#azeotrope
Explore tagged Tumblr posts
Text

The scientific research journals of S. Sunkavally. Page 105.
#rose#dew#solute#dilution#hydrogen ion stabilization#vapour pressure#endothermic reaction#ethanol-water mixture#coral#low tide#glycerol#volatilization#fermentation#hydrochloric acid#azeotrope#sebaceous gland#oil#pheromones#metal ion cofactors#enzyme flexibility#L-alanine#isomerization#eyes#crystallins#electroconvulsive shock#endergonic reactions#gas giants#magnetic field#metallic hydrogen#nicotinamide adenine dinucleotide
3 notes
·
View notes
Text
azeotropes. my least favorite thing ever
holy shit I hate azeotropes so much
why cant I just. distill the water away from hydrazine
no.
I have to react the hydrazine with sulfuric acid to make hydrazinium sulfate, then I have to separate that out from the water, then I have to react that with sodium hydroxide in alcohol, then filter it out again, then boil off the alcohol. holy shit I hate azeotropes. but they kinda cool though
#chemblr#chemistry#science#fire#hydrazine is sooo cool but suuuuuuch a nightmare#fuck you azeotropic hydrazine hydrate#i wish to kill god for deciding to make azeotropes
13 notes
·
View notes
Text
my favourite annoying thing to do as a science-scarred student is randomly name drop physics/chemistry stuff where they literally couldn't belong less just for the cool ring and the kicks. oh my god you are a viscous drag. she's so optically dense. he's such a bromoamide bore - uh, bro. i have such a goddamn sinusoidal personality when will I have my DC or even my pulsating DC era damnit. for the love of vinylic ketals. my brain's young's modulus decreases exponentially every day. damn we are such incoherent wavefronts. stop being a bernoulli bitch. what the fuck it's 315 kelvin outside. we are so azeotropic <3
#my drafts are a hellscape#liveblogging.pdf#chemistry my beloathed </3#physics my tsundere straight girl crush </3
29 notes
·
View notes
Text
I'm trying to find the boiling point of a water + ethyl lactate azeotrope, but the only thing I can find in literature is that they do indeed form an azeotrope. The boiling point simply does not exist.
2 notes
·
View notes
Text
Chapter 9 Trivia
The back of Tsukasa's sword here reminds me of fish fins…

The smoke signal is a double-edged sword, you say?
…A double-edged sword like Tsukasa's, perhaps? 🤔

The 4th use of calcium carbonate (CaCO3) mentioned here isn't that obvious since it's not a component of gunpowder, but rather it's involved in the process I described in last week's trivia here:

Wood ash is mostly CaCO3, but mortar can also be used in the niter-bed instead, as Senku's version is also made of CaCO3 (with sand, clay, and lime added).
There's also the 5th usage for sea shells: pretty necklaces!

Tsukasa only hears the second set of explosions (the 3 in quick succession) rather than the first explosion.
Was he out of range? Not much time had passed, and we didn't see Tsukasa sleep, so did he run the whole distance in a day?


Tsukasa cuts off Yuzuriha's ribbon here, but the edge tears rather than slices cleanly, making me think it's not a very sharp knife but a jagged and serrated one.
The "knife" is also his spear from earlier, broken.


Tsukasa dropped his coat somewhere between grabbing Yuzuriha and confronting Senku, as usual.


To cut hair, you generally want the sharpest tools you can find so you can make a clean cut without ripping the hair and causing split ends.
Even using modern razors, tension still needs to be placed on the hair for it to cut properly, which Tsukasa doesn't do here.



As shown earlier, Tsukasa's knife rips rather than cuts, so to do what he does here, he has to saw at the hair.
(I tested this; neither my serrated nor straight-edged knives managed to cut my hair at all. They're also not that sharp, more similar to Tsukasa's than a stylist's.)
If the knife was sharp enough to slice it all off at once, then Yuzuriha holding it like this would probably have made her bleed.

Alcohol can't be purer than ~95.6% because it forms an azeotrope (=a situation where the liquids cannot be separated any further via distillation as their vapor properties are the same), thus Senku is asking for pure alcohol.

This is possible to do with his primitive setup, either by using multiple distillations or a drying agent.
Nital for etching is generally a 1-10 : 100 mixture of nitric acid to ethanol. Any higher than this and it becomes explosive.
Senku is somehow making a 3 : 7 mixture which I'm pretty sure is either impossible or extremely dangerous.

Kohaku doesn't use the Japanese word for three (三, pronounced "san") and instead uses 3, (pronounced "surī") the English version.
I wonder if the reasoning behind this has any future significance…


11 notes
·
View notes
Text


Some impressions from me making some nitric acid for the first time; went much better than expected, the distillate seems to be of slightly over azeotropic concentration, while the dilute part from the little NO2 "scrubber" is some single digit percentage. I was really surprised by how little NO2 was produced, as I used a bisulfate in place of sulfuric acid, which requires a higher temperature (where I would expect more of the nitric to decompose). Idk why that happened, but for now I'm just happy that it worked out.
Overall the experiment yielded about 40g of the acid; tho there is almost certainly more left in the distillation flask, I just ran out of time, and thus didn't finish the distillation; maybe a project for later.
#chem#stem#science#science side of tumblr#chemistry#chemblr#lab#stemblr#ochem#organic chemistry#inorganic chemistry#molecules#sciblr
4 notes
·
View notes
Text

day 15 // 100dop
on @diaryofastemstudent's suggestion, i'm finally testing out forest! i planted 5 trees today and i didn't have to use the app the whole day to focus 😊 hopefully i'll get more and more used to not looking at my phone and eventually not have to use the app at all anymore!
but...even though i was focusing better, i still didn't finish everything i wanted to today...which tbh makes me a bit sad and scared, even though my goals were probably unrealistic, because i have to go through all the material and understand all of it by the end of this week to give myself at least the minimum 3 days to review for the final 🙈🙈🙈
but there's still tomorrow, so- BUT STILL!!! i have a lab report (always takes me longer than i'd like to write it and make sure it makes sense), a quiz, an assignment, and 2 chapters + the tail end of a 3rd chapter to go through!!!
sigh. "somehow it'll work out okay" is quickly becoming my mantra 😅 i have no idea how but somehow it will.
I had to break up a really long section on liquid-vapor equilibrium into several pages. I'm almost done with that section, just need to finish notes on azeotropic mixtures and their graphs...somehow that really threw me for a loop.
Then I need to get through eutectics and Henry's law, and get through the last two chapters which...sorta seem to make more common sense??? I think??? I hope????
I have no idea how I made it through high school. That was a breeze compared to this 😩
update after some thought: i think it was in part because i had great teachers who always guided you through every step of the logic AND because i always had math in high school and chemistry has sm math so i guess it's much easier when you're not out of practice. i have not touched math since high school. so 🤷♀️
Day 9: What is your favourite song in your study playlist?
don't have an actual study playlist but lately i've been loving slow-ish bass-y lofi, the music in this study with me video, and studio ghibli music!
#photo from unsplash as always#100dop#100 days of productivity#stem academia#stemblr#chemblr#studyblr#stem student#chemistry#studio ghibli#study music#my neighbor totoro#subatomicstudychallenge#raise your hand if you're a sleepy tired scared student#✋
9 notes
·
View notes
Note
Hydroiodic acid (or hydriodic acid) is an aqueous solution of hydrogen iodide (HI). It is a strong acid, i.e. an acid that is ionized completely in an aqueous solution. It is colorless. Concentrated solutions are usually 48% to 57% HI.
IUPAC Name: Iodane
Properties
Chemical formula: HI(aq)
Molar mass127.91g/mol
Appearance: colorless liquid
Odor: acrid
Density: 1.70 g/mL, azeotrope(57% HI by weight)
Boiling point: 127 °C (261 °F; 400 K) 1.03 bar, azeotrope
Solubility in water :Aqueous solution
Acidity (pKa)-9.3
Source Wikipedia
This acid also has a cool almost black colour
(gold anon)
Notes, if behind-closed-doors-ask gets an ask talking about what seeks goop is it should mention HI, b'cos that was me
Hey b-c-d-a hope you didn't find that too weird
YAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAY THANKYOU
2 notes
·
View notes
Text
KLEA R410a REFRIGERANT GAS
A modern, non-ozone-depleting, and efficient hydrofluorocarbon (HFC) refrigerant blend widely used in air conditioning and heat pump systems
Klea R-410A is a near-azeotropic blend of two HFC refrigerants, R-32 and R-125 (50/50 by weight), developed as a replacement for R-22 in residential and commercial air conditioning systems. It operates at higher pressures than R-22, providing improved energy efficiency and greater cooling capacity. R-410A has zero ozone depletion potential (ODP) but does have a high global warming potential (GWP), making it a transitional solution as the industry moves toward low-GWP alternatives.
Key Properties:
Composition: 50% R-32 / 50% R-125
Ozone Depletion Potential (ODP): 0
Global Warming Potential (GWP): ~2088
Applications: Split AC systems, heat pumps, rooftop units
Lubricant Compatibility: Requires synthetic POE (polyolester) oils
Color: Colorless, non-flammable under normal conditions

#hvac#alramiz#wholesale#machines#rewinding materials#thermostat#tools & safety#heater & element#are#acsparta#KleaR410A#R410A#Refrigerant#HVAC#AirConditioning#HeatPump#Cooling#Heating#HFCRefrigerant#NonOzoneDepleting#EnvironmentalRefrigerant#HVACR#RefrigerantGas#HVACIndustry#AirConditioningService#HeatPumpInstallation#Klea#ClimateControl#SustainableHVAC#RefrigerantManagement
0 notes
Text
girls there is a possibility i have fucked up >render a pint of chicken fat a couple days ago >kinda old-fryer smell, impurities settling to the bottom >decide ill clean it >heard you can do that with baking soda and/or salt >warm up the fat and some water in the microwave, bring to low boil on the stove >forgot to add baking soda and salt >dump them in, not sure how much to use, probably use too much >passes sodium bicarbonate through a hotter-than-212 oil (anhydrous) >remembers what happens when you add strong bases to fats >ohshitdidijustmakesoap.jpg anyways im still boiling it to see what happens. its slightly emulsified but i wonder if i can boil off the water at this point or if its formed some kind of azeotrope. we shall see if i accidentally made chicken soap
0 notes
Text
Why is cleaning needed
Harmful contaminants such as solder and adhesive residues, flux, and dust and debris from other manufacturing processes and handling are often formed during the course of electronics manufacture, and the primary purpose of cleaning is to remove these contaminants at regular intervals. This ultimately leads to increased lifetime of the electronic product by ensuring good surface resistance and by preventing current leakage due to PCB failure.
With the constantly evolving cleaning market to meet the demands of the ever-expanding electronics industry, it is of paramount importance that the level of cleanliness required be clearly defined. A correct method must then be used to ensure that the level of cleanliness achieved meets the standard specified by the electronics engineer.
When to perform cleaning
There are many stages where cleaning is required:
Before stencilling and soldering to remove contaminants from the previous production stages
After stencilling to remove excess solder/adhesive
After soldering to remove corrosive flux residues and any excess solder
How is cleaning performed
Precise application of solder is often achieved using a stainless steel stencil over the printed circuit board. Once the circuit design has been finalised, it is mostly purchased as a set of a stencil and many PCBs (a single stencil can be used on thousands of PCBs). The solder is applied on the stencil through which it flows precisely onto the PCB below.

The ultimate goal of cleaning is to remove unwanted residue from the surface and under components. This is achieved by considering selection of components, board material compatibility, placement and defining solder mask in the Design Phase. The cleaning agent must be according to the solder alloy and flux composition with proper heat exposure. The Cleaning Agent must be selected with reuse, environmental, temperature, use rate and Health and Safety considerations in mind. Any Cleaning Machine used must perform proper fluid management, give a good throughput and consume less energy.
Types of cleaning in electronics manufacturing
Proper cleaning can be categorised into PCB Cleaning, Stencil Cleaning and Maintenance Cleaning.
PCB cleaning This can be further categorised into inline and batch aqueous sprays for in-air cleaning, ultrasonic and batch immersion cleaning, manual PCB and benchtop cleaning and vapour degreasing.
While inline washers use high flow, energy and deflective forces, batch cleaning machines are designed to wash, rinse and dry assemblies of smaller footprint. In ultrasonic and batch immersion cleaning, the product being washed is completely immersed in the cleaning agent using either ultrasonic energy or spray-under-immersion forces. Manual PCB and benchtop cleaning is required in rework and repairs to production assemblies and after hand placement of BGAs, connectors or other surface mount components and this is achieved by using an aerosol can or a pump dispenser and ensuring the right cleaning chemistry. In vapour degreasing, the engineered cleaning fluid is a blend of solvents which behave like an azeotrope to produce a constant boiling system at a specific temperature range.
Stencil cleaning
According to some estimates, up to 70% of solder defects are attributed to the stencil printing process. Stencil cleaning is categorised into under-stencil wiping to remove soils, ultrasonic cleaning to remove trace levels of solder paste from stencil openings, solvent-based cleaning to clean wet solder paste, adhesives and flux residues from stencils, mis-printed PCBs, wave soldering pallets, tools and fixtures, spray-in-air aqueous wash/rinse to rapidly dissolve the solder paste, hand-held stencil cleaning to remove trace levels of solder paste from the apertures, and misprint cleaning to address misprints due to issues such as clogged apertures, stencil out of alignment and solder paste rheology shifts.
Maintenance cleaning
MELSS brings you cleaning solutions from KYZEN, the global leader in advanced electronics assembly cleaning technologies, who develop and deliver electronics manufacturing cleaning products and services for improved reliability, constantly innovating to match the changing requirements of the electronics industry.
0 notes
Text
Felicitazotropibility (noun) / fəˌlisɪtəˌzoʊˌtrəˈbɪlɪti /
The quality or state of being both felicitous and harmoniously adaptable in the presence of conflicting tendencies, especially in relation to achieving balance within complex systems or relationships.
The capacity to willfully navigate and resolve azeotropic dilemmas—situations where two or more elements are inseparably intertwined—while fostering mutual benefit and satisfaction.
Example: "Her felicitazotropibility in mediating the dispute left both parties not only content but aligned in their future goals."
0 notes
Text
Experimental Measurement and Thermodynamic Modelling of Vapor-Liquid Equilibria Correlations for Prediction Azeotropic Behavior and Fitting Multicomponent Mixtures Data_Crimson Publishers
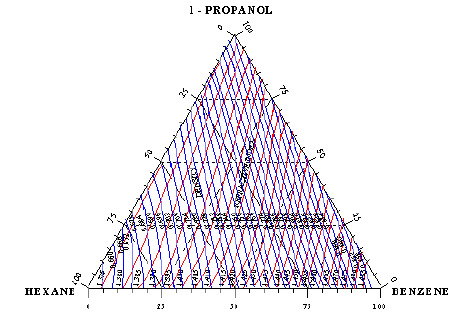
Abstract:
In this study, isobaric vapor-liquid equilibrium for two ternary systems: "1-Propanol-Hexane-Benzene” and its binaries "1-Propanol-Hexane, Hexane-Benzene and 1-Propanol-Benzene” and the other ternary system is "Toluene-Cyclohexane-iso-Octane (2,2,4-Trimethyl-Pentane)” and its binaries "Toluene-Cyclohexane, Cyclohexane-iso-Octane and Toluene-iso-Octane” have been measured at 101.325KPa. The measurements were made in re-circulating equilibrium still with circulation of both the vapor and liquid phases. The ternary system "1-Propanol-Hexane-Benzene” which contains polar compound (1-Propanol) and the two binary systems "1-Propanol-Hexane and 1-Propanol-Benzene” form a minimum azeotrope, the other ternary system and the other binary systems do not form azeotrope.
Correlation equations for expressing the boiling temperature as direct function of liquid composition have been tested successfully and applied for predicting azeotropic behavior of multi component mixtures and the kind of azeotrope (minimum, maximum and saddle type) using modified correlation of Gibbs-Konovalov theorem. Also, the binary and ternary azeotropic point has been detected experimentally using graphical determination on the basis of experimental binary and ternary vapor-liquid equilibrium data.
All the data passed successfully the test for thermodynamic consistency using McDermott-Ellis test method [1]. The maximum likelihood principle is developed for the determination of correlations parameters from binary and ternary vapor-liquid experimental data which provides a mathematical and computational guarantee of global optimality in parameters estimation for the case where all the measured variables are subject to errors and the non ideality of both vapor and liquid phases for the experimental data for the ternary and binary systems have been accounted. The agreement between prediction and experimental data is good. The exact value should be determined experimentally by exploring the concentration region indicated by the computed values.
Read More About this Article: https://crimsonpublishers.com/pps/fulltext/PPS.000508.php
Read More Articles: https://crimsonpublishers.com/pps/index.php
#crimson publishers#progress in petrochemical science#chemical engineering#petroleum#open access journals#peer review journals
0 notes
Text
From Distillation to Distribution: Navigating the Industrial Alcohol Landscape

Uses of Industrial Alcohol in Different Industries Production of Industrial liquor Industrial liquor is primarily produced through the process of fermentation. In fermentation, sugar is converted into ethanol by yeasts, bacteria, or a combination of both. The most common form of sugar used is starch from various grains like corn, wheat, barley etc. The starch is first converted into fermentable sugars like glucose through a process called saccharification. The sugars are then fermented by yeast to produce ethanol and carbon dioxide. The ethanol content of the fermented mash is further concentrated into anhydrous or denatured alcohol by means of distillation. Additional steps may also involve molecular sieves or azeotropic distillation to remove trace amounts of water. Chemical Properties of Industrial Liquor Ethanol or ethyl alcohol is a volatile, colorless, flammable oxygenated hydrocarbon. It has a characteristic odor and acts as a central nervous system depressant in humans. Chemically it is an aliphatic alcohol with a molecular formula C2H5OH. Industrial liquor has a molecular weight of 46.07 g/mol and an octanol-water partition coefficient value of -0.35. It is completely miscible with water and other polar organic compounds. However, it is not miscible in non-polar solvents like hydrocarbons. Industrial ethanol has a flash point of 78°F or 26°C. Uses in Chemical Industry A key use of industrial alcohol is as an intermediate in the production of other chemicals. It is used in the synthesis of ethyl acetate, diethyl ether, glycol ethers, chloroform, ethyl amines among others. These derived chemicals in turn find applications as solvents, resins, synthetic fibers, detergents, cosmetics etc. Various ethyl esters produced from ethanol also serve as important solvents or artificial fruit essences. Industrial ethanol also acts as a denaturant for other alcohols like isopropyl alcohol to deter human and animal consumption. Fuels Applications Transportation fuels like gasohol utilize industrial ethanol as a gasoline octane booster and oxygenate. E10 gasoline blends comprise 10% anhydrous ethanol with 90% gasoline. Higher ethanol-gasoline blends are also gaining popularity as alternative fuels. Industrial ethanol further serves as a feedstock for production of ethyl tert-butyl ether, an anti-knock additive used with unleaded petrol. Ethanol can also be mixed with natural gas to create motor vehicle fuels. With advances in flexible fuel vehicles, high ethanol gasoline blends up to E85 are being increasingly used. Industrial Solvent Demand Owing to its low toxicity and cost effectiveness, industrial ethanol finds wide application as a solvent and extractant. It is used for cleaning and degreasing applications in many industries including metal treatment, electronics manufacturing, precision instruments etc. It is also used as an extraction solvent in the food industry to produce flavors, colors, essences etc. from plant materials. Pharmaceutical, cosmetic and personal care sectors utilize ethanol as an emollient or solubilizer for creams and ointments. It is also commonly employed as a solvent for resins, gums, waxes and fats. Use in Chemical Production of Dyes, Inks and Coatings Ethanol serves as an important co-reactant in chemical production processes. It is used as a starting material in manufacturing various dyes and pigments. In paint, ink and coating industries as well, it acts as a co-reactant and solvent. With advances in flexo and digital printing technologies, the demand for high-purity industrial ethanol as a carrier solvent for inks and coatings has risen substantially. Continuous introduction of new coating, laminating and printing processes by various industries will also boost its future consumption.
0 notes