#eCTD
Explore tagged Tumblr posts
Text
AfriSummit 2024: Uniting for a Healthier Africa
Uniting Masterminds and Industry leaders to Shape the future of Regulatory Landscape in Pharmaceutical sector of the African continent. CAIRO, EGYPT – (AfricaNewswire.Net) — AfriSummit 2024, an initiative by PRA Consultancy, organized by Hubplus Events in collaboration with Pioneers, will take place from November 3-6, 2024, at the Grand Nile Tower in Cairo, Egypt. This significant event brings…
#African continent#AfriSummit 2024#Cairo#eCTD#Grand Nile Tower#healthcare#healthcare regulations#Healthier Africa#medical device industries#pharmaceutical industry#Pharmacovigilance#regulations
0 notes
Text
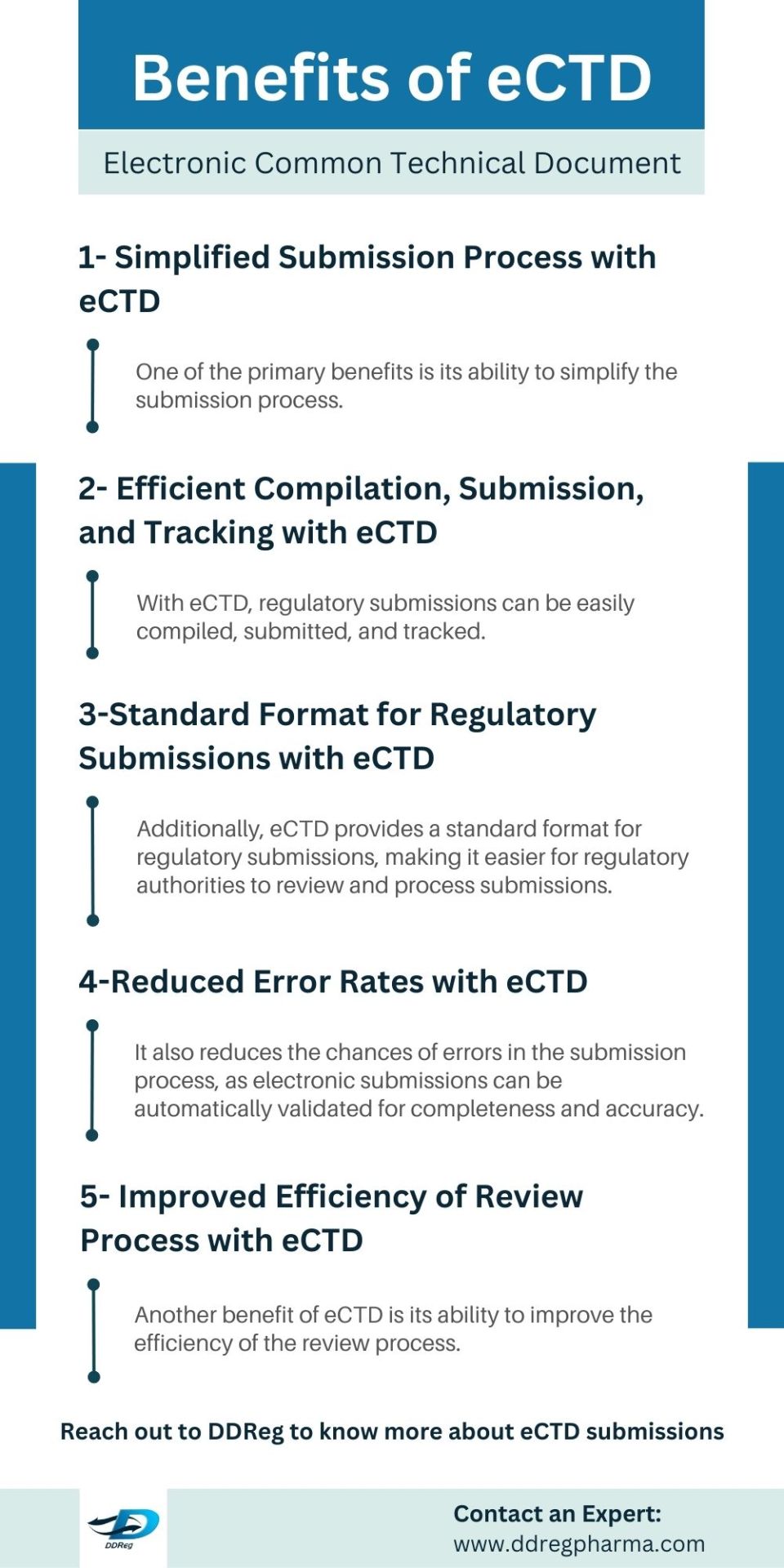
The Benefits of eCTD- DDreg Pharma
The eCTD offers numerous advantages compared to traditional paper-based submissions. It simplifies the submission process by allowing easy compilation, submission, and tracking of regulatory submissions. Electronic submissions reduce errors and improve review efficiency, leading to faster approvals and cost savings for pharmaceutical companies. To learn more about eCTD submission, read our blog.
0 notes
Text
IDOM BEAT BIGBIRD YEEEEESSSSSSSSS
#HELL FUCKING YEAH!!!!!!#USA USA USA USA#I want Idom to win ECTD so fucking bad yall#Yeah I'm like 20 minutes behind in the strean
0 notes
Text
Freyr SUBMIT Pro is one of the best eCTD software tool which helps Life science companies in various regulatory submissions to global health Authorities.
#ectd software#ectd tool#ectd format#ectd submission software#ectd publishing software#cloud-hosted ectd software
0 notes
Text
Integrating CTIS with eCTD Systems

The regulatory landscape in the life sciences industry is rapidly evolving, with increased emphasis on harmonization, transparency, and efficiency. The integration of the Clinical Trial Information System (CTIS) with electronic Common Technical Document (eCTD) systems represents a strategic move toward streamlining regulatory submissions and improving documentation management.
This blog delves into the importance of this integration, the benefits it offers, and key considerations for successful implementation.
Significance of Integrating CTIS with eCTD Systems
Regulatory submissions are complex processes that demand meticulous organization and compliance with region-specific requirements. CTIS, established under the EU Clinical Trials Regulation (CTR), is designed to centralize the submission and assessment of clinical trial data. On the other hand, eCTD systems are the gold standard for managing regulatory documentation globally.
Integrating these two systems bridges the gap between clinical trial management and regulatory submission, enabling seamless data exchange and reducing redundancies. It ensures consistency, improves traceability, and accelerates the approval timeline.
How is the Integration Beneficial?
Improved Efficiency: By synchronizing CTIS and eCTD systems, life sciences companies can eliminate manual data entry, automate workflows, and streamline document management. This reduces the time and resources required for regulatory submissions.
Enhanced Compliance: Integration ensures that data submitted through CTIS aligns with eCTD requirements, mitigating the risk of errors or discrepancies that can delay approvals.
Centralized Data Access: Integrated systems provide stakeholders with a unified platform to access clinical and regulatory data, facilitating collaboration across departments and geographies.
Real-Time Updates: With integrated systems, any updates made in CTIS automatically reflect in the eCTD, ensuring that documentation remains up-to-date throughout the submission lifecycle.
Challenges in Integration
While integration offers numerous benefits, it also comes with challenges, including:
System Compatibility: Ensuring that CTIS and eCTD platforms are compatible and can communicate effectively.
Data Migration: Transferring existing data from siloed systems to the integrated platform without losing integrity.
Regulatory Requirements: Adhering to region-specific guidelines while implementing integration.
These challenges can be addressed by involving cross-functional teams, leveraging robust integration tools, and working closely with regulatory authorities.
Best Practices for Successful Integration
To maximize the benefits of CTIS-eCTD integration, companies should:
Conduct a Gap Analysis- Evaluate existing processes and systems to identify areas that can be optimized through integration.
Adopt a Modular Approach- Implement integration in phases, starting with the most critical workflows, to minimize disruptions.
Invest in Training- Equip teams with the skills and knowledge needed to operate the integrated systems effectively.
Collaborate with Experts- Partner with regulatory consultants who can provide insights into best practices and ensure compliance with global standards.
As regulatory processes become increasingly digitalized, the integration of CTIS with eCTD systems is poised to become a cornerstone of modern regulatory strategy. This integration not only simplifies the submission process but also supports the broader goals of regulatory agencies to enhance transparency and efficiency.
By embracing this technology-driven approach, life sciences companies can position themselves for success in an ever-evolving regulatory environment.
Conclusion
The integration of CTIS with eCTD systems is a transformative step in streamlining regulatory submissions. It offers unparalleled efficiency, reduces the risk of non-compliance, and fosters a collaborative ecosystem for managing regulatory documentation. Companies that prioritize this integration will be better equipped to navigate the complexities of regulatory approval, ensuring faster time-to-market for their innovations.
#medical device regulatory consulting#medical devices#regulatoryaffairs#regulatoryapproval#regulatorycompliance#regulatoryrequirements
0 notes
Text
Pharma Regulatory Consultants: Simplifying Compliance for the Pharmaceutical Industry
The pharmaceutical industry operates under strict regulations to ensure the safety, efficacy, and quality of medicines and healthcare products. Navigating these complex regulatory requirements can be a daunting task for pharmaceutical companies. This is where Pharma Regulatory Consultants come into play, offering specialized expertise to streamline compliance and facilitate market entry.
What Are Pharma Regulatory Consultants?
Pharma regulatory consultants are experts who assist pharmaceutical companies in meeting the legal and regulatory requirements for drug development, approval, manufacturing, and marketing. They provide guidance on domestic and international regulations, helping companies avoid delays and achieve compliance with agencies like the CDSCO in India, FDA in the USA, or EMA in Europe.
Key Services Offered by Pharma Regulatory Consultants
Regulatory Strategy Development
Assessing regulatory requirements based on the target market.
Designing a compliance roadmap for drug approvals.
Regulatory Submissions
Preparing and submitting dossiers, such as Common Technical Documents (CTD) or eCTD, to regulatory bodies.
Managing applications for Investigational New Drugs (IND), New Drug Applications (NDA), or Abbreviated New Drug Applications (ANDA).
Clinical Trial Support
Assisting with clinical trial approvals and ensuring compliance with Good Clinical Practice (GCP) guidelines.
Liaising with ethics committees and regulatory authorities for trial documentation.
Quality Assurance and Audits
Ensuring compliance with Good Manufacturing Practices (GMP) and ISO standards.
Conducting internal audits and preparing for regulatory inspections.
Regulatory Intelligence
Monitoring changes in global pharmaceutical regulations.
Providing insights into new guidelines, trends, or market-specific requirements.
Post-Marketing Compliance
Assisting with pharmacovigilance activities, including adverse event reporting.
Managing lifecycle maintenance, such as renewals, amendments, and labeling updates.
Why Do Companies Need Pharma Regulatory Consultants?
Expertise in Complex Regulations
Regulatory frameworks differ significantly between regions. Consultants possess in-depth knowledge of these complexities, ensuring accurate compliance.
Faster Time-to-Market
With their expertise, consultants minimize errors and delays in submissions, accelerating approval processes.
Cost Efficiency
Avoiding compliance pitfalls and regulatory rejections saves significant resources.
Global Market Access
Consultants help pharmaceutical companies meet international standards, opening doors to global markets.
Industries That Benefit from Pharma Regulatory Consultants
Pharmaceutical Manufacturing: For drug approvals and GMP compliance.
Biotechnology Firms: To navigate biologics and biosimilars regulations.
Medical Device Companies: To meet requirements for combination products.
OTC and Nutraceutical Companies: For compliance with labeling, claims, and advertising standards.
Choosing the Right Pharma Regulatory Consultant
Experience: Look for consultants with proven expertise in your specific domain (e.g., small molecules, biologics, or generics).
Regulatory Knowledge: Ensure familiarity with target market regulations, such as CDSCO, FDA, or EMA requirements.
Client References: Check testimonials or case studies from similar projects.
Proactive Communication: Select a consultant who provides regular updates and clear guidance.
Conclusion
Pharma regulatory consultants are invaluable partners for companies aiming to thrive in a highly regulated industry. From ensuring compliance to streamlining market entry, their expertise helps businesses stay ahead in the competitive pharmaceutical landscape.
Whether you’re a startup developing innovative drugs or an established company expanding globally, a skilled regulatory consultant can make the journey smoother, faster, and more efficient. By partnering with the right consultant, you can focus on delivering quality healthcare solutions while they handle the intricacies of regulatory compliance.
0 notes
Text
Skycare Pharmaceuticals is hiring Regulatory Affairs Executives to join their growing team. If you have a B.Pharm or M.Pharm degree and 1-3 years of relevant experience, this could be the perfect role for you. Job Overview Position: Regulatory Affairs Executive Location: Ahmedabad, Gujarat Qualifications: B.Pharm, M.Pharm Experience: 1-3 years in regulatory affairs As a Regulatory Affairs Executive, you will play a key role in dossier filing and ensuring the company’s products comply with global pharmaceutical regulations. You will work closely with internal teams and external stakeholders, helping guide the company through the regulatory landscape. Key Responsibilities dossier filing Develop and implement regulatory strategies to ensure compliance with local and Minimum knowledge of CTD, ACTD,ECTD filing and in depth knowledge of international regulations and guidelines for pharmaceutical products. Preparation and submission of dossiers, including drug applications, variations, renewals etc. Stay updated on changes in regulatory requirements and communicate these changes to relevant stakeholders within the company. Coordinate with cross-functional teams, including R&D, quality assurance, and marketing, to ensure regulatory compliance throughout the product lifecycle. Develop and maintain relationships with key stakeholders Provide regulatory input for product development, including reviewing and approving labeling, packaging, and promotional materials. [caption id="attachment_106707" align="aligncenter" width="930"] Skycare PharmaceuticalsRecruitment - Job vacancies[/caption] Required Skills and Qualifications Degree: B.Pharm or M.Pharm is mandatory for this role. Experience: 1-3 years of hands-on experience in regulatory affairs, with specific experience in dossier preparation and submission. Knowledge: Familiarity with CTD, ACTD, eCTD guidelines, and a deep understanding of international pharmaceutical regulations. Skills: Strong organizational skills, attention to detail, and the ability to work with cross-functional teams. How to Apply If you are a qualified candidate with experience in regulatory affairs, Skycare Pharmaceuticals wants to hear from you! To apply, send your resume to [email protected] or reach out via WhatsApp at +91 98750 11522.
0 notes
Text
eCTD Publishing Best Practices: Streamlining Regulatory Submissions.
Discover essential eCTD publishing best practices to ensure successful regulatory submissions, from planning and software validation to consistent structuring and thorough reviews. https://www.pleasepublish.com/blog/ectd-publishing-best-practices/
1 note
·
View note
Text
Regulatory Services
The electronic Common Technical Document (eCTD) is a standardized electronic format used for submitting applications, amendments, supplements, and reports to regulatory authorities such as the United States Food and Drug Administration (USFDA), the European Medicines Agency (EMA), and other Health Authorities (HAs) worldwide. eCTD submissions streamline the creation and review of Electronic Regulatory Publishing data, offering the flexibility to incorporate metatags, hyperlinks, and bookmarks. This format enables efficient assessment and lifecycle management of submissions, ultimately expediting market approvals/authorizations.
With extensive expertise in global eCTD publishing trends and submission formats (e.g., eCTD/Non-eCTD Electronic Regulatory submissions [NeeS]), Freyr facilitates multi-country filings, data compilation, publishing, and dossier dispatch.
We have a team of experts proficient in a wide range of technologies including:
Insight Publisher, Validator
Veeva Vault RIM Suite (Submissions, Submissions Archive, Publishing), Veeva Vault Promomats & Veeva Quality Docs
Lorenz Docubridge, eValidator
Extedo EURS Validator
eCTDXpress
ISIS Publisher/Tollbox
Our team is equipped to provide comprehensive support across these technologies to meet your needs effectively.Health Authority by CountryApplication and Submission TypeSubmission Format Food and Drug Administration (FDA) USAInvestigational New Drug (IND), New Drug Application (NDA), Abbreviated New Drug Application (ANDA), Biological License Application (BLA), Over-the-Counter (OTC), Drug Master Files (DMF Submissions), Structured Product Labeling (SPL) Submissions, and Supplemental New Drug Application (SNDA Submissions)
Originals, Amendments, Annual Report Submissions, Labeling Supplement Submissions, Periodic Adverse Drug Experience Report (PADERS), Briefing Book, eCTD Baseline Submissions, Ad Promo Submissions, and eCTD Submissions for Lifecycle Management (LCM)eCTD European Medicinal Agency (EMA)Centralized Procedure (CP), Decentralized Procedure (DCP), Mutual Recognition Procedure (MRP), and National Procedure (NP)
Clinical Trial Application (CTA), Originals, Variations, AtoQs, Renewals, ASMF eCTD Submissions, and Medical Device SubmissionseCTD Health CanadaNew Drug Submission (NDS), Supplement to a New Drug Submission (SNDS), and Supplement to a New Drug Submission – Confirmatory (SNDS-C)
Abbreviated New Drug Submission (ANDS)
Supplement to Abbreviated New Drug Submission (SANDS)eCTD
0 notes
Text
Special access scheme (SAS) medicines
Unlock your success with Regulatory Affairs Consultants in Australia We specialize in eCTD submissions and Reference Listed Drug RLD approvals, ensuring streamlined regulatory processes.
0 notes
Text
The 10th edition of the GCC Regulatory Affairs Pharma Summit is set to convene in Dubai in 2025, marking a significant milestone in the pharmaceutical industry.
This prestigious event will bring together over 650+ professionals, including regional governmental officials and industry experts. Dubai, UAE – (ARAB NEWSWIRE) — The 10th edition of the GCC Regulatory Affairs Pharma Summit, themed “A Decade of Excellence,” will take place from February 17-21, 2025, at the Mövenpick Grand Al Bustan in Dubai, UAE. The summit will start with the GCC Regulatory…
#Dubai#eCTD Training#GCC#GCC Regulatory Affairs Pharma Summit#Medical Tender#Mövenpick Grand Al Bustan#Orphan Drugs#Pharma Summit#Pharmaceutical#Pharmaceutical Industries#Track Trace#UAE
0 notes
Text
Life Science Solutions
Life Science Translation
Willingjet provides premium translation services for pharmaceutical, biotech, and medical device companies and CROs with high-quality.
We have provided language solutions to the life science industry for more than two decades, our professional experts and native-speaking translators have more than ten years of experience, covering more than 100 languages. Willingjet has aquired ISO 17100 , ISO 9001:2015 and ISO 13485:2016 certifications to guarantee our medical translation services complying with the stringent regulatory requirements worldwide.
Medical translation document types
Documents included in drug registration dossier:
M2: Quality summary, non-clinical review and summary, clinical review and summary, etc.;
M3: Manufacturing information, specification, analytical methods and validation, stability study, container closure system for drug substance and drug product;
M4: Pharmacology, pharmocodynamic, pharmacokinetic, toxicology research reports, etc.;
M5: Clinical study protocol, clinical study report, investigator brochure, ccds, package insert, etc.
Documents from the medical device company:
Patient Information
Patient Reported Outcomes (PROs)
Medical device safety and pharmacovigilance document
Clinical trial protocol
Product labeling and packaging
Documents submitted to regulatory agencies
Instructions for use
Technical manual
Medical literature
Medical equipment instructions and installation manuals
Marketing and advertising
Websites, software, mobile apps and IoT
Online learning and training
Legal, financial documents
Documents from the medical device company:
Documents from CROs:
Managing Multi-regional clinical trial in many languages
Clinical trial documents
Patient engagement and recruitment
Contracts and POs
Registration dossier
Contact Us
Professional DTP
We have self-developed DTP software and are good at using industry-standard desktop typesetting and editing tools, including the eCTD format of drug/medical device registration dossier, user manuals, technical documents, and software GUI layout. Our DTP experts have professional knowledge of specific language environments and can produce high-standard dtp design service for any language product.

0 notes
Text
Damn everyone at ECTD is so sleepy it sounds like.
I'm tired too though ngl.
But like damn that crowd is Asleep. 😂
2 notes
·
View notes
Text
#Regulatory Medical Writing#publishing and submissions#ISE submissions#Clinical Trial#ISE#ISS#eCTD Specification#New Drug#Medical writers
0 notes
Text
Global Regulatory Publishing and eCTD submission Services | MakroCare
MakroCare Regulatory Publishing and eCTD Submission Services help you to reduce the cost and effort involved in converting paper-based documentation into eCTD.
0 notes
Text
Data Security and Confidentiality in eCTD Publishing Tools: Safeguarding Sensitive Information
In the current era of digitalization life sciences has seen a major change in the way that regulatory submissions are developed and presented. Electronic Common Technical Document (eCTD) publishing has transformed the process of submission by making it faster and more efficient. However, with the ease that digital technologies offer comes the vital responsibility of ensuring security and privacy.
This article will dive into the crucial role of security for data in eCTD publishing and discuss methods to safeguard sensitive information during the entire submission process.
The Imperative of Data Security
Security of data isn't an unimportant checkbox on the list of regulatory compliance. The eCTD Software format permits biotech and pharmaceutical companies to prepare electronic submissions of their application that has numerous advantages like a reduction in paper use speedier reviews and improved collaboration. However, it also presents risks if not managed properly.
• Protection of intellectual Property: Pharmaceutical companies invest large amounts of money for developing and research. Data security is essential to prevent unauthorised access to confidential information, thereby protecting intellectual property rights of the company.
• Patient Privacy: Data from clinical trials and patient information should be handled with utmost security to ensure privacy for patients and to comply with laws on protection of data.
• Regulation Compliance: A lot of regulatory eCTD Submission Software agencies require a strict adherence to standards regarding data security in their submission specifications. Failure to adhere to these standards can lead to delays or even rejections.
• Reputation and Trust: Keeping secure and confidential data builds trust with regulators and partners as well as others. Any breach could damage the image of a company irreparably.
Strategies for Safeguarding Sensitive Information
• Secure encryption: Use strong encryption methods to safeguard the data during transport and in rest. By using encryption, even in the event of unauthorized access the data is unreadable and inaccessible.
• Access Controls: Set strict access control that restricts the access of users to view or edit sensitive information. Multi-factor authentication adds a new layer of protection to the user's access.
• Audit Trails: Keep precise audit trails that record every step performed inside the eCTD publishing system. This does not just increase accountability, but also assists in identifying any suspicious actions.
• Secure Infrastructure: Select eCTD publishing tools built on secured infrastructure, frequently updated to fix security holes and are armed with intrusion prevention systems and firewalls.
• Users Training: Offer extensive training for employees who are engaged within participating in the eCTD publishing procedure. Be sure that they are aware the security protocols and best practices for avoiding unintentional breach.
• Data Minimization: Only include the information required in submissions, thus reducing the chance of disclosing sensitive information. This improves the efficiency of the process of reviewing.
• Vendor Due Diligence: When you are using an external third party eCTD software for publishing, perform thorough due diligence regarding the security measures they use and their certifications.
• Periodic Audits and Evaluations: Perform periodic security assessments and audits in order to discover vulnerabilities and then take action to address these.
The Final Thought!!
The life sciences industry continues to adopt eCTD Publishing Tools for submissions to regulatory agencies and submissions, the need to secure sensitive data has never been more important. Data breaches can result in a variety of implications, from regulatory repercussions and irreparable harm to a business's reputation.
When they prioritize the security and confidentiality of data pharmaceutical and biotech companies will be able to traverse the cyber world with confidence, build trust with regulatory agencies as well as others, and guarantee the continuous success of their data submissions.
0 notes