#and instead of oxygen we produce carbon monoxide
Explore tagged Tumblr posts
Text
if i was contributing to the Europa ice war memes it would be with 5 paragraphs about the complexity and process of getting all this stuff there and how that is by far the biggest problem. by mass driver maybe, because the expense of building the infrastructure for that would be worth it with the amount of stuff you'd have to transport (instead of current type of rocket launches). and build one on Europa too for the return. and at what speed can that realistically travel? At light speed it's like half an hour but we have no way of achieving that now or anytime soon. At current fastest rocket speeds it's ~90 days to mars and therefore like. A year to Europa? (Europa Clipper, which is en route right now, will take 5 years but there was a direct trajectory option that would have taken less than 3 years, but I'm imagining in this war scenario we are obviously in a big rush).
so the issue here is is "the resources are too far away from the front and it is likely impossible to get them there even within a *month*" and even if there is a lot of development on Europa by this point, even if food and fuel (everything's nuclear-powered in this setting that's my headcanon and i'm right) and missiles etc can somehow be created there, i cannot imagine that the entire global supply chain is replicated there. they are not building entire rockets on Europa because it's simply too difficult-- some of the materials to make the rockets are created on earth. the whole process can't happen from nothing. there are minerals they don't have and infrastructure they don't have. a self-sufficient colony (which i'm assuming is present) is different from one capable of producing all those weapons and aircraft and so on. and people, of course. no reinforcements are coming sooner than a year!
the point is that the self-sufficiency of the Europa colony would be so much more precarious than self-sufficiency on Earth, even if it was built with a lot of redundancy. you need to create food and water and breathable air and then have access to it. if your access to that is cut off somehow you can't create a way to access it again (unlike on Earth where food and water can be obtained from many different places and the air is free.) my first impulse was "the biggest deal on Europa (and on Earth!) must become who has control of the space launch platform! you would inevitably rely a lot on resources from Earth!" but then i realized the travel time is so long it's near-useless. the real biggest deal is running out of stuff. the conflict needs to be over as quickly as possible. you'd have to be SO intentional with it. and you better be really fucking good at predicting what will be needed far ahead of time and then defending it. you can communicate with Earth at light speed (~30-minute delay) but you can't receive anything physical from them without some light-speed technology we don't have yet and i feel we are unlikely to develop anytime soon.
knowing this, i imagine there wouldn't be like, one single electrolysis plant that produces the oxygen because that'd be really vulnerable. there would be a whole lot of them. even though current technology for doing that (decomposing CO2 into oxygen and carbon monoxide) is energy-intensive and expensive and requires rare earth metals.
but now think about how it's not just creating oxygen that would require redundancy, it's every life support process. food, water, ensuring atmospheric pressure, protection from the radiation of space, etc. so something, somewhere, is the bottleneck where there's just a couple targets that could be taken out to prevent continued life on Europa from being possible. of course there is storage but again, how distributed could that be? one storage facility would obviously be disastrous but even 5 is few enough that they could all be destroyed or at least be held by an enemy so you no longer have access to them. so identify which resource is this bottleneck and then defend it like it will be what kills you because it will be. a month ago i wrote some delirious 1am post abt how one of the cool things about a Dyson sphere is denying access to essentially any form of energy to anyone you don't want to have access to it, since basically everything on a planet relies on the light from the star (barring i guess geothermal and nuclear both of which would be impossible to set up without *starting* with something that comes from the light from a star). inescapable death sentence in a way that nothing on Earth really compares to. no one who is not in with you good enough that they are specifically allotted some of the energy produced will live.
tl;dr: Europa is really, really far away and inhospitable, and imo everything about a conflict there would be about that
that's my post you're welcome for utterly missing the point of this meme <3
#cal txt#europa ice war#now that definitely should have gone on my sideblog but it arguably isn't “politics”? at least not real life politics
93 notes
·
View notes
Text
april fools jokes on social media are just like
Everyone here in the kingdom of Plantae has been keeping a close eye on your feedback, and as a result, we've decided oxygen isn't the way to go anymore. From now on, we will produce carbon monoxide instead. We hope you're looking forward to this bold new direction in photosynthesis!
0 notes
Text
Green Air Purifiers: The Future of Fresh Air
Introduction
As the world grapples with rising air pollution and climate change, the need for innovative solutions to ensure clean and breathable air has never been more urgent. Air purification technologies have advanced significantly, and among the most promising developments are green air purifiers. These eco-friendly alternatives not only filter out harmful particles but also contribute to environmental sustainability.
This article explores the future of green air purifiers, their benefits, technologies, and potential to transform indoor and outdoor air quality. We will delve into how they work, the various types available, their impact on health and the environment, and their role in shaping a cleaner, healthier future.
Understanding Air Pollution and Its Impact
Air pollution is a global concern affecting millions of people. It comprises particulate matter (PM), volatile organic compounds (VOCs), carbon monoxide, nitrogen oxides, sulfur dioxide, and other hazardous pollutants. Prolonged exposure to poor air quality can lead to respiratory illnesses, cardiovascular diseases, and neurological disorders.
Indoor air pollution is equally dangerous, with sources including household cleaning agents, cooking emissions, furniture, and inadequate ventilation. The demand for air purifiers has surged in response to growing awareness about indoor air quality (IAQ) and its health implications.

Air Purification Technology
The Evolution of Air Purification Technology
Traditional air purifiers rely on high-efficiency particulate air (HEPA) filters, activated carbon filters, and UV light to remove pollutants. While effective, these methods often consume significant amounts of energy and require frequent filter replacements, contributing to waste.
Green air purifiers have emerged as a sustainable alternative, integrating natural and energy-efficient technologies. These purifiers harness the power of plants, photocatalysis, and other eco-friendly mechanisms to cleanse the air with minimal environmental impact.
Types of Green Air Purifiers
1. Plant-Based Air Purifiers
Living plants act as natural air purifiers by absorbing carbon dioxide and releasing oxygen. Certain species, such as peace lilies, spider plants, and snake plants, are particularly effective at removing toxins like formaldehyde and benzene. Advanced plant-based purifiers enhance this natural filtration process through bioengineered soil, microbial ecosystems, and smart sensors that optimize purification efficiency.
2. Photocatalytic Air Purifiers
These devices use a photocatalyst, often titanium dioxide, activated by ultraviolet (UV) light to break down pollutants into harmless byproducts like carbon dioxide and water. Unlike traditional filters, photocatalytic purifiers do not trap particles but instead neutralize them at the molecular level, offering a sustainable and long-lasting solution.
3. Electrostatic and Ionization Purifiers
Electrostatic air purifiers charge airborne particles, causing them to stick to a collection plate, effectively removing them from circulation. Ionizers release negative ions that attach to pollutants, making them heavy enough to settle on surfaces. Some models combine ionization with additional filtration technologies to enhance efficiency without excessive energy consumption.
4. Activated Carbon and Bamboo Charcoal Filters
Traditional activated carbon filters are widely used to remove odors and VOCs. Bamboo charcoal, a sustainable alternative, offers similar benefits while being biodegradable and renewable. These filters efficiently trap harmful gases and moisture, preventing mold growth and enhancing indoor air quality.
5. Algae-Based Air Purifiers
A cutting-edge approach to green air purification involves algae-based systems that absorb CO2 while producing oxygen. These bioreactors leverage photosynthesis, significantly improving air quality while promoting carbon sequestration. Researchers are exploring scalable applications, including urban air purification towers and household units.

Green Air Purifiers
The Benefits of Green Air Purifiers
1. Reduced Environmental Impact
Unlike traditional purifiers that rely on disposable filters and high energy consumption, green air purifiers use sustainable materials and low-energy processes. Many models integrate renewable energy sources like solar power, further minimizing their carbon footprint.
2. Health Benefits
Green air purifiers effectively remove allergens, pathogens, and harmful chemicals from the air. By utilizing plant-based and natural filtration mechanisms, they contribute to overall well-being without releasing ozone or other harmful byproducts.
3. Energy Efficiency
Many green purifiers operate without electricity or use minimal power, making them cost-effective and energy-efficient. Passive purification methods, such as plant-based and charcoal filtration, do not require continuous operation, further reducing energy demands.
4. Aesthetic and Psychological Advantages
Incorporating living plants or algae into air purification systems enhances indoor aesthetics and promotes psychological well-being. Studies suggest that indoor greenery reduces stress, improves mood, and increases productivity, creating a more comfortable and inviting environment.
The Role of Smart Technology in Green Air Purification
Advancements in smart technology have revolutionized air purification. Many green purifiers now feature IoT-enabled sensors that monitor air quality in real time, adjusting purification settings accordingly. Mobile applications allow users to track pollution levels, control settings remotely, and receive maintenance alerts, ensuring optimal performance.
Machine learning and artificial intelligence (AI) are also being integrated into purification systems, enabling predictive analysis and automated adjustments based on historical data and environmental conditions.
The Future of Green Air Purifiers
1. Urban Applications
As cities become more polluted, large-scale green air purification systems are being deployed in public spaces. Vertical gardens, algae-infused air towers, and smart purification infrastructure are gaining traction as sustainable urban solutions. These innovations contribute to cleaner air while enhancing urban aesthetics and biodiversity.
2. Integration with Renewable Energy
Future green air purifiers will increasingly incorporate renewable energy sources, such as solar panels and kinetic energy systems. This shift will further reduce dependency on conventional electricity, making air purification more accessible and environmentally friendly.
3. Enhanced Biotechnological Applications
Researchers are exploring genetic modifications in plants and algae to enhance their air-cleaning capabilities. Bioengineered species with superior pollutant absorption rates could revolutionize air purification, offering a naturally efficient and scalable solution.
4. Affordable and Accessible Solutions
As technology advances, the cost of green air purifiers is expected to decrease, making them accessible to a wider audience. Governments and organizations may incentivize their adoption through subsidies, tax benefits, and awareness campaigns, promoting cleaner air globally.
Conclusion
Green air purifiers represent a promising frontier in the quest for cleaner air and a healthier planet. By leveraging nature-inspired technologies and sustainable practices, they offer an effective, eco-friendly alternative to conventional air filtration systems. As research and innovation continue, these purifiers will play a crucial role in combating air pollution, improving public health, and ensuring a breathable future for generations to come.
0 notes
Text
Train Talk Tuesday

Train Talk Tuesday: As promised, this post will be about Hydrogen trains, because god fucking damnit, I have had enough of this hydrogen bullshit in trains, and I figured it would make for a great post. In this, I will cover what hydrogen trains are, a few examples in the wild/proposed, why they're incredibly dumb and bad, and what we could actually invest in instead of these half-baked attempts at rail-based transportation, featuring the dumbfucks from the California Department of Transportation, or CalTrans, and the United Kingdom. If you're ready, then stand clear from the closing doors, for the train is now ready to depart.
First off, what is a hydrogen train?
Talkin About The (H2) Flow

True to its name, a hydrogen train is a train that is powered purely off of Hydrogen gas, the lightest element on the Periodic Table. You also sometimes see them referred to as Hydrail or Zero Emission Multiple Units, or ZEMUs (Which is a bit of a dumb definition, but more on that later). The Hydrogen gas stored in these trains can be used to power it in a few different ways, either by burning the gas through an internal combustion engine, or using fuel cells to generate electricity from the gas, which in turn powers electric motors which drive the train, in a similar vein to diesel-electrics. The big selling point about these so-called "ZEMUs" is that both methods of powering the train yield no harmful pollutants, as burning Hydrogen gas only creates water vapor, due to how combustion works, and fuel cells generate electricity via reactions with hydrogen and oxygen, hence their marketing as "zero-emissions". Some notable examples of hydrogen trains you can see in the real world include the Stadler FLIRT H2 built for Metrolink (see above), the Alstom Coradia iLint (see below), and an upcoming conversion of a Class 60 diesel locomotive to hydrogen combustion (with some...quirks, but more on that later)

Now, I want to make it clear here that something like this on-paper is something I'd fully support. I am not a conspiracy theory crackhead who thinks that climate change is a myth, so something that could provide necessary transportation with no pollution involved is something I would support. But, notice I said on-paper. In practice, hydrogen trains have a myriad of issues which don't paint them as the environmentally friendly solution you would think they would be, and on top of that, there's an older technology we have that makes these things completely irrelevant. But, let's not get too ahead of ourselves. Let's talk about the problems.
A Grey Green Lie

Now once again, from the surface, its easy to think that hydrogen is a clean alternative to fossil fuels, because hey, no greenhouse gases emitted during burning, unlike those fossil fuels, and we can just get hydrogen from water! It's the perfect fuel source!
Yeahhhh. I wish.

Unfortunately, a large portion of the hydrogen we source involves Methane gas, which is commonly sourced from natural gas deposits. The most common method of hydrogen production, Steam-Methane Reforming, involves using high-pressure steam and methane gas and forcing them to react with each other, which derives hydrogen from the methane. Now, if it wasn't bad enough that this process involved fossil fuels, this process does yield some amounts of Carbon Monoxide and Carbon Dioxide, the latter of which is to blame for climate change occurring. So even if the desired gas itself doesn't produce greenhouse gases while being used for energy, its production already involves not only a fossil fuel, but it produces the greenhouse gases that the desired gas was trying to avoid. (For additional reading, this is a good source)
The only source of hydrogen that has the potential to be clean all the way through is Green Hydrogen, which relies on electrolysis to derive hydrogen from water. The major problem with this approach is that compared to Grey Hydrogen, it is a much more expensive and resource intensive method of obtaining hydrogen, requiring more capital and energy overall to even make feasible compared to SMR with Grey Hydrogen, hence why this option is usually passed up. And even then, Green Hydrogen only has the potential to be 100% clean. The Office of Energy Efficiency and Renewability has stated that the current grid isn't ready for mass-hydrogen production yet, as most of the electricity generated for the United States still comes from non-renewable sources, which would negate the "clean" benefit of Green Hydrogen entirely. (Source for anyone curious) And even with a purely green powered grid, there's a certain other option regarding powering trains which blows hydrogen away to the far reaches of the universe. But, odd, I don't seem to be able to remember what it could be. Eh, maybe it will come back to me later.
So, what about that fancy hydrogen-powered FLIRT ordered by CalTrans? Well, its more than likely going to be fueled up by that Grey Hydrogen stuff, so any notion of it being a "clean" alternative is null and void. And on top of that, these dumbfucks have the AUDACITY to even call it a so-called "Zero Emission Multiple Unit". I-I just...WHYYYYYYY. I absolutely despise that name, because all it is for is to convince the general population that its this "clean" solution to transit woes and will bring on an "era of sustainability". Its a pure marketing stunt, and for that, I despise it with every fiber of my being.
But even that pails in comparison to the UK choosing to convert one of their old diesel locomotives to burn hydrogen. But, being the UK, they have to have some kind of stupid and weird quirk with it, and in this case, the locomotive itself won't be using hydrogen to power the train directly. Rather, its going to use hydrogen to heat water, in order to power a fucking STEAM TURBINE, to generate the electricity to power the locomotive.
I-I just-I don't even know what to say anymore.
There's honestly a lot more about hydrogen trains that I could rant about in this post, but so far, I haven't mentioned anything about what's the solution to this hydrogen craze. All I've talked about are what they are and why they suck. It is clear that we are in desperate need of a clean solution to wean ourselves off of fossil fuels which isn't these meme machines. I just cannot seem to figure out watt would fit the bill, though. What we need is something so truly electrifying, so powerful, something which would truly shock the minds of those in charge and would fix all of our current issues regarding our dependencies on fossil fuels for transit, and could be implemented on a scale so wide there would be next to no resistance to it being the undisputed key to the sustainability we so crave. If only...

Oh, but there is.
The Shock You Didn't Expect

That's right, every single one of the problems I mentioned with hydrogen trains we have already solved using regular electrification. Electric trains not only produce zero emissions while in operation, but they can also be powered by 100% renewable energy, making Green Hydrogen completely irrelevant. Trains can come in either locomotive hauled or multiple unit varieties, so if you wanted a true "Zero Emission Multiple Unit", we have that covered in the form of Electric Multiple Units, or EMUs. On top of that, an electric train doesn't have to carry any kind of fuel with it, meaning it can not only run for a theoretical indefinite period, not requiring any downtime for refueling, but it will always be inherently more efficient than anything which requires onboard fuel storage, due to a lower axle weight caused by freeing up space taken up by the fuel storage. Electrification is something that the United States is no stranger to, with the notable examples of early electrification being the Baltimore & Ohio railroad being the first railroad in the continental United States to open an electrified line for revenue service in 1895, to the great Pennsylvania Railroad, who's legacy of electrified rail corridors survive to this day as parts of Amtrak's Northeast Corridor and Keystone Corridor, to even recent examples in California, where up in the Bay Area, Caltrain has finished electrifying a large portion of their main line in preparation for electric revenue service. There are no obstacles to electrification, no excuses, no issues. This nation is fully capable of doing these kinds of projects.
So, conclusion time?
On the surface, a train powered by hydrogen seems like a logical step forward for rail-based transport in the pursuit for a greener future. Yet this green solution that we praise as our savior hides within a dark secret, for it has a heart of methane, and it will only further our dependencies on fossil fuels. Meanwhile, the true green solution, electrification, continues to be shunned and shoved aside as we chase down new, unproven technologies in our thirst for sustainability salvation. If we are to truly perfect our transportation and achieve a status of carbon neutral, the path we must take won't be some unproven technology like hydrogen and batteries, but the path we took in the past, being proper electrification. It will definitely take a while for us to move on from our tie to fossil fuels, but if we know to avoid false promises like this hydrogen bullshit, then the move will be just that little bit faster.
0 notes
Text
Neither water nor atmosphere
Neither water nor atmosphere, the surface is filled with only 95% carbon dioxide,
that red planet. Mars is called red planet
because the entire planet is covered with rusty dust.
Where there is no sign of oxygen and water, how does rust and iron dust form there? Carbon is produced because burning turns into ash.
But on Mars, oxygen is only 0.13%, yet carbon dioxide is 95%.
Can carbon dioxide be present in large quantities due to any process other than reaction with oxygen?
Venus also has large quantities of carbon dioxide.
2.5 billion years ago, the earth also had an atmosphere of carbon dioxide.
It is said that there is no oxygen anywhere in the solar system.
Even in space, there is no oxygen.
Mars does not have it.
How can carbon dioxide be present on Mars apart from oxygen?
The Martian atmosphere is an oxidized atmosphere. The photochemical reactions in the atmosphere tend to oxidize the organic species and turn them into carbon dioxide or carbon monoxide.
With Mars Methane Mystery Unsolved, Curiosity Serves ...
NASA (.gov)
https://www.nasa.gov › missions › with-mars-methane-...
How can carbon dioxide be present on Mars apart from oxygen? from www.nasa.gov
12 Nov 2019
With Mars Methane Mystery Unsolved, Curiosity Serves Scientists a New One: Oxygen
For the first time in the history of space exploration, scientists have measured the seasonal changes in the gases that fill the air directly above the surface of Gale Crater on Mars. As a result, they noticed something baffling: oxygen, the gas many Earth creatures use to breathe, behaves in a way that so far scientists cannot explain through any known chemical processes.
Over the course of three Mars years (or nearly six Earth years) an instrument in the Sample Analysis at Mars (SAM) portable chemistry lab inside the belly of NASA’s Curiosity rover inhaled the air of Gale Crater and analyzed its composition. The results SAM spit out confirmed the makeup of the Martian atmosphere at the surface: 95% by volume of carbon dioxide (CO2), 2.6% molecular nitrogen (N2), 1.9% argon (Ar), 0.16% molecular oxygen (O2), and 0.06% carbon monoxide (CO). They also revealed how the molecules in the Martian air mix and circulate with the changes in air pressure throughout the year. These changes are caused when CO2 gas freezes over the poles in the winter, thereby lowering the air pressure across the planet following redistribution of air to maintain pressure equilibrium. When CO2 evaporates in the spring and summer and mixes across Mars, it raises the air pressure.
Within this environment, scientists found that nitrogen and argon follow a predictable seasonal pattern, waxing and waning in concentration in Gale Crater throughout the year relative to how much CO2 is in the air. They expected oxygen to do the same. But it didn’t. Instead, the amount of the gas in the air rose throughout spring and summer by as much as 30%, and then dropped back to levels predicted by known chemistry in fall. This pattern repeated each spring, though the amount of oxygen added to the atmosphere varied, implying that something was producing it and then taking it away.
“The first time we saw that, it was just mind boggling,” said Sushil Atreya, professor of climate and space sciences at the University of Michigan in Ann Arbor. Atreya is a co-author of a paper on this topic published on November 12 in the Journal of Geophysical Research: Planets.
As soon as scientists discovered the oxygen enigma, Mars experts set to work trying to explain it. They first double- and triple-checked the accuracy of the SAM instrument they used to measure the gases: the Quadrupole Mass Spectrometer. The instrument was fine. They considered the possibility that CO2 or water (H2O) molecules could have released oxygen when they broke apart in the atmosphere, leading to the short-lived rise. But it would take five times more water above Mars to produce the extra oxygen, and CO2 breaks up too slowly to generate it over such a short time. What about the oxygen decrease? Could solar radiation have broken up oxygen molecules into two atoms that blew away into space? No, scientists concluded, since it would take at least 10 years for the oxygen to disappear through this process.
“We’re struggling to explain this,” said Melissa Trainer, a planetary scientist at NASA’s Goddard Space Flight Center in Greenbelt, Maryland who led this research. “The fact that the oxygen behavior isn’t perfectly repeatable every season makes us think that it’s not an issue that has to do with atmospheric dynamics. It has to be some chemical source and sink that we can’t yet account for.”
To scientists who study Mars, the oxygen story is curiously similar to that of methane. Methane is constantly in the air inside Gale Crater in such small quantities (0.00000004% on average) that it’s barely discernable even by the most sensitive instruments on Mars. Still, it’s been measured by SAM’s Tunable Laser Spectrometer. The instrument revealed that while methane rises and falls seasonally, it increases in abundance by about 60% in summer months for inexplicable reasons. (In fact, methane also spikes randomly and dramatically. Scientists are trying to figure out why.)
With the new oxygen findings in hand, Trainer’s team is wondering if chemistry similar to what’s driving methane’s natural seasonal variations may also drive oxygen’s. At least occasionally, the two gases appear to fluctuate in tandem.
“We’re beginning to see this tantalizing correlation between methane and oxygen for a good part of the Mars year,” Atreya said. “I think there’s something to it. I just don’t have the answers yet. Nobody does.”
Oxygen and methane can be produced both biologically (from microbes, for instance) and abiotically (from chemistry related to water and rocks). Scientists are considering all options, although they don’t have any convincing evidence of biological activity on Mars. Curiosity doesn’t have instruments that can definitively say whether the source of the methane or oxygen on Mars is biological or geological. Scientists expect that non-biological explanations are more likely and are working diligently to fully understand them.
Trainer’s team considered Martian soil as a source of the extra springtime oxygen. After all, it’s known to be rich in the element, in the form of compounds such as hydrogen peroxide and perchlorates. One experiment on the Viking landers showed decades ago that heat and humidity could release oxygen from Martian soil. But that experiment took place in conditions quite different from the Martian spring environment, and it doesn’t explain the oxygen drop, among other problems. Other possible explanations also don’t quite add up for now. For example, high-energy radiation of the soil could produce extra O2 in the air, but it would take a million years to accumulate enough oxygen in the soil to account for the boost measured in only one spring, the researchers report in their paper.
“We have not been able to come up with one process yet that produces the amount of oxygen we need, but we think it has to be something in the surface soil that changes seasonally because there aren’t enough available oxygen atoms in the atmosphere to create the behavior we see,” said Timothy McConnochie, assistant research scientist at the University of Maryland in College Park and another co-author of the paper.
The only previous spacecraft with instruments capable of measuring the composition of the Martian air near the ground were NASA’s twin Viking landers, which arrived on the planet in 1976. The Viking experiments covered only a few Martian days, though, so they couldn’t reveal seasonal patterns of the different gases. The new SAM measurements are the first to do so. The SAM team will continue to measure atmospheric gases so scientists can gather more detailed data throughout each season. In the meantime, Trainer and her team hope that other Mars experts will work to solve the oxygen mystery.
“This is the first time where we’re seeing this interesting behavior over multiple years. We don’t totally understand it,” Trainer said. “For me, this is an open call to all the smart people out there who are interested in this: See what you can come up with.”
By Lonnie Shekhtman
NASA’s Goddard Space Flight Center, Greenbelt, Md.
Translate Hindi
न पानी न वायुमंडल सिर्फ 95 प्रतिशत कार्बन डाइऑक्साइड भरी हुई सतह है वो लाल ग्रह
वैसे मंगल ग्रह लाल ग्रह इसलिए
क्योंके पुरा ग्रह जंग भरी धूल भरी हुई है
जहां ऑक्सीजन और पानी का कोई चिह्न नहीं है वहां जंग लोहे वाली धूल कैसे बनते है
कार्बन की उत्पत्ति क्योंके जलने से राख बनता है
लेकिन मंगल ग्रह में ऑक्सीजन सिर्फ 0.13 प्रतिशत है फिरभी कार्बन डाइऑक्साइड 95 प्रतिशत
क्या ऑक्सीजन के साथ विक्रिया अलावा कुछ और प्रकृया में कार्बन डाइऑक्साइड की मात्रा भारी हो सकता है
शुक्र ग्रह में भी कार्बन डाइऑक्साइड का मात्रा भारी है
2.5 अरब वर्ष पहले धरती भी कार्बन डाइऑक्साइड का माहौल ही था
ऐसा कहा जाता है स��रमंडल की कहीं भी ऑक्सीजन नहीं होता है
यहां तक की स्पेस में भी ऑक्सीजन होता ही नहीं है
मंगल ग्रह में भी नहीं है
ऑक्सीजन अलावा कार्बन डाइऑक्साइड कैसे हो सकता है मंगल ग्रह में
मंगल ग्रह का वायुमंडल ऑक्सीकृत वायुमंडल है। वायुमंडल में होने वाली फोटोकैमिकल अभिक्रियाएँ कार्बनिक प्रजातियों को ऑक्सीकृत कर उन्हें कार्बन डाइऑक्साइड या कार्बन मोनोऑक्साइड में बदल देती हैं।
मंगल ग्रह के मीथेन रहस्य का पता न चलने पर, क्यूरियोसिटी ने...
NASA (.gov)
https://www.nasa.gov › missions › with-mars-methane-...
मंगल ग्रह पर ऑक्सीजन के अलावा कार्बन डाइऑक्साइड कैसे मौजूद हो सकता है? www.nasa.gov से
12 नवंबर 2019
मंगल ग्रह के मीथेन रहस्य का पता न चलने पर, क्यूरियोसिटी ने वैज्ञानिकों को एक नया सुराग दिया: ऑक्सीजन
अंतरिक्ष अन्वेषण के इतिहास में पहली बार, वैज्ञानिकों ने मंगल ग्रह पर गेल क्रेटर की सतह के ठीक ऊपर हवा में भरी गैसों में मौसमी परिवर्तनों को मापा है। परिणामस्वरूप, उन्होंने कुछ चौंकाने वाली बात देखी: ऑक्सीजन, वह गैस जिसका उपयोग पृथ्वी के कई जीव सांस लेने के लिए करते हैं, इस तरह से व्यवहार करती है जिसे अब तक वैज्ञानिक किसी भी ज्ञात रासायनिक प्रक्रिया के माध्यम से नहीं समझा पाए हैं।
तीन मंगल वर्षों (या लगभग छह पृथ्वी वर्षों) के दौरान नासा के क्यूरियोसिटी रोवर के पेट के अंदर मंगल ग्रह पर नमूना विश्लेषण (एसएएम) पोर्टेबल रसायन विज्ञान प्रयोगशाला में एक उपकरण ने गेल क्रेटर की हवा में साँस ली और इसकी संरचना का विश्लेषण किया। एसएएम ने जो परिणाम निकाले, उनसे सतह पर मंगल ग्रह के वायुमंडल की संरचना की पुष्टि हुई: मात्रा के हिसाब से 95% कार्बन डाइऑक्साइड (CO2), 2.6% आणविक नाइट्रोजन (N2), 1.9% आर्गन (Ar), 0.16% आणविक ऑक्सीजन (O2), और 0.06% कार्बन मोनोऑक्साइड (CO)। उन्होंने यह भी बताया कि मंगल ग्रह की हवा में अणु पूरे वर्ष हवा के दबाव में बदलाव के साथ कैसे मिलते और घूमते हैं। ये परिवर्तन तब होते हैं जब सर्दियों में ध्रुवों पर CO2 गैस जम जाती है इस वातावरण में, वैज्ञानिकों ने पाया कि नाइट्रोजन और आर्गन एक पूर्वानुमानित मौसमी पैटर्न का पालन करते हैं, जो हवा में मौजूद CO2 की मात्रा के सापेक्ष पूरे वर्ष गेल क्रेटर में सांद्रता में वृद्धि और कमी करता है। उन्हें उम्मीद थी कि ऑक्सीजन भी ऐसा ही करेगी। लेकिन ऐसा नहीं हुआ। इसके बजाय, वसंत और गर्मियों के दौरान हवा में गैस की मात्रा 30% तक बढ़ गई, और फिर गिरावट में ज्ञात रसायन विज्ञान द्वारा अनुमानित स्तरों पर वापस गिर गई। यह पैटर्न हर वसंत में दोहराया जाता है, हालांकि वातावरण में शामिल ऑक्सीजन की मात्रा अलग-अलग होती है, जिसका अर्थ है कि कुछ इसे पैदा कर रहा था और फिर इसे दूर ले जा रहा था।
एन आर्बर में मिशिगन विश्वविद्यालय में जलवायु और अंतरिक्ष विज्ञान के प्रोफेसर सुशील आत्रेय ने कहा, "पहली बार जब हमने यह देखा, तो यह आश्चर्यजनक था।" आत्रेय 12 नवंबर को जर्नल ऑफ जियोफिजिकल रिसर्च: प्लैनेट्स में प्रकाशित इस विषय पर एक पेपर के सह-लेखक हैं। जैसे ही वैज्ञानिकों ने ऑक्सीजन की पहेली को खोजा, मंगल ग्रह के विशेषज्ञों ने इसे समझाने की कोशिश में काम करना शुरू कर दिया। उन्होंने सबसे पहले गैसों को मापने के लिए इस्तेमाल किए जाने वाले SAM उपकरण की सटीकता की दोहरी और तिहरी जांच की: क्वाड्रुपोल मास स्पेक्ट्रोमीटर। उपकरण ठीक था। उन्होंने इस संभावना पर विचार किया कि CO2 या पानी (H2O) के अणु वायुमंडल में टूटने पर ऑक्सीजन छोड़ सकते हैं, जिससे अल्पकालिक वृद्धि हो सकती है। लेकिन अतिरिक्त ऑक्सीजन का उत्पादन करने के लिए मंगल के ऊपर पाँच गुना अधिक पानी की आवश्यकता होगी, और CO2 इतनी कम समय में इसे बनाने के लिए बहुत धीमी गति से टूटती है। ऑक्सीजन में कमी के बारे में क्या? क्या सौर विकिरण ने ऑक्सीजन के अणुओं को दो परमाणुओं में तोड़ दिया होगा जो अंतरिक्ष में उड़ गए? नहीं, वैज्ञानिकों ने निष्कर्ष निकाला, क्योंकि इस प्रक्रिया के माध्यम से ऑक्सीजन को गायब होने में कम से कम 10 साल लगेंगे। मैरीलैंड के ग्रीनबेल्ट में नासा के गोडार्ड स्पेस फ़्लाइट सेंटर की ग्रह वैज्ञानिक मेलिसा ट्रेनर ने कहा, "हम इसे समझाने के लिए संघर्ष कर रहे हैं।" "यह तथ्य कि ऑक्सीजन का व्यवहार हर मौसम में पूरी तरह से दोहराया नहीं जा सकता है, हमें लगता है कि यह कोई ऐसा मुद्दा नहीं है जिसका वायुमंडलीय गतिशीलता से कोई लेना-देना है। यह कुछ रासायनिक स्रोत और सिंक होना चाहिए जिसका हम अभी तक हिसाब नहीं लगा पाए हैं।" मंगल ग्रह का अध्ययन करने वाले वैज्ञानिकों के लिए, ऑक्सीजन की कहानी मीथेन की कहानी से काफी मिलती-जुलती है। मीथेन लगातार गेल क्रेटर के अंदर हवा में इतनी कम मात्रा में (औसतन 0.00000004%) मौजूद है कि मंगल पर सबसे संवेदनशील उपकरणों द्वारा भी इसे पहचान पाना मुश्किल है। फिर भी, इसे SAM के ट्यूनेबल लेजर स्पेक्ट्रोमीटर द्वारा मापा गया है। उपकरण ने खुलासा किया कि मीथेन मौसमी रूप से बढ़ता और घटता है, लेकिन गर्मियों के महीनों में यह अस्पष्ट कारणों से लगभग 60% तक बढ़ जाता है। (वास्तव में, मीथेन भी अनियमित रूप से और नाटकीय रूप से बढ़ता है। वैज्ञानिक इसका कारण जानने की कोशिश कर रहे हैं।) ऑक्सीजन के नए निष्कर्षों के साथ, ट्रेनर की टीम सोच रही है कि क्या मीथेन के प्राकृतिक मौसमी बदलावों को चलाने वाले रसायन विज्ञान के समान ही ऑक्सीजन के भी कारक हो सकते हैं। कम से कम कभी-कभी, दोनों गैसें एक साथ उतार-चढ़ाव करती दिखाई देती हैं। अत्रेय ने कहा, "हम मंगल वर्ष के एक बड़े हिस्से के लिए मीथेन और ऑक्सीजन के बीच इस लुभावने संबंध को देखना शुरू कर रहे हैं।" "मुझे लगता है कि इसमें कुछ है। मेरे पास अभी तक इसका उत्तर नहीं है। किसी के पास नहीं है।" ऑक्सीजन और मीथेन जैविक रूप से (उदाहरण के लिए, सूक्ष्मजीवों से) और अजैविक रूप से (पानी और चट्टानों से संबंधित रसायन विज्ञान से) दोनों तरह से उत्पादित किए जा सकते हैं। वैज्ञानिक सभी विकल्पों पर विचार कर रहे हैं, हालांकि उनके पास मंगल पर जैविक गतिविधि का कोई ठोस सबूत नहीं है। क्यूरियोसिटी के पास ऐसे उपकरण नहीं हैं जो निश्चित रूप से कह सकें कि मंगल पर मीथेन या ऑक्सीजन का स्रोत जैविक है या भूवैज्ञानिक। वैज्ञानिकों को उम्मीद है कि गैर-जैविक स्पष्टीकरण अधिक संभावित हैं और वे उन्हें पूरी तरह से समझने के लिए लगन से काम कर रहे हैं।
ट्रेनर की टीम ने मंगल ग्रह की मिट्टी को वसंत ऋतु में मिलने वाली अतिरिक्त ऑक्सीजन का स्रोत माना। आखिरकार, यह हाइड्रोजन पेरोक्साइड और परक्लोरेट्स जैसे यौगिकों के रूप में तत्व से भरपूर मानी जाती है। वाइकिंग लैंडर्स पर दशकों पहले किए गए एक प्रयोग से पता चला कि गर्मी और नमी मंगल ग्रह की मिट्टी से ऑक्सीजन छोड़ सकती है। लेकिन वह प्रयोग मंगल ग्रह के वसंत ऋतु के वातावरण से काफी अलग परिस्थितियों में हुआ था, और यह अन्य समस्याओं के अलावा ऑक्सीजन की कमी की व्याख्या नहीं करता है। अन्य संभावित व्याख्याएँ भी अभी पूरी तरह से सही नहीं हैं। उदाहरण के लिए, मिट्टी का उच्च-ऊर्जा विकिरण हवा में अतिरिक्त O2 उत्पन्न कर सकता है, लेकिन मिट्टी में पर्याप्त ऑक्सीजन जमा होने में दस लाख साल लगेंगे, जो केवल एक वसंत में मापी गई वृद्धि के लिए जिम्मेदार होगी, शोधकर्ताओं ने अपने शोधपत्र में बताया। यूनिवर्सिटी ऑफ मैरीलैंड इन कॉलेज पार्क के सहायक अनुसंधान वैज्ञानिक और पेपर के एक अन्य सह-लेखक टिमोथी मैककोनोची ने कहा, "हम अभी तक एक भी ऐसी प्रक्रिया नहीं खोज पाए हैं जो हमें आवश्यक मात्रा में ऑक्सीजन पैदा कर सके, लेकिन हमें लगता है कि यह सतह की मिट्टी में कुछ ऐसा होना चाहिए जो मौसमी रूप से बदलता हो क्योंकि वायुमंडल में पर्याप्त ऑक्सीजन परमाणु उपलब्ध नहीं हैं जो हमारे द्वारा देखे जाने वाले व्यवहार को बना सकें।" धरती के पास मंगल ग्रह की हवा क�� संरचना को मापने में सक्षम उपकरणों के साथ एकमात्र पिछला अंतरिक्ष यान नासा के जुड़वां वाइकिंग लैंडर थे, जो 1976 में ग्रह पर पहुंचे थे। हालांकि, वाइकिंग प्रयोगों ने केवल कुछ मंगल ग्रह के दिनों को कवर किया, इसलिए वे विभिन्न गैसों के मौसमी पैटर्न को प्रकट नहीं कर सके। नए SAM माप ऐसा करने वाले पहले हैं। SAM टीम वायुमंडलीय गैसों को मापना जारी रखेगी ताकि वैज्ञानिक प्रत्येक मौसम में अधिक विस्तृत डेटा एकत्र कर सकें। इस बीच, ट्रेनर और उनकी टीम को उम्मीद है कि अन्य मंगल विशेषज्ञ ऑक्सीजन रहस्य को सुलझाने के लिए काम करेंगे। ट्रेनर ने कहा, "यह पहली बार है जब हम कई वर्षों में इस दिलचस्प व्यवहार को देख रहे हैं। हम इसे पूरी तरह से समझ नहीं पाए हैं।" "मेरे लिए, यह उन सभी बुद्धिमान लोगों के लिए एक खुला आह्वान है जो इसमें रुचि रखते हैं: देखें कि आप क्या कर सकते हैं।" लोनी शेखटमैन द्वारा नासा का गोडार्ड स्पेस फ़्लाइट सेंटर, ग्रीनबेल्ट, मैरीलैंड।
0 notes
Text
Air is the invisible mixture of gases that surrounds Earth. Air contains important substances, such as oxygen and nitrogen, that most species need to survive. Human beings (Homo sapiens), of course, are one of those species. Sometimes, the word "atmosphere" is used instead of the word "air." Standard Dry Air is the composition of gases that make up air at sea level. It is a standard scientific unit of measurement. Standard Dry Air is made up of nitrogen, oxygen, argon, carbon dioxide, neon, helium, krypton, hydrogen, and xenon. It does not include water vapor because the amount of vapor changes based on humidity and temperature. Because air masses are constantly moving, Standard Dry Air is not accurateeverywhere at once. Nitrogen and oxygen make up about 99 percent of Earth’s air. People and other animals need oxygen to live. Carbon dioxide, a gas that plants depend on, makes up less than 0.04 percent. Plants and animals each produce the gases that the other needs to live. Plants need carbon dioxide—people and other animals exhale carbon dioxide as a waste product. People and other animals need oxygen—plants produce oxygen during an important process called photosynthesis, which turns the sun’s energy into nutrients. Water vapor in the air is sometimes visibleas clouds. Water enters the atmosphere through the water cycle. The water cycle also brings molecules in the air into oceans, lakes, and rivers. Some gases in the air come from volcanic eruptions. Volcanic eruptions eject gases from the interior of Earth. The most common gas emitted by volcanoes is water vapor. Other gases, such as carbon monoxide and sulfur dioxide, are toxic to most organisms. A few organisms, however, thrive on these gases. At the bottom of the ocean are bacteria that do not need oxygen or sunlight to survive. In other words, they do not need air. These strange organisms create their own nutrients using hydrogen sulfide, not carbon dioxide. The hydrogen sulfide comes from cracks, or vents, in Earth’s crust.
The air is different as you move higher and higher into the atmosphere. The air gets "thinner" as elevation climbs because there are fewer air molecules up there. Mountain climbers often have to use canisters of oxygen as they climb above 3,800 meters (12,500 feet) because there is not enough oxygen in the atmosphere for most people to breathe. High mountains such as Mount Everest (8,848 meters, or 29,035 feet), in Nepal and China, are littered with empty oxygen canisters that climbers discard when they are used up. High in the stratosphere, a layer of Earth’s atmosphere, is a special air molecule called ozone. Ozone is made up of three atoms of oxygen. The massive collection of these molecules is called the ozone layer. The ozone layer blocks harmful ultraviolet, or UV, rays so the sun’s powerful radiation does less damage to living things on Earth. Unfortunately, air pollution has a negative effect on the air we breathe. Air pollution happens when harmful byproducts, like exhaust from cars, enter the air. These pollutants can clog the atmosphere with smog, a combination of smoke and fog. They can also create toxic clouds of dust. Other air pollutants, such as methane and excess amounts of carbon dioxide, can upset the balance of molecules in the air, contributing to global warming.
air
9 notes
·
View notes
Text
Whether electric cars can protect the environment

There have been many controversies about whether electric cars are environmentally friendly. Click here to learn more about electric cars. One of the biggest controversies is that although electric cars can achieve zero emissions, coal-fired thermal power plants account for a very high proportion of China's electricity energy mix, so electric cars only transfer pollution to the power plants.
Based on this analysis, there were many people who expressed the view that "electric cars are not environmentally friendly." In particular, the head of Toyota, Akio Toyoda, recently used the same logic to "demonize" electric cars as not environmentally friendly. In a press conference held by the Japan Automobile Manufacturers Association at the end of the year, Akio Toyoda said that promoting electric cars in a country like Japan, where most of the electricity comes from coal and natural gas (70% of Japan's total electricity generation comes from fossil fuels), is not good for the environment. With this statement, many people once again began to attack electric cars as not being environmentally friendly.
At first glance, this logic seems to make sense. In 2019, for example, the nation's interregional power plants generated 7.14 trillion kWh of electricity, up 3.5% from the previous year, while thermal power plants generated 5.16 trillion kWh, up 1.9% or 72%. Coal is not a clean energy source. Its combustion produces dust, nitrogen oxides, sulfur dioxide, carbon monoxide, carbon dioxide, and other pollutants that are more serious than the pollution caused by oil. Electric vehicles are currently powered mostly by thermal energy, and using electricity as a substitute for fuel seems only to shift pollutant emissions.
Is this view correct? A few years ago, Tsinghua University researchers Ou Xunmin, Zhang Xiliang, Qin Yining, and Qi Tianyu used the Well-to-Wheels (WTW) module of Tsinghua University's CA3EM model in an article titled "A full life-cycle analysis of future coal-powered electric vehicles." In this paper, the life-cycle energy consumption and greenhouse gas emissions of five coal-powered electric vehicle routes are quantified using the Well-to-Wheels (WTW) module of the Tsinghua-CA3EM model, with 2020 as the target year, and compared with integrated grid-powered routes and conventional gasoline vehicle routes.
Based on the analysis of the electricity use chain, the energy consumption and emissions of the resource extraction, transportation, and electricity transmission and distribution phases were fully considered. The results show that: the life cycle energy consumption of electric vehicles is 1123-1. 592 kJ/km and the greenhouse gas equivalent CO2 is 131-162 g/km; compared with the gasoline vehicle route, the advantages of the electric vehicle route in terms of energy saving and emission reduction are obvious, with energy saving of more than 35% and emission reduction of about 20%; while the use of integrated coal gasification The advanced power supply technology of combined power generation and carbon capture and storage can reduce greenhouse gas emissions by up to 80% and energy consumption by up to 40% compared with the gasoline route.
On March 28 of this year, Nature Sustainability, a sub-publication of Nature, published a major research study that also refutes the claim that "electric cars are not environmentally friendly." A new study by researchers at Radboud University in the Netherlands and Cambridge University in the United Kingdom concludes that fears that electric vehicles will increase carbon emissions are almost always unfounded. Dr. Florian Knobloch, corresponding author of the study and from the Department of Environmental Sciences at Radboud University in the Netherlands, says, "We counted data from cars and heating systems around the world, and even in the worst case, carbon emissions (from electric cars) are reduced."
It is true that coal is not a clean source of energy in the traditional sense, but the country has made great strides in generating electricity from coal thanks to the promotion and application of advanced technologies. At the end of 2016, all existing coal-fired power plants in China completed dedusting, desulfurization and denitrification, and the emission concentrations of the three pollutants soot, sulfur dioxide and nitrogen oxides are no higher than 10 mg / m3, 35 mg / m3 and 50 mg / m3 with a base oxygen content of 6%. Xie Kechang, an academic from the Chinese Academy of Engineering, said in 2019 that "ultra-low-emission technology can minimize particulate matter emissions from coal-fired power generation through end-of-pipe treatment," and China's operational data has shown that the fine dust emissions are greatly reduced after reaching the ultra-low emissions and that some pollutants are lower than the natural gas emissions. China's coal-fired power generation is now the world leader in terms of pollutant emissions.
Although thermal power has always been absolutely dominant in China's electricity-energy mix, China has made very rapid progress in the generation of renewable energies in recent years. Electricity generation from renewable energies reached a value of 2.04 trillion kilowatt hours in 2019, which corresponds to an increase of around 176.1 billion kilowatt hours compared to the previous year; the share of renewable energies in total electricity generation was 27.9% and thus 1.2 percentage points higher than in the previous year. Including hydropower 1.3 trillion kWh, an increase of 5.7% compared to the previous year; Wind energy 405.7 billion kWh, an increase of 10.9% compared to the previous year; Photovoltaics 224.3 billion kWh, an increase of 26.3% compared to the previous year; Electricity generation from biomass 111.1 billion kWh, an increase of 20.4% compared to the previous year. While the growth rate of thermal energy in the same period was only 1.9%. As China's installed capacity of renewable energy generation continues to grow, the future of electric vehicles in "clean energy" will naturally be more and more.
In addition, China's energy structure has long been characterized by "a lot of coal, little oil, little gas". China's coal resources total 5.9 trillion tons, which is 94% of total primary energy resources, while oil and gas resources are only 6%, and it is difficult to increase production and the degree of foreign dependency is high (im In 2019, the degree of foreign dependency for crude oil is up to 70%). China's coal reserves are much larger than the country's proven oil reserves, and coal production is also much larger than oil production, and the gap between China's coal and oil production has gradually widened in recent years. That is why "coal instead of oil" has become an important energy strategy for China. Electric vehicles that use electricity as an energy source are clearly one way to implement this strategy.
Some people may fear that the use of electricity in cars will lead to a scarcity of electricity in society as a whole. In fact, the explosive growth of all-electric vehicles will not have much of an impact on electricity supply. According to the current vehicle ownership in China, about 200 million calculations, even if an electric car average 50 km per day, power consumption of 16 degrees (this is already the mainstream electric car 100 km power consumption), 200 million is only 3.2 billion kilowatt hours. And since the country's daily electricity generation is now around 25 billion kWh, 3.2 billion kWh is less than a fraction of the total electricity generated. It will take about 20 years for electric vehicles to become fully popular in China, and by then the country's power generation will surely increase even more. It can be said that there is enough power for electric vehicles even with the power supply.
In summary, it can be said that we should look at the question of whether or not electric cars are environmentally friendly from a development-policy and systematic perspective. Currently, the technology of coal-fired power generation is improving, the technology of electric vehicles is improving, the power consumption of electric vehicles per kilometer is falling, and the proportion of clean energy is increasing. ...... From many perspectives, electric vehicles will only become more environmentally friendly in the future.
1 note
·
View note
Text
TAFAKKUR: Part 291
THE MIRACULOUS WORLD OF OXYGEN
All living organisms require oxygen to live. As humans, we breathe to take in oxygen; if we were not to do this we would die as we would not be able to meet our energy needs. Eighteen times more energy is extracted from glucose, a basic carbohydrate, in the presence of oxygen than without it. Just as we tend to underestimate the beauty and miracles found around us everyday, so we take oxygen and the breathing process for granted. In this article, we will illustrate several aspects of the miraculous world of oxygen.
The air that we breathe consists of 78% nitrogen, 21% oxygen and 1% other gases, such as water, carbon dioxide, and carbon monoxide. First of all, the level of oxygen in the air is of extreme importance; that is, if the air were to contain 40% oxygen instead of the normal 21%, then there would be no life on Earth. Most living organisms, if not all, would die due to oxygen poisoning. Their proteins and DNA would be oxidized and become non-functional. Metals would be corroded and trees would burn at slightly higher temperatures than normal.
Vertebrates have been equipped with two principal mechanisms to supply their cells with an adequate and continuous flow of oxygen. The first one is the circulatory system and the second one is oxygen-carrying molecules; hemoglobin in the red blood cells and myoglobin in the muscles. The air we breathe is filtered even before it reaches our lungs. Then, it dissolves in the mucus, a highly viscous material, which coats the inside of our lungs. Next, the dissolved oxygen diffuses into the blood through alveolar cells and the walls of the capillary vessels. Finally, the oxygen is picked up by the red blood cells; these are what make our blood red. The red color is due to a molecule called heme that is present in hemoglobin and myoglobin. Every heme molecule in hemoglobin can bind four oxygen molecules together. Every oxygen molecule bound to hemoglobin increases the affinity of hemoglobin to bind to another oxygen molecule. The hemoglobin becomes saturated if the dissolved oxygen is above a certain level; this can be seen in the lungs. If the level of dissolved oxygen drops below a certain level, as can be seen in tissues like the muscles, brain, and liver, then the oxygen molecules start to dissociate from the hemoglobin. Likewise, every dissociating oxygen molecule facilitates the dissociation of another oxygen molecule from the hemoglobin. This is one miraculous design that is known to us: a molecule devoid of any wisdom and intelligence grasps a very crucial cargo where it is abundant, carries it to a place where the cargo is most needed and less abundant, and releases it. The myoglobin in the muscle tissue then binds the oxygen and serves as an oxygen backup resource for times when there is inadequate oxygen supply during exertion.
Fetuses have their own specific hemoglobin, called hemoglobin-F, which is different from that of adult hemoglobin, hemoglobin-A. Before birth, the fetus gets its oxygen from the mother’s blood through the placenta. The higher affinity of hemoglobin-F than hemoglobin-A to oxygen makes the oxygen exchange between the maternal and fetal blood possible. It is interesting to note that right around the time of birth the fetus switches the production of hemoglobin-F to hemoglobin-A, as this is more efficient under normal breathing conditions. Our current knowledge is insufficient to completely understand how this switch-over occurs and how it is regulated. Future studies will shed light on this complex but magnificent mechanism of regulation and this superb design.
Why are we so dependent on oxygen? In fact, our energy metabolism is completely dependent on oxygen. The chemical breakdown of nutrients by a dozen enzymes releases energy, which as is cannot be stored or transferred to the places where it is required. We are equipped with a second mechanism, which involves another set of different proteins that converts the released chemical energy to a more useful and transferable molecular form, called ATP. ATP, which we can think of as small packages of energy, is the main form of energy within our cells that can be readily used by all reactions that require energy. The first set of enzymes abstracts electrons from the nutrients during their chemical breakdown. These so called high-energy electrons are transferred from one protein to the next by the second set of proteins that form the electron transport chain. The final acceptor of these electrons is molecular oxygen. If oxygen were not there to pick up the electrons at the end of this chain, the last protein (cytochrome oxidase) would lead to a dead end, as it would be rendered inactive with the electrons that it is carrying. This would make this superb design of complex mechanism useless and wasteful; the synthesis of every new and active cytochrome oxidase would require more energy than is produced in one cycle of an electron transfer in the absence of oxygen.
It has been known for some time that cells can sense the level of oxygen in their environment. They are equipped not only with a sensing mechanism, but also with a response mechanism, by which they can survive for a short period of time. In 1995, a protein called HIF (Hypoxia Inducible Factor) was identified and was shown to regulate cellular response to hypoxia, i.e., a reduced oxygen level. HIF is a transcription factor, which induces the expression of a set of genes that are required for survival under hypoxia. Several genes encoding glycolytic enzymes are regulated under hypoxia; this allows cells to produce ATP even without oxygen. Nevertheless, oxygen-independent energy generation is very inefficient and the yield is insufficient. Another set of genes induce angiogenesis (vascularization), or the making of new capillary vessels. VEGF (vascular endothelial growth factor) is one of the best known HIF target genes that induces the formation of new vessels where expressed.
One of the most remarkable aspects of HIF-based oxygen sensing is that under normal oxygen levels the HIF protein is simultaneously synthesized and degraded. Only under low levels of oxygen does HIF accumulate and induce its target genes. At first sight, this continuous production and degradation of HIF may look wasteful, whereas in reality it is a very well designed precautionary mechanism. The HIF protein is marked and sent for degradation by a class of enzymes called HPH/PHD. These enzymes also use the oxygen molecule to tag the HIF protein. If there were not enough oxygen around, HPH/PHD enzymes would not be able to tag HIF. As a result HIF accumulates and induces its target genes to ensure the adequate supply of oxygen. With this mechanism cells can quickly adapt and survive. Therefore, continuous production and degradation of HIF turns out to be a necessary precautionary measure which is taken against the risk of death arising from a low oxygen level.
This article is by no means a complete picture of the miraculous world of oxygen, perhaps it is no more than a brush stroke on the entire picture. Yet, even this incomplete glimpse is enough to help us realize how perfectly we have been created, and how well we are taken care of. We do not have even the slightest control over any of these aforementioned mechanisms. We breathe day and night, and every breath should be taken in gratitude to God, who created us as this masterpiece.
#allah#god#prophet#Muhammad#quran#ayah#islam#muslim#muslimah#hijab#help#revert#convert#religion#reminder#hadith#sunnah#dua#salah#pray#prayer#welcome to islam#how to convert to islam#new convert#new muslim#new revert#revert help#convert help#islam help#muslim help
3 notes
·
View notes
Text
Actually, good summary, excepting cast iron is _too_much_ carbon - usually about 5-6%. For carbon steels, you want between 0.7-2%, if that, which is the reason for cast iron then going to the oxygen blast furnaces. Basically, there were historically two ways to get iron - Bloomery Smelter, which was the smallish charcoal fueled cylindrical stack furnace used by most iron age cultures throughout the world, and which only went out of fashion in the 18th century, and higher bellows efficiency Blast Furnaces, which were initially a thing with the Chinese, and stayed there, and weren't really a thing anywhere else until the 16th century and later, and what we call a Blast Furnace didn't really turn up until Bessemer made a breakthrough in the early 19th century.
The principle reason for the difference between Asian Blast furnace technology and Indo-Euro-Afro Bloomery furnace technology is that the first people in China who thought 'How do we make a thing to blow air at our fire to make it hotter' solved that problem by making a disc on a stick piston pump out of bamboo, while the first people who had that thought in India/Africa/Europe made a bag out of an animal skin and squeezed it. Both technologies then improved their system by putting inlet and outlet flap valves, tubes and refractory tuyere/nozzles on that, and pointing it at their furnace, and Yay, you can have a bronze age, and then later, an iron age. Away we go.
But, and this is crucial, the Chinese bamboo piston pump could have inlet and outlet flap valves at either end, so it was _double_acting_, whichever way the piston was moving, air was being pushed into the fire with very little pause between strokes. This made it more efficient, and very scaleable, because you can build a very big wooden box with a piston and inlet/outlet valves at either end with very little other tool and infrastructure needed. Whereas if you're making bellows like all the rest of us, out of a couple of animal skins, well, the rest of us quite quickly changed our skin bag to 'two wooden flaps and a hinge and a flap valve, and if you want continuous air flow, use two or more at once', and that bellows style of 'two big wooden panels with handles on the end and a leather bag' sorta limited the size and efficiency and stayed with us to the early blast furnace era in the 17th-18th century and then finally went out of style as we got steam power and could use fans instead. But over in China, those big bamboo piston derived double action box bellows meant that in their bronze age, they were doing tonnes of bronze at once, whereas everyone else was mostly doing much smaller kilos to tens of kilos or hundreds of kilos, not tonnes to tens of tonnes, all because of the more efficient bellows technology in China.
But that meant, as the knowledge that you could get a useful metal out of rusty ochre ores spread out of Anatolia, when that knowledge got to China, well, they chucked a whole lot of it in one of their big ass high oxygen blast box bellows furnaces, and got a molten, high carbon, brittle cast iron out of it. And said 'This stuff is shit, use it to cast hoes and stuff for farming and munitions grade weapons, we'll keep to bronze for fancy weapons and important stuff,' and muddled on that way for some (centuries!? I think) before realising that the reason the iron from the other guys was better was because their bag bellows furnaces were shittier and lower temperature and so were not getting hot enough to melt the ore completely and produce liquid carburised cast iron, but instead were getting 1100-1200 degree ish reducing conditions that were barely liquefying the ore, stripping the oxygen off it with carbon monoxide, and a spongey mass of iron would grow in the molten silicate and iron slag bath at the base of the stack below the bellows nozzle. Which then had to be hammer wrought to weld it all together and squeeze the slag out to produce a workable wrought iron, and steel was a little understood thing that happened sometimes in smelting, but usually by cementation/case hardening, but was hit and miss. But iron was everywhere, so wrought iron for all and sometimes steel was better than bronze for some. So the Chinese had to work backwards to make lower efficiency furnaces to get useful sponge iron bloomery furnaces (still with box bellows, just not going as hard on the blast, and smaller). Roman bloomery furnaces, for example, sometimes got big enough to accidentally produce cast iron, and they'd throw it out as a waste product.
But the problem is that bloomery iron production, although giving a product that was a workable iron, made small lumps - kilos to tens of kilos at a time, and even at their largest, never really more than hundreds of kilos at a time, and each bloom a bit different, so hard to get uniform product, and nobody really understood how to get rid of things like phosphorus, so you get the 'Ok, iron from this region is ok for farm tools and wrought work and goes black and doesn't corrode, but is too brittle for cutting edges' (high phosphorus ore) and 'Ok, iron from that region is good for steel and doesn't get as brittle, use that for cutting edges and weapons' (low phosphorus ore, etc)
Now, lots of cultures kept, or started, as they got big bellows technologies, producing cast iron in blast furnaces, because large volumes of uniform quality cast iron is great for cheap tools, cookpots, and once you understand how to get the bubbles out of it, cannons, and also cos big bellows blast furnaces are also great for doing large volumes of copper alloy stuff for all your cupro alloy needs, but also self aggrandisment statues or Vatican Doors or again, Cannons. But we were all stuck with small batch bloomery stack furnaces to make workable wrought iron for steel until the 17th-18th century, maxing them out to as big as they'd go without making too much accidental cast iron. By the mid 18th century, I think, puddling techniques had turned up for slowly turning high carbon cast iron into workable low carbon iron - really awful job, standing with a long iron rake in front of an open blast hearth stirring a puddle of molten cast iron around to burn off enough carbon that a lump of malleable sponge iron began to form under your rake, which you could then turn into steels or wrought iron. Various versions of this process were independently developed. (There's some evidence that there were Chinese versions of malleable white cast iron developed back in the Han dynasty, and were used intermittently to the Tang dynasty, too).
But the modern blast process turned up from people like Bessemer, Kelly, Naysmith, etc, in Europe, UK and America, all around the 1840s, as multiple groups experimented with improving that awful (and worker health destroying) puddling furnace process, with different versions of blowing an air blast, oxygen blast etc, over, around or through your vessel of molten high carbon cast iron, to burn off just enough carbon to turn it into steel, but not so much that you burnt it all off and your useful alloying components too. That couple decades between the 1840s to 1860s solved the base problems of turning high carbon but large volume cast iron blast furnace product into useful steel and iron alloys to allow us to escape the small batch production bottleneck of the two and a half thousand year old bloomery process, which @iamthepulta has covered above.
But of course now we're cooking ourselves, so we need to shift as many of our necessary materials production and use processes to carbon neutral, or carbon negative. In the case of steel, while arc furnaces are now a mature technology for alloying and recycling, it's hard to get the carbon and oxygen intensive reactions out of iron smelting to produce your arc furnace feedstock, but it looks like hydrogen is the way to go: Fe2O3 + 3H2 = 2Fe + 3H2O. People have been fiddling with that reaction since the 1950s, but the problem is that it's endothermic, and wants 95.8 kJ/mol to run in the direction we like. So you have to put in A Lot of energy with your hydrogen, and that means you need to do this sort of hydrogen reduction of iron ores at locations of large availability of hydropower or other non fossil fuel electricity, and where you can store large volumes of electrolytically produced hydrogen (preferably in salt mine cavities). The HYBRIT process seems to be the people most successful, but the problem is that it's around 30% more expensive than even the most efficient carbon based iron reduction processes:
success, everybody, i thought about something other than vampires for like a twenty minute stretch. the something was: electric arc furnaces
about 7% of us coal consumption is metallurgical coal, which is used (after being coked) as fuel for blast furnaces. blast furnaces smelt ore and scrap metal, usually to make steel. most coal in the united states is used for the power grid & must be replaced with renewable sources, but it's a little more straightforward to see how that swap needs to go; we need better batteries & genuine investment, there are questions about where & how those renewable sources should be generated, & i do think that our power consumption needs to fall. it's less obvious how we might replace metallurgical coal, though, because we still need steel. electric arc furnaces are efficient, cheaper, smaller, and more capable of running variable loads than blast furnaces, but almost all of them are for the scrap metal -> steel process, they're not for iron ore -> iron -> steel. but we are getting better at making them! so i read through part of a DoE powerpoint & glowered at links to mckinsey reports about it. i don't know anything really about metals mining, mostly i've just read about coal & all of that from a labor safety perspective, but i'm very curious about the, like. engineering problems (and also still labor safety & environmental problems) presented by trying to genuinely transition away from coal, which we absolutely must do, like guys even UMWA is out here like 'we gotta stop pulling this shit out of the ground' [official position of union president cecil roberts is that coal miners & their communities need a 'just transition' away from coal]
#Iron rant#I hope this helps#I haven't even covered crucible steel#because that's a whole other story#But really fucking cool story at that.#But I have to talk about solvus curves and eutectics for that and it's late and my brain is hurting.
30 notes
·
View notes
Text
I wanted to share some tips for surviving in the cold. As a Finn this is something I experience every year. Unfortunately that experience doesn't make me the best person to talk about survival tips as people in here tend to take all that for granted, but here's what I thought of.
Here we live in houses with thick walls and insulation (I live in an old house though so the heat is not such a given), but your house probably wasn't built with freezing temperatures in mind, so this is what you can do:
Gather all your family, pets and plants in a single room. Preferably an inner room that doesn't have any walls in touch with the cold air outside. A bathroom or a room directly connected to a bathroom would be the best for obvious reasons, if possible.
Gather everything you (and your family and pets) need in that room so you won't need to open the door unnecessarily as you want to keep the air in that one room warm and keep the warm air inside that room.
If it's an inner room, the rooms around it will protect it from the cold wind and act as a type of insulation. But in any case you want to seal all the gaps in the doors and windows, if there are any. Materials for this can be anything you can think of from newspapers to towels, or wool or even bubblewrap. You can use tape to seal the gaps. Only open the door if there is an emergency, like if the room really runs out of air.
Gather as many blankets and towels you can find. If your room has windows, cover them with blankets and towels to keep the heat from escaping through the glass. If your walls are really thin and can't keep the heat inside, you might want to cover them with blankets or towels as well.
The floor is going to be cold, so don't sit on the bare floor. If your room has a bed or a couch, sit on that. If not, bring a mattress.
Wear as many layers of clothing as you need. But not too much, as sweat will make your clothes damp and the moisture draws heat out of your body. So if your feet are cold, put more socks on. Multiple shirts or multiple coats, or whatever you need. If you have any blankets left, now is the time to use them to stay warm. Feel free to live in a pile of blankets now. Wear a hat or a hood or a scarf on your head to keep your ears warm. Headphones can do the trick too. Now isn't the time to think about fashion.
If you have electricity (a generator or batteries work too), and a small heater, bring that to your room of choice. Some electronics, like computers produce plenty of heat too, so if you have electricity and the use isn't restricted you can take advantage of that too.
I've never tried it, but the tips about candles under an earthenware pot should work as a heat source quite well (works similar to a sauna stove that heats the rocks on top of the fire, just in smaller scale). Support the pot upsidedown slightly above the ground level with something fire-resistant to allow air to get in and out and place the candle under it. Just be very careful when dealing with fire and don't leave it unattended. Also, don't use too many candles in your sealed room as the fire will use the oxygen and you don't want to run out of air to breathe.
Bring battery-powered flashlights to the room with you if there is no electricity (and just in case it goes out).
Just like your house, your water pipes probably weren't made with freezing temperatures in mind. Water will freeze if it's standing still and that might break the pipes so keep them running – leaving them dripping just a little should do the trick. You can have them drip into a bucket and use the water later for whatever you need.
Avoid going outside, but if you absolutely must, wear even more layers of clothing. Your worst enemies are the wind and getting wet because that will make the cold much worse.
Wear boots if you can, and have your pants tucked inside the boots. The outer layer of your clothes should be something made to resist the wind. A rain coat will do in an emergency (but seriously, that's just the outer layer, you should have a lot of warm clothes under it!). Cover your ears and hands. Gloves and mittens are great, but if you don't own any, you can totally wear socks on your hands! Long sleeves are great too. Hands stay warmer as fists so keep your fingers together!
If the snow isn't too deep, it's better for walking than icy roads. But a thin layer of powderlike snow on an icy road is super treacherous so beware of that. It's going to be slippery! Same goes for a layer of water on an icy road. If you have to walk on ice, step carefully and place your whole foot down. If you fall, it's safer to crawl to a less slippery ground than try to stand up right away. This is no time to feel ashamed! Also, avoid going out alone. It’s easy to break bones if you fall on ice and you’ll need someone to help you. Keep your mobile available and charged at least, and keep it warm. Electronics don’t handle cold well so you might want to keep your phone inside your shirt or in your sleeve instead of a pocket so it works when you need it.
You can make your driveway less slippery by sprinkling some sand or gravel over the ice. Saw dust will work too.
Avoid driving a car, and if you must, go slowly and avoid any sudden movements such as braking or turning too fast because the car will just keep sliding forward or start spinning out of control. If your car is spinning, let go of the pedals and don't try to steer. The car will stop on its own. When it has stopped, take a deep breath and if there is no damage you can start driving very slowly. But really, don't drive a car unless it's an emergency. In Finland we basically have spikes on the tires during winter, but you don't so driving is just way more risky there!
Also, if you get stranded outside with no buildings nearby, your car is probably the safest place for you to wait for help. In such a situation it's like that small room and you want to keep it as insulated and warm as possible. Have some blankets inside in case of an emergency so you can insulate the windows from the inside as well as keep yourself warm. A layer of snow on the car can help keep the warmth inside, but never idle the car for warmth, especially if you are stuck and covered in snow because it will keep the toxic Carbon Monoxide in as well! (In some cases it’s best to get the snow off the car so the rescuers can find you more easily. A car that’s covered in snow is hard to find in snowy environment. Use common sense to decide what’s best in your situation.)
If you get stranded in the cold without a car, create your ”small room” from the snow. It will protect you from the freezing wind and at least keep you warmer than you'd be outside.
Don't walk on the ice over a body of water unless you know what you are doing. But if you end up falling through the ice, try to stay calm. Call for help. Push yourself forward, not up. When you get your body on the ice again, don't stand up. Crawl back to safety. Being wet is your worst enemy so get rid of your wet clothes and replace them with dry clothes asap!
For this same reason, don't go outside with wet hair, it will literally freeze. I know because I don't follow my own advice, but I'm a crazy Finn and we also swim in freezing waters so unless you'd do that too, you don't want to go out wet.
You can also use the cold to your advantage, especially if the electricity runs out by keeping your food products outside so they stay cold. One idea is to place them in a bucket or a plastic box with a lid and covering it with snow.
So yeah. Stay safe and warm out there!
#long post is long#this is all I could think of right now#there is definitely more#survival#JeMiChi talking#all of this is common knowledge where I live#I hope this helps someone who doesn't have so much experience with freezing temperatures
1 note
·
View note
Text
WEEK 1 - 2
Syllabus
Research on Water Contamination and ways to prevent it. Research Materials - Get in touch with Foli.
Progress:
Spoke to Foli and there are two priorities:
Temporary and/or Permanent covering for the well to prevent contamination and pollution.
Collection of rainwater for future use.
The idea that we discussed was to create a funnel through which water can be collected in a cistern. Foli initially preferred Aluminium and bamboo in the making of the funnel but after research on materials I came to realise how Aluminium might not be the safest way to transport water and pose a health risk.
Week 3- Week 4
Research on additional materials.
Progress:
Researched a number of techniques that are used to filter water without electricity or expensive filtration systems.
Number of options found:
Bamboo Charcoal - this is created through heating bamboo and creating a charcoal of the plant, which when placed in water is able to absorb most of the bacteria in the water. This is extremely helpful and sustainable for a place like NDOR eco village.
Second option was the inside gel of a cactus plant which can be put inside the contaminated water and once boiled is able to absorb most of the bacteria. The only problem with this is you need cactus plants for this and they are not natives to the region.
Last method I thought would be a good match for the village was clay pots. Clay pots have perforations of about 0.2 ml in them so once water is poured in them, it slowly starts to leak from the bottom. The water that leakes is clean from almost any bacteria and is healthy and drinkable for infants and adults.
I have decided to do a combination of both points 1 and 3. I will create a funnel which will be attached to an underground cistern. In the underground cistern I will place bamboo charcoal to first put the water through initial filtration.
Secondly, whatever water is extracted from the cistern for drinking use will go through pots into a plastic/or any other material container they have present.
Polycarbonate corrugated roofing - Foli needs me to use.
Week 5 - Week 7
Collect water and start testing.
Method:
Bamboo charcoal, you’ve heard of it, you’ve heard of the great things it can do. But really, does it work? I think so. I’m not 100% sure, but I allowed several people to try my “bamboo water” and tea brewed with it alongside normal Chicago tap water and the results were positive. I have no scientific evidence, and I don’t need any – I like it, it tastes good, and I’m going to use it — and that’s enough for me (want technical? read this).
If you are interested in making bamboo charcoal, Pyro Energen has put together this pretty neat guide to making your own at home, with bamboo chopsticks at that!: http://www.pyroenergen.com/articles/how-to-make-bamboo-charcoal.htm
The idea behind bamboo charcoal, and any charcoal for that matter is the fact that it is extremely porous and will absorb impurities in water. It really isn’t “filtering” water if you set a stick in the water, in order to filter the water, the water must pass through granulated charcoal or some other medium (this is how Brita filters work).
Once you have obtained bamboo charcoal, follow these steps:
Sterilize the bamboo charcoal by boiling it for 10 minutes in water.
Allow the charcoal to dry for 24 hours.
Soak it in your tea water for 24 hours, then use the water to brew tea.
After about a month or so, reboil the charcoal, dry it, then use it again.
How Foli can do this on his own:
How to Make Bamboo Charcoal in a Simple Way
This time, a small part of the bamboo branch will be used instead for charcoal making.
Materials to prepare:
Few sticks of dried bamboo branch
Aluminum foil
Spirit (alcohol) lamp
Bamboo charcoal procedures:
Wrap the small bamboo branches with aluminum foil as seen below.
Probably, wrap the bamboo several times with aluminum foil to seal and protect it (air intact).
You can use an ice pick to make a tiny hole to the wrapped aluminum foil, to prevent it from bursting when the trapped air in it is expanded by the heat.
Bamboo Charcoal
Place a wire mesh on top of the spirit lamp and position the aluminum-wrapped bamboo on top of it.
Light up the lamp, this is done to dry the bamboo inside the foil. White smoke will come out from the tiny hole, and will turn to yellow. This might produce an unpleasant smell, so you will need to open your windows or do it outdoors.
Bamboo Charcoal
When the color of aluminum foil turns yellow, it means the job is done. The yellowish color is caused by the bamboo tar. Do not open the aluminum foil until it's cooled. Heat can break the charcoal easily.
Another Simple Way in Making Bamboo Charcoal
Bamboo Charcoal 1
Prepare an aluminum foil and a few bamboo sticks (I used bamboo chopsticks).
Bamboo Charcoal 2
Bamboo sticks, aluminum foil, wire mesh, and stove are the only thing you need.
Bamboo Charcoal 3
Wrap the bamboo sticks with aluminum foil. Make sure the wrapping has no holes.
Bamboo Charcoal 4
At the end of the wrapping, make a small hole. This is to let the accumulated gas to escape and not to blast.
Bamboo Charcoal 5
Place the wire mesh on the gas stove. Place the bamboo sticks on top of it.
Bamboo Charcoal 6
First, put up a small and weak flame. Soon, white steam-like gases will come out. Increase the flame after few minutes (steam will turn bluish). If it turns into a whitish smoke, then turn off the gas stove.
Bamboo Charcoal 7
Sink the aluminum foil into a water basin for few minutes.
Bamboo Charcoal 8
Now, open the aluminum foil and you'll see hard bamboo charcoals.
Hard and good charcoals do not make your hands dirty black.
Soft charcoals are caused by oxygen present during processing. These are poor charcoals and we can't use them for our purposes.
Another Procedure in Making Bamboo Charcoal
Bamboo carbonization can be divided into four stages according to temperature and products situation in a kiln.
First stage drying: the temperature is below 120°C and the speed of carbonization is slow. Heat is used to evaporate the water in bamboo, and the chemical composition of the bamboo is still intact.
Second stage precarbonization: the temperature is in the range of 120°C to 260°C and there is a distinct chemical reaction in bamboo. The unstable chemical compounds begin to decompose and carbon dioxide and carbon monoxide are released.
Third stage carbonization: the temperature is in the range of 260°C to 450°C, and the bamboo is decomposed into liquid and gas products. Liquid products contain much acetic acid, methanol and bamboo tar. Flammable methane and ethylene in gas products are increasing while carbon dioxide production is reduced.
Fourth stage calcinations (refining stage): the temperature is over 450°C. The bamboo becomes charcoal by providing a mass of heat, emitting the volatile substances and to enhance nonvolatile carbon. Based on the temperature in this stage, the bamboo charcoal can be divided into three groups (low-temperature, middle-temperature and high-temperature charcoal). The quality and properties of bamboo charcoal differs with different temperatures during the refining stage.
Lastly the bamboo is left to cool down and depending on the weather; this process may take from five to eight days in big volume.
During the above process, you can extract alcohol, tar, vinegar, medicinal liquid (water form), and many other products. Products from bamboo charcoal have countless uses - from skin diseases, allergies, influenza, heart diseases, stomachaches, insecticides, pesticides, germicides, bactericides, deodorants, disinfectants, gardening, cosmetics, cooking, washing, and hundreds of others.
Start small prototype - testing
Week 8
Necessary changes / revisions
Week 9 - Week 10
Start Filteration Research
Week 11 and 12
Make initial prototype
Week 13
Texting prototype / water testing
Week 14
Revisions/feedback
Project Assessment and next steps:
As with any project, the best way to test the success of your design and to ensure it works is through physically visiting the actual site, so my hope is to get the opportunity to actually go visit and test out the design on site and make necessary changes if required. Through the feedback that Foli provides I would like to continue to finesse the design till it works flawlessly for their system. I would also hope to continue to expand on the LifeStraw concept ,if it works for the Ndor Eco-Village and create bigger products that last longer for families in need of drinkable water.
Research on Water Contamination and ways to prevent it. Research Materials - Get in touch with Foli.
Progress:
Spoke to Foli and there are two priorities:
Temporary and/or Permanent covering for the well to prevent contamination and pollution.
Collection of rainwater for future use.
The idea that we discussed was to create a funnel through which water can be collected in a cistern. Foli initially preferred Aluminium and bamboo in the making of the funnel but after research on materials I came to realise how Aluminium might not be the safest way to transport water and pose a health risk.
1 note
·
View note
Text
A Full Guide to Inflammation: Foods that cause Inflammation and the anti-inflammatory diet
“the absolute worst piece of advice we have ever gotten, is to eat a low fat diet. Mother’s milk is 50% fat and nature makes no mistakes.” – Dr- Bill Seers
Inflammation is the immune system’s response to specific stimuli such as joint injuries or allergic reactions. It is essential in small amounts, as it’s an indispensable part of our immune systems response to foreign invaders.
Once the invader has been dealt with and the system functions adequately, the immune system removes the inflammation. In chronic inflammation, the situation is different. Chronic inflammation is most commonly caused by the food we eat.
In fact, most chronic diseases are caused by continuous low-grade inflammation usually caused by food. In many cases, this level of inflammation is not easily perceptible, and goes unnoticed until serious pathologies such as diabetes, cardiovascular disease, obesity, autoimmune disorders, etc. are developed.
Inflammatory foods, which many of us eat on a daily basis, cause the immune system to induce inflammation. Over time, this leads to weight gain, skin problems, digestive problems and with time can leads to severe chronic diseases.
Those who have sluggish digestion, low energy, are unable to lose weight will benefit from replacing all inflammatory foods with anti-inflammatory and wholesome foods.
Taking care of our Mitochondria
Mitochondria are the powerhouses of our cells. They help turn the energy from food into energy we can use. Mitochondria may very well be the most important part of our biology and keeping them healthy is essential for health and wellness as all levels. Dr. Bill Seers, an internationally well-known paediatrician and author of over 45 books says “Inflammation is what matters more than anything else. What causes inflammation? Mitochondrial dysfunction. You cannot have inflammation unless you have mitochondrial dysfunction or at least damage or stress”.
He continues to say, “the absolute worst piece of advice we have ever gotten, is to eat a low fat diet. Mother’s milk is 50% fat and nature makes no mistakes.” The human brain is 60% fat and our mitochondria thrive on good wholesome fats. So a diet high in good and wholesome fats is indispensable to enjoy good health.
What causes Decay
The second law of thermodynamics says that everything eventually decays. This is a universal law, which is applicable to everything from planets to our very own bodies. For this very reason, life itself causes decay within the body. For instance, when we inhale carbon monoxide from cars in traffic. The carbon monoxide creates a free radical within the body, which is basically just an oxygen atom which has lost its electron. Such atoms, stick to anything and then oxidise it. Just like steel gets rusted when oxidised, oxidative damage made by free radicals is the main cause of decay within the body. It is also the main cause of inflammation in the body, as the immune system actively tries to fight the free radical.
These are regular events, which the body is able to handle through the immune system and temporary inflammation provided the adequate nutrients are present within the body. However, if we eat inflammatory foods, then it becomes very difficult for the immune system to fight external invaders.
Foods that cause Inflammation
1. Sugar
Table sugar is 50% glucose and 50% fructose. It cannot be processed quickly enough by our digestive system, so it releases pro-inflammatory messengers called Cytokines. Also, sugar suppresses the effectiveness of white blood cells, which weakens our immune system making us vulnerable to infection. Remember sugar is not only in sodas and sweet foods, it is added to many foods such as cereal bars, pre-packaged fruit juices, some salad dressings, cooking sauces, white bread, etc.
Avoid: Table sugar, fructose syrup. Instead opt for low glycemic alternatives, such as whole grains and foods with healthy fats, proteins and fibers.
2. Artificial Trans Fats
Artificial trans fats are created by adding hydrogen to unsaturated fats. Sometimes they’re referred to as hydrogenated fat. The unsaturated fats are liquid, so they’re hydrogenated to give them the stability of a solid fat. They aren’t natural so our body does not have the ability to metabolise and break them down adequately. Our immune system registers them as foreign invaders, stimulating an immune reaction which triggers systemic inflammation.
Most margarines contain trans fats, and they are often added to processed foods in order to extend shelf life. Trans fats cause inflammation, lower good (HDL) cholesterol and impair the functioning of the endothelial cells lining the arteries.
Avoid fries, microwave popcorn, margarines, packaged cakes, cookies, pastries, processed foods, soybean oil, palm oil, sunflower oil.
3. Saturated Fats
Fat cells secrete hormones which bind to themselves and are either pro-inflammatory or anti-inflammatory, which happens when they’re in balance. However high intake of saturated fats triggers white adipose or fat tissue inflammation. This tissue stores energy rather than burning it, which makes the fat cells get bigger and bigger. When fat cells grow too much, they release pro-inflammatory hormones which result in systemic inflammation.
Avoid: Pizza, cheese, full-fat dairy, grain-based desserts and red meat.
4. Vegetable and Seed Oils
Vegetable oils are highly concentrated in omega-6 (inflammatory fat), and low in the omega-3 (anti-inflammatory fat). Omega-6 is an essential fatty acid that the body needs for normal growth and development, however taking care of the Omega6-to-Omega3 ratio is of upmost importance when it comes to health. The ratio should be a 1:1 ratio, however people eating vegetable oils on a daily basis can sometimes have ratios of up to 20:1. When there are too many omega6’s, they eat up all the enzymes, preventing the Omega3s from getting into the cells, triggering the body to produce pro-inflammatory chemicals.
Avoid oils such as corn, sunflower, safflower, grape-seed, soy, peanut, vegetable and mayonnaise.
5. Cooking with oil
When oils are heated they get oxidised. Oils such as extra virgin olive oil should always be eaten raw. When used for cooking, it is best to use oils which are solid at room temperature such as coconut oil.
Avoid: Cooking with oil. Instead, cook with organic coconut oil, organic grass-fed butter or organic-grass fed ghee.
6. Fried Foods
Vegetable-oil fried foods are high in AGEs, which are produced whenever food is fried.
AGEs are toxic compounds which are produced when proteins or fats combine with sugar in the bloodstream, a process also known as Glycation. AGEs also form in foods, especially in foods that have been fried, grilled, toasted or exposed to high temperatures. When too many AGEs are consumed, the body is unable to eliminate them and the immune system immediately responds with inflammation.
Oil used in fried food is usually highly oxidised, which double up the resulting inflammation on the body. More importantly, fried foods block endothelial cells from normal functioning.
Avoid: All fried foods. Try steaming, pressure cooking or low temperature baking.
7. Refined Flour
Refined wheat flours have their slow-digesting fibre and many of their nutrients removed. This means the body digests them too quickly, which makes blood sugar levels spike. This in turn triggers a spike in insulin levels which causes an inflammatory response. Refined Flour is one of the main drivers of escalating rates of obesity and other chronic conditions. It is also considered one of the biggest causes of cancer. These high-glycaemic index foods fuel the production AGEs and products that stimulate inflammation.
It is also important to note that fibre promotes fullness, improves blood sugar control and feeds the beneficial bacteria in the gut.
Avoid: Candy, bread, pasta, pastries, some cereals, cookies, cakes, sugary soft drinks and processed food that contains added sugar or flour.
8. Alcohol
Breaking down alcohol generates toxins that damage liver cells, promote inflammation and weaken the body’s immune system. Besides, alcohol increases all the inflammatory markers within the body. Consuming over one glass of wine can lead to bacterial toxins moving from the colon into the body, which can drive widespread inflammation within the body.
Avoid: Drinking over one glass of wine or beer a day. Avoid spirits and cocktails.
9. Grain-fed meat
Most cattle, chicken and other farm animals bred for human consumption are now grain-fed. However, this is an unnatural process, which means they have to be fed antibiotics to prevent them from getting diseases due to their artificial diet or the way they are confined in small spaces.
Also, most farm animals are either fed corn or soy, which results in meats high in saturated inflammatory fats with greater levels of omega-6s, creating an imbalance in our omega6-to-omega3 ratio. Furthermore, the levels of antibiotics and growth hormones present in the meat, trigger an inflammatory immune response.
Avoid: grain-fed meat. Limit your meat consumption as much as possible, unless you are aware of the farm it comes from and know how the animal is treated. Organic meat is not necessarily good for you, as the animals could have still been fed antibiotics, growth-hormones and organic grain.
10. Processed Meat
Processed meats are even worse the grain-fed meats. They are usually grain-fed and contain high levels of AGEs which are created when the meats are processed – when they’re dried, smoked, pasteurised or cooked at high temperatures. Also, most processed meats have preservatives, colourings and other artificial additives which our immune systems also consider a foreign invader.
Processed meat is associated with an increased risk of heart disease, diabetes, stomach cancer and colon cancer.
Avoid: All processed meats, sausages, cold meats, etc.
11. MSG and Artificial Additives
Artificial foods are not natural, so the body has no way to metabolise them. An immune response is triggered when artificial colourings, emulsifying agents and other additives are ingested, which activates an inflammatory reaction.
Mono-sodium glutamate (MSG) is a flavour enhancing additive most commonly found in prepared Asian food, soy sauce, fast foods, prepared soups and soup mixes and salad dressings. It can trigger two important pathways of chronic inflammation.
Avoid: avoid fast food, prepared meals, dressings, sauces, foods with emulsifiers, etc.
12. Gluten and Casein
Many store-bought breads have very short periods of fermentation which reduces the amount of gluten the yeast can predigest for us. This makes digesting gluten in bread much harder, causing inflammation in the intestines. People who have joint pain and are sensitive to gluten – found in wheat, barley and rye – or casein – found in dairy products – may find relief by avoiding them. There may be an overlap in which some people with arthritis also have a gluten sensitivity or also have celiac disease.
Avoid: store-bought packaged breads, white breads and excessive gluten.
13. Aspartame and artificial sweeteners
Aspartame is a non-nutritive, intense artificial sweetener found in over 4000 products worldwide. The body reacts to the foreign substance by attacking it, in turn triggering an inflammatory response.
This is also the case with many other artificial sweeteners, which are also one of the top five leading causes of cancer. When artificial sweeteners are ingested, the body releases cytokines.
Avoid: Artificial sweeteners. Instead try 100% natural stevia or raw honey.
14. Dairy products (sometimes)
Saturated fats in dairy are a common cause of inflammation if taken often. Dairy is also a common allergen; millions of people worldwide are intolerant to dairy. All allergens can cause inflammatory reactions by releasing histamines.
Avoid: Dairy products if you’re intolerant or feel bloated after ingesting dairy products. (Except grass-fed organic butter and grass-fed organic ghee)
15. Packaging in fast foods and drinks
Phthalates – which are endocrine-disrupting toxins and are found in most plastic packaging – get filtered into the food covered by the packaging. Phthalates and BPAs in plastics cause immediate inflammation, as the toxin is considered a threat by our immune system.
Avoid: Vegetables, fruits and other foods pre-packaged in plastic
Foods that are natural anti-inflammatory.
1. Berries
Berries – mainly strawberries, blueberries, raspberries and blackberries – are high in anthocyanins, which have an overall anti-inflammatory effect on the body.
2. Cruciferous Vegetables: Broccoli, Cauliflower, Kale and Brussels sprouts.
Cruciferous vegetables are high in antioxidants which lower cytokines.
3. Avocado
Avocados are loaded with potassium, magnesium, fibre and healthy monounsaturated fats. They offer many beneficial compounds, which protect against inflammation.
4. Green Tea
Green tea has antioxidant and anti-inflammatory properties. Epigallocatechin-3-gallate (EGCG) is a substance which inhibits inflammation by reducing cytokine production and damage to the fatty acids in our cells.
5. Peppers
Bell peppers and chili peppers are rich in vitamin C and antioxidants which have powerful anti-inflammatory effects.
6. Grapes
Grapes contain anthocyanins, which reduce inflammation
7. Turmeric
Turmeric is a spice which contains curcumin, a powerful anti-inflammatory. It is highly effective in reducing inflammation related to arthritis and diabetes.
8. Extra Virgin Olive Oil (Raw)
Extra-virgin olive oil is packed with monounsaturated fats and contains oleocanthal, an antioxidant sometimes compared to ibuprofen. However, when cooked, olive oil becomes oxidised and induces inflammation. It must always been eaten raw.
9. Dark Chocolate
Dark chocolate is rich in inflammation-fighting antioxidants called Flavanols. Make sure the chocolate is at least 75% cocoa.
10. Tomatoes
Tomatoes are high in vitamin C, potassium and a powerful antioxidant called lycopene.
11. Cherries
Cherries are packed with anthocyanins and catechins which are strong anti-inflammatories.
Vegetarians and inflammation
People following a vegetarian diet have higher levels of plasma AA, a marker of overall health that is directly associated with lower levels of inflammation and heart disease.
Inflammation and Stress
One of the often overlooked causes of inflammation, is the communication between the immune system and the central nervous system. When under chronic stress, the nervous system can activate the pro-inflammatory pathways. Moreover, stress also promotes ingesting inflammatory and unhealthy foods, which over time further aggravates stress and creates adiposity.
It is therefore recommendable to practice regular meditation and deep breathing, in order to reduce stress levels and ensure effective communication between the immune system and the central nervous system.
Conditions that can be improved by an anti-inflammatory diet
Arthritis, psoriasis, asthma, crohns disease, colitis, inflammatory bowel disease, diabetes, obesity, metabolic syndrome, heart disease, esophagitis, lupus and certain cancers.
6 notes
·
View notes
Text
What exactly are coffee bags? How can you use them?

Coffee beans are visual, aural, tactile, and aromatic, even before they are ground and brewed into a delicious drink. They need proper packaging and storage bags to retain the aroma and freshness throughout its journey till the drinker.
When coffee is first roasted, it releases carbon dioxide for days after being roasted. To be vacuum sealed, the coffee has to first release all of its CO gases, or it will burst the bag. The vacuum bag does help preserve coffee longer while it ships and maybe sits on a store shelf, but before it ships, it sits around for a while before it is "sealed for freshness". Vacuum sealing is best for pre-ground coffee, which we already know is not going to taste as good as fresh-ground coffee.
Instead, fresh-roasted coffee can be packaged in valve-sealed coffee bags, which allow the carbon monoxide gases to escape while still capturing and preserving the flavor soon after roasting. Packaged like this, coffee tastes best about 48 hours after roasting, but still remains fresh long after being bagged.
A few easy tips for serving the best tasting coffee:
Buy whole beans directly from a local coffee roaster.
Look for valve-sealed hermetic packages or bags.
Store your coffee beans in a sealed container in a dark and dry place.
Grind your beans just before brewing.
Enjoy your flavorful cup of coffee!
Ecotact Bags- for coffee storage and packaging:
Manufactured with precision, Ecotact bags are one of the pioneers in multi-layered hermetic packaging solutions. In a world-class manufacturing infrastructure we produce food grade storage facilities for dried grains like cocoa, green coffee beans, barley,etc. Our bags have a uniform thickness profile with a minimum variation of 2 sigma quality, excellent stretch, cling, and puncture resistance with high clarity recyclable film.
Some significant features of a typical Ecotact hermetic bag are:
It has:
High level of temperature tolerance (- 30 degrees C to + 90 degrees C)
Aroma and moisture level maintained
High resistance to oil and solvents
Hydrocarbon free
Hydrocarbon free
Sensitive food and vitamin content protected
Protects flavour and quality
Holds excellent forming and can be used with any secondary bags of jute/PP
Easy to transport crops to any part of the world
Customized branding is available
Recycling solutions are monitored globally
With extra-clear transparent bags for clean visibility
Optimal usage of coffee bags can vary according to quantity and purpose.
Troiseal Bags - These are tamper-proof hermetically sealed bags designed especially for green coffee & other coffee beans. The flexibility of the bag makes it a perfect match for small and home grown roasters.
Hermetic Sampler bags -Sampling of green coffee by coffee producers/millers/traders/roasters is one of the most critical exercises in the coffee industry. These little bags are perfect for sampling use.
Sterile vacuum bags -These high-barrier multi-layered plastic bags are strong & flexible and can preserve food grains for a longer duration. They are space convenient and can be easily packed into corrugated cartons wherein branding can be done in the most attractive ways.
Multilayered Hermetic Storage Bags - These bags are used inside burlap sacks as they can resist high oxygen and moisture. It is used to store green coffee throughout the supply chain and storage.
For further enquiries or information related to storage and packaging just visit our site https://www.ecotactbags.com/.
0 notes
Text
Why Does Oxygen Not Absorb Infrared Radiation?
How Do Carbon Dioxide Absorbs and Emits Infrared radiation?
There are a lot of theories about infrared radiation and carbon dioxide absorption but no one is certain as to which one of them is correct.
The absorption of carbon dioxide by the infrared light comes from the absorption of infrared photons.
The absorption of carbon dioxide is not seen, nor is it believed to be absorbed by carbon dioxide.
If carbon dioxide were to absorb infrared light then the theory says that we would lose our sense of smell and taste and so would die.
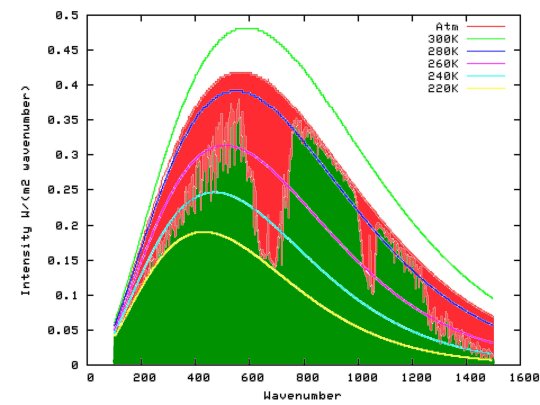
However there is evidence that suggests carbon dioxide absorption does happen.
It has been measured in a number of different situations and has been found to be true in some cases but false in others.
The truth may lie somewhere in between. The main theory that explains absorption is that the carbon dioxide reacts with the water molecules in the air to create a compound called oxalate.
This compound in turn combines with the protein fluorine in the hemoglobin so that the red blood cells can get the oxygen they need which they do when we are breathing.
However, the amount of carbon dioxide that combines with the protein will depend upon the concentration of carbon dioxide in the air.
When there is a large concentration of carbon dioxide then absorption is going to take place.
If the concentration is low then there will not be any absorption.
If the concentration is high then there will be.
So measuring the concentration is not always the best way to measure absorption. However, it still is an important part of understanding carbon dioxide absorption and understanding what the effect of increasing the concentration in the atmosphere will have on us.
A Look at the Interacting Molecule of Carbon Dioxide and Re-Emitted As Infrared Radiation
Now, the next question in mind would be how carbon dioxide can be an energy interacting molecule?
This is because carbon dioxide reacts to photons coming from an atom or molecule which absorbs energy.
The more carbon dioxide present, the more it absorbs energy and the more infrared radiation are emitted as a result.
Now, there are many other known interacting molecules with different atomic structures.
There is another molecule which is known as fluorine.
This one is found at high temperatures and very reactive to light. If there is less carbon dioxide in the Earth’s atmosphere then fluorine will interact with it and will emit a lot of infrared radiation as a result.
Therefore, it is believed that every time there is less carbon dioxide in the Earth’s atmosphere there is more fluorine present and more infrared radiation emitted as a result.
Now that you know that carbon dioxide is a very important greenhouse gas which leads to global warming and climate change, you might be concerned as to how you can reduce your carbon dioxide emissions.
You can do this by changing your lifestyle and buying green products.
In addition, you can also use energy efficient appliances and cars.
However, even if you use green products and make every effort to be environmentally friendly, you cannot stop carbon dioxide emission completely.
So, instead of looking for ways on how you can reduce your carbon dioxide emissions go ahead and look for ways on how you can better protect the Earth from global warming and its effects.
Why Carbon Dioxide Absorbs and Emits Infrared Radiation
The reason why carbon dioxide absorbs infrared radiation is because the carbon dioxide in our air combines with the water vapor that is present to become denser and as a result infrared radiation cannot be absorbed.
When carbon dioxide absorbs infrared radiation, it creates a warm blanket around the body that acts similar to that of a sauna.
It has been suggested that taking a brisk walk after you have your swim can help to increase the absorption of the infrared radiation by as much as forty per cent.
However, there are many factors involved when it comes to the absorption rates and it is difficult to give an exact figure.
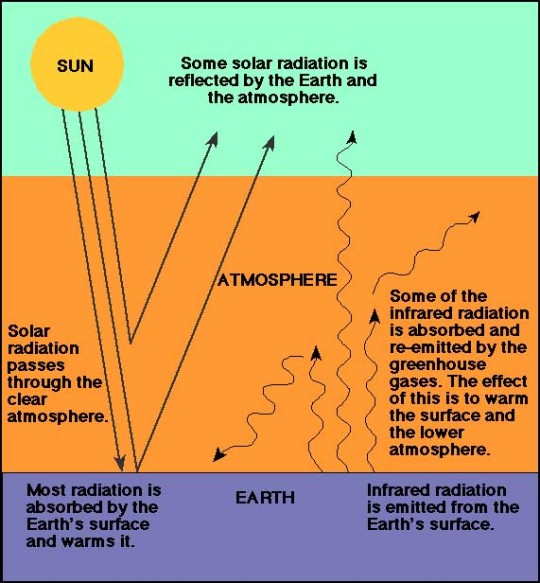
There are various other reasons why carbon dioxide does not reflect infrared radiation.
Some of the more common ones are that when swimming, it is possible for the carbon dioxide in the water to mix with the oxygen that is in the swimming pool causing the water to become cloudy and this can reduce the absorption rate significantly.
Also, it is possible that the swimming pool is surrounded by a glass window that has some sort of barrier preventing most of the heat from escaping.
If this is the case then the amount of time that you spend in the pool will also be decreased as the amount of heat that is able to escape will be reduced.
Energy Interacting Molecules of Carbon Dioxide and Re Emitted As Infrared Radiation
We are all aware of the fact that carbon dioxide is one of the three most common elements in our atmosphere. The other two are water and oxygen.
These are present in the earth as they are being continuously produced by the nature’s inexhaustible process, while carbon dioxide is being present since millions of years from the beginning of the earth’s existence.
So, if carbon dioxide is not supposed to be there with us, then where did it disappear without our knowing?
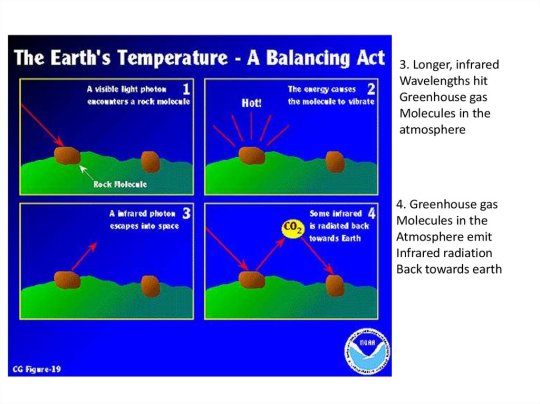
Carbon dioxide is one of the twenty-four natural compounds which makes up the majority of the earth’s atmosphere.
This is being continuously produced by the earth’s metabolism and, through the food chain, by plants, animals, bacteria and even insects.
But, at some point, some of these molecules may become colliding with one another causing an excess of carbon dioxide to be produced and thus causing the earth to warm up and create the greenhouse effect which is otherwise known as the greenhouse effect.
When this happens, the earth tends to absorb more heat than it used to before and the result is that, on top of the already warm temperatures, a rise in ground temperature also takes place, sometimes accompanied by rainfall, and this may set the stage for the creation of clouds.
The greenhouse effect, or climate change, has become one of the biggest problems that the world is facing.
One of the major causes for this is the increased use of fossil fuels such as coal, oil and gas, which are used in large quantities in the human endeavor to satisfy the energy needs of the global community.
Because of this, there is a constant rise in the concentration of carbon dioxide in the atmosphere.
There is another equally important cause for climate change and that is the burning of fossil fuels like oil, coal and gas for producing heat.
Thus, even if the carbon dioxide is not immediately removed from the atmosphere, gradual removal will occur as the extra amounts of these gases are burned off. This will in time have a negative effect on the earth’s climate and consequently on its climate in general.
How the Faster Motion of a Molecule That Eventually Results in IR photons Being Absorbed by a Carbon Monoxide Molecule
In the search for an answer to the question how the faster motion of a molecule that ultimately results in IR photons being absorbed by a carbon monoxide molecule, many scientists have come up with some interesting theories and have made some surprising observations.
One theory is that the faster motion of molecules may result in the absorption of more infrared photons than slow molecules.
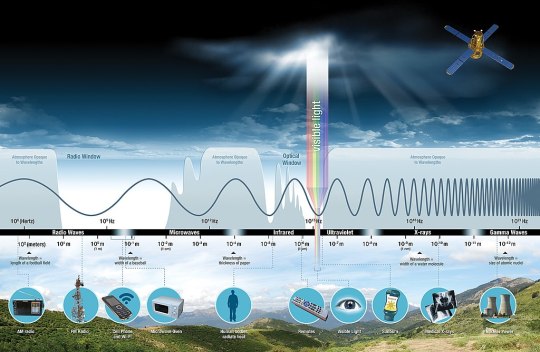
How Can We Reduce the Absorption of Carbon Dioxide?
The absorption and emission of carbon dioxide is a natural process that is occurring in the Earth’s atmosphere.
When carbon dioxide is breathed in by human, it combines with the oxygen present in the air and gives off heat, known as the greenhouse effect.
When this process occurs, the Earth’s average surface temperature raises above freezing. If the average temperature continues to rise for many years, scientists predict that the ice will start to build up on the poles and that the planet will experience further climate change.
Because carbon dioxide is believed to be a major cause of global warming, the key to limiting this long-term cycle is looking into ways of reducing the amount of carbon dioxide in the atmosphere.
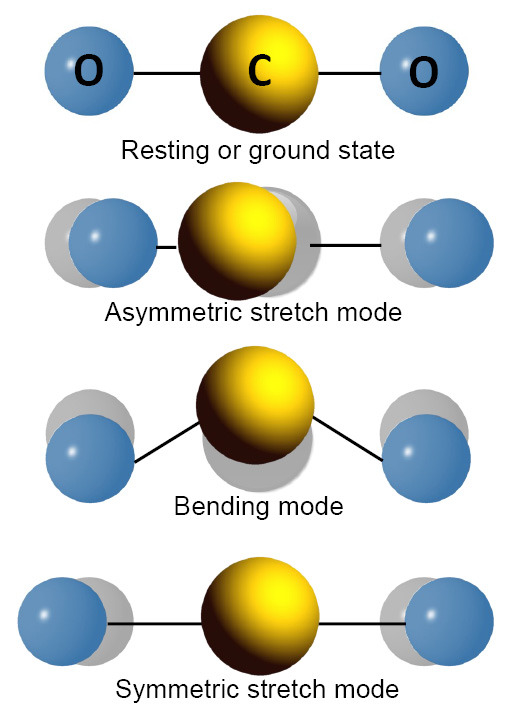
The absorption of carbon dioxide can take place in a number of different ways.
One of the more familiar processes that is thought to allow carbon dioxide absorption is melting ice.
This is a very natural process, however, it can also happen when the Earth is under high pressure or when clouds are blocking the sun’s rays.
While it may seem like a very complicated process to understand, there are a number of technological advancements currently being tested to find better ways of removing carbon dioxide from the air.
For instance, some research is currently being done to develop new scrubbers that can remove carbon dioxide from sewage sludge.
Another way that we can reduce the absorption of carbon dioxide is through venting.
This refers to taking any excess gases outside of a building, such as a store, an industrial plant, or even a home.
A lot of this excess gas is created by businesses when they ventilate. It is estimated that around 20% of all the carbon dioxide in our atmosphere comes from venting.
As technology advances, we can expect this number to drop significantly.
Co2 Molecule – How Does It Relate To Heat Energy Generation?
So you have driven the car long enough and now come to a complete stop. You see a cop car heading your way at high speeds, and you now get to realize that you do not have the right for stopping this car. The cop car stops the car on the side right away. How does this work?
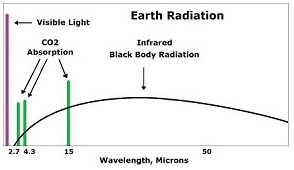
If you put a molecule of pure carbon dioxide together with hydrogen and oxygen atoms, you would soon find that these two would be attracted to each other due to their orbital radii around the nucleus of the carbon dioxide molecule.
The presence of oxygen would push the carbon dioxide molecule outwards thereby making it emit photons in the infrared spectrum.
This absorption of photons by the carbon dioxide molecule would result in emission of infrared light which is detected by infrared cameras or infrared thermometers.
What is so interesting about this phenomenon?
How could the carbon dioxide emission from a working engine possibly be harnessed by man?
If the Carbon Dioxide emission is harnessed, what are its uses?
Well you would find that the carbon dioxide emission from an engine plays a very important role in giving an uphill push towards the speedometer.
By placing the fuel injectors near the engine’s combustion chambers, you can increase the speed at which the fuel burns. Now you will surely understand the importance of understanding the basic chemistry of the universe we live in.
How the Energy From the photon Causes the Voltage in the Fiber
How the energy from the photon changes is described by the Heisenberg constant, H=0.
The photons have a very high frequency, and as they travel through the medium, they emit tiny amounts of energy as they move.
This energy is measured by the Coefficient of Conversion, or CoC.
It is well known that as the frequency of the photons increases, so does the power of the electric field. The higher the Coefficient of Conversion, the stronger the electric field.
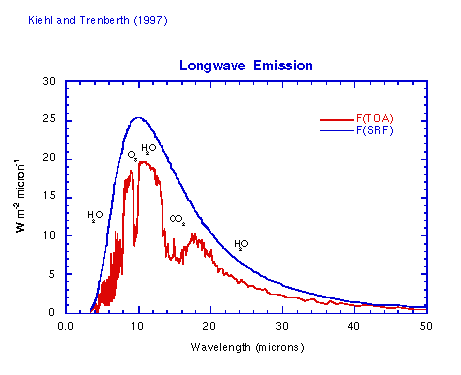
The energy from the photon that we can see and hear is not the only energy that makes up what we call light.
Radio waves, gamma rays, x-rays, and infrared are also absorbed and emitted radiation.
In fact, almost everything has an invisible radiation field. The energy from the photon causes the highest amounts of radiation, and therefore the Coefficient of Conversion is most sensitive to these wavelengths.
When we measure the energy levels, we use two different ways to calculate the energy.
One method is based on how many photons are emitted per second.
The other method measures the wavelength of the photons, and then calculates the amount of energy that is emitted as the product of the wavelength and the energy levels. The result is the amount of energy in cells that can be measured with a spectroscope.
Molecules of Carbon Dioxide
Carbon dioxide molecules of varying density are absorbed by the infrared radiation in the atmosphere.
The absorption is not complete but the infrared energy that is emitted is of very low energy, very much like the light that travels in the ultraviolet rays.
These molecules absorb carbon dioxide and emit infrared energy. Molecules of carbon dioxide that are very similar to the types of molecules that absorb infrared radiation are HHO molecules.
There is no doubt that HHO molecules have a higher affinity for carbon dioxide than ordinary carbon dioxide molecules do.
A lot of research has been done on this subject and a great deal of progress has been made.
It has been proved theoretically that the absorption and emission of infrared radiation from these molecules are actually taking place.
For this to be so, the molecule must have a high electrical charge and it must be polarised. If the polarisation state of the molecule is changed then the absorption and emission of infrared radiation also changes.
If the change in the hydrogen bonding percentage is less than twenty per cent then the carbon dioxide will not absorb any of the infrared energy.
There are many experiments that have been carried out to prove this fact.
All the results show that the presence of HHO molecules increases the absorption and emission of infrared radiation substantially.
Many scientists believe that the emergence of this new kind of molecule is the main reason for the recent global warming scare. Perhaps we need to start concentrating more on the creation of pure HHO molecules to save this planet that we call home.
A Significant Greenhouse Gas is Chlorine and Other greenhouse gases
The greenhouse gases, which are the most important cause for global warming are water vapor, methane, nitrous oxides, and ozone.
Each one of these has a much smaller effect on global warming compared to carbon dioxide.
Although all four are released into the atmosphere, they make up a lesser amount of the gases that are scattered around the globe.
Carbon dioxide is the most important greenhouse gas, but only a small amount of the gases are actually trapped within the Earth’s atmosphere.
Other less effective greenhouse gases include carbon monoxide, chlorofluorocarbons, and nitrous oxide. Each of them has a varying concentration in the atmosphere.
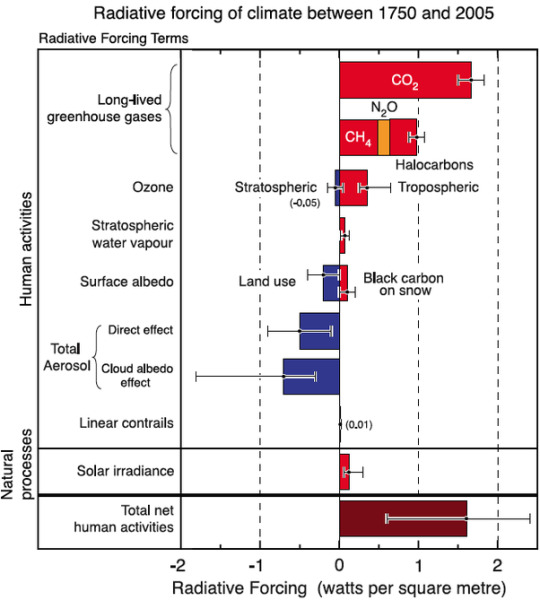
The greenhouse gases that are the most potent have a high concentration in the atmosphere, and thus they are the main cause for global warming.
Water vapor has a high concentration in the atmosphere as well, but its concentration is a bit lower than that of carbon dioxide.
The greenhouse gas methane is a very potent greenhouse gas, as it combines with other elements to form thole gases, which trap heat in the atmosphere.
When there is enough of this gas in the air, it will absorb heat from the sun and send the air rushing through the greenhouse, absorbing heat and slowing down the process of warming up the planet.
A decrease in the concentration of water vapor in the atmosphere will result in an increase in the concentration of methane, which is a more potent greenhouse gas.
A decrease in the concentration of chlorine in the atmosphere will result in an increase in the concentration of hydrogen sulfide.
The presence of sulfur in the atmosphere is what makes HHO gas so potent.
This gas is made when you combine oxygen and hydrogen to produce an unlimited amount of energy.
If this method were used to replace fossil fuels, then we could expect a significant reduction in greenhouse gas emissions.
Have Burning Fossils Watered the Earth’s Climate?
Over the last several decades scientists have concluded that burning fossil fuels have begun to warm the climate of the Earth at a troubling rate.
In fact, recent studies by the National Academy of Sciences show that recent increases in global temperatures are unprecedented in the past history of the planet.
While most people have begun to accept that man has contributed to the rising temperatures of the earth’s climate, very few people seem to know what to do about it.
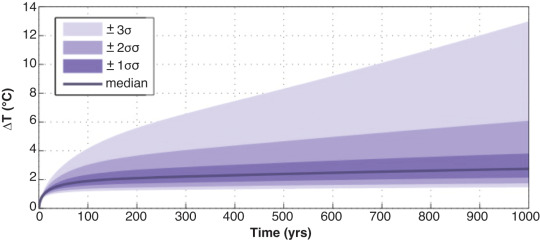
The burning of fossil fuels have begun to warm the Earths climate at a troubling rate.
Man is the cause of all the pollutants which are the major cause of global warming.
These pollutants have also resulted in changing the Earths climate system, which in turn has begun to warm the Earths climate at a troubling rate.
Although man has not altered the composition of the atmosphere, they have been able to alter the way it functions.
This alteration has created problems for plant and animal life.
The burning of fossil fuels have begun to warm the Earths climate at a troubling rate. It is extremely important to utilize alternative sources of energy.
We have discovered that we can produce clean, plentiful energy from wind, water and sun.
Utilizing these resources will not only save our environment, but it will also save our lives. If we continue to utilize the burning of fossil fuels as our source of energy, we will soon discover that they will not be around much longer.
The Problem of Excess Ozone Emissions From Human Activities
Excess emissions of carbon dioxide and other greenhouse gases from human activities are a major cause for concern.
Carbon dioxide is a naturally occurring gas that is essential for life but has harmful effects upon the environment if it is released into the atmosphere in high concentration.
This is true both in direct and indirect ways.
Direct emissions are those which are produced directly by the burning of fossil fuels like petrol, natural gas, coal and so on.
Indirect emissions occur through alteration of the earths atmospheric condition through the use of chemical means such as through carbon dioxide released into the atmosphere or through the exhaust of vehicles running on roads.
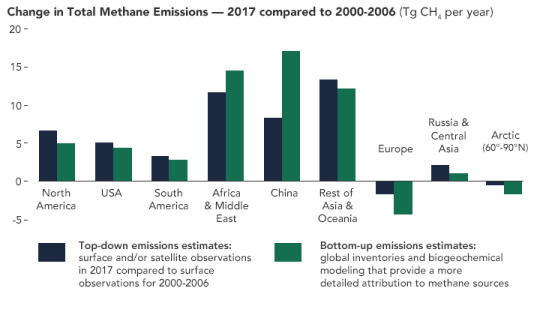
There are many other greenhouse gases that are released from human activity. methane and chlorofluorocarbons are two such gases, which are believed to be the cause of ‘the greenhouse effect’.
These two substances are considered to be the result of the ‘greater efficiencies of chemical reaction’ i.e. byproducts of chemical reactions are formed.
Other substances believed to be the result of these reactions include nitrous oxides, carbon dioxide and water vapor.
The problem of greenhouse gases is not evenly spread around the world.
Although Africa has relatively high levels of emissions, Europe and Asia-Pacific have low ones.
Asia-Pacific countries, in particular, have been identified as major emitters of carbon dioxide, methane and chlorofluorocarbons.
Developed economies have been identified as the main cause of carbon dioxide emissions from land, ocean and atmosphere.
Thus, a joint effort by governments in Asia-Pacific and other developing countries can only bring a solution to the problem of greenhouse gases by reducing greenhouse gas emissions.
youtube
Why Greenhouse Gas Emissions Is So Important – And What Can Be Done To Overcome The Problem?
Without greenhouse gases our planet would be a frozen ball of lava that would have no liquid surface. But we have greenhouse gases, so we need to use them.
In fact, if we do not use them today then sometime in the future they will.
For most of us it is a very important issue as the average earth year is less than one hundred years.
We have already committed ourselves to exhaust all natural fossil fuels which are currently being used to extract oil and gas, and this would only take us to around the twenty-first century if we continue to use these resources.

How would we solve the problem of how to live without greenhouse gases by having “green house” technology?
I am sure you have heard of carbon sequestration by using water and charcoal to create a kind of vacuum.
Another solution would be to build a “geothermal power plant” where the sun shines and heat is trapped.
Or maybe we could simply design better buildings so that the warm air from the sun does not escape and warm the interior of the house.
This would not solve the problem but it might make a difference.
Some think that if we go big like going solar or building huge facilities that we can save the planet. Maybe we could, but what about all the other problems with global warming, like the ice caps melting and ocean pollution.
It seems to me, for our long term survival, to not go down that path. I believe we should try to find other means to allow the growth of greenhouse gases without depleting the earth’s supply.
youtube
Information on the Relationship Between CO2 and Climate Change
Theory of everything states that the human body is composed of molecules. These molecules are made up of amino acids, lipids, proteins and small inert gases called free radicals.
One of the most perplexing questions in the chemistry of the human body is how carbon dioxide is able to become part of some organic compounds and become part of a different kind of molecule.
Theoretical chemists believe that it is due to the interaction between co2 molecules and the infrared photon, which make these co2 molecules highly reactive to the infrared energy.
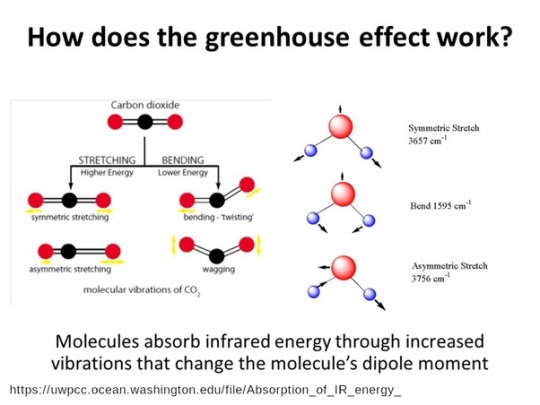
The existence of co2 molecules was demonstrated experimentally in the 1970s with the use of gas sensors.
These sensors could detect the presence of co2 molecules in the greenhouse gases. There are predictions that further studies will confirm that co2 is indeed one of the major drivers behind global warming.
Global warming is believed to be one of the greatest challenges humanity has ever faced. The sooner we can solve this problem, the better for future generations.
If you are concerned about global warming and its effects on future generations, the study of the role of CO2 molecules in affecting climate change should interest you.
What Is Nitrogen?
Infrared is a type of radiation that is actually invisible to the human eye, but when it is absorbed it produces heat. We can detect this heat by looking at infrared spectrums, which are emitted when the atmosphere is warmed by the sun or by lightning.
It has been calculated that every square meter area of the earth contains at least five billionths of a molecule of nitrogen, which is roughly the same amount as the gas nitrogen gas found in stars and on the earth’s moon.
This means that nitrogen is very common, and that it accounts for about eighty percent of the earth’s atmosphere.
Since nitrogen is the dominant element in the atmosphere, scientists have speculated that a planet might be capable of supporting life.
Nitrogen is one of the four gases emitted during combustion, along with oxygen and carbon dioxide. It has many uses and is vital to plant growth and development.
Nitrogen is used for curing agricultural problems by encouraging the growth of more plants and slowing down plant photosynthesis so that more nitrogen is present in the soil for food production.
Nitrogen is also used in the process of fossil fuel extraction, since it forms part of the composition of fossil fuels.
Fossil fuel is also said to contain a large amount of nitrogen and scientists believe that nitrogen is responsible for the warming of the earth caused by the earth’s core.
Although nitrogen has many important uses it is difficult to extract from certain types of foods, and this means that most of the nitrogen in the diet comes from vegetables and fruits.
Greenhouse gases like carbon dioxide do play an important role in climate change, greenhouse gas emissions are the main cause of global warming.
In fact the earth absorbs carbon dioxide from the air, and this process happens constantly.
However, it is the absorption of nitrogen from the air that leads to the greenhouse effect.
How Absorption and Reception of Infrared Energy Make Co2 an Effective Greenhouse Gas
A new study by NASA and the NASA Center for Infrared Earth Science has revealed that absorption of carbon dioxide and its subsequent release makes a large portion of greenhouse gases in the atmosphere.
Carbon dioxide is said to be the largest contributor to global warming.
The absorption and the emission process is said to occur when layers of soil carbon dioxide become hydrated through a process called capillary adsorption.
When this happens, this process allows carbon dioxide to absorb infrared energy from the surrounding environment, which in turn makes carbon dioxide absorb energy and move out into the atmosphere.
The study says that this process is also used in the production of vitamin D, one of the most important natural antioxidants to prevent health problems such as obesity, heart disease and skin ageing.
The ability to absorb and to emit infrared energy makes CO2 a very effective greenhouse gas.
It is also capable of absorbing other greenhouse gases, such as methane, which has been widely recognised as a major cause of global warming.
It is estimated that methane may already be in the atmosphere at such high levels that it is currently contributing to climate change.
The ability to absorb and to emit infrared energy makes CO2 extremely powerful greenhouse gas, making it an essential part of our efforts to reduce greenhouse gases and climate change.
There are other greenhouse gases, which are said to be curbing the effects of global warming. methane is believed to be the number 1 most important greenhouse gas and is released as a result of the digestion of plants.
This means that planting of trees and changing land use can both reduce methane emissions.
One of the ways in which you can reduce greenhouse gas emissions is by planting more trees. In addition to being nature’s green energy, plants are capable of storing up carbon dioxide for many years and in doing so they offer a long-term solution to storing up carbon dioxide.
The post Why Does Oxygen Not Absorb Infrared Radiation? appeared first on Infrared for Health.
source https://infraredforhealth.com/why-does-oxygen-not-absorb-infrared-radiation/
0 notes
Text
7 Ways to Improve Your Health Through Proper Breathing
"When you can't breathe, nothing else matters," is a popular saying among asthma sufferers, alluding to the desperation of someone who can't inhale the life-giving air. Is breathing all about the life-giving air? This article examines common breathing advice and explains why it works. It goes even farther by supplying crucial factors in breathing technique for improved health.
Breathing is one of the most centrally integrated autonomous behaviors, involving far more than just filling the lungs. In 2011, Garcia AJ writes:
"Breathing is triggered by complicated network connections involving neurons all over the neurological system. The respiratory rhythm producing network is made up of micro-networks that operate within bigger networks to generate distinct breathing rhythms and patterns."
When a person is confronted with powerful emotions such as fear or wrath, the results of Garcia's research are most visible.
The conventional breathing advice is to override autonomous control and actively inhale deeply through the nose and exhale gently through the mouth with pursed lips.
Dr. Carla Naumburg Ph.D., author of "Ready, Set, Breath," suggests that breathing exercises might help you be more attentive in your daily life. By remembering to breathe, a space is established that allows for the restoration of calm and the reduction of blood pressure and stress hormones, allowing for situation control.
Professor Konstantin Buteyko (Russia, 1923-2003) is credited with inventing a breathing technique that combines slow, reduced breathing with spaced periods of no breathing, allowing Carbon Dioxide to build up until bursting point.
Yoga practice includes a lot of breathing. Yoga breathing techniques are frequently combined with a variety of positions or a kind of meditation. As a result, it's tough to pinpoint the cause of the result and ascribe it just to the breathing, poses, or meditation.
Pandit JJ studied three breathing methods for maximum oxygen uptake in 2003:
For three (3) minutes, do tidal breathing
2. In under 30 seconds, take four (4) deep breaths.
3. For 60 seconds, take eight (8) deep breaths.
Item 1 and 3 exhibited similar oxygen uptake and efficacy to Item 2, but Item 2 had a higher efficacy. His work emphasizes the necessity of proper breathing.
Nitric oxide (NO), a colorless gas, has a short half-life. Nitric oxide (NO) was selected "molecule of the year" by Science Magazine in 1994.
Furchgott, PhD, Ferid Murad, MD, PhD Robert F, and Louis J. Ignarro, PhD, were awarded the Nobel Prize in Physiology or Medicine in 1998 for their discoveries of the role of Nitric Oxide (NO) as a signaling molecule in the cardiovascular system.
NO relaxes the smooth muscle in arteries, allowing more blood to flow through, lowering blood pressure and delivering more nutrients to where they are required. It is impossible to overestimate the relevance of NO in human biological functions. Despite the fact that thousands of study papers have been created, world research continues. NO has been linked to heart health, reduced blood pressure, improved sleep quality, and even erectile dysfunction.
NO is produced in the sinuses, with the maxillary sinuses on either side of the nose being the largest. Except for a little soft-tissue aperture called the ossium, which opens the olfactory airways, they are closed chambers.
There is no correct or incorrect method to breathe; the autonomous brain activity ensures that you get enough oxygen. There are, however, techniques to breathe in order to get the most NO into your system. Here are seven tips to help you get this wonderful gas into your system.
1. INHALE QUICKLY THROUGH YOUR NOSE.
A negative pressure in the airways is maintained by nose hair and restricted nose ducting. The sinuses transmit a little amount of NO-laden air into your inhaled breath as a result of the partial vacuum. The more NO the sinuses deliver, the harder you breathe in.
2. BREATHE IN AND BLOCK ONE NOSTRIL.
By blocking one nostril and then the other, a partial vacuum is created, allowing NO-laden air to be pumped into your inhaled breath.
3. BLOCK BOTH NOSTRILS WHILE ATTEMPTING TO BREATHE IN.
Close both nostrils and take a deep breath. This creates the most vacuum in your respiratory system, allowing NO-laden air to be drawn out of your sinuses. You may only do this for a brief period of time before returning to normal breathing.
4. EXHALE CAREFULLY THROUGH YOUR MOUTH.
It takes time for NO to enter your bloodstream. As a result, it is beneficial to hold your breath for as long as possible. Exhale gently instead to give the lungs time to absorb the NO.
5. SING OR HUM
In 2003, Lundberg et colleagues discovered that humming increases exhaled NO by 700%. During humming, other researchers discovered an even larger rise in exhaled NO. The issue is that inhaling while humming is tough. As a result, the recommended sequence is to hum for 3 seconds before inhaling.
6. ACT AS IF YOU'RE SLEEPING
To overcome the problem of humming and inhaling at the same time, it is suggested that you pretend to snore by generating a snoring sound. The natural frequencies of the maxillary sinuses are around 110 to 350 Hz, which correspond to the snoring sound frequencies. When the maxillary sinuses are allowed to resonate, NO-laden air is pulsated into the inhaled breath volume. Because I snore,
7. MANOEUVRE DE VALSALVA
The Valsalva manoeuvre is frequently used to avoid headaches during a descent in an airplane. This maneuver is shutting both nostrils and trying to exhale until the ear drums 'snap.' This pressurizes the sinuses, which then release the pressure and inject NO-laden air into the olfactory airways with successive inhalation.
FAQ's
A. NO is a finite resource in the sinuses that can be exhausted. What can be done to replenish it? Eat plenty of nitrate-rich foods, such as beets, fenugreek, and other legumes, and give your body time to convert the nitrates to NO.
B. Why not inhale NO gas, as is done for babies with pulmonary hypertension? In a medical environment, the dosage of NO is carefully monitored. NO has induced lethargy, unconsciousness, and death in animals.
C. Why not sit in a heavily trafficked place and inhale the NO produced by automobiles? NO is present in the exhaust emissions of automobiles. Exhaust gases, on the other hand, are a hazardous combination of various gases, including Carbon Monoxide. The risk of poisoning outweighs any potential benefits.
We discovered a necessity to verify supply is according to the following after roughly 5 years of buying women's clothes from China, India, Thailand, Bangladesh, and Indonesia:
• No child labor is used.
• No Azo dyes (cancer-causing colors)
• No harsh chemicals that are damaging to the environment are used in the processing.
• Materials made from sustainable natural resources
• Fabric with a built-in fire-resistance
We took it a step further and asked ourselves the following question:
What can we add to our line of apparel to make it more beneficial to the wearer's health?
We came up with some surprising results. Keep a close eye on it.
Aerobics Cardio from Blogger https://ift.tt/3gMJous via IFTTT
0 notes
Text
TAFAKKUR: Part 136
A Miraculous Molecule: Hemoglobin
Do you know what hemoglobin does? You should, because it has been perfectly created to keep you alive!
Hemoglobin is one of the miraculous molecules in the human body. While its most important function is to carry oxygen in the blood, it also plays a role in maintaining the body’s acid-base balance. It also carries carbon monoxide in the blood, though to a much lesser amount than oxygen. Hemoglobin is not yet free to roam in the blood; it is carried in red blood cells, which act as hemoglobin sacs. Red blood cells have almost no other function but to carry hemoglobin. They lose all their organelles, including the nucleus, to be able to carry more hemoglobin – and hence, our oxygen.
Hemoglobin is produced in the bone marrow by combining the “hem” molecule with the “globin” molecule. The hem part is produced in the mitochondria of the red blood cell. Two succynil coenzyme “A”s and two glycine amino acids are combined to form pyrrole. Four pyrroles are then combined to form protoporphyrin, which is combined with the iron atom to produce the hem. Four hems are combined with four globins, a kind of protein, to produce one hemoglobin molecule. Since each hem has one iron atom, every hemoglobin has four iron atoms. And since an oxygen molecule binds with each iron atom, every hemoglobin can carry a total of four oxygen molecules – or eight oxygen atoms.
The makeup of hemoglobin along with its production steps is very complicated and it features a precise and intricate design.
Hemoglobin functions much like a truck that hauls oxygen. The blood circulates between the lungs and tissues thanks to the continuous work of the heart. As hemoglobin moves through the lungs it binds with oxygen and as it flows through tissues it releases the oxygen.
For this process to work, the bond between oxygen and hemoglobin can be neither too strong nor too weak. If it were too strong, oxygen would not be able to break free in the tissues, and the tissues would go without oxygen. If the bond were too weak, hemoglobin would not be able to bind with enough oxygen in the lungs, in which case the tissues would again not get oxygen.
There are basically two kinds of hemoglobin. The first type is found in fetuses, and it is called fetal hemoglobin (Hb-F). The other is found in adult humans and is called adult hemoglobin (Hb-A). Before being born, the fetus gets oxygen from the mother’s womb. To get more oxygen from the mother, fetal hemoglobin is designed to bind more strongly with oxygen. One is tempted to ask: Because fetal hemoglobin binds so strongly with oxygen, will the fetus’ tissues not be deprived of oxygen? Yet there is no need to worry. Because there is less oxygen in fetuses than in adult humans, this different hemoglobin easily breaks free from the oxygen in the low-oxygen environment. After the baby is born, the body produces Hb-A instead of Hb-F because it starts to breathe through its own lungs.
Hemoglobin is charged with carrying 97% of the oxygen carried in the blood. 3% of the oxygen is carried in dissolved form in plasma. Because an increase in dissolved oxygen leads to oxygen poisoning, it is not desirable at all. If one breathes from a tube containing 100% oxygen instead of atmospheric air containing 80% nitrogen, the amount of dissolved oxygen in the blood increases, causing oxygen poisoning.
Hemoglobin is designed to store some oxygen, too. At rest, there is 20 ml of oxygen in the hemoglobin of 100 ml of arterial blood. Only 5 milliliters of this oxygen will be given to cells. The remaining 15 milliliters remains in the hemoglobin. In other words, not all oxygen in the hemoglobin is transferred to the body’s tissues. This is a security measure against possible risks. In the event that blood does not come from the lungs, this stored oxygen is used so that life can continue, though for a short time. The same situation is experienced when we do not breathe for a long time.
Exercise
During exercise or work that requires physical effort, the amount of oxygen demanded by the body increases twenty-fold. The heart works faster as does circulatory system. Hemoglobin is supposed to get 20 times more oxygen from the lungs so that it can take 20 times more oxygen to the cells. Body temperature increases, too. Hemoglobin is designed to respond to all these changes. During exercise, hemoglobin starts to give almost all the oxygen to the tissues. When it gets back to the lungs, it is like an empty truck and can load more oxygen.
The exercising person breathes more deeply and quickly; thus, the lungs work faster, the heart pumps blood with greater force and speed, and the design of the hemoglobin is just good enough to carry more oxygen to the tissues.
Inflammatory diseases
Cellular metabolism speeds up when a person has an inflammatory disease. More oxygen is needed because the chemical reactions in cells gain speed. The body’s temperature increases; so, too, the amount of acids and carbon dioxide in the blood due to increase in metabolic rate. In addition, the amount of a very important molecule in the blood increases. This molecule is called diphosphoglycerate. It is charged with protecting the cell during inflammatory diseases and preventing cell death. Diphosphoglycerate coaxes hemoglobin to send more oxygen to the body’s tissues. As we can see, the body has been perfectly created to ensure it receives enough oxygen, even during illnesses!
Transfer of carbon dioxide
Hemoglobin is also assigned the task of carrying carbon dioxide. Carrying carbon dioxide in the blood is easier than carrying oxygen, as carbon dioxide is twenty times more water-soluble than oxygen. Doctors, therefore, do not have to make an extra effort to reduce the amount of carbon dioxide in blood, and it is enough to give oxygen to patients with breathing problems. If carbon dioxide were not easy to dispose of, we would face an enormous problem, because there is no way of disposing of carbon dioxide and it is virtually impossible to develop one.
A vast amount of the carbon dioxide in the blood (70%) is carried in the form of bicarbonate. First, carbon dioxide combines with water, as a result of which carbonic acid, and then bicarbonate, are produced. After these reactions, carbon dioxide hides in bicarbonate (HCO3) and arrives at the lungs. Then bicarbonate combines with H+, by which carbonic acid (H2CO3) is made. Then water (H2O) and carbon dioxide (CO2 ) are produced. The carbon dioxide that breaks up from the bicarbonate is discharged through the lungs into the atmosphere. 7% of the dissolved carbon dioxide in the blood is carried in plasma and 23% in hemoglobin. That is to say, as hemoglobin travels from the lungs to the cells, it carries oxygen, and as it returns from the cells it loads off some of the carbon dioxide.
Acid-base balance
The pH value of human blood is 7.4, on average. It is vital for cells that the pH of the blood remain stable. The regulation of the acid (H+) ion concentration in body fluids is called the acid-base balance. Little changes in H+ ions cause enormous changes in cellular chemical reactions. The regulation of the balance of H+ ions is therefore crucial for the body’s internal balance.
Hemoglobin plays an important role in the acid-base balance of blood. If strong acids find their way into the bloodstream because of certain diseases, hemoglobin help prevent an increase in the amount of acid (and a decrease in pH) by binding with the acid or carbon dioxide. A similar role is also true for times when the amount of base rises.
When we study hemoglobin and the chemical substances and reactions it is involved in, we can see that they are all arranged extremely precisely, and everything is in its proper place.
#allah#god#muhammad#prophet#sunnah#hadith#quran#ayah#islam#muslim#muslimah#hijab#help#revert#convert#religion#reminder#dua#salah#pray#prayer#welcome to islam#how to convert to islam#new muslim#new revert#new convert#revert help#convert help#islam help#muslim help
4 notes
·
View notes