#bioanalytical research
Explore tagged Tumblr posts
Text
intro! ^_^
hello! i'm eli, i use any pronouns, and i'm a senior chemistry college student with a double major in biochemistry & molecular biology.
i (sometimes) post daily logs of what i do academically, book reviews, will probably post the occasional guide or tutorial, and i reblog other people's daily logs, aesthetic posts, journaling, and more!
i made this blog partially to hold myself accountable and stay on top of things! it will also help to serve as an archive for some of the projects that i work on.

i'll tag everything with the relevant class(es)! other tags below the cut :)
spring 2425 schedule:
upcoming
*main blog is @sleepy-zoroark !

from the horse's mouth (original posts)
gently used posts (reblogs)
stack of books (reblogs and posts about reading, also where i might save titles to read)
each post will be tagged with the relevant term - fall/winter/spring 2x2y
ARCHIVE:
spring 2223: biochemistry, technical communications, comparative anatomy and physiology, potions and poisons, research rotation
fall 2324 (INACTIVE): physical chemistry i, evolution & diversity, research rotation, tutor training, baroque classical and romantic music
winter 2324 (INACTIVE): bioanalytical chemistry, biochemistry ii, physical chemistry ii, biochemical pharmacology
#studyblr#study motivation#study tips#studying#studyspo#studyinspo#study inspo#study blog#studying blog#chemistry#lab work#research#research project#learning#education#college student#university student#student life#gently used posts#stack of books#from the horse's mouth#bioanalytical chemistry#biochemistry ii#physical chemistry ii#biochemical pharmacology
7 notes
·
View notes
Video
youtube
CEKG Grand Ceremony---2024
In 2024, we will be better.
#youtube#2024#LabEquipment#Research Bioanalytics Therapeutics#laboratorylife biotechnology bioprocessing biotech startup bioprocess
1 note
·
View note
Link
According to the new market research report "Bioanalytical Testing Services Market by Type, Application (Oncology, Neurology, Infectious Diseases, Gastroenterology, Cardiology), End User and Region (North America, Europe, APAC, Latin America, MEA) - Global Forecast to 2027", The global bioanalytical testing services market is projected to reach USD 6.0 billion by 2027 from an estimated USD 2.9 billion in 2022, at a CAGR of 15.6% during the forecast period.
#marketsandmarkets research pvt. ltd.#bioanalytical testing services#bioanalytical testing market#bioanalytical testing service#bioanalytical testing industry
0 notes
Text
64 | Sex In Space
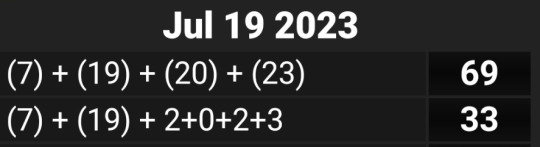
NASA literally changed the world and perceptions in '69 with the moon landing.
And as for 33 that speaks for itself, but of course:


The 19 in reverse is 91, much like the value for 'Space'
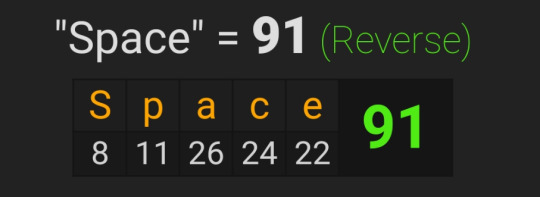
July 19, 2023, leaves 165 days left in the year
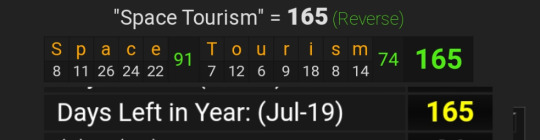
Lastly

1 note
·
View note
Text
Best CRO Companies in Hyderabad
Introduction
Hyderabad has emerged as a major hub for Contract Research Organizations (CROs), offering cutting-edge research and development services to the pharmaceutical and biotech industries. With its strong infrastructure, skilled workforce, and favorable government policies, Hyderabad has become a preferred destination for CRO companies that support drug discovery, clinical trials, and regulatory compliance.
Understanding CROs and Their Role
Contract Research Organizations (CROs) play a crucial role in pharmaceutical research by assisting companies in conducting clinical trials, preclinical research, and regulatory affairs. These organizations help pharmaceutical firms bring new drugs to market efficiently by handling complex research processes, ensuring compliance with regulatory guidelines, and reducing development costs. The services offered by CROs range from early-stage drug discovery to post-marketing surveillance, making them vital players in the healthcare and life sciences industries.
Why Hyderabad is a Hub for CRO Companies
Hyderabad, often referred to as the ‘Genome Valley of India,’ has become a hotspot for pharmaceutical and biotech research. The city boasts state-of-the-art laboratories, research parks, and biotechnology clusters that attract global pharmaceutical giants. Additionally, Hyderabad is home to renowned research institutions, universities, and a skilled talent pool, making it an ideal location for CRO companies. The Indian government’s proactive support, including tax incentives and funding initiatives, further strengthens Hyderabad’s position as a leading CRO destination.
Leading CRO Companies in Hyderabad
Several CRO companies in Hyderabad are renowned for their expertise in clinical research, bioanalytical testing, and regulatory affairs. Some of the top players in the industry include:
Syngene International – A leading CRO specializing in drug discovery, development, and manufacturing solutions.
GVK BIO (Aragen Life Sciences) – Offers integrated research services across the drug development spectrum.
Sai Life Sciences – Focuses on preclinical and clinical research for global pharmaceutical companies.
Parexel – A global CRO with a strong presence in Hyderabad, providing clinical trial management and regulatory support.
These companies contribute significantly to drug development by ensuring the safety, efficacy, and compliance of new pharmaceutical products.
Services Offered by CRO Companies in Hyderabad
CROs in Hyderabad provide a comprehensive range of services tailored to the needs of pharmaceutical and biotech companies. Some key services include:
Preclinical Research – Laboratory testing to evaluate the safety and effectiveness of new drug compounds.
Clinical Trials – Conducting Phase I-IV trials to assess drug safety and efficacy before regulatory approval.
Regulatory Affairs – Assisting with documentation, submission, and compliance with global regulatory authorities.
Bioanalytical Testing – Quality control and analytical testing to ensure drug consistency and purity.
These services help pharmaceutical companies streamline the drug development process while maintaining high standards of research and compliance.
Factors to Consider When Choosing a CRO in Hyderabad
Selecting the right CRO is critical for the success of a research project or clinical trial. Companies should consider the following factors when choosing a CRO in Hyderabad:
Experience & Expertise – A well-established CRO with a strong track record in the industry.
Regulatory Compliance – Adherence to international guidelines such as FDA, ICH, and GCP standards.
Technology & Infrastructure – Availability of advanced laboratories, equipment, and research facilities.
Client Reviews & Success Stories – Positive testimonials and proven success in delivering results.
By carefully evaluating these factors, pharmaceutical companies can partner with the right CRO to accelerate their drug development process.
Growth and Future Prospects of the CRO Industry in Hyderabad
The CRO industry in Hyderabad is poised for significant growth as global pharmaceutical companies continue to outsource research and development to India. With increasing investments in biotechnology, government support, and advancements in medical research, CRO companies in Hyderabad are expected to expand their capabilities and strengthen their global presence. This growth will not only benefit the pharmaceutical industry but also create employment opportunities and drive innovation in healthcare research.
Conclusion
Hyderabad continues to solidify its reputation as a premier destination for CRO services, offering world-class research and development support to pharmaceutical and biotech companies. With a strong ecosystem, skilled professionals, and a growing demand for drug innovation, CRO companies in Hyderabad are playing a crucial role in shaping the future of global healthcare.
0 notes
Text
Understanding the Importance of Preclinical Research Labs in Drug Development
The pharmaceutical and biotech industries rely heavily on preclinical research labs to ensure the safety and efficacy of new drugs before they proceed to clinical trials. These labs play a crucial role in assessing the pharmacokinetics, pharmacodynamics, and toxicology of potential drug candidates. A reliable preclinical company provides comprehensive preclinical lab services to meet regulatory requirements and accelerate drug discovery and development.

What Are Preclinical Research Labs?
Preclinical research labs are specialized facilities where scientists conduct experiments to evaluate the biological effects of new compounds. These studies include in vitro (cell-based) and in vivo (animal-based) testing to assess safety and effectiveness before advancing to human trials. Preclinical testing services ensure that only the most promising drug candidates move forward in the pipeline.
Key Services Offered by a Preclinical Company
A leading preclinical company offers various preclinical lab services to support drug development. These services include:
1. Toxicology Studies
Toxicology assessments are critical to determining the potential risks associated with a drug. Preclinical research labs conduct acute, subchronic, and chronic toxicity studies to evaluate adverse effects.
2. Pharmacokinetics and Pharmacodynamics (PK/PD)
Understanding how a drug is absorbed, distributed, metabolized, and excreted (ADME) is essential in drug development. Preclinical testing services provide detailed insights into these processes, helping researchers optimize drug formulations.
3. Safety Pharmacology
Preclinical lab services include safety pharmacology studies to examine the potential impact of a drug on major organ systems, such as the heart and nervous system.
4. Efficacy Testing
Before moving to clinical trials, preclinical company experts conduct efficacy studies to determine the therapeutic potential of a drug. These studies involve disease models to assess how well a compound treats a specific condition.
5. Bioanalytical Testing
Preclinical testing services also include bioanalytical assays to quantify drug concentrations in biological samples, ensuring precise dose selection for clinical trials.
The Role of Preclinical Testing Services in Drug Development
Preclinical testing services serve as the foundation for regulatory approvals by organizations such as the FDA and EMA. Without rigorous preclinical research labs, it would be impossible to predict how a drug might behave in humans. The data generated from preclinical lab services support Investigational New Drug (IND) applications, paving the way for clinical studies.
Challenges in Preclinical Research Labs
Despite their importance, preclinical research labs face several challenges, including:
Regulatory Compliance: A preclinical company must adhere to stringent regulatory guidelines, such as Good Laboratory Practice (GLP), to ensure the reliability of study data.
High Costs: Conducting comprehensive preclinical lab services can be expensive, requiring advanced technology and skilled professionals.
Animal Welfare Concerns: Preclinical testing services often involve animal studies, which require ethical considerations and adherence to humane testing protocols.
Data Reproducibility: Ensuring that experimental data is consistent and reproducible is a critical aspect of preclinical research labs to avoid failures in later stages of drug development.
How to Choose the Right Preclinical Company?
Selecting a trustworthy preclinical company is crucial for successful drug development. Here are some factors to consider:
Accreditation & Compliance: Ensure the company follows GLP and other regulatory guidelines.
Expertise & Experience: Look for a preclinical company with a strong track record in preclinical testing services.
Advanced Technology: A well-equipped preclinical research lab should have state-of-the-art instrumentation for accurate testing.
Customized Services: Choose a provider that offers tailored preclinical lab services to meet your specific project requirements.
Timely Delivery: Speed is essential in drug development. A reliable preclinical company should offer efficient turnaround times without compromising quality.
The Future of Preclinical Research Labs
Advancements in technology are transforming preclinical research labs, making drug testing more efficient and reliable. Innovations such as artificial intelligence (AI), organ-on-a-chip technology, and in silico modeling are reducing the need for animal testing while improving the accuracy of preclinical testing services. These developments are helping preclinical companies provide faster and more ethical solutions for drug discovery.
Conclusion
Preclinical research labs are the backbone of the pharmaceutical industry, ensuring that new drugs are safe and effective before entering human trials. A reputable preclinical company provides top-notch preclinical lab services, including toxicology studies, pharmacokinetics, safety pharmacology, and efficacy testing. By utilizing comprehensive preclinical testing services, biotech and pharmaceutical firms can accelerate drug development and bring innovative treatments to market faster.Choosing the right preclinical company is essential for success in drug discovery. Partnering with an experienced provider of preclinical lab services ensures regulatory compliance, high-quality data, and efficient study execution. As technology continues to evolve, preclinical research labs will play an even more critical role in shaping the future of medicine.
#preclinical research labs#preclinical company#preclinical lab services#preclinical testing services
0 notes
Text
Healthcare Analytical Testing Services Market Forecast: Future Growth Prospects and Projections
The global healthcare analytical testing services market is experiencing significant growth, driven by the increasing complexity of pharmaceutical products and the stringent regulatory requirements necessitating rigorous testing protocols. Valued at USD 7.37 billion in 2023, the market is projected to reach USD 19.14 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.21% over the forecast period from 2024 to 2032.
Market Segmentation:
The healthcare analytical testing services market is segmented based on type and end-user:
By Type:
Medical Device Analytical Testing Services
Pharmaceutical Analytical Testing Services
By End-User:
Contract Research Organizations (CROs)
Medical Device Companies
Pharmaceutical and Biopharmaceutical Companies
Get Free Sample Report @ https://www.snsinsider.com/sample-request/1048
Regional Analysis:
North America currently dominates the market, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows, with significant contributions from countries like Germany and the UK. The Asia-Pacific region is anticipated to witness the fastest growth, driven by increasing healthcare investments and the expansion of pharmaceutical manufacturing capabilities.
Key Players and Their Products in Healthcare Analytical Testing Services
Eurofins Scientific – Microbial Testing, Pharmaceutical Testing, Biotech Services, Analytical Chemistry, Environmental Testing, Stability Testing
Laboratory Corporation of America Holdings – Drug Development Services, Clinical Trial Services, Bioanalytical Testing, Stability Studies, Compounding & Analytical Testing, Genomic Services
SGS S.A. – Analytical Chemistry, Stability Testing, Biocompatibility Testing, Microbial Testing, Method Validation, Regulatory Compliance Services
Charles River Laboratories – Safety Assessment, Biologics Testing Solutions, Microbial Testing, Product Development Services, Bioanalytical Services, In-Vitro Testing
WuXi AppTec Co. Ltd. – Drug Discovery and Development Services, Biologics Testing, Cell-Based Assays, Analytical Chemistry, Stability Testing, Preclinical Testing
Element Materials Technology – Analytical Testing Services, Chemical Testing, Microbial Testing, Biocompatibility Testing, Environmental Testing, Validation Services
Thermo Fisher Scientific, Inc. – Analytical Instruments, Clinical Testing, Genomic Analysis, Bioanalytical Services, Stability Studies, Method Development
Pace Analytical Services LLC – Pharmaceutical Analytical Testing, Environmental Testing, Stability Testing, Bioanalytical Services, Chemical Analysis
Intertek Group plc – Analytical Chemistry, Bioanalytical Testing, Stability Studies, Regulatory Compliance Services, Safety Testing, Environmental Testing
IQVIA Inc. – Pharmaceutical Analytics, Clinical Research Services, Drug Development Testing, Market Research Analytics, Data Analytics Solutions
Merck KGaA – Laboratory Equipment, Bioanalytical Services, Stability Studies, Chemical Analysis, Microbial Testing, Process Validation
Source BioScience – DNA/RNA Testing, Pathology Services, Stability Testing, Microbial Testing, Pharmaceutical Analytical Services
Almac Group – Clinical Trial Services, Analytical Testing, Stability Studies, Bioanalytical Services, Drug Development
ICON Plc – Clinical Research Services, Bioanalytical Testing, Stability Testing, Clinical Trial Management Services
Frontage Laboratories, Inc. – Bioanalytical Services, Preclinical Testing, Analytical Chemistry, Stability Testing, Formulation Development
STERIS Plc – Sterility Testing, Biocompatibility Testing, Microbial Testing, Analytical Chemistry, Validation Services
Sartorius AG – Bioanalytical Testing, Process Development Services, Stability Studies, Quality Control Services, Cell & Gene Therapy Services
ALS Life Science – Pharmaceutical Analytical Services, Stability Testing, Biotech & Biopharma Testing, Environmental & Chemical Testing
Syneos Health, INC – Clinical Trial Testing, Analytical Testing Services, Biopharmaceutical Testing, Regulatory Compliance, Drug Development Services
Key Highlights:
The shift towards personalized medicine is increasing the need for specialized assays and biomarker-based testing, supporting targeted therapies.
The rising demand for biosimilars and generic medications necessitates comprehensive analytical testing to ensure bioequivalence and compliance with regulatory standards.
Technological advancements, such as automated liquid chromatography systems and AI-driven predictive analytics, are enhancing testing efficiency and accuracy.
Future Outlook:
The healthcare analytical testing services market is poised for robust growth, driven by ongoing pharmaceutical innovations and the escalating demand for precise and efficient testing solutions. As the industry continues to evolve, service providers are expected to expand their capabilities, incorporating advanced technologies to meet the diverse needs of pharmaceutical and biopharmaceutical companies.
Conclusion:
The global healthcare analytical testing services market is on a promising trajectory, offering significant opportunities for stakeholders, including CROs, medical device companies, and pharmaceutical manufacturers. Continuous advancements and the increasing complexity of healthcare products underscore the critical role of analytical testing services in ensuring product safety, efficacy, and regulatory compliance.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Digital PCR-dPCR Market Size
Electronic Medical Record (EMR) Systems Market Size
3D Printing Medical Devices Market Size
#Healthcare Analytical Testing Services Market#Healthcare Analytical Testing Services Market Share#Healthcare Analytical Testing Services Market Size#Healthcare Analytical Testing Services Market Trends
0 notes
Text
Central Lab Market Size, Growth Outlook 2035
The Central Lab Market Size was estimated at 2.67 (USD Billion) in 2023. The Central Lab Industry is expected to grow from 2.81 (USD Billion) in 2024 to 4.29 (USD Billion) by 2032. The Central Lab Market CAGR (growth rate) is expected to be around 5.43% during the forecast period (2024 - 2032).
Market Overview
The Central Lab Market is expanding rapidly due to the increasing complexity of clinical trials, advancements in diagnostic technologies, and growing demand for centralized testing services. Central labs play a crucial role in drug development and clinical research by providing high-quality laboratory services, including biomarker testing, pharmacokinetics analysis, and pathology assessments.
With the rising prevalence of chronic diseases and the need for more efficient clinical trials, pharmaceutical companies and contract research organizations (CROs) are increasingly outsourcing laboratory services to central labs. The adoption of automated laboratory workflows and AI-driven data analytics is further enhancing lab efficiency and precision.
Market Size and Share
The Central Lab Market Size was estimated at 2.67 (USD Billion) in 2023. The Central Lab Industry is expected to grow from 2.81 (USD Billion) in 2024 to 4.29 (USD Billion) by 2032. The Central Lab Market CAGR (growth rate) is expected to be around 5.43% during the forecast period (2024 - 2032).

North America holds the largest market share due to strong research funding, a well-established pharmaceutical industry, and high adoption of centralized laboratory services. Asia-Pacific is anticipated to witness the fastest growth, driven by increasing clinical trial activities and the expansion of research infrastructure.
Market Drivers
Growing Complexity of Clinical Trials: The increasing number of multi-site global trials has led to higher demand for centralized laboratory services to ensure standardization.
Rising Prevalence of Chronic Diseases: The surge in oncology, cardiovascular, and infectious disease research has boosted the need for specialized lab testing.
Advancements in Laboratory Automation: AI-powered clinical trial sample management systems and automated sample analysis are improving efficiency.
Increasing Outsourcing of Lab Services: Pharmaceutical companies and contract research organizations (CROs) are increasingly relying on central labs for biospecimen testing and drug development.
Growing Focus on Personalized Medicine: The demand for genetic testing and biomarker analysis in precision medicine is accelerating the adoption of central lab services.
Challenges and Restraints
Regulatory and Compliance Challenges: Strict guidelines for clinical trial sample testing can slow down approvals and increase operational complexity.
Data Management and Cybersecurity Concerns: Handling vast amounts of clinical trial data requires robust cybersecurity measures to ensure patient confidentiality.
High Costs of Advanced Diagnostic Technologies: Implementing AI-driven pathology analysis and next-generation sequencing (NGS) technologies can be expensive.
Market Trends
Adoption of AI & Machine Learning in Laboratory Analysis: AI-driven biomarker identification and pathology assessment are enhancing efficiency.
Expansion of Decentralized and Hybrid Clinical Trials: While central labs remain critical, decentralized trial models are being integrated with remote patient monitoring.
Increasing Demand for Genetic & Molecular Testing: NGS-based diagnostic services and liquid biopsy analysis are gaining traction in clinical trials.
Growth in Bioanalytical Testing Services: The demand for pharmacokinetic and pharmacodynamic analysis is increasing for new drug approvals.
Regional Analysis
North America: Dominates the market with strong CRO presence, high R&D investments, and regulatory support for clinical trials.
Europe: Significant market growth due to advancements in biopharmaceutical research and increased adoption of centralized clinical trial services.
Asia-Pacific: Fast-growing market with rising clinical trial activities, government support for research infrastructure, and increasing pharmaceutical outsourcing.
Rest of the World: Latin America and the Middle East are witnessing growth in clinical trial investments and biomarker testing services.
Segmental Analysis
By Service Type:
Biomarker Testing
Genetic & Molecular Testing
Microbiology Testing
Pathology & Histology Services
Bioanalytical Services
By End-User:
Pharmaceutical & Biotechnology Companies
Contract Research Organizations (CROs)
Academic & Research Institutes
Hospitals & Diagnostic Centers
Key Market Players
Qiagen
HoffmannLa Roche Ltd.
BioMérieux
Merck Co., Inc.
Agilent Technologies, Inc.
Danaher Corporation
Siemens Healthineers
Recent Developments
Strategic Acquisitions: Labcorp expanded its central lab services by acquiring a leading bioanalytical testing company.
Advancements in AI-Powered Diagnostics: Eurofins Scientific launched an AI-driven pathology assessment tool for clinical trials.
Partnerships for Biomarker Research: ICON plc collaborated with biotech firms to enhance biomarker discovery in oncology trials.
For more information, please visit us at marketresearchfuture.
#Central Lab Market Size#Central Lab Market Share#Central Lab Market Growth#Central Lab Market Analysis#Central Lab Market Trends#Central Lab Market Forecast#Central Lab Market Segments
0 notes
Text
Clinical Research Companies in Hyderabad: A Hub of Innovation
Introduction
Hyderabad has become one of the leading cities in India for clinical research, with a growing number of organizations offering services that support the development of new medicines, treatments, and healthcare innovations. The city’s emerging clinical research landscape has positioned it as an attractive destination for both national and international pharmaceutical and biotechnology companies looking to conduct clinical trials, preclinical studies, and drug development.
The Growing Clinical Research Landscape in Hyderabad
Over the past decade, Hyderabad has attracted global attention as a major hub for clinical research, driven by factors such as a skilled workforce, cost efficiency, and regulatory advancements. With its rich infrastructure, robust educational institutions, and proximity to top healthcare providers, the city has become a focal point for pharmaceutical companies seeking to accelerate the development of new treatments and therapies. This growth has been supported by favorable government policies and investments, which continue to drive the sector’s expansion.
Top Clinical Research Companies in Hyderabad
Syngene International
Syngene International is a leading Contract Research Organization (CRO) that offers integrated services to the pharmaceutical, biotechnology, and healthcare sectors. With a comprehensive suite of services including drug discovery, development, and manufacturing support, Syngene is at the forefront of clinical research in Hyderabad. The company works with global clients to streamline the drug development process, making it one of the city’s top CROs.
IQVIA
IQVIA provides innovative healthcare solutions and conducts clinical trials to ensure the safety and efficacy of new therapies. The company offers a range of services including clinical development, market access, and patient safety monitoring. With a strong presence in Hyderabad, IQVIA contributes significantly to India’s growing role in global clinical trials and healthcare data analytics.
GVK BIO
GVK BIO offers comprehensive drug discovery and development services and is a key player in India’s growing CRO sector. With its state-of-the-art facilities and expertise in areas like bioanalytical testing, clinical pharmacology, and toxicology, GVK BIO plays a crucial role in supporting pharmaceutical and biotech companies in the development of novel therapies.
Medpace
Medpace is a global, full-service clinical contract research organization that offers clinical trial services in Hyderabad for clinical and regulatory support. Medpace focuses on providing high-quality clinical trials and regulatory services to ensure that new therapies meet global standards. The company’s ability to navigate complex regulatory landscapes and support international studies has made it an important player in the field.
Factors Contributing to the Growth of Clinical Research in Hyderabad
Hyderabad’s rapid growth as a clinical research hub is due to several factors, including its highly educated workforce, large patient pool, and favorable business environment. The city is home to a large number of medical schools, research institutes, and training programs, producing a steady supply of highly qualified clinical research professionals. Additionally, the city’s large, diverse population provides an ideal environment for patient recruitment, which is critical for the success of clinical trials. Supportive government policies, such as tax incentives and investment in healthcare infrastructure, also contribute to Hyderabad’s growth as a clinical research destination.
Challenges in Clinical Research in Hyderabad
While Hyderabad’s clinical research industry is booming, there are challenges such as regulatory hurdles, recruitment difficulties, and maintaining the quality of clinical trials. India’s complex regulatory environment, while improving, can still be difficult to navigate, which sometimes leads to delays in trial approval or regulatory compliance. Additionally, recruiting patients for clinical trials can be time-consuming, and maintaining consistent trial quality across a large population can prove challenging.
Future Outlook for Clinical Research in Hyderabad
With technological advancements, partnerships with international organizations, and a strong focus on personalized medicine, the future of clinical research in Hyderabad looks promising. The city is increasingly adopting digital technologies such as electronic data capture (EDC), artificial intelligence (AI), and telemedicine to improve the efficiency and accuracy of clinical trials. Furthermore, Hyderabad’s collaboration with global pharmaceutical companies and research institutes positions it well for continued growth in clinical research. Personalized medicine, with a focus on genetic-based treatments, is also expected to drive innovation and shape the future of clinical trials.
Conclusion
Hyderabad continues to solidify its position as a global leader in clinical research, playing a crucial role in advancing medical science and improving global healthcare outcomes. The city’s dynamic CRO landscape, combined with its skilled workforce, strong infrastructure, and favorable regulatory environment, makes it an attractive destination for pharmaceutical and biotech companies looking to develop and test new therapies. As the industry evolves and technology continues to improve, Hyderabad will remain at the forefront of clinical research and innovation.
0 notes
Link
Asia Pacific is anticipated to observer the fastest growth rate over the forecast period. At present the world’s second biggest economy, China is a market...
0 notes
Text
Data Scientist (multi-omics) Technical University of Munich Join TUM as a #DataScientist in #bioinformatics to tackle #cancer with multi-omics, #machinelearning, and cutting-edge proteomics research! See the full job description on jobRxiv: https://jobrxiv.org/job/technical-university-of-munich-27778-data-scientist-multi-omics/?feed_id=91645 #bioinformatics #data_science #mass_spectrometry #multi_omics #proteomics #ScienceJobs #hiring #research
0 notes
Link
According to the new market research report "Bioanalytical Testing Services Market by Type, Application (Oncology, Neurology, Infectious Diseases, Gastroenterology, Cardiology), End User and Region (North America, Europe, APAC, Latin America, MEA) - Global Forecast to 2027", The global bioanalytical testing services market is projected to reach USD 6.0 billion by 2027 from an estimated USD 2.9 billion in 2022, at a CAGR of 15.6% during the forecast period.
#marketsandmarkets research pvt. ltd.#bioanalytical testing services#bioanalytical testing market#bioanalytical testing service#bioanalytical testing industry
1 note
·
View note