#corona vaccine biontec
Explore tagged Tumblr posts
Text
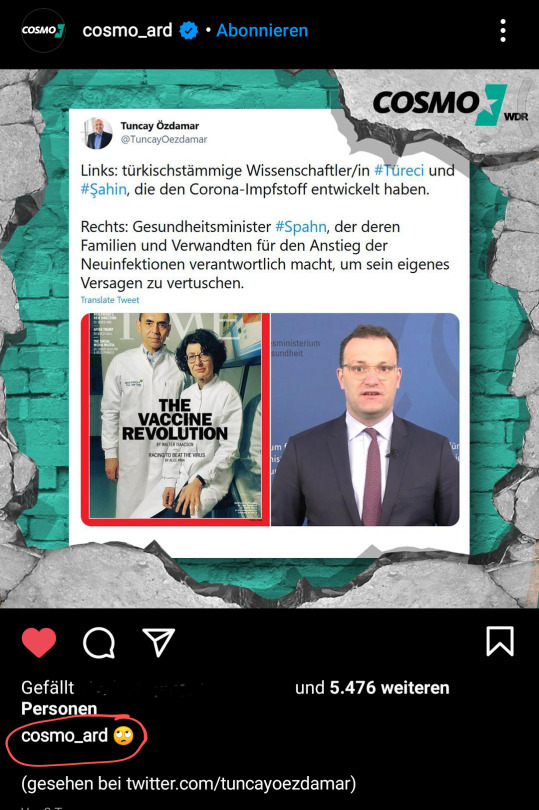
Für sowas zahle ich gerne GEZ
#also nicht ich sondern meine Eltern#aber es lohnt sich#unabhängige Berichterstattung ist wichtig#und politische Bildung auch#deutsche politik#jens spahn#corona vaccine biontec#ard cosmo
18 notes
·
View notes
Text
I received my first dose of the biontech vaccine. If you have any questions, feel free to ask! I will always get vaccinated and hope to answer some questions of people who are unsure.
#covid treatment#covid#covid19#corona#covid lockdown#covid mention#covid 19#vaccine#ask me and i'll tell you#ask me#vaccination#biontech#corona vaccine biontec#please get vaccinated#pro vax#pro vaccination#pro vaccines
1 note
·
View note
Text
कोरोना खत्म नहीं होगा: बायोएनटेक के सीईओ बोले- कम से कम 10 साल तक वायरस हमारे बीच रहेगा
कोरोना खत्म नहीं होगा: बायोएनटेक के सीईओ बोले- कम से कम 10 साल तक वायरस हमारे बीच रहेगा
Ads से है परेशान? बिना Ads खबरों के लिए इनस्टॉल करें दैनिक भास्कर ऐप लंदनएक घंटा पहले कॉपी लिंक ब्रिटेन में कोरोना का नया स्ट्रेन सामने आने के बाद कई देशों ने आवाजाही पर पाबंदियां लगा दी हैं। इंग्लैंड और फ्रांस के बॉर्डर पर ट्रैफिक रोकते सुरक्षाकर्मी। दुनियाभर में कोरोना के नए और ज्यादा खतरनाक स्ट्रेन को लेकर चिंता जताई जा रही है। इस बीच फाइजर के साथ कोरोना वैक्सीन बनाने वाली कंपनी बायोएनटेक…

View On WordPress
#BioNTec CEO Ugur Sahin#corona vaccine#corona vaccine BioNTec#Coronavirus in India#Coronavirus in World
0 notes
Text
खत्म नहीं होगा कोरोना: बायोएनटेक के सीईओ बोले- कम से कम अगले 10 साल तक वायरस हमारे साथ रहेगा
खत्म नहीं होगा कोरोना: बायोएनटेक के सीईओ बोले- कम से कम अगले 10 साल तक वायरस हमारे साथ रहेगा
Ads से है परेशान? बिना Ads खबरों के लिए इनस्टॉल करें दैनिक भास्कर ऐप लंदन11 मिनट पहले कॉपी लिंक ब्रिटेन में कोरोना का नया स्ट्रेन सामने आने के बाद कई देशों ने आवाजाही पर पाबंदियां लगा दी हैं। इंग्लैंड और फ्रांस के बॉर्डर पर ट्रैफिक रोकते ब्रिटिश सुरक्षाकर्मी। दुनियाभर में कोरोना के नए और ज्यादा खतरनाक स्ट्रेन को लेकर चिंता जताई जा रही है। इस बीच फाइजर के साथ कोरोना वैक्सीन बनाने वाली कंपनी…

View On WordPress
#BioNTec CEO Ugur Sahin#corona vaccine#corona vaccine BioNTec#Coronavirus in India#coronavirus in world
0 notes
Text
The Biontech/Pfizer vaccine has been tested on 16+ year old and has been approved by the EU
Quick reminder that vaccines for under-18s is not a viable option. None of the vaccines have been fully tested on under-18s yet.
Also, whilst the government are fucking around with the vaccination schedule, getting vaccinated is no guarantee of protection.
39 notes
·
View notes
Text
Kuwait: 4 vaccine approved for "Corona" passport
Kuwait: 4 vaccine approved for “Corona” passport
Kuwait: 4 vaccine approved for “Corona” passport Kuwait has approved four vaccines to be included in the “Corona” passport, in case it is approved. According to the sources, there are currently only 4 vaccines approved in Kuwait ie Pfizer / Biontec – AstraZeneca / Oxford – Moderna – Johnson & Johnson, while the DGCA will be notified of updates to the list of approved vaccines. Read Kuwait:…

View On WordPress
#expats in kuwait#kuwait#kuwait airport#Kuwait City#Kuwait coronavirus#kuwait latest news#kuwait latest updates#Kuwait News#kuwait news latest#kuwait news today#Kuwait: 4 vaccine approved for "Corona" passport#kuwaiti#latets kuwait news
0 notes
Photo

CORONA cases fluctuate – 1,332 infected
KUWAIT CITY, March 15, (Agencies): Kuwait reported 1,332 coronavirus cases and seven deaths, raising the total number of cases to 210,855 and fatalities to 1,179 on Monday. In a statement to KUNA, the Ministry of Health’s official spokesperson Dr Abdullah Al-Sanad said there are 219 patients in ICUs, adding that 14,169 patients are still receiving medical care. The number of swabs reached 7,365 in the past 24 hours with infection rate of 18.09 percent, raising the total number of swabs to 1,914,579, he noted. Earlier today, the ministry announced that 1,332 people recovered from the virus, raising the total number of recoveries to 210,855. While the number of people who have taken the injection for the prevention of COVID-19 in Kuwait increased to 408,000 people, at a rate of 10% of the population, Al-Sabah Specialized Medical District has begun to take an inventory and prepare lists of the names of patients who are hospitalized at centers affiliated to the hospital for long periods to prepare them for the corona vaccine.
The Al-Seyassah daily has learned from well-informed health sources that the head of the Public Health Services Department in the Al-Sabah Specialized Medical District, Dr Samira Al-Qabandi, in an address to the Director of Al- Sabah Medical District, Dr Ahmed Al-Shatti and the directors of hospitals and specialized centers has called for making a list of all patients who have been hospitalized for long periods of time to receive one of the two vaccines against the corona pandemic either Pfizer Biontec or Astra- Zeneca ‘Oxford’. However, Al-Qabandi said for the immunization of patients the approval of the attending physician is needed to know if the patient’s health status allows him/her to be vaccinated, and to write a medical report on each case separately.
Approval This is in addition to the patient’s family giving approval for vaccination. Meanwhile, a recent scientific study published in the British medical journal ‘Biomed’, ophthalmologist consultant at the Ministry of Health, Dr Yousef Al-Dhafiri, demonstrated the effectiveness of using sonar to treat intraocular pressure during cataract operation. Al-Dhafiri said in a press statement he conducted a scientific research on 60 patients in Kuwait. He added the period of research lasted two years and the intraocular pressure was reviewed for all patients with the use of drops to treat intraocular pressure or without the use of those drops, and it was found during the observation period the eye pressure decreased significantly, most of who were dispensed with eye drops.
Al Dhafiri advised that ultrasound and cataract should be performed in one operation in the event of eye pressure because of its great benefit in the long term, as well as reducing the use of ‘glaucoma’ drops, which may cause damage to the optic nerve. Director General of the Public Authority for Manpower (PAM) Ahmed Al-Mousa confirmed that at present, the authority has no restriction for the private sector to recruit the medical staff they need, reports Al-Rai daily.
Al-Mousa explained in a statement to the daily that the private medical sector can hire the medical professionals they need through the new procedures which are in place in light of the repercussions of the corona pandemic by submitting a formal request to the corona Emergency Ministerial Committee which has been tasked to assess the situation. He pointed out that once the request is approved, the committee’s approval will be attached to the necessary documents for PAM to issue the work permit and the Ministry of Interior to issue the visa; thereby, completing the recruitment process.
The post CORONA cases fluctuate – 1,332 infected appeared first on ARAB TIMES - KUWAIT NEWS.
Read full article: https://expatimes.com/?p=19147&feed_id=38261
0 notes
Text
Cebuano News: Sinovac gitandi sa bakuna sa Moderna, Pfizer, Astra
#PHinfo: Cebuano News: Sinovac gitandi sa bakuna sa Moderna, Pfizer, Astra
TAGBILARAN CITY, Bohol, Jan. 26 (PIA) -- Samtang ang mga dagko ug inila nga mga kompanya sa kalibutan nagkamolo pa og tiwas sa katapusang hugna sa medical trials alang sa ilang bakuna batok sa corona virus disease (COVID-19), dagway og ang China, daku na og pagka abante sa ilang duha ka mga bakuna pinaagi sa Sinovac ug sa Sinopharm.
Unsa man ang kinaiya sa sa mga bakuna nga ginama sa China kon itandi sa mga bakuna nga mugna sa Russia, America, Britanya ug uban pang nasud nga nangandoy nga makahimo sa ilang kaugalingon nga bakuna batok sa sakit nga COVID?
Sebun sa pagpanuki-duki, ang Sinovac ginama sa usa ka biopharmaceutical company nga Sinovac nga nakabase sa Beijing kinsa maoy nagparehistro sa ilang bakuna nga gitawag og CoronaVac, bakuna nga migamit sa gipaluya nga kagaw sa SARS COV 2.
Ang kagaw nga patay na, ug dili na makatakod, maoy gigamit sa Sinovac aron may paon nga maoy dali makit-an dayun sa immune system sa lawas, apan wala nay ikasukol pa, mao nga dili na maka-ingon sa sakit.
Sa laing bahin, ang bakuna nga Moderna ug sa Pfizer BioNTech nga gihimo sa Amerika migamit sa teknolohiya sa messenger RNA (mRNA) sa ilang bakuna.
Pinaagi sa messenger RNA, kini ang magbitbit sa genetic code sa coronavirus sa higayon nga itupok kini sa lawas.
Ang mensahe nga nakasuwat sa RNA mao ang mandu nga magsugo sa paghimo sa antibodies nga may mga viral proteins, aron dili na kinahanglan pa nga sudlan pa og mismo kagaw o parte sa kagaw nga maoy itupok, aron mabansay ang immune system sa lawas sa pag-asdang sa kagaw nga mosulod.
Ang pamaagi sa Coronavac subay sa kinaraang paagi sa paghimo og bakuna nga una na nga malampusong gigamit sa kapid-an na nga panahon, sama sa bakuna sa rabies, polio.
Sa laing bahin, ang "mRNA vaccines bag-ong matang sa bakuna ug wala pay malampusong ehemplo nga masulti niining bag-ong teknolohiya, ilabi na sa epekto niini sa mga katawhan.
Samtang ang mga bahuna nga mitumaw karon, gipadali aron may dinali-an nga magamit, kini silang tanan kinahanglang mosubay sa tulo ka hugna nga medical testing.
Samtang nagpadayun ang ikatulo ug katapusang hugna sa mga testing sa kasagarang bakuna, wala pa man makita ang epekto niini, gipagawasan na kini sa mga gobernor sa Emergency Use Authorization aron madapat na kini.
Ang ikatulo nga hugna sa pagtesting sa mga bakuna mogamit sa labing menos 30 mil ka mga tawo sa nagkadaiyang edad, lugar, kahimtang sa lawas ug sex aron ilhon ang epekto niini alang sa tanan.
Segun sa mga dokumento sa unang mga medical testing sa Sinovac, mahimo ang bakuna nga tipigan sa ordinary nga refrigerator sa 2-8 degrees Celsius, sama sa bakuna sa AstraZeneca nga migamit sa genetically engineered nga kagaw nga maog kinaingnan sa sip-on aron maoy sakyan sa timailhan sa COVID.
Ang bakuna sa Moderna magkinahanglan nga tipigan sa -20 degrees Celsius samtang ang bakuna sa Pfizer tipigan sa -70 degrees Celsius.
Niini, ang bakuna sa Sinovac ug ang sa Oxford-AstraZeneca mas sayun nga magamit sa mga pobre nga nasud ilabi na niadtong nakumalisud sa pagpalit sa mga freezers nga sudlan sa daghang mga bakuna.
Labing lisud usab sa mga modeno nga bakuna ang pagpadala niini sa mga lagyo nga lugar, aron ikapanghatag didto, kon itandi sa uban nga mga bakuna. (rahc/PIA-7/Bohol)
***
References:
* Philippine Information Agency. "Cebuano News: Sinovac gitandi sa bakuna sa Moderna, Pfizer, Astra." Philippine Information Agency. https://pia.gov.ph/news/articles/1065017 (accessed January 27, 2021 at 07:55PM UTC+08).
* Philippine Infornation Agency. "Cebuano News: Sinovac gitandi sa bakuna sa Moderna, Pfizer, Astra." Archive Today. https://archive.ph/?run=1&url=https://pia.gov.ph/news/articles/1065017 (archived).
0 notes
Text
The expected vaccine
The expected vaccine
The World Health Organization says that there is a race to produce a very fast vaccine against the emerging coronavirus, which first appeared in China late last year and soon swept various countries.
So far, more than 100 scientific projects have been announced to produce a vaccine in a short period. Some of these projects are promising and are awaited by the Medical community, and the following are the most prominent vaccines that are on top: "Oxford University" Vaccine and "AstraZeneca" It is considered the most promising vaccine in the world and developed by the University of Oxford and produced in partnership with the British "AstraZeneca", it has shown a "strong immune response" on more than a thousand patients. Chinese "Cansino Biologic" vaccine It is being developed with the support of the Chinese "Cansino Biology", and has achieved good results in the production of antibodies in 500 patients in a separate trial. The German-American Biontec-Pfizer vaccine and the French Valneva vaccine Earlier, the British government announced an agreement to produce 90 million doses of other vaccines under development, manufactured by the German-American "Biontec-Pfizer" coalition and the French "Valneva" laboratories. These two vaccines are at the forefront among potential vaccines. The agreement of the concerned parties provides for the production of 30 million doses for the German-American consortium and 60 million for the French group. "American Moderna Vaccine" American Moderna announced, in turn, that clinical trials of its vaccine have entered the final stages, making it the first company to reach this advanced stage. This announcement places Moderna at the forefront of the global race to achieve a vaccine. "Russian Ministry of Defense vaccine" It is the first Russian vaccine that was jointly invented and produced by military specialists and scientists from the "Gamali" Research Center, and it is also ready, according to the Russian Ministry of Defense. According to the World Health Organization, 23 anti-corona virus vaccines are being tested around the world. “To be practical, we expect that by mid-2021 there will be a vaccine that can be widely deployed,” said chief scientist of the World Health Organization, Somaya Swaminathan.
from Blogger https://ift.tt/3aJOO4J via IFTTT
0 notes