometry, you can gain insights into the biological roles of proteins and how they contribute to various cellular processes.
The field of post-translational modifications is vast and continuously expanding, with researchers discovering new types of modifications and their effects on protein function. Understanding these modifications is crucial for unraveling the complexities of cellular signaling pathways and regulatory mechanisms.
Applications in Proteomics Research
Biomarker Discovery and Disease Diagnosis
Now, 2D electrophoresis is a powerful tool for biomarker discovery and disease diagnosis in proteomics research. By analyzing protein expression patterns in different samples, you can identify potential biomarkers that are specific to certain diseases or conditions. These biomarkers can be used for early detection, monitoring disease progression, and developing targeted treatments.
2D electrophoresis allows you to compare protein profiles between healthy and diseased samples, enabling you to pinpoint differences that may indicate the presence of a particular disease. By identifying and validating biomarkers using this technique, you can contribute to advancements in personalized medicine and improve patient outcomes.
Cellular Signaling Pathways and Protein Interactions
Any proteomics research focused on cellular signaling pathways and protein interactions can benefit greatly from 2D electrophoresis. This technique allows you to map out complex protein networks within cells, unraveling the intricacies of signaling cascades and protein interactions. By analyzing changes in protein expression and modifications, you can gain valuable insights into how cells respond to stimuli and communicate with each other.
Proteomics research utilizing 2D electrophoresis can uncover key players in cellular signaling pathways, such as signaling kinases, phosphatases, and transcription factors. By understanding how these proteins interact and regulate cellular processes, you can unravel the molecular mechanisms underlying various physiological and pathological conditions. This knowledge is imperative for developing targeted therapies that modulate specific protein interactions to treat diseases effectively.
Advantages of 2D Electrophoresis in Protein Analysis
Despite the many techniques available for protein analysis, 2D electrophoresis stands out as a powerful tool with unique advantages. One key benefit is the high-throughput analysis and automation capabilities it offers.
High-Throughput Analysis and Automation
Analysis with 2D electrophoresis allows for the simultaneous separation of a large number of proteins in a single gel. This means you can analyze multiple samples at once, saving you time and enabling you to generate comprehensive protein profiles efficiently. Additionally, advancements in automation technology have streamlined the process, making it easier to handle high sample volumes and ensuring reproducibility in results.
To wrap up
Now, as you have learned, 2D electrophoresis is a powerful protein analysis tool that allows you to separate and visualize a large number of proteins in a sample. This technique provides valuable information about the proteins present in a sample, such as their size, charge, and abundance. With its high resolution and ability to detect post-translational modifications, 2D electrophoresis is a versatile tool that can be used in a wide range of applications, from biomarker discovery to studying protein-protein interactions.
By understanding the principles behind 2D electrophoresis and the ways in which it can be optimized for different research questions, you can harness the full potential of this technique in your own protein analysis experiments. Armed with this knowledge, you are well-equipped to take on the challenges of studying the complex world of proteins and their functions, opening new doors for discovery and innovation in the field of proteomics.
Original Source: https://kendricklabs.blogspot.com/2024/07/what-makes-2d-electrophoresis-powerful.html
0 notes
Text
(Recall from chapter 11 that the smaller the pKa the more acidic is the group. At lower pH, carboxylic acids are found in the RCOOH form and amines are found in the RNH3+ form. At higher pH, the opposite is true; carboxylic acids are present as the salt RCOO- and amines are present as uncharged RNH2. Figure 24.3 on p. 1061 shows how this looks at different pH.)
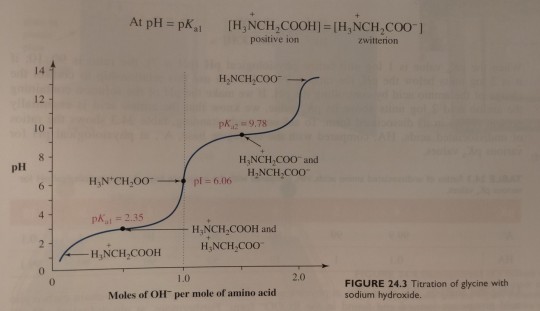
Next, the solution is titrated with 1.00 M NaOH; the volume of base added and the pH of the resulting solution are recorded and then plotted as shown in figure 24.3. (...) By examining the titration curve (figure 24.3), you can see that the isoelectric point for glycine falls halfway between the pKa values for the carboxyl groups and the ammonium ion:
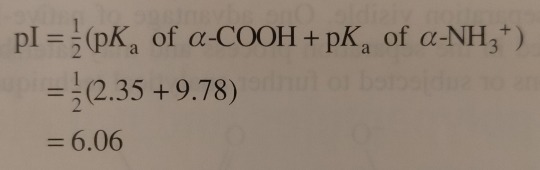
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#acidic#carboxylic acid#ph#salt#amine#titration#glycine#sodium hydroxide#carboxyl#ammonium#ions
1 note
·
View note
Text
IJMS, Vol. 24, Pages 14267: High Recovery Chromatographic Purification of #mRNA at Room Temperature and Neutral pH
Messenger #RNA (#mRNA) is becoming an increasingly important therapeutic modality due to its potential for fast development and platform production. New emerging #RNA modalities, such as circular #RNA, drive the need for the development of non-affinity purification approaches. Recently, the highly efficient chromatographic purification of #mRNA was demonstrated with multimodal monolithic chromatography media (CIM® PrimaS), where efficient #mRNA elution was achieved with an ascending pH gradient approach at pH 10.5. Here, we report that a newly developed chromatographic material enables the elution of #mRNA at neutral pH and room temperature. This material demonstrates weak anion-exchanging properties and an isoelectric point of 5.3. It enables the baseline separation of #mRNA (at least up to 10,000 nucleotides (nt) in size) from parental plasmid DNA (regardless of isoform composition) with both a NaCl gradient and ascending pH gradient approach, while #mRNA elution is achieved in a pH range of 5–7. In addition, the basic structure of the novel material is a chromatographic monolith, enabling convection-assisted mass transfer of large #RNA molecules to and from the active surface. This facilitates the elution of #mRNA in 3–7 column volumes with more than 80% elution recovery and uncompromised integrity. This is demonstrated by the purification of a model #mRNA (size 995 nt) from an in vitro transcription reaction mixture. The purified #mRNA is stable for at least 34 days, stored in purified H2O at room temperature. https://www.mdpi.com/1422-0067/24/18/14267?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text

WELL. DO I HAVE A RAMBLE FOR YOU!
I’m studying rn and i’m on “break” while i switch topics and you have just triggered an unskippable cut scene. Welcome to combat!
So there’s multiple ways to purify a protein and it all depends on the protein it self, which depends on the properties of the amino acids found within it, which depends on the functional groups found within that amino acid.
Think of it as how you’d think of food as spicy, savory, sweet or what ever based on the way the food is served, what the main ingredient is, and what seasonings are put to make that main ingredient taste the way it does.
The method then is chosen by those features.
The first one i’ll explain is that of SDS gels because they’re the first thing that comes to mind. Basically, you’ll coat the protein with SDS which gives it a negative charge. That charge is then like a magnet that pulls the protein to the positive side. The larger the protein, the harder it is for it to move through the gel to the other side. there’s a variation of this gel called “native gel” that doesn’t use SDS and so charge is also taken into account when being pulled.
Then there’s also Isoelectric focusing where a tube with a gradient of pH’s is created and different proteins are placed within it. The protein will gain or lose charges depending on the pH it’s in. A charge will pull it into a direction until it reaches a neutral charge/ zero net charge. Think of it as a battery, once it reaches 0, it stops working. If it doesn’t have a charge, it doesn’t move. This is important because that means that specific pH is where that protein’s isoelectric point is. Which is important because of solubility (how easily that protein can dissolve in water).
Now there’s also “Salting out” proteins which rely on said solubility of proteins. The more protein A can dissolve in water the less that protein B can dissolve in water. Basically you’re changing how much salt is in the solution to kick one protein out of its home.
Remember how I said some proteins have different characteristics? Well some are more negatively charged than others and some are more positively charged. This helps in the technique of Ion exchange. (Ions are when a molecule is charged). If the protein is positively charged, we use a bead that’s negatively charged so the two hold hands and don’t escape together. The opposite is true if the protein is negatively charged. All other proteins will run through the tube pretty easily, leaving the one you want still in there. You then can stop the two from holding hands with salt or acid.
(Sorta the same concept for hydrophobic technique with the more water loving proteins will run right through while the hydrophobic (non polar) proteins will HATE it it whole time.)
Gel Filtration uses size and small little holes in the beads that trap the smaller proteins and let the big ones go through.
Affinity chromatography gives little ID markers to specific chemical groups so they get stuck like that of the Ion one. Theres two different attachments that can be added a GST tag or a 6x HIS tag and both require different methods to attach them and to remove them.
From there you keep repeating the purification process until you get the purity percent that you want
the scariest part about being a stem major is starting to get good at being a stem major ™
#speck rambles#long post#being silly with the mutuals#biology#biochemistry#uh. oops#learning with speck
55 notes
·
View notes
Text
What Are Peptides
Peptides Advantages - What Are They?
Content
Iaaf Values Board Choice: Timeline
Blood Sugar Level Ranges.
Peptides.
Is It Worth Taking Ostarine? Results And Advantages Of Ostarina.

Rite-Flex collagen peptides is produced via Rousselot's cutting-edge procedures with an unsinkable dedication to top quality, safety and sustainability. 80-- 90% are either type I, II or III, with kind I being one of the most common. The characteristic impacts of specific amino acid deposits on peptide fragmentation behavior have been examined carefully by Papayannopoulos. An internal piece with just a solitary side chain developed by a combination of a kind as well as y type cleavage is called an immonium ion. These ions are identified with the 1 letter code for the corresponding amino acid. Typically, these are developed by a combination of b kind andy type bosom to create the detailed framework, an amino-acylium ion. Sometimes, inner bosom ions can be formed by a mix of a type and y kind cleavage, an amino-immonium ion.
Does Vitaminhoppe sell SARMs?
SARMs are drugs and they are not dietary supplements. Using the internet one can easily find SARMs for sale. The bigger retail stores like GNC and Vitamin Shoppe do not carry them, but the smaller independently owned supplement stores are notorious for distributing SARMs.
The major teams of glycoconjugates are the glycoproteins, glycopeptides, peptidoglycans, glycolipids and lipopolysaccharides. The very first three of tbese are considered in the here and now file.
Iaaf Ethics Board Choice: Timeline
BNP dimension is utilized to look for proof of cardiac arrest as well as are used as an analysis tool. There are two different sorts of BNP levels which can be determined BNP and also NT Pro-BNP, worths for these are rather different. The normal actions of the natriuretic peptides are to help the heart in dealing with quantity overload as well as stretch. As the most plentiful healthy protein in the body, collagen serves a number of crucial architectural features. Nutritional collagen is harder for our body to absorb, as compared to hydrolysed variations.

If the pores of the gel are big, the amino acids will certainly relocate at a much faster price to the electrodes contrasted to in a gel which has smaller pores. If the temperature of the system is high, the kinetic energy of the amino acids boosts and hence the amino acids take a trip to the billed electrode at a quicker rate. Amino acids that have smaller sized alkyl chains connected to them relocate at a much faster rate than amino acids that have a bigger alkyl chain attached to them. Amino acids that have a bigger fee will move at a quicker rate contrasted to the amino acids that have a smaller charge. The fee is not because of the amino acid itself but as a result of the charge on the side chain.
Blood Sugar Level Varieties.
An additional little research study discovered that taking a day-to-day collagen peptide supplement for 6 months brought about renovations in nail toughness and development. Nevertheless, this only included 25 individuals, as well as there was no sugar pill team-- which reduces the stamina of this proof. There is some emerging evidence which recommends that taking hydrolysed collagen supplements may help to reduce creases and also boost collagen degrees in the skin, in addition to skin hydration as well as skin flexibility. Nevertheless, there are problems with some of the studies around, as an example some are very small, and do not utilize a control group.
Plumps, firms as well as reduces the appearance of great lines by boosting collagen production. Oily skin can be a problem and also the hardest skin type to deal with. Typically, you are glossy with a lot of oily spots, your pores are bigger and there are a great deal of blackheads and acnes.
Peptides.
Inner pieces are labelled with their 1 letter amino acid code. In the liver the majority of collagen is either kind 1 or type 3. The removal of the propeptides advertises advancement of collagen fibrils. The pro-peptides may either be preserved in the matrix or released into the blood circulation. Fibrosis occuring in the liver leads to the deposition of collagen and launch of propeptides, mainly P3NP. In information BPC157 Italy , 52 individuals with osteo arthritis of the knee were offered 10 mg bioactive undenatured type II collagen or glucosamine hydrochloride plus chondroitin daily for three months.
DRS-Labs Launches New Research Line of SARMs - Markets Insider
DRS-Labs Launches New Research Line of SARMs.
Posted: Mon, 13 Aug 2018 07:00:00 GMT [source]
However, to date, there are no researches on the metabolic engineering of the proteolytic system from lactobacilli. Numerous strategies have actually likewise been attempted to increase the return of peptide production by CEPs.
Is It Worth Taking Ostarine? Impacts As Well As Benefits Of Ostarina.
It is also important to note that collagen supplements are usually derived from fish-- therefore any person that has a fish allergic reaction need to prevent this sort of supplement. Vegan collagen supplements are offered, which are made from genetically modified bacteria and also yeast. There is very little proof to suggest that collagen supplements boost digestive tract health and wellness.
GTx Announces Results from Preclinical Studies of SARMs in Duchenne Muscular Dystrophy Models Published in Human Molecular Genetics - Business Wire
GTx Announces Results from Preclinical Studies of SARMs in Duchenne Muscular Dystrophy Models Published in Human Molecular Genetics.
Posted: Wed, 03 May 2017 07:00:00 GMT [source]
According to the "barrel-stave version," the peptides insert perpendicularly right into the bilayer while recruitment of additional peptides subsequently results in development of a peptide-lined transmembrane pore. In this pore, the peptides are aligned with the hydrophobic side dealing with the lipid core of the membrane and the hydrophilic areas facing the indoor area of the pore. According to the "toroidal-pore version," insertion of peptides compels the phospholipid to flex continually from one brochure to the other, leading to a pore lined by both peptides and also the head groups of the phospholipids. Finally, in the "carpet model," build-up of peptides on the membrane surface area triggers tension in the bilayer that eventually causes disruption of the membrane layer and formation of micelles. AMPs are transformative saved in the genome as well as produced by all life forms, from prokaryotes to humans. In higher microorganisms, AMPs make up important elements of the innate immunity, shielding the host versus infections.
A buffer remedy resists the adjustment in the pH of the option. The barrier service is maintained the Isoelectric Factor of one known amino acid. The amino acids with Isoelectric Factors greater than the pH of the buffer solution become positively billed. The amino acids with Isoelectric Factors lower than the pH of the buffer service end up being adversely billed. The amino acids with the Isoelectric factor that amounts to the pH of the buffer option stay uncharged.
What is the safest SARM to take?
A three-week trial at Boston University demonstrated that LGD-4033, a SARM developed by Ligand Pharmaceuticals, was safe and tolerable in healthy men, producing “significant gains in muscle mass and strength” without raising levels of a protein linked to prostate cancer.
In the condensed system of symbols for sugar residues the common arrangement and ring size are indicated in the sign. Hence, Girl signifies D-galactopyranose; Man, D-mannopyranose; Fuc, L-fucopyranose; GlcNAc, 2-acetamido-2-deoxy-D-glucopyranose or N-acetyl-D-glucosamine; Neu5Ac N-acetylneuraminic acid. The symbol Sia stands for sialic acid, a general term that can likewise be utilized when the precise structure is unidentified. Whenever the setup or ring size is found to differ from the common one it should be suggested by using the ideal icons for the extensive system.
The longer the chain of the peptide, the longer the hydrolysis process will certainly happen. This is due to the fact that even more time is required to damage the peptide bond as well as other bonds like Van der Wall forces of attraction between the atoms. Isoelectric Factor is the pH worth of the amino acids when they are added in water. This is due to the dissociation of the H+ ion from the amino acids. Our collagen peptides are made from the finest pasture-raised bovine hides. Studies reveal that 5g to 10g of collagen peptides daily have shown statistically significant benefits. Peptan is the worlds leading collagen brand generated by Rousselot.
The classification of glycolipids has been the topic of an earlier record, which is now under alteration. Identification of enhanced strain versions or even metabolic engineering is an additional approach.
UK Sarms ostarine has given me excellent recomp outcomes at 10mg/day and also helped me push past weights I was previously stuck at for months.
Selective androgen receptor modulators are a class of androgen receptor medicines, which have a high capacity to be performance boosters in human and also animal sports.
In this study, we have actually clarified as well as confirmed the chemical structure of 2 major equine arylpropionamide-based SARM metabolites utilizing a mix of chemical synthesis and fluid chromatography-mass spectrometry (LC-MS) analysis.
you can find more information on workflow rules on peptides-uk crm's help pages here. was so amazed with the ostarine that I have actually recently gotten RAD-140 for my following cycle as I currently require to focus on structure muscle mass in advance of my following comp.
Arylpropionamides are among the major SARM classes as well as get swiftly metabolized substantially making complex easy detection of transgression in blood or urine example evaluation.
Particular drug-derived metabolites are called for as references due to a short half-life of the parent compound however are typically lacking.
However, these researches contrasted the effect of resistance training plus a collagen supplement, with resistance training with no type of healthy protein after training. As protein is essential for muscle mass fixing, these researches suggest that collagen is better than consuming no healthy protein after resistance training. However there isn't adequate proof to recommend that collagen is better than various other types of protein in terms of muscle mass growth and also repair work. In 2011 the European Food Security Authority reported there was insufficient proof to declare that hydrolysed collagen aids in maintaining joint wellness in energetic individuals. Since then, a few tiny research studies have located that eating collagen may aid in lowering joint discomfort related to work out. There is likewise some proof that collagen supplements can aid with minimizing pain related to osteoarthritis in the short-term. Nonetheless, these research studies don't supply proof as to whether collagen supplements help in athletic efficiency, or the recovery from joint injuries.
On top of that, pathogen insult will bring about growth of dendritic cells and succeeding initiation of flexible resistance. Up- or down-regulation of feedbacks by AMPs is shown by environment-friendly arrows. Remarkably, the membrane-destabilizing activity of AMPs is likewise made use of in so called Artilysins, which have actually just recently revealed potential to properly target immune and persistent Gram-negative infections.
youtube
A small number of human studies have actually also discovered that taking collagen supplements may assist to maintain and enhance bone mass. However, comparable to the research studies associated with muscular tissue mass, we can not rule out that the favorable effect of collagen in these studies may result from a rise in total protein consumption, as opposed to a specific advantage pertaining to collagen.
As an example, enzymatic immobilization (cross-linked CEP accumulations) was established to increase task and also security of CEPs. This not only supports the concept that lactobacilli can produce a huge selection of BAPs, however also highlights the need to carefully pick the pressures that will be used for BAP manufacturing. The rapid bactericidal activity of AMPs makes them appealing prospects for therapeutic anti-infectives. Schematic illustration of immunomodulatory tasks of AMPs.
Although icons such as Gal as well as Man work in representing oligosaccharide structures they ought to not be made use of in the text to stand for monosaccharides. Various sorts of substance containing carbs covalently related to various other types of chemical component are categorized under the general name of glycoconjugates.
#BPC157 EU#BPC157 Europe#information BPC157 EU#information BPC157 Europe#EU BPC157 how does it work#Europe BPC157 how does it work
1 note
·
View note
Text
Sarms Supplements
Uk Sarms
Content
The Remarkable All-natural Power Of Flower Acids From Hibiscus.
Where To Buy Sarms Online.
Exploring The Very Best Market Access Approach For Reagents And Methods Connecting To Peptide.
Clients That Saw This Item Likewise Watched.
Food proteins are currently being researched past their nutritional features, for their positive effect on human health pertaining to certain sequences encrypted right into the indigenous protein, called bioactive peptides. Research on BAPs provides insightful details on the effect of dietary healthy proteins on health. Basically, electrophoresis is a biochemical analytical strategy which can be made use of as a test to learn if the healthy protein is a peptide or an amino acid. Although electrophoresis can be utilized for peptides, polypeptides, amino acids, as well as even various healthy proteins as well, for quality, we will only review amino acids. Nonetheless, the same concepts can be put on all kinds of molecules discussed above.
my diete was 2200 calorie each day for get shreds and also i take strenght and keep the muscle i think its not phony ostarine.
Generally, a medication with a 24-hour fifty percent would certainly take a week to get to constant state I believe so an option I employ is two days of two caps at the start instead of the normal one a day truly obtains that study as much as degree quickly.
Massively boosted strength in the gym, can bench an extra 20kg from day 1.
I gained about 2 kg in general in 8 weeks while visibly loosing fat.
Also excellent pumps throughout training as well as raised vascularity.
Total I think I got around 4-5 Kilos of lean muscle mass as well as lost 3 kilos of fat with no diet regimen constraint.
The first week changeover was enormous, with no question a residual result of the creatine and beta alanine, as well as the choice I made to front tons the research study for ostarine for two days just.
To satisfy their nitrogen need, Lactobacillus types have actually developed a proteolytic system, which hydrolyzes the healthy proteins and also supplies the amino acids. Healthy protein hydrolysis is started by CEPs that cleave the healthy proteins into peptides varying from 4 to 30 amino acids. Especially, healing peptides, also synthetic ones, are usually less immunogenic than recombinant healthy proteins and antibodies. Lastly, neighborhood management, which is one of the most usual distribution course for AMPs, even more reduces the threat for any type of systemic toxicology issues. The role healthy proteins play within the body associates with the actions of 4 categories of peptides. Many peptides are lab-enhanced as well as are derived from all-natural plant, keratin, wheat, milk casein, rice, potatoes, and yeast. So, exactly how precisely do protein pieces as well as amino acid chains enhance the look as well as general health of the skin?
The Incredible All-natural Power Of Flower Acids From Hibiscus.
When peptides connect together, they develop the basis for all proteins. Peptides additionally have the exceptionally crucial work to regulate the activity of other particles, offer antibiotic advantages and also aid in hormone balance.
Nonetheless, they are considered as meticulous bacteria as a result of their auxotrophy for countless amino acids. In order to locate the amino acids required for their development, lactobacilli hydrolyze healthy proteins in their environment through their proteolytic system, and, extra specifically, via the activity of enzymes called cell envelope proteinases. The most meaningful instances of BAPs created by Lactobacillus types are the antihypertensive tripeptides, Ile-Pro-Pro and also Val-Pro-Pro, created from casein hydrolysis by different Lb.
Where To Purchase Sarms Online.
Finally, a last concern to be born in mind is that as an item designed for direct usage, fermented food will certainly be sent to GID. The influence of GID on a fermented food must, as a result, be examined to obtain an accurate prediction of the actual in vivo tasks of the food. GID is even, on its own, one of the major procedures for BAP manufacturing from food proteins. Usually, for ACE inhibitory peptides, GID enhances the release of BAPs from fermented food, as healthy proteins that were not hydrolyzed during the fermentation will be digested throughout GID.

Enzyme inhibitor peptides impede the task of enzymes that deteriorate architectural proteins like collagen, slowing down the loss of quantity and flexibility in the skin. A presumptive artificial injection for foot-and-mouth condition has actually verified much less successful in a host species, cattle, than predicted by cause a small-animal version. Feasible reasons for this consist of non-recognition by T cells influenced by significant histocompatibility facility -connected immune feedback gene control. It is now feasible to kind for human leucocyte antigen DR-like bovine MHC course II polymorphisms with a one-dimensional isoelectric focusing technique. Utilizing this approach 14 unrelated livestock were chosen with eight different BoLA class II IEF types.
Exploring The Best Market Entrance Method For Reagents And Also Strategies Associating With Peptide.
The effect of GID can be assessed in vitro by treating the fermented product with stomach and pancreatic enzymes, as well as comparing the task of the resulting item with the activity of a non-fermented sample. Collagen makes up for 25-35% of the whole body's healthy protein web content. To day, 29 different sorts of collagen have been determined that contribute to various jobs in the skin, as well as a variety of peptides are called for to keep these collagens undamaged and healthy and balanced. As we age, the body loses 1% of collagen every year as well as thinning of ageing skin happens at the rate of regarding 6% every one decade! Collagen manufacturing is important to healthy and balanced skin, so think of peptides like 'body building' but also for the skin.
Do SARMs shrink balls?
Androgenic steroids are known to increase muscle development but are accompanied by a host of undesirable effects. For men, this often means things like acne, breast development (gynecomastia), enlarged prostate, and shrinking of the testicles.
After that, the process of antigen presentation using MHC class II particles generally follows the exact same pattern as for MHC course I discussion. Bicyclic peptides are known to have the capability of being employed as an effective alternative to complicated particles, such as antibodies, or little particles. This review gives a recap of the recent progress on the types, synthesis as well as applications of bicyclic peptides. A lot more particularly, natural and synthetic bicyclic peptides are presented with their different manufacturing approaches and relevant applications, including medicine targeting, imaging and diagnosis. Their usages as antimicrobial agents, as well as the healing functions of various bicyclic peptides, are likewise talked about. Lactobacillus species are auxotrophic for countless amino acids. An exterior resource of nitrogen is needed for their development, particularly in milk where focus of amino acids are low.
Consumers Who Saw This Product Also Saw.
Peptides are brief chains of amino acids that take place normally in our body. When amino acids connect with each other, the chain they create is called an amino peptide.

After immunization with FMDV15, 13 cattle generated a T-cell feedback to FMDV15. Nevertheless, the great uniqueness and magnitude of the response was related to BoLA class II type. The non-response by one animal and reduced action by 2 other animals were related to two of the BoLA class II kinds. Action to the region was immunodominant and animals which did not react to this area had reduced actions to the whole peptide. The constraint patterns of the lines suggested that the IEF method does not distinguish all practical polymorphisms. At the very least two of the IEF-defined types could each be split right into 2 distinctive uniqueness and exposed that the 3 sets of pets with identical IEF enters reality expressed unique limitation elements.
MHC class II particles bind to peptides that are originated from proteins degraded in the endocytic pathway. MHC course II complicateds includes α- and also β-chains that are set up in the Emergency Room and also are stabilised by regular chain. The facility of MHC class II and Ii is transferred via the Golgi right into an area which is labelled the MHC class II compartment. As a result of acidic pH, proteases cathepsin S and also cathepsin L are triggered and absorb Ii, leaving a residual course II-associated Ii peptide in the peptide-binding groove of the MHC class II. Later on, Buy peptides Direct Slovakia is exchanged for an antigenic peptide stemmed from a protein degraded in the endosomal path. This process calls for the chaperone HLA-DM, and, in the case of B cells, the HLA-DO particle. MHC class II particles packed with international peptide are then transported to the cell membrane to offer their cargo to CD4+ T cells.
youtube
Peptides currently exist normally in the body (mixes of amino-acids); healthy protein is ingested with the diet, permitting people to acquire these necessary amino-acids. Amino acids after that incorporate in particular series that result in peptides that perform a selection of functions-- one feature is producing collagen. Signal peptides can send out skin cells solid regrowth signals, triggering them to synthesise even more healthy proteins such as collagen and elastin which keep the skin company and also supple. An additional source of BAP diversity is the type of protein made use of as substrate by the germs. On the whole, Lactobacillus pressures are mostly utilized for milk fermentation and a lot of the BAPs characterized so far were isolated from milk societies. Nonetheless, also milk proteins are not the same, and the same Lactobacillus strain will create different peptides when hydrolysing caseins from cow milk, goat milk, camel milk, or mare milk. Several types of nanocarriers have been evaluated for delivery of AMPs with encouraging outcomes.
Lots of studies focused on ACE prevention peptides, possibly as a result of the ease of usage of in vitro anti-ACE assays. The popular Val-Pro-Pro and Ile-Pro-Pro peptides are created during milk fermentation by some Lb. The resulting fermented milk showed antihypertensive activities in animal and human clinical researches, with a considerable decline in systolic blood pressure. An extra ACE inhibitory peptide sequence (Ala-Ile-Pro-Pro-Lys-Lys-Asn-Gln-Asp) was likewise recognized in milk fermented by Lb. Additionally, various other Lactobacillus types might also release brand-new ACE repressive peptides, as reported for some Lb. The LABORATORY constitute a varied group of Gram-positive, catalase-negative bacteria generating lactic acid as the main end-product of carb fermentation. With greater than 231 valid species and also 29 subspecies, Lactobacillus genus is definitely the main and also most varied LABORATORY group.
It needs to be noted that 30-- 70% of proteins are immediately degraded after synthesis (they are called DRiPs-- malfunctioning ribosomal products, as well as they are the outcome of malfunctioning transcription or translation). This process enables viral peptides to be offered very quickly-- for example, flu infection can be identified by T cells approximately 1.5 hours post-infection. When Buy Peptides Products bind to MHC course I particles, the chaperones are released and also peptide-- MHC course I facilities leave the Emergency Room for presentation at the cell surface area. Sometimes, peptides fail to relate to MHC course I and also they need to be gone back to the cytosol for destruction. Some MHC class I molecules never bind peptides and they are additionally deteriorated by the ER-associated healthy protein deterioration system.
It keeps skin communication as well as anchors the epidermis to the dermis. Imagine the skin as plates held together by a series of chain web links. If among these web links becomes weak as well as breaks, home plates will certainly slip. As the healthy protein composed web links, such as laminin as well as integrin comes to be weak within the DEJ, the skin starts to droop, and loses elasticity ultimately developing a line or wrinkle.
In SARM's way: Why USADA has altered its stance on Shayna Jack substance - Sydney Morning Herald
In SARM's way: Why USADA has altered its stance on Shayna Jack substance.
Posted: Sat, 17 Aug 2019 07:00:00 GMT [source]
Especially, numerous AMPs are currently under clinical advancement for healing indicators besides antimicrobials or antifungal representatives. The mechanisms through which LL-37 promotes injury recovery are not completely understood, yet are likely to include several wound fixing parts such as re-epithelialization, angiogenesis, and swelling. One more AMP presently in scientific growth for its residential or commercial properties other than anti-infection is PXL01. A collection of amazing items created for mix to oily skin types. Created with powerful active ingredients that consist of Hyaluronic acid, Liquid oxygen as well as seaweed extracts such as Undaria algae, Laminaria and Marine peptides that aid to bring back the skins Ph equilibrium and also aid decrease aging signs and symptoms. These technologically advanced peptides are a lot more complicated chains of amino acids as well as have the capacity once more to address a wide variety of aging problems however more successfully. The DEJ holds the skin together, boosting its compactness, firmness and elasticity.
Neuropeptides are tiny proteinaceous cell-cell signaling molecules generated and launched by nerve cells. They vary from peptide hormonal agents in that they are secreted from nerve cells and act locally on adjoining nerve cells, whereas peptide hormones are secreted in to the blood by neuroendocrine cells as well as act at remote sites. Neuropeptides are the most varied class of indicating molecules in the brain, and also are involved in a broad range of mind features, consisting of analgesia, recreation, discovering as well as memory, benefit, food intake and also even more. Electropherograms can aid compare the various sorts of healthy proteins, peptides and amino acids. This is a wonderful natural choice with this powder likewise being loaded with amino acids in addition to respectable healthy protein degrees in each serving and also being extremely easy to mix right into healthy protein trembles or various other drinks without including any kind of awful flavours like some can. This will last around a month based on the recommended offering recommendation so for a suitable collagen powder it's really not bad worth either and also one I would certainly more than happy to continue utilizing. MHC class II molecules are expressed by APCs, such as dendritic cells, macrophages and also B cells (and, under IFNγ stimuli, by mesenchymal stromal cells, fibroblasts and endothelial cells, along with by epithelial cells as well as enteric glial cells).
#buy peptides direct eu#buy peptides direct europe#eu peptides best buy#europe peptides best buy#buy best quality peptides direct eu#buy best quality peptides direct europe
1 note
·
View note
Photo

How To Electrospin Electrospun Casein Nanofibers Mats?
#electrospin #electrospun #casein #nanofibers #mats
Casein is the major precipitated protein of milk at the temperature of 20℃ and a pH value of 4.6. Casein is a phosphorus-containing protein, which has a high content and nutrient value. Bovine casein is made up of as1-, as2-, k-, and b-casein. These four monomers constitute the spatial structure of casein via a-helix, b-sheet, and b-turn. b-Casein from bovine can be used as a superior emulsifier and colloid stabilizer. The pH value is 4.8, which is the isoelectric point of casein. Casein has excellent steric stability to dispersed oil/fat droplets under neutral pH.
A solution containing only casein could not be electrospun due to its strong intermolecular force and 3D structure. However composite dispersions blending PEO or PVA with casein could be electrospun successfully as indicated in Figure. The 10% (w/w) casein solutions containing 80% PEO or 50% PVA are used to electrospin with average fiber diameters ranging from 100 to 500 nm.
1 note
·
View note
Text
Basic properties of amino acids

What are the basic properties of amino acids? What is the difference between a basic and an acidic amino acid? The basic properties are those of the side chain. In a molecule, amino acid side chains are known as peptides. There are two forms of the peptide bond: - trans forms - cis forms In addition to being optically active, amino acids have other important properties. This article will discuss the basic properties of amino acids and how these properties affect the function and structure of proteins.
Acid-base properties of amino acids

An amino acid's acid-base properties can be found in its structure and composition. The basic properties of amino acids are essentially neutral ions, with only one -NH2 group and one -COOH group. These groups are the basis of amino acid reactions, which change the pH of a solution. An amino acid's isoelectric point is 7 because it is neither acidic nor alkaline. Amino acids are classified according to their structure, with one type having a carboxyl end and another with an a-carbon. These acids are also referred to as secondary amines. The difference between a-amino acids and b-amino acids lies in the side chain called R group. There are four types of amino acids: alkyl-amino acid, branched-chain peptides, and isomerized amino acids. The acid-base properties of amino acids can be demonstrated using a potentiometric titration. In this process, the acidic form of alanine is titrated by adding NaOH to a solution. A graph is then plotted that shows the change in pH as the amino acid molecules are added. Both the pH and the volume of the titrant are recorded. In this way, one can determine the pH of a solution without having to worry about determining the concentration of an acid. Despite the fact that these compounds are neutral, there are still many misconceptions about their acids. While they are used in everyday life, they also have industrial applications. Their chemical properties and applications range from the production of drugs to biodegradable plastics. One way to understand their properties is to try the reactions they are involved in with food and beverages. Aside from that, amino acids are often used in the production of biodegradable plastics and chiral catalysts.
Colorless

The simplest basic properties of amino acid is glycine, which is a sweet-tasting, colorless crystalline solid. It is nonvolatile and melts at temperatures above 200degC. These temperatures are higher than those of organic acids and amines. The chemical structure of an amino acid consists of one positively and one negatively charged group, known as a zwitterion. It is therefore considered the simplest type of amino acid. The chirality of an amino acid is determined by its R-substituent, which is a three-carbon chain attached to an alpha-carbon ring. The chiral nature of amino acids is one of the main factors determining their colorlessness and their structural differences. Cys is the only natural colorless amino acid. The chiral structure of the acid is based on Cahn-Ingold-Prelog priority rules, which are widely used in chemistry.
Crystalline

Amino acids are crystalline solids that melt at temperatures of 200degC or higher. They are more like inorganic salts than organic acids or amines because of the positive and negative charge on the amine group. Crystalline ammonium acetate has a zwitterion structure. As a result, the amino acid has a higher melting point than other organic acids. In addition, amino acids are insoluble in non-polar organic solvents. In addition to their molecular mass, amino acids are chiral. They do not have a plane of symmetry and have two stereoisomers. This gives rise to their crystalline nature. This is also true of their structure. They have been investigated by spectroscopy and piezoelectric response at temperatures from 120 K to 320 K. And finally, they were studied by optical second harmonic generation and optical activity. Each amino acid has a different structure. It is distinguished by its amine and acid functional groups. Despite these differences, each amino acid has special common names, such as a-amino-proline. The abbreviations for residues with multiple identities are included in blue. The termination codon is shown in red. This structure can be useful for identifying the type of amino acid required for a particular species. Aside from being the building blocks of proteins, amino acids also determine a wide range of biochemical properties. The amino acid cysteine, for example, can be converted into a crystalloid form by replacing a portion of soybean meal with a crystalline form. This synthetic form is 100 percent digested and decomposed by bacteria. This means that crystalline amino acids are ideal for the production of proteins since they are non-toxic to humans and the environment.
Physical properties of amino acids

There are two main types of amino acids: polar and nonpolar. The polar amino acids are those that contain a hydrocarbon side chain, and the nonpolar ones are those that lack this ring structure. Nonpolar amino acids include asparagine, cysteine, leucine, and phenylalanine, as well as any amino acid with an aromatic ring. Depending on the amino acid, nonpolar or polar amino acids may be more or less acidic. Each amino acid consists of five different atoms - H, C, N, and O. The polarity of an amino acid is determined by its alkyl groups, with the higher the number, the less polar it is. Valine and methionine are two examples of nonpolar amino acids, which are water-fearing. However, polar amino acids are not inherently anti-magnetic. Amino acids are classified based on their location in protein molecules, as well as their degree of exposure to solvent. Amino acids with hydrophobic side chains are usually buried, while those with polar or charged residues are exposed to greater extents. In addition, polar amino acids have higher pKa values than nonpolar acids. However, the chemical reactions involving amino acids require the extraction of a proton. Glycine is a common amino acid that is found in coils and loops. This amino acid gives a polypeptide chain its high flexibility, which is important for sharp turns in loop regions. On the other hand, proline is a nonpolar amino acid that imparts rigidity to a polypeptide chain. Its side chain forms a covalent bond with the main chain and constrains the polypeptide's phi-angle.
Hydrophobic
Quantitative characterization of the hydrophobic basic properties of amino acids is an important prerequisite for predicting the structures and functionalities of proteins in a biological environment. These properties of amino acids can be measured using phase-partitioning behavior of molecular fragments. The corresponding values for hydrophobic amino acid fractions can be obtained by calculating the partitioning coefficients of the aliphatic and phenolic hydroxyls of the amino acids. The hydrophobic basic properties of amino acids are associated with their lack of polarity in their side chains. These amino acids generally reside in the hydrophobic core of proteins and lipid portions of membranes. These properties are describe by authors including M.J. Betts, R.B. Russell, and M.R. Barnes. I.C. Gray is the editor of Bioinformatics for Geneticists. The hydrophobicity of a macroscopic planar surface is typically measured by the contact angle of water droplets, but this cannot be extended to proteins due to their nanometer-scale amino acids and inherent nonplanar structure. Hydrophobic solvation is characterized by numerous parameters. However, a consistent pattern cannot be established for all parameters. Hydrophobic basic properties of amino acids can be characterized using computational data. A combination of Pronase and Peptidase R shows the highest selectivity for hydrophobic amino acids. The enzymes increased hydrophobic amino acid concentrations by twofold and doubled their selectivity. This enzyme-based method is applicable to agro-industrial residues. It have been shown that Pronase and Peptidase R can increase the hydrophobic basic content of proteins by combining them. https://www.youtube.com/watch?v=8J-vYTfHMaA Read the full article
0 notes
Text
Reverse Phase Chromatography
The ability to purify samples and isolate specific proteins is an important first step for studying proteins. If you want to get the 3D structure of a protein or an amino acid sequence or gain any understanding of how a protein interacts with something, then a basic requirement is having a pure sample. But let's face it your sample has a bunch of other undesirable junk, because nothing can be too convenient in science. Even if you chemically synthesize the protein instead of stealing it from the original source (like a dead anemone) you still have to purify the sample and get your target protein.
How do you do this?
There are a variety of liquid chromatography techniques used for purifying proteins such as anion exchange chromatography (separates proteins based on charge), affinity chromatography (this is really specific since the column will only bind to the antibody or ligand you choose, not just any randy that passes by), Size Exclusion Chromatography (separates proteins based on size) and Reverse-phase chromatography. These techniques exploit a specific property of the protein such as charge, size, isoelectric points etc. to separate proteins. Reverse-phase liquid chromatography (RP-HPLC) is one the most important and commonly used forms of liquid chromatography. It separates proteins based on how hydrophobic (water hating) or hydrophilic (water loving) a protein is.
There are two phases in all types of chromatography. The stationary phase (or the lazy phase which clings to things) and the mobile phase, which slowly seeps through the stationary phase. For Reverse-phase the stationary phase is non-polar (or hydrophobic). The interaction between the stationary phase and analyte is mostly hydrophobic. So, if your protein is really hydrophobic (i.e. it has a lot of valine and alanine resides) then it will cling to stationary phase for its dear life until it is eluted by something more hydrophobic. Typically, the stationary phase in Reverse-phase columns have little beads made of silica gel and you have alkyl groups (hexyl, butyl, or ethyl groups) covalently linked to the beads. These little extensions are non-polar and they play a part in the hydrophobic interaction with your sample. The types of alkyl groups attached vary depending on the type of column you’re using and the kind of protein you're purifying. Of course, there are other types of beads like polymer based beads, which are better if you have the pH of your sample or solvent is above 7. BUT they are not as efficient at separating things.

Figure 1: Silica resin with C18 chains.
Source: http://chem-net.blogspot.com/2013/11/reversed-phase-chromatography.html
The second player is the mobile phase or the hydrophobic solvent you will use to elute your protein. Usually as reverse phase happens you increase the hydrophobicity of the solvent and over time the proteins are eluted based on hydrophobic they are. The hydrophilic proteins come out first and the most hydrophobic proteins come out last because you need a higher concentration of your non-polar solvent. Most of the time, a mix of acetonitrile and trifluoroacetic acid (TFA) is used for the mobile phase.
Acetonitrile (ACN) is a non-polar solvent. If you want to make the mobile phase more hydrophobic to elute the more hydrophobic proteins, then you increase the concentration of ACN. TFA is a weak hydrophobic ion-pairing reagent which helps maintain a low pH for our silica resin and helps reduce ionic interactions between the peptide/protein and the stationary phase (because there is always a small chance of something annoying like this happening). You can use other non-polar solvents like methanol, ethanol and isopropanol. Isopropanol works better for ridiculously large and hydrophobic proteins but it places a lot of pressure on the column. If the pressure is too high, your incredibly expensive column will die a horrible and resin-crushing death (Pro-tip: Always check the manual for the maximum pressure limit!).
You can tell how well your purification went based on the chromatogram. A chromatogram shows the UV absorbance for your sample against the retention time. Acetonitrile absorbs UV light very well which is why it's a great solvent for reverse phase. The peptide bonds in proteins absorb UV light at 215 nm whereas, aromatic amino acids like tryptophan absorb UV light at 280nm. UV absorbance can be used to find the concertation of your protein or it can help determine the resolution for your feeble attempts at purification.
If you have ‘peaks’ or ‘mini mountains’ that are well separated then your peptides are probably separated pretty well. But if your all of peaks on the chromatogram merge into an indistinguishable and horrifying blob—you have very poor resolution and the eluted sample you get at the end won't be that pure. You can take all of the eluted samples and try purifying them with even more reverse phase or other methods, but you will lose a bit of protein at each purification step. Or you can tweak your method to improve the resolution for reverse phase.

Figure 2: What a chromatogram looks like in an ideal world where the experiment actually works. The “peaks” are sharp, narrow and distinct.

Figure 3: A blob monster of my own making. I have probably spent more time doing Reverse phase than socialising at this point and I still get chromatograms like this.
Stuff you need to worry about:
What kind of column your using: Sometimes you want to purify lots of your proteins in one go so you have to pick a column that can handle a high load. Other times you are analysing the purity of your sample and using a sensitive analytical column makes more sense. If you want to isolate a really hydrophilic protein then using a c18 column is a solid option because it has more alkyl chains that can bind better to proteins that are not so hydrophobic. BUT since you are introducing a really strong hydrophobic interaction it can mess up the structure of your protein to the point where it loses its bioactivity. If you want to protect the bioactivity of your protein using a C4 or C5 column is a decent option.
Pore size: Each column will have its own pore size (the size of the gap between the beads) and you have pick the pore size based on the size of the target protein. Small pore sizes work for smaller proteins (<10kDa) but it most cases a pore size 300 Å works for a wide variety of protein sizes.
Temperature: A high temperature can speed up chromatography and give you better resolution (AKA the sharp and sexy peaks). All of this will come at the cost of your protein being denatured—of course.
pH: Proteins are pH sensitive since the amine, carboxylic and R groups of the amino acids can change charge depending on the pH. Silica columns have a pH of 2-7, so the basic amino acids exist as positive ions. You need a hydrophobic anionic (negatively charged) ion-pairing reagent (like TFA) that will pair up with the positively charged basic amino acids, since you don't want any electrostatic interaction between the column and the peptide. (remember we only want to separate the proteins based on how hydrophobc they are)
Flow rate: A faster flow rate will allow a better separation. (Pro-Tip: Check the manual for the maximum flow rate. Usually the big, thick columns tend to have a higher flow rate than the thin, small and dainty columns)
Gradient: This is the most important factor which impacts the resolution. If you have a gradual increase in ACN concentration or hydrophobicity of your solvent (AKA a shallower gradient), you tend to get better resolution and higher peaks. However, if the run time is too long then your protein can get damaged since it’s being exposed to harsh chemicals (e.g. Acetonitrile) for a longer period of time.
There are other applications for Reverse-phase aside from protein purification. It isn't just used to purify and analyse proteins but it can work for drugs, metabolites, fatty acids, aldehydes and ketones. I hope this was a helpful introduction to Reverse-phase for anyone who is interested in the technique or actually has to use this technique.
Next time, I’ll dive into Size Exclusion Chromatography (SEC).
Primary sources:
Dorsey, J. G. & Dill, K. A. The Molecular Mechanism of Retention in Reversed-Phase Liquid Chromatography. Chem. Rev. 89, 331–346 (1989).
Nagy, K. & Vékey, K. Separation methods. Med. Appl. Mass Spectrom. 61–92 (2008). doi:10.1016/B978-044451980-1.50007-0
Recommended readings:
Chromatography, R. Reverse-Phase Chromatography. Proteins 26–34 (2009). doi:10.1201/NOE1420084597.ch409
Bradshaw, T. P. A Users Guide: Introduction to Protein and Peptide HPLC. (2006).
#Protein chemistry 101#Reverse phase#RP-HPLC#Lab techniques#liquid chromatography#protein purification#biochemistry
2 notes
·
View notes