#dimethylsulfoxide
Explore tagged Tumblr posts
Text
something annoying that has been happening to me for a few years now, is that raw meat smells awful to me no matter what. it can be completely fresh, way before the sell by, and perfectly refrigerated. makes me gag. I made orzo with ground beef and tomato today and took Lu out for a walk and came back in and just got super paranoid, sniffing around my apartment for something rotten but I think it was just lingering meat smell from the packaging so I took out the trash and put on a candle but my nose is just. as soon as I get a bad whiff I can't get rid of it. dog pee, dimethylsulfoxide, other sulfonyls and amides and amines, I have such a low detection limit that I can tell when someone's been working with them a mile away.
it's fine when it's chemicals but MEAT is a problem 😖 I need to cook!!!!! without making myself sick from the smell 😖
11 notes
·
View notes
Note
what is your favorite chemical and which touhou would you kin assign it?
i don't think chemicals have deep mental connections to fictional characters, so i wouldn't kin assign any character to any chemical. that said, i can tell you my favorite chemical and the touhou i think fits it best!
or at least i could, but asking a chemist their favorite chemical is like asking a meteorologist their favorite cloud. i'll give you an unordered list of five faves and their touhous.
Carbon tetrachloride. Terrible for absolutely everyone, it's highly carcinogenic, hepatotoxic, mildly neurotoxic, and depletes the ozone layer. However, it was used for many years as a refrigerant, dry cleaning agent, and fire extinguisher, because it's inert and has a convenient boiling point. It was also used as a NMR solvent for many years because it doesn't have any hydrogen atoms, and therefore won't interfere with the hydrogen spectrum of your analyte molecule. Nowadays, though, deuterated compounds are much more common, so those are used as solvents instead (deuterium has a different gyromagnetic ratio to hydrogen). Common ones are deuterated chloroform, deuterated dimethylsulfoxide, and heavy water. The touhou who I think fits this best is Eirin. She's very helpful and has a number of unique skills you might want to go to her for, but thanks to her crap personality and her vague disregard for life the consequences are likely to outweigh the benefits of her lending her your aid.
Tungsten carbide (Ni-alloyed). What can I say about this guy that hundreds of others haven't already said? It's extremely hard, still fairly good at not cracking under pressure, and- here's the awesome thing- entirely non-magnetic. If you want to service anything in a room with several dozen Teslas of magnetic field strength running around and your titanium or aluminum bronze tools are stripping because some guy doesn't know that screws only need to be so tight, tungsten carbide is your guy. Just be careful to only use the Ni alloy- Co and Fe are obviously ferromagnetic. The touhou which this fits best is Reimu, because it'll do basically anything in its purview with a little grumbling, but keeping it happy is quite expensive.
Acetone. It's not a universal solvent, but it's cheap as hell and useful for practically anything that soap won't get. Its only problem as a household cleaner is that it's so good at dissolving things it'll also partially dissolve most plastics, but it's such a friend in the lab (and on hard surfaces) that I'll not dock it points. The touhou that this best fits is Marisa. Ordinary, applicable to practically any situation, but not always the best at it. Still, helpful practically everywhere.
Dimethyl mercury. King of reminding you that just because you work in an analysis lab doesn't mean you don't have to practice lab safety (though the chloroform really should do that already.) If you're unaware, dimethyl mercury killed a prodigious toxicologist and NMR spectroscopist at Dartmouth way back in the day. She was trying to create a standard to measure frequencies against, you see, but it got on her glove. At the time, the permeability of dimethyl mercury through polymers was not well known, so she and her colleagues assumed that as she had been wearing gloves, she would be fine. However, nowadays we know that the only gloves suitable for wearing when one works with dimethyl mercury are Silver Shield type. By the time she found out she had mercury poisoning, unfortunately, she didn't respond to chelation therapy and passed away. The touhous (plural) that this chemical is best represented by are the entire Hifuu Club. If you poke around in unknown places, you may discover useful and fascinating things (such as ways to treat mercury-contaminated water) but because these things are unknown, there's always the possibility that you poke around a bit too far and never come back.
[Polymer redacted so I don't doxx myself for being in one of three labs that works on it]. The touhou that this is best represented by is Nue Houjuu, for the transformation powers and difficulty of understanding it.
13 notes
·
View notes
Text
NOOOO NOOOOOOO THAT'S SO MANY HYDROGENS. YOU WON'T BE ABLE TO SEE ANYTHING.
Please dissolve your Bocchis in deuterated chloroform or deuterated dimethylsulfoxide.
dissolving bocchi in thf and putting her in the mri machine
42 notes
·
View notes
Photo

DMSO (Dimethyl Sulfoxide) Online India
Asterveda’s dmso(dimethyl sulfoxide) is 100% natural, manufactured using tree extracts. It is intended to be served as a solvent only. The choice of purpose and administration method is the sole responsibility of the user. We have the best DMSO online in India.
#DMSO#dimethylsulfoxide#Best dmso online#Online dmso#best supplement for pain relief#dmso 99.995% pure dmso#dmso liquid#dmso for pain#dmso for joint pain#dmso for skin#dmso for toe toe fungus#dmso for bladder#best dmso in India#best dmso supplement online#best dmso supplement in India#best dmso online india#best dmso online in india#best dmso supplement#buy dmso online#buy best dmso online#buy best dmso india
0 notes
Link
#msm40#msm60#msmforhorses#dmso2#dimethylsulfoxide#methylsulfonylmethane#msm#msmcrystals#msmpowder#msmpure#dimethylsulfone
0 notes
Text
Journals on Medical Microbiology

Inhibition of CK1 affects Viability and Survival of Glioblastoma Cells by Joachim Bischof in Biomedical Journal of Scientific & Technical Research (BJSTR) https://biomedres.us/fulltexts/BJSTR.MS.ID.000735.php
For more articles on biomedical open access journals please click here: https://biomedres.us/index.php
For bjstr journals
#biomedicalsciencejournal#Glioblastoma#dysregulated#difluoro-dioxolo#Dimethylsulfoxide#Diphenyltetrazolium
0 notes
Text
MSM


Scientific Names: Methylsulfonylmethane Other Common Names: Crystalline DMSO (dimethylsulfoxide), dimethyl sulfone, DMSO2, methyl sulfone, sulfonyl sulfur, mineral sulfur Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Seasonal Allergic Rhinitis: 😐 At this time, only one case series has examined the effects of MSM. Although results were promising, the evidence is insufficient to recommend MSM for this indication. Usual dose is 2600-5200 mg per day in divided doses.
Osteoarthritis: 😐 After a tiny pilot study demonstrated some benefit with 2250 mg//day, two trials have noted benefit for OA symptoms with doses of 500 TID (with and without glucosamine) and up to 3 gm BID. At all doses, side effects were minimal. More research is warranted to determine extent of efficacy and long-term safety. At this time, evidence is insufficient to support a general recommendation for use, especially in MSM in light of safer and more well-studied dietary supplement options, such as glucosamine. Patients taking combination glucosamine and MSM products do not necessarily need to be discouraged from using but should be closely monitored. The usual dose is 1500-3000 mg per day in divided doses.
Chemistry/Pharmacology: MSM is the crystalline oxidation product and metabolite of DMSO (dimethylsulfoxide), an industrial solvent that also has FDA approval for treatment of interstitial nephritis. The compound occurs in animal and human adrenal glands, milk, and urine, as well as many food plants and fruits. As dimethyl sulfoxide, MSM is considered a sulfur source for amino acid synthesis. It is thought that sulfur donation may be responsible for effects in arthritis and other connective tissue diseases. Animal and in vitro studies of DMSO suggest free radical scavenger properties, as well as a reduction in synovial proliferation, and decreases prostacyclin and prostaglandin synthesis. It is unknown if oral MSM possesses these same properties.
Drug Interactions: No known drug interactions exist.
Contraindications/Precautions: Use with caution during pregnancy and lactation due to unknown effects. Use with caution in patients with a sulfa allergy. Note: Commercially available MSM may be prepared from industrial-grade DMSO, rather than from pharmaceutical-grade DMSO, resulting in potential for impurities.
Adverse Effects: May cause gastrointestinal distress, cramping, and diarrhea, pruritus, an increase in allergy symptoms, and headache.
#sigler dietary supplement drug cards#2nd edition#msm#methylsulfonylmethane#crystalline dmso (dimethylsulfoxide)#dimethylsulfone#dmso2#methyl sulfone#sulfonyl sulfur#mineral sulfur#drug facts
1 note
·
View note
Link
0 notes
Text
Chemical Warfare in Shadowtech for Shadowrun (1st Edition)
DMSO
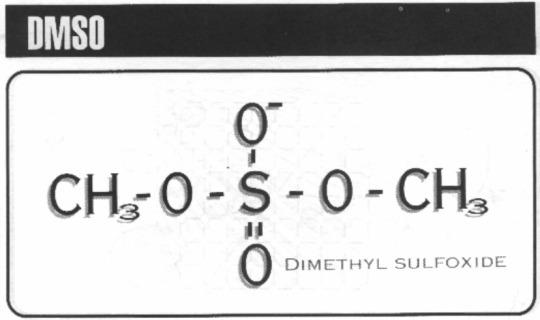
Mmm… just check out that pair of methyl groups…
Also known as DiMethylSulfOxide, also known as (CH3)2SO, this chemical acts as a skeleton key to your skin’s pores, allowing for rapid transdermal delivery of whatever substances are mixed with the delivery chemical. You can either patiently wait for your target to stand still while you gently brush it on, or violently splat them with the…
Ares Squirt
Just as a warming to readers, if you do an image search for “Ares Squirt” you get two DRASTICALLY different types of results, only one of which is relevant for our purposes.

This particular weapon wouldn’t get a picture until the 2nd Edition Corporate Security Handbook, and I certainly am not searching for the Ares Supersquirt after what I’ve seen.
This is, literally, a squirt gun. A messier alternative to the Narcoject Pistol.
Atropine

Atropine is a tranquilizing nerve agent derived form the belladonna (nightshade) plant.

That’s right. Another Anthrax reference.
ACTH
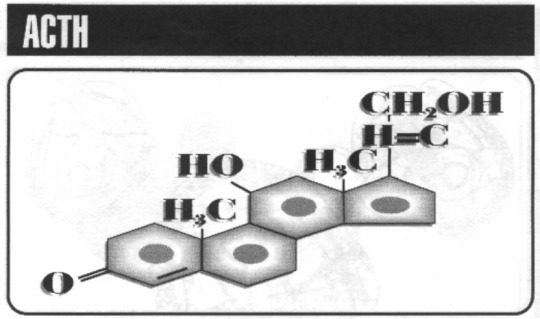
The AdrenoCortiTrophic Hormone is what’s responsible for stimulating the adrenal gland in the human body, or in the Sixth World, for kick-starting the Adrenal Pump.
Because there’s nothing more badhoop than hitting an inhaler before combat.
MAO

No, not that one.
There we go. MonoAmine Oxidase is an enzyme that oxidizes monoamines.
Duh.
If ACTH is the “On” switch for your Adrenal Pump, then MAO is the “Off” switch. Regardless of bioware, a successful hit with MAO reduces Reaction by 1 and takes away an Initiative die – which if you’re not cybered, severely slows you down.
8 notes
·
View notes
Text
chemistry blog
1. Introduction
Today, over 90% of the world‘s organic chemicals are produced from petroleum, and 85% of crude oil is used for the production of transportation fuel. Due to the depletion of fossil resources and global warming concern, considerable attention was focused on the conversion of renewable biomass to chemicals and fuels. Among these chemicals, 2,5- furandicarboxylic acid (FDCA) has received significant attention as potential replacement for terephthalic acid for the production of poly (ethylene terephthalate) (PET). A FDCA-based polymer poly(ethylene-2,5-furandicarboxylate) (PEF) has been prepared and investigated, which showed comparable properties to PET. FDCA was also listed as one of the 12 key value-added chemicals from biomass by the US Department of Energy.
Currently, there is a growing attention on the production of fuels and commodity chemicals from the renewable resources. Biomass is one of the most abundant renewable resources in the earth. Through bio refinery, both liquid fuels and organic chemicals can be generated from biomass.
5-Hydroxymethylfurfural (HMF), one of the 12 top value added chemicals, has received great attention for the past decades. HMF owns one hydroxyl group, one aldehyde group and furan ring, and thus it can be served as versatile precursor for the synthesis of a variety of chemicals and value added fuels. For example, the catalytic hydrogenation of HMF can generate 2,5-dimethylfuran, which has a high energy density. Through the catalytic oxidation reactions, several kinds of furanic compounds with very important application in many fields can be produced, including 2,5-diformylfuran (DFF), 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), 5-formyl-2-furancarboxylic acid (FFCA).
HMF can be obtained from the dehydration product of C6 based carbohydrates, such as fructose, glucose, cellulose even lignocelluloses. The dehydration of carbohydrates (mainly fructose) has been early performed by the use of mineral acids as the catalysts. However, the use of mineral acids exhibited many drawbacks such as their corrosive and non-recyclable nature, and its wastes to environment, which is unsuitable in the chemical industry. Catalytic conversion of carbohydrates into HMF over heterogeneous catalysts can overcome the drawbacks caused by the use of homogeneous catalysts. Besides to the catalyst, the dehydration of HMF is also reported to be affected by the reaction solvent. Water is a green solvent for chemical reaction, but it is not suitable for the dehydration of carbohydrates to HMF, as the water can accelerate many side products such as levulinic acid (LA), formic acid (FA) and humins. In contrast to water, ionic liquids with unique physicochemical properties are proved to be good reaction medium for the conversion of carbohydrates into HMF. However, the large-scale synthesis of HMF is limited by the high cost of ionic liquids. Encouragingly, dimethylsulfoxide (DMSO) shows high ability to dissolve carbohydrates, stabilize HMF and reduce side-reactions. Thus, good results of the dehydration of carbohydrates can also be attained in DMSO.
The production of HMF has been extensively reviewed by many researchers, including some analyses of the catalytic systems used and the underlying mechanisms. The availability of the functional groups, C=O, C-O and furan ring, allows HMF some flexibility in its conversions by multifunctional catalysts and in the future production of many value-added chemicals.
Hydrogenolysis of the C=O and C-O groups of the side chains can lead to 2,5-dimethylfuran (DMF), a promising fuel candidate with similar properties to commercial gasoline.Hydrogenation of the C=O bonds and the furan ring offers access to useful monomers for bio-based polyesters, including 2,5-dihydromethylfuran (DHMF) and 2,5- dihydromethyltetrahydrofuran (DHMTHF). Etherification of HMF can afford 5-ethoxymethylfurfural (EMF), which was an excellent additive for diesel. Opening of the furan ring and subsequent hydrogenation can produce 1,6-hexanediol, an important diol for the synthesis of caprolactone and caprolactam. Long-chain alkanes with targeted molecular weight can be obtained by aldolization/crotonization of the side-chain C=O group followed by complete hydrogenation. 3-(Hydroxymethyl)cyclopentanone (HCPN), a raw material for producing fragrances, drugs and solvents, can be produced by hydrogenation and ring rearrangement reactions. A DielsAlder reaction of DMF can yield p-xylene (PX). Finally, oxidation of the C-O/C=O bonds gives 2,5-diformylfuran (DFF) and 2,5-furandicarboxylic acid (FDCA), which serve as building blocks for polymers.
FDCA can be prepared by oxidation of HMF, while HMF is prepared by acid-catalyzed dehydration of sugars or cellulose. HMF could be oxidized to FDCA with stoichiometric oxidants, metal catalysts, or enzyme. Among these, the catalytic conversion of HMF with oxygen is more attractive. The most studied catalysts are Au, and Pt usually with base additive and at elevated temperature. High or even quantitative yield of FDCA can be obtained. However, the product obtained from these catalytic systems under basic condition is the salt form of FDCA which cannot be directly used in polymer industry. The separation of FDCA from aqueous system usually requires the addition of strong mineral acids such as HCl and H2SO4 pH =1, where FDCA will then be precipitated from the solution as white precipitate. In contrast, the conversion of HMF to FDCA under base-free conditions would eliminate the need to convert FDCA salt to FDCA, making the system greener with less waste generated.
In fact, our study for the conversion of HMF to FDCA with Ru/C catalyst started with the addition of equivalent amount of base. It was found that stronger base led to lower FDCA yield. For example, when NaOH was used as base, it gave only 69% FDCA yield. The color of the reaction mixture turned brownish due to the degradation of HMF at higher pH. To minimize this side effect, weak bases were tested in the reaction system. It is clear that the weaker the base used, the higher the FDCA yield was obtained.
On the other hand, with limited fossil resources, the search for chemicals and fuels from renewable sources has recently attracted considerable interest. Biomass is the only carbon containing renewable resource. 5-Hydroxymethylfurfural (HMF) is regarded as a value-added platform chemical. Several important furan chemicals can be obtained by the oxidation of HMF including 5-hydroxymethyl2-furancarboxylic acid (HMFCA), 2,5-diformylfuran (DFF), and FDCA. In contrast to HMFCA and DFF, FDCA has received a great deal of attention as an excellent candidate as a monomer for the synthesis of polymers. FDCA has the ability to replace terephthalic acid in the synthesis of polyamides, polyesters, and polyurethanes. There are many reports on the oxidation of HMF into FDCA. In one such report, homogeneous metal acetate catalysts including Co(OAc)2, Mn(OAc)2, and HBr were applied for the synthesis of FDCA from HMF at 70 bar air pressure in acetic acid. Recently, a similar reaction system was also developed for the transformation of HMF into FDCA in the presence of homogeneous catalysts [Co(OAc)2/Zn- (OAc)2/NaBr]. However, it is difficult to recycle homogeneous catalysts. Recently, heterogeneous catalysts have been widely utilized for chemical reactions. Among various heterogeneous catalysts, supported gold nanoparticles have been extensively used for the oxidation of HMF into FDCA. Although some of the reported methods produced high FDCA yields, some drawbacks were still present, including the high cost of the noble catalyst, high oxygen pressure, and use of base additives.
Extensive research on the hydrothermal conversion of organic waste and low-value organic materials into fuels and chemicals has demonstrated that hydrothermal reactions can convert various natural organic materials directly and efficiently into useful chemicals, synthetic organic materials like plastics into oil and ultra-heavy crude oil like bitumen into light oil. Some works on the hydrothermal conversion of biomass, the chemical reactions for specified compounds and the roles of HTW in chemical reactions have been described in review articles. Tester et al. reviewed hydrothermal technology for converting biomass into liquid and gaseous fuels, and Watanabe et al. reviewed chemical reactions for C1 compounds in sub- and supercritical water. Akiya and Savage reviewed the role of HTW in chemical reactions.
HTW exhibits properties that are very different from those of ambient liquid water. The ion product (Kw) at 250–300 C is about three orders of magnitude higher than that of ambient liquid water. The natural abundance of hydronium and hydroxide ions suggests that some acid- and base-catalyzed reactions may proceed easily in HTW without the addition of a catalyst. Research into the catalytic role of acids and bases in sub- and supercritical water has studied model acid/base catalyzed reactions, such as ether reactions, the hydrolyses of esters, the dehydration of alcohol and alkylation reactions. This section outlines the base catalytic role of HTW in the conversion of carbohydrates into lactic acid and the acid catalytic role in the conversion of carbohydrates into 5-hydroxymethyl2-furaldehyde (HMF).
Research into the acid catalytic role of HTW is more active than into the base catalytic role. Many studies have found that a large quantity of HMF, a product of the facile acid catalyzed dehydration of hexoses, is formed in the hydrothermal treatment of carbohydrates without addition of an acid catalyst. The formation of HMF may then be attributed to either an acid catalytic role of subcritical water or autocatalysis by acidic product formation. Experiments in the presence of formic and acetic acids, 500– 1000 ppm each, with the aim of recognizing the autocatalytic potential of formic and acetic acids showed that acetic and formic acids do not significantly catalyze the reaction. It has been suggested that HTW may act both as an acid and base catalyst. Experimental results give support to this thinking, as it has been shown that lactic acid is observed in experiments where 0.02 mol L1 H2SO4 is used, whereas HMF and 2-furaldehyde (2-FA) are observed when 0.02 mol L1 NaOH is used.
The examination of the effect of temperature on the yields of lactic acid and HMF show that the highest yields of both HMF and lactic acid are obtained in the temperature range of 280–300 C. High yields at these temperatures imply that the ionization constant has a strong influence on the reaction chemistry.
Generally, hexoses undergo dehydration to form HMF, whereas pentoses undergo dehydration to form 2-furaldehyde (2-FA). Many researchers have found, however, that 2-FA is formed during hydrothermal treatment of hexoses without the addition of catalyst. The mechanism of 2-FA formation from hexoses is unclear. One possible mechanism is that hexose is first degraded to HMF, which then loses a –CH2O group to form 2-FA. The second possible mechanism is that hexose is first degraded to pentose, which then forms 2-FA.
Luijkx et al. have reported that 2-FA can be formed from HMF in supercritical water. In our experiments with HMF at 400 C, the production of a small amount of 2-FA was observed. However, the production of 2-FA was not observed at 300 C. Furthermore, experiments at 300 C both without and with the addition of HMF to the solution after the hydrothermal reaction of glucose were performed to examine the possibility of intermediate products that might form in the hydrothermal treatment of glucose, but results showed that HMF produces minimal 2-FA. These results may indicate that formation of 2-FA via HMF does not readily proceed in subcritical water.
2. Chapter one: the selective oxidation of HMF to DFF.
1.Classification of catalysts:
A. Heterogeneous catalysis:
Serious study of heterogeneous catalysis has proceeded with ever-in- ^ creasing intensity for some 70 years; by contrast, serious study of homogeneous catalysis by transition metal salts and complexes began only a decade ago. It is scarcely surprising therefore that heterogeneous catalysis has achieved a cardinal position in chemical industry, whereas the application of homogeneous catalysis (although not slow to start) has yet to achieve a similar prominence. Among the considerations discussed in this volume is the question of whether homogeneous catalysis may in a generation have relegated many heterogeneous catalytic operations to the lumber-rooms of chemical technology.
Although heterogeneous catalysis has a good head start in its applications and usefulness because of its chronological advantage, theoretical understanding of its phenomena has not progressed as rapidly. It is no exaggeration that some homogeneously catalyzed reactions are understood as well after five years study as some heterogeneously catalyzed ones are after 50. The reasons for this are not hard to find: the application of modern physical methods (particularly spectroscopic ones) to detecting intermediates, and the simplicity and reproducibility of homogeneous systems, permits the specification of reaction mechanisms with a facility which is the envy of those who are restrained to multiphase systems. Those so restrained have hoped, not unreasonably, that the rapid advances we are witnessing in understanding homogeneous mechanisms will assist the resolution of some of the seemingly intractable problems in the heterogeneous field.
Heterogeneous Catalytic Properties of the Group VIII Metals In addition to their ability to atomize molecular hydrogen ( an ability widely shared by other d metals although not to the same extent by sp metals), the Group VIII metals are outstanding in their propensity to catalyze the hydrogénation of unsaturated functions, for example C=C , C=C , C=0 , C=N , NNN , 0=0 , N02 etc. Between the metals there are however considerable differences in activity, selectivity, and stereospecifity shown; thus, all the Group VIII metals catalyze the hydrogénation of oxygen, olefins, and acetylenes, while the facility to synthesize ammonia is limited to the Fe, Ru, Os Group. Hydrogenolysis of carbonhalogen bonds and, under more vigorous conditions, of C— C bonds is also catalyzed by these metals. A practical difficulty often encountered is the lack of specificity shown by heterogeneous catalysts; for example, supported palladium catalysts will convert a chloronitrobenzene to a mixture of chloroaniline and aniline, and undesirable selective poisoning procedures often must be adopted to obtain the desired result. Some of the Group VIII metals have uses as oxidation catalysts. A l l except platinum do however tend to oxidize under vigorous conditions, such as are used in ammonia oxidation and the Andrussow process, for which only platinum and its alloys are acceptable catalysts. Under milder conditions both platinum and palladium have somewhat limited applications in liquid-phase oxidation processes, as for example in the carbohydrate field.
B. Homogeneous catalysis:
The type of catalysis in which both the reactants and the catalyst are in the same phase with each other. Like if we have reactants in the gaseous phase, the catalyst which is to bring about the reaction is also in the gaseous state. That is they share the physical state with each other.
The main areas which have commanded attention to the present are olefin isomerization, hydrogénation, oxidation, carbonylation, and polymerization. Olefin isomerization has been widely studied, mainly because it is a convenient tool for unravelling basic mechanisms involved in the interaction of olefins with metal atoms. The reaction is catalyzed by cobalt hydrocarbonyl, iron pentacarbonyl, rhodium chloride, palladium chloride, the platinum-tin complex, and by several phosphine complexes; a review of this field has recently been published. Two types of mechanism have been visualized for this reaction. The first involves the preformation of a metal-hydrogen bond into which the olefin (probably already coordinated ) inserts itself with the formation of a σ-bonded alkyl radical. On abstraction of a hydrogen atom from a different carbon atom, an isomerized olefin results.
The ability of solutions of salts and complexes of the Group VIII metals to catalyze homogeneous hydrogénation is also widespread; once again hydridic species probably play an important role.
Among the complexes which may function in this way are pentacyanocobaltate ion, iron pentacarbonyl, the platinum-tin complex, and iridium and rhodium carbonyl phosphines. It has been suggested that with tristriphenylphosphine Rh(I) chloride, a dihydride is formed and that concerted addition of the two hydrogen atoms to the coordinated olefin occurs . There are few examples of the homogeneous reduction of other functional groups besides C=C , C=C , and C=C—C=C ; pentacyanocobaltate incidentally is specific in reducing diolefins to monoolefins. Among the several types of homogeneously catalyzed reactions, oxidation is perhaps the most relevant and applicable to chemical industry. The well-known Wacker oxidation of ethylene to ethylene oxide is the classic example, although this is not a true catalytic process since the palladium (II) ion becomes reduced to metallic palladium unless an oxygen carrier is present. Related to this is the commercial reaction of ethylene and acetic acid to form vinyl acetate, although the mechanism of this reaction does not seem to have yet been discussed publicly. Attempts to achieve selective oxidation of olefins or hydrocarbons heterogeneously do not seem very successful.
Unsaturated compounds have been carbonylated by their reaction with carbon monoxide under pressure in the presence of palladous chloride, but supported palladium catalysts will also perform this function. This is perhaps the clearest illustration of a class of reactions which proceeds both homogeneously and heterogeneously with apparent comparable facility. Rhodium chloride catalyzes the polymerization of butadiene with high stereospecificity to trans-poly( 1,4-butadiene) and also the dimerization of ethylene and other olefins . Although certain oligomerizations are catalyzed by solid palladium and rhodium catalysts, polymerization to high molecular weight products is not generally observed.
In one field, although restricted, there is a reasonably close analogy between the reactivity of olefins under reducing conditions in both homogeneous and heterogeneous catalytic systems. We now turn our attention to possible explanations of the observed anomalies and to the causes of the different behaviors shown by the two systems in oxidation and polymerization. The major anomaly in olefin reactions is the superior ability of Fe, Co, and N i to act as hydrogénation catalysts in comparison with expectations based on the strength of olefin bonding in complexes. Olefins are quite strongly chemisorbed by these metals; we must therefore infer that the presence of several metal atoms in proximity sometimes confers a binding ability not possessed by single atoms. Whether this effect is of particular importance with these metals or whether it will prove to have general significance is not certain at this time and will clearly be a matter for future debate. Structure determinations of organometallic complexes performed in recent years have vastly widened our concepts of chemical bonding, and of particular relevance to our problem are the recently investigated complexes of alkynes with multi atom clusters. It will be interesting to see whether such multi atom clusters have catalytic properties.
We now consider homogeneously catalyzed oxidation and polymerization—both of which do not have strict heterogeneous counterparts. Taking the Wacker oxidation of ethylene to acetaldehyde as an example, it appears that the central role of the Pd atom is to act as an electron acceptor, permitting the first formed (HOCH 2 C H 2 PdCl 3 ) 2 ~ species to form the HOCH 2 C H 2 + carbonium ion, which subsequently rearranges to CH 3 C H O and H + . The accumulation of negative charge on the PdCl 3 moiety is released only by disruption to Pd° and 3C1". Strict analogy in a heterogeneous system is therefore not to be expected since surface metal atoms cannot similarly be reduced, and some alternative means of releasing the negative charge would have to be found.
C. Noble metal catalysts:
Metals that are corrosion resistant and offer oxidation in moist are known as noble metal catalysts. Noble metals include platinum (Pt), Osmaium (Os), palladium (Pd), ruthenium (Ru), silver (Ag), iridium (Ir), and gold (Au).
All of the catalysts presently used for purification of automotive exhaust contain the noble metals Pt, Pd or Rh as active components. Because of the high cost and limited availability of these metals, it is important to use as low a noble metal concentration in these catalysts as feasible. This is accomplished by keeping the active metals at a high degree of dispersion. Two patents claimed that this can be achieved by adding a promoter such as Ce02. It is important, however, to understand the ways in which efforts to reduce the noble metal content of the catalysts affect catalytic activity. It is well known, for example, that the degree of dispersion, the nature of the catalyst support, and the presence of the promoter affect the specific catalyst activity, the selectivity, and the kinetic parameters. Furthermore, catalysts are routinely exposed to high temperatures of 600°C and above for varying periods of time. This thermal aging can cause severe sintering and decreased dispersion. Thus the kinetics of the reaction over an aged catalyst may be different from those of a fresh catalyst. We have made a detailed study of the kinetic parameters of the total oxidation of CO and the model hydrocarbons as a function of the following variables: (1) nature and concentration of noble metal, Pt, Pd, and Rh; (2) bulk metal versus supported catalysts; (3) degree of dispersion; (4) thermal aging; (5) presence of the promoter, CeO,; (6) effect of pretreatment; and (7) operating conditions, e.g., oxidizing or reducing.
The results for CO and olefin oxidation over the supported catalysts with or without CeOz are complex. The kinetic parameters suggest that there is more than one type of catalytic sites, each having a different set of kinetic parameters. For convenience, we call the kinetic behavior that closely resembles that obtained over the wire samples as type I kinetics. The conversion curves for this type of kinetics generally show the light-off phenomenon, a sharp rise in conversion from 20 to 100% within a very narrow range of temperature. The kinetics of CO and olefins oxidation over many low PM concentration catalysts, particularly those containing CeOz, differ sharply from those of the wires. In general, they are less inhibited by CO or hydrocarbon (over Pt or Pd) or by O2 (over Rh) and show a smaller partial reaction order with respect to the other reactant. The activation energies for CO oxidation are also much lower.
Comparison of the catalytic activity of the various catalysts can only be made among catalysts with approximately the same kinetic parameters. Among the PM/ A1203 catalysts exhibiting predominantly type I kinetics and for which the approximate surface areas are known, the rate of CO oxidation per unit PM surface areas varies within 0.2 to 0.6 of the value found for the respective wire. In view of the fact that the areas of the wires are underestimated (the geometrical area without correction for the roughness was used) and the large range of metal dispersions covered in the results, the specific rate of CO oxidation can be considered to be independent of the presence of the A&O3 support. The specific oxidation rates of C3H6 over the type I sites of the PM/A1203 catalysts are at least one order of magnitude lower than those over the PM wire. This may be rationalized by the size effect, i.e., C3H6 is a larger molecule than CO and may require several adjacent sites for adsorption, thus not all the surface metal sites are usable for C3H6 oxidation.
D. Transition metal catalysts
Transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to adsorb other substances on to their surface and activate them in the process.
Ziegler-Natta (Z-N) and metallocene-alumoxane type catalyst systems for the polymerization of olefins are well known in the art. Recently a new, ionic pair type of catalyst has been developed which yields polymers of improved properties compared to those made with conventional type catalysts systems. It discloses new cyclopentadienyl based catalyst systems comprising an ionic compound wherein the cyclopentadienyl transition metal component or metallocene is reacted with an activator comprising an anion and a cation; the cation being one which is reactable with a non-cyclopentadienyl ligand of the cyclopentadienyl moiety to yield as the reaction product a neutral ligand derivative, and a cationic metallocene species to which the anion of the activator compound is essentially non-coordinating.
This invention relates to new poly anionic non-coordinating anions or activator moieties comprising a plurality of metal or metalloid atom-containing non-coordinating anionic groups pendant from and chemically bonded to a core component, which can be used to prepare a wide variety of new ionic catalysts compositions which are useful in the polymerization of olefins, di olefins and/or acetyl enically unsaturated monomers. These non-coordinating anions are stabilized by a sufficient number of cations to balance charge on the composition or catalyst formed. When exposed to unsaturated monomers, the polymerization catalyst of this invention yield a wide variety of homo or copolymers having variable molecular weight, molecular weight distribution and comonomer content.
In one application, the poly anionic activators are used to prepare a catalyst system of enhanced performance by immobilizing the catalyst on a catalyst support material. The heterogeneous, or supported, catalyst of this invention can be used in a wide variety of commercial processes including gas phase, slurry or fixed bed reactors.
Catalytic oxidation of HMF to DFF
Selective oxidation of 5-hydroxymethyl-2-furfural (HMF) to 2,5-diformylfuran (DFF) toward industrial production was studied over Ru supported g-alumina catalyst using molecular oxygen as an oxidant. From the solvents screening, considering recyclability after reaction, toluene was found to be the best solvent and gave maximum conversion of 99% with 97% DFF selectivity at 130 8C and 40 psi O2 pressure. Catalyst was washed with NaOH solution of pH = 12 to remove the adsorbed polymer impurities and then reused up to 5 cycles. The product could be purified by simple evaporation of the solvent, which could add advantage for industrial process.
DFF is among highly potential chemicals which can be applied to furanic polymers, a precursor for pharmaceuticals and antifungal agents, and renewable furan-urea resin. In addition, DFF is a readily handled intermediate of FDCA known as a promising alternative to petroleum-based terephthalic acid (TPA) owing to its poor solubility in most of the organic solvents which renders its application. Several attempts were made to prepare DFF in one step from fructose (one of the biomass-derived carbohydrates) using both homogeneous and heterogeneous catalysts in aprotic polar solvents like DMSO and DMF. However, high boiling points of those solvents have caused the difficulty in isolation of DFF and reuse of solvents. Therefore, selective oxidation of HMF to DFF was preferably explored with a range of oxidizing agents and solvent system. Homogenous metal bromide catalyst (MBr2, M = Co(II)/Mn(II)/Zr(II)) in acetic acid solvent at 70 bar oxygen pressure gave 99.7% of HMF conversion with 61% of DFF selectivity. Oxidation of HMF in water solvent always led to carboxylic acid byproducts which are difficult to separate from DFF. Due to strong oxidative power in water. Homogenous Mn(III) salen complexes gave maximum of 86% DFF yield in buffer–CH2Cl2 system in room temperature using NaOCl as an oxidant and this system found difficulty in reusability. Vanadyl-pyridine complexes (PVP) in homogeneous reaction in DMSO solvent gave 81% conversion with >99% DFF selectivity, however SBA supported vanadyl-pyridine complexes (PVP) gave only 50% conversion with 98% DFF selectivity in 10 bar air pressure. With this regard, the selective oxidation of HMF under recyclable solvent system is still challenging particularly in industrial area.
100 mL of 0.5 mM solution of RuCl3 was taken to which 2 g of gAl2O3 (350–500 mm) was added and stirred at room temperature for 3 h. Filtered and washed with copious amount of water and dried in vacuum oven at 30 8C. All the reactions were carried out in a 50 mL Parr reactor; HMF, solvent, and catalyst were added and reactor was flushed with O2 for 3 times. Pressurized with O2 again and heated up to the desired temperature, the operating pressure was maintained at 40 psi (unless otherwise mentioned); all the reactions were carried for 4 h with 650 rpm stirring. Immediately after the reaction reactor was cooled to room temperature and samples were analyzed with HPLC (Agilent Technologies 1200 series) using 0.001 N H2SO4 buffer and Bio-Rad Aminex HPX-87 H (300 mm 7.8 mm) column.
Powder X-ray diffraction analysis of g-Al2O3 and Ru/g-Al2O3 materials showed that the crystalline nature of the material is slightly increased during ruthenium loading. There are no distinct reflections for the Ru (0) or RuO2 was observed and Ru/g-Al2O3 has the BET surface area of 101 m2 /g. ICP analysis of the samples showed that 1.8 wt% of Ru was supported in g-Al2O3. For screening most suitable reaction media for selective oxidation of HMF, solvent variation studies were performed with a range of readily recyclable solvents with moderate boiling points using Ru/g-Al2O3 at a constant pressure of 40 psi.
Great selectivity toward DFF was shown in alcohol-type solvents including ethanol, 1-butanol, and IPA showed (99%), however, conversion was unsatisfactory (18% in ethanol, 3% in 1- butanol, 40% in IPA). Low boiling point aprotic acetonitrile also gave low conversion of HMF less than 30% though it showed 99% DFF selectivity. DMF and MIBK gave a modest level of conversion (35% in DMF and 44% in MIBK), and on the other hand they also gave FFCA as an over-oxidation product and LA as a deformylated product by along with DFF. The highest oxidation power occurred in water and FDCA that is most oxidized product of HMF was obtained as a major product (24% in selectivity). Good HMF conversion of 88% with DFF selectivity of 40% appeared using 1,4- dioxane as a solvent and it was however observed that (through GCMS) decomposition of 1,4- dioxane took place under this reaction conditions, which led to several decomposed products. Attempts were made to overcome decomposition of 1,4-dioxane by low temperature and low pressure but failed to obtain good conversion. Non-polar toluene showed HMF conversion of 93% with DFF selectivity of 98% that is the highest conversion reported for readily recyclable solvents with a moderate boiling point.
Further to improve the yield of DFF in toluene, optimization of reaction temperature and substrate concentration were carried out. As expected, HMF conversion decreased for low temperature reactions. HMF conversion was less than 70% at 80 8C but 99% HMF conversion could be achieved at 130 8C within 4 h. The DFF selectivity was >97% in all different temperatures and this result was the uniqueness of the toluene solvent.
2 notes
·
View notes
Photo

DMSO Helps Reduce Inflammation
DMSO (Dimethyl Sulfoxide) is used as an anti-inflammatory agent to reduce pain and speed the healing of burns, wounds and musculoskeletal injuries.It may also help to relieve the pain in various conditions such as headache, inflammation, rheumatoid arthritis, osteoarthritis and facial pain. At Asterveda Healthcare one can find the best dmso online in India — 99.9% pure pharma grade.
#DMSO#dimethylsulfoxide#Best dmso online#Online dmso#best supplement for pain relief#dmso 99.995% pure dmso#dmso liquid#dmso for pain#dmso for joint pain#dmso for skin#dmso for toe toe fungus#dmso for bladder#best dmso in India#best dmso supplement online#best dmso supplement in India
0 notes
Link
#msm40#msm60#msmforhorses#dmso2#dimethylsulfoxide#methylsulfonylmethane#msm#msmcrystals#msmpowder#msmpure#dimethylsulfone
0 notes
Video
How to use DMSO | DMSO Uses | DMSO benefits | dimethylsulfoxid | Pain re...
0 notes
Photo

DMSO Gel 75% | Natürliche Heilcremes und Spray, DMSO #Dimethylsulfoxid #DMSOGel online kaufen. Unsere Heilcremes und Gele werden aus rein natürlichen Inhaltsstoffen hergestellt. #DMSO #DMSOcreme #DMSOgel #NatürlicheCreme #dmsospray (at Germany) https://www.instagram.com/p/BuLxDaHBNLI/?utm_source=ig_tumblr_share&igshid=okv4jsezbk4u
0 notes
Text
DMSO (Dimethylsulfoxid) bald nicht mehr verfügbar in der EU?
DMSO (Dimethylsulfoxid) bald nicht mehr verfügbar in der EU?
Die Produktion des einzig, pharmazeutischen reinen DMSO (Dimethylsulfoxid 99,9 vol.%) in Europa wurde eingestellt. Der Grund dafür ist der Aufkauf der herstellenden Fabrik und die Verlagerung des Standortes von Europa nach Texas. (more…)

View On WordPress
0 notes
Text
DMSO -Hybri-Max™ | Dimethyl sulfoxide | D2650 | SIGMA |رهاشیمی
https://www.rahach.com/?p=4190 DMSO -Hybri-Max™ | Dimethyl sulfoxide | D2650 | SIGMA |رهاشیمی ---------------------------------------- ☎️: 02166569720 🌐: https://www.rahach.com/?p=4190 ----------------------------------------- #dimethylsulfoxide #dimethylsulfoxide(dmso) #dimethylsulfoxideionicorcovalent #dimethylsulfoxidemerck #dimethylsulfoxidemsds #dimethylsulfoxideouterworlds #dimethylsulfoxidesds #dimethylsulfoxidesigma #dimethylsulfoxidestructure #dimethylsulfoxideuses #dmso #dmsocellculture #dmsoinnmr #dmsoinpcr #dmsomerck #dmsomsds #dmsonmrpeak #dmsosigma #dmsosolvent #dmsostructure #رهاشیمی #آزمایشگاهی #محصولاتسیگما
0 notes