#generic lenalidomide
Explore tagged Tumblr posts
Text
Generic Lenalidomide: A Game Changer in Affordable Cancer Treatment
Cancer treatment has come a long way in recent years, and medications like Lenalidomide have played a crucial role in improving the quality of life and survival rates for patients with serious conditions like multiple myeloma and other blood-related cancers. However, with the success of drugs like Lenalidomide comes a hefty price tag that can create significant financial burdens for patients and their families. This is where generic Lenalidomide comes into play, offering an affordable alternative without compromising on quality or efficacy.
In this article, we’ll explore everything you need to know about generic Lenalidomide, its effectiveness, safety, and how it provides a viable solution for patients in need of life-saving treatment without the staggering costs associated with brand-name versions like Revlimid.
What Is Lenalidomide?
Lenalidomide is an immunomodulatory drug (IMiD) that works by altering the body’s immune response, helping to kill cancer cells, reduce inflammation, and prevent tumors from forming new blood vessels. It has gained approval for treating several cancers, including:
Multiple Myeloma: A type of blood cancer that affects plasma cells in the bone marrow.
Myelodysplastic Syndromes (MDS): A group of conditions that cause the body to produce abnormal blood cells.
Mantle Cell Lymphoma (MCL): A rare type of non-Hodgkin lymphoma.
Lenalidomide has become a cornerstone in the treatment of these cancers, helping to extend the lives of thousands of patients worldwide. However, like many advanced cancer therapies, the high cost of the drug has made it difficult for many to afford long-term treatment, especially in countries where healthcare coverage may be limited.
The Need for Generic Lenalidomide
The high cost of brand-name Lenalidomide (Revlimid) has been a significant barrier for patients, with monthly treatments sometimes costing tens of thousands of dollars. For patients undergoing extended therapy, these costs quickly add up, often leaving families struggling to manage the financial burden.
In 2019, the patent for Revlimid began to expire in certain regions, opening the door for generic versions of the drug to enter the market. Generic drugs are just as effective and safe as their brand-name counterparts, but they are available at a fraction of the cost.
Generic Lenalidomide provides the same level of treatment but at a more accessible price, making it a game-changer for cancer patients who otherwise might not be able to afford the medication. The availability of a generic option allows more patients to continue with their prescribed treatment regimens, improving their chances of a better outcome.
How Does Generic Lenalidomide Work?
Generic Lenalidomide works in the same way as the brand-name version by modulating the immune system. The drug performs several functions that help in the fight against cancer:
Immune System Stimulation: It enhances the immune system’s ability to detect and destroy abnormal cancer cells.
Inhibition of Angiogenesis: The drug prevents the growth of new blood vessels that tumors need to expand, essentially starving the cancer cells.
Anti-inflammatory Action: It reduces inflammation, which can be a contributing factor in cancer growth.
Induces Apoptosis: Lenalidomide can induce cancer cells to undergo apoptosis, a form of programmed cell death.
These actions make Lenalidomide a powerful tool in cancer therapy, especially in treating blood cancers such as multiple myeloma and MDS. The generic version of the drug maintains these same therapeutic benefits, offering patients a more affordable way to continue their treatment.
The Cost Difference Between Generic and Brand-Name Lenalidomide
The most significant advantage of generic Lenalidomide over brand-name Revlimid is the cost savings. In general, generic drugs are priced much lower than their brand-name counterparts. This is because the manufacturers of generics do not need to invest heavily in research, development, or marketing, as the brand-name drug already did that work. Instead, they can focus on producing a bioequivalent version of the drug, meeting the same strict regulatory standards for safety and effectiveness.
For example, a month’s supply of brand-name Revlimid can cost upwards of $20,000, depending on the dosage and location. In contrast, generic versions of Lenalidomide can be priced 50-80% lower, potentially saving patients thousands of dollars per month. This significant cost reduction makes cancer treatment more accessible to patients who might not have been able to afford it otherwise.
Is Generic Lenalidomide as Effective as Revlimid?
One of the most common concerns patients have about generic medications is whether they are as effective as the brand-name versions. The short answer is yes.
Generic Lenalidomide must meet the same stringent standards set by regulatory bodies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). These organizations require that generic medications be bioequivalent to the original drug, meaning that they must:
Contain the same active ingredient in the same dosage.
Be administered in the same form (e.g., oral capsules).
Provide the same therapeutic benefits.
Bioequivalence ensures that the generic drug works just as well as the brand-name version, with no difference in safety, efficacy, or quality. This means that patients who switch from Revlimid to generic Lenalidomide can expect the same results in their treatment.
How to Access Generic Lenalidomide
With the increasing availability of generic Lenalidomide, patients now have more options to manage the financial aspects of their cancer treatment. There are several ways to access the drug at an affordable price:
1. International Pharmacies
Many patients turn to international pharmacies to access lower-priced generic medications. Countries like Canada and India often offer medications at a fraction of the cost compared to the U.S. Patients should ensure that they are purchasing from reputable, licensed pharmacies to guarantee the quality and safety of their medication.
2. Patient Assistance Programs
Many pharmaceutical companies and non-profit organizations offer patient assistance programs (PAPs) that provide discounts or free medications to eligible patients. These programs can help offset the cost of generic Lenalidomide for patients who meet specific financial criteria.
3. Insurance Coverage
In some cases, health insurance plans may cover the cost of generic Lenalidomide, either fully or partially. Patients should check with their insurance providers to determine what level of coverage is available for generic cancer medications.
Safety and Side Effects of Generic Lenalidomide
Like all medications, generic Lenalidomide can cause side effects. The most common side effects include:
Fatigue
Diarrhea
Rash
Low blood cell counts
Nausea
Patients should consult their healthcare providers for guidance on managing side effects and report any unusual or severe symptoms. It’s essential to follow the prescribed dosage and treatment plan closely to ensure the best possible outcomes.
Conclusion: Why Generic Lenalidomide Is a Smart Choice
The availability of generic Lenalidomide represents a major breakthrough in making cancer treatment more accessible and affordable. Patients now have a cost-effective alternative to the high-priced brand-name Revlimid without sacrificing quality or efficacy. For those battling cancers like multiple myeloma or MDS, generic Lenalidomide offers a lifeline that can make all the difference in maintaining their health and quality of life.
Switching to generic Lenalidomide can significantly reduce the financial burden on patients and their families, allowing them to focus on recovery instead of worrying about exorbitant medication costs. For more information on how to access affordable Lenalidomide, visit Generic Lenalidomide.
This game-changing alternative offers hope and relief to countless cancer patients seeking both effective treatment and financial sustainability.
0 notes
Text
Buy Lenalid 10mg Lenalidomide Capsule Online in the Philippines
Lenalid 5 mg, Lenalid 10 mg, Lenalid 15 mg, and Lenalid 25 mg capsules Price Philippines – Affordable Cancer Treatment
Are you looking for Lenalidomide Capsules in the Philippines? LetsMeds offers Lenalidomide 5 mg, Lenalidomide 10 mg, Lenalidomide 15 mg, and Lenalidomide 25 mg capsules at wholesale prices.Lenalidomide capsule online price Philippines
Why Choose LetsMeds for Lenalidomide in the Philippines?
Affordable Pricing: Competitive costs for generic Lenalidomide capsules from trusted manufacturers like Natco.
Multiple Strengths Available: Choose from Lenalid 5, Lenalid 10, Lenalid 15, and Lenalid 25 mg capsules.
Fast Delivery: Reliable shipping to Manila, Quezon City, Makati, Cebu, Davao, and across the Philippines.
Trusted Quality: Genuine and effective cancer medications at competitive prices.
Seamless Online Ordering: Easily purchase Lenalidomide capsules online with quick customer support.
How Much Does Lenalidomide Cost in the Philippines?
The price of Lenalid 10 mg Lenalidomide capsules is affordable when ordered through LetsMeds. Whether you need Natco Greyish Capsule Lenalid 10 mg Lenalidomide or other brands, we ensure cost-effective solutions for your cancer treatment needs.
Order Now
Call/WhatsApp: +91-7428091874
Email: [email protected]
WeChat/Skype: @LetsMeds
Website: www.letsmeds.com
For more information on Lenalidomide, prices, and delivery in the Philippines, visit LetsMeds today!
#How much does lenalidomide cost?#lenalidomide price philippines#Natco Greyish Capsule Lenalid 10 Mg Lenalidomide#generic lenalidomide cost manila#buy_lenalidomide_capsules#generic_lenalidomide_capsules#lenalid_lenalidomide_capsules#lenalidomide_alternative_supplier#lenalidomide_brands_price#lenalidomide_capsules_online#natco_lenalid_supplier
0 notes
Text
Budget-Friendly Lenalidomide: Explore Generic Prices for Cost-Effective Treatment
Find affordable solutions for your medical needs with generic Lenalidomide. Explore cost-effective options for effective treatment, ensuring quality care without breaking the bank.
For more info visit our website https://www.lenalidomidecost.com/lenalidomide-price-in-usa.
0 notes
Text
From Diagnosis to Treatment: What Every Multiple Myeloma Patient Should Know
The diagnosis of multiple myeloma can be overwhelming and confusing; however, you can make informed decisions about your health journey by knowing more about this complex blood cancer. To overcome this challenge head-on, one must understand the symptoms, diagnosis, and treatment options of multiple myeloma.
What is Multiple Myeloma?
Multiple myeloma is a type of blood cancer that affects plasma cells in the bone marrow. Plasma cells play a crucial role in the immune system by producing antibodies that help the body fight infections. However, in multiple myeloma, these plasma cells become malignant and multiply uncontrollably, crowding out healthy blood cells in the bone marrow.
Its symptoms may include:
Fatigue
Easy bruising
Loss of appetite
Constipation
High calcium levels in their blood
Renal (kidney) problems
Anemia (low red blood cell count)
Bone damage and fractures
Multiple myeloma is staged to determine the extent and severity of the disease, which helps guide treatment decisions. The staging system typically includes three main stages:
Stage
Smoldering
Asymptomatic stage, where there are no symptoms or only very mild symptoms.
Blood tests might show abnormal levels of proteins or abnormal plasma cells in bone marrow, but not enough to cause symptoms or organ damage.
Stage I
Low levels of abnormal proteins in blood and/or urine.
Few abnormal plasma cells in bone marrow (less than 10%).
No signs of organ damage or related symptoms.
Stage II
Intermediate stage with moderately abnormal protein levels.
Moderate levels of abnormal plasma cells in bone marrow (between 10-30%).
May show specific features suggestive of potential organ damage.
Stage III
High levels of abnormal proteins in blood and/or urine.
High levels of abnormal plasma cells in bone marrow (more than 30%).
Increased likelihood of organ damage or related symptoms.
Diagnosis of Multiple Myeloma
The diagnosis of multiple myeloma involves a series of tests and examinations aimed at confirming the presence of the disease and determining its stage. Early detection plays a pivotal role in formulating an effective treatment plan. Here's an overview of the diagnostic process:
Physical Examination: Your healthcare provider begins by conducting a thorough physical examination, discussing your medical history, and noting any symptoms you might be experiencing, such as bone pain, fatigue, or recurrent infections.
Blood and Urine Tests: Blood tests are performed to check for abnormal levels of specific proteins, calcium, kidney function, and blood cell counts. Urine tests specifically look for abnormal proteins, known as Bence Jones proteins, that may indicate multiple myeloma.
Bone Marrow Biopsy: A bone marrow biopsy is a crucial diagnostic test. It involves extracting a small sample of bone marrow, often from the hip bone, using a needle. This sample is then examined under a microscope to check for the presence of abnormal plasma cells, which are characteristic of multiple myeloma. The biopsy helps confirm the diagnosis and provides information about the stage and aggressiveness of the disease.
Imaging Studies: X-rays, CT scans, MRIs, or PET scans may be performed to evaluate bone damage, identify lesions or fractures, and assess the extent of the disease in bones or other organs. These imaging tests help determine the stage of multiple myeloma and guide treatment decisions.
Other Tests: In some cases, additional tests like genetic studies, flow cytometry, or cytogenetic tests may be conducted to analyze the genetic makeup of the cancer cells. This information helps doctors understand the disease's behavior and may influence treatment choices.
Staging: Once multiple myeloma is diagnosed, it is staged to determine the extent and spread of the cancer. Staging involves categorizing the disease as smoldering, stage I, stage II, or stage III, based on factors like specific proteins, kidney function, bone damage, and the number of plasma cells in the bone marrow.
Treatment Options
Treatment can include chemotherapy, stem cell transplants, and targeted therapy. Patients need to receive regular check-ups and follow-ups with their healthcare providers. Early detection and proper treatment can help slow the progression of multiple myeloma and improve patients' quality of life.
Lenalid 25 (lenalidomide 25 mg capsules) is one of many medicines that can be used to kill cancer cells. This medication treats white blood cell cancer (multiple myeloma) and myelodysplastic syndrome.
Contrary to conventional chemotherapy, Lenalid lenalidomide 25 mg capsules belong to the category of immunomodulatory drugs (IMiDs). Its anticancer efficacy is achieved through dual mechanisms:
It strengthens your immune system against cancer cells by enhancing the activity of specific immune cells, such as T cells and natural killer (NK) cells.
Lenalid 25 also inhibits the formation of new blood vessels (angiogenesis), which is crucial for tumor growth and survival.
Besides this, Lenalidomide 25 mg capsules have a direct cytotoxic effect on cancer cells, inducing apoptosis (programmed cell death) and stopping the growth of cancer cells.
While Lenalid lenalidomide capsules 25 mg share some characteristics with traditional chemotherapy drugs regarding their impact on cancer cells, its mode of action is more targeted and immunomodulatory. You can purchase this anti-cancer drug online from The Lotus Biotech. This reputable pharmaceutical products wholesaler offers high-quality products at affordable prices.
#lenalid 25#lenalidomide 25 mg capsule#lenalidomide 25 mg india price#lenalidomide 25 mg price#lenalidomide 25 mg price in india#lenalidomide capsules 25 mg#lenalidomide 25 mg brands in india#lenalidomide brand name#buy lenalidomide online#lenalidomide cost in india#lenalidomide generic name#lenalidomide generic price"
0 notes
Text
Global Lenalidomide Market Size,Growth Rate,Industry Opportunities 2025-2031
According to our (Global Info Research) latest study, the global Lenalidomide market size was valued at US$ million in 2024 and is forecast to a readjusted size of USD million by 2031 with a CAGR of %during review period.
Lenalidomide, sold under the trade name Revlimid among others, is a medication used to treat multiple myeloma (MM) and myelodysplastic syndromes (MDS). For MM it is used after at least one other treatment and generally together with dexamethasone.
In China, Lenalidomide key players include Celgene, SL Pharma. In terms of product, 25 mg Capsules is the largest segment, with a share over 40%. And in terms of application, the largest application is Multiple myeloma (MM), followed by Myelodysplastic syndromes (MDS).
Our Lenalidomide Market report is a comprehensive study of the current state of the industry. It provides a thorough overview of the market landscape, covering factors such as market size, competitive landscape, key market trends, and opportunities for future growth. It also pinpoints the key players in the market, their strategies, and offerings.
The report offers an in-depth look into the current and future trends in Lenalidomide, making it an invaluable resource for businesses involved in the sector. This data will help companies make informed decisions on research and development, product design, and marketing strategies. It also provides insights into Lenalidomide’ cost structure, raw material sources, and production processes. Additionally, it offers an understanding of the regulations and policies that are likely to shape the future of the industry. In essence, our report can help you stay ahead of the curve and better capitalize on industry trends.
The research report encompasses the prevailing trends embraced by major manufacturers in the Lenalidomide Market, such as the adoption of innovative technologies, government investments in research and development, and a growing emphasis on sustainability. Moreover, our research team has furnished essential data to illuminate the manufacturer's role within the regional and global markets.
The research study includes profiles of leading companies operating in the Lenalidomide Market:
The report is structured into chapters, with an introductory executive summary providing historical and estimated global market figures. This section also highlights the segments and reasons behind their progression or decline during the forecast period. Our insightful Lenalidomide Market report incorporates Porter's five forces analysis and SWOT analysis to decipher the factors influencing consumer and supplier behavior.
Segmenting the Lenalidomide Market by application, type, service, technology, and region, each chapter offers an in-depth exploration of market nuances. This segment-based analysis provides readers with a closer look at market opportunities and threats while considering the political dynamics that may impact the market. Additionally, the report scrutinizes evolving regulatory scenarios to make precise investment projections, assesses the risks for new entrants, and gauges the intensity of competitive rivalry.
Lenalidomide Market by Type: 5 mg Capsules、10 mg Capsules、15 mg Capsules、25 mg Capsules
Lenalidomide Market by Application: Multiple myeloma (MM)、Myelodysplastic syndromes (MDS)
Key Profits for Industry Members and Stakeholders:
The report includes a plethora of information such as market dynamics scenario and opportunities during the forecast period. Which regulatory trends at corporate-level, business-level, and functional-level strategies. Which are the End-User technologies being used to capture new revenue streams in the near future. The competitive landscape comprises share of key players, new developments, and strategies in the last three years. One can increase a thorough grasp of market dynamics by looking at prices as well as the actions of producers and users. Comprehensive companies offering products, relevant financial information, recent developments, SWOT analysis, and strategies by these players. The content of the study subjects, includes a total of 15 chapters: Chapter 1, to describe Lenalidomide product scope, market overview, market estimation caveats and base year. Chapter 2, to profile the top manufacturers of Lenalidomide, with price, sales, revenue and global market share of Lenalidomide from 2020 to 2025. Chapter 3, the Lenalidomide competitive situation, sales quantity, revenue and global market share of top manufacturers are analyzed emphatically by landscape contrast. Chapter 4, the Lenalidomide breakdown data are shown at the regional level, to show the sales quantity, consumption value and growth by regions, from 2020 to 2031. Chapter 5 and 6, to segment the sales by Type and application, with sales market share and growth rate by type, application, from 2020 to 2031. Chapter 7, 8, 9, 10 and 11, to break the sales data at the country level, with sales quantity, consumption value and market share for key countries in the world, from 2020 to 2024.and Lenalidomide market forecast, by regions, type and application, with sales and revenue, from 2025 to 2031. Chapter 12, market dynamics, drivers, restraints, trends and Porters Five Forces analysis. Chapter 13, the key raw materials and key suppliers, and industry chain of Lenalidomide. Chapter 14 and 15, to describe Lenalidomide sales channel, distributors, customers, research findings and conclusion.
Global Info Research is a company that digs deep into global industry information to support enterprises with market strategies and in-depth market development analysis reports. We provides market information consulting services in the global region to support enterprise strategic planning and official information reporting, and focuses on customized research, management consulting, IPO consulting, industry chain research, database and top industry services. At the same time, Global Info Research is also a report publisher, a customer and an interest-based suppliers, and is trusted by more than 30,000 companies around the world. We will always carry out all aspects of our business with excellent expertise and experience.
0 notes
Text
"Global Elotuzumab Market Report: Trends, Forecast & Insights (2024-2034)"
The Elotuzumab Market: Trends, Challenges, and Future Prospects
Introduction
In the evolving landscape of oncology, targeted therapies are transforming the way we approach cancer treatment. Elotuzumab, an innovative monoclonal antibody, is making waves in the treatment of multiple myeloma, a malignancy of plasma cells. Known under the brand name Empliciti, Elotuzumab has shown significant promise in clinical settings, particularly when combined with other therapies. This blog will delve into the current state of the Elotuzumab market, exploring its growth drivers, challenges, and future outlook.
Request Sample PDF Copy:
https://wemarketresearch.com/reports/request-free-sample-pdf/elotuzumab-market/1494
What is Elotuzumab?
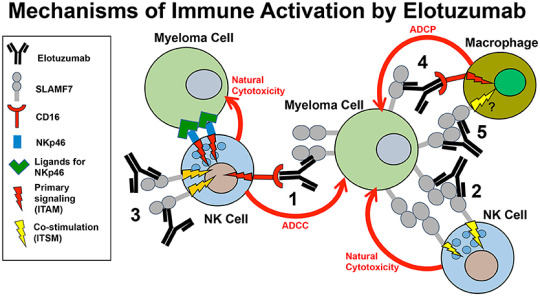
Elotuzumab is a monoclonal antibody designed to target and stimulate the immune system against cancer cells. Specifically, it binds to a protein called SLAMF7 (Signaling Lymphocytic Activation Molecule Family member 7) found on multiple myeloma cells and natural killer (NK) cells. By enhancing the immune system's ability to attack cancer cells, Elotuzumab helps improve outcomes in patients with multiple myeloma, particularly when used in combination with other drugs such as lenalidomide and dexamethasone.
Current Market Landscape
Elotuzumab Market Growth Drivers
Increasing Incidence of Multiple Myeloma: The rising prevalence of multiple myeloma, driven by an aging population and improved diagnostic techniques, is a major factor propelling the Elotuzumab market. With the global incidence of multiple myeloma on the rise, the demand for effective therapies like Elotuzumab continues to grow.
Advancements in Combination Therapies: Elotuzumab's efficacy is significantly enhanced when used in combination with other drugs, leading to better patient outcomes. This synergistic effect has made Elotuzumab a valuable component of combination regimens, contributing to its growing market adoption.
Improved Patient Outcomes: Clinical studies have demonstrated that Elotuzumab, in combination with standard treatments, can lead to prolonged progression-free survival and improved overall survival rates in multiple myeloma patients. These positive outcomes are driving its acceptance and usage in clinical practice.
Ongoing Research and Development: Research into expanding Elotuzumab's indications beyond multiple myeloma, including its potential use in other cancers and earlier stages of the disease, could open new market opportunities and broaden its therapeutic reach.
Elotuzumab Market Challenges
High Cost of Therapy: Elotuzumab is a high-cost medication, and its price can be a barrier to access for some patients. The high cost of treatment may impact market growth, particularly in regions with limited healthcare budgets.
Competition from Other Therapies: The multiple myeloma treatment landscape is highly competitive, with several other therapies and new entrants vying for market share. Elotuzumab faces competition from other monoclonal antibodies, proteasome inhibitors, and novel agents, which can affect its market positioning.
Side Effects and Safety Concerns: While generally well-tolerated, Elotuzumab can cause side effects such as infections and infusion-related reactions. Managing these side effects is crucial for patient adherence and overall treatment success, and any safety concerns can impact market acceptance.
Elotuzumab market Opportunities
Expansion into New Indications: Exploring new therapeutic indications and combinations could significantly expand the market for Elotuzumab. Ongoing research into its use in other hematological malignancies or solid tumors could provide new opportunities for growth.
Emerging Markets: As healthcare infrastructure improves in developing regions, there is potential for growth in these markets. Strategic partnerships and pricing strategies could enhance Elotuzumab’s reach and accessibility in these areas.
Innovative Combination Therapies: Developing and optimizing new combination therapies involving Elotuzumab could lead to better patient outcomes and increased market demand. Collaboration with other pharmaceutical companies and research institutions could drive innovation in this space.
Future Outlook
The Elotuzumab market is poised for continued growth, driven by increasing disease prevalence, advancements in combination therapies, and ongoing research and development. However, challenges such as high treatment costs and competition from other therapies will require strategic planning and adaptation.
Looking ahead, the future of Elotuzumab will likely involve efforts to expand its therapeutic indications, improve patient access, and navigate the competitive landscape. Continued innovation and research will be crucial in realizing the full potential of this promising therapy.
Conclusion
Elotuzumab Market has established itself as a significant player in the treatment of multiple myeloma, offering hope for improved patient outcomes through its unique mechanism of action. As the market evolves, addressing challenges and seizing emerging opportunities will be key to maximizing its impact. Stay tuned to our blog for the latest updates on the Elotuzumab market and other developments in the pharmaceutical industry.
#ElotuzumabMarket#Elotuzumab#MultipleMyelomaTreatment#MarketForecast#GlobalMarketAnalysis#HealthcareTrends
0 notes
Text
Innovations in Oncology Drugs 2025 Launch Preview

The oncology landscape is constantly evolving, with new therapies emerging to offer hope to patients battling various types of cancer. As research in cancer treatment continues to progress, 2025 is expected to bring some groundbreaking oncology drug launches that could transform the treatment paradigm. From novel targeted therapies to advanced immunotherapies, these upcoming drugs are poised to change the way oncologists approach cancer care. Here’s a look at the top 10 oncology drugs expected to launch in 2025.
1. Tisotumab Vedotin (Tivdak) for Ovarian Cancer
Tisotumab vedotin, developed by Seagen and Genmab, is an antibody-drug conjugate (ADC) that targets the tissue factor receptor, often overexpressed in ovarian cancer. After showing promising results in clinical trials, this therapy is anticipated to provide a new treatment option for patients with advanced ovarian cancer, especially those who have exhausted traditional chemotherapy options.
2. Pirtobrutinib (Loxo-305) for Relapsed/Refractory CLL
Pirtobrutinib is a next-generation Bruton's tyrosine kinase (BTK) inhibitor being developed by Loxo Oncology. With a unique mechanism of action that enables it to work in patients who have developed resistance to other BTK inhibitors, it is expected to become a valuable treatment option for chronic lymphocytic leukemia (CLL), particularly in the relapsed and refractory settings.
3. Niraparib (Zejula) for Early-Stage Ovarian Cancer
Niraparib, an oral PARP inhibitor from GSK, has already shown success in treating advanced ovarian cancer. The expected approval in 2025 for its use in earlier stages of ovarian cancer could significantly change the treatment landscape by providing a first-line option for patients with this aggressive disease, particularly in combination with chemotherapy.
4. Atezolizumab (Tecentriq) in Combination for Triple-Negative Breast Cancer
While Atezolizumab is already approved for some cancers, its use in combination with chemotherapy for triple-negative breast cancer (TNBC) is being closely watched. If approved for this indication in 2025, it could become a game-changer in the treatment of this difficult-to-treat cancer, providing a new hope for patients with limited treatment options.
5. Kymriah (tisagenlecleucel) for Solid Tumors
Approved for certain hematologic cancers, Kymriah from Novartis is now being studied in solid tumors. If its use expands to solid tumor cancers like ovarian or non-small cell lung cancer in 2025, it could open up a new frontier in immunotherapy by harnessing CAR-T cell technology to target and destroy cancer cells.
6. Lenalidomide (Revlimid) for Myelodysplastic Syndromes
Lenalidomide, a widely used drug in multiple myeloma and lymphoma, is being tested for its efficacy in myelodysplastic syndromes (MDS). This therapy is expected to receive regulatory approval in 2025, providing a new treatment avenue for MDS patients, a group that has limited therapeutic options.
7. Bemarituzumab (ABBV-181) for Gastric Cancer
Developed by AbbVie, Bemarituzumab is a promising monoclonal antibody targeting FGFR2b, which is implicated in the growth of gastric cancer cells. With strong clinical data suggesting its potential as a first-line treatment for advanced gastric cancer, it is poised to be one of the top oncology therapies to watch in 2025.
8. Magrolimab for Acute Myeloid Leukemia (AML)
Magrolimab, an anti-CD47 monoclonal antibody from Gilead Sciences, has shown potential in treating acute myeloid leukemia (AML). The drug works by blocking the “don’t eat me” signal that cancer cells use to evade the immune system. If approved for AML in 2025, it could become an essential part of the treatment landscape for this aggressive form of leukemia.
9. Selpercatinib (Retevmo) for RET-Altered Cancers
Selpercatinib, developed by Eli Lilly, is already approved for RET-altered non-small cell lung cancer (NSCLC) and medullary thyroid cancer. With expanding indications for RET-altered solid tumors, its broader application in cancers such as breast and colon cancer is expected to launch in 2025, offering more patients targeted therapy options based on their genetic mutations.
10. T-DXd (Trastuzumab Deruxtecan) for HER2-Positive Colorectal Cancer
T-DXd, developed by AstraZeneca and Daiichi Sankyo, is an ADC targeting HER2-positive cancers. Already approved for HER2-positive breast cancer, the upcoming approval for its use in HER2-positive colorectal cancer in 2025 is highly anticipated. It could provide a much-needed treatment option for patients with metastatic colorectal cancer who overexpress HER2.
Conclusion
The oncology landscape is set for a revolution in 2025 with these top 10 oncology drugs that are poised to transform cancer treatment across various indications. As research and innovation continue to advance, these new therapies bring hope to patients and healthcare providers, offering improved efficacy, reduced side effects, and the potential for better long-term outcomes. With the global demand for cancer therapies increasing, the year 2025 will undoubtedly be a pivotal moment in the battle against cancer.
Latest Healthcare Market Research Reports:
Trichomoniasis Market | Typhoid Fever Market | Venous Leg Ulcer Market | Adrenocortical Carcinoma Market | Anesthesia Workstation Machines Market | Bronchiectasis Market | Conductive Hearing Loss Market | Erythema Market | Homocystinuria Market | Idiopathic Interstitial Pneumonias Market | Metabolic Syndrome Market | Muscle Invasive Bladder Cancer Market | Myofascial Pain Syndrome Market | Opioid Use Disorder Market | Orthopedic Trauma Devices Market | Post-polycythemia Vera Myelofibrosis Market | Primary Open-angle Glaucoma Market | Seborrhea Market
#Oncology Drug#Oncology Drug Market#Oncology Drug Forecast#Oncology Drug Companies#Oncology Drug Therapies#Oncology Drug Epidemiology#Oncology Drug Pipeline#Oncology Drug Market Size#Oncology Drug Market Trends
0 notes
Text
Which is Better Pomabuzz 2 mg or Lenalidomide?
The choice between pomalidomide and lenalidomide depends on the patient’s specific condition and response to treatment. Both drugs belong to the same class of immunomodulatory agents and are used in the management of multiple myeloma. However, lenalidomide is typically used earlier in treatment, while pomalidomide, such as Pomabuzz 4 mg capsule from Chawla Medicos, is often prescribed for relapsed or refractory multiple myeloma after patients have become resistant to lenalidomide. Clinical studies suggest that pomalidomide can be more effective in these advanced cases, as it is a second-generation drug with enhanced potency. Ultimately, the decision should be made by a healthcare provider based on the patient’s unique health status and previous treatments. For high-quality pomalidomide options, trust Pomabuzz 4 mg capsule from Chawla Medicos.
#Pomabuzz 4 mg uses#pomabuzz 4 mg capsule#pomabuzz 4 mg cost#pomabuzz 4 mg side effetcs#Pomalidomide 4 mg#Pomalidomide 4 mg uses#Pomabuzz 4 mg price#Pomalidomide 4 mg capsule#Pomaidomide 4 mg cost
0 notes
Text
The Evolution of MDS Treatment: A Cross-Regional Examination of Advances in the U.S., Europe, and China
Myelodysplastic Syndrome (MDS) represents a diverse group of bone marrow disorders characterized by ineffective blood cell production and an increased risk of leukemia. As research and technology advance, treatment approaches for MDS have evolved significantly, reflecting regional differences in healthcare systems, available therapies, and patient needs. This blog explores the evolution of MDS treatment across the U.S., Europe, and China, highlighting key advancements and regional approaches that are shaping the future of care for this complex condition.
The Evolution of MDS Treatment: A Global Overview
The management of MDS has seen remarkable progress over the past few decades. Initially, treatment options were limited to supportive care and basic chemotherapy. However, the landscape has transformed with the development of targeted therapies, advancements in genetic understanding, and improved supportive care strategies. Understanding how these advancements are applied across different regions provides insight into the global approach to managing MDS.
Advances in the U.S.
In the United States, the approach to treating MDS has evolved through a combination of cutting-edge research, personalized medicine, and a focus on patient-centered care.
1. Personalized Risk Stratification
One of the most significant advancements in the U.S. has been the development of risk stratification systems such as the International Prognostic Scoring System (IPSS) and its updated versions. These tools categorize patients based on factors like genetic mutations, blood cell counts, and chromosomal abnormalities, guiding treatment decisions. The move towards personalized treatment plans allows for more tailored therapies, improving patient outcomes and minimizing unnecessary side effects.
2. Hypomethylating Agents
The introduction of hypomethylating agents, such as azacitidine and decitabine, has been a game-changer in U.S., Europe, and China Myelodysplastic Syndrome (MDS) Treatment. These drugs work by modifying DNA methylation patterns, which can lead to improved blood cell production and reduced disease progression. They are now a standard treatment for patients with intermediate to high-risk MDS, offering a significant improvement over traditional chemotherapy.
3. Targeted Therapies and Clinical Trials
The U.S. is at the forefront of developing and testing new therapies through extensive clinical trials. Advances in genetic research have led to the identification of specific mutations that drive MDS, resulting in targeted therapies designed to address these genetic abnormalities. For example, drugs like lenalidomide are used for patients with deletion 5q, a specific genetic abnormality associated with MDS.
4. Stem Cell Transplantation
Allogeneic stem cell transplantation remains one of the most promising treatment options for high-risk MDS patients. The U.S. has developed sophisticated transplantation techniques and supportive care measures to improve patient outcomes and reduce complications associated with the procedure. However, due to the high risk and complexity of stem cell transplants, this option is generally reserved for younger, healthier patients.
Advances in Europe
In Europe, the treatment of MDS has evolved with a focus on standardized guidelines, integration of new research, and access to innovative therapies. The European approach emphasizes both risk-based treatment and inclusion in clinical trials.
1. European LeukemiaNet (ELN) Guidelines
The ELN provides comprehensive guidelines for the diagnosis and treatment of MDS, which are widely adopted across European countries. These guidelines incorporate risk stratification tools similar to those used in the U.S. and offer a framework for managing MDS based on the latest research and clinical evidence.
2. Access to New Therapies
European countries have been proactive in incorporating new therapies into clinical practice. For example, hypomethylating agents and targeted therapies are widely used in Europe, with many countries following similar protocols to those in the U.S. However, variations in drug availability and healthcare policies can impact the accessibility of these treatments.
3. Clinical Trials and Research
Europe is known for its robust clinical trial network, which provides patients with access to innovative treatments that may not yet be available elsewhere. European researchers are also involved in international collaborations, contributing to the global understanding of MDS and the development of new therapies.
4. Supportive Care and Palliative Options
European healthcare systems place a strong emphasis on supportive care and palliative options for MDS patients. This includes a focus on managing symptoms, improving quality of life, and providing psychological support. This approach ensures that patients receive comprehensive care, addressing both the physical and emotional aspects of living with MDS.
Advances in China
China's approach to treating MDS reflects a blend of traditional practices and modern medical advancements. The rapid development of healthcare infrastructure and increasing investment in medical research are driving significant changes in MDS treatment in the region.
1. Improved Diagnosis and Access to Care
China has made strides in improving the diagnosis of MDS through enhanced awareness and better access to diagnostic tools. Efforts to increase awareness and improve early detection are critical in a country with a large and diverse population.
2. Integration of Western Medicine and Traditional Chinese Medicine (TCM)
In China, there is a growing interest in combining Western medical treatments with Traditional Chinese Medicine (TCM). Some patients use TCM practices such as herbal remedies and acupuncture alongside conventional therapies to manage symptoms and improve overall well-being. While TCM is not considered a cure for MDS, it offers complementary benefits that can enhance the patient’s quality of life.
3. Access to Modern Therapies
China has seen improvements in access to modern treatments like hypomethylating agents and targeted therapies. The availability of these drugs can vary depending on the region, but overall, there has been progress in making these advanced treatments more accessible to patients.
4. Stem Cell Transplantation and Research
China has a large stem cell donor registry and has made significant advancements in stem cell transplantation. This procedure is increasingly available to MDS patients, particularly those with high-risk disease. Additionally, China’s investment in research is contributing to the development of new therapies and treatment strategies for MDS.
Common Challenges and Future Directions
Despite advancements in MDS treatment across the U.S., Europe, and China, there are common challenges that need to be addressed globally:
Access to Care: Disparities in access to advanced treatments and supportive care remain a challenge, particularly in low-resource settings or rural areas. Efforts to improve access and equity in healthcare are essential for ensuring that all patients receive the care they need.
Cost of Treatments: The high cost of modern therapies, including hypomethylating agents and stem cell transplantation, can be a barrier to treatment for some patients. Addressing the cost of care and exploring ways to make treatments more affordable are important considerations.
Research and Innovation: Continued investment in research is crucial for developing new therapies and improving treatment outcomes. International collaboration and sharing of research findings can accelerate progress and lead to more effective treatments for MDS.
The treatment of Myelodysplastic Syndrome (MDS) has evolved significantly, with advancements driven by research, innovation, and regional healthcare practices. The U.S., Europe, and China each contribute unique approaches to managing MDS, reflecting their distinct healthcare systems and patient needs. As global research and collaboration continue to advance, the future of MDS treatment looks promising, with new therapies and strategies on the horizon. By learning from each region’s experiences and addressing common challenges, the global medical community can work towards improving outcomes and quality of life for individuals living with MDS.
Get more insights on U.S., Europe, And China Myelodysplastic Syndrome (MDS) Treatment
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights.
(LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )

#U.S.#Europe#And China Myelodysplastic Syndrome (MDS) Treatment#Lenalidomide#Decitabine#Azacitidine#Luspatercept#Chinese MDS Treatment#Global MDS Treatment
0 notes
Text
Velcade Market Size, Share, Industry Trends, and Forecast 2032
Introduction
Velcade, also known by its generic name bortezomib, is a proteasome inhibitor used primarily in the treatment of multiple myeloma and mantle cell lymphoma. Since its approval, Velcade has significantly impacted the oncology market, offering a promising therapeutic option for patients with these malignancies. The global Velcade market is characterized by its dynamic nature, driven by advancements in oncology research, increasing incidence of hematologic cancers, and a growing focus on personalized medicine.
Market Size and Growth Dynamics
Velcade Market Size was estimated at 1.76 (USD Billion) in 2023. The Velcade Market Industry is expected to grow from 1.83(USD Billion) in 2024 to 2.5 (USD Billion) by 2032. The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). This growth is fueled by the rising prevalence of multiple myeloma and other related cancers, which are increasingly being diagnosed due to advances in diagnostic technologies. Furthermore, the aging population, which is more susceptible to cancer, is also contributing to the expanding market.
North America holds the largest market share, attributed to the region's advanced healthcare infrastructure, high adoption rates of new therapies, and a strong focus on research and development. Europe follows closely, driven by similar factors. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, spurred by increasing healthcare expenditures, improving access to cancer treatments, and rising awareness about hematologic cancers.
Market Share Analysis
Velcade, originally developed by Millennium Pharmaceuticals and marketed by Takeda Oncology, has maintained a dominant position in the proteasome inhibitor segment. Its efficacy, safety profile, and first-mover advantage have contributed to its strong market share. However, with the expiration of key patents, the market has seen the entry of generic versions, which has led to increased competition and a shift in market dynamics.
The introduction of generics has made the treatment more accessible, particularly in developing regions, but it has also put pressure on the market share of branded Velcade. Despite this, the brand continues to hold a significant share due to its established presence and the trust it has garnered among healthcare professionals.
Industry Trends
Several key trends are shaping the Velcade market:
Rise of Combination Therapies: The use of Velcade in combination with other drugs, such as lenalidomide and dexamethasone, is becoming increasingly common. These combination therapies have shown improved efficacy and are becoming a standard of care in multiple myeloma treatment protocols.
Focus on Personalized Medicine: With the growing emphasis on personalized medicine, there is a trend towards tailoring treatments based on individual patient profiles, which includes genetic makeup and disease characteristics. This approach is expected to drive demand for Velcade as part of personalized treatment regimens.
Increased Research and Development: The oncology sector continues to see significant investment in research and development, leading to the discovery of new therapeutic targets and treatment options. While this fosters innovation, it also intensifies competition as new drugs enter the market.
Patent Expirations and Generic Competition: The expiration of Velcade’s patents has opened the market to generic competition, leading to reduced prices and increased accessibility. This trend is expected to continue, particularly in cost-sensitive markets.
Expanding Indications: Research is ongoing to explore the potential of Velcade in treating other cancers and conditions beyond multiple myeloma and mantle cell lymphoma. If successful, these efforts could lead to new indications, further driving market growth.
Forecast Through 2032
The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). Key drivers of this growth include the increasing global cancer burden, advancements in cancer therapy, and the expansion of healthcare infrastructure in emerging markets.
However, challenges such as the rising cost of cancer treatment, the availability of alternative therapies, and regulatory hurdles may pose risks to market expansion. Additionally, the shift towards biosimilars and generics is likely to impact the market dynamics, particularly in terms of pricing and market share distribution.
The forecast period is also expected to witness a greater emphasis on real-world evidence and outcome-based reimbursement models, which could influence the adoption of Velcade and its competitors.
Conclusion
The Velcade market is poised for significant growth in the coming years, driven by a combination of factors including rising cancer incidence, advancements in treatment protocols, and the expanding reach of healthcare services. While challenges such as generic competition and pricing pressures exist, the overall outlook remains positive, with opportunities for growth in emerging markets and through the development of new therapeutic indications. As the oncology landscape continues to evolve, Velcade is expected to remain a key player, contributing to improved outcomes for patients worldwide.
0 notes
Text
Advancement in Healthcare Industry: Myelodysplastic Syndrome Treatment Options

Myelodysplastic syndromes are a group of hematopoietic stem cell malignancies characterized by inefficient production of blood cells. The specific treatments for MDS are tailored based on the severity and subtype of disease, as well as the patient's overall health and preferences. A few options can be considered for managing MDS. Myelodysplastic Syndrome Treatment: Bone Marrow Transplantation Bone marrow transplantation, also known as hematopoietic stem cell transplantation, is the only potential cure for MDS. It involves destroying the abnormal bone marrow using high-dose chemotherapy and replacing it with healthy stem cells from another person, usually a relative or unrelated donor. Blood stem cells can also be collected from the donor's bloodstream. Candidates must be in relatively good health and have a matched donor. Due to donor availability and transplant-related risks, it is only considered for certain subtypes and higher-risk cases. Myelodysplastic Syndrome Treatment: Drug Therapy Several drugs can be used to treat low- and intermediate-risk MDS. These include: - Azacitidine (Vidaza) and decitabine (Dacogen): These are DNA methyltransferase inhibitors that help restore normal blood cell production. They are recommended as initial therapy for most intermediate-risk and selected low-risk MDS cases. - Lenalidomide (Revlimid): An immunomodulatory drug, lenalidomide alone or with azacitidine can benefit those with chromosome 5q deletions or ring sideroblasts. - Erythropoietin-stimulating agents: Drugs like epoetin alfa and darbepoetin alfa may improve anemia in low-risk cases with ring sideroblasts or specific chromosome abnormalities. - Immunosuppressive drugs: Cyclosporine and antithymocyte globulin can be tried in syndromes associated with previous cytopenias or medications. Myelodysplastic Syndrome Treatment: Supportive Care When a patient is not a transplant candidate or has lower-risk disease, supportive care focuses on managing complications through: - Blood transfusions: Regular red blood cell and platelet transfusions help control anemia and bleeding risks. - Antibiotics: Infection prophylaxis with antibiotics is commonly used due to impaired immunity from low white blood cell counts. - Growth factors: Granulocyte colony-stimulating factors may be prescribed to boost white cell production during infection recovery. Clinical Trials Clinical trials investigate new drug therapies and treatment strategies. Eligible patients should consider enrolling in trials exploring additional treatment options. Some promising MDS therapies in trials include: - Hypomethylating agents combined with lenalidomide or immune checkpoint inhibitors - Bcl-2 inhibitors targeting MDS stem cells - Monoclonal antibodies blocking cellular targets involved in leukemia development - Next-generation epigenetic drugs with novel mechanisms of action Careful monitoring under a hematologist is imperative for ongoing care, managing side effects from any treatment received, and determining if intervention is needed due to disease progression or complications. The optimal approach aims to improve blood counts and quality of life while minimizing risks.
#Myelodysplastic Syndrome Treatment Analysis#Myelodysplastic Syndrome Treatment Trend#Myelodysplastic Syndrome Treatment Demand.
0 notes
Text
0 notes
Text
Where to Buy Affordable Lenalidomide Capsules in the Philippines – LetsMeds is Your Trusted Source

LetsMeds provides affordable, high-quality Lenalidomide capsules for the treatment of multiple myeloma and related blood disorders, ensuring fast, reliable delivery across the Philippines, including cities like Manila, Cebu, and Davao, Among other locations, we provide medicines to the US, UK, Russia, Philippines, Singapore, Thailand, Vietnam, Hong Kong, Japan, South Korea, Poland, Australia, and Italy with a commitment to trusted service, competitive pricing, and convenient online ordering for patients in need of effective cancer care solutions.Order now for competitive prices and reliable service.call/Whatsapp: +91-7428091874 | Email:
#buy lenalidomide capsule philippines#lenalidomide price philippines#lenalidomide price Thailand#Generic lenalidomide capsule#Buy Lenalidomide capsules online#Where to buy Lenalidomide Thailand#Buy Revlimid alternative online#Lenalidomide for cancer treatment#Lenalidomide 25 mg price
0 notes
Text
LenalidomideCost Unveils Competitive Lenalidomide Generic Price, Empowering Patients with Affordable Access to Vital Medication
LenalidomideCost, a leading online pharmaceutical platform committed to enhancing healthcare accessibility, proudly announces the launch of a cost-effective Lenalidomide generic, catering to the growing needs of patients seeking affordable solutions for their medical treatment.
Lenalidomide, a crucial medication used in the treatment of various conditions, has become more accessible than ever with the introduction of LenalidomideCost's competitively priced generic alternative. The platform aims to address the financial challenges faced by patients while ensuring the highest quality and efficacy of the medication.

In response to the increasing demand for affordable healthcare options, LenalidomideCost has strategically positioned itself as a reliable source for patients seeking a budget-friendly yet reliable alternative. The Lenalidomide generic price available on the platform aligns with the mission of making essential medications accessible to all.
We understand the financial burden that healthcare expenses can impose on individuals and families. Our commitment is to bridge the gap between quality healthcare and affordability, stated Richy Rosatio, the spokesperson for LenalidomideCost. The introduction of our competitively priced Lenalidomide generic is a testament to our dedication to improving the lives of those in need. We believe that everyone should have access to effective medications without compromising on their financial well-being.

LenalidomideCost's Lenalidomide generic not only offers a cost-effective solution but also maintains the highest standards of quality, meeting regulatory requirements to ensure the safety and efficacy of the medication. By providing an affordable alternative, the platform aims to empower patients to prioritize their health without the added stress of exorbitant medical costs.
Patients can now experience the benefits of Lenalidomide without sacrificing financial stability. LenalidomideCost is committed to fostering a community where healthcare is accessible to all, irrespective of economic constraints.

The spokesperson further emphasized, "Our goal is to create a positive impact on the lives of patients by offering them affordable options for their healthcare needs. We believe that this initiative will not only benefit individuals directly but also contribute to a healthier and more resilient society."
LenalidomideCost invites individuals seeking a budget-friendly Lenalidomide generic to explore their website and discover the cost-effective solution that aligns with their healthcare needs.
0 notes
Text
Multiple Myeloma: Symptoms, Causes and Prognosis
Multiple Myeloma is a complex and challenging blood cancer that affects plasma cells, a type of white blood cell found in the bone marrow. Comprehending the condition's signs, causes, and prognosis is essential for patients and their loved ones. Furthermore, recent developments in medicine, including Lenalid 10mg Lenalidomide Capsules, have given people with this illness fresh hope.
Introduction
What is Multiple Myeloma?
Symptoms of Multiple Myeloma
Causes and Risk Factors
Prognosis and Staging
Lenalid 10mg Lenalidomide Capsules: A Breakthrough Treatment
Side Effects and Considerations
Patient Support and Lifestyle Considerations
Conclusion
What is Multiple Myeloma?
The malignancy known as multiple Myeloma affects the plasma cells, which are in charge of creating antibodies that aid the body in fighting infections. These malignant plasma cells can displace healthy blood cells in the bone marrow, which can result in several problems.
Symptoms of Multiple Myeloma
Identifying the symptoms of Multiple Myeloma early is essential for a timely diagnosis and course of treatment. Typical signs and symptoms include:
Bone Pain: One common early indication is persistent pain, usually in the back or ribs. The buildup of aberrant plasma cells in the bones is the cause of this.
Fatigue: Anemia brought on by multiple myeloma might result in weakness and exhaustion.
Frequent Infections: An increased vulnerability to infections can be the outcome of impaired immune function.
Kidney Issues: Myeloma cell-produced aberrant proteins have the potential to harm the kidneys and cause issues such as renal failure.
Anemia: Pale skin and dyspnea are two signs that may indicate a reduction in red blood cells.
Hypercalcemia: High blood calcium levels can result in symptoms such as frequent urination, intense thirst, and constipation.
Unexplained Weight Loss: Unintentionally losing weight quickly could be a sign of multiple Myeloma early on.
Causes and Risk Factors
Although the precise etiology of multiple Myeloma is unknown, there are several risk factors that may raise the illness's chance of developing:
Age: People over 65 are more likely to develop multiple myeloma.
Gender: The risk of developing multiple myeloma is slightly higher in men than in women.
Family History: A higher risk may apply to those with a family history of multiple Myeloma.
Certain Medical Conditions: Several illnesses, including monoclonal gammopathy of unknown significance (MGUS), may make it more likely for multiple Myeloma to develop.
Prognosis and Staging
The age of the patient, general health, and the stage at which the illness is discovered all affect the prognosis for Multiple Myeloma. Determining the disease's stage is essential for assessing its severity, and it is frequently categorized as:
Smoldering (Asymptomatic) Myeloma: Early stages, when there may not be any indications of the illness and no need for emergency care.
Stage I-III Myeloma: Gradual escalation of symptoms and problems in severity.
Relapsed or Refractory Myeloma: The illness could recur or develop treatment resistance.
Patient outcomes have been markedly improved by advances in treatment, such as immunomodulatory medications and targeted therapies.
Lenalid 10mg Lenalidomide Capsules: A Breakthrough Treatment
A highly promising advancement in treating Multiple Myeloma is the utilization of Lenalid 10mg Lenalidomide Capsules. This therapy, which is in the class of immunomodulatory medications, has demonstrated impressive success in treating the illness.
Mechanism of Action: The mechanism of action of Lenalidomide is to boost the immune system's capacity to combat and eradicate cancer cells. It also prevents aberrant cells in the bone marrow from proliferating.
Treatment in Newly Diagnosed Patients: Lenalidomide has shown promise in treating newly diagnosed Multiple Myeloma patients. It is frequently used in conjunction with other drugs. This combo strategy aims to produce more profound and long-lasting reactions.
Maintenance Therapy: Lenalidomide may occasionally be recommended as maintenance medication after the first treatment to extend durations of remission.
Relapsed or Refractory Cases: Lenalidomide offers an alternate therapeutic option for patients with resistant Multiple Myeloma or those who have had a relapse.
Reducing Bone Marrow Abnormalities: Improved results have been attributed to Lenalidomide's efficacious reduction of bone marrow abnormalities associated with Multiple Myeloma.
Side Effects and Considerations
Despite the fact that Lenalid 10mg Lenalidomide Capsules provide many advantages, it's essential to be aware of any possible adverse effects, such as:
Risk of Blood Clots: Lenalidomide may raise the risk of blood clots, necessitating vigilant observation and maybe preventative actions.
Bone Marrow Suppression: This drug may cause a brief suppression of bone marrow function, which would decrease blood cell counts.
Fatigue and Weakness: Weakness and exhaustion are possible side effects of Lenalidomide for certain persons.
Peripheral Neuropathy: The hands and feet can become numb or tingly, which calls for close observation.
Patient Support and Lifestyle Considerations
Living with Multiple Myeloma requires both medical care and Holistic care. Patient support groups, counseling, and lifestyle modifications significantly impact the general well-being of people with multiple Myeloma.
Support Groups: For those with Multiple Myeloma, joining a support group can offer community, information sharing, and emotional support.
Healthy Lifestyle: A balanced diet and frequent exercise are two aspects of a healthy lifestyle that can improve general well-being.
Regular Check-ups: Frequent check-ups with the doctor are necessary to track the disease's progression and modify treatment programs as required.
Counseling Services: Patients and their families can benefit from professional counseling services to help them manage the psychological and emotional effects of having Multiple Myeloma.
Conclusion
Even though Multiple Myeloma is difficult, there is still hope. Patient outcomes and quality of life have improved dramatically due to new medicines like Lenalid 10mg Lenalidomide Capsules and advances in knowledge of the condition. For people navigating the difficulties of Multiple Myeloma, a more optimistic future is being shaped by early discovery, extensive medical care, and continuous research initiatives. As with any medical problem, consulting healthcare professionals is essential to receiving individualized treatment and support.
#lenalid 10 mg#lenalid 10 mg natco#lenalidomide 10 mg capsule#lenalidomide 10 mg price in india#lenalidomide 10 mg cost#lenalidomide capsules 10mg#lenalidomide capsules 10mg price#lenalidomide brand name#buy lenalidomide online#lenalidomide cost in india#lenalidomide generic name#lenalidomide generic price#Lenalidomide Capsules#Lenalid 10mg Capsules
0 notes
Text
Farydak Capsule Dosage Guidelines

In the realm of cancer treatment, precision and efficacy are paramount. One groundbreaking option in this landscape is Farydak capsules. Understanding the dosage guidelines for Farydak is crucial for both patients and healthcare professionals seeking optimal outcomes. Let's delve into the dosage intricacies of Farydak capsules.
What are Farydak Capsules?
Farydak, with the generic name Panobinostat, belongs to a class of medications called histone deacetylase (HDAC) inhibitors. It is indicated for the treatment of multiple myeloma, a type of cancer affecting plasma cells in the bone marrow.
Initiating Farydak Treatment: Dosage Initiation
Initiating Farydak treatment involves meticulous dosage titration to ensure safety and efficacy. The recommended starting dosage is 20 mg orally once every other day for three doses per week on Days 1, 3, 5, 8, 10, and 12 of a 21-day cycle. This dosage regimen is accompanied by dexamethasone and either bortezomib or lenalidomide.
Adjusting Dosage: Individualized Approach
Dosage adjustments may be necessary based on individual patient tolerability and treatment response. Healthcare providers should closely monitor patients for adverse reactions, particularly gastrointestinal and hematologic toxicities. Depending on the severity of adverse effects, dosage modifications, interruptions, or discontinuations may be warranted.
Optimizing Treatment: Maintenance Dosage
Once an optimal therapeutic response is achieved and adverse effects are managed, maintenance dosing of Farydak is essential to sustain treatment benefits. The maintenance dosage typically involves continuing the last tolerated dosage level or adjusting based on individual patient response.
Dosage Modifications: Special Considerations
Special populations, such as patients with hepatic or renal impairment, may require dosage adjustments to mitigate potential risks. Additionally, concomitant use of medications with known interactions should be carefully evaluated, and dosage modifications may be necessary to prevent adverse drug interactions.
Patient Education: Empowering Patients
Empowering patients with comprehensive knowledge about Farydak dosage guidelines is integral to treatment success. Patients should be educated on proper administration techniques, potential side effects, and the importance of adhering to prescribed dosing schedules. Open communication between patients and healthcare providers fosters a collaborative approach to treatment management.
Conclusion
In the landscape of cancer treatment, Farydak capsules offer a promising therapeutic option for patients with multiple myeloma. Understanding and adhering to Farydak dosage guidelines are imperative for optimizing treatment outcomes and minimizing adverse effects. With a personalized approach to dosing and vigilant monitoring, Farydak holds the potential to unlock new avenues in the fight against cancer.
Frequently Asked Questions [FAQs]
What is the recommended dosage of Farydak?
The recommended dosage of Farydak (panobinostat) typically involves a 20 mg capsule taken orally once daily on Days 1, 3, 5, 8, 10, and 12 of a 21-day cycle, followed by a 7-day rest period. This regimen is usually repeated for up to 8 cycles unless otherwise directed by your healthcare provider.
What should I do if I miss a dose of Farydak?
If you miss a dose of Farydak, take it as soon as you remember on the same day. If it's already the next day, skip the missed dose and resume your regular dosing schedule. Do not take extra capsules to make up for a missed dose. Always consult your healthcare provider if you have any questions or concerns about missed doses.
Are there any special instructions for taking Farydak capsules?
Yes, Farydak capsules should be swallowed whole with a glass of water, preferably with food. It's important not to crush, chew, or open the capsules. Additionally, avoid grapefruit and grapefruit juice while taking Farydak, as it can increase the risk of side effects.
Can the dosage of Farydak be adjusted based on individual factors?
Yes, your healthcare provider may adjust your Farydak dosage based on various factors such as your overall health, any side effects experienced, and how you respond to the treatment. Never adjust your dosage on your own without consulting your healthcare provider.
What should I do if I experience side effects from Farydak?
If you experience any side effects from taking Farydak, it's important to notify your healthcare provider immediately. They can provide guidance on managing side effects and may adjust your dosage or recommend other interventions to help alleviate symptoms. Do not stop taking Farydak without consulting your healthcare provider, as this could affect the effectiveness of your treatment.
0 notes