#cdm records
Explore tagged Tumblr posts
Text
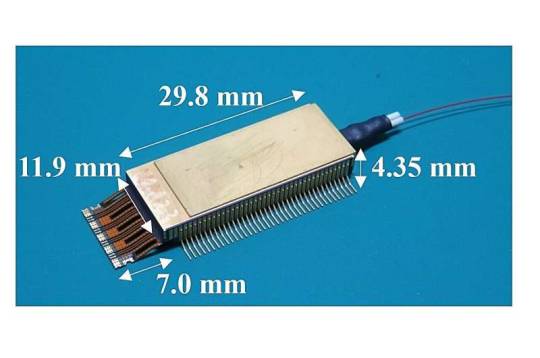
New InP-based modulator with record-high bit rates could help move more data faster
As data traffic continues to increase, there is a critical need for miniaturized optical transmitters and receivers that operate with high-order multi-level modulation formats and faster data transmission rates. In an important step toward fulfilling this requirement, researchers have developed a new compact indium phosphide (InP)-based coherent driver modulator (CDM) and showed that it can achieve a record high baud rate and transmission capacity per wavelength compared to other CDMs. CDMs are optical transmitters used in optical communication systems that can put information on light by modulating the amplitude and phase before it is transmitted through optical fiber. "Services that require data capacity, such as video distribution and web conferencing services, have become widespread, and services that more enrich our lives are expected to be introduced in the future," said Josuke Ozaki from NTT Innovative Devices Corporation in Japan.
Read more.
12 notes
·
View notes
Text
CDM Presents: An Interview with Curta’n Wall


Matt talks to black metal/dungeon synth act Curta’n Wall’s Abysmal Spectre about summarising your life with 21 symbols, using Shrek as medieval inspiration, creating their own coat of arms and their new album YR GWYDDBWYLL, out now on Grime Stone Records.
Read the full interview and listen to the new album:
https://cavedwellermusic.net/interviews/cdm-presents-an-interview-with-curtan-wall
6 notes
·
View notes
Text
Autogate Singapore: 10 Factors To Consider When Choosing

The demand for autogates in Singapore has surged in recent years due to their unmatched convenience and security features. These systems have become an undeniable solution for controlling access and ensuring safety from residential to commercial properties. However, with many available options, it can take time to decide which Singapore autogate system is best suited for your requirements.
This blog will explore ten essential factors when selecting an autogate Singapore system.
What is an autogate?
Autogate Singapore systems are an access control solution that automatically manages entry and exit to a residential or commercial property. Typically installed at the entrance or exit, such as a driveway or parking lot, it regulates traffic flow. The system can be activated using a remote control, keypad, or other access control devices.
An autogate system typically comprises a gate, motor, control panel, and access control device. The gate is the physical barrier restricting access to the property, while the motor opens and closes the gate. The control panel manages the gate’s operation and receives signals from the access control device.
Overall, autogate Singapore systems provide property owners with both convenience and security by enabling them to control who can access their property and when.
How to choose the right autogate
Choosing the right autogate system is crucial as it can affect the safety and accessibility of your property. This blog post will explore the ten factors to consider when choosing an autogate in Singapore.
Factor 1: Type of autogate Singapore systems
There are three types of autogate systems — sliding, swing, and folding.
Sliding autogates are ideal for properties with limited space.
Swing autogates are more suitable for wider entrances.
Folding autogates are a combination of sliding and swing and are ideal for properties with limited space and uneven terrain.
Each type of autogate system has pros and cons, and choosing the right style for your property is essential based on your needs and available space.
Factor 2: Material and durability
The material used for the autogate is an essential factor to consider because it affects the durability and lifespan of the gate. Aluminum, stainless steel, and wrought iron are common materials used for autogates. Because aluminium is lightweight and resistant to rust and corrosion, it is often the most desirable option. Another common option is stainless steel, which is sturdy, long-lasting, bulkier and more costly. Traditional wrought iron has a timeless appearance but is heavy and needs routine upkeep to avoid rust and corrosion.
The quality of the manufacturing and installation processes are other aspects that influence an autogate system’s longevity. When looking for an autogate in Singapore, pick a reputable manufacturer with premium materials and a solid track record of delivering robust and long-lasting autogate Singapore systems. Furthermore, a thorough installation is necessary to ensure that the gate operates as intended and is spared from damage brought on by a poor installation.
Factor 3: Size and weight
The performance of an autogate can be significantly impacted by its size and weight. A too-big or tiny gate might hinder its movement or harm both the gate and the motor. Similarly, an overly heavy gate might stress the motor, shortening their lives.
Before deciding, you should also consider the weight and size of your car and the space available to install your autogate Singapore system. Your vehicle should be able to fit through the gate in terms of its roof height and width. In addition, make sure the motor can support the gate’s weight without strain.
Factor 4: Security features
Security features are an essential consideration when choosing an autogate Singapore system. Autogates are often used as a security measure to prevent unauthorised entry into a property, so choosing a gate with reliable security features is important.
Common security features include:
Electronic locks — An effective way to secure the gate and prevent unauthorized entry.
Security cameras — Provide additional surveillance to monitor the gate and the surrounding area.
Motion sensors — Detect movement and alert the owner of any potential threats.
It is essential to think about your unique security requirements when selecting security features and select those that satisfy them. Confirming that the security mechanisms are trustworthy and have a solid track record of preventing illegal entrance is crucial.
Factor 5: Accessibility and ease of use
An autogate Singapore system must be simple to operate and available to everyone requiring it. This is especially important for families with young children or senior citizens. Keypads, remote controls, and sensors that can identify the presence of a person or a vehicle are a few elements that can enhance usability and accessibility.
The position of your Singapore autogate system and the surroundings must also be taken into account. An autogate system that is weather-resistant and can survive severe circumstances may be necessary if the location is prone to flooding or excessive rains.
Factor 6: Power source
Singapore autogate systems can be powered by either AC or DC sources. AC-powered systems are generally more powerful and suitable for larger gates but require a constant electricity supply. While DC-powered systems may run on battery power during power outages, they are more energy-efficient.
It’s crucial to consider power needs and the availability of a power supply when selecting an autogate Singapore system. A DC-powered system will be better if you are in a region where power outages are common. But, if you have a big gate that needs a lot of power, an AC-powered system could be more suited.
Factor 7: Maintenance and repair
Like any mechanical system, autogate Singapore systems require regular maintenance to ensure optimal performance and longevity. Typical maintenance tasks include lubricating moving parts, tightening bolts and screws, and checking for wear and tear.
It’s crucial to select an autogate system with simple maintenance requirements and accessible replacement components. When estimating its cost, it is crucial to account for the cost of maintenance and repair during an autogate system’s lifespan.
Factor 8: Noise level
Autogate systems can produce noise during operation, which can concern some homeowners. The type of motor, the grade of the materials, and the gate layout are all variables that might impact an autogate Singapore system’s noise level.
It is essential to consider the noise level and select a quiet autogate system when making your selection. This is particularly crucial for houses situated in peaceful residential areas.
Factor 9: Price and affordability
An autogate system’s price can vary significantly based on size, construction, and functionality. In addition to the initial purchase price, it is crucial to consider the long-term expenditures of an autogate system, such as maintenance and repair expenses.
Finding a balance between price and quality when picking an autogate Singapore system is crucial. While a cheaper system could save you money short term, it may not last long nor be as dependable as one that costs you more. Another thing to remember is the manufacturer’s reputation and the standard of customer service they provide.
Factor 10: Warranty and customer support
Finally, thinking about an autogate’s warranty and customer support alternatives is critical. A strong guarantee may provide confidence and safeguard your investment in case of flaws or malfunctions. Also, effective customer service may make resolving problems and locating new components simpler.
When selecting an autogate in Singapore, choose a producer with a thorough guarantee and dependable customer assistance. This can ensure that you obtain the maximum long-term value from your investment.
Install your autogate Singapore system today
Investing in the right Singapore autogate system ensures your property’s security, convenience, and ease of access. You can decide which autogate system will best meet your needs by considering the ten factors outlined in this blog.
At CDM Engineering, we are committed to providing top-notch services that exceed your expectations. Contact us today to learn more about our Singapore autogate solutions and how we can help you secure your property.
2 notes
·
View notes
Text

A name badge from the 97th annual convention of the Georgia Education Association (now the Georgia Association of Educators), held in 1964.
Delegate's name removed for privacy.
Found in an abandoned home in Eastern Georgia.
A record of this convention can be found below, for those who find historical documents interesting. The proceedings of this convention begin on page 15. The delegate whom this name badge belonged to is not named (or pictured, to my knowledge) in this document.
Georgia Association of Educators. "Georgia Education Journal, 1964-04." Georgia State University. Special Collections. 1964-04, http://digitalcollections.library.gsu.edu/cdm/ref/collection/gae/id/10658.
Having found this source, I almost wonder if any copies of it exist at the site this photo was taken... I suppose I'll have to take a peek sometime!
That aside, the GEA (white educators) and the Georgia Teachers and Education Association (black educators) were merged in 1969 to form the modern-day Georgia Association of Educators, under a desegregation resolution passed by the National Education Association in 1964.
6 notes
·
View notes
Text
Comprehensive Guide to Site Engineer Jobs in the UK
Site Engineer Jobs UK are critical roles within the construction and civil engineering industries, combining technical expertise, project management, and leadership skills. In this guide, we delve into what it takes to excel in this role, the qualifications needed, and the opportunities available across the UK.
What is a Site Engineer?
A site engineer acts as the linchpin in construction projects, bridging the gap between design and execution. Responsibilities include:
Supervising construction activities.
Managing on-site operations and workers.
Ensuring adherence to project timelines, budgets, and quality standards.
Communicating effectively with project managers, architects, and other stakeholders.
Conducting site inspections to maintain safety and compliance.
Essential Qualifications and Skills
To secure a Site Engineer Jobs UK , candidates typically need the following:
Educational Background
A degree in civil engineering, construction management, or a related field.
Membership in professional bodies like ICE (Institution of Civil Engineers) or CIOB (Chartered Institute of Building).
Key Skills
Technical Proficiency: Expertise in CAD software and engineering principles.
Problem-Solving: Ability to tackle unforeseen challenges on-site.
Communication: Clear interaction with teams and stakeholders.
Leadership: Managing and motivating site teams effectively.
Compliance Knowledge: Understanding health and safety regulations like CDM (Construction Design and Management).
Types of Projects for Site Engineers
Site engineers contribute to diverse projects, including:
Residential Construction: From housing estates to apartment complexes.
Infrastructure Projects: Bridges, roads, and rail systems.
Commercial Developments: Office buildings and retail spaces.
Industrial Projects: Factories, warehouses, and plants.
Each project type demands unique skills, offering varied challenges and opportunities.
How to Find Site Engineer Jobs in the UK
1. Online Job Portals
Popular platforms like Indeed, TotalJobs, and Reed list hundreds ofSite Engineer Jobs UK vacancies daily.
2. Specialist Recruitment Agencies
Agencies such as Hays Construction and Randstad specialize in construction and engineering roles, providing tailored job listings.
3. Networking and Referrals
Join professional groups and attend industry events to connect with hiring managers.
4. Company Websites
Direct applications to leading construction firms like Balfour Beatty, Kier Group, and Laing O’Rourke often yield excellent results.
Salary Expectations
The average salary for site engineers in the UK ranges between £30,000 and £50,000 per year. Senior site engineers or those working on major projects can earn upwards of £60,000 annually. Salaries vary by region, project scope, and level of experience.
Day-to-Day Responsibilities
The daily tasks of a site engineer include:
Reviewing technical designs and blueprints.
Setting out and marking locations on-site.
Supervising and directing construction teams.
Maintaining project records and documentation.
Coordinating material deliveries and managing resources.
Growth Opportunities
Site engineers have ample opportunities for career progression:
Senior Site Engineer: Leading larger teams and projects.
Project Manager: Overseeing entire project lifecycles.
Consultant Roles: Advising on specialized engineering solutions.
Director-Level Positions: Heading departments or divisions.
Challenges Faced by Site Engineers
While rewarding, the role comes with challenges such as:
Managing tight deadlines and budgets.
Resolving disputes among teams.
Adapting to changing project scopes.
Staying updated on regulations and technologies.
0 notes
Text
Clinical Data Management with SAS: From Fundamentals to Advanced Techniques
Clinical Data Management (CDM) is an essential discipline in the management and oversight of clinical trial data, ensuring that data is collected, validated, and ready for analysis. This training program focuses on how Clinical SAS (Statistical Analysis System) plays an instrumental role in managing, analyzing, and reporting clinical data. The use of SAS in clinical trials has become a gold standard due to its powerful tools for data manipulation, cleaning, and analysis.
This Clinical SAS Training course will equip participants with the necessary knowledge and skills to effectively manage clinical data, utilize SAS for data handling, and prepare clinical trial results for submission to regulatory bodies.
Introduction to Clinical Data Management and SAS in Clinical Trials
In clinical trials, data is collected from patients, including their demographic details, medical history, lab results, and adverse event reports. Ensuring that the data is clean, organized, and compliant with industry standards is critical to the success of the trial. SAS is a versatile tool that allows clinical data managers to efficiently process and analyze this data.
This course begins by explaining the critical role of clinical data management in the life cycle of a clinical trial. It introduces participants to the various stages of a clinical trial, from protocol design to final analysis. A deep dive into the regulatory standards, such as Good Clinical Practice (GCP) guidelines and data management standards like the Study Data Tabulation Model (SDTM), will help participants understand the legal and ethical aspects of data management.
The course also covers essential skills in SAS programming for clinical data management, such as how to use SAS to clean and transform clinical trial data. SAS's extensive features, including Data Step and PROC SQL, are essential for cleaning raw data and transforming it into meaningful information. This training also includes hands-on sessions to familiarize participants with SAS’s interface and coding structure.
SAS Programming Basics for Clinical Data Management
SAS programming is a vital skill for anyone involved in clinical data management. Through this course, participants will gain hands-on experience in using SAS for data manipulation, transformation, and analysis. The training starts with an introduction to SAS programming syntax, the basics of data steps, and procedures:
Data Steps and Procedures: Data Steps allow users to create and modify datasets, while SAS Procedures (PROCs) are used for analysis. The participants will be taught how to import clinical data into SAS, manipulate it, and apply transformation techniques.
Basic SAS Syntax: Participants will become comfortable with common SAS commands, variables, and data handling techniques that are commonly used in clinical data management tasks. These skills are vital when working with large clinical datasets.
Data Cleaning and Preprocessing for Clinical Trials
Data cleaning is a critical component of clinical data management because the quality of the trial data directly impacts the outcome. SAS provides powerful tools for identifying issues like missing values, duplicate records, and outliers that may skew the results of the analysis.
Participants will learn how to:
Identify and Handle Missing Data: SAS offers several techniques to handle missing data in clinical trials. This course will cover methods such as imputation techniques, and exclusion of incomplete records, which ensure that the data used for analysis is robust and accurate.
Identify Duplicates and Inconsistencies: Ensuring the dataset is free from duplicates and inconsistencies is essential for the integrity of clinical trial results. Using SAS procedures, participants will learn how to spot and handle duplicate records efficiently.
Standardize Clinical Data: Clinical data collected from multiple sites may follow different formats. Standardization is essential to make the data usable across all analysis platforms. Participants will learn to transform this data into standardized formats that comply with SDTM and ADaM (Analysis Data Model) formats.
Statistical Analysis in Clinical Trials Using SAS
The ability to perform statistical analysis on clinical trial data is vital for making data-driven decisions and generating valid conclusions. SAS is recognized for its robust statistical procedures, which are commonly used in clinical trials for analyzing patient outcomes and treatment effects.
In this part of the training, participants will learn:
Descriptive Statistics: The first step in analyzing clinical trial data is to calculate summary statistics. SAS procedures such as PROC MEANS and PROC FREQ help participants calculate means, medians, frequencies, and other descriptive statistics that summarize the data.
Survival Analysis: Clinical trials, especially in fields like oncology or cardiology, often involve survival data, such as the time to disease progression or patient survival. SAS’s PROC LIFETEST and PROC PHREG are used for Kaplan-Meier survival curves and Cox Proportional Hazards Models, allowing participants to understand the time-related behavior of clinical data.
Advanced Statistical Techniques: This course also introduces advanced statistical techniques like longitudinal analysis, mixed-effects models, and Bayesian methods, which can be applied in more complex clinical trials.
Creating Reports for Regulatory Submissions
Once data is cleaned, analyzed, and interpreted, it needs to be presented in a clear and concise manner for regulatory submission. SAS is the go-to tool for creating comprehensive clinical trial reports that meet the regulatory requirements of organizations like the FDA and EMA.
Participants will learn how to:
Generate Tables, Listings, and Figures (TLFs): These reports are an integral part of clinical trial submissions. Participants will learn how to use SAS’s PROC REPORT, PROC TABULATE, and PROC PRINT procedures to generate high-quality, compliant tables, listings, and figures.
Prepare Final Reports for Regulatory Bodies: The course will also cover how to format these TLFs to ensure that they adhere to the reporting standards required for FDA and EMA submissions. This includes creating reports that are clear, concise, and easy for regulatory bodies to interpret.
#sas programming#sas tutorial#clinical data management training#sas certification training#clinical sas training
0 notes
Text
Data science in clinical data management
Data science has revolutionized clinical data management (CDM) by enhancing the collection, processing, analysis, and interpretation of clinical trial data. It integrates advanced analytical tools and methodologies to ensure data accuracy, consistency, and compliance with regulatory standards, contributing to faster and more reliable decision-making in healthcare research.
Role of Data Science in Clinical Data Management:
Efficient Data Collection:
Data science enables the use of electronic data capture (EDC) systems and mobile health technologies to gather real-time data from patients.
These tools improve data accuracy by reducing manual errors.
Data Cleaning and Validation:
Advanced algorithms identify and rectify inconsistencies, missing values, and outliers in datasets.
Automation of these processes ensures quicker turnaround times and higher-quality data.
Integration of Diverse Data Sources:
Data science facilitates the integration of clinical trial data with data from electronic health records (EHR), genomics, wearable devices, and patient-reported outcomes.
This holistic approach provides richer insights for research.
Predictive Analytics and AI:
Predictive modeling helps forecast clinical outcomes, patient responses, and potential risks.
AI algorithms identify patterns in large datasets, uncovering insights that would be difficult to discern manually.
Improved Decision-Making:
Data visualization tools like dashboards and graphs provide stakeholders with an easy-to-understand view of clinical trial progress.
Insights from data science drive more informed decisions regarding drug efficacy and safety.
Regulatory Compliance:
Data science ensures that clinical trial data adheres to international standards like GCP (Good Clinical Practice) and regulatory requirements such as FDA and EMA guidelines.
Benefits of Data Science in CDM:
Faster Clinical Trials: Accelerates the drug development process by streamlining data workflows.
Cost Efficiency: Reduces manual effort and operational costs.
Better Patient Outcomes: Enhances the understanding of treatment effectiveness and patient safety.
Scalability: Handles large and complex datasets efficiently.
Challenges:
Data Privacy: Ensuring compliance with data protection regulations like GDPR and HIPAA is critical.
Data Standardization: Managing data from diverse sources requires consistent formats and structures.
Skill Gaps: The need for skilled professionals in both data science and clinical research domains remains a challenge.
Conclusion:
Data science has become a cornerstone of modern clinical data management. Its ability to handle and analyze complex datasets ensures the reliability of clinical trials, reduces development timelines, and supports innovation in healthcare. As data science continues to evolve, it promises to transform CDM, leading to more efficient research processes and improved patient outcomes.
0 notes
Text
Clinical Data Management: What Are The Key Challenges And How To Navigate Them?
Formerly, clinical research institutions employed paper-based methods to record patient information. Clinical Data Management Systems, for example, are being developed to streamline the procedure. They are meant to improve the speed and quality of clinical research data collecting by utilizing electronic systems to save, manage, and store data.
What challenges do clinical data management systems currently face?
The volume of data to be handled is one of the most difficult challenges that clinical data management faces. With growing volumes of patient information being available, CDM software frequently struggles to keep up. Additionally, many CDM systems are neither user-friendly nor interactive, making it difficult for patients to get the most out of these systems.
Clinical data management is also confronted by:
Clinical Trial Complexity
The modern design of clinical trials necessitates real-time data modeling and simulation to provide reliable data that allows for faster judgments and reduces the time to develop expenses and research failures in the late stages. Many clinical trials are now considered adaptive, which means they may be changed throughout the trial and the information gained during the study is utilized to determine the next steps. Some therapeutic areas and settings, such as immuno-oncology and multi-arm investigations, are also complicating clinical trials.
The ability to adapt to changing conditions and needs is the future of clinical data management. A CDM system must be able to manage large amounts of data while also being user-friendly in order to be effective. It should also leverage Artificial Intelligence to assist in the automation of manual processes. Using an EDC in clinical data management can prove to be game-changing.
Mid-Study Changes
Clinical data management is a difficult task. It has many stakeholders, ranging from researchers to CROs and sponsors. This complicates CDM, particularly with relation to mid-study adjustments (MSCs). Changes in procedures and study management plans are examples of mid-study alterations (SDMPs). Mid-study changes might be caused by one or more of the following factors:
Modifications to the inclusion/exclusion criterion
An increase in the frequency or amount of medicine administration
New patient subpopulation exclusion/inclusion
New devices/therapeutic agents are either excluded or included.
Changes in the primary and secondary outcomes measures (SO).
According to a Tufts University survey, almost 70% of respondents felt that unanticipated mid-study alterations are the most major cause of trial delays. The planned adjustments are more difficult since they need substantial planning prior to implementation to ensure that they do not interrupt existing trials or other initiatives.
The essential revisions in the study offer a considerable challenge for CDM. The biggest cause of trial delays is unplanned mid-study adjustments. As a result, a system that can handle rapid mid-study adjustments and is incredibly simple to create and faster to adopt is required. Instead of requiring many systems to make modifications, the CDM system must be capable of processing all essential changes in a single location.
Does the role of clinical Data Managers change?
Clinical data management has advanced significantly in the last several years. What was once a minor division inside a research organization has turned into a highly specialized and critical responsibility. Before, clinical data managers were in responsible of cleansing and data input and quality control in clinical data management. When electronic data capture (EDC) became more common in the mid-1990s, the CDM's function shifted. The CDM was in responsible of building up and implementing the EDC systems, as well as producing and handling database queries.
Clinical data managers are now in responsible of establishing and implementing data management plans, ensuring completeness and correctness, and safeguarding data.
What is the future of clinical data management?
The future of healthcare data management is dependent on systems and regulations. There should be clear procedures on patient data ownership and information exchange among entities engaged in a research. It is also vital to standardize the formats used to record patient data and papers linked with studies. This eliminates any ambiguity regarding who holds the papers or information at any given time.
The future of data management is predicted to be increasingly automated, with more artificial machine learning and intelligence used to comb through data to discover patterns and trends across websites, patients, and studies, which can help speed up the drug development process. These new technologies will lead to a better knowledge of illnesses and improved patient outcomes, which will improve the accuracy and quality of the data even more.
To grasp the significance of the huge and expanding quantity of data being generated, CDM roles are already requiring expertise of analytics and data science. CDMs may need to be able to interact with machine learning and artificial intelligence systems in the near future to expedite data management duties and improve data quality.
Octalsoft is a forward-thinking firm that is always proposing creative methods to improve our settings, like Octalsoft's eClinical suite, to better enable mid-study adjustments. Choose a solution that can respond to mid-study adjustments at scale and has the functionality to lead your clinical data management efforts to set your research up for success.
Set up a Free Demo with one of Octalsoft's specialists now to see how our systems can increase the flexibility of your clinical trials and augment the efficiency of clinical trial data management.
#clinical data management#clinical trial data management#clinical data management software#clinical data management system
0 notes
Text
e-Clinical Solutions Market Size, Share, Trends, Growth Opportunities, Key Drivers and Competitive Outlook
"Global e-Clinical Solutions Market – Industry Trends and Forecast to 2030
Global e-Clinical Solutions Market, By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File Systems, Regulatory Information Management Solutions, and Others), Delivery Mode (Web- hosted (On- demand) Solutions, Licensed Enterprise (On- Premises) Solutions, and Cloud-Based (SAAS) Solutions), Clinical Trial Phase (Phase I, Phase II, Phase III, and Phase IV), Organization Size (Small & Medium and Large), User Device (Desktop, Tablet, Handheld PDA Device, Smart Phone, and Others), End User (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, Consulting Service Companies, Medical Device Manufacturers, Hospitals, and Academic Research Institutes), Industry Trends and Forecast to 2030.
Access Full 350 Pages PDF Report @
**Segments**
- **Product**: The e-Clinical Solutions market can be segmented based on the product offering, including electronic data capture (EDC), clinical trial management systems (CTMS), clinical data management systems (CDMS), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), and others. These products are crucial in streamlining clinical trial processes, improving data collection accuracy, and enhancing overall efficiency.
- **Delivery Mode**: Another important segmentation of the e-Clinical Solutions market is by delivery mode, which includes web-hosted (cloud-based) solutions and licensed enterprise solutions. Cloud-based solutions are gaining traction due to their scalability, cost-effectiveness, and flexibility, allowing organizations to access data securely from anywhere, anytime.
- **Clinical Trial Phase**: The market can also be segmented by the clinical trial phase, such as Phase I, II, III, and IV trials. Each phase has specific requirements and data management needs, with e-Clinical Solutions playing a vital role in ensuring compliance, data integrity, and regulatory adherence throughout the clinical trial process.
**Market Players**
- **Oracle Corporation**: A leading player in the e-Clinical Solutions market, offering a comprehensive suite of products for clinical trial management, data collection, and analytics.
- **Medidata Solutions (a Dassault Systèmes company)**: Known for its innovative cloud-based solutions for clinical research, Medidata provides a range of offerings to improve trial efficiency and data quality.
- **IBM Corporation**: With its Watson Health platform, IBM offers advanced analytics and insights for clinical trials, helping organizations make informed decisions and drive better patient outcomes.
- **BioClinica (Evolent Health)**: Specializing in imaging and eClinical solutions, BioClinica provides technologies to enhance trial imaging, data management, and regulatory compliance.
- **ERT (eResearch Technology)**: ERT focuses on eCOA solutions and cardiac safety services, supporting clinical trials with reliable data collectionThe e-Clinical Solutions market is a dynamic and competitive landscape with key players such as Oracle Corporation, Medidata Solutions, IBM Corporation, BioClinica, and ERT leading the way with innovative products and services. These market players offer a variety of solutions targeting different segments of the market. Oracle Corporation stands out with a comprehensive suite of products catering to clinical trial management and data collection needs. Medidata Solutions, a Dassault Systèmes company, is known for its cloud-based offerings that enhance trial efficiency and data quality. IBM Corporation's Watson Health platform provides advanced analytics and insights for clinical trials, empowering organizations to make data-driven decisions and improve patient outcomes. BioClinica, now part of Evolent Health, specializes in imaging and eClinical solutions, offering technologies that enhance trial imaging, data management, and regulatory compliance. ERT focuses on eCOA solutions and cardiac safety services, ensuring reliable data collection and valuable insights for clinical trials.
As the e-Clinical Solutions market continues to evolve, market players are increasingly focusing on innovation, efficiency, and compliance to meet the growing demands of the pharmaceutical and healthcare industries. With the increasing complexity of clinical trials and regulatory requirements, there is a rising need for advanced solutions that can streamline processes, improve data accuracy, and ensure regulatory compliance. Cloud-based solutions are gaining popularity due to their scalability, cost-effectiveness, and flexibility, enabling organizations to access and manage data securely from anywhere at any time. These solutions also offer seamless integration with existing systems, providing a more efficient and collaborative environment for clinical trial management.
In terms of product segmentation, the e-Clinical Solutions market offers a range of products such as electronic data capture (EDC), clinical trial management systems (CTMS), clinical data management systems (CDMS), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), among others. These products play a crucial role in enhancing data collection accuracy, improving process efficiency, and ensuring regulatory compliance**Segments**
- **Product**: The e-Clinical Solutions market is segmented based on the product offering, including electronic data capture (EDC), clinical trial management systems (CTMS), clinical data management systems (CDMS), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), and others. These products play a vital role in streamlining clinical trial processes, improving data collection accuracy, and enhancing overall efficiency in the pharmaceutical and healthcare industries.
- **Delivery Mode**: Another crucial segmentation of the e-Clinical Solutions market is by delivery mode, which includes web-hosted (cloud-based) solutions and licensed enterprise solutions. Cloud-based solutions are gaining traction due to their scalability, cost-effectiveness, and flexibility, enabling secure data access from anywhere, anytime, ensuring streamlined processes and regulatory compliance.
- **Clinical Trial Phase**: The market can also be segmented by the clinical trial phase, facilitating Phase I, II, III, and IV trials requirements and data management needs. e-Clinical Solutions play a vital role in ensuring compliance, data integrity, and regulatory adherence throughout the clinical trial process, driving efficiency and improved patient outcomes.
**Global e-Clinical Solutions Market, By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization, and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File
Key points covered in the report: -
The pivotal aspect considered in the global e-Clinical Solutions Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global e-Clinical Solutions Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global e-Clinical Solutions Market.
The Global e-Clinical Solutions Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
Table of Content:
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Global e-Clinical Solutions Market Landscape
Part 04: Global e-Clinical Solutions Market Sizing
Part 05: Global e-Clinical Solutions Market Segmentation by Product
Part 06: Five Forces Analysis
Part 07: Customer Landscape
Part 08: Geographic Landscape
Part 09: Decision Framework
Part 10: Drivers and Challenges
Part 11: Market Trends
Part 12: Vendor Landscape
Part 13: Vendor Analysis
Reasons to Buy:
Review the scope of the e-Clinical Solutions Market with recent trends and SWOT analysis.
Outline of market dynamics coupled with market growth effects in coming years.
e-Clinical Solutions Market segmentation analysis includes qualitative and quantitative research, including the impact of economic and non-economic aspects.
Regional and country level analysis combining e-Clinical Solutions Market and supply forces that are affecting the growth of the market.
Market value data (millions of US dollars) and volume (millions of units) for each segment and sub-segment.
and strategies adopted by the players in the last five years.
Browse Trending Reports:
18g Substrate Materials Market Cloud Application Programming Interface Api And Management Platforms And Middleware Market Abscisic Acid Aba Market Benign Mesonephroma Market Cancer Supportive Care Products Market Data Center Interconnect Market Potash Fertilizers Market Private Label Food And Beverage Market Relational Database Market Commercial Lighting Market Ethoxylates Market Eclinical Solutions Market Vaccines Market Spark Plug Market High Visibility Clothing Market Gas Turbine Services Market Dessert Mix Market Lipid Nutrition Market Barrier Films Flexible Electronics Market Nasal Polyposis Drugs Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
0 notes
Text
NEW CDM PODCAST OUT NOW!!!
We sit down with the guys from New Haven, Connecticut technical/progressive death metal band Xenosis to discuss their new single Altar of the Hound and their upcoming work. We also talk about working with Transcending Obscurity Records, the band's history, and the Connecticut music and food scene. We also talk about why slam is underrated, working progressive and psychedelic elements into extreme metal, where in the US actually has the best pizza and much more.
Spotify:
YouTube:
youtube
Soundcloud:
#podcast#music podcast#metal podcast#death metal#extreme metal#technical death metal#prog metal#cave dweller music#xenosis#Spotify#Youtube#SoundCloud
3 notes
·
View notes
Text
Interview I did last year.
It was more focused on dungeon synth music, but it was fun to answer the questions.
https://cavedwellermusic.net/interviews/cdm-presents-an-interview-with-jani-of-misantropia-records/
1 note
·
View note
Text
Cdm Consultants: Unlocking Project Potential with Expertise
Construction Design and Management (CDM) consultants play a crucial role in ensuring the safety, health, and welfare of all individuals involved in a construction project. From the initial planning stages to the completion of the project, CDM consultants provide valuable expertise and guidance to ensure compliance with relevant regulations and best practices.
Understanding the Responsibilities of CDM Consultants
CDM consultants are responsible for advising and assisting the client in meeting their duties under the Construction (Design and Management) Regulations. These regulations are in place to ensure that health and safety considerations are integrated into the planning and management of construction projects.
Some key responsibilities of CDM consultants include:
Providing advice on health and safety issues during the design and planning stages of a project.
Assisting in the identification and management of potential risks throughout the project lifecycle.
Collaborating with designers, contractors, and other stakeholders to ensure compliance with CDM regulations.
Developing and maintaining relevant documentation, including the health and safety file.
Conducting regular site inspections and audits to monitor compliance and identify areas for improvement.
Benefits of Engaging CDM Consultants
By engaging CDM consultants, clients can benefit from their specialized knowledge and experience in managing health and safety considerations within the construction industry. Some of the key benefits include:
Enhanced safety culture: CDM consultants contribute to the development of a strong safety culture within construction projects, leading to a reduction in accidents and incidents.
Compliance assurance: With their in-depth understanding of CDM regulations, consultants help clients navigate complex compliance requirements and avoid potential penalties.
Risk management: CDM consultants assist in identifying and mitigating risks, ultimately contributing to the successful delivery of projects within budget and schedule.
Efficiency and cost-effectiveness: Through proactive risk management and compliance oversight, CDM consultants support efficient project delivery and cost control.
Expert guidance: Clients benefit from expert guidance on health and safety matters, ensuring that these considerations are integrated into every phase of the project.
Choosing the Right CDM Consultants
When selecting CDM consultants for a construction project, it is essential to consider their qualifications, experience, and track record in delivering successful outcomes. Factors to evaluate include:
Professional credentials and certifications related to health and safety management.
Relevant experience in overseeing similar construction projects, including their approach to risk management and compliance.
Client references and testimonials that demonstrate the consultant's ability to add value to construction projects.
Effective communication and collaboration skills, as CDM consultants often work closely with diverse project stakeholders.
Conclusion
In conclusion, CDM consultants play a vital role in ensuring the safe and successful delivery of construction projects. Their expertise in health and safety management, risk mitigation, and compliance oversight contributes to the overall quality and efficiency of construction endeavors. By engaging qualified and experienced CDM consultants, clients can benefit from enhanced safety, regulatory compliance, and efficient project delivery.

0 notes
Text
Navigating Safety and Compliance: The Essential Role of CDM Consultants in Construction Projects
In the world of construction, ensuring safety and efficiency is paramount. Construction Design and Management (CDM) Consultants play a crucial role in this domain, providing expert advice and overseeing the application of health and safety regulations. This article delves into the functions and significance of CDM Consultants in the construction industry. What is a CDM Consultant? CDM Consultants are professional advisors who specialize in the Construction (Design and Management) Regulations 2015, a key legal framework in the UK designed to enhance the health and safety of construction environments. These consultants are pivotal in planning, managing, and monitoring construction projects to ensure all legal safety standards are met. Key Responsibilities of CDM Consultants CDM Consultants shoulder a variety of responsibilities that contribute to safer construction sites: • Risk Assessment and Management: They identify potential hazards and develop strategies to mitigate them, ensuring a safer work environment. • Health and Safety Planning: Consultants draft detailed health and safety plans that outline critical safety procedures and emergency protocols. • Regulatory Compliance: Their deep understanding of CDM regulations ensures that projects adhere to all legal requirements, helping to avoid penalties. • Collaboration and Communication: Effective communication with all project stakeholders is facilitated to maintain a clear flow of safety-related information. • Site Inspections: Regular inspections help monitor adherence to safety plans and identify any deviations. • Documentation: Maintaining thorough records of safety procedures, assessments, and incidents to safeguard against legal issues and improve future safety measures. The Value Added by CDM Consultants Hiring a CDM Consultant can significantly impact the safety and success of a construction project. Their expertise not only minimizes the risk of accidents but also enhances the project's compliance with safety regulations, which can save costs and enhance the project’s reputation for safety and reliability. Services Offered by CDM Consultants The range of services provided by CDM Consultants is comprehensive: • Principal Designer: Acting as key advisors and coordinators in managing health and safety during the pre-construction phase. • CDM Advisory Services: Offering guidance to ensure compliance with CDM regulations throughout the project. • Compliance Audits: Conducting thorough inspections and evaluations to ensure ongoing compliance. • Support for Principal Contractors: Assisting contractors in understanding and fulfilling their legal health and safety responsibilities. Choosing the Right CDM Consultant Selecting the right CDM Consultant is crucial for ensuring project success. Key factors to consider include: • Expertise and Experience: Knowledge of specific industry sectors and familiarity with local regulations. • Communication Skills: Ability to effectively communicate with various project stakeholders. • Track Record: Evidence of successfully guiding previous projects to comply with health and safety standards. The Importance of Early Engagement Engaging a CDM Consultant during the early stages of project planning is critical. Early involvement allows for a comprehensive assessment of potential risks and the establishment of robust safety measures, setting the stage for a smooth and compliant project execution. Conclusion CDM Consultants are indispensable in the construction industry, playing a critical role in ensuring projects are safe, compliant, and efficient. Their expertise not only protects workers but also safeguards the project owner from potential legal repercussions and financial losses. Choosing and involving a CDM Consultant early in your project planning can be one of the most important decisions in ensuring project success.
0 notes
Text
Match Review: Manchester United 3-2 Newcastle United
A win's a win. It wasn't comfortable, but at times it was pretty - with a bit of luck thrown in for good measure...

I don't want to be too lazy with this review, because it was a game that needs a legitimate commentary, but the tweets help flavour what was an interesting and peculiar match in a despairing and peculiar season.
United started modestly. Possession play was attempted and reasonably successful, but Garnacho was ponderous on the ball and there were a few errant passes from Casemiro at CB. He was also at fault for some random surges out into midfield and more missed/wild interception attempts.
Newcastle's threats of Gordon, Isak and Guimaraes played well and their speed, guile, and intelligence kept United honest, but chances did fall to our front three of Garnacho, Bruno and Amad.

United took the lead in the 31st minute through Kobbie Mainoo, who was perplexingly unmarked in the centre of the box thanks to Kieran Trippier rushing out to Amad Diallo. The Ivorian did what Antony always does - cutting in from the right - but unlike our expensive Brazilian he actually decided that 4 bodies wasn't worth a shot and played a cheeky bobbling throughball perfectly through to the waiting Mainoo for a cool right-foot finish into the bottom left of Dubravka's net.




The love-fest for Amad was on, Mainoo's back in action having had doubters lately, but the return of Capitão Bruno was a much needed bit of quality, drive and creativity to really wake United up. If this man is unfit and recovering then damn, he really is a machine.


One more player deserving plaudits though was Sofian Amrabat, who did make a couple of mistakes but generally looked assured in the CDM position. He pressed at the right times, put in some inch perfect slide tackles to deal with Newcastle's pacey counter attacking, and otherwise kept his game simple - be strong in the duels, press smartly, recycle possession to the creators. Amazing how much better we look with a proper pivot.


United went in 1-0 at half time then, which felt dangerous given our Premier League record of never losing a game at home we led at half time.
Cue Anthony Gordon in the 47th minute, smashing home a Murphy cross in from the right flank and finding the LW at back post to bundle it home.

You can argue that Casemiro isn't used to being a centre-back and this is a necessity time, so small errors will happen. That's fair. But it's still poor that his marking target isn't within arm's reach of him.
The lack of positional awareness from Wan Bissaka is an issue too, and has been forever. He was in a weird void between both Newcastle attackers.
Even with last season's improved attacking output, he hasn't improved that much - nor seems to have the confidence of a real elite "top of the league" player. An old interview with him revealed he thought he moved from Palace a year too early, but also he's a very reserved guy. Shy is fine, but there's a line where shy becomes timid, and I do think we might part ways in the summer. That said, he'd be a great buy for most teams for his defensive work - perhaps for someone like Bayern where their LB bombs up so much he's needed to help be a back 3.

Newcastle back in the mix kept on the counter, and should have scored when Gordon and Isak broke 2v1 against Amrabat only for Isak to sky over. This aside, United were fairly comfortable in possession and got the deserved second goal - courtesy of the boy wonder Amad Diallo.
A United corner was headed away at the near post by Newcastle, only to drift perfectly into the gulf of space held by Amad just inside the box. He then hits a peach of a shot into the mid left of the net; something Dubrakva should perhaps have saved but he's not Pope and it was a crowded box.
A triple sub with 8 minutes to go saw Hojlund, Martinez and Rashford all come on for Mainoo, Garnacho and the booked Amad, and almost immediately there was impact; Bruno Fernandes playing a tidy ball into a pocket of space for Hojlund who picked up, drifted right twice to get shot space, and tucked home a crafty finish through the defender's legs to beat the full stretched Dubravka. 1 chance, 1 goal. Good return rate that.
6 chance creations in 9 games though, compared to Haaland's 6 chances provided him versus just Wolves - Erik Ten Hag has work to do to make the team pass to Rasmus more. The good news as well is that Rooney scored only 2 more than Hojlund in 2 more games. We have Brighton and City left this season - can the Dane match a club legend?

Inevitably United would then go and fuck up a bit and let Newcastle back into the swing of things; a 92nd minute goal for Hall beamed into the mid right of the net past Onana. His second goal of the season, his second goal of the season at Old Trafford. Sake.
How did it come about though? Eriksen. His lack of legs and his lack of game speed coming on so late led to some lapse decision making.



Only Brighton remains now before our league campaign is over, and without Chelsea getting wrecked by Bournemouth we can't steal two places and get the Europa League - and we only have ourselves to blame by throwing away a win against them and being shit versus others so often this season. Wild times.
Instead, we need to win and hope Newcastle don't win away at Brentford, and that bags us Europa Conference. The upside? Less serious so we could blood more of the kids and new signings, but also a new competition which we are yet to win. It's no big cup, but it'd be a new one in the annals of our history.
7th is definitely achievable, but given our form against Brighton? It's going to be tough. That said, there's still the FA Cup route to the Europa League. If Erik Ten Hag wants the players to galvanise and believe we really have turned a corner (so many false dawns this season) then this is the perfect time to fix up confidence. Stick with roughly this lineup vs Brighton, get another win over our bogey team, and go in on a two-game streak to the FA Cup Final in the hope we can upset Shitty and steal a Europa League spot. Time to clutch up.
youtube
#manchester united#man u#man united#man utd#manchester reds#erik ten hag#marcus rashford#casemiro#old trafford#christian eriksen#kobbie mainoo#amad diallo#bruno fernandes#lewis hall#anthony gordon#alexander isak#bruno guimaraes#sofian amrabat#aaron wan bissaka#Youtube
1 note
·
View note
Text
SAS IN HEALTHCARE ANALYTICS

The Statistical Analysis System (SAS) is one of the most common and effective methods used in data analysis and statistical modelling. It is one of the fastest and most robust advanced analytics, multivariate analysis, business intelligence, data mining, data management, report writing, statistical analysis, application development, business modeling, and data warehousing software. SAS is an asset in various labor markets, as it holds the largest market share in advanced analytics jobs. Position of SAS in the healthcare sector: SAS is extensively used in the pharmaceutical and clinical research companies for clinical trial data analysis. SAS programmers play a key role in the study of data from clinical trials.
• A SAS programmer addresses the Healthcare industry's technical needs. • Implementation of information technology in the healthcare delivery system helps gain valuable knowledge from data, and insight in recommending action or guiding decision-making, and improving the quality and practice of medicine in patient care. • SAS analytical tools help accomplish corporate goals related to revenue generation, cost reduction, and strategic performance management in healthcare. • To improve the overall quality of patient care, SAS instruments are used to investigate clinical outcomes and risk tolerances.
Role of SAS Developer:
Statisticians provide data analysis tools, apply analytical methods on the data obtained, and provide statisticians and or clinicians with the research overview tables, graphs, and data listing for writing study report.
SAS programmers work closely with statisticians and data managers to connect the raw data to the analysis.
SAS programmers can build the database in only a few businesses. To read database data to SAS data sets, SAS programmers must write programs and also generate a library of SAS formats.
SAS programmers can also write SAS programs to clean the data from the database according to CDM's edit requirements.
Through identifying the clinical trial to regulatory approval, SAS plays a significant role. The report for the clinical trial is compiled using the data produced in the SAS context.
SAS also plays a role in the development of protocols, the randomization process, developing CRFs, recording adverse events etc.
Future in SAS: • Over the last few decades, the clinical SAS programming industry in India has seen rapid germination and the trend appears to continue for the next few years due to cost advantage and the availability of skilled labour.
• Because of the growing demand for professional services, programmers' desires have taken a different turn towards diversifying access, excessive wage growth due to various incentives and high expectations of career advancement.
• SAS analytics skills are, according to a recent survey, the most important skills to acquire in today's job market. The conventional health care model extends the career opportunities for SAS programmers. SAS has become a valuable tool to gage the current state and future vision of healthcare analytics.
• While inside the SAS programming environment there were a number of professionals working, they did everything but code. Another interesting fact about this data analytical tool is that most of the professionals who actually worked with it, were not Data Scientists, they actually weren't even Data Analysts. They were also the decision-makers, the highest tier of the companies who were responsible for making decisions and would rather have to code for them a qualified specialist to help them accomplish their goals.
• Fast forward to today when the Data Science industry's SAS programmers get paid out as handsomely as ever. Over the last two, three decades, a Data Analyst's average annual compensation has risen twofold to nearly threefold. Because the compensation has increased, the obligations a specialist needs to take care of have not. Compared to those who have worked with SAS codes in the past , today there is a substantial increase in both the demands and roles of today's professionals. Today, SAS programmers are doing much more than just coding today, with the advent of big data, data science and big data analytics concepts.
• Now that we've effectively overcome our past and current questions about SAS programming status, let's concentrate more on the future of SAS programming. Over the last few years, the demand for this career option has increased significantly. Most importantly, this has resulted in such a progressive image is the knowledge of SAS programming and the field of data science. SAS is popularly regarded as one of the industry's most popular data analytics tools. At the same time, it is also a licensed program which means that downloading is not free or easy. This is why many businesses this opt for SAS must buy the license to use the same.
• Although there are those who say it's a wonderful opportunity to work with SAS and the future looks pretty promising for the same. At the same time, however, there are several questions about the use of open sourced data analytics resources such as R programming. So much so that there are lots of professionals who are currently employed with SAS but are still expected to work inside R. It may seem like a threat to the future of SAS programming but then again there may be far less chances of a survival problem.
About Rang Technologies: Headquartered in New Jersey, Rang Technologies has dedicated over a decade delivering innovative solutions and best talent to help businesses get the most out of the latest technologies in their digital transformation journey. Read More...
0 notes