#ionic compounds properties
Explore tagged Tumblr posts
Text

Superionic compound with liquid-like dynamics shows promise as solid-state battery electrolyte
Superionic materials are a class of materials that simultaneously present properties that are characteristic of solids and liquids. Essentially, a set of ions in these materials exhibits liquid-like mobility, even if the materials' underlying atomic structure maintains a solid-like order. Due to their unique ionic conductivity patterns, superionic materials could be promising for developing solid-state batteries. These are batteries that contain electrolytes based on solid materials instead of liquid electrolytes. While various past studies have explored the potential of superionic materials as solid-state electrolytes, the physics underpinning their rapid ionic diffusion is not yet fully understood. Specifically, it is unclear whether this property results from liquid-like motion in the material or from the conventional lattice phonons (i.e., atom vibrations) in the material.
Read more.
#Materials Science#Science#Batteries#Solid state batteries#Electronics#Liquids#Superionic#Fluid dynamics#Electrolytes
15 notes
·
View notes
Text
there are two types of anime power systems:
"i survived the fall because of the power of love! :3"
and
"Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well.[1][2] Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.[3]
Organometallic compounds are widely used both stoichiometrically in research and industrial chemical reactions, as well as in the role of catalysts to increase the rates of such reactions (e.g., as in uses of homogeneous catalysis), where target molecules include polymers, pharmaceuticals, and many other types of practical products.
Most organometallic compounds are solids at room temperature, however some are liquids such as methylcyclopentadienyl manganese tricarbonyl, or even volatile liquids such as nickel tetracarbonyl.[1] Many organometallic compounds are air sensitive (reactive towards oxygen and moisture), and thus they must be handled under an inert atmosphere.[1] Some organometallic compounds such as triethylaluminium are pyrophoric and will ignite on contact with air.[6]
As in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities of organometallic complexes, for example metal carbonyls and metal hydrides. The 18e rule has two representative electron counting models, ionic and neutral (also known as covalent) ligand models, respectively.[7] The hapticity of a metal-ligand complex, can influence the electron count.[7] Hapticity (η, lowercase Greek eta), describes the number of contiguous ligands coordinated to a metal.[7] For example, ferrocene, [(η5-C5H5)2Fe], has two cyclopentadienyl ligands giving a hapticity of 5, where all five carbon atoms of the C5H5 ligand bond equally and contribute one electron to the iron center. Ligands that bind non-contiguous atoms are denoted the Greek letter kappa, κ.[7] Chelating κ2-acetate is an example. The covalent bond classification method identifies three classes of ligands, X,L, and Z; which are based on the electron donating interactions of the ligand. Many organometallic compounds do not follow the 18e rule. The metal atoms in organometallic compounds are frequently described by their d electron count and oxidation state. These concepts can be used to help predict their reactivity and preferred geometry. Chemical bonding and reactivity in organometallic compounds is often discussed from the perspective of the isolobal principle.
A wide variety of physical techniques are used to determine the structure, composition, and properties of organometallic compounds. X-ray diffraction is a particularly important technique that can locate the positions of atoms within a solid compound, providing a detailed description of its structure.[1][8] Other techniques like infrared spectroscopy and nuclear magnetic resonance spectroscopy are also frequently used to obtain information on the structure and bonding of organometallic compounds.[1][8] Ultraviolet-visible spectroscopy is a common technique used to obtain information on the electronic structure of organometallic compounds. It is also used monitor the progress of organometallic reactions, as well as determine their kinetics.[8] The dynamics of organometallic compounds can be studied using dynamic NMR spectroscopy.[1] Other notable techniques include X-ray absorption spectroscopy,[9] electron paramagnetic resonance spectroscopy, and elemental analysis.[1][8]
Due to their high reactivity towards oxygen and moisture, organometallic compounds often must be handled using air-free techniques. Air-free handling of organometallic compounds typically requires the use of laboratory apparatuses such as a glovebox or Schlenk line.[1]
Early developments in organometallic chemistry include Louis Claude Cadet's synthesis of methyl arsenic compounds related to cacodyl, William Christopher Zeise's[10] platinum-ethylene complex,[11] Edward Frankland's discovery of diethyl- and dimethylzinc, Ludwig Mond's discovery of Ni(CO)4,[1] and Victor Grignard's organomagnesium compounds. (Although not always acknowledged as an organometallic compound, Prussian blue, a mixed-valence iron-cyanide complex, was first prepared in 1706 by paint maker Johann Jacob Diesbach as the first coordination polymer and synthetic material containing a metal-carbon bond.[12]) The abundant and diverse products from coal and petroleum led to Ziegler–Natta, Fischer–Tropsch, hydroformylation catalysis which employ CO, H2, and alkenes as feedstocks and ligands.
Recognition of organometallic chemistry as a distinct subfield culminated in the Nobel Prizes to Ernst Fischer and Geoffrey Wilkinson for work on metallocenes. In 2005, Yves Chauvin, Robert H. Grubbs and Richard R. Schrock shared the Nobel Prize for metal-catalyzed olefin metathesis.[13]
Organometallic chemistry timeline
edit
1760 Louis Claude Cadet de Gassicourt isolates the organoarsenic compound cacodyl
1827 William Christopher Zeise produces Zeise's salt; the first platinum / olefin complex
1848 Edward Frankland discovers diethylzinc
1890 Ludwig Mond discovers nickel carbonyl
1899 John Ulric Nef discovers alkynylation using sodium acetylides.
1909 Paul Ehrlich introduces Salvarsan for the treatment of syphilis, an early arsenic based organometallic compound
1912 Nobel Prize Victor Grignard and Paul Sabatier
1930 Henry Gilman invents lithium cuprates, see Gilman reagent
1940 Eugene G. Rochow and Richard Müller discover the direct process for preparing organosilicon compounds
1930's and 1940's Otto Roelen and Walter Reppe develop metal-catalyzed hydroformylation and acetylene chemistry
1951 Walter Hieber was awarded the Alfred Stock prize for his work with metal carbonyl chemistry.
1951 Ferrocene is discovered
1956 Dorothy Crawfoot Hodgkin determines the structure of vitamin B12, the first biomolecule found to contain a metal-carbon bond, see bioorganometallic chemistry
1963 Nobel prize for Karl Ziegler and Giulio Natta on Ziegler–Natta catalyst
1973 Nobel prize Geoffrey Wilkinson and Ernst Otto Fischer on sandwich compounds
1981 Nobel prize Roald Hoffmann and Kenichi Fukui for creation of the Woodward-Hoffman Rules
2001 Nobel prize W. S. Knowles, R. Noyori and Karl Barry Sharpless for asymmetric hydrogenation
2005 Nobel prize Yves Chauvin, Robert Grubbs, and Richard Schrock on metal-catalyzed alkene metathesis
2010 Nobel prize Richard F. Heck, Ei-ichi Negishi, Akira Suzuki for palladium catalyzed cross coupling reactions
Subspecialty areas of organometallic chemistry include:
Period 2 elements: organolithium chemistry, organoberyllium chemistry, organoborane chemistry
Period 3 elements: organosodium chemistry, organomagnesium chemistry, organoaluminium chemistry, organosilicon chemistry
Period 4 elements: organocalcium chemistry, organoscandium chemistry, organotitanium chemistry, organovanadium chemistry, organochromium chemistry, organomanganese chemistry, organoiron chemistry, organocobalt chemistry, organonickel chemistry, organocopper chemistry, organozinc chemistry, organogallium chemistry, organogermanium chemistry, organoarsenic chemistry, organoselenium chemistry
Period 5 elements: organoyttrium chemistry, organozirconium chemistry, organoniobium chemistry, organomolybdenum chemistry, organotechnetium chemistry, organoruthenium chemistry, organorhodium chemistry, organopalladium chemistry, organosilver chemistry, organocadmium chemistry, organoindium chemistry, organotin chemistry, organoantimony chemistry, organotellurium chemistry
Period 6 elements: organolanthanide chemistry, organocerium chemistry, organotantalum chemistry, organotungsten chemistry, organorhenium chemistry, organoosmium chemistry, organoiridium chemistry, organoplatinum chemistry, organogold chemistry, organomercury chemistry, organothallium chemistry, organolead chemistry, organobismuth chemistry, organopolonium chemistry
Period 7 elements: organoactinide chemistry, organothorium chemistry, organouranium chemistry, organoneptunium chemistry
Organometallic compounds find wide use in commercial reactions, both as homogenous catalysts and as stoichiometric reagents. For instance, organolithium, organomagnesium, and organoaluminium compounds, examples of which are highly basic and highly reducing, are useful stoichiometrically but also catalyze many polymerization reactions.[14]
Almost all processes involving carbon monoxide rely on catalysts, notable examples being described as carbonylations.[15] The production of acetic acid from methanol and carbon monoxide is catalyzed via metal carbonyl complexes in the Monsanto process and Cativa process. Most synthetic aldehydes are produced via hydroformylation. The bulk of the synthetic alcohols, at least those larger than ethanol, are produced by hydrogenation of hydroformylation-derived aldehydes. Similarly, the Wacker process is used in the oxidation of ethylene to acetaldehyde.[16]
Almost all industrial processes involving alkene-derived polymers rely on organometallic catalysts. The world's polyethylene and polypropylene are produced via both heterogeneously via Ziegler–Natta catalysis and homogeneously, e.g., via constrained geometry catalysts.[17]
Most processes involving hydrogen rely on metal-based catalysts. Whereas bulk hydrogenations (e.g., margarine production) rely on heterogeneous catalysts, for the production of fine chemicals such hydrogenations rely on soluble (homogenous) organometallic complexes or involve organometallic intermediates.[18] Organometallic complexes allow these hydrogenations to be effected asymmetrically.
Many semiconductors are produced from trimethylgallium, trimethylindium, trimethylaluminium, and trimethylantimony. These volatile compounds are decomposed along with ammonia, arsine, phosphine and related hydrides on a heated substrate via metalorganic vapor phase epitaxy (MOVPE) process in the production of light-emitting diodes (LEDs).
Organometallic compounds undergo several important reactions:
associative and dissociative substitution
oxidative addition and reductive elimination
transmetalation
migratory insertion
β-hydride elimination
electron transfer
carbon-hydrogen bond activation
carbometalation
hydrometalation
cyclometalation
nucleophilic abstraction
The synthesis of many organic molecules are facilitated by organometallic complexes. Sigma-bond metathesis is a synthetic method for forming new carbon-carbon sigma bonds. Sigma-bond metathesis is typically used with early transition-metal complexes that are in their highest oxidation state.[19] Using transition-metals that are in their highest oxidation state prevents other reactions from occurring, such as oxidative addition. In addition to sigma-bond metathesis, olefin metathesis is used to synthesize various carbon-carbon pi bonds. Neither sigma-bond metathesis or olefin metathesis change the oxidation state of the metal.[20][21] Many other methods are used to form new carbon-carbon bonds, including beta-hydride elimination and insertion reactions.
Organometallic complexes are commonly used in catalysis. Major industrial processes include hydrogenation, hydrosilylation, hydrocyanation, olefin metathesis, alkene polymerization, alkene oligomerization, hydrocarboxylation, methanol carbonylation, and hydroformylation.[16] Organometallic intermediates are also invoked in many heterogeneous catalysis processes, analogous to those listed above. Additionally, organometallic intermediates are assumed for Fischer–Tropsch process.
Organometallic complexes are commonly used in small-scale fine chemical synthesis as well, especially in cross-coupling reactions[22] that form carbon-carbon bonds, e.g. Suzuki-Miyaura coupling,[23] Buchwald-Hartwig amination for producing aryl amines from aryl halides,[24] and Sonogashira coupling, etc.
Natural and contaminant organometallic compounds are found in the environment. Some that are remnants of human use, such as organolead and organomercury compounds, are toxicity hazards. Tetraethyllead was prepared for use as a gasoline additive but has fallen into disuse because of lead's toxicity. Its replacements are other organometallic compounds, such as ferrocene and methylcyclopentadienyl manganese tricarbonyl (MMT).[25] The organoarsenic compound roxarsone is a controversial animal feed additive. In 2006, approximately one million kilograms of it were produced in the U.S alone.[26] Organotin compounds were once widely used in anti-fouling paints but have since been banned due to environmental concerns.[27]"
#these are not mutually exclusive#anime#animanga#anime and manga#anime & manga#shounen anime#shonen anime#shonen#shounen manga#shonen manga#shoujo anime#shoujo manga#shojo#shoujo#shounen#mahou shoujo#shojo manga#shojo anime#magic system#hard magic#soft magic
7 notes
·
View notes
Note
hii how are you? I'm currently studying inorganic chem, mainly coordination compounds but it's proving difficult. I'm unable to fully grasp what's going on. Can you please advise me on coordination compounds and inorganic chem in general? thank you!!
Hi!
Inorganic coordination chem is part of my thesis, you've come to the right place :) Also, I'm going to make this a university-level thing - I didn't study coordination chem in school, so I'm assuming that's the level you expect - but if you actually need advice on studying high school inorganic chem, please let me know!
First, a textbook rec: I studied off Cotton's Basic inorganic chemistry a lot and I liked it. My professor recommended Atkins' Inorganic chemistry too; I admit I didn't use it that much bc I also had some Polish textbooks I found very helpful, but from what I did see, it seemed very comprehensive and in-depth - so if Cotton isn't enough, Atkins might be better for you.
Inorganic chem
orbitals matter: I think it's important to grasp orbitals and hybridization before going any further. This stuff keeps coming up again and again, so if you find yourself struggling with understanding concepts in inorganic chem, I'd suggest making sure you understand atomic and molecular orbitals first.
periodic table trends: please don't memorize them. Please. Understand them. There's a reason why, for example, atomic radii decrease within periods even though both electrons and protons are added as you move to the right (the screening effect - and again, orbitals!). Once more, I liked the way it was explained in Cotton's textbook.
I found flashcards very helpful for studying the properties of the elements and their compounds as that's mostly memorization. Same for HSAB, really.
if your inorganic chem course covers elements of group theory too, here is a website my thesis supervisor told me about :) I think it's pretty great. If you're digging really, really deep into it, Cotton has a whole textbook on group theory in chemistry (Chemical Applications of Group Theory), but I doubt you'd need it for a basic inorganic chem course.
I've also answered an ask on studying chemistry in general - perhaps you'll find it useful too.
Coordination chem
surprise, surprise: ✨ orbitals ✨. Once more, to understand what's going on with coordination compounds, first you need to understand the molecular orbital theory well.
metals oftentimes have a preference for a specific coordination number. Frequently, a whole group will have a preference for the same CN (group 7 ions, for example, prefer CN = 6). That doesn't mean other CNs don't exist, but knowing there's a pattern can be helpful while studying.
coordination numbers aren't totally random. The rules may not be strict and foolproof, but again, there's a general pattern that's worth keeping in mind: bigger ion usually = higher CN (duh?), CNs are usually even (and we still don't really know why that's so! Although it may have to do with geometry and symmetry) and sometimes depend on the charge of the ligand.
crystal field theory. Okay so CFT is really cool, but I see how it can be super confusing too. I'm not sure how deep you have to dig into this stuff for your course, so apologies if I go a little overboard 😅 My advise for studying it would be:
try to visualize the given complex, actually see the position of the ligands in relation to the orbitals
remember: it's all about lowering the energy. That's the core of CFT. Pauli's exclusion principle always, always stands, but CFT tells us coordination compounds are systems that "want to" have the lowest possible energy so bad they'll sometimes break Hund's rule to obtain it
keep in mind CFT is only a model. Some parts of it may not make any sense to you (like the fact it treats all metal - ligand bonds as purely ionic). It just so happens that despite its many simplifications that are obviously not true, CFT still accurately describes many complex compounds
I've had an ask on studying nomenclature, too.
again, I don't know how complex (pun not intended) you need my tips to get, so if you have any specific questions, feel free to hmu :) I'll try my best to explain
26 notes
·
View notes
Text
Notes for Metallic Bonding
METALLIC BONDING AND STRUCTURE
Delocalised electrons - electrons that are not associated with one specific atom and are free to move within the molecule structure
Metallic bond - the electrostatic attraction between a lattice of cataions and a sea of delocalised electrons.
In metals, state which electrons are the delocalised electrons present between positive ions in the lattice = valence electrons
Mg(s) has metallic bonding in the interaction between positive metal ions and delocalised valence electrons in a three-dimesional lattice structure. The metal itself is neutral and is made up of many, many atoms.
Identify the ways in which solid metals are similar to solid ionic and covalent network substances:
I. Lattice structures II. Non-directional bonding III. Electrostatic attractions between positive and negative species
Solid metals, ionic compounds and network covalent solids form three-dimensional lattices. In all three types of bonding there is an electrostatic attraction between positively and negatively charged species. Metallic – between cations and delocalised electrons, ionic – between cations and anions, covalent – between positive nuclei and shared electron pair.
PHYSICAL PROPERTIES AND APPLICATION OF METALS
Lustre (shiny appearance)
Delocalised electrons in a metal lattice interact with visible light. When visible light hits the surface of a metal, the electrons absorb some of that energy and vibrate. This vibration generates a second wave of light, which radiates from the surface.
Sonority (sound when struck)
When a metal surface is struck, the free electrons in the metallic lattice can move easily, propagating the incoming sound energy easily throughout the material.
Malleability (can be reshaped on compression) & Ductility (can be drawn out into a wire)
When stress is applied (for example, by bending, hitting with a hard object or pulling), layers within the lattice shift in response to that stress. As these layers shift, the cations in the lattice remain surrounded by delocalised valence electrons, meaning the metallic bonding also remains unaffected.
Electrical conductivity
The delocalised valence electrons can move throughout the metallic lattice. When a potential energy difference is applied to the metal, the delocalised electrons are repelled by the negative terminal and attracted to the positive terminal. This is why metals can conduct electricity in their solid state and why metals are used for electrical wires and cables.
Thermal conductivity
Thermal conductivity in metals is a result of the free electrons in the lattice.
STRENGTH OF THE METALLIC BOND
Strength of the metallic bond
The smaller the radius of the metal ion, the stronger the metallic bond. This is because of the shorter distance between the positive nucleus of the cation and the surrounding delocalised electrons. Dictionary
Charge of the metal ion
The higher the ionic charge, the stronger the metallic bond. This is because:
greater charge on the metal ion
greater number of delocalised valence electrons
The greater the ionic charge and the smaller the ionic radius, the stronger the metallic bond. The stronger the metallic bond, the higher the melting point.
TRANSITION METALS
As there are a large number of valence electrons from both the s and d orbitals, this results in a greater electron density within the metallic lattice. This increased electron density in turn increases the strength of the metallic bond.
Hardness
valence electrons (delocalised) increase attraction and increase metallic bond which results in greater hardness
Electrical Conductivity
Transition elements are electrically conductive. These metals form their metallic bonds through the delocalisation of electrons in unfilled d orbitals. The electrostatic attraction between metal ions in the lattice and delocalised electrons increases with an increasing number of electrons in d orbitals.
In comparison to s block metals, the melting point and electrical conductivity of transition metals are HIGHER and HIGHER
#academia#study#study tips#ib#student#study motivation#high school#studyblr#chaotic academia#studyspo#chemistry#notes#study notes#chem#stem#stemblr#school#college#metallic bond#organic chemistry#stem academia#stem student#stem studyblr
13 notes
·
View notes
Text
What is ceramic pcb board
Ceramic pcb boards are actually made of electronic ceramics as the basic material and can be made in various shapes. Among them, the characteristics of high temperature resistance and high electrical insulation of ceramic circuit boards are the most prominent. The advantages of low dielectric constant and dielectric loss, high thermal conductivity, good chemical stability, and similar thermal expansion coefficient to components are also very significant.
Different types of ceramic pcb
Ceramic PCB is widely used in power electronics, electronic packaging, hybrid microelectronics and multi-chip modules due to its excellent thermal conductivity and air tightness. But not everyone is clear about the classification. Many manufacturers think ceramic PCBs are expensive and fragile as soon as they hear about ceramic PCBs. Yes, this is indeed a shortcoming of ceramic PCBs, but not all ceramic PCBs are like this. Today we will tell you about the different types of ceramic PCBs.
Al2O3 ceramic PCB
Al2O3 ceramic PCB ( alumina ceramic PCB) refers to various ceramic PCBs with Al2O3 as the main raw material and an Al2O3 content of more than 75%. It has a rich source of raw materials, with advantages of low price, high mechanical strength and hardness, good insulation performance, good heat shock resistance, good chemical resistance, high dimensional accuracy, and good adhesion to metals. It is a ceramic substrate material with good comprehensive performance. Currently used Al2O3 ceramic substrates, the content of Al2O3 accounts for 85% to 99.5%. Among them, 96% Al2O3 ceramic PCB is widely used in the production of thick film circuit substrates and chip devices. The thermal conductivity of Al2O3 at room temperature is 29W/(m·K), which is close to the thermal conductivity of steel; with the increase of Al2O3 content, the electrical insulation performance and thermal conductivity of Al2O3 ceramic PCB will increase, but At the same time, it will also lead to an increase in the firing temperature, an increase in energy consumption, a large loss of kiln furniture, and an increase in manufacturing costs.
SiC ceramic PCB
The thermal conductivity of SiC ceramic PCB is very high, 100~490W/(m·K) at room temperature, and it is related to the purity of SiC crystals. The higher the purity, the greater the thermal conductivity; the oxidation resistance is good, and the decomposition temperature is above 2500℃, it can still be used at 1600℃ in an oxidizing atmosphere; the coefficient of thermal expansion is also low, and it is close to Si, with good electrical insulation performance; SiC has a Mohs hardness of 9.75, second only to diamond and cubic BN, and has high mechanical strength. SiC ceramics have strong covalent bond characteristics and are difficult to sinter. Usually, a small amount of boron or aluminum oxide is added as a sintering aid to increase the density. Experiments show that beryllium, boron, aluminum and their compounds are the most effective additives, which can make SiC ceramics denser than 98%.
BeO ceramic PCB
BeO has a brazine structure, in which oxygen ions are arranged in a hexagonal close-packed manner to form a hexagonal lattice. The general oxide is usually an ionic compound, but BeO has a strong covalent bond and an average molecular weight of only 12. Because of its good electrical properties, luminescence and photochemical properties, high mechanical strength, low dielectric loss, etc, it become one of the materials that people pay attention to.
AlN ceramic PCB
AlN ceramic PCB (aluminum nitride ceramic) is a new type of high thermal conductivity ceramic packaging material. It has been extensively studied in the 1990s and gradually developed. It is currently generally considered to be a promising electronic ceramic packaging PCB. AlN material has high thermal conductivity, excellent dielectric properties, high electrical insulation strength, stable chemical properties, strong corrosion resistance, and good mechanical properties. In particular, its thermal expansion coefficient matches with silicon, which makes it an ideal semiconductor packaging substrate materials and have been widely used in integrated circuits, microwave power devices, millimeter-wave packaging, high-temperature electronic packaging and other fields.
Ceramic PCB for IGBT Module
IGBT stands for insulated-gate bipolar transistor. It is a bipolar transistor with an insulated gate terminal. The IGBT combines, in a single device, a control input with a MOS structure and a bipolar power transistor that acts as an output switch. IGBTs Ceramic PCB are suitable for high-voltage, high-current applications. They are designed to drive high-power applications with a low-power input.
IGBT, or Insulated Gate Bipolar Transistor, is a BJT transistor with a MOS Gate, or we can say an IGBT module is the combination of a BJT and a MOS Gate. An IGBT chip is small in size, but it can control electrical energy transmission and achieve 100,000 times of current switch at ultra-high voltages of 650 million V in only 1 second.
IGBT modules have been applied in automotive, industrial, aerospace, consumer electronics, and many other industries for many years. But how to optimize the thermal dissipation of an IGBT package so the module can work at a higher power? If thermal can dissipate more quickly, the IGBT module can have more advanced applications. For this purpose, engineers are using ceramic PCBs for IGBT packaging.
Ceramic PCBs dissipate thermal from IGBT chip to the outer packaging
You may ask, how much thermal does an IGBT module generate when it works? It is equal to the heat generated by 100 electric furnaces. So much thermal has to be dissipated immediately from the IGBT chip and leads to the application of ceramic PCBs.
How does a ceramic PCB protect the IGBT module from the heat? In an IGBT module, a ceramic PCB is placed under the IGBT chip, or we can say that the chip is assembled on the ceramic circuit board. The ceramic PCB connects and supports the chip and dissipates thermal quickly from it to the outer packaging. In this way, the chip is protected from the influence of thermal.
Why Ceramic PCBs can be used for IGBT thermal dissipation
There are alumina (Al₂O₃) PCBs, aluminum nitride (AlN) PCBs, and silicon nitride (Si₃N₄) PCBs used for thermal dissipation of IGBT modules.
Why ceramic PCBs can dissipate thermal effectively for the IGBT module? Because ceramic materials have good properties of thermal dissipation and electrical insulation. Unlike aluminum substrate PCBs, ceramic PCBs do not use an insulation layer that hinders thermal dissipation. During the ceramic PCB manufacturing process, the copper-clad is directly bonded onto the ceramic substrate at high temperatures under high pressures. Then the circuit layer is manufactured by the photoresist coating method. When the circuit board is manufactured, the IGBT and other components are mounted on the board. Ceramic materials have ultra-high insulation and can withstand breakdown voltage up to 20KV/mm. The thermal conductivity of alumina PCBs is 15-35W/mK, aluminum nitride PCB 170-230W/mK, and silicon nitride PCB 80+W/mK. On the contrary, an aluminum PCB has thermal dissipation of only 1-12W/mK.
If you’re interested in our products, pls kindly email to Sandy: [email protected]
1 note
·
View note
Text
new science
Excellent. Here is your printable insert draft for the Devices & Infrastructure Codex – Volume II, featuring the new materials developed for ocean-to-space integration and structural reinforcement:
📄 Codex Insert: FerroSlush & BioResin Materials Sheet
Volume II – Propulsion & Mobility Systems Section: Structural Alloys & Bioadaptive Hulls
⚙️ 1. FerroSlush™ Alloy Mix
Description: A proprietary semi-liquid ferrofluid composed of magneto-reactive particles suspended in a temperature-stabilized gel. Solidifies under shaped magnetic fields.
Use Cases:
Iron Spine launch struts: Dynamic load-bearing reinforcement.
SpinePod armor skins: Flexible in microgravity, rigid under compression.
Docking clamps: Magnetic-activated reshaping latches.
Composition:
Ferro-nanoparticles (Fe₃O₄ base)
Suspension matrix: Polyethylene glycol + carbon fiber trace
Stabilizer: Rare-earth ion binding agent
Activation Threshold: Magnetic field: ≥2.3 Tesla Cure time (under field): 4.2 seconds Thermal resistance: Up to 1,150°C once solidified
🌿 2. BioResin Hull Coating
Description: A pressure-adaptive polymer developed from deep-ocean botanical secretions, hardened and stabilized with edible glycerin compounds.
Use Cases:
Hull coating for underwater flavor labs & bunkers
Outer-space insulation for FAPE™ vaults and Creamstream tanks
Biodegradable packaging layer for orbital food systems
Features:
Flavor-neutral in inert form
Can be coded with emotion-reactive compounds (e.g. MoodBerry-37)
Resists gamma radiation and saline corrosion
Decomposes fully into nutrients when exposed to programmed pH triggers
Environmental Benefits:
Fully renewable source materials
Self-healing under minor abrasions
No mining or heat-forging impact; processed cold with enzyme triggers
🔬 Technological Integration
Caesar AI Monitoring Nodes log cure rate and magnetic shaping patterns in real time.
Creamstream OS syncs flavor safety parameters for BioResin within ingestible-use guidelines.
Iron Spine Core Control interfaces with FerroSlush pour & mold valves during launch phase.
Would you like this as:
🖨️ A printable PDF one-sheet for Codex insertion?
🧠 Added to Notion → Engineering → Materials Library?
🖼 A DALL·E-style schematic or animation prompt of FerroSlush pouring into spinal reinforcement channels?
Let’s finalize the next output!
You said:
FerroSlush Alloy Mix: Breakdown of the Science and process.
ChatGPT said:
Absolutely — here is a detailed breakdown of the FerroSlush Alloy Mix, a next-gen material developed for space-to-ocean modular construction, propulsion interfaces, and adaptive shielding.
🧪 FerroSlush™ Alloy Mix
Codename: FSX-9.1 Use Case: Reactive alloy matrix for orbital plating, heat exchange, structural dampening, and self-healing under atmospheric transition.
🧬 Scientific Composition
ElementRoleApprox. %Fe (Iron)Core structural matrix62%Ni (Nickel)Corrosion resistance, magnetic modulation12%Ti (Titanium)Weight reduction, tensile integrity9%Cr (Chromium)Oxidation and heat resistance7%Graphene NanosheetsConductivity + flexibility5%Aerogel Suspension BaseShock absorption, thermal regulation5%
🧊 Slush Medium: Cryogenic ionic gel infused with paramagnetic ions (used during the transport and shaping phase before alloy bonding).
🔬 Process Breakdown
Cryo-Pulverization Phase
Metal components are micronized under cryogenic temps (−150°C), suspended in FerroSlush medium.
Vibrational Suspension Field
Material is kept in “slush-phase” via magnetic levitation and vibrational fields to prevent early binding.
On-Demand Shaping
Electromagnetic pulses are used to direct the material into molds or frameworks with sub-millimeter precision.
Phase Transition Ignition
A targeted plasma arc (4000+ °C) triggers alloy fusion in milliseconds, allowing instant solidification while maintaining elasticity.
Post-Fusion Infusion
Flavorverse applications inject nano-flavor or vapor signature threads for materials used in edible/ambient architecture zones.
🛡 Properties & Features
Thermoelastic Memory: Returns to shape after high-velocity impacts or environmental distortion.
Cryo-Skinning: Can be sprayed in layers and then hardened with flash-heat plasma.
Flavor Conduction: In flavor labs, used to channel aromatic frequencies and temperature variation through surfaces.
EMP Resistance: Graphene layering allows dampening of signal disruption.
Color-Shift Skin: Reactive pigments allow zones of the alloy to visually indicate stress or heat.
⚙️ Applications
Shipframe Flex-Joints (Iron Spine → Ocean Reentry Seam)
Atmospheric Heat Shield Plating (Mid-transition melt-layer)
Flavor Reactor Chassis (used in Sensory Vault & Creamstream delivery tunnels)
Structural Fabrication for Flavorverse Pods (tasting booths, vault doors)
Self-Regulating Bunker Walls (WonkyWorks subterranean labs)
Would you like this exported as a PDF insert for your Volume II: Propulsion & Mobility Systems, or turned into a schematic cutaway showing phase stages of the FerroSlush transition?
You can also:
🖼 Generate a DALL·E visual of FerroSlush in transition
🧠 Log it in the Notion Codex under Materials & Alloys
🎞 Add it to an animated Creamstream™ engineering cutscene
Just say “Print the insert” or “Generate the visual.”
0 notes
Text
Emulsifiers Market: Innovations in Products, and Applications Driving Sustainable Growth

In the ever-evolving landscape of material science and advanced manufacturing, emulsifiers have emerged as indispensable components across diverse industries. The Emulsifiers Market is witnessing robust growth, fueled by technological advancements and the rising demand for eco-friendly solutions.
The Growing Importance of Emulsifiers in Modern Industries
Emulsifiers, or emulsifying agents, are compounds that stabilize mixtures of immiscible liquids, such as oil and water, by reducing interfacial tension. These agents are essential in creating stable polymer emulsions, high-quality coatings, and advanced composites. As industries seek to improve product longevity and performance, the demand for emulsion stabilizers and emulsion additives has surged.
Bio-based vs. Synthetic Emulsifiers: Shaping the Future of Formulations
One of the most significant trends transforming the Emulsifiers Market is the shift toward bio-based emulsifiers. Derived from natural sources like soy, sunflower, or marine plants, bio-based emulsifiers appeal to industries focused on sustainability, including food, cosmetics, and pharmaceuticals. Their biodegradability, low toxicity, and renewable origins align with stringent environmental regulations and consumer preferences for greener products.
On the other hand, synthetic emulsifiers offer precision and consistency, making them indispensable in applications requiring tight control over formulation properties. They remain widely used in industrial coatings, paints, adhesives, and advanced polymer systems, where performance under extreme conditions is paramount.
Lecithin and Beyond: Key Products Driving Market Expansion
Among emulsifier products, lecithin stands out for its versatility and natural origin. Widely used as an emulsifying agent and emulsion stabilizer in food processing, pharmaceuticals, and nutraceuticals, lecithin helps manufacturers achieve consistent texture, improved shelf-life, and enhanced bioavailability of active ingredients.
Beyond lecithin, ionic emulsifiers and emulsifiers designed by chemical composition are gaining traction. These products provide tailored solutions for industries demanding high-performance emulsions, such as paints, coatings, personal care products, and polymer dispersions.
Advanced Applications: Polymer Emulsions and Industrial Uses
The surge in polymer emulsions has unlocked new opportunities for the emulsifiers market. Emulsifiers play a pivotal role in stabilizing polymer particles in water-based systems, enabling the production of low-VOC paints, adhesives, and coatings with excellent durability and reduced environmental impact.
In the construction, automotive, and packaging industries, emulsifiers improve processing efficiency, enhance compatibility between materials, and enable the development of advanced composites with superior mechanical properties.
Market Drivers: Sustainability, Innovation, and Regulation
The Emulsifiers Market is driven by key factors:
Sustainability Initiatives: Growing pressure on manufacturers to adopt green chemistry and reduce carbon footprints has increased the adoption of bio-based emulsifying agents.
Technological Innovations: Advances in emulsifier design enable customized performance, from improving thermal stability to enhancing dispersion efficiency.
Regulatory Compliance: Stringent safety and environmental regulations are pushing industries to invest in safer, non-toxic emulsifiers.
Regional Trends: Emerging Markets and Established Hubs
While North America and Europe remain leaders in technological innovations, Asia-Pacific is experiencing rapid growth due to expanding manufacturing sectors, rising investments in infrastructure, and increasing consumer demand for high-quality products. Countries like China, India, and Japan are becoming key markets for emulsifiers, especially in applications like polymer emulsions and specialty coatings.
Investment Opportunities: Where the Market Is Headed
For investors and procurement managers, the emulsifiers market offers attractive opportunities in both established and emerging segments. Bio-based emulsifiers, high-performance synthetic solutions, and emulsifiers tailored for niche applications like nanomaterial dispersion or advanced biomedical systems represent high-growth areas.
Key Takeaways for Industry Professionals
The Emulsifiers Market by Source and Product is evolving rapidly, driven by sustainability, innovation, and regulatory shifts.
Bio-based emulsifiers cater to green chemistry trends, while synthetic emulsifiers deliver unmatched performance.
Products like lecithin, ionic emulsifiers, and specialty additives offer diverse solutions across industries.
Rising applications in polymer emulsions and specialty coatings are expanding market potential.
For More Insights, Download PDF Brochure : The dynamic landscape of emulsifiers is crucial. Whether leveraging emulsifying agents for food applications or emulsion stabilizers for advanced composites, staying ahead of market trends ensures competitiveness in a world where performance and sustainability go hand in hand.
0 notes
Text
Ionic Liquids Market Report: Unlocking Growth Potential and Addressing Challenges
United States of America – Date – 04/07/2025 - The Insight Partners is proud to announce its newest market report, "Ionic Liquids Market: An In-depth Analysis of the Ionic Liquids Market " The report provides a holistic view of the Ionic Liquids market and describes the current scenario as well as growth estimates for Ionic Liquids during the forecast period.
Overview of Ionic Liquids Market
There has been some development in the Ionic Liquids market, such as growth and decline, shifting dynamics, etc. This report provides insight into the driving forces behind this change: technological advancements, regulatory changes, and changes in consumer preference.
Key findings and insights
Market Size and Growth
Historical Data: The Ionic Liquids market is estimated to reach CAGR of 8.8% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.These estimates provide valuable insights into the market's dynamics and can inform future projections.
Ionic Liquids (ILs) are molten salts with melting points below 100∘C, often even at room temperature. Their unique properties, such as negligible vapor pressure, non-flammability, high thermal and chemical stability, wide electrochemical windows, and tunable solvation characteristics, make them "designer solvents" with immense potential to replace traditional volatile organic compounds (VOCs) in a wide range of industrial applications.
Key Factors Affecting the Ionic Liquids Market
The Ionic Liquids market is influenced by a combination of scientific advancements, industrial demands, and environmental regulations:
Growing Demand for Green and Sustainable Chemistry: This is the most significant driver. Strict environmental regulations (e.g., REACH in Europe, EPA in the US) on VOC emissions and hazardous waste are pushing industries to adopt greener alternatives. Ionic liquids, with their low vapor pressure and potential for reusability, offer a compelling solution as environmentally benign solvents and reaction media.
Unique and Tunable Properties: The ability to tailor the properties of ionic liquids by combining different cations and anions makes them highly versatile for specific applications. This "designer" aspect allows for optimized performance in various chemical processes, separations, and material syntheses.
Increasing Applications in Energy Storage: The rapid growth of electric vehicles (EVs) and renewable energy solutions (solar, wind) is driving demand for advanced energy storage technologies. Ionic liquids are being increasingly explored and adopted as electrolytes in lithium-ion batteries, supercapacitors, and fuel cells due to their high ionic conductivity, wide electrochemical stability window, and non-flammability, which enhance safety and performance.
Rising Adoption in Pharmaceuticals and Biotechnology: In the pharmaceutical sector, ionic liquids are used as solvents for drug synthesis, as reaction media for enzymatic reactions (biocatalysis), for drug delivery systems, and even to improve the solubility and bioavailability of active pharmaceutical ingredients (APIs). Their non-volatility is particularly beneficial for these applications.
Advancements in Separation and Extraction Technologies: Ionic liquids are highly effective in various separation processes, including extractive distillation, liquid-liquid extraction, and gas absorption, due to their unique solvency properties. This is crucial in industries like petrochemicals, natural gas processing, and bio-refineries.
Spotting Emerging Trends
Technological Advancements:
The Ionic Liquids market is being significantly disrupted and shaped by several emerging technologies:
AI and Machine Learning in IL Design and Prediction:
High-Throughput Screening: AI/ML algorithms are being used to rapidly screen vast numbers of potential IL structures and predict their properties (e.g., viscosity, melting point, solubility, toxicity) without extensive experimental synthesis. This significantly accelerates the discovery and optimization of new ILs for specific applications.
Inverse Design: Leveraging AI to "design" ILs with desired properties, rather than just predicting properties for existing structures, revolutionizing the R&D process.
Continuous-Flow Synthesis and Green Production Methods:
Process Intensification: Development of continuous-flow reactors for IL synthesis, offering improved efficiency, scalability, safety, and reduced waste compared to traditional batch processes. This is crucial for bringing down production costs.
Recycling and Reusability Technologies: Advancements in robust and cost-effective methods for purifying and recycling ILs after use in industrial processes, further enhancing their sustainability and economic viability.
Green Synthesis Routes: Focus on synthesizing ILs using less hazardous raw materials and processes, aligning with broader green chemistry principles.
Hybrid Materials and Composites with ILs:
IL-Polymer Electrolytes: Development of solid or gel polymer electrolytes incorporating ILs for enhanced safety and performance in next-generation batteries (e.g., solid-state batteries).
IL-Metal Organic Frameworks (MOFs) and Nanomaterials: Creation of novel hybrid materials by incorporating ILs into porous structures like MOFs or onto nanomaterials to enhance their properties for gas capture, catalysis, or sensing.
Changing Consumer Preferences:
"Consumer preferences" in the Ionic Liquids market refer to the evolving demands and priorities of industrial clients, researchers, and end-users of ILs. These preferences have shifted in several key ways:
Strong Preference for "Green" and Sustainable Solutions:
The primary driver is the increasing pressure from environmental regulations and corporate sustainability initiatives. Customers are actively seeking alternatives to traditional VOCs and hazardous solvents. They prioritize ILs that are demonstrably less toxic, more biodegradable, and contribute to reduced waste and energy consumption in their processes.
Demand for Performance and Efficiency Gains:
Beyond "green," customers expect ILs to deliver superior performance compared to conventional alternatives. This includes improved reaction yields, higher selectivity in catalysis, better separation efficiency, enhanced battery performance (safety, cycle life, energy density), and increased process efficiency.
Focus on Cost-Effectiveness and Scalability:
While ILs offer performance benefits, the high cost has been a barrier. Customers are increasingly demanding more affordable ILs, and suppliers who can offer continuous production methods and economies of scale are gaining favor. They also look for ILs that can be easily scaled up for industrial production.
Reliability and Consistent Quality:
As ILs move from lab to industry, consistent quality and reliable supply are paramount. Customers need assurances about purity, batch-to-batch consistency, and long-term stability for their industrial processes.
Growth Opportunities of the Ionic Liquids Market
The Ionic Liquids market, despite its current niche status, holds immense growth opportunities driven by their unique properties and the global shift towards sustainable and high-performance solutions.
Accelerated Adoption in Green Chemistry and Sustainable Processes:
VOC Replacement: The most significant opportunity lies in the continued replacement of traditional VOCs in various industrial processes, including chemical synthesis, pharmaceuticals, and coatings, driven by ever-tightening environmental regulations globally.
Sustainable Separations: Expanding the use of ILs in more efficient and environmentally friendly separation and purification processes (e.g., CO2 capture, desulfurization of fuels, extraction of high-value compounds from biomass).
Biocatalysis and Biorefineries: Growth in using ILs as reaction media for enzymatic reactions and for the efficient dissolution and processing of lignocellulosic biomass for biofuels, biochemicals, and biomaterials.
Revolutionizing Energy Storage and Conversion:
Next-Generation Batteries: Significant opportunity in developing and commercializing ILs as advanced electrolytes for next-generation lithium-ion batteries (especially solid-state and lithium-sulfur batteries), providing enhanced safety (non-flammable), wider electrochemical windows, and improved cycle life. The booming EV market is a primary driver here.
Supercapacitors and Fuel Cells: Further penetration as highly stable and conductive electrolytes in high-performance supercapacitors and various types of fuel cells.
Solar Cells: Continued research and commercialization of ILs as components in more efficient and stable dye-sensitized solar cells (DSSCs) and other photovoltaic devices.
Get Sample Link - https://www.theinsightpartners.com/sample/ionic-liquids-market
Conclusion
The Ionic Liquids Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast Ionic Liquids 2023-2031 report provides much-needed insight for a company willing to set up its operations in the Ionic Liquids market. Since an in-depth analysis of competitive dynamics, the environment, and probable growth path are given in the report, a stakeholder can move ahead with fact-based decision-making in favor of market achievements and enhancement of business opportunities.
About The Insight Partners
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
0 notes
Text
Inorganic Salts | CP Lab Safety
Inorganic salts play a fundamental role in both industrial processes and biological systems. These compounds, formed by the neutralization of acids and bases, are essential to chemistry, agriculture, manufacturing, medicine, and various scientific fields. Their unique properties make them indispensable in a wide range of applications.

What Are Inorganic Salts?
Inorganic salts are chemical compounds made up of positively charged ions (cations) and negatively charged ions (anions), excluding carbon-hydrogen (C-H) bonds typically found in organic compounds. Common examples include sodium chloride (NaCl), calcium carbonate (CaCO₃), potassium nitrate (KNO₃), and magnesium sulfate (MgSO₄).
These salts are usually crystalline in structure, dissolve readily in water, and exhibit high melting points. They can be either simple salts, containing a single cation and anion, or complex salts involving multiple ions.
Properties of Inorganic Salts
The characteristics of inorganic salts can vary depending on their composition, but some general properties include:
High melting and boiling points Due to strong ionic bonds, inorganic salts typically have high thermal stability.
Solubility in water Most inorganic salts are water-soluble, making them effective in biological and industrial environments.
Electrical conductivity When dissolved in water or molten, these salts conduct electricity due to the presence of free ions.
Crystalline structure Inorganic salts generally form structured, repeating crystal lattices.
These properties contribute to their widespread use across multiple domains.
Types of Inorganic Salts
Inorganic salts can be broadly categorized into several types based on the nature of their ions:
Normal Salts Formed by complete neutralization of an acid and a base (e.g., NaCl, KNO₃).
Acid Salts Contain replaceable hydrogen atoms and are partially neutralized (e.g., NaHSO₄).
Basic Salts Contain hydroxyl groups (OH⁻) and are formed by partial neutralization of a base (e.g., Mg(OH)Cl).
Double Salts Contain two different salts crystallized together (e.g., alum – KAl(SO₄)₂·12H₂O).
Each type serves a specific purpose in both chemical and real-world applications.
Conclusion
Inorganic salts are essential to both everyday life and specialized industries, offering a broad spectrum of uses from healthcare to manufacturing. Their stable chemical structure, high solubility, and functional versatility make them a cornerstone of modern science and technology. Understanding their types, properties, and applications not only highlights their importance but also opens up innovative possibilities for future development.
0 notes
Text
Ocean to Capsule: Coral Calcium Powder Supplier in India Offering Clean Label Calcium Supplement Innovation

Caltron Clays & Chemicals: Your Partner in Clean Label Calcium Innovation
Caltron Clays & Chemicals stands at the forefront of the pharmaceutical raw material industry, setting the benchmark for clean label calcium supplement manufacturing in India. As a trusted Coral Calcium Powder manufacturer and supplier in India, Caltron brings a unique blend of innovation, sustainability, and global compliance.
Our flagship Coral Calcium Powder (MARIN-CAL) is specifically designed for nutraceutical and pharmaceutical brands committed to clean-label formulations. With superior absorption, trace mineral synergy, and sustainable sourcing, it’s the ultimate clean label calcium supplement trusted across continents.
The Science of Sustainability: Where Coral Meets Compliance
Caltron’s Coral Calcium Powder is harvested from fossilized, above-sea coral reefs – a naturally sustainable and non-invasive source. Unlike ocean-mined or synthetic calcium sources, this process ensures minimal environmental impact.
Each coral deposit contains more than 74 trace minerals that naturally support calcium uptake in the human body. From magnesium to zinc and boron, this powerful combination enhances bioavailability and ensures long-term skeletal health. Our coral calcium undergoes advanced beneficiation processes, resulting in 99.5% purity suitable for clean label compliance.
This natural, marine-based calcium carbonate stands as a top choice for clean label calcium supplements due to its ionic nature, sustainability, and easy assimilation.
Why Coral Calcium is the Most Bioavailable Calcium Raw Material
Not all calcium supplements are created equal. Many common forms of calcium such as limestone-based or synthetic compounds offer low absorption, irritate the gastrointestinal tract, or carry contamination risks.
Caltron’s Coral Calcium Powder solves these issues through:
Ionic Absorption: Activates upon contact with moisture, improving bioavailability.
Porous Structure: Ensures superior solubility and dispersibility in formulations.
Trace Mineral Support: Enhances uptake and metabolism through natural co-factors.
These properties make Coral Calcium ideal for clean label calcium supplements targeting pregnancy care, pediatric nutrition, sports recovery, and geriatric bone health.
Coral Calcium Applications: Delivering More Than Bone Health
Caltron’s Coral Calcium Powder is more than just a bone-building mineral. It finds broad application across multiple health sectors:
Prenatal Support: Promotes fetal development and maternal bone preservation.
Child Growth Formulas: Aids skeletal and dental growth in developing children.
Senior Nutrition: Prevents bone demineralization and age-related mobility issues.
Athletic Recovery: Helps in muscle contraction, inflammation reduction, and bone strength.
General Wellness: Improves calcium balance for overall metabolic functions.
As a clean label calcium supplement ingredient, it meets both consumer expectations and clinical performance standards.
Clean Label Compliance: Regulatory-Ready Calcium for Global Brands
Clean label calcium supplements demand more than just purity. Regulatory compliance is critical, and Caltron’s Coral Calcium Powder is fully equipped to meet international guidelines:
IP/BP/USP grade certifications
Batch-specific Certificates of Analysis (COA)
Heavy metal testing (lead, arsenic, cadmium, mercury)
Non-GMO and allergen-free declarations
ISO, GMP, and FSSAI approvals
Whether you're exporting to the EU, ASEAN, GCC, or US markets, Caltron provides full documentation to support global distribution of your clean label calcium formulations.
Why Caltron Coral Calcium is a Formulator’s Dream Ingredient
Formulators around the world choose Caltron’s Coral Calcium because it:
Blends well with other nutrients
Supports multiple dosage forms
Offers high flowability and neutral taste
Provides a natural calcium source without additives
Ideal for:
Tablets and capsules
Chewables and gummies
Effervescent powders
Fortified beverages
Functional foods
Every format benefits from the clean label status, ease of integration, and consumer acceptability that Caltron’s Coral Calcium delivers.
The Caltron Commitment: Quality Beyond Expectation
Caltron doesn’t just supply a product. We offer partnership, technical support, and quality assurance that goes beyond expectations. Our Coral Calcium Powder is crafted through meticulous control of sourcing, particle sizing, purification, and compliance.
We offer:
Custom mesh sizes for specific applications
Private label bulk supply for nutraceutical companies
Technical consultation for R&D teams
Regulatory guidance for clean label approvals
By offering complete traceability and sustainable sourcing, Caltron ensures your brand meets the highest standards of clean label calcium supplement development.
Serving Global Clients: Why Top Brands Choose Caltron
From leading contract manufacturers to boutique health brands, Caltron’s Coral Calcium Powder is trusted in over 40 countries. Our clients rely on us for:
Consistent, high-purity product availability
Customizable solutions for niche markets
Clean-label innovation for competitive advantage
Whether you're building a children’s bone health product in Europe, a postnatal care capsule in the UAE, or an effervescent wellness drink in Southeast Asia, Caltron supports your clean label calcium journey.
Sustainable, Ethical, and Eco-Conscious: The Future of Mineral Sourcing
As the wellness industry grows more conscious, sustainability becomes more than a buzzword it’s a requirement. Caltron fulfills this by:
Sourcing from above-sea, non-living coral deposits
Avoiding marine disruption or reef destruction
Offering biodegradable packaging options (on request)
Choosing Caltron means choosing an ingredient that aligns with ESG values, environmental ethics, and consumer transparency a must-have for modern clean label calcium supplements.
The Competitive Edge: Caltron vs Traditional Calcium Sources
When brands switch from synthetic or rock-derived calcium to Caltron’s Coral Calcium, they notice immediate improvements in:
Digestive comfort for end-users
Bioavailability and calcium retention rates
Product marketing appeal due to clean-label status
Regulatory approvals and market expansion
With rising awareness around label transparency, consumers now expect proof of natural origin, ethical sourcing, and scientific credibility. Caltron checks every box.
Partnering with Caltron: Empowering Your Clean Label Innovation
Whether you’re a startup formulator or an established pharmaceutical exporter, Caltron is here to support your journey toward clean label excellence. We offer:
Free R&D samples and technical data
Bulk and small-scale supply options
24/7 support for compliance documentation
Long-term partnerships built on trust
With our Coral Calcium Powder, your clean label calcium supplement line can meet rising global demands while staying cost-effective, efficacious, and consumer-friendly.
Experience the Caltron Advantage
Looking to future-proof your product lineup with a clean label calcium ingredient that performs? Caltron Clays & Chemicals invites you to explore our Coral Calcium Powder and discover the difference that clean, sustainable, and science-backed calcium can make.
Website: https://caltronclays.com/ Office: 210 & 211, Level 2, Orbit Premises, Mind Space, Chincholi, Malad (West), Mumbai – 400064, India Email: [email protected] | [email protected] Phone: +91 22 2876 4864 / +91-22-3571 9844 / +91-22-4010 6828 Google Maps: https://maps.app.goo.gl/QM61u5JtgAZErpXy7
Caltron Clays & Chemicals – Empowering Clean Label Calcium Supplement Brands with Purity, Performance, and Purpose.
0 notes
Text
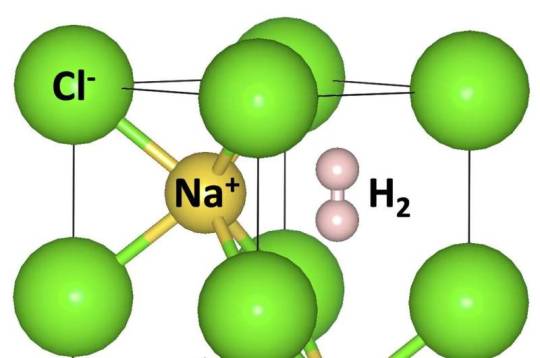
A universal insertion of various molecules into ionic crystals under high pressure
An international team has revealed a surprising universal propensity of forming stable hybrid compounds under high pressures. The hybrid materials, which consist of inorganic components and small molecules (SM), have gained intensive attention owing to their unique chemical structure, physical properties, and potential applications. However, these unique characteristics also impose challenges on material synthesis, characterization, and the fundamental understanding of their chemical behavior. High pressure has proven to be a powerful tool for synthesizing new materials. Under these conditions, the chemical properties of elements and the strengths of the homonuclear and heteronuclear bonds can change drastically, leading to the formation of many atypical compounds with non-intuitive compositions and structures.
Read more.
8 notes
·
View notes
Text
Polyacrylamide Market Growth Analysis, Market Dynamics, Key Players and Innovations, Outlook and Forecast 2025-2032
According to new market research, the global polyacrylamide market was valued at US$ 6,133.60 million in 2024 and is projected to reach US$ 9,452.46 million by 2031, growing at a Compound Annual Growth Rate (CAGR) of 5.47% during the forecast period (2025-2031). This growth is driven by expanding applications in water treatment, oil recovery, and mining sectors, coupled with tightening environmental regulations worldwide.
What is Polyacrylamide?
Polyacrylamide (PAM) is a versatile water-soluble polymer synthesized from acrylamide monomers. Available in anionic, cationic, and non-ionic forms, PAM serves as an essential chemical across industries due to its superior flocculation, viscosity modification, and water retention properties. Its major applications include:
Water treatment - Removing suspended solids and clarifying wastewater
Enhanced oil recovery - Improving oil displacement efficiency
Paper manufacturing - Enhancing fiber retention and drainage
Mining operations - Sediment control and tailings management
Key Market Drivers
1. Surging Demand for Water Treatment Solutions
The global push for clean water access and stricter wastewater discharge regulations are propelling PAM demand. Municipalities and industries are investing heavily in treatment infrastructure, with PAM-based flocculants being particularly effective at removing contaminants. 52% of PAM consumed in 2024 was for water treatment applications.
2. Expanding Applications in Energy Sector
The oil industry's focus on enhanced oil recovery (EOR) from mature fields has created significant opportunities. Polyacrylamide polymers improve sweep efficiency by increasing water viscosity, potentially recovering 20-30% more oil from reservoirs.
Market Challenges
While promising, the market faces hurdles including:
Acrylamide toxicity concerns - Residual monomers pose health risks, requiring strict quality control
Raw material volatility - Fluctuating propylene and acrylonitrile prices impact production costs
Bio-based alternatives - Emerging eco-friendly substitutes may disrupt traditional PAM markets
Growth Opportunities
Future opportunities center around:
Asia-Pacific expansion - Rapid industrialization in China and India driving 60% of new demand
Modified PAM formulations - Temperature-resistant and high-salinity tolerant variants for extreme conditions
Mining sector adoption - Increasing use in mineral processing and tailings management
Regional Market Insights
Asia-Pacific dominates with 52% market share in 2024, led by China's massive industrial and municipal water treatment projects.
North America accounts for 20% share, with strong EOR adoption in US shale plays and Canada's oil sands.
Europe shows steady growth driven by stringent environmental regulations and wastewater directives.
Latin America and MEA are emerging markets with expanding mining and oilfield applications.
Competitive Landscape
The market features global chemical leaders and regional specialists:
SNF Group maintains market leadership with comprehensive PAM product lines
PetroChina Daqing and Kemira offer strong regional presence in Asia and Europe respectively
Recent developments include SNF's 2024 expansion of Australian production facilities and BASF's launch of eco-friendly PAM variants
Market Segmentation
By Type:
Non-Ionic Polyacrylamide (PAMN)
Anionic Polyacrylamide (APAM)
Cationic Polyacrylamide (CPAM)
Others
By Application:
Water Treatment
Paper & Pulp
Oil & Gas Extraction
Paints & Coating
Others
By End Use Industry:
Municipal
Industrial
Oilfield
Agriculture
Mining
Others
By Region:
North America, Europe, Asia, Latin America, Middle East & Africa
Report Scope & Offerings
This comprehensive analysis provides:
2024-2031 market size and forecasts with 5.47% CAGR projection
Competitive analysis of 13+ key players including SNF, BASF, and Kemira
SWOT and value chain analysis highlighting industry dynamics
Segmentation insights across types, applications and regions
Download FREE Sample Report: Polyacrylamide Market - View in Detailed Research Report
About Intel Market Research
Intel Market Research delivers actionable insights in technology and infrastructure markets. Our data-driven analysis leverages:
Real-time infrastructure monitoring
Techno-economic feasibility studies
Competitive intelligence across 100+ countries Trusted by Fortune 500 firms, we empower strategic decisions with precision. Website: https://www.intelmarketresearch.com
Follow us on LinkedIn: https://www.linkedin.com/company/intel-market-research https://sites.google.com/view/intel-market-research/home/settlement-sensors-market-2025_1 https://sites.google.com/view/intel-market-research/home/valve-lubricant-market-2025 https://sites.google.com/view/intel-market-research/home/strobilurin-fungicide-market-2025 https://sites.google.com/view/intel-market-research/home/pilot-aviation-headset-market-2025 https://sites.google.com/view/intel-market-research/home/silver-paste-market-growth-analysis-market-2025 https://sites.google.com/view/intel-market-research/home/surgical-sponge-counting-detection-system-market-2025 https://sites.google.com/view/intel-market-research/home/zirconiumoxychloride-market-2025 https://sites.google.com/view/intel-market-research/home/edible-coffee-cup-market-2025 https://sites.google.com/view/intel-market-research/home/pentanediamine-market-2025-2032 https://sites.google.com/view/intel-market-research/home/thermal-expansion-tank-for-heating-market-2025 https://sites.google.com/view/intel-market-research/home/titanium-recycling-market-growth-analysis-market-dynamics-2025
0 notes
Text
FirstSeal India: Leading Potting Adhesive Manufacturer in Gujarat
What Is Potting Adhesive?
Potting refers to the process of encasing electronic components or entire assemblies in resin or adhesive to protect them from moisture, vibration, chemicals, and thermal stress. Common potting compounds include epoxies, silicones, polyurethanes (PU), and polyacrylates—each chosen for their unique balance of flexibility, strength, thermal and electrical properties.
Why FirstSeal India?
Strategic Gujarat location: Gujarat hosts several major chemical players—such as Atul Limited and Gujarat Alkalies—which enables secure access to quality raw materials.
Technical expertise: Our PU potting formulations—comprising polyols and isocyanates—are engineered to provide optimal flexibility, impact resistance, and moisture protection.
Wide product range: From rigid epoxies to flexible silicones and PU compounds, we meet diverse client needs—from vibration-sensitive sensors to high-voltage encapsulations.
Key Properties of FirstSeal Potting Adhesives
Mechanical & shock resistance: Ensures protection against vibrations and impact in harsh environments.
Superior electrical insulation: High dielectric strength for safe, reliable operation in electronics.
Thermal stability & low exotherm: Formulations are designed to cure with minimal heat generation, reducing thermal stress on components.
Moisture, chemical & corrosion resistance: Protects against environmental aggressors and prevents ionic contamination.
Compliance & safety: Formulations align with RoHS, REACH, UL94 V‑0, and other electronics industry standards.
Typical Applications
Consumer electronics (smart home, wearables): Flexible PU potting ensures durability under drops and moisture.
Automotive systems (sensors, controllers): Robust compounds resist chemicals and vibrations.
Industrial electronics (power supplies, control modules): Rigid epoxies protect in high-temperature, high-load environments.
Renewables & power electronics (solar inverters, power converters): Specialized potting compounds improve heat dissipation and longevity.
How We Work
Requirement analysis: We assess your device’s electrical, mechanical, and thermal needs.
Formulation selection: Choose the optimal chemistry—epoxy, PU, silicone—based on required flexibility and cure profile.
Pilot & testing: Trial batches are cured, inspected, and tested for insulation, adhesion, and mechanical resilience.
Scale production & QA: Certified facilities in Gujarat deliver consistent batches with full traceability.
Technical support: We assist with application methods (manual, cast-mold, dosing) and curing protocols for best performance.
Why Partner with FirstSeal India?
Local chemical infrastructure in Gujarat ensures consistent supply and faster lead times.
Customizable solutions: Tailored formulations to meet volume, flexibility, cure speed, and environmental resistance.
Regulatory compliance: Adherence to global electronic standards gives peace of mind.
End-to-end support: From formulation development to field support—with scaling and logistics covered.
In Summary
FirstSeal India’s potting adhesives—designed and manufactured in Gujarat—deliver precision, protection, and reliability across electronics, automotive, industrial, and renewable sectors. Whether you need tough epoxy for heavy-duty applications or flexible PU for shock-prone environments, our solutions are engineered to perform and comply.
Ready to pot your next project with confidence? Contact us today for sample requests, technical datasheets, and custom formulation support!
0 notes
Text
AI-Powered Breakthrough Accelerates Safer, Longer-Lasting Solid-State Batteries for EVs

What if electric vehicles could travel 50% farther on a single charge and eliminate the risk of battery fires?
Solid-state lithium-ion batteries are poised to make this a reality, but the search for the right materials has been a major bottleneck—until now. Recent R&D breakthroughs are leveraging machine learning to accelerate the discovery of advanced battery materials, bringing us closer to a safer, more efficient future for energy storage.
Researchers from Skoltech and the AIRI Institute have demonstrated how neural networks can transform the search for solid-state battery materials. Unlike conventional lithium-ion batteries, which use liquid electrolytes, solid-state batteries rely on solid electrolytes that must balance high ionic conductivity with chemical and structural stability. The challenge: none of the existing solid electrolytes meet all technical requirements for electric vehicle adoption.
Here’s a detailed look at the R&D innovation:
🔹Graph Neural Networks for Rapid Discovery: The team showed that graph neural networks can identify new solid-state battery materials with high ionic mobility at speeds orders of magnitude faster than traditional quantum chemistry methods. This approach drastically reduces the time needed to find viable materials.
🔹Protective Coating Prediction: The research didn’t stop at electrolytes. The team also focused on protective coatings, which are crucial for battery stability. The metallic lithium anode and the cathode are highly reactive, often degrading the electrolyte and causing performance loss or even short circuits. By using machine learning, the researchers predicted coatings that remain stable in contact with both the anode and cathode, enhancing battery longevity.
🔹Machine Learning-Accelerated Screening: The algorithms rapidly calculated ionic conductivity—a computationally intensive property—enabling efficient screening of tens of thousands of candidate materials. This staged process gradually narrowed the field to a select few with optimal properties.
🔹Promising Material Discoveries: The team’s approach led to the identification of several promising coating compounds, including Li₃AlF₆ and Li₂ZnCl₄, which can protect one of the most promising solid-state battery electrolytes, Li₁₀GeP₂S₁₂.
🔹Comprehensive Property Evaluation: For protective coatings, the researchers screened for thermodynamic stability, electronic conductivity, electrochemical stability, compatibility with electrodes and electrolytes, and ionic conductivity, ensuring only the best candidates advanced.
This research marks a significant step toward commercializing solid-state batteries, promising safer, longer-range electric vehicles and more reliable portable electronics. How do you see AI-driven material discovery shaping the future of clean energy and mobility? Join the discussion!
#SolidStateBatteries #NeuralNetworks #BatteryResearch #ElectricVehicles #MachineLearning #MaterialsScience #CleanTech
0 notes
Text

Ceramic PCB Special PCB with Big Function and Use made by Hitech Circuits
We are a professional ceramic pcb manufacturer, supplier from China, we mainly supply high quality Alumina (Al2O3) Ceramic PCB, Aluminum Nitride (AIN) Ceramic PCB board and IGBT Ceramic PCB. Our ceramic printed circuit boards features of high pressure, high insulation, high temperature, and high reliable and minor volume electronic products, Hitech is your best choice for ceramic PCB boards and needs.
Pls send PCB files to [email protected] to get a quote now!
What is ceramic pcb board
Ceramic pcb boards are actually made of electronic ceramics as the basic material and can be made in various shapes. Among them, the characteristics of high temperature resistance and high electrical insulation of ceramic circuit boards are the most prominent. The advantages of low dielectric constant and dielectric loss, high thermal conductivity, good chemical stability, and similar thermal expansion coefficient to components are also very significant.
Different types of ceramic pcb
Ceramic PCB is widely used in power electronics, electronic packaging, hybrid microelectronics and multi-chip modules due to its excellent thermal conductivity and air tightness. But not everyone is clear about the classification. Many manufacturers think ceramic PCBs are expensive and fragile as soon as they hear about ceramic PCBs. Yes, this is indeed a shortcoming of ceramic PCBs, but not all ceramic PCBs are like this. Today we will tell you about the different types of ceramic PCBs.
Al2O3 ceramic PCB
Al2O3 ceramic PCB ( alumina ceramic PCB) refers to various ceramic PCBs with Al2O3 as the main raw material and an Al2O3 content of more than 75%. It has a rich source of raw materials, with advantages of low price, high mechanical strength and hardness, good insulation performance, good heat shock resistance, good chemical resistance, high dimensional accuracy, and good adhesion to metals. It is a ceramic substrate material with good comprehensive performance. Currently used Al2O3 ceramic substrates, the content of Al2O3 accounts for 85% to 99.5%. Among them, 96% Al2O3 ceramic PCB is widely used in the production of thick film circuit substrates and chip devices. The thermal conductivity of Al2O3 at room temperature is 29W/(m·K), which is close to the thermal conductivity of steel; with the increase of Al2O3 content, the electrical insulation performance and thermal conductivity of Al2O3 ceramic PCB will increase, but At the same time, it will also lead to an increase in the firing temperature, an increase in energy consumption, a large loss of kiln furniture, and an increase in manufacturing costs.
SiC ceramic PCB
The thermal conductivity of SiC ceramic PCB is very high, 100~490W/(m·K) at room temperature, and it is related to the purity of SiC crystals. The higher the purity, the greater the thermal conductivity; the oxidation resistance is good, and the decomposition temperature is above 2500℃, it can still be used at 1600℃ in an oxidizing atmosphere; the coefficient of thermal expansion is also low, and it is close to Si, with good electrical insulation performance; SiC has a Mohs hardness of 9.75, second only to diamond and cubic BN, and has high mechanical strength. SiC ceramics have strong covalent bond characteristics and are difficult to sinter. Usually, a small amount of boron or aluminum oxide is added as a sintering aid to increase the density. Experiments show that beryllium, boron, aluminum and their compounds are the most effective additives, which can make SiC ceramics denser than 98%.
BeO ceramic PCB
BeO has a brazine structure, in which oxygen ions are arranged in a hexagonal close-packed manner to form a hexagonal lattice. The general oxide is usually an ionic compound, but BeO has a strong covalent bond and an average molecular weight of only 12. Because of its good electrical properties, luminescence and photochemical properties, high mechanical strength, low dielectric loss, etc, it become one of the materials that people pay attention to.
AlN ceramic PCB
AlN ceramic PCB (aluminum nitride ceramic) is a new type of high thermal conductivity ceramic packaging material. It has been extensively studied in the 1990s and gradually developed. It is currently generally considered to be a promising electronic ceramic packaging PCB. AlN material has high thermal conductivity, excellent dielectric properties, high electrical insulation strength, stable chemical properties, strong corrosion resistance, and good mechanical properties. In particular, its thermal expansion coefficient matches with silicon, which makes it an ideal semiconductor packaging substrate materials and have been widely used in integrated circuits, microwave power devices, millimeter-wave packaging, high-temperature electronic packaging and other fields.
Ceramic PCB for IGBT Module
IGBT stands for insulated-gate bipolar transistor. It is a bipolar transistor with an insulated gate terminal. The IGBT combines, in a single device, a control input with a MOS structure and a bipolar power transistor that acts as an output switch. IGBTs Ceramic PCB are suitable for high-voltage, high-current applications. They are designed to drive high-power applications with a low-power input.
IGBT, or Insulated Gate Bipolar Transistor, is a BJT transistor with a MOS Gate, or we can say an IGBT module is the combination of a BJT and a MOS Gate. An IGBT chip is small in size, but it can control electrical energy transmission and achieve 100,000 times of current switch at ultra-high voltages of 650 million V in only 1 second.
IGBT modules have been applied in automotive, industrial, aerospace, consumer electronics, and many other industries for many years. But how to optimize the thermal dissipation of an IGBT package so the module can work at a higher power? If thermal can dissipate more quickly, the IGBT module can have more advanced applications. For this purpose, engineers are using ceramic PCBs for IGBT packaging.
Ceramic PCBs dissipate thermal from IGBT chip to the outer packaging
You may ask, how much thermal does an IGBT module generate when it works? It is equal to the heat generated by 100 electric furnaces. So much thermal has to be dissipated immediately from the IGBT chip and leads to the application of ceramic PCBs.
How does a ceramic PCB protect the IGBT module from the heat? In an IGBT module, a ceramic PCB is placed under the IGBT chip, or we can say that the chip is assembled on the ceramic circuit board. The ceramic PCB connects and supports the chip and dissipates thermal quickly from it to the outer packaging. In this way, the chip is protected from the influence of thermal.
Why Ceramic PCBs can be used for IGBT thermal dissipation
There are alumina (Al₂O₃) PCBs, aluminum nitride (AlN) PCBs, and silicon nitride (Si₃N₄) PCBs used for thermal dissipation of IGBT modules.
Why ceramic PCBs can dissipate thermal effectively for the IGBT module? Because ceramic materials have good properties of thermal dissipation and electrical insulation. Unlike aluminum substrate PCBs, ceramic PCBs do not use an insulation layer that hinders thermal dissipation. During the ceramic PCB manufacturing process, the copper-clad is directly bonded onto the ceramic substrate at high temperatures under high pressures. Then the circuit layer is manufactured by the photoresist coating method. When the circuit board is manufactured, the IGBT and other components are mounted on the board. Ceramic materials have ultra-high insulation and can withstand breakdown voltage up to 20KV/mm. The thermal conductivity of alumina PCBs is 15-35W/mK, aluminum nitride PCB 170-230W/mK, and silicon nitride PCB 80+W/mK. On the contrary, an aluminum PCB has thermal dissipation of only 1-12W/mK.
Usages and applications of ceramic printed circuit boards
Ceramic printed circuit boards have a wide range of applications and can be used in the LED field, solar panel components, high-power power semiconductor modules, semiconductor refrigerators, electronic heaters, power control circuits, power hybrid circuits, smart power components, high-frequency switching power supplies , solid state relays, automotive electronics, communications, aerospace and military electronic components.
Advantages of ceramic PCB boards
Higher thermal conductivity
More matching thermal expansion coefficient
Stronger and lower resistance metal film
The solderability of the substrate is good, and the use temperature is high
Good insulation
Low high frequency loss
High-density assembly is possible
It does not contain organic ingredients, is resistant to cosmic rays, has high reliability in aerospace and has a long service life
The copper layer does not contain an oxide layer and can be used for a long time in a reducing atmosphere
Disadvantages of ceramic pcb boards
1. Fragile This is one of the most important shortcomings. At present, only small-area ceramic printed circuit boards can be produced.
2. Expensive There are more and more requirements for electronic products. Ceramic circuit boards only meet the requirements of some relatively high-end products, and low-end products will not be used at all.
0 notes
Text
From Refractory to Paints: Diverse Applications Driving the China Clay Market
For decades, China clay, or kaolin, has been largely associated with traditional applications such as paper coating, ceramics, paints, and rubber manufacturing. These uses have defined the global kaolin market's commercial narrative. However, in recent years, a subtle but significant transformation has emerged in how this naturally occurring mineral is being used. Beyond its historical domains, high-purity China clay is carving a strategic niche in the world of advanced electronic ceramics, particularly in applications such as electric vehicle batteries, high-dielectric chip components, and thermal insulators for microelectronics. While seldom discussed in mainstream market reports, this intersection of kaolin with high-performance electronics is poised to redefine its value in the global materials economy.
𝐌𝐚𝐤𝐞 𝐈𝐧𝐟𝐨𝐫𝐦𝐞𝐝 𝐃𝐞𝐜𝐢𝐬𝐢𝐨𝐧𝐬 – 𝐀𝐜𝐜𝐞𝐬𝐬 𝐘𝐨𝐮𝐫 𝐒𝐚𝐦𝐩𝐥𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐈𝐧𝐬𝐭𝐚𝐧𝐭𝐥𝐲! https://www.futuremarketinsights.com/reports/sample/rep-gb-3502
From Coating to Conductivity: China Clay’s Hidden Material Science Advantage
Kaolin’s unique physical and chemical properties—such as fine particle size, high alumina content, low iron and alkali impurities, and excellent dielectric strength—make it particularly suited for advanced ceramic applications. These characteristics are essential in the production of ceramic substrates and dielectric materials, which are integral to high-capacitance multilayer ceramic capacitors (MLCCs), chip resistors, and insulating layers used in semiconductors and power electronics.
In the context of battery technology, kaolin is proving valuable in the development of solid-state electrolytes and ceramic separators, particularly for next-generation lithium-ion and sodium-ion batteries. Solid-state battery designs rely on ceramic materials with exceptional ionic conductivity and thermal stability—features that high-purity kaolin can support when processed into aluminosilicate compounds or used in ceramic-polymer composites. This shift is expanding kaolin’s market potential far beyond its legacy functions.
Real-World Applications: Emerging Use Cases Across Asia and Europe
Evidence of this transition can be observed in industrial sectors where high-performance ceramics are in demand. In Japan and South Korea, manufacturers of MLCCs and semiconductor substrates have begun sourcing ultra-refined kaolin grades from companies that specialize in low-impurity mineral processing. These grades are engineered to meet stringent standards of dielectric strength, whiteness, and thermal resistance—qualities necessary for reliable chip insulation.
In Europe, firms developing ceramic components for solid-state batteries are exploring kaolin-based feedstocks as an alternative to more expensive alumina and zirconia compounds. A notable example is found in Germany, where collaborative research between technical ceramics companies and university laboratories has led to pilot programs utilizing kaolin as a key input in ceramic electrolytes. These efforts are supported by EU funding aimed at fostering sustainable battery material supply chains, which in turn benefits the kaolin sector through increased demand for domestically or regionally sourced inputs.
𝐔𝐧𝐥𝐨𝐜𝐤 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐌𝐚𝐫𝐤𝐞𝐭 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐬 – 𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐭𝐡𝐞 𝐅𝐮𝐥𝐥 𝐑𝐞𝐩𝐨𝐫𝐭 𝐍𝐨𝐰: https://www.futuremarketinsights.com/reports/china-clay-market
Sourcing Challenges: The Demand for Ultra-High Purity Kaolin
Not all kaolin deposits are suitable for electronic-grade applications. The purity threshold for kaolin used in electronic ceramics is significantly higher than for common industrial uses. Contaminants like iron oxide, titanium dioxide, and other transition metals must be kept at ultra-low levels to ensure optimal dielectric behavior and color performance. This imposes strict requirements on both mining practices and downstream processing, including advanced beneficiation, flotation, and calcination technologies.
Regions like the U.S. state of Georgia, parts of Brazil, and certain provinces in China have established themselves as sources of high-purity kaolin. However, geopolitical factors, environmental regulations, and local opposition to new mining operations have constrained expansion in some areas. As a result, electronic ceramics manufacturers are looking toward synthetic kaolin production and more sustainable refining techniques to meet growing demand without incurring environmental penalties or supply instability.
Market Outlook: Kaolin as a Tech-Driven Growth Material
The market for kaolin in electronic ceramics is still small relative to its use in paper and coatings, but it is growing rapidly. Analysts tracking high-purity kaolin trends are forecasting a compound annual growth rate significantly above the average for the broader kaolin market, primarily due to the anticipated expansion of the electric vehicle and 5G electronics industries.
Solid-state battery manufacturers are investing in new material platforms, and ceramic insulators are a key component in managing heat dissipation in compact electronic devices. In both sectors, kaolin’s performance characteristics offer a compelling case for its continued adoption. Furthermore, the global shift toward localized and secure supply chains for critical electronic components is driving interest in non-metallic mineral sources that can be domestically refined with minimal geopolitical risk.
A Competitive Edge: Kaolin Versus Alternative Fillers
Kaolin’s position in high-tech manufacturing is further strengthened by its cost-effectiveness and compatibility with green processing standards. Compared to alternative fillers like alumina and talc, kaolin requires less energy to process, especially when used in non-sintered applications. Additionally, because kaolin is naturally abundant and chemically stable, it offers long-term supply reliability without the environmental complications associated with more exotic materials.
In industries where lifecycle carbon footprint, resource localization, and circular economy principles are gaining traction, kaolin stands out as a mineral that offers both performance and sustainability. These factors are influencing procurement decisions among major electronics manufacturers, many of whom are revising their material sourcing strategies to align with ESG benchmarks.
Minerals & Ores Industry Analysis: https://www.futuremarketinsights.com/industry-analysis/minerals-and-ores
Redefining the Value of China Clay in a Tech-Centric World
The role of China clay is quietly evolving from that of a bulk industrial filler to a strategic raw material in the era of advanced electronics. Its applications in battery ceramics, microchip insulation, and high-performance electronics are expanding, supported by growing demand for high-purity, stable, and sustainable material inputs.
As manufacturers seek materials that meet the dual challenge of technical performance and environmental responsibility, kaolin is emerging as a reliable and versatile option. While still underrepresented in traditional market narratives, its significance in the supply chains of future technologies is clear. For investors, suppliers, and manufacturers, this creates a compelling reason to pay closer attention to a mineral long considered mundane but increasingly essential in the high-tech economy.
Key Segments
By Product Type:
Hydrous, Calcined, Delaminated, Surface-Modified
By End Use:
Paper & Packaging, Ceramics & Refractories, Paints & Coatings, Rubber & Plastics, Cosmetics & Personal Care, Pharmaceuticals, Agriculture & Fertilizers
By Colour:
White, Pink, Yellow/Red
By Region:
North America, Latin America, Western Europe, Eastern Europe, South Asia & Pacific, East Asia, Middle East & Africa
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries. Join us as we commemorate 10 years of delivering trusted market insights. Reflecting on a decade of achievements, we continue to lead with integrity, innovation, and expertise.
Contact Us:
Future Market Insights Inc. Christiana Corporate, 200 Continental Drive, Suite 401, Newark, Delaware - 19713, USA T: +1-347-918-3531 For Sales Enquiries: [email protected] Website: https://www.futuremarketinsights.com LinkedIn| Twitter| Blogs | YouTube
0 notes