#U.S. pharmaceutical analytical testing outsourcing market size
Explore tagged Tumblr posts
Text
The U.S. pharmaceutical analytical testing outsourcing market size was exhibited at USD 4.50 billion in 2023 and is projected to hit around USD 9.07 billion by 2032, growing at a CAGR of 8.1% during the forecast period 2023 to 2032.
0 notes
Text
The U.S. pharmaceutical analytical testing outsourcing market size was exhibited at USD 4.50 billion in 2023 and is projected to hit around USD 9.07 billion by 2032, growing at a CAGR of 8.1% during the forecast period 2023 to 2032.
0 notes
Text
Bioanalytical Testing Services Market Outlook: Key Drivers Shaping the Future of Pharmaceutical and Healthcare Research
The global bioanalytical testing services market size is anticipated to reach USD 7.93 billion by 2030 and is projected to grow at a CAGR of 9.0% from 2024 to 2030, according to a new report by Grand View Research, Inc.An increasing rate of outsourcing R&D activities by several biopharmaceutical companies to streamline their core competencies is one of the major factors supporting the market growth. In addition, the outbreak of COVID-19 has further boosted demand for pharma-analytical testing services as the rapid surge in SARS-CoV-2 infection cases has significantly augmented the production of a larger batch of COVID-19 vaccines across the globe.
Furthermore, the growing demand for pharmaceutical products across the globe has resulted in a surge of pipeline drugs that require bioanalytical testing for development, thus augmenting the market growth. In addition, the market is expected to witness considerable growth over the forecast period owing to an increasing number of contract manufacturing companies expanding their development capacities across the globe. For instance, in September 2023, Cerba HealthCare announced the acquisition of CIRION BioPharma Research, a Canadian contract research laboratory, to expand its bioanalytical capabilities and reduce the time required for implementing intricate clinical trials.
Increasing awareness about product quality, environmental issues, and the safety of consumers are driving companies to outsource tests as a means to ensure that their products comply with the aforementioned aspects. This helps them build brand image and protects as well as enhances reputation. The U.S. FDA has conducted workshops to increase awareness regarding bioanalytical testing and related regulations.
Key operating companies are undertaking various strategic initiatives to strengthen their market presence. For instance, in August 2023, Pace Analytical Services announced the acquisition of Alpha Analytical, adding new capabilities such as advanced hydrocarbon analytical support along with expanded sediment & tissue testing. Moreover, in April 2022, Charles River Laboratories acquired Explora BioLabs, a prominent player in contract research services, for USD 295 million in cash. This has broadened the company’s operating capabilities in the market.
Bioanalytical Testing Services Market Report Highlights
Small molecule segment accounted for the largest revenue share of over 55.6% in 2023. The high demand for generic drug development boosts the segment growth
Large moleculesegment is anticipated to witness a significant CAGR of 9.61% during the forecast period. This is attributed to the increased investment and focus on the development of biopharmaceuticals
The bioequivalence segment is estimated to witness the fastest CAGR during the forecast period. This is attributed to the growing production and consumption of generics & biosimilars
The sample preparation segment held a significant market share in 2023 and is estimated to expand further at the fastest CAGR from 2024 to 2030
North America was the largest regional market in 2023 due to the strong presence of several pharmaceutical players in the U.S.
On the other hand, the market in Asia Pacific is projected to register the fastest CAGR from 2024 to 2030 owing to increasing pharmaceutical & biotechnology activities, rising healthcare expenditure
Bioanalytical Testing Services Market Segmentation
Grand View Research has segmented the global bioanalytical testing services market based on molecule, test, workflow, and region:
Bioanalytical Testing Services Molecule Outlook (Revenue, USD Billion, 2018 - 2030)
Small Molecule
Large Molecule
LC-MS Studies
Immunoassays
PK
ADA
Others
Others
Bioanalytical Testing Services Test Outlook (Revenue, USD Billion, 2018 - 2030)
ADME
In-Vivo
In-Vitro
PK
PD
Bioavailability
Bioequivalence
Others
Bioanalytical Testing Services Workflow Outlook (Revenue, USD Billion, 2018 - 2030)
Sample Preparation
Protein Precipitation
Liquid-Liquid Extraction
Solid Phase Extraction
Sample Analysis
Hyphenated technique
Chromatographic technique
Electrophoresis
Ligand Binding Assay
Mass Spectrometry
Nuclear Magnetic Resonance
Other Workflow Processes
Bioanalytical Testing Services Application Outlook (Revenue, USD Billion, 2018 - 2030)
Oncology
Neurology
Infectious Diseases
Gastroenterology
Cardiology
Other Applications
Bioanalytical Testing Services End Use Outlook (Revenue, USD Billion, 2018 - 2030)
Pharma & BioPharma Companies
CDMO
CRO
Bioanalytical Testing Services Regional Outlook (Revenue, USD Billion, 2018- 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Netherlands
Switzerland
Asia Pacific
India
China
Japan
South Korea
Australia
Thailand
Indonesia
Malaysia
Singapore
Taiwan
Latin America
Brazil
Mexico
Argentina
Colombia
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
Israel
List of Key Players
ICON Plc
Thermo Fisher Scientific Inc (PPD, Inc.)
Charles River Laboratories International
Covance, Inc.
IQVIA
Syneos Health
SGS SA
Labcorp (Toxikon)
Intertek Group Plc
Pace Analytical Services LLC
Order a free sample PDF of the Bioanalytical Testing Services Market Intelligence Study, published by Grand View Research.
0 notes
Text
U.S. Pharmaceutical Analytical Testing Outsourcing Market Worth $6.8 Billion By 2030
U.S. Pharmaceutical Analytical Testing Outsourcing Market Worth $6.8 Billion By 2030
U.S. Pharmaceutical Analytical Testing Outsourcing Market Growth & Trends The U.S. pharmaceutical analytical testing outsourcing market size is expected to reach USD 6.8 billion by 2030, according to a new report by Grand View Research, Inc. The market is anticipated to expand at a CAGR of 8.0% from 2022 to 2030. Technological advancements in the healthcare industry and an increase in end-users…
View On WordPress
0 notes
Text
U.S. Pharmaceutical Analytical Testing Outsourcing Market Worth $6.8 Billion By 2030
U.S. Pharmaceutical Analytical Testing Outsourcing Market Worth $6.8 Billion By 2030
The U.S. pharmaceutical analytical testing outsourcing market size is expected to reach USD 6.8 billion by 2030, according to a new report by Grand View Research, Inc. The market is anticipated to expand at a CAGR of 8.0% from 2022 to 2030. Technological advancements in the healthcare industry and an increase in end-users are the key factors driving the growth of the U.S. market. Analytical…
View On WordPress
0 notes
Text
Knowledge Process Outsourcing (KPO) Market Statistical Forecast and Competitive Analysis Report till 2025
June 22, 2021: The global Knowledge Process Outsourcing (KPO) market size was valued at USD 28.94 billion in 2016 and is anticipated to reach USD 124.29 billion by 2025. The huge availability of data is one of the key factors that have enabled the growth as it needs to be analyzed and processed. The KPO market also observed the transformation of the market into a consultative model. Though, rules set by several governments regarding compliance and legal clauses are one of the major challenges faced by the global KPO market.
With global perceptions on outsourcing business activities, companies are looking for more specialist and analytical expertise, hence KPOs are seen to be gradually fulfilling such requirement of enterprises that are keen to outsource third party service providers. With competitive market scenario and increasing globalization the time for commercialization of services and products are thinning down. For example, in BFSI capital markets, with the modification in demand for complex global products, investment managers are needed for making faster strategic decisions. This has driven the demand for middle office outsourcing as it has improved from cost driven abilities to strategic platforms, thus letting investment managers to perform business strategies easily.
With respect to end users, the global market for KPO is segmented into Healthcare, BFSI, and Retail. Healthcare sector is the fastest growing segments in the outsourcing for knowledge intensive services. Bio-tech and pharmaceutical are the sub industries of healthcare segment for drug discovery and clinical research. Types of services carried out by KPO include consultancy, research and development, legal and medical services, intellectual property research for patent applications, market research, business research, and training.
Request a Free Sample Copy of this Report @ https://www.millioninsights.com/industry-reports/knowledge-process-outsourcing-kpo-market/request-sample
Geographically the global market for KPO is segmented into North America, Asia Pacific, Middle East and Asia, Europe and Latin America. Emerging economies such as Latin America and Asia Pacific have been able to appeal KPO service providers owing to the advantages such as favorable business environment, availability of trained professionals combined with high productivity, and low labor costs. These advantages are not prevailing in developed countries, and as a result, many companies are setting up businesses in developing markets.
The future forecasts for KPO in India are immense as KPO is applicable to several industry sectors in which India’s technically educated professionals and highly skilled workers have developed particularized expertise. These sectors include engineering, law, education, publishing, media, entertainment, industrial machinery, textiles, automotive, aerospace, software, electronics, insurance, biotechnology, healthcare, pharmaceuticals, and finance.
Although India has traditionally been a KPO destination for Australian, British and North American companies, a growing number of European companies are looking to Eastern Europe, especially the Baltic countries, to satisfy their KPO needs. Several U.S. businesses have already made successful ventures into the KPO field in India to leverage India’s knowledge class including Caterpiller, General Motors, Ford, United Airlines, Morgan Stanley, Reuters, Goldman Sachs, McKinsey, Citigroup, JP Morgan, Motorola, Philips, Sun Microsystems, Texas Instruments, Cisco, Oracle, Intel, Microsoft, HP, IBM, and GE. In the pharmaceutical industry, global pharmaceutical firms such as Eli Lilly, GlaxoSmithKline, AstraZeneca, Novartis, and Pfizer have moved part of their clinical drug testing to India to tap India’s diverse and vast population of highly skilled and low wage demanding labors. This can considerably accelerate the time to market for new drugs, and offers potential cost savings of up to 50.0 % relative to the U.S.
The key vendors for global KPO market include Wipro, RR Donnelley & Sons, Genpact, Mphasis, while other prominent vendors include Cognizant, Adventity, Syntel, The smart cube, Mu Sigma, Aranca, Accenture, EXL Services, Inductis, Infosys, McKinsey, Pangea3, Pulsar knowledge center, Oracle, SAP, TechBooks, Grail Research, Moody's, TCS, WNS, EXL Service, Pipal Research, RocSearch, and IP Pro.
Browse Full Research Report @ https://www.millioninsights.com/industry-reports/knowledge-process-outsourcing-kpo-market
Genpact are a global leader in digitally powered business process management and services. They architect the Lean Digital enterprise through their patented Smart Enterprise Processes framework that reimagines their clients operating models end-to-end, including the back and middle offices. This creates intelligent operations that help in transforming, designing and running. The impact on clients is a high return on investments through business agility, growth and efficiency.
Market Segment:
Service Outlook (Revenue, USD Million; 2014 - 2025)
• Analytics & Market Research
• Customer & Marketing Analytics
• Operations & Supply Chain Analytics
• Competitive Intelligence & Industry Analysis
• Engineering & Design
• Financial Process Outsourcing
• Legal Process Outsourcing
• Publishing Outsourcing
• Research & Development Outsourcing
• Others
Application Outlook (Revenue, USD Million; 2014 - 2025)
• BFSI
• Healthcare
• IT & Telecom
• Manufacturing
• Pharmaceutical
• Retail
• Others
Regional Outlook (Revenue, USD Million; 2014 - 2025)
• North America
• U.S.
• Canada
• Europe
• Germany
• UK
• Asia Pacific
• China
• India
• Philippines
• Latin America
• Brazil
• Middle East & Africa
Get in touch
At Million Insights, we work with the aim to reach the highest levels of customer satisfaction. Our representatives strive to understand diverse client requirements and cater to the same with the most innovative and functional solutions.
Contact Person:
Ryan Manuel
Research Support Specialist, USA
Email: [email protected]
0 notes
Text
Clinical Reference Laboratory Market 2021 Analysis by Size, Trends, Growth, Regional Demands, Top Companies, Segmentation and Forecast by 2027
Market Synopsis
The global clinical reference laboratories market is considered to demonstrate 5.8% CAGR during the forecast period (2016-2027) owing to the usage of advanced robotics technology in reference laboratories, asserts Market Research Future (MRFR). Reference laboratories are referred to as commercial as well as private facilities which perform high-volume and specialty testing routinely. Physicians’ clinics, offices, government agencies, hospitals, pharmaceuticals, insurance companies, and biotechnology companies generally outsource the specialty to reference laboratories.
Free Sample Copy With Considering Coivd19 Impact on this Market @https://www.marketresearchfuture.com/sample_request/1190
Drivers and Constraints Impacting the Market
The global clinical reference laboratories market is progressively growing and is estimated to continue its growth in the coming years. With adoption of value-based services gaining prominence, hospitals are outsourcing some part or even all of their radiology and pathology diagnostic services. One of the major reasons for rising outsourcing by the hospitals is the constrained budgets due to Medicare reimbursements. The cut down in the rates of clinical laboratories reimbursements under Medicare has impacted the U.S. lab industry.
The U.S. clinical labs which depend on Medicare for 20% of their billings and for certain communities it is 80% of the business, got affected. To protect the hospitals, CEOs are outsourcing value-based services. Such factors have augmented the market for these independent reference laboratory services. Moreover, technologies which are capable of performing repetitive activities at high speed has developed to improve the consistency, quality, and effectiveness. The test results on samples which used to consume several hours to process can be found in a few minutes. Automation has impacted histopathology and microbiology to a great extent. Development of molecular technologies and genetics into the mainstream medicine being the most significant innovation, has impacted the health of the population, management of patients, and clinical practice. Moreover, launch of cost-effective, time-saving, and new diagnostic procedures owing to the accelerating number of FDA approvals is considered to contribute to the market growth.
On the flip side, illegible and incorrect sample labelling are some of the major factors considered to slowdown the market growth during the appraisal period.
Global Clinical Reference Laboratories Market: Segmental Analysis
The global clinical reference laboratories market has been segmented on the basis of service providers and application.
By mode of service providers, the global clinical reference laboratories market has been segmented into hospital based, stand alone, and clinical based. Among these, the stand-alone service providers are presumed to dominate the global market. With rising opportunities, presence of a new-range of condition-specific markers, availability of advanced technologies, and tests with advances in proteomics and genomics are some of the key factors likely to drive the segment.
By mode of application, the global clinical reference laboratories market has been segmented into clinical trials, laboratory medicine, and others.
Regional Insights
Geographically, the clinical reference laboratories market spans across regions namely, Europe, America, Asia Pacific, and the Middle East & Africa.
Among all the regions, America is considered to be the largest market for clinical reference laboratories. North America with countries such as Canada, and the United States lead the global market.
The European region occupies the second-largest share of the global market and is anticipated to continue its dominance in the coming years. The growth is credited to the increasing geriatric population, rising private and public funding for clinical laboratory test research, and growing incidences of chronic diseases. On the other hand, Asia Pacific is anticipated to expand at the fastest rate during the assessment period.
Industry Updates
January 18, 2019: One of the leading healthcare bio-analytic solution providers, Invitrocue has recently announced its first laboratory agreement in Germany in order to make its Onco-PDO personalized cancer screening service which will be available to the physicians and patients in Germany.
Competitive Dashboard
The prominent players operating the global clinical reference laboratories market are Quest Diagnostics, Clinical Reference Laboratory, ARUP Laboratories, Sonic HealthCare Limited, Unilabs, Laboratory Corporation of America, Bioreference Laboratories, KingMed Diagnostics, Aurora Diagnostics, Synlab International GmbH, and others.
Click here to get the short-term and long-term impact of COVID-19 on this Market: https://www.marketresearchfuture.com/reports/clinical-reference-laboratories-market-1190
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services.
MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by Components, Application, Logistics and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions.
In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.
Contact:
Market Research Future
Office No. 528, Amanora Chambers
Magarpatta Road, Hadapsar,
Pune – 411028
Maharashtra, India
+1 646 845 9312
Email: [email protected]
NOTE : Our team of researchers are studying Covid19 and its impact on various industry verticals and wherever required we will be considering covid19 footprints for a better analysis of markets and industries. Cordially get in touch for more details.
0 notes
Text
Metagenomic Sequencing Market To Witness Astonishing Growth With Key Players
The global Preclinical Cro market was valued at US$ xx million in the year 2019. The market is estimated to be valued at US$ xx million in the year 2020, and is expected to reach US$ xx million by the year 2027, with an estimated CAGR of xx% during the forecast period (2021-2027). The research study also includes exhaustive information about market dynamics such as drivers, restraints, opportunities, technological advancements, future trends, supply chain analysis, patent landscape, pricing analysis, regulatory and reimbursement framework for precise market estimations and forecasts.

Additionally, the business intelligence study encompasses the COVID-19 scenario as follows:
· Long-Term, Mid-Term, and Short-Term Impact of COVID-19
· Pre- and Post-COVID-19 Scenario
· Disruptions in the Supply Chain
Get Free PDF Brochure Of This Report @ https://www.fostermarketresearch.com/product/industry/10/339/Pdf%20Brochure/
Competitive Insights
Leading and emerging companies of the global Preclinical Cro market were identified based on their current offerings. Further, the Preclinical Cro industry is one of the most competitive industries, with the leading players actively competing against each other to gain a greater share in the industry. Some of the major companies in the Preclinical Cro market are: Envigo, WuXi AppTec, IQVIA, MD Biosciences, ICON PLC, Pharmaceutical Product Development, Parexel International Corporation, PRA Health Sciences, Charles River, Laboratory Corporation of America Holdings, Eurofins Scientific, and Medpace, among others.
The competitive landscape of the Preclinical Cro industry exhibits an inclination towards emerging strategies including product launches, partnerships, collaborations and contracts, acquisitions, funding, and other developments to achieve the objectives faster.
Our reports fill up the gaps and provide you with all the detailed analysis of competitive companies involving:
· Analyzing competitive strategies and techniques
· Competitive positioning of key players
· PORTER’s and SWOT analysis for competitive risk analysis
· Competitive Share Analysis
· Financial analysis of players to determine their withstand capacity
· Analyzing their sales path and product study
For More Information Of This Report @ https://www.fostermarketresearch.com/product/industry/10/339/
Key Segments Covered in the global Preclinical Cro market report are:
· By Service: Toxicology Testing, Bioanalysis & Drug Metabolism & Pharmacokinetics (DMPK) Studies, and Others
· By Application: Oncology, Central Nervous System (CNS) Disorders, Cardiovascular Diseases, Respiratory Diseases, Immunological Disorders, Diabetes, Infectious Diseases, and Others
· By End User: Medical Device Companies, Pharmaceutical and Biopharmaceutical Industries, and Academic Institutes
Regional Coverage:
The global Preclinical Cro market segregates into five regions including North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa. North America followed by Europe held the major share of the global market (in terms of value) in 2020. However, Asia-Pacific region exhibit highest CAGR (%) during the forecast period (2021-2027). Our research study further sub-divides regions into countries as follows:
· North America - the U.S. and Canada
· Europe - Germany, the U.K., France, Spain, Italy, Russia, and Rest-of-Europe
· Asia-Pacific - China, India, Japan, South Korea, Australia, ASEAN, and Rest-of-Asia-Pacific
· Latin America - Mexico, Brazil, and Rest-of-Latin America
· Middle East and Africa - Gulf Cooperation Council (GCC) Countries, South Africa, Israel, and Rest-of-Middle East and Africa
Market Dynamics:
The preclinical CRO market has been significantly benefited by the thrive in research and development expenditure, outsourcing non-core functions, and surging the number of drugs in the preclinical phase. The companies largely depend on the preclinical CRO and outsourcing to develop the drugs due to the efficacy of safety before launching it in the market. For instance, in July 2016, the American Pharmaceutical Review stated that about 55% of the services were outsourced by the emerging companies in the first phase of the clinical trials, while the medium-sized biotech and pharmaceutical companies outsourced close to 69% in the second phase of the clinical trials.
Buy Now This Business Strategic Report To Improve Your Profits @ https://www.fostermarketresearch.com/product/buy/10/339/
About Foster Market Research:
Foster Market Research is a global market intelligence and advisory firm engaged in providing data-driven research extract from rigorous analysis, to the clients to make critical business decisions and execute them successfully. Foster connects over various distribution channels and numerous markets for great understanding of the trends and market to deliver our clients with accurate data.
Our focus is on providing market research that delivers a positive impact on your business. We work continuously to provide our clients with the most accurate analytics data and research reports without any delay so as to improve their business strategies and provide them with rich customer experience.
Contact Us:
1701 Royal Lane,
#1306, Dallas
Tx-75229
Phone: +1 469 4981929
Email:
0 notes
Text
Knowledge Process Outsourcing Market Analysis, Growth Forecast by Manufacturers, Regions, Type and Application to 2025
15th March 2021 – The global Knowledge Process Outsourcing (KPO) market size was valued at USD 28.94 billion in 2016 and is anticipated to reach USD 124.29 billion by 2025. The huge availability of data is one of the key factors that have enabled the growth as it needs to be analyzed and processed. The KPO market also observed the transformation of the market into a consultative model. Though, rules set by several governments regarding compliance and legal clauses are one of the major challenges faced by the global KPO market.
With global perceptions on outsourcing business activities, companies are looking for more specialist and analytical expertise, hence KPOs are seen to be gradually fulfilling such requirement of enterprises that are keen to outsource third party service providers. With competitive market scenario and increasing globalization the time for commercialization of services and products are thinning down. For example, in BFSI capital markets, with the modification in demand for complex global products, investment managers are needed for making faster strategic decisions. This has driven the demand for middle office outsourcing as it has improved from cost driven abilities to strategic platforms, thus letting investment managers to perform business strategies easily.
With respect to end users, the global market for KPO is segmented into Healthcare, BFSI, and Retail. Healthcare sector is the fastest growing segments in the outsourcing for knowledge intensive services. Bio-tech and pharmaceutical are the sub industries of healthcare segment for drug discovery and clinical research. Types of services carried out by KPO include consultancy, research and development, legal and medical services, intellectual property research for patent applications, market research, business research, and training.
Access Knowledge Process Outsourcing (KPO) Market Report with TOC @ https://www.millioninsights.com/industry-reports/knowledge-process-outsourcing-kpo-market
Geographically the global market for KPO is segmented into North America, Asia Pacific, Middle East and Asia, Europe and Latin America. Emerging economies such as Latin America and Asia Pacific have been able to appeal KPO service providers owing to the advantages such as favorable business environment, availability of trained professionals combined with high productivity, and low labor costs. These advantages are not prevailing in developed countries, and as a result, many companies are setting up businesses in developing markets. The future forecasts for KPO in India are immense as KPO is applicable to several industry sectors in which India’s technically educated professionals and highly skilled workers have developed particularized expertise. These sectors include engineering, law, education, publishing, media, entertainment, industrial machinery, textiles, automotive, aerospace, software, electronics, insurance, biotechnology, healthcare, pharmaceuticals, and finance.
Although India has traditionally been a KPO destination for Australian, British and North American companies, a growing number of European companies are looking to Eastern Europe, especially the Baltic countries, to satisfy their KPO needs. Several U.S. businesses have already made successful ventures into the KPO field in India to leverage India’s knowledge class including Caterpiller, General Motors, Ford, United Airlines, Morgan Stanley, Reuters, Goldman Sachs, McKinsey, Citigroup, JP Morgan, Motorola, Philips, Sun Microsystems, Texas Instruments, Cisco, Oracle, Intel, Microsoft, HP, IBM, and GE. In the pharmaceutical industry, global pharmaceutical firms such as Eli Lilly, GlaxoSmithKline, AstraZeneca, Novartis, and Pfizer have moved part of their clinical drug testing to India to tap India’s diverse and vast population of highly skilled and low wage demanding labors. This can considerably accelerate the time to market for new drugs, and offers potential cost savings of up to 50.0 % relative to the U.S.
The key vendors for global KPO market include Wipro, RR Donnelley & Sons, Genpact, Mphasis, while other prominent vendors include Cognizant, Adventity, Syntel, The smart cube, Mu Sigma, Aranca, Accenture, EXL Services, Inductis, Infosys, McKinsey, Pangea3, Pulsar knowledge center, Oracle, SAP, TechBooks, Grail Research, Moody's, TCS, WNS, EXL Service, Pipal Research, RocSearch, and IP Pro. Genpact are a global leader in digitally powered business process management and services. They architect the Lean Digital enterprise through their patented Smart Enterprise Processes framework that reimagines their clients operating models end-to-end, including the back and middle offices. This creates intelligent operations that help in transforming, designing and running. The impact on clients is a high return on investments through business agility, growth and efficiency.
Request a Sample Copy of Knowledge Process Outsourcing (KPO) Market Report @ https://www.millioninsights.com/industry-reports/knowledge-process-outsourcing-kpo-market/request-sample
0 notes
Text
Contract Research Outsourcing Market Share & Analysis, By Product Type, By End Use, Forecasts to 2026
The Global Contract Research Outsourcing Market report gives a comprehensive overview of the Contract Research Outsourcing market scenario to present accurate forecasts of the upcoming years with special focus on the competitive landscape, market segmentation, current and emerging trends, and strategic recommendations to help readers gain a robust footing in the market. The report also focuses on the comprehensive analysis of the competitive landscape along with descriptive company profiles, market share, product portfolio, financial standings, market reach, global position, and strategic business expansion plans. Market Size – USD 38.18 Billion in 2018, Market Growth - CAGR of 7.4%, Market Trends – Product launches and research for advanced Contract Research Outsourcing Market

The report is furnished with the latest market scenario pertaining to the COVID-19 pandemic and its after-effects on the Contract Research Outsourcing industry and the key segments. The pandemic has disrupted the workflow of the industry and created financial difficulties. The report assesses the complete impact of the pandemic on the market and offers key insights into the market scenario along with trends and demands disruptions. The report also offers an outlook on the market scenario in the forecast timeline.
Get the sample copy of the report @ https://www.reportsanddata.com/sample-enquiry-form/1846
Some of the players profiled in the report are Charles River Laboratories, Parexel International, IQVIA, Covance, PRA Health Sciences, LabCorp, Pharmaceutical Product Development (PPD), Clinitec, ICON plc, Medidata Solutions, and EPS International.
The Global Contract Research Outsourcing Market is segmented as follows:
Product Type (Revenue, USD Million; 2016–2026)
Early Phase Development Services
Clinic Research Services (CRS)
Laboratory Services
Physical Characterization
Toxicology Testing
Stability Testing
Raw Material Testing
Other Analytical Testing
Consulting Services
Pharmacokinetics/Pharmacodynamics (PK/PD)
Batch Release Testing
Others
Discovery Studies
Chemistry, Manufacturing & Control (CMC)
Preclinical Services
Phase I CRS
Phase II CRS
Phase III CRS
Phase IV CRS
Bioanalytical Testing
Analytical Testing
Therapeutic Area Type (Revenue, USD Million; 2016–2026)
Oncology
Immunological Disorders
Central nervous system (CNS) Disorders
Cardiovascular Diseases
Infectious Diseases
Respiratory Disorders
Diabetes
Other
End Use (Revenue, USD Million; 2016–2026)
Pharmaceutical and Biopharmaceutical Companies
Medical Device Companies
Academic Institutes
Others
Request a discount on the report @ https://www.reportsanddata.com/discount-enquiry-form/1846
Market Segmentation by Regions:
North America (U.S., Canada)
Europe (U.K., Italy, Germany, France, Rest of EU)
Asia Pacific (India, Japan, China, South Korea, Australia, Rest of APAC)
Latin America (Chile, Brazil, Argentina, Rest of Latin America)
Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of MEA)
Key Point Summary of the Report:
The global Contract Research Outsourcing market research report is an investigative study offering key insights into the latest growth trends, developments, technological and product advancements, and the research and development scenario. The report also covers the market aspects that directly influence the growth of the market. These features include strategies undertaken by the prominent players, their expansion tactics, and the product portfolios of the companies, and micro and macro-economic factors.
Access complete report description, toc, chart, etc.@ https://www.reportsanddata.com/report-detail/contract-research-outsourcing-market
The study also analyses the crucial market aspects, including R&D, product launches and brand promotions, mergers and acquisitions, collaborations, joint ventures, and the growth pattern on both regional and global levels. The report offers an in-depth evaluation of factors such as cost, capacity, rates of production and consumption, gross revenue, profit margin, demand and supply ratio, import/export, market share, market size, and market trends.
The Global Contract Research Outsourcing Market includes relevant and verified information relating to the overall market, key players, and their market position and financial standing. The report utilizes advanced analytical tools such as SWOT analysis, Porter’s Five Forces Analysis, investment return analysis, and feasibility analysis to offer a comprehensive view of the market position of the major players of the industry.
Request customization of the report @ https://www.reportsanddata.com/request-customization-form/1846
To summarize everything stated above, the report offers key insights into the Contract Research Outsourcing market to allow the reader to gain a complete understanding of the Global Contract Research Outsourcing Market through accurate estimations, a panoramic view of the market scenario, competitive landscape, factors influencing the growth of the market, driving factors, restraints, regulatory framework, growth prospects and opportunities, and factors propelling the market forward. The research study offers an in-depth view of the industry to offer a competitive edge to the reader and help them in formulating beneficial investment plans. The report provides a comprehensive overview of the market with facts relating to the past, present, and future of the Global Contract Research Outsourcing Market.
Thank you for reading the report. The report can be customized as per the requirements of the clients. For further information or queries about customization options, please reach out to us, and we will offer you the report best suited for your needs.
About Us:
Reports and Data is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target and analyze consumer behavior shifts across demographics, across industries and help client’s make a smarter business decision. We offer market intelligence studies ensuring relevant and fact-based research across a multiple industries including Healthcare, Technology, Chemicals, Types, and Energy. We consistently update our research offerings to ensure our clients are aware about the latest trends existent in the market.
Contact Us:
John W Head of Business Development Direct Line: +1-212-710-1370 E-mail: [email protected] Reports and Data | Web: www.reportsanddata.com News: www.reportsanddata.com/market-news Connect with us: Facebook | Google+ | LinkedIn | Twitter
0 notes
Text
Global Pharmaceutical Testing and Analytical Services Market Research Report 2021 Analysis and Forecast 2027
Global Pharmaceutical Testing and Analytical Services Market is valued approximately USD 3.50 billion in 2019 and is anticipated to grow with a healthy growth rate of more than 11.00 % over the forecast period 2020-2027. Pharmaceutical testing and analytical services serve a variety of purposes and used to identify the chemical composition and molecular structure of each individual substance contained in a medication. This approach is mainly used to provide insights into a drugs performance and safety considerations. Also, these services can predict the medications ability to reach to specific target within a body. Presently, the outbreak of coronavirus has put an immense pressure on life sciences sector to innovate in treatments and vaccines. Also, there is an unprecedented demand for technical support pertaining to finished product and raw material testing. As a result, companies in the pharmaceutical testing and analytical services market are meeting demand pertaining to the discovery and development of small-molecule drugs and vaccines. Also, the key players are focusing on strategies such as product launch, innovation and merger & acquisition to sustain themselves amidst fierce competition. Growing need for the development and cost reduction of core competencies by pharmaceutical companies and the rising acceptance of outsourcing would drive the growth of the market during the forecast period of 2020-2027. As per Pharmaceutical Research and Manufacturers of America report of 2018, biopharmaceutical research companies in U.S. are developing 200 medicines for heart diseases, 537 medicines for neurological disorders, 260 vaccines enhancing research and development activities. As per the Canadian Government, total research and development expenditure in scientific research by Canada accounted for $7,612 million in 2017/2018. Hence, pertaining the above facts, OBRC analysis finds North America as the second most favorable region for investment over the forecast period (2017-2024). However, the lack of skilled professional is hampering the growth of the market during the forecast period of 2020-2027.
Get Sample Copy of the Report @https://www.wiseguyreports.com/sample-request/6071396-global-pharmaceutical-testing-and-analytical-services-market-size
The regional analysis of global Pharmaceutical Testing and Analytical Services market is considered for the key regions such as Asia Pacific, North America, Europe, Latin America and Rest of the World. North America is the leading/significant region due to the well-established healthcare infrastructure and familiarity with the regulatory scenario. Also, the dominance of the region is witnessed owing to the growing need for the development and cost reduction of core competencies by pharmaceutical companies. Whereas Asia-Pacific is also anticipated to exhibit highest growth rate / CAGR over the forecast period 2020-2027.
Major market player included in this report are: Eurofins Scientific Laboratory Corporation of America Holdings Charles River Laboratories International, Inc. SGS S.A Intertek Group plc RD Laboratories, Inc WuXi AppTec Co., Ltd. DYNALABS LLC (Infinity Laboratories) ARL Bio Pharma
The objective of the study is to define market sizes of different segments & countries in recent years and to forecast the values to the coming eight years. The report is designed to incorporate both qualitative and quantitative aspects of the industry within each of the regions and countries involved in the study. Furthermore, the report also caters the detailed information about the crucial aspects such as driving factors & challenges which will define the future growth of the market. Additionally, the report shall also incorporate available opportunities in micro markets for stakeholders to invest along with the detailed analysis of competitive landscape and product offerings of key players. The detailed segments and sub-segment of the market are explained below:
By Service Type: Bioanalytical Testing Method Development & Validation Raw Material Testing Stability Testing Microbial Testing Others
By End-User: Medical Device Companies Pharmaceutical & Biopharmaceutical Companies Others
By Region: North America U.S. Canada Europe UK Germany France Spain Italy ROE
Asia Pacific China India Japan Australia South Korea RoAPAC Latin America Brazil Mexico Rest of the World
Furthermore, years considered for the study are as follows:
Historical year - 2017, 2018 Base year - 2019 Forecast period - 2020 to 2027
Target Audience of the Global Pharmaceutical Testing and Analytical Services Market in Market Study:
Key Consulting Companies & Advisors Large, medium-sized, and small enterprises Venture capitalists Value-Added Resellers (VARs) Third-party knowledge providers Investment bankers Investors
Continuous...
For further information on this report, visit -https://www.wiseguyreports.com/reports/6071396-global-pharmaceutical-testing-and-analytical-services-market-size
CONTACT US:
Wiseguy Research Consultants
+44 203 500 2763
+1 62 825 80070
0 notes
Text
U.S. Pharmaceutical Analytical Testing Outsourcing Market Worth $6.8 Billion By 2030
U.S. Pharmaceutical Analytical Testing Outsourcing Market Worth $6.8 Billion By 2030
U.S. Pharmaceutical Analytical Testing Outsourcing Market Growth & Trends The U.S. pharmaceutical analytical testing outsourcing market size is expected to reach USD 6.8 billion by 2030, according to a new report by Grand View Research, Inc. The market is anticipated to expand at a CAGR of 8.0% from 2022 to 2030. Technological advancements in the healthcare industry and an increase in end-users…
View On WordPress
0 notes
Text
Contract Research Outsourcing Market Size, Share, Industry Growth, Trend, Business Opportunities, Challenges, Drivers and Restraint Research Report by 2026
Rising number of R&D outsourcing activities, growing demand and number of clinical trials, emphasis on developing novel products, emphasis on R&D activities, Elevated demand for Specialized testing services, technological innovations and development, and emerging new markets in Asia Pacific are key factors contributing to high CAGR of Contract Research Outsourcing Market during forecast period.
the global Contract Research Outsourcing market was valued at USD 38.18 Billion in 2018 and is expected to reach USD 68.14 Billion by the year 2026, at a CAGR of 7.4 %.
The study covers Contract Research Outsourcing – An organization that offers comprehensive services to biotechnology, pharmaceutical, and medical devices industries. Contract Research Outsourcing offers a wide range of support services like project management, clinical trial data management, database design & build, preclinical research, biopharmaceutical development, data entry & validation, biologic assay development, medicine and disease coding, safety and efficacy summaries, and others.
Get a sample of the report @ https://www.reportsanddata.com/sample-enquiry-form/1846
The report initially offers market introduction, market overview, product scope, market opportunities, growth prospects, risks, limitations, and key drivers. The report also covers a comprehensive analysis of the competitive landscape with extensive profiling of the key competitors. It covers recent technological advancements, product developments, product launches, mergers and acquisitions, collaborations, joint ventures, and partnerships.
Key Companies in the market include:
Charles River Laboratories, Parexel International, IQVIA, Covance, PRA Health Sciences, LabCorp, Pharmaceutical Product Development (PPD), Clinitec, ICON plc, Medidata Solutions, and EPS International.
Regional segmentation comprises of a current and forecast estimation of the market in the key geographical regions such as North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. The report also covers the scope of individual applications and types in each region. The report also covers details about production and consumption patterns, technological developments, revenue growth, market size, market share, key trends and demands influencing market growth in the region, and robust presence of key players in the region.
Market segment analysis:
Product Type (Revenue, USD Million; 2016–2026)
Discovery Studies
Chemistry, Manufacturing & Control (CMC)
Preclinical Services
Phase I CRS
Phase II CRS
Phase III CRS
Phase IV CRS
Bioanalytical Testing
Analytical Testing
Therapeutic Area Type (Revenue, USD Million; 2016–2026)
Oncology
Immunological Disorders
Central nervous system (CNS) Disorders
Cardiovascular Diseases
Infectious Diseases
Respiratory Disorders
Diabetes
Other
End Use (Revenue, USD Million; 2016–2026)
Pharmaceutical and Biopharmaceutical Companies
Medical Device Companies
Academic Institutes
Others
Request a discount on the report @ https://www.reportsanddata.com/discount-enquiry-form/1846
Regional analysis further covers a country-wise analysis to offer insights into key trends and demands in each major country that might affect the growth of the market in the region.
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
U.S.
Canada
Mexico
Germany
U.K.
Italy
France
BENELUX
Rest of Europe
China
India
Japan
South Korea
Rest of APAC
Brazil
Rest of LATAM
Saudi Arabia
A.E.
South Africa
Rest of MEA
To know more about the report @ https://www.reportsanddata.com/press-release/global-contract-research-outsourcing-market
Key Features of the Global Contract Research Outsourcing Market Report:
Comprehensive analysis of the key players operating in the market along with their SWOT analysis, their business profiles, business overview, market share, global position, and market value
Identification and analysis of significant trends and factors driving revenue growth of the market
Analysis of the competitive landscape along with strategic mergers, expansions, agreements, partnerships, joint ventures, acquisitions, and product launches
Assessment of each market segment along with their growth trends and market revenue contribution
Study of the key regions to pinpoint growth potential and study opportunities, threats, limitations, and risks
Request a customization of the report @ https://www.reportsanddata.com/request-customization-form/1846
Thank you for reading our report. Customization of this report is available according to the client’s needs. Please get in touch with us to know more about the customization feature and our team will ensure the report is tailored as per your requirements.
0 notes
Text
GMP Cell Banking Services Market – Insights
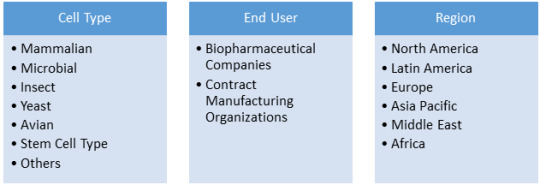
Cell banking involves storing of cells of specific genome for the purpose of future use in a product or medicinal needs. Mammalian cells, microbial cells, insect cell lines, yeast cells, avian cells, and stem cells are some of the cells that are stored in cell banks for various purposes. Mammalian cells are isolated from specific tissues such as skin, liver, and glands for production of vaccines and various proteins.
Statistics:
The global GMP cell banking services market is estimated to account for US$ 1,326.8 Mn in terms of value by the end of XXX.
Global GMP Cell Banking Services Market: Drivers
Increasing funding for R&D in rare diseases is expected to boost growth of the global GMP cell banking services market over the forecast period. For instance, the U.S. Food and Drug Administration (FDA) funds research in rare diseases through congressionally mandated programs such as the Orphan Products Grants Program that supports natural history studies and clinical trials for rare diseases.
Moreover, establishment of stem cell banking resource centers is also expected to aid in growth of the market. For instance, in March 2020, Stemlife Berhad, a cord blood bank in Malaysia, started a Stem Cell Banking Resource Center in Jerudong Park Medical Center, Brunei.
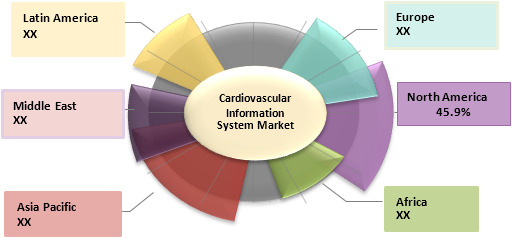
North America region held dominant position in the global GMP cell banking services market in 2019, accounting for 45.9% share in terms of value, followed by 2027.
Figure 1. Global GMP Cell Banking Services Market Share (%), by Region, 2019
Global GMP Cell Banking Services Market: Restraints
Stringent regulatory requirements are expected to hinder growth of the global GMP cell banking services market. Quality of the master and working cell banks must be monitored and confirmed on a continuous basis to justify the continuation of the production processes. The data collected from these analytical tests is a regulatory requirement for maintaining the biologic license and distribution of marketed product. The European Medicines Agency (EMA) and the U.S. FDA, in collaboration with other regulatory agencies worldwide, formed the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), which set forth guidelines for good manufacturing practices. Specifically, ICH guidelines Q5, Q6 and Q7 are applicable to cell banks used in production processes.
Moreover, high costs of services due to nature of material is also expected to limit growth of the market. The costs involved in GMP cell banking services are considerably high, due to the complex processes involved in generation, storage, maintenance, and testing of GMP cell banks and expanses in regulatory adherence.
Global GMP Cell Banking Services Market: Opportunities
Several pharmaceutical and biotechnology companies are increasingly seeking full service in the form of global partners that can be outsourced the entire manufacturing process. This in turn is expected to offer lucrative growth opportunities for players in the market.
Moreover, focus on fast growing emerging markets is also expected to aid in growth of the market. There has been a shift from investment in R&D and technology, especially in the healthcare industry, from North America and Europe to Asia Pacific. Developing economies such as India have ample skilled labor and qualified manpower at affordable costs, thereby increasing the scope for outsourcing of biotech projects.

Biopharmaceutical Companies segment in the global GMP cell banking services market was valued at US$ 315.4 Mn in 2019 and is expected to reach US$ 993.8 Mn by 2027 at a CAGR of 15.4% during the forecast period.
Market Trends/Key Takeaways
The demand for ready-to-use bioassay banks has significantly increased. Ready-to-use bioassay banks are used in situations where cells are used straight from the vial in the bioassay, thereby eliminating the cell expansion step. Ready-to-use bioassay banks are typically large in size, commonly ranging between 400-1000 vials and have large cell densities per vial.
Major players in the market are focused on adopting M&A strategies to expand their product portfolio. For instance, in January 2020, Charles River Laboratories International, Inc. acquired HemaCare Corporation for around US$ 380 million in cash.
Regulations
U.S.
Section 501(a)(2)(B) of the FD&C Act; 21 CFR parts 210 and 211; and 21 CFR part 600
All manufacturing facilities to ensure compliance with cGMP regulations
cGMP includes the implementation of oversight and controls over the manufacture of drugs to ensure quality, including managing the risk of and establishing the safety of raw materials, materials used in the manufacturing of drugs, and finished drug products.
21 CFR 211.22(d)
Requirement of the quality unit of a pharmaceutical company to monitor adherence to regulations by a CMO
When a pharmaceutical company uses a contract facility, their quality unit is legally responsible for approving or rejecting drug products manufactured by the contract facility, including for final release. The regulations require that the quality unit’s responsibilities and procedures be in writing and that they be followed. Quality agreements should clearly describe the materials or services to be provided, quality specifications, and communication mechanisms between the owner and contract facility.
https://www.coherentmarketinsights.com/insight/request-sample/3634
https://www.coherentmarketinsights.com/insight/request-pdf/3634
Global GMP Cell Banking Services Market: Competitive Landscape
Major players operating in the global GMP cell banking services market include, WuXi AppTec Group, Charles River Laboratories International, Inc., Eurofins Scientific, Merck KGaA, Lonza Group Ltd., SGS Ltd., ViruSure GmbH, Austrianova, Goodwin Biotechnology Inc., and Paragon Bioservices, Inc.
Global GMP Cell Banking Services Market: Key Developments
Major players in the market are focused on adopting collaboration strategies to expand their product portfolio. For instance, in March 2020, Lonza collaborated with Stanford University School of Medicine, Fred Hutchinson Cancer Research Center, and Parker Institute for Cancer Immunotherapy for R&D in cell therapy.
Major players in the market are also focused on adopting various marketing strategies to expand their customer base. For instance, in January 2020, Charles River Laboratories International, Inc. presented at the 38th Annual J.P. Morgan Healthcare Conference in San Francisco, California, U.S.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/gmp-cell-banking-services-market-3634
0 notes
Text
Knowledge Process Outsourcing Market Research 2020-2025 Report by Outlook, Demand, Size Estimation and Upcoming Trend
Knowledge Process Outsourcing Market size was valued at USD 28.94 billion in 2016 and is anticipated to reach USD 124.29 billion by 2025. The huge availability of data is one of the key factors that have enabled the growth as it needs to be analyzed and processed. The KPO market also observed the transformation of the market into a consultative model. Though, rules set by several governments regarding compliance and legal clauses are one of the major challenges faced by the global KPO market.
Request a Sample PDF Copy of This Report @ https://www.millioninsights.com/industry-reports/knowledge-process-outsourcing-kpo-market/request-sample
The key driving factors responsible for the growth of Knowledge Process Outsourcing market :
With global perceptions on outsourcing business activities, companies are looking for more specialist and analytical expertise, hence KPOs are seen to be gradually fulfilling such requirement of enterprises that are keen to outsource third party service providers. With competitive market scenario and increasing globalization the time for commercialization of services and products are thinning down. For example, in BFSI capital markets, with the modification in demand for complex global products, investment managers are needed for making faster strategic decisions. This has driven the demand for middle office outsourcing as it has improved from cost driven abilities to strategic platforms, thus letting investment managers to perform business strategies easily.
With respect to end users, the global market for KPO is segmented into Healthcare, BFSI, and Retail. Healthcare sector is the fastest growing segments in the outsourcing for knowledge intensive services. Bio-tech and pharmaceutical are the sub industries of healthcare segment for drug discovery and clinical research. Types of services carried out by KPO include consultancy, research and development, legal and medical services, intellectual property research for patent applications, market research, business research, and training.
Geographically the global market for KPO is segmented into North America, Asia Pacific, Middle East and Asia, Europe and Latin America. Emerging economies such as Latin America and Asia Pacific have been able to appeal KPO service providers owing to the advantages such as favorable business environment, availability of trained professionals combined with high productivity, and low labor costs. These advantages are not prevailing in developed countries, and as a result, many companies are setting up businesses in developing markets.
The future forecasts for KPO in India are immense as KPO is applicable to several industry sectors in which India’s technically educated professionals and highly skilled workers have developed particularized expertise. These sectors include engineering, law, education, publishing, media, entertainment, industrial machinery, textiles, automotive, aerospace, software, electronics, insurance, biotechnology, healthcare, pharmaceuticals, and finance.
Although India has traditionally been a KPO destination for Australian, British and North American companies, a growing number of European companies are looking to Eastern Europe, especially the Baltic countries, to satisfy their KPO needs. Several U.S. businesses have already made successful ventures into the KPO field in India to leverage India’s knowledge class including Caterpiller, General Motors, Ford, United Airlines, Morgan Stanley, Reuters, Goldman Sachs, McKinsey, Citigroup, JP Morgan, Motorola, Philips, Sun Microsystems, Texas Instruments, Cisco, Oracle, Intel, Microsoft, HP, IBM, and GE. In the pharmaceutical industry, global pharmaceutical firms such as Eli Lilly, GlaxoSmithKline, AstraZeneca, Novartis, and Pfizer have moved part of their clinical drug testing to India to tap India’s diverse and vast population of highly skilled and low wage demanding labors. This can considerably accelerate the time to market for new drugs, and offers potential cost savings of up to 50.0 % relative to the U.S.
View Full Table of Contents of This Report @ https://www.millioninsights.com/industry-reports/knowledge-process-outsourcing-kpo-market
0 notes
Text
Chromatography Instrument Market Forecast To 2025| Post Impact Of Worldwide Covid-19 Spread Analysis
Chromatography Instrument Market is Valued at USD 8.04 Billion in 2018 and Expected to Reach USD 12.76 Billion by 2025 with a CAGR of 6.8% Over the Forecast Period.
Chromatography Instrument Market: Global Size, Trends, Competitive, Historical & Forecast Analysis, 2020-2025. Advancement In chromatography technologies and increasing demand in pharmaceutical and biotechnological industries driving the chromatography instrument market.
Scope of Chromatography Instrument Market -
Chromatography instrument is technique to separate various compounds from the natural solution or mixture of sample through the use of multiple chromatographic techniques by using chromatography instruments. This is done by passing the mixture in sample solution through a specific medium in which the components move at different rates. The chromatography instrument comprises of pumps, gels, columns, detectors, and software for the system used among various chromatography types. It comprises separation of biomolecules on the basis of type, size, and other attributes that needs mobile phase, stationary phase, and elutants to successful separation of component. The analytical chromatography is done usually with smaller amounts of material and is for measuring the relative proportions or establishing the presence or of analytes in a sample mixture. However, it is important to know the sample type for choosing the chromatography instruments.
The chromatography instruments have benefits in research due to their ability of separation, analysis, and purification of molecules in an effective manner. The chromatography can be analytical chromatography which is used in environmental labs and preparative chromatography which is used in pharmaceutical industry. The chromatography instruments are used in various industries such as biotechnology, pharmaceuticals, chemical, environmental testing, food and beverages, drug discovery, and semiconductor industries. Moreover, recently the application of chromatography has increased in many fields, especially in pharmaceutical and food and beverage industries. The growing use of chromatography in the pharmaceutical industry is for the separation of chiral compounds. These compounds have molecules that differ slightly in the way their atoms are oriented in space.
Chromatography instrument market report is segmented based on system type, consumable & instruments and end users. Based upon system type, chromatography instrument market is classified into gas chromatography system, ion exchange chromatography, affinity chromatography, super critical fluid chromatography, column chromatography, liquid chromatography system, high pressure liquid chromatography, ultra high pressure liquid chromatography and low pressure liquid chromatography. Based upon consumable & instruments, chromatography instrument market is classified into tubes, detectors, columns, vials, auto samplers, pumps, fraction collectors and others. Based upon end users, the chromatography instrument market is classified into biotechnology and pharmaceutical industries, hospitals, research laboratories and others.
The regions covered in this chromatography instrument market report are North America, Europe, Asia-Pacific and Rest of the World. On the basis of country level, market of chromatography instrument is sub divided into U.S., Mexico, Canada, U.K., France, Germany, Italy, China, Japan, India, South East Asia, GCC, Africa, etc.
Chromatography Instrument Manufactures-
Chromatography instrument market report covers prominent players like Agilent technologies, THERMO- FISHER SCIENTIFIC incorporated, PerkinElmer Incorporated, Shimadzu Corporation, Novasep Holdings S.A.S., PALL corporation, Jasco Incorporated, GL Sciences Incorporated, PHENOMENEX Inc., Waters corporation GE Healthcare, Becton, Sigma-Aldrich Corporation, Dickinson and Company, Phenomenex, Inc., Life Technologies Corporation, Regis Technologies, Tosoh Corporation, Bio-Rad Laboratories, Helena Laboratories, Pall Corporation, VWR International and among others.
Chromatography Instrument Market Dynamics –
The chromatography instruments market has witnessed various technological advancements driving the growth of market. Increasing the demand of advanced chromatography technologies for biotechnology, chemical, biopharmaceutical companies, semiconductor industries, food and beverage and environmental testing. These advancements have led to the development of miniaturized, automated, and computerized devices. Moreover, raised government investments in the last few decades for academic, research institutes and life science industries, are the main source of revenue for the chromatography instruments manufacturers and expected to increase near future fuelling the growth of the chromatography instrument market. Additionally, increased number of CROs and generics owning to increasing demand for drug development and new convenient methods developments are likely to encourage chromatography instrument market growth.
On the other flip, chromatography instruments are significantly high in price and lack of availability of these products due to technological insufficiencies in areas may restrain the market growth. Nevertheless, increasing collaboration of private companies with research institutes biotechnology and pharmaceuticals industries for the development of advancement technologies to gain precious result expected to create the huge opportunity in the market over the forecast period.
Chromatography Instrument Market Regional Analysis –
North America is dominating the chromatography instrument market followed by Europe, due to the presence of key players in the region and expected to continue over the forecast period. Additionally, the increase in funding for R&D activities and environmental testing in the U.S. are some other factors driving the growth of the market in the U.S. Moreover, rise of the Canadian food testing industry and the rising investment and initiatives by the government to improve laboratory infrastructure in Canada will also fuel market growth during the forecast period.
Asia Pacific is anticipated to grow at a highest CAGR over forecast period due to outsourcing of drug & development services, expansion of major chromatographic companies in Asia Pacific is expected to drive the growth of chromatography instruments market in this region. Additionally, recent progression in instrumentation, increasing investment in biotechnology, emerging economic conditions, pharmaceutical and academic and research organizations, and technological advancements in the developing countries of Asia-Pacific. These are factor fueling the growth of market and expected to show same rate near future.
Key Benefits for Chromatography Instrument Market Reports –
Global Market report covers in depth historical and forecast analysis.
Global Market research report provides detail information about Market Introduction, Market Summary, Global market Revenue (Revenue USD), Market Drivers, Market Restraints, Market opportunities, Competitive Analysis, Regional and Country Level.
Global Market report helps to identify opportunities in market place.
Chromatography Instrument Market Segmentation –
By System Type: Gas chromatography system, Ion Exchange chromatography, Affinity Chromatography, Super critical fluid chromatography, Column chromatography, Liquid chromatography system, High pressure liquid chromatography, Ultra high pressure liquid chromatography, Low pressure liquid chromatography, Others
By consumables and instruments: Tubes, Detectors, Columns, Vials, Auto samplers, Pumps, Fraction collectors, Others
By end user: Biotechnology and pharmaceutical industries, Hospitals, Research laboratories, Others
Regional & Country Analysis: North America, U.S., Mexico, Canada , Europe, UK, France, Germany, Italy , Asia Pacific, China, Japan, India, Southeast Asia, South America, Brazil, Argentina, Columbia, The Middle East and Africa, GCC, Africa, Rest of Middle East and Africa.
About Us:
Brandessence Market Research and Consulting Pvt. ltd.
Brandessence market research publishes market research reports & business insights produced by highly qualified and experienced industry analysts. Our research reports are available in a wide range of industry verticals including aviation, food & beverage, healthcare, ICT, Construction, Chemicals and lot more. Brand Essence Market Research report will be best fit for senior executives, business development managers, marketing managers, consultants, CEOs, CIOs, COOs, and Directors, governments, agencies, organizations and Ph.D. Students. We have a delivery center in Pune, India and our sales office is in London.
Contact us at: +44-2038074155 or mail us at [email protected]
0 notes