#chemistry101
Explore tagged Tumblr posts
Text
A Normal Wednesday
Wiley+: Your Hw grade is a 1.25 :)
Me: *explodes painfully*
Wiley+: It is due Thursday :DD
Me: ~_~
#i fall for it every time#for those who don’t have the honor of wily+ hw#it will show you your grade for the assignment once you come back to finish but it’s not finalized till the due date#so unanswered questions count as wrong and gives me a heart attack#😩😩😩#Wiley+#science#chemistry101#high school#homework
0 notes
Text
Simplification of Precipitation equations: Mole Course (14)
Fundamental Chemistry: Moles and Concentration Calculations Course (14) Precipitation reactions occur when aqueous solutions react to produce a solid precipitate. These equations can be simplified similarly to ion equations. Initially, we list the ions participating in the reaction, then remove the ions that don’t get involved in the formation of the precipitate. Exercise 1 Write the…

View On WordPress
#atom#britishdiploma#chem#chemical reaction#chemilearn#chemistry#chemistry101#chemistry301#chemistrybook#chemistrycourse#chemistryexplained#chemistrylectures#chemistrynotes#chemistrytutor#chemistryundergrade#Course#crushcourse#Discover#Education#environmental#equation#explainedchemistry#Explore#free#freecourse#freelearning#Fundamental chemistry#generalchemistry#IGCSE#learn
0 notes
Text
Published on YouTube: Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules
Published on YouTube: Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules https://reymcauley.blogspot.com/2024/11/published-on-youtube-examples-of.html 20 Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules Are you struggling to understand the rules for writing and naming chemical compounds? 🌟 In this video, we dive deep into 20 clear examples of writing and naming chemical compounds, covering the fundamental chemical formula basics you need! Perfect for beginners in chemistry or those needing a refresher, this guide includes explanations for common compounds like water (H₂O), magnesium chloride (MgCl₂), ammonium sulfate ((NH₄)��SO₄), and many more. Understanding chemical compounds, the charges of cations and anions will help build foundations and basics for naming chemical formulas and also help with writing net ionic compounds. Understanding chemistry naming rules will help your basics in writing chemical equations and also strengthen your understanding fundamental and basic chemistry questions which pivot around chemical formulas and compounds. We'll walk you through essential rules, such as balancing charges in ionic compounds, using prefixes in covalent compounds, and naming acids based on their anion endings. Learn how to master the rules for: 1) Writing chemical formulas for ionic and covalent compounds 2) Naming compounds with multiple charges 3) Using prefixes like "di-" and "tri-" for covalent compounds 4) Correctly naming hydrates and acids 🎬 Make sure to watch till the end for a solid foundation on naming and writing formulas confidently! 📌 Key Examples Covered in this Video: 1. Water (H₂O) 2. Magnesium chloride (MgCl₂) 3. Ammonium sulfate ((NH₄)₂SO₄) 4. Dinitrogen tetroxide (N₂O₄) 5. Hydrochloric acid (HCl) 6. Iron(III) chloride (FeCl₃) 7. Phosphorus pentachloride (PCl₅) 8. Copper(II) sulfate pentahydrate (CuSO₄•5H₂O) …and many more! 👉 If you find this video helpful, don’t forget to like, subscribe, and share with fellow chemistry enthusiasts or classmates. #ChemicalFormulas #NamingChemicalCompounds #ChemistryBasics #HowToNameCompounds #ChemistryForBeginners #WritingChemicalFormulas #IonicCompounds #CovalentCompounds #AcidNaming #HydrateFormulas #LearnChemistry #ChemistryLessons #chemistry #chemistrybasics #chemistry101 Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules published first on https://www.youtube.com/@waqademy12 / from WAqademy https://waqademy.blogspot.com/2024/11/published-on-youtube-examples-of.html via https://www.youtube.com/@waqademy12 via Ray McCauley https://reymcauley.blogspot.com/ November 02, 2024 at 07:22AM
0 notes
Text
Published on YouTube: Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules
Published on YouTube: Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules https://meybellreed.wordpress.com/2024/11/02/published-on-youtube-examples-of-writing-and-naming-chemical-compounds-chemical-formula-basics-chemistry-naming-rules/ 20 Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules Are you struggling to understand the rules for writing and naming chemical compounds? In this video, we dive deep into 20 clear examples of writing and naming chemical compounds, covering the fundamental chemical formula basics you need! Perfect for beginners in chemistry or those needing a refresher, this guide includes explanations for common compounds like water (H₂O), magnesium chloride (MgCl₂), ammonium sulfate ((NH₄)₂SO₄), and many more. Understanding chemical compounds, the charges of cations and anions will help build foundations and basics for naming chemical formulas and also help with writing net ionic compounds. Understanding chemistry naming rules will help your basics in writing chemical equations and also strengthen your understanding fundamental and basic chemistry questions which pivot around chemical formulas and compounds. We’ll walk you through essential rules, such as balancing charges in ionic compounds, using prefixes in covalent compounds, and naming acids based on their anion endings. Learn how to master the rules for: 1) Writing chemical formulas for ionic and covalent compounds 2) Naming compounds with multiple charges 3) Using prefixes like “di-” and “tri-” for covalent compounds 4) Correctly naming hydrates and acids Make sure to watch till the end for a solid foundation on naming and writing formulas confidently! Key Examples Covered in this Video: 1. Water (H₂O) 2. Magnesium chloride (MgCl₂) 3. Ammonium sulfate ((NH₄)₂SO₄) 4. Dinitrogen tetroxide (N₂O₄) 5. Hydrochloric acid (HCl) 6. Iron(III) chloride (FeCl₃) 7. Phosphorus pentachloride (PCl₅) 8. Copper(II) sulfate pentahydrate (CuSO₄•5H₂O) …and many more! If you find this video helpful, don’t forget to like, subscribe, and share with fellow chemistry enthusiasts or classmates. #ChemicalFormulas #NamingChemicalCompounds #ChemistryBasics #HowToNameCompounds #ChemistryForBeginners #WritingChemicalFormulas #IonicCompounds #CovalentCompounds #AcidNaming #HydrateFormulas #LearnChemistry #ChemistryLessons #chemistry #chemistrybasics #chemistry101 Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules published first on https://www.youtube.com/@waqademy12 / from WAqademy https://waqademy12.wordpress.com/2024/11/02/published-on-youtube-examples-of-writing-and-naming-chemical-compounds-chemical-formula-basics-chemistry-naming-rules/ via https://www.youtube.com/@waqademy12 via Maybell Reed https://meybellreed.wordpress.com November 02, 2024 at 06:39AM
0 notes
Text
youtube
20 Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules Are you struggling to understand the rules for writing and naming chemical compounds? 🌟 In this video, we dive deep into 20 clear examples of writing and naming chemical compounds, covering the fundamental chemical formula basics you need! Perfect for beginners in chemistry or those needing a refresher, this guide includes explanations for common compounds like water (H₂O), magnesium chloride (MgCl₂), ammonium sulfate ((NH₄)₂SO₄), and many more. Understanding chemical compounds, the charges of cations and anions will help build foundations and basics for naming chemical formulas and also help with writing net ionic compounds. Understanding chemistry naming rules will help your basics in writing chemical equations and also strengthen your understanding fundamental and basic chemistry questions which pivot around chemical formulas and compounds. We'll walk you through essential rules, such as balancing charges in ionic compounds, using prefixes in covalent compounds, and naming acids based on their anion endings. Learn how to master the rules for: 1) Writing chemical formulas for ionic and covalent compounds 2) Naming compounds with multiple charges 3) Using prefixes like "di-" and "tri-" for covalent compounds 4) Correctly naming hydrates and acids 🎬 Make sure to watch till the end for a solid foundation on naming and writing formulas confidently! 📌 Key Examples Covered in this Video: 1. Water (H₂O) 2. Magnesium chloride (MgCl₂) 3. Ammonium sulfate ((NH₄)₂SO₄) 4. Dinitrogen tetroxide (N₂O₄) 5. Hydrochloric acid (HCl) 6. Iron(III) chloride (FeCl₃) 7. Phosphorus pentachloride (PCl₅) 8. Copper(II) sulfate pentahydrate (CuSO₄•5H₂O) …and many more! 👉 If you find this video helpful, don’t forget to like, subscribe, and share with fellow chemistry enthusiasts or classmates. #ChemicalFormulas #NamingChemicalCompounds #ChemistryBasics #HowToNameCompounds #ChemistryForBeginners #WritingChemicalFormulas #IonicCompounds #CovalentCompounds #AcidNaming #HydrateFormulas #LearnChemistry #ChemistryLessons #chemistry #chemistrybasics #chemistry101 Examples of Writing and Naming Chemical Compounds | Chemical Formula Basics | Chemistry Naming Rules published first on https://www.youtube.com/@waqademy12 /
0 notes
Text


test for lipids—our last experiment for the semester 🥰
9 notes
·
View notes
Text
Feeling kinda nostalgic today
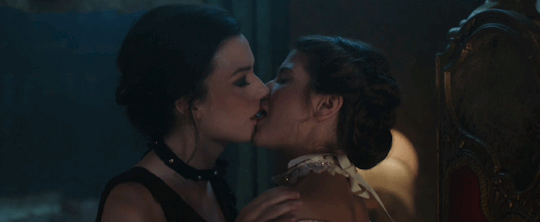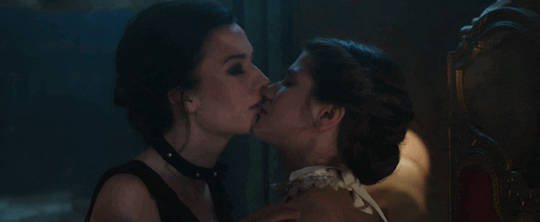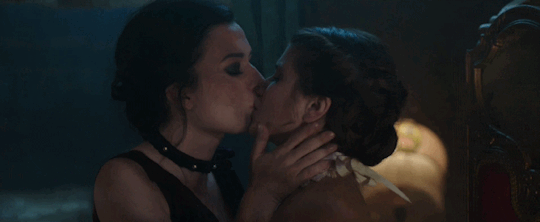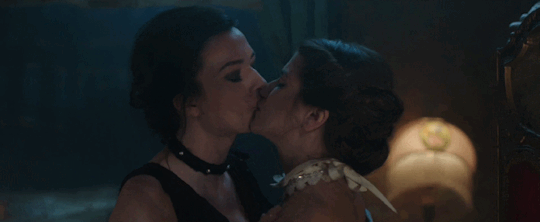




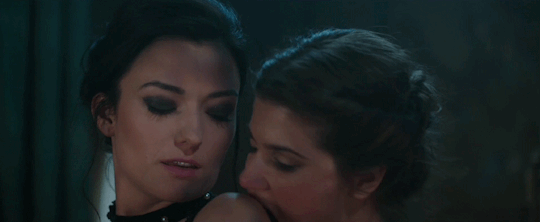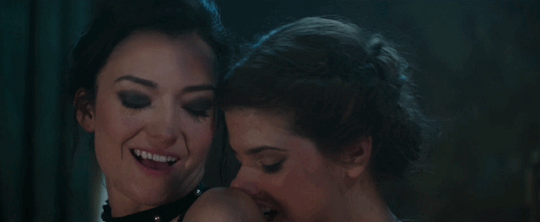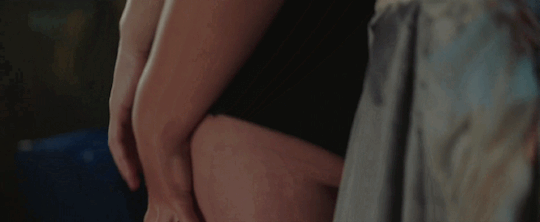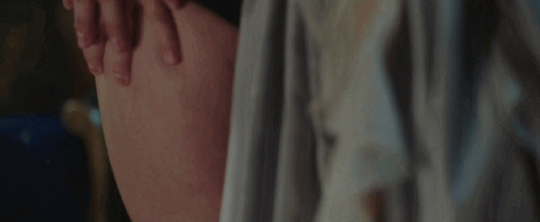
"40 minutes to get you in to that, huh? Bet I can get you out of it faster."
1K notes
·
View notes
Photo

Chemistry 101 #longliveharrilychua #everywheremolmol #chemistry101 #mole #mol #tuition #awesometutors #backtoschool https://www.instagram.com/p/B3rkk8NADFS/?igshid=1rvn4tob150nx
0 notes
Photo

Because a good wife deserves flowers 💐 every day. #happywifehappylife #chemistry101 (at Marlbrook, Worcestershire) https://www.instagram.com/p/BtqAkSmDFaIwo59xbK69BzgSuBcQQl4l2Swj440/?utm_source=ig_tumblr_share&igshid=g278rtqpzt8k
0 notes
Text







Clexa is my OTP always & forever.
I combined one of my favorite song lyrics, Like A Star by Corinne Bailey Rae with the gifs from the awesome video made by Lexa Heda X
36 notes
·
View notes
Photo

Lmao I LOLd too hard at this 😂 #chemistry101 #lol (at Alkali Metals Ltd.)
0 notes
Text
sp2 Hybridization: Covalent Bonds Course (19)
Course Chemical Bonds: Covalent Bonding and Shapes of Molecules (19) Hybridization sp2 Hybrid Orbitals—Bond Angles of Approximately 120° In sp2 hybridization, the s orbital hybridizes with two p orbitals. We say “2” indicating the number of involved p orbitals. Notice, this leaves one p orbital unhybridized. This orbital typically forms the pi covalent bond. Here, carbon is sp2 hybridized,…

View On WordPress
#atom#britishdiploma#chem#Chemical bonding#chemilearn#chemistry#chemistry101#chemistry301#chemistryAlevel#chemistrybook#chemistrycourse#chemistryexplained#chemistrylectures#chemistrynotes#chemistrytutor#chemistryundergrade#crushcourse#Education#explainedchemistry#freecourse#freelearning#generalchemistry#IGCSE#learn#learnchem#learnchemi#learnchemistry#learning#lectures#Molecular geometry
0 notes
Video
This is all I think about... . #StayInYourLane #TropicalGlitzTakeOver #TheGameIsToBeSoldNotTold #TheHussleIsReal #Chemistry101
0 notes
Text
chemistry101
take home quize of chemistry anyone is godd with it DEADLINE : 10 hr
View On WordPress
0 notes
Text
Copy and paste ..
Ccto to the owner
FAI ..."THE CURE"
DO YOU WANT TO LEARN THE BASIC CHEMISTRY BEHIND FAI SECRET ALCOHOL MECHANISM IN DISSOLVING TOXINS THAT ENTERS OUR PURE & HEALTHY BLOOD VIA TOXIC VACCINES?
The DEAE or di-ethylaminoethanol is a primary alcohol, the key component that is hidden in Procaine that can only be produce in the bloodstream because of one key component present in the blood plasma, what is that? It's called "cholinesterase" or the blood plasma enzyme responsible for the hydrolysis reaction of Procaine to break it's esters into PABA or para-aminobenzoic-acid and DEAE.
Water is a universal polar solvent, meaning it can dissolve most water soluble components but not the "lipids and some proteins that are non-polar". See the presentation of the video of the FAI made recently, the DEAE "denatures lipids and proteins", why? Because DEAE is a primary alcohol like ethanol.
CHEMISTRY101, this are the properties of ethanol why it is a second universal solvent.
1. Ethanol is a very polar molecule due to its hydroxyl (OH) group, with the high electronegativity of oxygen allowing hydrogen bonding to take place with other molecules.
2. Ethanol therefore attracts polar and ionic molecules.
3. The ethyl (C2H5) group in ethanol is non-polar.
4. Ethanol therefore attracts non-polar molecules.
Thus, ethanol can dissolve both polar and non-polar substances.
In industrial and consumer products, ethanol is the second most important solvent after water.
Ethanol is the least toxic of the alcohols (it is only poisonous in large amounts), which makes it more suitable for use in industry and consumer products.
Ethanol is a common solvent in:
Cosmetics (such as perfumes).
Food colourings and flavourings (such as vanilla essence).
Medicinal preparations (such as antiseptics).
Some cleaning agents.
Now, imagine the toxic ingredients in the vaccines, can water dissolve this toxins that are produce & grown in aborted fetal cells, human and animal kidneys called HEK-293, and other lipids and proteins that are not water soluble. How can the blood excretes this toxins in our bodies if it cannot be dissolve by the blood plasma water alone?
The human cells,tissues, and organs contaminated by these toxic vaccines will produce unregulated number of human viruses or exosomes to dissolve all this toxins but in the process will also hurt the immune system because viruses or exosomes contains RNA that can be hydrolise in blood plasma water to produce "phosphoric acid" to dissolve toxins in the damaged cells. RNA & DNA are acidic in nature because of phosphate bond, and the presence of too much exosomes is not healthy in our immune system that's why "antibodies" are produced to locked-in the viruses or exosomes receptors for it to prevent entering the damaged & healthy cells for replication.
Do you understand now, why the "safe alcohol mechanism" of FAI is the answer to immediately dissolve the toxins in the vaccines before it can damage or contaminate our healthy cells and tissues?
In the near future, when we can no longer avoid the vaccines because our government seems supporting the New World Order vision coming from Bill Gates on Global Vaccination. Do not worry anymore, because FAI is the perfect counter medicine to help us immediately dissolve the toxic vaccines ingredients that enters our bloodstream.
Thank you Dr.Ruben G. Fabunan for your great contribution to humanity.
Can the New World Order prevent the use of FAI, the God given cure for humanity in the whole world? YES or NO?
The biblical answer is NO!, remember this powerful words in scriptures;
"If God is for us who can be against us" and remember that "Greater is He that is in me that he is in the world".
We will win this battle, NEW FAI WARRIORS & SUPPORTERS!
(Read and ponder John1:5)
My Jesus mercy. S&IHMMP4us.Amen
https://easychem.com.au/production-of-materials/renewable-ethanol/ethanol-as-a-solvent/#:~:text=The%20ethyl%20(C2H,most%20important%20solvent%20after%20water.
0 notes
Text
UGH I HATE STOICHIOMETRY 😡
1 note
·
View note