#ethan does not want to have to explain human biology to them
Explore tagged Tumblr posts
Photo

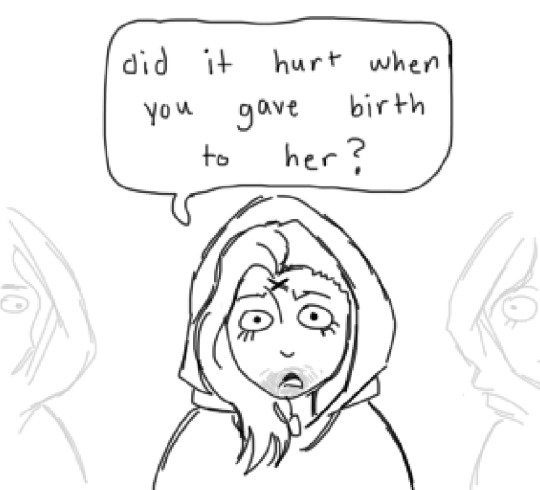
misunderstanding
#dimitrescu daughters#daniela dimitrescu#cassandra dimitrescu#bela dimitrescu#ethan winters#rose winters#rosemary winters#'i cant believe the manthing is a single mother hes so strong'#resident evil 8#re8#resident evil village#rebhfun#resident evil#resident evil fanart#ethan does not want to have to explain human biology to them
3K notes
·
View notes
Text
Fankids With Gold/Silver Demo Pokemon: Noelle & Draco
Noelle and Cruz/Aqua/Aquaria

Being from another world where the demo pokemon are canon, Noelle is familiar with a different pokedex than everyone else. For the most part, the pokemon she knows are the same as what’s found in her current world, but there are a few that are different. In her homeworld, as the demo starters were canon, trainers who wanted to pick a Water-type starter from Professor Elm would receive a Cruz instead of a Totodile. For Noelle however, her Cruz was actually her fourth pokemon. She already had a Dratini from Lance and two Charmander siblings from Liza, but she also wanted to “do things properly” when she set out on her journey and pick one of the traditional Johto starters as well.
Arriving at the Newbark Lab, Noelle knew that she wanted either Chikorita or Cruz. She already had two Fire-types on her team and was planning on following in her father’s footsteps to train Dragon-types and similar pokemon. Meganium and Aquaria both resemble reptiles or dragons of a sort, so either one would be a good addition to the three pokemon she already had. In fact, she found that she was having quite a bit of trouble deciding which one she actually wanted. Both were so cute, and would grow up to be so strong… she couldn’t choose! Luckily, she didn’t have to. Cruz, getting impatient at how long this prospective trainer was deliberating, decided to show off her strength by leaping off the table and picking a fight with Noelle’s Dratini, who had been minding his one business at his trainer’s side. Once the fight was broken up, Noelle couldn’t help but be impressed at Cruz’s boldness, so she was confident in choosing the Water-type to bring with her.
Years later, when Noelle Falls, Cruz, now fully evolved into Aquaria, comes with her along with the rest of Noelle’s pokemon. Once Noelle is taken in by Interpol, there’s a bit of fuss over Aquaria since, while they have encountered various strange pokemon that came from other worlds, Aquaria is the first of her kind recorded. Noelle is a bit nervous to let these guys study her partner, but once she’s assured she can be there the whole time - and probably should be since she knows Aquaria better than any of the scientists at this point - she agrees to let them take some observations and tell them what she knows about Aquaria’s biology and the like. Interpol has a list of other-world pokemon that is sent out to the various Leagues so Noelle doesn’t have to worry about getting questioned for having such an unusual pokemon, but they still get plenty of stares in public. Aquaria on her part, loves the attention, preening at the fact she’s considered rare and special, the only one of her kind to exist here. Noelle finds her pokemon’s attitude pretty amusing and will answer any questions people may have about her, always happy to show off her beloved pokemon.
Draco and Kingdra

While Noelle having a pokemon from another world makes perfect sense, somehow all of Ethan’s other children end up with them as well. Whether this is merely coincidence or some kind of cosmic conspiracy is a well-debated subject in the family. In Draco’s case, it’s his Kingdra that turns out to be from another world, though he has no idea of this until he evolves and grows arms and small wings. Draco initially panics, wondering if somehow he interfered while Seadra was evolving and that messed things up, but once he calms down a bit and tries to think through what could have happened, he remembers that Ethan has a strange Tangrowth-like pokemon that supposedly evolved from a normal Tangela. At this point, Draco is a sullen teenager who currently despises his father for a variety of mostly petty reasons, so he immediately blames Ethan and Gela for what happened to Kingdra. Somehow, Gela must have corrupted Seadra or something since they did always like playing with each other.
Draco storms home to confront his father, but before he can do so, he runs into Noelle, who’s visiting home during a break from work. When she sees her little brother’s Kingdra, she exclaims that it looks just like the Kingdra did back in her original world and is shocked when Draco explains that this is what his Seadra evolved to look like. Later on, after dinner, the whole family sits down to try and figure out just how exactly this whole ordeal came to pass. After some debate, it’s Ethan who supplies the most likely answer, recalling how Draco first met his Horsea. When Draco was just a wee child of 7, he was quite jealous of how his cousin Arethusa had a Horsea and he did not. He wanted one of his own and begged and begged his parents to take him to Kanto so he could catch one. After all, Arethusa had gotten her Horsea from Clair, so if Draco could catch his own, he’d prove that he was better than her. Eventually, they relent, and Ethan and Draco head out to Route 12 for a day trip that weekend, ready to do some fishing.
It turns out that Horsea are fairly rare, however, so the trip ends up entailing quite a bit of sitting and waiting around. For Draco, it’s incredibly frustrating and boring. They’ve tried fishing, placing food in the water to lure pokemon, they even borrowed a nearby fisher’s Starmie that knew Surf to head out onto the water to search, but to no avail. The only pokemon they’ve found are the usual Magikarp and Tentacool. Just as Draco is beginning to give up hope, he notices a nearby commotion. Gela, who had been sitting on the edge of the dock splashing water with her tentacles seems to be interacting with another pokemon. Draco looks for his father, but Ethan is busy talking to a fisherman, so he heads over by himself. Lo and behold, Gela is playing with a Horsea! Draco can scarcely believe his luck as he fumbles for one of the pokeballs Ethan gave him and tries to carefully approach the pair. Horsea notices the incoming boy and beings to dart away, scared off from his new playmate by the approaching human, but pauses when he hears Draco’s desperate cries for him to come back, “Please! Please don’t go! I really want to be your friend!” Moved by this human’s sincerity, Horsea turns around and slowly approaches the docks again, as Draco himself is doing his best puppy-dog eyes in the hopes that Horsea will know just how much he wants to befriend him. Horsea glances at Gela, who nods encouragingly, then slowly approaches Draco’s outstretched hand and bumps against the pokeball, successfully being caught. Draco runs back to Ethan with his new pokemon, babbling excitedly about how he succeeded, and the two return home triumphant, Draco getting to ride on his father’s shoulders, pokeball in one hand and the ice cream cone Ethan bought him as a special treat in the other.
After recounting those fond memories of simpler, pre-sullen teen times, Ethan concludes that Horsea must have recognized Gela as being a pokemon from his own homeworld and approached her as a result. Draco has to admit that that explanation makes the most sense, but… on the one hand, he doesn’t want to acknowledge that Ethan was right about something so is grumpy, while on the other hand, this does prove that Seadra evolving into an odd Kingdra was Ethan’s fault after all so he wants to be smug… in the end he just mumbles something incoherent and sulks out of the room, though deep down he’s quite pleased to have such a rare and unique pokemon. Down the line, once the grumpy teen years are over and Draco is admitting he loves his father again, he does thank Ethan for being the reason he and Kingdra ultimately got to meet, and apologizes for blaming him for “breaking” Kingdra and thinking that Ethan’s Gelanla was weird, as his pokemon is perfect the way he is, just like Gela.
#pkmn fankids#Noelle#Noelle's Cruz#Noelle's Aquaria#Noelle's Pokemon#Draco#Draco's Kingdra#Draco's Pokemon#Ethan#Ethan's Gelanla#Ethan's Pokemon#Lance#Liza F#Long Post
5 notes
·
View notes
Note
Edit: removed part about infections as I realize I got that wrong
I’m on mobile so forgive me if I’m a bit over the place or miss something. I scrolled back quite a bit but I own I may miss something :)
I remember those docs from re7 and that’s not really my issue. It’s just that is super high level and doesn’t actually get to the nitty gritty of why it’s just such a weird and hard concept for me to accept mainly considering Ethan is considered technically dead. Not a strict infection. A dead man revised with mold however we want to call it that is now maintaining his body. An infection that surely wasn’t progressed enough to maintain what he experienced (but maybe that’s an issue w this retcon and not the functionality of his body.) Personally, if the infection route RE8 had been taken, I think my issues with this may not be here because it makes him more human as a highly infected or controlled individual. But he’s supposed to be dead.
Because it’s not just DNA. It’s cells. So what cells? What kind of takeover? To what an extent? It has to be most of them for him to be functioning yes? That’s why I still maintain my how does this work? For Ethan and biologically related functions. I guess I could just accept that the infected cells act just like the human cells but that route just aggravates me. Probably again my own irritation w this twist. Mainly because If I am creating a bio weapon, I would assume I wouldn’t want it to be held hostage to human short comings but maybe they would so they blend in better.
I’m in agreement w Zoe and Jack and Lucas. Although I do really find bringing Jack back created some issues lol (because how is anyone dead dead now loool) and I know Zoe was under some influence re hallucinations in a way but obviously she fought off Evie better. Or evie didn’t care. It isn’t necessarily clear imo. But evie seemed to want the parents most.
I’m okay w the BSAA knowing about Ethan but if they were informing Miranda, I’m unsure why she was surprised he came back in the end. She should know as well imo. She seems thoroughly unprepared to handle him. But yeah it’s the weakest part probably that Capcom probably didn’t spend so much time on.
Re Ethans will: Travis still became molded whereas Ethan didn’t. And I think he’s the only technically dead one that didn’t become molded as the bakers were alive when Evie got ahold of them. Now I can concede they’re dead in a way by re7 but also they’re very progressed in the infection. Which kinda takes me back to the whole Ethan didn’t have the time to become infected enough to jump back up as he supposedly did. But maybe Evie did it but if she did it then why did she struggle to manipulate him in a way until the end ? Because she got the others right away. And we’re back to my issue with this twist. How long does it take for infections to take over
I also agree whatever we want to call Ethan in re8 that is fully 100% Ethan. I will absolutely die on that hill.
I def understand translation issues. It’s still sci-fi because I mean it can be pushed pretty far lol there’s still a connection even tho it’s foreign in some ways to our own biology. I promise I’m not trying to be difficult; I just...maybe I’m just poorly explaining myself because I agree with you on most of these things! I think it largely starts with Ethan being dead and murdered by Jack. Because he has things like a heart monitor and I’m like why tho? And if it’s because a molded man can perfectly emulate a human man through DNA, cells, and functionality, then I guess I just have to accept it. I just don’t find as is the game made that incredibly clear.
well... Mia was injected with the antidote and so that probably did something
I apologize if I misunderstand but is this re Mias pregnancy? I suppose that could be the answer. But wouldn’t it in a way counter balance the mold? Or am I totally misunderstanding this ask? Or is this how Rose survives?
I’m still very much feel like there has to be more of this stuff out there. Or they’re successfully growing it elsewhere? I dunno. It makes my brain hurt. Maybe Rose is partially human enough to survive? Or she is just sentient? I still don’t understand how Mia could be pregnant. I’m gonna avoid to many details to stay sfw but this whole process has me....curious to say the least.
Again apologies if I totally misunderstood
30 notes
·
View notes
Text
Mice, dice and copycats: Metaphors for gene drives in mammals
When you hear the word ‘gene drive’, you will either be baffled or you will think about mosquitoes, engineered to eradicate insect-born disease like malaria, Dengue fever, or Zika for example. But gene drive research has now moved from insects to mammals.
Mammals
On the 23rd of January, researchers at University of California, San Diego, led by Kimberly Cooper, announced that they had developed a method for controlling inheritance in mice using the gene-editing tool CRISPR Cas9.
Such a method, generally called ‘gene drive’, had, so far, only been demonstrated in insects, not mammals. Hannah A. Grunwald, Valentino M. Gantz, Gunnar Poplawski, Xiang-ru S. Xu, Ethan Bier, and Kimberly L. Cooper, published their paper on ‘Supermendelian inheritance’ in Nature on the 23rd. Two press releases were issued on that day, one by UC San Diego (UCSD) (which was used widely, e.g. by Science Daily), and another syndicated by the Press Association.
Grant Jacobs, a computational biologist and science communicator, published a blog post the following day, which is worth a read, as it provides a clear and detailed explanation of this achievement. He explains: “A gene drive is the informal name given to a process where a genetic variation is set up so that it will be inherited more often in the offspring than it would by chance. In a gene drive each generation has a better than 50% chance of inheriting the new variant, so over time the chosen variant becomes the dominant variant of that gene in the population.” Jacobs goes on to tease out what the new research demonstrates and does not demonstrate, what it has achieved and what its limitations are.
Another good overview of the background to this research can be found in a Nature News article published on 6 July 2018, together with a Science article from 13 July 2018. These articles appeared at the time of the submission of a pre-peer-reviewed version of the paper on the preprint server bioRxiv.
Mice and models
The Science article summarises the research as follows: “The team from the University of California, San Diego, used the genome editor CRISPR to put a gene in the mice that modifies their coat colors. But they also engineered these same mice to pass on the genes that create CRISPR itself so that progeny edit their own genomes to carry the coat color–modifying gene. When they mated an engineered mouse to a normal one, it should have created a pup with the coat color–modifying gene in one of two chromosomes. But because the pup also inherited CRISPR, it altered the unmodified chromosome passed down from the normal parent so that it, too, had the gene.”
The scientists did not set out to find a way of eradicating diseases or pests, in this case rodents, but to demonstrate ways in which gene drives can be used to bias inheritance in order to “transform the use of rodent models in basic and biomedical research” (Nature article), to “make mammals models of complex human genetic diseases, like arthritis and cancer, that are not currently possible” (Press Association, 23, January), to control “the inheritance of multiple genes in mice” (Cooper quoted in City News Service, 23 January), and even to understand “the mechanisms of evolution” (UCSD, Press release, 23 January) and of the ”origins of mammalian diversity” (ibid.).
As a secondary outcome it my “decrease the time, cost and number of animals needed to advance biomedical research on human diseases and to understand other types of complex genetic traits” (ibid.).
Media
How was this new research reported after the official publication? To see what is out there, I consulted the news database Nexis and downloaded all articles published in English Language News between 23 and 28 January. After removing duplicates, there were, astonishingly only nine items left, and of these only four were published in mainstream newspapers, of which Ian Sample’s article for The Guardian was the most interesting. One article by Cooper herself appeared in The Conversation on the 24th of January (republished in MENA English, Middle East and North Africa Financial Network). Others mainly reproduced what the two press releases (UCSD and Press Association) had said.
To this small sample I added one article published in New Scientist, another in Scientific American (reprinting an article from Quanta Magazine) and a third in the Financial Times.
In this blog post I’ll pick out a few metaphors used in this coverage, but I will not cover ethical legal and social aspects in any detail. This will have to wait for a bigger and more detailed study.
Metaphors
Loading dice and flipping coins
The first two words of the Nature title, “Super-Mendelian inheritance mediated by CRISPR–Cas9 in the female mouse germline”, are already slightly metaphorical or rather hyperbolic and point to a nexus of metaphors explicating how inheritance works and is reworked in gene drives. The phrase ‘supermendelian inheritance’ had previously been employed to report on research on gene drives in mosquitoes.
One of the most used phrases (see Press Association, 23 January) was that of ‘increasing the odds’. This was in the context of using a specially designed gene drive to increase the chance of mouse offspring to inherit two copies of a mutation causing them to be born with white instead of black fur. But how does this work? To understand this, we have to go back to the beginnings of genetics.
The Press Association article explains: “Under normal circumstances, genes come in pairs, on inherited from each parent. Gregor Mendel, the ‘Father of Genetics’, discovered this fundamental principle of heredity in the 19th century as he experimented with pea plants. It means an offspring has an equal chance of inheriting a particular genetic variation, or mutation, form either its mother or father. A gene drive loads the dice, making it more likely that the offspring will be born with a chosen trait, such as fur colour.”
In her Conversation article, Cooper has provided one of the most detailed explanations of this type of inheritance . Here she talks about ‘uber-inheritance’, which ‘boosts the odds’ of a trait or several being inherited. To explain things, she uses the analogy of flipping or tossing a coin: “Each animal has two versions of each gene. Each parent will pass only one version to each offspring. Inheritance of different genetic traits is therefore a bit like a coin toss where a particular version is inherited 50 percent of the time.” This gets us to the uber- or supermendelian aspect of the research:
“Creating a mouse that inherited mutated versions of three disease-causing genes from each parent has the same likelihood as six simultaneously flipped coins all landing on ‘heads.’ But what if the coins could be unevenly weighted so that they have a higher probability of falling heads up?
The concept of stacking the odds in favor of one of the two versions of a gene underlies efforts to engineer gene drives. A gene drive is simply defined as a piece of DNA that is inherited more often than can be explained by random chance over several generations so that it sweeps through a population.”
Surprisingly, none of the articles on gene drive in mice used card analogies instead of dice or coin ones, despite the fact that card games can be used quite successfully to explain Mendelian inheritance – some people even talk about the card game of life!
Scissors and pens
To make their gene drive work, the Californian team used a now ubiquitous tool, namely a gene-editing tool called Crispr/Cas9. As quite a few articles said: “the system relies on ‘molecular scissors’ to make highly precise changes to DNA.” (Press Association, 23 January; The Conversation, 24 January)
One article in The Guardian was entitled “Scientists rewrite mice DNA so genes can be spread through species” – echoing older synthetic biology metaphors of rewriting the book of life. The article also puts this new research in the context of existing gene drive research using insects, where gene drives are used for “rewriting the genetic makeup of mosquitoes that carry the malaria parasite” (Ian Sample, 23 January).
Copycats
Now we come to the special way in the researchers used gene-editing and to the fun name they chose for the molecular element that was employed. It was called ‘CopyCat’. This “was spliced into the gene for an enzyme controlling fur colour. Gene editing allowed CopyCat inserted into one copy of the inherited gene to be duplicated in the other during egg production in females. This increased the chances of CopyCat being passed on to the next generation.” (Press Association, 23 January)
In another article, CopyCat is called a “self-replicating DNA sequence” (UPI.com, 24 January). It was said to be “copying and pasting the same genetic coding from one chromosome to the other”. The article goes into more detail about the various mechanisms involved.
Donors and recipients
Crispr and CopyCat are used, it seems, between donor genes and recipient or target genes. As Cooper explains in The Conversation: “In a gene drive, a donor gene, which is the version we want to introduce into the animal, is engineered to use these components so that it can replace the non-engineered version, or the so-called recipient gene. When the non-engineered recipient gene is cut, the donor gene repairs the cut by copying itself into the recipient site so that there are two identical copies of the donor gene.
The donor gene therefore acts like the find and replace feature of a word processing program. The recipient gene is converted so that a mosquito, for example, would have two copies of the engineered donor gene to pass to its offspring.”
In the context of the new research, it turned out that the “donor gene was inherited as much as 86 percent of the time – a heavily weighted coin – compared to just the usual 50 percent.” This was relatively low though and only worked in females.
Selfish genes and cheating gene drives
Some articles also employ more creative metaphors. “Gene drives are pieces of DNA that cheat. They ‘copy and paste’ themselves from one genome copy to a target sequence in the other copy.” (New Scientist, 23 January).
This article also tells readers that some people call gene drives ‘active genetics’ (see also Scientific American). The term was apparently coined by two of Cooper’s colleagues, Valentino Gantz and Ethan Bier (see Scientific American, 28 January).
Such active gene drives are not only ‘super’ or ‘uber’, they are also selfish. As a Nature News article said: “Gene drives work by ensuring that a higher proportion of an organism’s offspring inherit a certain ‘selfish’ gene than would happen by chance, allowing a mutation or foreign gene to spread quickly through a population.” (6 July, 2018). I am starting to wonder whether there will ever by selfish self-driving gene drives…
Mice and ice-nine
The Scientific American article also quotes a scientist who framed gene drives more in scifi terms: “Fred Gould, an entomologist and evolutionary biologist at North Carolina State University, likens gene drives to the fictional substance ice-nine in Kurt Vonnegut’s novel Cat’s Cradle: a bizarre form of ice that freezes all other water it touches. Gene drives spread fast because they are sets of genetic elements that spontaneously copy themselves from a maternal chromosome to a matching paternal one or vice versa. In the process of copying itself, the gene drive can also add, delete or modify genes at its insertion point.”
Death and destruction
One short article published in The Times on the 24th of January, focuses on the possibility of using gene drives to eradicate disease carrying insects or invasive mammal species. It uses words like ‘wipe out’, ‘erase’, or ‘wreck’ (ecosystems). Such war metaphors were otherwise absent from the coverage of this particular research into mice, but are widely used when dealing with gene drives and insects.
Possibilities and responsibilities
However they framed it, most articles called this research ‘controversial’. It changes the ‘germline’ of mammals and one therefore has to proceed with extreme care, as this can “alter entire species” (Press Association, 23 January) and can lead to “unintended consequences” (ibid.).
Researchers and commentators alike expressed a need for caution, with everybody treading a fine line between excitement and restraint. One comment reported in the Financial Times summarises this quite well: “Bruce Whitelaw, professor of animal biotechnology at Edinburgh’s Roslin Institute who has also investigated gene drives in mice, said the UCSD project was ‘important as it both demonstrates mammalian gene drive for the first time and starts to clarify the aspects and limitations of the process’. He added: ‘The authors correctly state how this approach could make a huge positive . . . impact for laboratory animal use while pointing the way to the still far-off but feasible application in wild animals.’”
Scientific American pointed out: “For at least some time to come, these kinds of ‘active genetics’ technologies may be more useful as laboratory tools than as instruments for remaking nature.”
This means keeping, at present, an eye on ethics and responsibility in the context of labs and lab animal use, while also scanning ethical horizons for potential dangers ahead, especially with regard to using gene drives ‘in the wild’.
At the same time, it is also important to keep an eye on the language used, which, at the moment, seems to be quite restrained, mostly using metaphors for explanation not hype.
Image: Pixabay
The post Mice, dice and copycats: Metaphors for gene drives in mammals appeared first on Making Science Public.
via Making Science Public http://bit.ly/2Bqfp69
0 notes
Photo
To be fair. He has been HCed many times to be a trans man so like. It's not the worse thing Daniela could have said.



misunderstanding
#dimitrescu daughters#daniela dimitrescu#cassandra dimitrescu#bela dimitrescu#ethan winters#rose winters#rosemary winters#'i cant believe the manthing is a single mother hes so strong'#resident evil 8#re8#resident evil village#rebhfun#resident evil#resident evil fanart#ethan does not want to have to explain human biology to them
3K notes
·
View notes